Abstract
Cutaneous warts are known to be recurrent and often resistant to therapy. Resistant warts may reflect a localized or systemic cell mediated immune (CMI) deficiency to HPV. Many modalities of treatment are in use; most of the provider-administered therapies are destructive and cause scarring, such as cryotherapy, chemical cauterisation, curettage, electrodessication and laser removal. Most patient-applied agents like podophyllotoxin have the risk of application-site reactions and recurrence. Thus immunotherapy is a promising modality which could lead to resolution of warts without any physical changes or scarring and in addition would augment the host response against the causative agent, thereby leading to complete resolution and decreased recurrences. Immunomodulators can be administered systemically, intralesionally or intradermally, and topically. A few agents have been tried and studied extensively such as cimetidine and interferons; others are new on the horizon, such as Echinacea, green tea catechins and quadrivalent HPV vaccine, and their efficacy is yet to be completely established. Though some like levamisole have shown no efficacy as monotherapy and are now used only in combination, other more recent agents require large and long term randomized placebo-controlled trials to clearly establish their efficacy or lack of it. In this review, we focus on the immunomodulators that have been used for the treatment of warts and the studies that have been conducted on them.
Keywords: Cimetidine, imiquimod, immunomodulator, levamisole, resistant, warts
What was known?
Immunotherapy is a promising modality for recurrent and/or resistant warts which could lead to resolution without any physical changes or scarring and in addition would augment the host response against the causative agent, thereby leading to complete resolution and decreased recurrences.
Immunomodulators can be administered systemically, intralesionally or intradermally, and topically.
Some agents like Cimetidine, Levamisole and Zinc have been studied in few randomized trials and their efficacy or lack of it has been established.
Newer immunomodulator agents have been arriving on the scene and studies are required to define their role more clearly.
Introduction
Cutaneous infections caused by human papillomavirus (HPV) are usually recurrent and are among the most troublesome conditions presenting to dermatologists.
HPV causes a myriad of infectious lesions, out of which common warts are the most prevalent. Warts are usually self-limiting but spontaneous resolution may take months to years. Spontaneous clearance rates are also painfully low (23% at 2 months, 30% at 3 months and 65-78% at 2 years), hence underlining the need for intervention.[1] The fact that they can recur even after complete physical removal makes them extremely frustrating both for the patient and the physician. Recalcitrant warts may reflect a localized or systemic cell-mediated immune (CMI) deficiency to HPV. Various reasons like lack of production of memory T cells to target HPV infection, failure of clonal expansion of lymphocytes to adequate stimulation, inability of T lymphocytes to traffic to sites of infection and weak effector response mechanism have been hypothesized.[2] Consequently, warts are particularly exuberant in patients with Hodgkin's disease, AIDS and those on immunosuppressants.
Genital warts pose an even bigger challenge to dermatologists. Firstly, because of the reluctance of patients to consult a physician, and secondly, because of their propensity to relapse. Individuals with frequent relapses suffer a substantial psychological morbidity. Thus, drugs with immune-stimulating properties are potentially useful agents in them.
Many modalities of treatment are in use; most of the provider-administered therapies are destructive and cause scarring, such as cryotherapy, chemical cauterisation, curettage, electrodessication and laser removal. Most patient-applied antimitotic agents like podophyllotoxin have the risk of application-site reactions such as erythema, edema and ulceration. Recurrence rates are also high due to the possibility of some microbial organisms remaining after physical destruction of the visible lesions. Recurrence rates of warts up to 30% have been reported with cryotherapy, probably due to a lack of immune response. Thus, immunotherapy is a potential logical modality which could lead to resolution without any physical changes or scarring and in addition would augment the host response against the causative agent, thereby leading to complete resolution and decreased recurrences. Though many immunomodulators have been tried, none of them has been found to be ideal, due to inadequate sample sizes in studies, impracticality of use, adverse effects or limited efficacy.[3] Hence, the search for the ideal drug still continues! Table 1 lists the various agents that have been tried as immunomodulators in the treatment of warts. However, the treating physician must remember that none of the treatments discussed in the review are FDA approved (except Polyphenon - E) for the treatment of warts.
Table 1.
The various immunomodulatory agents that have been used in the treatment of warts
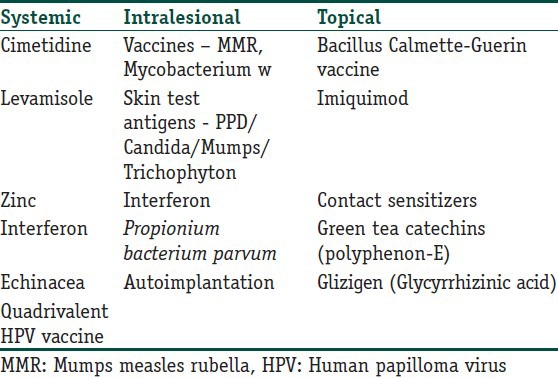
Systemic agents
H2 antagonists (strength of evidence D, I)
H2 receptor antagonists are believed to possess immunomodulatory properties, but only cimetidine and ranitidine have been documented to have clinically significant effects.[4,5,6] Both have been tried, but ranitidine has shown only limited efficacy in a single open-label trial by Karaman et al.[7] At high doses (25-40 mg/kg/d), cimetidine stimulates IL-12 and decreases IL-10, leading to an increase in Th1 response and suppression of Th2 cells.[4,8,9] It also increases IL-2 production thus stimulating lymphocyte proliferation which in turn increases the CMI. It prevents histamine-induced stimulation of T suppressor cells.[5,6]
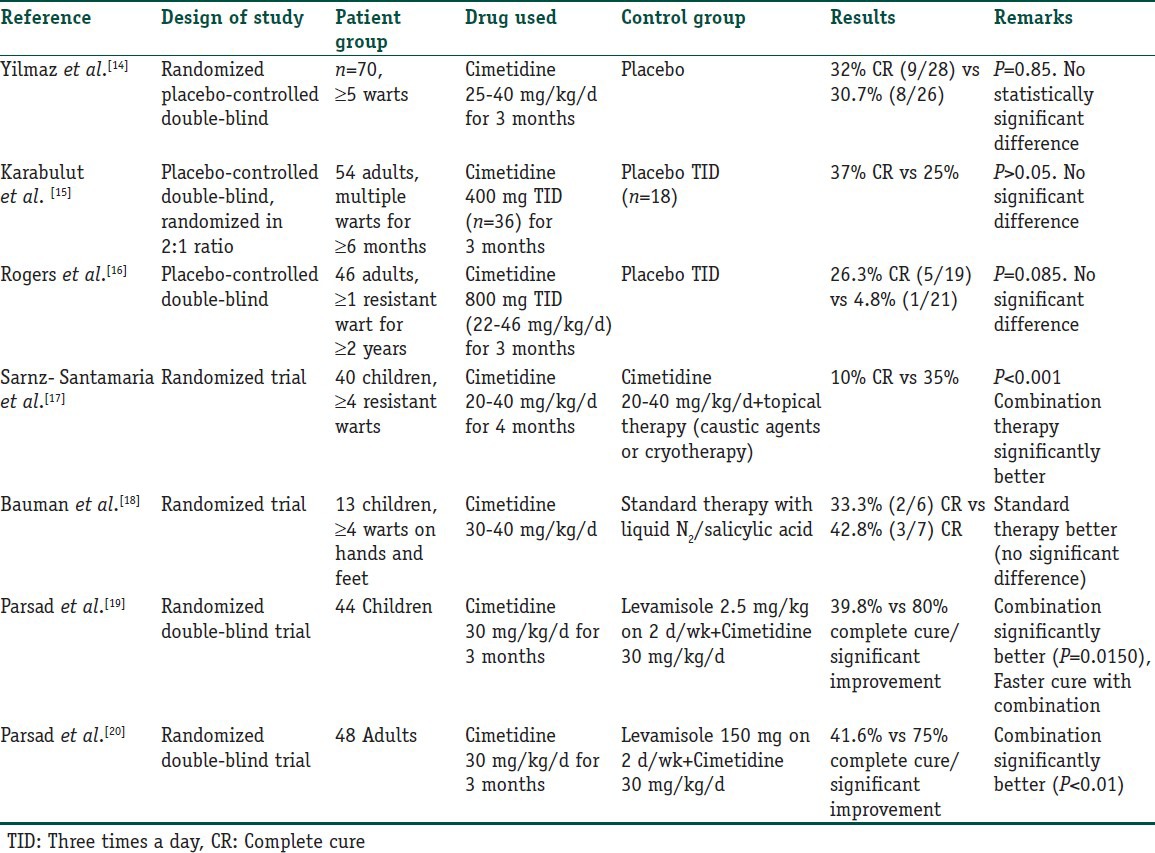
Cimetidine has been used in various trials in warts. Four open label studies were done and reported a complete response (CR) rate ranging from 48.8% to a very impressive 81.8%.[10,11,12,13] However, the randomized placebo controlled trials conducted later [Table 2] did not support such high clearance rates and showed rather disappointing results.
Table 2.
Randomized controlled trials for Cimetidine in the treatment of verruca vulgaris
It has also been used in HSV infections, herpes zoster virus (HZV) in immunocompromised individuals and in mucocutaneous candidiasis and common variable immunodeficiency disease (CVID).[21,22,23,24,25,26,27] Dohil et al. treated 13 children with Molluscum Contagiosum with cimetidine 40 mg/kg/d.[28] Nine of them cleared completely while one showed partial clearance. Facial MC lesions responded more promptly than body lesions.
Its side effects include headache, dizziness, diarrhea, rash, urticaria, alopecia, gynecomastia, breast soreness, arthralgias and myalgias. A higher dose up to 40 mg/kg/day (with a maximum of 3500 mg/day) has been seen to correlate with an increased response in an open label study in adults. The FDA-approved maximum dose is 2400 mg/day, so the risk-benefit ratio has to be clearly evaluated before using higher doses. It is not officially approved in children less than 16 years of age, but continues to be used.[29]
Levamisole
Levamisole is an immunomodulator used in many patients with extensive viral infections such as warts and molluscum contagiosum. It alters polymorphonuclear leukocyte (PMN) chemotactic responsiveness.[30] It stimulates phosphodiesterase breakdown of cyclic AMP while inhibiting destruction of cyclic GMP-this appears to correlate with increased chemotactic responsiveness.[31] An increase in adenosine deaminase and a “scavenger” effect on free radicals are also believed to play a role in its action. It stimulates delayed type hypersensitivity (DTH) preferentially, involving upregulation of Th1 cells and IL-2, 12, IFN-gamma and downregulation of Th2 cells with a concomitant effect on IL-4,5,10.[32] It is prescribed in a dose of 2.5 mg/kg two to three times weekly.
Though in combination with cimetidine it has shown promise in treatment of warts, monotherapy has not. Only one double-blind placebo-controlled trial by Amer et al. in 40 patients with warts treated with 5 mg/kg on 3 consecutive days fortnightly for 5 months showed 60% cure rate (vs 5% for placebo) but other randomized trials have not shown much efficacy (Saul et al., Schou et al.).[33,34,35]
Most common side effects are nausea, taste alteration, rash, alopecia and a flu-like illness. Patients need to be monitored for the rare but potentially fatal agranulocytosis (<20% PMNs), which is reversible initially. The bone marrow is not damaged permanently and patients with HLA-B27 genotype are more susceptible. Cases of multifocal leukoencephalopathy have also been described.[36]
Zinc (strength of evidence - C, II)
Zinc is perhaps the most important trace element for immune function. Kitamura et al. proposed that toll-like receptor (TLR)-mediated regulation of zinc homeostasis influences dendritic cell function.[37] Zinc deficiency has been shown to cause decreased immunity to cutaneous infections.[38,39] It also has specific anti-viral activity; firstly, by cross-linking the double helix of viral DNA so that it is unable to undergo the scission necessary for viral replication, and secondly, by inactivating the viral surface glycoproteins thus interfering with penetration into a susceptible host cell.
Zinc sulfate is the most well-tolerated compound with highest bioavailability. Each 100 mg capsule of zinc sulfate contains 22.5 mg elemental zinc. Side effects are nausea, vomiting and mild epigastric distress.
A placebo-controlled (PC) trial was attempted using oral zinc sulfate 10 mg/kg (2.5 mg/kg/d elemental zinc) once daily for treatment of recalcitrant warts.[40] Complete clearance was seen in 87% of the treatment group versus 0% of the placebo group. In another randomized double-blind placebo-controlled (DB PC) trial, oral zinc sulfate 10 mg/kg/d was given up to 2 months in patients with recalcitrant warts.[41] After 2 months, complete clearance rate was 76.9% (10/13) in the treatment group versus 7.8% (1/13) in the placebo group. The regression of the warts was not asymptomatic as occurs in the natural evolution of the disease. Instead, it was associated with itching, increase in size and number of lesion for the first 2 weeks followed by subsidence. However, Lopez-Garcia et al. conducted a DB PC trial in 50 patients with ≥ 5 resistant warts.[42] They found similar clearance rates with zinc sulfate and placebo (28% vs 24%) and pointed out that none of the patients in either group had low baseline zinc levels. Stefani et al. conducted a randomized double-blind trial in 18 patients where they compared zinc sulfate (10 mg/kg/d) and cimetidine (35 mg/kg/d) for 3 months for resistant warts. They found zinc to be more effective than cimetidine.[43]
Zinc has also been found useful in cutaneous leishmaniasis, recurrent ENL and common variable immunodeficiency.[40,44,45]
Interferon
Interferons (IFNs) are a class of small (15-28 kD) protein and glycoprotein cytokines produced by T cells, fibroblasts, and other cells in response to viral infection and other biologic and synthetic stimuli.[46] As a curative drug, IFN are divided into three major classes (alpha, beta, and gamma) on the basis of physicochemical properties, cells of origin, mode of induction, and antibody reactions.[47] IFN has been shown to be active against HPV by three mechanisms: Antiviral, antiproliferative and immunostimulation.[48,49,50,51] It is reported that IFNs exert their activities mainly by binding to specific membrane receptors on the cell surface and initiating specific intracellular events, including the induction of enzymes, suppression of cell proliferation, enhancement of macrophage phagocytosis, augmentation of lymphocytic cytotoxicity for target cells, and inhibition of virus replication in virus-infected cells.
Yang et al. conducted a systematic review of 12 randomized control studies on IFN in treatment of genital warts, which involved a total of 1445 patients. Five studies compared systemic IFN and placebo.[46] They found that there was no significant difference in the clearance rates of the two groups. They concluded that locally used IFN was more efficacious for genital warts than systemic IFN. The most common reported adverse reaction of systemic IFN was a flu-like syndrome, (the simultaneous occurrence of two of the following: Fever/chills, headache, malaise/fatigue, and myalgias/muscle aches).
Echinacea
Echinacea (purple coneflower) is a member of the compositae family.[52] Three main medically important species are E. purpurea, E. augustifolia and E. pallida. Earlier it was mostly used for prevention and treatment of common cold and upper respiratory tract infections. Coeugni and Kuhnast reported a chance finding of decreased recurrences of vaginal candidiasis during the 6-month monitoring period following treatment of URTIs.[53] It was then tried in cutaneous infections. It influences immune function through T-cell activation, increase in number and activity of macrophages, production of TNF and IFN-γ, and inhibition of hyaluronidase produced by bacteria and viruses.[52]
Zedan et al. compared Propolis (Bee Propolis®) (Pollen Assiut, Egypt) 500 mg, Echinacea purpurea 600 mg and placebo all single oral dose for 3 months or till complete cure for treatment of plane, plantar and common warts.[52] They observed significant difference between Propolis and Echinacea in common and plane warts (P < 0.05 for each) and significant difference between Propolis and placebo in common and plane warts (P < 0.01 and P < 0.05, respectively). However, there was no significant difference between Echinacea and placebo in the treatment of any type of wart.
Cassano et al. used Echinacea in an oral supplement (OS) formulation for resistant warts.[54] The nutraceutical OS (ImmunoSkin Plus® tablets, Morgan Pharma s.r.l., Vicenza, Italy) consisted of a cocktail of Echinacea augustifolia, Echinacea purpurea, methionine, inulin, probiotics, taurine, vitamins C, A, B3, coenzyme Q10 and zinc gluconate. They divided their patients into two groups: one receiving conventional standard therapy (CST) alone and the other receiving CST plus OS. CST consisted of liquid nitrogen cryotherapy or topical salicylic acid 15% + lactic acid 15% continued until complete remission. The OS was prescribed as 1 tablet once daily for 20 days every month initiated concomitantly with CST continued for four consecutive months. The authors observed complete remission of warts in 86% of the CST + OS group versus 54.5% in the CST group (P < 0.001). Development of new warts was significantly reduced in the CST + OS group (9%) versus the CST group (25%) (P = 0.004). The absence of the OS was also more likely to be associated with treatment failure (8% in the CST + OS group versus 37% in the CST group) (P < 0.001).
No significant adverse effects, except mild allergic reactions have been reported.
Quadrivalent human papillomavirus vaccine
The quadrivalent HPV vaccine was first used by Venugopal and Murrell for the treatment of recalcitrant warts in an adult male.[55] Ault hypothesized that the vaccine had potential to show cross protection against strains other than HPV types 6, 11, 16 and 18.[56] Common capsid epitopes and significant homology of L1 between various HPV types are believed to result in the cross protection. Subsequently there have been a few more case reports of the successful use of the vaccine for treating recalcitrant common as well as plantar warts.[57,58] The vaccine is administered intramuscularly in the arm at 0 (or 1), 2 and 6 months. No significant adverse reactions have been reported by any author. Obviously, the vaccine cannot be recommended for more extensive use unless backed by larger controlled trials, but these results are surely encouraging.
Intralesional agents
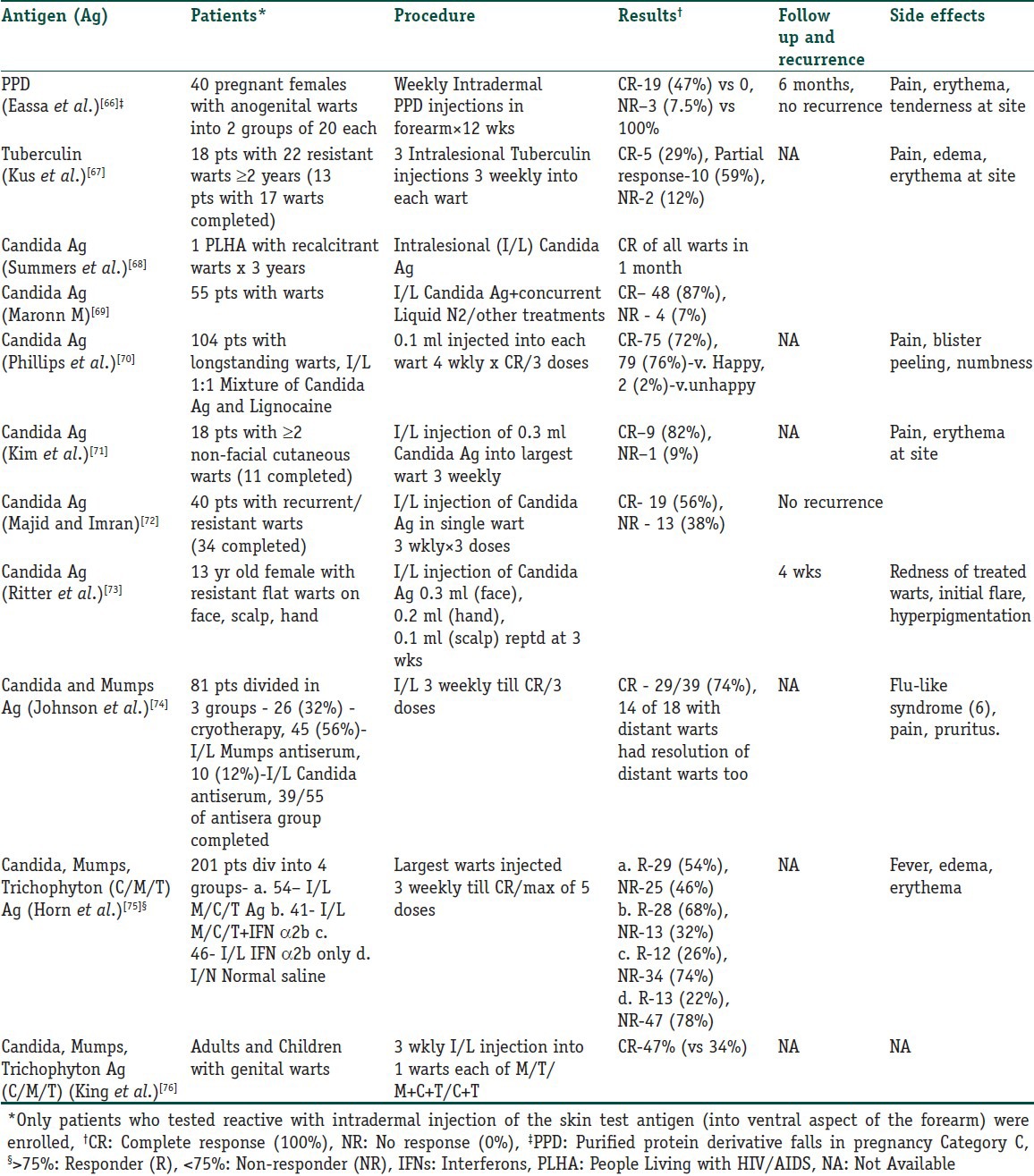
Intralesional immunotherapy utilizes the ability of the immune system to mount a delayed type hypersensitivity response to various antigens or wart tissue. It leads to production of Th1 cytokines which stimulate cytotoxic T cells and natural killer cells to eradicate HPV infection. What is interesting is that this immune attack has a potential to resolve the distant warts as well and not the wart alone that has been primarily injected.[59,60] Many authors have used different immunotherapeutic agents for intralesional injection. These include, Candida antigen, mumps antigen, MMR vaccine, trichophytin skin test antigen, tuberculin, BCG vaccine, Mycobacterium w vaccine autologous wart tissue and IFN-alpha and -gamma injection. This procedure utilizes the fact that there is a high prevalence of immunity to these antigens in the general population.[57] However, this procedure is not suitable for those with hypersensitivity to any of these antigens, pregnant females and immunosuppressed individuals.[61] The antigens can be injected in normal skin or into the wart tissue itself. In case of multiple warts, it is usually the mother wart or the wart that appeared first that is injected. Tables 3 and 4 detail the studies that have been conducted with vaccines and skin test antigens in immunotherapy of warts.
Table 3.
Intralesional vaccines used in immunotherapy of warts
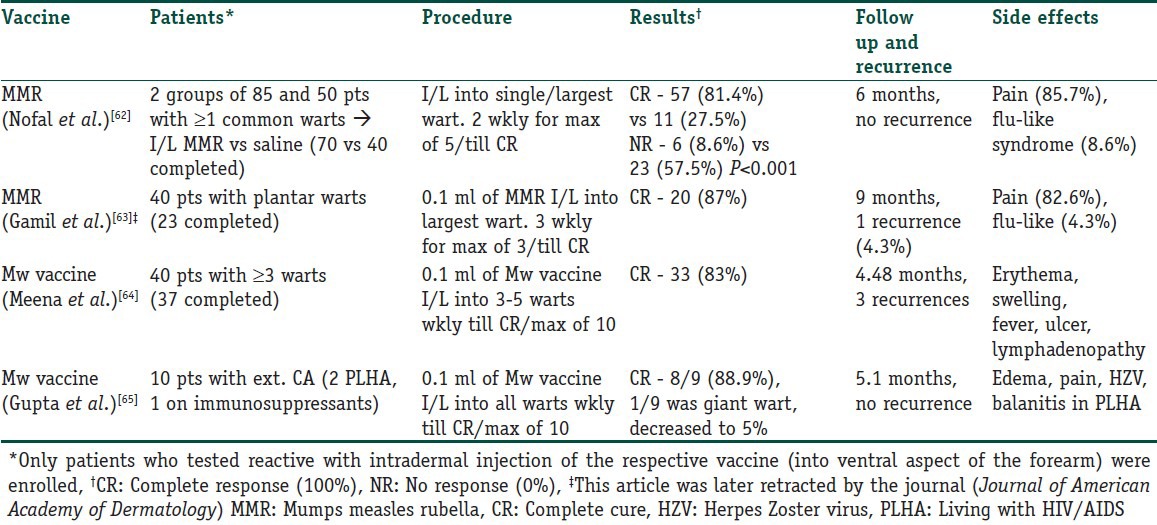
Table 4.
Intralesional/intradermal skin test antigens used in immunotherapy of warts
Intralesional interferon
The basis of IFN therapy in warts is the observation that a T-helper lymphocyte deficiency associated with an inversion of T4/T8 ratio is seen to exist in warts, and it improves after IFN therapy.[77] Intralesional IFN-alpha (IFN-α) has been tried in a randomized double-blind placebo-controlled multicenter trial for recalcitrant genital warts where the warts were injected twice weekly for up to 8 weeks. Complete clearance was seen in 62% of the IFN group versus 21% of the placebo group.[78]
Yang et al. conducted a review of 12 studies on IFN used in genital warts, out of which 7 used local IFN.[46] They found out that the rate of complete response with IFN was significantly better than with placebo (44.4% vs 16.1%, P < 0.00001). The results demonstrated that HPV-infected patients given local IFN were less likely to relapse. Because genital warts are widely regarded as a local illness, it is probable that warts are more sensitive to local administration, optimizing suppression of viral replication and cellular proliferation. Also, systemic administration of IFN may result in much lower intralesional effects of IFN. Intralesional IFN-α has also been successfully used in the treatment of recurrent oral warts in AIDS patients.[79]
Application-site reactions, such as itching, burning sensation and pain, may occur in parts of patients treated with intralesional IFN.
Propioniumbacterium parvum
Propionium bacterium parvum, also known as Propionibacterium acnes or Coryneobacterium parvum is a gram-positive, pleomorphic, strictly anaerobic bacterium.[80,81,82] It has a potent stimulant effect on the reticuloendothelial system and has been used in recent years as an antibacterial and adjuvant immune stimulant to chemotherapy in numerous tumors.[83,84,85] It can be administered parenterally or topically.[82,85] It stimulates the activity of natural killer (NK) cells by releasing IFN and TNF.[80]
Nasser conducted a randomized DB PC trial in 28 volunteers with common warts, out of which 20 completed the study.[86] Intradermal application of 0.1 ml of the drug or placebo was done in one wart each time at intervals of 30-40 days for a total of up to five times. A mild local reaction developed in some warts, indicating the antigen-antibody complex formation. They found that in the group receiving the drug, 8/10 volunteers showed complete clearance of all their warts, 1 had a reduction in size and number of warts and 1 showed no change. On the other hand, none of the placebo group except one showed any change in their warts (P < 0.001) which was highly significant.
Autoimplantation therapy
Autoimplantation refers to injection of homologous wart tissue into untreated warts leading to resolution of the injected wart as well as distant lesions. Shivakumar et al., in 2009, used homologous autoimplantation in the treatment of multiple palmoplantar and common warts in 60 patients in an open trial.[87] They removed a chunk of wart tissue with an 18G needle and introduced it subcutaneously into a nick on the flexor aspect of the left forearm. A total of 73.3% patients showed total clearance, 91% within the first 2 months itself. They hypothesized that autoimplantation induced a CMI response. In 2010, Srivastava and Bajaj also evaluated autowart therapy.[88] However, they removed 3-4 mm of wart tissue with radiocautery, crushed it in distilled water and then injected the fine suspension into the gluteal area intramuscularly. Out of 53 patients, 35 (66%) had complete clearance. Though impressive results have been seen, RCTs with autoimplantation therapy are yet to be conducted.
Topical agents
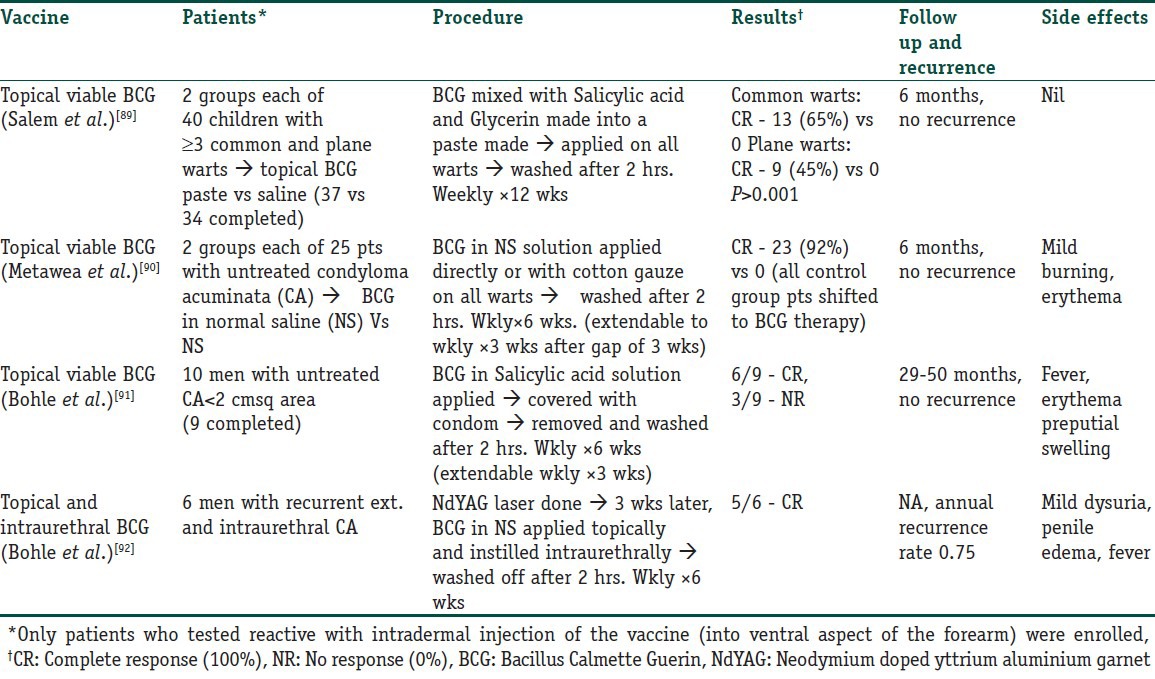
Immunotherapy in warts can be administered by various methods. The simplest method is topical application of certain inorganic molecules that are capable of eliciting a contact hypersensitivity reaction with secondary activation of an immunological response, or even topical applications of immune modulators like Imiquimod and BCG vaccine. Table 5 lists the studies that have been conducted with topical application of BCG vaccine.
Table 5.
Trials with topical BCG vaccine used in immunotherapy of warts
Imiquimod
Imiquimod is an immunomodulator that stimulates cytokines including IFN-α, IL-1,6 TNF-α, GM-CSF and GCSF.[93] It is US FDA approved for the treatment of external genital warts. However, its absorption through intact skin is minimal hence has not been used in cutaneous warts very often.[94] In an open-label, uncontrolled study, 5% Imiquimod cream was applied on five successive days a week and washed off in the morning.[95] It was continued up to 16 weeks or until warts were not visible. Complete clearance occurred in 30%, 26% had > 50% reduction in wart size. Follow up at 32 weeks revealed no recurrence in treated areas. Mild transient local inflammation was the only side effect. It has also been used in recalcitrant plantar, periungual, subungual warts.[96,97,98,99,100,101]
Contact sensitizers (strength of evidence C, IV)
Contact sensitizers are a mode of inducing a type IV hypersensitivity reaction thus making them a form of topical immunotherapy.[94] The three agents that have been used are dinitrochlorobenzene (DNCB), squaric acid dibutyl ester (SADBE) and diphencyprone (DCP). DNCB has been shown to be mutagenic and is no longer used. SADBE is costly and less stable in solution than DCP; hence, DCP is the preferred compound.
Buckley et al. reviewed resistant palmoplantar warts treated with DCP over 8 years.[102] Patients were sensitized with a 2% DCP solution on medial upper arm every 10-14 days until local erythema and vesiculation occurred. Treatment was repeated up to three times. Pared warts were then treated with stepwise concentrations of DCP: 0.01%, 0.05%, 0.10%, 0.25%, 0.50%, 1.0%, 1.5%, 2.0%, 3.0%, 4.0% and 6.0%. Treatments were applied every 1-4 weeks. They found that 42/48 patients completed treatment and exhibited 88% clearance rate. However, a large percentage of patients developed adverse effects (56%), including painful blistering at the site of sensitization and near warts, pompholyx like or generalized eczematous eruption, influenza like symptoms, vesiculation elsewhere due to passive transfer of DCP and inguinal lymphadenopathy. They concluded that patients with recalcitrant palmar, plantar, periungual and digital warts are good candidates for DCP therapy.[103]
In another trial, Rampen et al. applied DCP weekly for 8 weeks in 134 patients and obtained a response rate of 60% (complete clearance in 44% patients at 4 months).[104]
DCP is a potentially useful option as it is less destructive than most destructive modalities, less costly, less time consuming and can be used for multiple warts at a single time.
Green tea catechins (Polyphenon® E)
Polyphenon® E (MediGene AG, Munich, Germany) is a defined extract of catechins of green tea leaves of Camellia sinensis, a species of the Theaceae family. It contains mainly tea polyphenols, including a group of related flavonoids, particularly catechins (>80%). The lead catechin, (−)-epigallocatechin gallate (EGCG), comprises 50-72% of the catechin fraction. Tea catechins are very powerful antioxidants, and Rösl et al. demonstrated the inhibition of transcription of HPV viral proteins by antioxidants.[105,106,107,108] In addition, inhibition of viral binding to receptors, signal transduction and cell cycle modification, antiproliferative effects as well as induction of apoptosis by tea catechins may add to the pharmacological effect of Polyphenon E ointment.[109,110,111,112] These potential properties support its use in the treatment of warts, mainly anogenital warts.
It is a patient-applied modality to be applied three times daily. Mild local symptoms are the most common side effects. Rarely, herpes simplex infection, balanitis, phimosis and lymphadenitis have been reported.[113] Food and Drug Administration has recently approved Polyphenon E ointment for treatment of external genital warts in 2006.[114]
Gross et al. conducted a randomized, double-blind, placebo controlled study comparing a 15% ointment and a 10% cream formulation versus placebo in the treatment of external genital warts.[115] They concluded that the 15% ointment was more effective than the placebo while retaining a favorable safety profile too; however, the 10% cream formulation did not show any statistically significant benefit.
In another trial, Stockfleth et al. found that the use of Polyphenon E 15% and 10% ointment for external genital warts was associated with recurrence rates of 5.9% and 4.1%, respectively.[113] Other treatment modalities gave recurrence rates of 5%-65%. Cryotherapy, for instance, showed a convincing clearance rate but risk of recurrence is about 20-40%. Likewise, imiquimod 5% cream and podofilox demonstrated comparable efficacy rates but recurrence rates ranged from 13% to 19% and up to 91%, respectively.[116,117]
Thus, Polyphenon® E is a promising and relatively safe self-applied topical treatment with few advantages over the other self-applied modalities.
Glizigen (Glycyrrhizinic acid)
Glizigen has been developed by Catalysis Laboratories and the main ingredient is glycyrrhizinic acid, a substance found in the Glycyrrhiza glabra root (sweet root).[118] It is known to possess anti-inflammatory, antiulcerative and antiviral effects. It interacts with viral proteins leading to inactivation of extracellular virus, prevention of intracellular decapsulation of infectious particles and the deterioration of the assembling capacity of the virus particles. Gomez et al. conducted a study to evaluate its use in external genital warts.[118] They combined it with viusid, a food supplement that was immunostimulatory. They compared glizigen-viusid with podophyllin in two groups of 50 patients each. Podophyllin was applied weekly for 6 weeks by the physician. Glizigen was to be sprayed on the lesions by the patients themselves according to the surface area involved - 1-3.9cm2, three times a day; 4-6.9 cm2, four times a day; 7-10 cm2, five times a day, for 8 weeks. In addition, they were told to drink 30 ml Viusid syrup three times a day throughout the treatment period. They found that 84% of total patients had <5 cm2 involved. Of them, in the Glizigen group, 38 were cured while 5 were not; while in the podophyllin group, 36 were cured while 10 were not. Hence, Glizigen was slightly better and was also tolerated better. However, further larger and better planned studies would be needed before glizigen can be used more extensively.
The peak incidence of warts is in children aged 12 to 16 years; hence, the role of immunotherapy for warts in children needs to be studied in greater detail. Systemic drugs like cimetidine have had disappointing results in randomized controlled trials, but continue to be used by some practitioners in children due to their convenience of administration, lack of pain and local adverse effects.[119] Topical sensitizers like SADBE and DPC have been tried on the face and neck and imiquimod on external genital warts in children, both being painless and extremely convenient.[120,121] Intralesional vaccines and skin test antigens are the most popular kids on the block currently, and are being tried in different sites and patient populations, including children.[60,74] However, the attendant pain is less likely to find favor with children, unless their topical use (for example, topical BCG) is proven to have consistent documented success in large controlled trials.
Thus, a plethora of immunotherapeutic agents has been tried in recurrent warts. Some like intralesional or intradermal PPD may be an effective, well-accepted and very cost-effective treatment, especially in countries like India where vaccination against TB is performed routinely and mandatorily. That immunomodulators are effective and devoid of major adverse effects has been shown in open studies and small randomized trials. An added advantage is their potential to avoid recurrences. However, large-scale standardized studies with these agents are the need of the hour in this age of evidence-based dermatology for them to be used more widely and routinely.
What is new?
Many new agents have now become available such as Echinacea, green tea catechins and Propionium bacterium parvum, and few studies have been carried out on them. However, no definite role has yet been defined due to lack of larger randomized, placebo-controlled trials.
There is lack of standardization with regard to the dose, mode of administration, duration and interval of treatment with most of these agents; hence underlining the need for further evaluation.
Spontaneous resolution is a potential confounding factor in all trials on warts, and hence open uncontrolled trials are redundant as far as establishing the efficacy of an agent is concerned.
Footnotes
Source of support: Nil
Conflict of Interest: Nil.
Multiple Choice Questions
-
The maximum dose of cimetidine approved by FDA is:
- 1200 mg/day
- 2400 mg/day
- 3600 mg/day
- 4800 mg/day
-
The use of cimetidine is not approved for:
- Children under 4 years of age
- Children under 12 years of age
- Children under 16 years of age
- There is no such criterion
-
Mechanisms of action of levamisole include all except:
- It alters PMN chemotactic responsiveness
- It stimulates breakdown of cyclic AMP
- It increases adenosine deaminase levels
- It decreases delayed type hypersensitivity
-
Dose of levamisole used for immunostimulation is:
- 2.5 mg/kg once a week
- 2.5 mg/kg twice a week
- 5 mg/kg once a week
- 5 mg/kg twice a week
-
The most common reported side effect of systemic interferons is:
- Flu-like syndrome
- Chest pain
- Wheezing
- Diarrhea
-
Which is not one of the modes of action of Echinacea?
- B-cell activation
- Increase in number of macrophages
- Stimulation of production of IFN and TNF
- Inhibition of hyaluronidase produced by viruses
-
Intralesional agents are not recommended for all except:
- Pregnant females
- Immunosuppressed individuals
- Known hypersensitivity to the antigen
- Previous exposure to similar agent
-
Which of the following statements for imiquimod is incorrect:
- Stimulates cytokines like IL-1
- Approved for treatment of external genital warts
- Absorption through intact skin is good
- It is used for treatment of recalcitrant plantar warts
-
Contact sensitizers induce:
- Type I hypersensitivity reaction
- Type II hypersensitivity reaction
- Type III hypersensitivity reaction
- Type IV hypersensitivity reaction
-
One of the following statements regarding Polyphenon® E is incorrect:
- It is extracted from tea leaves
- It contains antioxidant substances
- It is a physician applied modality
- The FDA has approved it for external genital wart treatment
Answers:
1. B
2. C
3. D
4. B
5. A
6. A
7. D
8. C
9. D
10. C
References
- 1.Sterling JC, Handfield-Jones S, Hudson PM. British Association of Dermatologists. Guidelines for the management of cutaneous warts. Br J Dermatol. 2001;144:4–11. doi: 10.1046/j.1365-2133.2001.04066.x. [DOI] [PubMed] [Google Scholar]
- 2.Scheinfeld N. Treatment of molluscum contagiosum: A brief review and discussion of a case successfully treated with adapalene. Dermatol Online J. 2007;13:15. [PubMed] [Google Scholar]
- 3.Stulberg DL, Hutchinson AG. Molluscum contagiosum and warts. Am Fam Physician. 2003;67:1233–40. [PubMed] [Google Scholar]
- 4.Elenkov IJ, Webster E, Papanicolaou DA, Fleisher TA, Chrousos GP, Wilder RL. Histamine potently suppresses human IL-12 and stimulates IL-10 production via H2 receptors. J Immunol. 1998;161:2586–93. [PubMed] [Google Scholar]
- 5.Gifford RR, Tilberg AF. Histamine type-2 receptor antagonist immune modulation. II. Cimetidine and ranitidine increase interleukin-2 production. Surgery. 1987;102:242–7. [PubMed] [Google Scholar]
- 6.Mitsuishi T, Iida K, Kawana S. Cimetidine treatment for viral warts enhances IL-2 and IFN- gamma expression but not IL-18 expression in lesional skin. Eur J Dermatol. 2003;13:445–8. [PubMed] [Google Scholar]
- 7.Karaman G, Sendur N, Sevk E. Ranitidine therapy for recalcitrant warts in adults: A preliminary study. J Eur Acad Dermatol Venereol. 2001;15:495–6. [PubMed] [Google Scholar]
- 8.Rogers CJ, Gibney MD, Siegfried EC, Harrison BR, Glaser DA. Cimetidine therapy for recalcitrant warts in adults: Is it any better than placebo? J Am Acad Dermatol. 1999;41:123–7. doi: 10.1016/s0190-9622(99)70421-4. [DOI] [PubMed] [Google Scholar]
- 9.Mitsuishi T, Iida K, Kawana S. Cimetidine treatment for viral warts enhances IL-2 and IFN-gamma expression but not IL-18 expression in lesional skin. Eur J Dermatol. 2003;13:445–8. [PubMed] [Google Scholar]
- 10.Glass AT, Solomon BA. Cimetidine therapy for recalcitrant warts in adults. Arch Dermatol. 1996;132:680–2. [PubMed] [Google Scholar]
- 11.Orlow SJ, Paller A. Cimetidine therapy for multiple viral warts in children. J Am Acad Dermatol. 1993;28:794–6. doi: 10.1016/s0190-9622(09)80278-8. [DOI] [PubMed] [Google Scholar]
- 12.Gooptu C, Higgins CR, James MP. Treatment of viral warts with cimetidine: An open-label study. Clin Exp Dermatol. 2000;25:183–5. doi: 10.1046/j.1365-2230.2000.00608.x. [DOI] [PubMed] [Google Scholar]
- 13.Fischer G, Rogers M. Cimetidine therapy for warts in children. J Am Acad Dermatol. 1997;37:289–90. doi: 10.1016/s0190-9622(97)80150-8. [DOI] [PubMed] [Google Scholar]
- 14.Yilmaz E, Alpsoy E, Basaran E. Cimetidine therapy for warts: A placebo-controlled double-blind study. J Am Acad Dermatol. 1996;34:1005–7. doi: 10.1016/s0190-9622(96)90279-0. [DOI] [PubMed] [Google Scholar]
- 15.Karabulut AA, Sahin S, Ekşioglu M. Is cimetidine effective for nongenital warts: A double-blind, placebo-controlled study. Arch Dermatol. 1997;133:533–4. doi: 10.1001/archderm.133.4.533. [DOI] [PubMed] [Google Scholar]
- 16.Rogers CJ, Gibney MD, Siegfried EC, Harrison BR, Glaser DA. Cimetidine therapy for recalcitrant warts in adults: Is it any better than placebo? J Am Acad Dermatol. 1999;41:123–7. doi: 10.1016/s0190-9622(99)70421-4. [DOI] [PubMed] [Google Scholar]
- 17.Sáenz-Santamaria MC, Gilaberte Y. Cimetidine and warts. Arch Dermatol. 1997;133:530–1. doi: 10.1001/archderm.1997.03890400136026. [DOI] [PubMed] [Google Scholar]
- 18.Bauman C, Francis JS, Vanderhooft S, Sybert VP. Cimetidine therapy for multiple viral warts in children. J Am Acad Dermatol. 1996;35:271–2. doi: 10.1016/s0190-9622(96)90351-5. [DOI] [PubMed] [Google Scholar]
- 19.Parsad D, Pandhi R, Juneja A, Negi KS. Cimetidine and levamisole versus cimetidine alone for recalcitrant warts in children. Pediatr Dermatol. 2001;18:349–52. doi: 10.1046/j.1525-1470.2001.01951.x. [DOI] [PubMed] [Google Scholar]
- 20.Parsad D, Saini R, Negi KS. Comparison of combination of cimetidine and levamisole with cimetidine alone in the treatment of recalcitrant warts. Australas J Dermatol. 1999;40:93–5. doi: 10.1046/j.1440-0960.1999.00328.x. [DOI] [PubMed] [Google Scholar]
- 21.Cohen PR, Kurzrock R. Herpes simplex virus infections and cimetidine therapy. J Am Acad Dermatol. 1988;19:762–3. doi: 10.1016/s0190-9622(88)80358-x. [DOI] [PubMed] [Google Scholar]
- 22.Kurzrock R, Auber M, Mavligit GM. Cimetidine therapy of herpes simplex viral infections in immunocompromised patients. Clin Exp Dermatol. 1987;12:326–31. doi: 10.1111/j.1365-2230.1987.tb02501.x. [DOI] [PubMed] [Google Scholar]
- 23.Truhan AP, Raab B. Cimetidine and recurrent genital herpes. J Am Acad Dermatol. 1985;13:313–4. doi: 10.1016/s0190-9622(85)80300-5. [DOI] [PubMed] [Google Scholar]
- 24.Mavligit GM, Talpaz M. Cimetidine for herpes zoster. N Engl J Med. 1984;310:318–9. doi: 10.1056/NEJM198402023100512. [DOI] [PubMed] [Google Scholar]
- 25.Jorizzo JL, Sams WM, Jr, Jegasothy BV, Olansky AJ. Cimetidine as an immunomodulator: Chronic mucocutaneous candidiasis as a model. Ann Intern Med. 1980;92:192–5. doi: 10.7326/0003-4819-92-2-192. [DOI] [PubMed] [Google Scholar]
- 26.White WB, Ballow M. Modulation of suppressor-cell activity by cimetidine in patients with common variable hypogammaglobulinemia. N Engl J Med. 1985;312:198–202. doi: 10.1056/NEJM198501243120402. [DOI] [PubMed] [Google Scholar]
- 27.Segal R, Dayan M, Epstein N, Zecler E, Peller S, Michalevitch R, et al. Common variable immunodeficiency: A family study and therapeutic trial with cimetidine. J Allergy Clin Immunol. 1989;84:753–61. doi: 10.1016/0091-6749(89)90305-9. [DOI] [PubMed] [Google Scholar]
- 28.Dohil M, Prendiville JS. Treatment of molluscum contagiosum with oral cimetidine: Clinical experience in 13 patients. Pediatr Dermatol. 1996;13:310–2. doi: 10.1111/j.1525-1470.1996.tb01247.x. [DOI] [PubMed] [Google Scholar]
- 29.Franco I. Oral cimetidine for the management of genital and perigenital warts in children. J Urol. 2000;164:1074–5. doi: 10.1097/00005392-200009020-00038. [DOI] [PubMed] [Google Scholar]
- 30.Scheinfeld N, Rosenberg JD, Weinberg JM. Levamisole in dermatology: A review. Am J Clin Dermatol. 2004;5:97–104. doi: 10.2165/00128071-200405020-00004. [DOI] [PubMed] [Google Scholar]
- 31.Hogan NA, Hill HR. Enhancement of neutrophil chemotaxis and alteration of levels of cellular cyclic nucleotides by levamisole. J Infect Dis. 1978;138:437–44. doi: 10.1093/infdis/138.4.437. [DOI] [PubMed] [Google Scholar]
- 32.Rivkin I, Rosenblatt J, Becker EL. The role of cyclic AMP in the chemotactic responsiveness and spontaneous motility of rabbit peritoneal neutrophils. The inhibition of neutrophil movement and the elevation of cyclic AMP levels by catecholamines, prostaglandins, theophylline and cholera toxin. J Immunol. 1975;115:1126–34. [PubMed] [Google Scholar]
- 33.Amer M, Tosson Z, Soliman A, Selim AG, Salem A, al-Gendy AA. Verrucae treated by levamisole. Int J Dermatol. 1991;30:738–40. doi: 10.1111/j.1365-4362.1991.tb02624.x. [DOI] [PubMed] [Google Scholar]
- 34.Saul A, Sanz R, Gomez M. Treatment of multiple viral warts with levamisole. Int J Dermatol. 1980;19:342–3. doi: 10.1111/j.1365-4362.1980.tb00355.x. [DOI] [PubMed] [Google Scholar]
- 35.Schou M, Helin P. Levamisole in a double-blind study: No effect on warts. Acta Derm Venereol. 1977;57:449–54. [PubMed] [Google Scholar]
- 36.Cheng YC, Po HL. Leukoencephalopathy after levamisole for the treatment of verrucae. Acta Neurol Taiwan. 2011;20:262–6. [PubMed] [Google Scholar]
- 37.Kitamura H, Morikawa H, Kamon H, Iguchi M, Hojyo S, Fukada T, et al. Toll-like receptor-mediated regulation of zinc homeostasis influences dendritic cell function. Nat Immunol. 2006;7:971–7. doi: 10.1038/ni1373. [DOI] [PubMed] [Google Scholar]
- 38.Ibs KH, Rink L. Zinc-altered immune function. J Nutr. 2003;133(Suppl 1):1452S–6S. doi: 10.1093/jn/133.5.1452S. [DOI] [PubMed] [Google Scholar]
- 39.Fraker PJ, King LE. Reprogramming of the immune system during zinc deficiency. Annu Rev Nutr. 2004;24:277–98. doi: 10.1146/annurev.nutr.24.012003.132454. [DOI] [PubMed] [Google Scholar]
- 40.Al-Gurairi FT, Al-Waiz M, Sharquie KE. Oral zinc sulphate in the treatment of recalcitrant viral warts: Randomized placebo-controlled clinical trial. Br J Dermatol. 2002;146:423–31. doi: 10.1046/j.1365-2133.2002.04617.x. [DOI] [PubMed] [Google Scholar]
- 41.Sadighha A. Oral zinc sulphate in recalcitrant multiple viral warts: A pilot study. J Eur Acad Dermatol Venereol. 2009;23:715–6. doi: 10.1111/j.1468-3083.2009.03169.x. [DOI] [PubMed] [Google Scholar]
- 42.López-Garcia DR, Gómez-Flores M, Arce-Mendoza AY, de la Fuente-Garcia A, Ocampo-Candiani J. Oral zinc sulphate for unresponsive cutaneous viral warts: Too good to be true? A double blind, randomized, placebo-controlled trial. Clin Exp Dermatol. 2009;34:e984–5. doi: 10.1111/j.1365-2230.2009.03623.x. [DOI] [PubMed] [Google Scholar]
- 43.Stefani M, Bottino G, Fontenelle E, Azulay DR. Efficacy comparison between cimetidine and zinc sulphate in the treatment of multiple and recalcitrant warts. An Bras Dermatol. 2009;84:23–9. doi: 10.1590/s0365-05962009000100003. [DOI] [PubMed] [Google Scholar]
- 44.Sharquie KE, Najim RA, Farjou IB, Al-Timimi DJ. Oral zinc sulphate in the treatment of cutaneous leishmaniasis. Clin Exp Dermatol. 2001;26:21–6. doi: 10.1046/j.1365-2230.2001.00752.x. [DOI] [PubMed] [Google Scholar]
- 45.Mahajan PM, Jadhav VH, Patki AH, Jogaikar DG, Mehta JM. Oral zinc therapy in recurrent erythema nodosum leprosum: A clinical study. Indian J Lepr. 1994;66:51–7. [PubMed] [Google Scholar]
- 46.Yang J, Pu YG, Zeng ZM, Yu ZJ, Huang N, Deng QW. Interferon for the treatment of genital warts: A systematic review. BMC Infect Dis. 2009;9:156. doi: 10.1186/1471-2334-9-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dianzani F. Viral interference and interferon. Ric Clin Lab. 1975;5:196–213. doi: 10.1007/BF02908284. [DOI] [PubMed] [Google Scholar]
- 48.Turek LP, Byrne JC, Lowy DR, Dvoretzky I, Friedman RM, Howley PM. Interferon induces morphologic reversion with elimination of extrachromosomal viral genomes in bovine papillomavirus- transformed mouse cells. Proc Natl Acad Sci USA. 1982;79:7914–8. doi: 10.1073/pnas.79.24.7914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Borden EC, Hogan TF, Voelkel JG. Comparative antiproliferative activity in vitro of natural interferons alpha and beta for diploid and transformed human cells. Cancer Res. 1982;42:4948–53. [PubMed] [Google Scholar]
- 50.Einhorn N, Ling P, Strander H. Systemic interferon alpha treatment of human condylomata acuminata. Acta Obstet Gynecol Scand. 1983;62:285–7. doi: 10.3109/00016348309155812. [DOI] [PubMed] [Google Scholar]
- 51.Gall SA, Hughes CE, Trofatter K. Interferon for the therapy of condyloma acuminatum. Am J Obstet Gynecol. 1985;153:157–63. doi: 10.1016/0002-9378(85)90103-6. [DOI] [PubMed] [Google Scholar]
- 52.Zedan H, Hofny ER, Ismail SA. Propolis as an alternative treatment for cutaneous warts. Int J Dermatol. 2009;48:1246–9. doi: 10.1111/j.1365-4632.2009.04184.x. [DOI] [PubMed] [Google Scholar]
- 53.Coeugniet E, Kuhnast R. Adjuvante immuntherapie mit verschiedenen Echinacin® -Darreichungsformen. Therapiewoche. 1986;36:1–19. [Google Scholar]
- 54.Cassano N, Ferrari A, Fai D, Pettinato M, Pellè S, Del Brocco L, et al. Oral supplementation with a nutraceutical containing Echinacea, methionine and antioxidant/immunostimulating compounds in patients with cutaneous viral warts. G Ital Dermatol Venereol. 2011;146:191–5. [PubMed] [Google Scholar]
- 55.Venugopal SS, Murrell DF. Recalcitrant cutaneous warts treated with recombinant quadrivalent human papillomavirus vaccine (types 6, 11, 16 and 18) in a developmentally delayed, 31-year-old white man. Arch Dermatol. 2010;146:475–7. doi: 10.1001/archdermatol.2010.71. [DOI] [PubMed] [Google Scholar]
- 56.Ault KA. Human papillomavirus vaccines and the potential for cross-protection between related HPV types. Gynecol Oncol. 2007;107(Suppl 1):S31–3. doi: 10.1016/j.ygyno.2007.08.059. [DOI] [PubMed] [Google Scholar]
- 57.Landis MN, Lookingbill DP, Sluzevich JC. Recalcitrant plantar warts treated with recombinant quadrivalent human papillomavirus vaccine. J Am Acad Dermatol. 2012;67:e73–4. doi: 10.1016/j.jaad.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 58.Daniel BS, Murrell DF. Complete resolution of chronic multiple verruca vulgaris treated with quadrivalent human papillomavirus vaccine. JAMA Dermatol. 2013;149:370–2. doi: 10.1001/jamadermatol.2013.1463. [DOI] [PubMed] [Google Scholar]
- 59.Mulhem E, Pinelis S. Treatment of nongenital cutaneous warts. Am Fam Physician. 2011;84:288–93. [PubMed] [Google Scholar]
- 60.Clifton MM, Johnson SM, Roberson PK, Kincannon J, Horn TD. Immunotherapy for recalcitrant warts in children using intralesional mumps or Candida antigens. Pediatr Dermatol. 2003;20:268–71. doi: 10.1046/j.1525-1470.2003.20318.x. [DOI] [PubMed] [Google Scholar]
- 61.Chandrashekar L. Intralesional immunotherapy for the management of warts. Indian J Dermatol Venereol Leprol. 2011;77:261–3. doi: 10.4103/0378-6323.79694. [DOI] [PubMed] [Google Scholar]
- 62.Nofal A, Nofal E. Intralesional immunotherapy of common warts: Successful treatment with mumps, measles and rubella vaccine. J Eur Acad Dermatol Venereol. 2010;24:1166–70. doi: 10.1111/j.1468-3083.2010.03611.x. [DOI] [PubMed] [Google Scholar]
- 63.Gamil H, Elgharib I, Nofal A, Abd-Elaziz T. Intralesional immunotherapy of plantar warts: Report of a new antigen combination. J Am Acad Dermatol. 2010;63:40–3. doi: 10.1016/j.jaad.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 64.Meena JK, Malhotra AK, Mathur DK, Mathur DC. Intralesional immunotherapy with Mycobacterium w vaccine in patients with multiple cutaneous warts: Uncontrolled open study. JAMA Dermatol. 2013;149:237–9. doi: 10.1001/jamadermatol.2013.866. [DOI] [PubMed] [Google Scholar]
- 65.Gupta S, Malhotra AK, Verma KK, Sharma VK. Intralesional immunotherapy with killed Mycobacterium w vaccine for the treatment of ano-genital warts: An open label pilot study. J Eur Acad Dermatol Venereol. 2008;22:1089–93. doi: 10.1111/j.1468-3083.2008.02719.x. [DOI] [PubMed] [Google Scholar]
- 66.Eassa BI, Abou-Bakr AM, El-Khalawany MA. Intradermal injection of PPD as a novel approach of immunotherapy in anogenital warts in pregnant women. Dermatol Ther. 2011;24:137–43. doi: 10.1111/j.1529-8019.2010.01388.x. [DOI] [PubMed] [Google Scholar]
- 67.Kus S, Ergun T, Gun D, Akin O. Intralesional tuberculin for treatment of refractory warts. J Eur Acad Dermatol Venereol. 2005;19:515–6. doi: 10.1111/j.1468-3083.2004.01176.x. [DOI] [PubMed] [Google Scholar]
- 68.Summers P, Richards-Altmon P, Halder R. Treatment of recalcitrant verruca vulgaris with candida antigen in patient with human immunodeficiency virus. J Drugs Dermatol. 2009;8:268–9. [PubMed] [Google Scholar]
- 69.Maronn M, Salm C, Lyon V, Galbraith S. One-year experience with Candida antigen immunotherapy for warts and molluscum. Pediatr Dermatol. 2008;25:189–92. doi: 10.1111/j.1525-1470.2008.00630.x. [DOI] [PubMed] [Google Scholar]
- 70.Phillips RC, Ruhl TS, Pfenninger JL, Garber MR. Treatment of warts with candida antigen injection. Arch Dermatol. 2000;136:1274–5. doi: 10.1001/archderm.136.10.1274-a. [DOI] [PubMed] [Google Scholar]
- 71.Kim KH, Horn TD, Pharis J, Kincannon J, Jones R, O’Bryan K, et al. Phase 1 clinical trial of intralesional injection of Candida antigen for the treatment of warts. Arch Dermatol. 2010;146:1431–3. doi: 10.1001/archdermatol.2010.350. [DOI] [PubMed] [Google Scholar]
- 72.Majid I, Imran S. Immunotherapy with intralesional Candida albicans antigen in resistant or recurrent warts: A study. Indian J Dermatol. 2013;58:360–5. doi: 10.4103/0019-5154.117301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ritter SE, Meffert J. Successful treatment of flat warts using intralesional candida antigen. Arch Dermatol. 2003;139:541–2. doi: 10.1001/archderm.139.4.541-c. [DOI] [PubMed] [Google Scholar]
- 74.Johnson SM, Roberson PK, Horn TD. Intralesional injection of mumps or Candida skin test antigens: A novel immunotherapy for warts. Arch Dermatol. 2001;137:451–5. [PubMed] [Google Scholar]
- 75.Horn TD, Johnson SM, Helm RM, Roberson PK. Intralesional immunotherapy of warts with mumps, Candida and Trichophyton skin test antigens: A single-blinded, randomized, and controlled trial. Arch Dermatol. 2005;141:589–94. doi: 10.1001/archderm.141.5.589. [DOI] [PubMed] [Google Scholar]
- 76.King M, Johnson SM, Horn TD. Intralesional immunotherapy for genital warts. Arch Dermatol. 2005;141:1606–7. doi: 10.1001/archderm.141.12.1606. [DOI] [PubMed] [Google Scholar]
- 77.von Krogh G. Management of anogenital warts (condylomata acuminate) Eur J Dermatol. 2001;11:598–604. [PubMed] [Google Scholar]
- 78.Friedman-Kien AE, Eron LJ, Conant M, Growdon W, Badiak H, Bradstreet PW, et al. Natural interferon alfa for treatment of condylomata acuminata. JAMA. 1988;259:533–8. [PubMed] [Google Scholar]
- 79.Lozada-Nur F, Glick M, Schubert M, Silverberg I. Use of intralesional interferon-alpha for the treatment of recalcitrant oral warts in patients with AIDS: A report of 4 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:617–22. doi: 10.1067/moe.2001.118905. [DOI] [PubMed] [Google Scholar]
- 80.Santana CF, Asfora JJ, Lins LP, Lopes CA, Santos ER. Efeitos imunoestimulantes do Corynebacterium parvum em pacientes portadores de neoplasias malígnas. Rev Inst Antib. 1979;19:137–41. [Google Scholar]
- 81.Megid J, Dias Junior JG, Aguiar DM, Nardi Júnior G, Silva WB, Ribeiro MG. Tratamento da papilomatose canina com Propionibacterium acnes. Arq Bras Med Vet Zootec. 2001;53:574–6. [Google Scholar]
- 82.Kalis C, Gumenscheimer M, Freudenberg N, Tchaptchet S, Fejer G, Heit A, et al. Requirement for TLR9 in the immunomodulatory activity of Propionibacterium acnes. J Immunolol. 2005;174:4295–300. doi: 10.4049/jimmunol.174.7.4295. [DOI] [PubMed] [Google Scholar]
- 83.Sljivić VS, Watson SR. The adjuvant effect of Corynebacterium parvum: T-cell dependence of macrophage activation. J Exp Med. 1977;145:45–57. doi: 10.1084/jem.145.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Adlam C, Scott MT. Lympho-reticular stimulatory properties of Corynebacterium parvum and related bacteria. J Med Microbiol. 1973;6:261–74. doi: 10.1099/00222615-6-3-261. [DOI] [PubMed] [Google Scholar]
- 85.Cantrell JL, Wheat RW. Antitumor activity and lymphoreticular stimulation properties of fractions isolated from Corynebacterium parvum. Cancer Res. 1979;39:3554–63. [PubMed] [Google Scholar]
- 86.Nasser N. Treatment of common warts with the immune stimulant Propionium bacterium parvum. An Bras Dermatol. 2012;87:585–9. doi: 10.1590/s0365-05962012000400011. [DOI] [PubMed] [Google Scholar]
- 87.Shivakumar V, Okade R, Rajkumar V. Autoimplantation therapy for multiple warts. Indian J Dermatol Venereol Leprol. 2009;75:593–5. doi: 10.4103/0378-6323.57721. [DOI] [PubMed] [Google Scholar]
- 88.Srivastava PK, Bajaj AK. Autowart injection therapy for recalcitrant warts. Indian J Dermatol. 2010;55:367–9. doi: 10.4103/0019-5154.74548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Salem A, Nofal A, Hosny D. Treatment of common and plane warts in children with topical viable Bacillus Calmette-Guerin. Pediatr Dermatol. 2013;30:60–3. doi: 10.1111/j.1525-1470.2012.01848.x. [DOI] [PubMed] [Google Scholar]
- 90.Metawea B, El-Nashar AR, Kamel I, Kassem W, Shamloul R. Application of viable bacille Calmette-Guérin topically as a potential therapeutic modality in condylomata acuminata: A placebo-controlled study. Urology. 2005;65:247–50. doi: 10.1016/j.urology.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 91.Böhle A, Büttner H, Jocham D. Primary treatment of condylomata acuminata with viable bacillus Calmette-Guerin. J Urol. 2001;165:834–6. [PubMed] [Google Scholar]
- 92.Böhle A, Doehn C, Kausch I, Jocham D. Treatment of recurrent penile condylomata acuminata with external application and intraurethral instillation of bacillus Calmette-Guerin. J Urol. 1998;160:394–6. [PubMed] [Google Scholar]
- 93.Arndt KA, Bowers KE, Alam M, Reynolds R, Tsao S, editors. Manual of Dermatologic Therapeutics. 6th ed. Philadelphia: Lippincott, Williams and Wilkins; 2002. Warts; pp. 241–51. [Google Scholar]
- 94.Leman JA, Benton EC. Verrucas. Guidelines for management. Am J Clin Dermatol. 2000;1:143–9. doi: 10.2165/00128071-200001030-00001. [DOI] [PubMed] [Google Scholar]
- 95.Hengge UR, Esser S, Schultewolter T, Behrendt C, Meyer T, Stockfleth E, et al. Self-administered topical 5% imiquimod for the treatment of common warts and molluscum contagiosum. Br J Dermatol. 2000;143:1026–31. doi: 10.1046/j.1365-2133.2000.03777.x. [DOI] [PubMed] [Google Scholar]
- 96.Micali G, Dall’Oglio F, Nasca MR. An open label evaluation of the efficacy of imiquimod 5% cream in the treatment of recalcitrant subungual and periungual cutaneous warts. J Dermatol Treat. 2003;14:233–6. doi: 10.1080/09546630310016763. [DOI] [PubMed] [Google Scholar]
- 97.Poochareon V, Berman B, Villa A. Successful treatment of butcher's warts with imiquimod 5% cream. Clin Exp Dermatol. 2003;28(Suppl 1):42–4. doi: 10.1046/j.1365-2230.28.s1.14.x. [DOI] [PubMed] [Google Scholar]
- 98.Sauder DN, Skinner RB, Fox TL, Owens ML. Topical imiquimod 5% cream as an effective treatment for external genital and perianal warts in different patient populations. Sex Transm Dis. 2003;30:124–8. doi: 10.1097/00007435-200302000-00006. [DOI] [PubMed] [Google Scholar]
- 99.Zamiri M, Gupta G. Plantar warts treated with an immune response modifier: A report of two cases. Clin Exp Dermatol. 2003;28(Suppl 1):45–7. doi: 10.1046/j.1365-2230.28.s1.15.x. [DOI] [PubMed] [Google Scholar]
- 100.Hesterberg U, Böhlen LM, Brand CU. Imiquimod in the treatment of recalcitrant warts: A new therapy option? Praxis (Bern 1994) 2003;92:535–9. doi: 10.1024/0369-8394.92.12.535. [DOI] [PubMed] [Google Scholar]
- 101.Arican O, Guneri F, Bilgic K, Karaoglu A. Topical Imiquimod 5% cream in external anogenital warts: A randomized, double-blind, placebo-controlled study. J Dermatol. 2004;31:627–31. doi: 10.1111/j.1346-8138.2004.tb00568.x. [DOI] [PubMed] [Google Scholar]
- 102.Buckley DA, Keane FM, Munn SE, Fuller LC, Higgins EM, Du Vivier AW. Recalcitrant viral warts treated by diphencyprone immunotherapy. Br J Dermatol. 1999;141:292–6. doi: 10.1046/j.1365-2133.1999.02978.x. [DOI] [PubMed] [Google Scholar]
- 103.Upitis JA, Krol A. The use of diphenylcyclopropenone in the treatment of recalcitrant warts. J Cutan Med Surg. 2002;6:214–7. doi: 10.1007/s10227-001-0050-9. [DOI] [PubMed] [Google Scholar]
- 104.Rampen FH, Steijlen PM. Diphencyprone in the management of refractory palmoplantar and periungual warts: An open study. Dermatology. 1996;193:236–8. doi: 10.1159/000246252. [DOI] [PubMed] [Google Scholar]
- 105.Hara Y. Influence of tea catechins on the digestive tract. J Cell Biochem Suppl. 1997;27:52–8. [PubMed] [Google Scholar]
- 106.Rice-Evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic Res. 1995;22:375–83. doi: 10.3109/10715769509145649. [DOI] [PubMed] [Google Scholar]
- 107.Tomita I, Sano M, Watanabe J, Miura S, Tomita T, Yoshino K, et al. Tea and its components as powerful antioxidants. In: Cutler RG, Packer L, Bertram J, Mori A, editors. Oxidative Stress and Aging. 1st ed. Basel: Birkhauser Verlag; 1995. pp. 355–65. [Google Scholar]
- 108.Rösl F, Das BC, Lengert M, Geletneky K, zur Hausen H. Antioxidant-induced changes of the AP-1 transcription complex are paralleled by a selective suppression of human papillomavirus transcription. J Virol. 1997;71:362–70. doi: 10.1128/jvi.71.1.362-370.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mukoyama A, Ushijima H, Nishimura S, Koike H, Toda M, Hara Y, et al. Inhibition of rotavirus and enterovirus infections by tea extracts. Jpn J Med Sci Biol. 1991;44:181–6. doi: 10.7883/yoken1952.44.181. [DOI] [PubMed] [Google Scholar]
- 110.Ahmad N, Chen P, Mukhtar H. Cell cycle dysregulation by green tea polyphenol epigallocatechin-3-gallate. Biochem Biophys Res Commun. 2000;275:328–34. doi: 10.1006/bbrc.2000.3297. [DOI] [PubMed] [Google Scholar]
- 111.Li HC, Yashiki S, Sonoda J, Lou H, Ghosh SK, Byrnes JJ, et al. Green tea polyphenols induce apoptosis in vitro in peripheral blood T lymphocytes of adult T-cell leukemia patients. Jpn J Cancer Res. 2000;91:34–40. doi: 10.1111/j.1349-7006.2000.tb00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang GY, Liao J, Kim K, Yurkow EJ, Yang CS. Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols. Carcinogenesis. 1998;19:611–6. doi: 10.1093/carcin/19.4.611. [DOI] [PubMed] [Google Scholar]
- 113.Stockfleth E, Beti H, Orasan R, Grigorian F, Mescheder A, Tawfik H, et al. Topical Polyphenon E in the treatment of external genital and perianal warts: A randomized controlled trial. Br J Dermatol. 2008;158:1329–38. doi: 10.1111/j.1365-2133.2008.08520.x. [DOI] [PubMed] [Google Scholar]
- 114.US Food and Drug Administration, Center for Drug Evaluation and Research. Approval letter VeregenTM Ointment, 15%, NDA 021902. U.S. Food and Drug Administration, Washington, DC. 2006. [Last accessed on 2010 Jan 3]. Available from: http://www.fda.gov/cder/foi/appletter/2006/021902s000ltr.pdf .
- 115.Gross G, Meyer KG, Pres H, Thielert C, Tawfik H, Mescheder A. A randomized, double- blind, four-arm parallel-group, placebo-controlled, phase II/III study to investigate the clinical efficacy of two galenic formulations of Polyphenone E in the treatment of external genital warts. J Eur Acad Dermatol Venereol. 2007;21:1404–12. doi: 10.1111/j.1468-3083.2007.02441.x. [DOI] [PubMed] [Google Scholar]
- 116.Beutner KR, Wiley DJ, Douglas JM, Tyring SK, Fife K, Trofatter K, et al. Genital warts and their treatment. Clin Infect Dis. 1999;28(Suppl 1):S37–56. doi: 10.1086/514722. [DOI] [PubMed] [Google Scholar]
- 117.Kodner CM, Nasraty S. Management of genital warts. Am Fam Physician. 2004;70:2335–42. [PubMed] [Google Scholar]
- 118.Domínguez Gómez J, Simón RD, Abreu Daniel A, Zelenkova H. Effectiveness of glycyrrhizinic acid (glizigen) and an immunostimulant (viusid) to treat aniogenital warts. ISRN Dermatol 2012. 2012 doi: 10.5402/2012/863692. 863692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dasher DA, Burkhart CN, Morrell DS. Immunotherapy for childhood warts. Pediatr Ann. 2009;38:373–9. doi: 10.3928/00904481-20090622-06. [DOI] [PubMed] [Google Scholar]
- 120.Brandt HR, Fernandes JD, Patriota RC, Criado PR, Belda Junior W. Treatment of human papillomavirus in childhood with imiquimod 5% cream. An Bras Dermatol. 2009;85:549–53. doi: 10.1590/s0365-05962010000400020. [DOI] [PubMed] [Google Scholar]
- 121.Weisshaar E, Neumann HJ, Gollnick H. Successful treatment of disseminated facial verrucae with contact immunotherapy. Eur J Dermatol. 1998;8:488–91. [PubMed] [Google Scholar]


