Abstract
Background:
The etiopathogenesis of psoriasis has not been clearly elucidated although the role of chronic inflammation, imbalance between pro- and anti-inflammatory cytokines, and many immunological events have been established. Endothelin 1 (EDN1) and endothelin receptor type-A (EDNRA) are implicated in the inflammatory process. The relationships between EDN1 and EDNRA polymorphisms with several diseases have been found.
Aims and Objectives:
This study examined the possible association of EDN1 (G5665T and T-1370G) and EDNRA (G-231A and G + 70C) single nucleotide polymorphisms (SNPs) with the occurence of psoriasis, and evaluated the relationship between genotypes and clinical/laboratory manifestation of psoriasis.
Materials and Methods:
We analyzed genotype and allele distributions of the above-mentioned polymorphisms in 151 patients with psoriasis and 152 healthy controls by real-time PCR combined with melting curve analysis.
Results:
We did not find significant differences in the genotype and allele distributions of EDN1 T-1370G, EDNRA G-231A, and EDNRA G+70C polymorphisms between patients with psoriasis and healthy controls. Psoriasis area and severity index (PASI) score of EDNRA –231 polymorphic A allele carrying subjects (AA and AA + AG) was higher than that of wild homozygotes (P = 0.044 and P = 0.027, respectively). In addition, EDN1 levels in EDNRA+70 polymorphic C allele carriers (CC + CG) were elevated when compared with GG genotype; however, the difference was at borderline significance (P = 0.05).
Conclusion:
Although there were no associations between studied polymorphisms and psoriasis susceptibility, the PASI score and EDN1 levels seem to be affected by EDNRA G-231A and G + 70C polymorphisms.
Keywords: Autoimmune, endothelin family, inflammation, polymorphism, psoriasis
What was known?
EDN1 and EDNRA are implicated in the inflammatory process. The relationships between EDN1 and EDNRA polymorphisms with several diseases have been found.
Introduction
Psoriasis is a chronic inflammatory and immune-mediated skin disease characterized by increased keratinocyte proliferation, hyperkeratinisation, leukocyte and T-lymphocyte chemotaxis, and neoangiogenesis. Chronic inflammation, imbalance between pro- and anti-inflammatory cytokines, as well as various immune system-related mechanisms have been proposed to play an important role in the etiopathogenesis.[1,2] Endothelins (EDNs) are vasoactive peptides implicated in the inflammatory process, and having important cardiovascular, mitogenic, and neuroregulatory functions.[3,4] There are three identified isopeptides – EDN1, 2, and 3. Endothelin receptors, type-A (EDNRA) and type-B (EDNRB), mediate the biological effects of EDNs. Among the three isopeptides, EDN1 is considered the most important vasoconstrictor with angiogenic and mitogenic properties. The EDN1 is mediated predominantly by EDN1-specific EDNRA located on vascular smooth muscle cells.[3,4] The relationships between EDN1 and EDNRA polymorphisms with several diseases have been found.[5,6,7,8,9] Till date there are only two studies in the literature regarding the relationship between psoriasis and polymorphisms of EDN1 gene (G8002A and -1343A/4A), reporting controversial results.[10,11] Therefore, the aim of this study was to investigate the relationship between psoriasis with polymorphisms of other loci of EDN1 gene [G5665T and T-1370G] and EDNRA gene (G-231A and G+70C), and also to evaluate whether there were relationships between genotypes and clinical/laboratory findings in psoriasis.
Materials and Methods
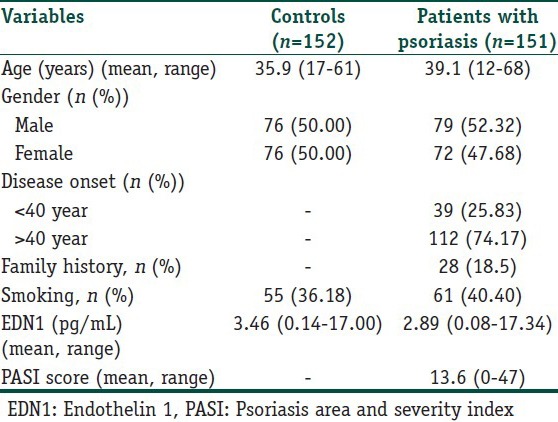
One hundred and fifty one patients with plaque-type psoriasis not previously diagnosed with psoriatic arthritis were included in this study. Diagnosis of psoriasis was based on clinical findings. Depending on the age at diseases onset, patients were classified as suffering from either early- (age < 40 years) or late-onset (age ≥ 40 years) psoriasis. For definition of the severity of the cutaneous manifestations of psoriasis, we used the psoriasis area and severiy index (PASI), which assesses erythema, infiltration, desquamation, and percentage of the involved body surface. The PASI total score ranges from 0 to 72. Higher scores indicate greater psoriasis severity. All patients were clinically assessed by the same dermatologist to establish disease severity using the PASI with the same standarts. The control group consisted of 152 individuals matched for age and sex. None of the controls had personal or family history of any dermatologic disease on examination. Exclusion criteria for patients and controls were the existence of any comorbid cardiac, autoimmune, infectious, musculoskeletal, or malignant disease and a recent history of operation or trauma. Patients were excluded if they were receiving concurrent systemic or topical antipsoriasis therapy. Characteristics of the patients with psoriasis and controls are shown in Table 1. The study was approved by the Institutional Review Board. Informed consent was obtained from each subject.
Table 1.
Characteristics of the patients with psoriasis and controls
Blood samples were taken in the morning subsequent to an overnight (12 h) fast. Peripheral venous blood samples were collected in plain tubes for EDN1 analysis, and in EDTA-K3 for genotype analysis. EDN1 levels were assayed using a commercially available ELISA kit (Biomedica, Wien, Austria).
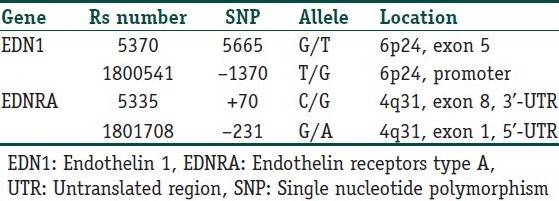
Genomic DNA was isolated from peripheral blood leukocytes by using High Pure PCR Template Preparation Kit (Roche Diagnostics GmbH, Mannheim, Germany). We selected the EDN1 G5665T (rs 5370), EDN1 T-1370G (rs1800541), EDNRA C+70G (rs5335), and EDNRA G-231A (rs1801708) polymorphisms [Table 2]. These single nucleotide polymorphisms (SNPs) were chosen according to the following criteria: associated with susceptibility of other diseases[5,6,7,8,9] and adequate frequency in Caucasian populations to perform evaluation. For detection of the mentioned polymorphisms, Light SNIP assays were used. Light SNIP assays are based on simple probe melting curve analysis. They consist of premixed primers and probes. They were developed and optimized according to NCBI “rs” numbers of mentioned SNPs by Tib MolBiol (Berlin, Germany). The detection of polymorphisms was performed in a LightCycler (Roche Diagnostics, Mannheim, Germany).
Table 2.
Single nucleotide polymorphisms (SNP) in the endothelin 1 and endothelin receptors type A genes
All statistical analyses were performed with SPSS 15.0 for Windows (Chicago, IL, USA). Differences in genotype distributions and allele frequencies in the cases and the controls were compared using the Chi-square (χ2) test. The deviations from Hardy–Weinberg Equilibrium (HWE) were determined using the Pearson χ2-test. Odds ratios (ORs) were calculated and given with 95% confidence intervals (CIs). The wild-type genotype/allele served as a reference category. Mann-Whitney U, Kruskal–Wallis, and Spearman correlation tests were used for the evaluation of clinical and biochemical parameters. In addition, the NCSS 2000 statistical package (Kaysville, Utah, USA) was used to evaluate the power analysis. We had a 89% power to detect an effect size (W) of 0.20 using a 2 degrees of freedom (α = 0.05).
Results
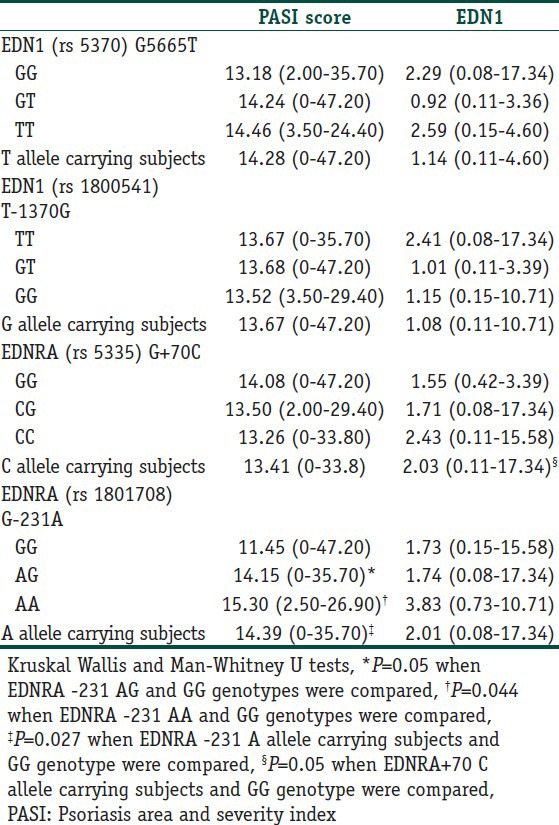
EDN1 (G5665T and T-1370G) and EDNRA (G-231A and G+70C) polymorphisms were analyzed in 151 patients with psoriasis (72 women and 79 men) and 152 unrelated healthy controls (76 women and 76 men). The clinical characteristics of controls and patients with psoriasis were given in Table 1. The genotypic and allelic distributions of EDN1 (G5665T and T-1370G) and EDNRA (G-231A and G+70C) polymorphisms for cases and controls are shown in Table 3. All genotype distributions were in accordance with the HWE among the controls and cases. The allelic frequencies of EDN1 G5665T G (0.79) and T (0.21); EDN1 T-1370G T (0.83) and G (0.17); EDNRA G-231A G (0.62) and A (0.38); EDNRA G+70C G (0.50) and C (0.50) found in our control population were similar to those reported for the English,[5,7] and German,[6] and Turkish[8] populations. No notable differences were observed in allele and genotype frequencies for EDN1 T-1370G, EDNRA G-231A and EDNRA G+70C polymorphisms among the groups. However, regarding EDN1 G5665T SNP, patients homozygous for T allele had two-fold increased risk for developing psoriasis according to GG homozygotes, although the difference did not reach a significant level. PASI score in EDNRA-231 polymorphic A allele carriers (AA and AA + AG) was higher than that of wild homozygotes (P = 0.044 and P = 0.027, respectively; Table 4). In addition, EDN1 levels in EDNRA+70 polymorphic C allele carrying subjects (CC + CG) were elevated in comparison with GG genotype; however, the difference was at borderline significance (P = 0.05). In addition, the possible relationships between the studied polymorphisms and certain clinical phenotypes, including age of onset and gender, were evaluated in patients with psoriasis, and no significant difference were found (data not shown).
Table 3.
Distribution of genotypes and allele frequencies for patients with psoriasis and control group
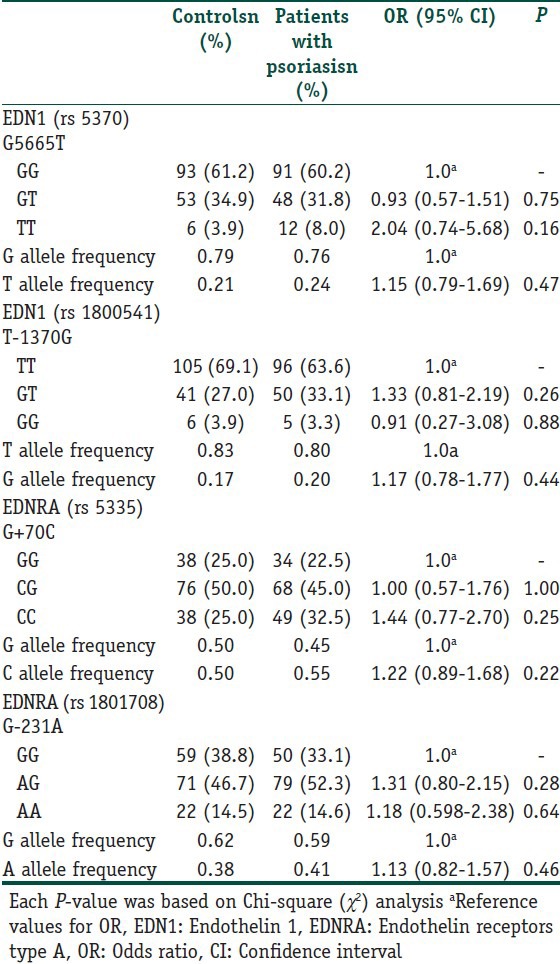
Table 4.
PASI score and endothelin 1 (pg/mL) levels in patients with psoriasis [mean (range)] according to genotypes of studied polymorphisms
Discussion
Psoriasis is a common chronic inflammatory skin disorder with multifactorial etiology. There is growing evidence that the disease is a consequence of interactions between genetic and environmental factors.[12] Recently, genome-wide association studies (GWAS) have been used to identify causative genes of psoriasis and other common diseases.[13] This study is the first one regarding for the association between EDN1 (G5665T and T-1370G) and EDNRA (G-231A and G+70C) polymorphisms and psoriasis susceptibility.
EDN1 is a vasoactive peptide synthesized by different cell types including monocyte, macrophage, vascular smooth muscle, and endothelial cells.[3,4] Besides its effects on the vasal tone, EDN1 acts as mitogen for keratinocytes, mediates proinflammatory pathways by both synthesis of cytokines such as IL -6, IL-8, and the activation of nuclear factor-κB (NF-κB).[14,15] Meanwhile, it upregulates the expression of intercellular adhesion molecule-1 on human dermal microvascular endothelial cells and stimulates the proliferation of T lymphocytes.[14,15,16,17,18] EDN1 has been shown to stimulate endothelial cell growth and to induce vascular smooth muscle cell and pericyte mitogenesis.[19] It could be involved in neoangiogenesis seen in psoriatic skin lesions because of its mitogenic and angiogenic properties. EDN1 can also induce vascular endothelial growth factor (VEGF) which is also a potent angiogenic factor and a mitogen for vascular endothelium.[20] It has been reported that EDN1 induces angiogenic responses through the EDNRA receptor and stimulates neovascularization in concert with VEGF.[20,21] Consequently, all these functions of EDN1 have been shown to contribute to the development of psoriatic lesions.[22] Indeed, increased plasma EDN1 concentrations have previously been found among psoriatic patients.[23,24] Moreover, the overexpression of EDN1 both at protein and mRNA levels has been demonstrated in psoriatic lesions compared with normal skin.[23]
Regarding polymorphisms of EDN1 gene, the G5665T polymorphism of the EDN1 gene (which predicts a lysine-asparagine change at amino acid 198 of EDN1 protein) is associated with several diseases.[5,6,7,8] In addition, the G5665T polymorphism of the EDN1 gene has been shown to affect EDN1 expression with increased plasma EDN1 concentrations in TT subjects.[25] In our study, patients homozygous for mutant T allele had two-fold increased risk for developing psoriasis than healthy controls, although the difference did not reach a significant level. The reason of this nonsignificant result probably is the low number of TT subjects (the number of TT patients was 12 and that of healthy controls were 6). In addition, we could not find any relationship between this polymorphism and plasma EDN1 levels in our patients.
T-1370G polymorphism of EDN1 gene (located in the promoter region of the EDN1 gene), it is well known that polymorphisms in the promoter region of respective gene have potential regulatory effects on gene expression and protein synthesis.[5,26] Elevated EDN1 levels among hypertensive patients with rheumatoid arthitis[5] and preeclampsia[25] carrying EDN1 -1370T allele has been reported. In our study, we did not find significant difference either in the allele and genotype distributions or in EDN1 levels according to EDN1-1370 polymorphism. However, EDN1 levels in patients with TT genotype are higher (but non-significant) than GT and GG genotypes.
With regards to EDNRA polymorphisms, G+70C is located at nucleotide 211 of exon8, which is within the 3’-untranslated regions (UTR). 3’-UTRs of eukaryotic mRNAs are often involved in posttranslational regulatory pathways and have been implicated in message stability.[27] G-231A, the other studied EDNRA polymorphism, is within the 5’-UTR of the gene. The functional consequences of 5’-UTR polymorphisms might be related to the regulatory sequences of gene transcriptions and be associated with differences in the level of gene expression.[7] Another possibility is that such polymorphisms may create novel splice sites and thus affect the function of the receptor. In addition, such changes may be associated with other functionally active, but unidentified, gene variants.[7] Therefore, these polymorphisms could have significant effects on mRNA message stability and interaction with EDN1. In our study, although no significant association between psoriasis and the variant allele of EDNRA +70 was observed, EDN1 levels seem to be affected by this polymorphism. EDN1concentrations in EDNRA +70 polymorphic C allele carrying subjects (CC + CG) were observed to be higher than GG genotype.
The PASI is the most commonly used and best validated psoriasis severity assessment tool. It is used to measure psoriatic plaques severity by evaluating the area erythema, scaliness, and thickness of plaques. The changes in PASI score reflect improvement or worsening of the disease. The PASI score is calculated before, during, and after the treatment to determine how well psoriasis responds to the treatment under test. Higher PASI score was found to be associated with poor quality of life, poorer satisfaction with skin condition, need for hospital admission, and use of systemic therapy.[28,29] It was found that CRP was correlated with PASI as well as with total leukocytes. The association of PASI with CRP was used to access psoriasis severity.[30] Bonifati et al.[23] found increased serum EDN1 concentrations psoriatic patients and reported a direct correlation between serum EDN1 levels and PASI score. They suggested that EDN1 was linked to the PASI score. In this study, there was no significant correlation between EDN1 levels and PASI scores in our psoriatic patients. However, PASI score values in polymorphic A allele carriers (AA and AA + AG) of EDNRA -231 polymorphism were significantly increased in comparison with GG genotype. Although no significant association between psoriasis susceptibility and the variant alleles of EDNRA -231 was observed, PASI scores seem to be affected by this polymorphism.
As a conclusion, this is first study regarding the association between EDN1 (G5665T and T-1370G) and EDNRA (G-231A and G+70C) polymorphisms and psoriasis susceptibility. There is no significant difference in the allele and genotype frequencies of the studied polymorphisms between patient with psoriasis and controls. However, the PASI score and EDN1 levels seems to be affected by EDNRA G-231A and G+70C polymorphisms. There is need for further larger-scale study including other loci of EDN1 and EDNRA genes to confirm our findings, and fully examine the possible relationship between EDN family and development of psoriasis.
What is new?
The PASI scores and EDN1 levels seems to be affected by EDNRA G-231A and G+70C polymorphisms. There is no significant difference in the allele and genotype frequencies of the studied polymorphisms between patient with psoriasis and controls.
Acknowledgements
This study was supported by the Research Fund of the University of Istanbul (Project No. 9727).
Footnotes
Source of support: Research Fund of the University of Istanbul (Project No. 9727)
Conflict of Interest: Nil.
References
- 1.Raychaudhuri SP. Recent advances in psoriasis: Bench to bedside. Indian J Dermatol. 2010;55:150. doi: 10.4103/0019-5154.62749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raychaudhuri SK, Raychaudhuri SP. Scid Mouse model of psoriasis: A unique tool for drug development of autoreactive T-cell and Th-17 cell-mediated autoimmune diseases. Indian J Dermatol. 2010;55:157–60. doi: 10.4103/0019-5154.62752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levin ER. Endothelins. N Engl J Med. 1995;333:356–62. doi: 10.1056/NEJM199508103330607. [DOI] [PubMed] [Google Scholar]
- 4.Ehrenreich H, Anderson RW, Fox CM, Rieckmann P, Hofman GS, Travis WD, et al. Endothelins: Peptides with potent vasoactive properties are produced by human macrophages. J Exp Med. 1990;172:1741–8. doi: 10.1084/jem.172.6.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panoulas VF, Douglas KM, Smith JP, Taffe P, Stavrapoulos-Kalinoglou A, Toms TE, et al. Polymorphisms of endothelin-1 gene associated with hypertesion in patients with rheumatoid arthitis. Endothelium. 2008;15:203–12. doi: 10.1080/10623320802228708. [DOI] [PubMed] [Google Scholar]
- 6.Bühler K, Ufer M, Müller-Marbach A, Brinkmann U, Laule M, Stangl V, et al. Risk of coronary arery disease as influenced by variants of the human endothelin and endothelin-converting enzyme genes. Pharmacogenet Genomics. 2007;17:77–83. doi: 10.1097/01.fpc.0000230118.26581.40. [DOI] [PubMed] [Google Scholar]
- 7.Fonseca C, Renzoni E, Sestini P, Pantelidis P, Lagan A, Bunn C, et al. Endothelin axis polymorphisms in patients with scleroderma. Arthritis Rheum. 2006;54:3034–42. doi: 10.1002/art.22036. [DOI] [PubMed] [Google Scholar]
- 8.Aydýn FA, Develi-İş S, Doğru-Abbasoğlu S, Vural P, Özderya A, Karadağ B, et al. Polymorphisms of endothelin 1 (G5665T and T-1370G) and endothelin receptor type A (C+70G and G-231A) in Graves’ Disease. Int Immunopharmacol. 2014;18:198–202. doi: 10.1016/j.intimp.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Barath A, Endeffy E, Bereczki C, Szücs B, Nemeth I, Turi S. Endothelin-1 gene and endothelial nitric oxide synthase gene polymorphisms in adolescents with juvenile and obesity-associated hypertension. Acta Physiol Hung. 2007;94:49–66. doi: 10.1556/APhysiol.94.2007.1-2.6. [DOI] [PubMed] [Google Scholar]
- 10.Vasku V, Vasku A, Tschöplova S, Izakovicova Holla L, Semiradova V, Vacha V. Genotype association of C(-735) T polymorphism in matrix metalloproteinase 2 gene with G (8002) A endothelin 1 gene with plaque psoriasis. Dermatology. 2002;204:262–5. doi: 10.1159/000063355. [DOI] [PubMed] [Google Scholar]
- 11.Weger W, Hofer A, Wolf P, El-Shabrawi Y, Renner W, Kerl H, et al. The angiotensis-converting enzyme insertion/deletion and the endothelin -134 3A/4A gene polymorphisms in patients with chronic plaque psoriais. Exp Dermatol. 2007;16:993–8. doi: 10.1111/j.1600-0625.2007.00620.x. [DOI] [PubMed] [Google Scholar]
- 12.Enamandram M, Kimball AB. Psoriasis epidemiology: The interplay of genes and the environment. J Invest Dermatol. 2013;133:287–9. doi: 10.1038/jid.2012.434. [DOI] [PubMed] [Google Scholar]
- 13.Oka A, Mabushi T, Ozawa A, Inoko H. Current understanding of human genetics and genetic analysis of psoriasis. J Dermatol. 2012;39:231–41. doi: 10.1111/j.1346-8138.2012.01504.x. [DOI] [PubMed] [Google Scholar]
- 14.Wilson SH, Simari RD, Lerman A. The effect of endothelin-1 on nuclear factor kapa B in macrophages. Biochem Biophys Res Commun. 2001;286:968–72. doi: 10.1006/bbrc.2001.5485. [DOI] [PubMed] [Google Scholar]
- 15.Browatzki M, Schmidt J, Kubler W, Kranzhofer R. Endothelin 1 induces interleukin-6 release via activation on the transcription factor NF-kappa B in human vascular smooth muscle cells. Basic Res Cardiol. 2000;95:98–105. doi: 10.1007/s003950050170. [DOI] [PubMed] [Google Scholar]
- 16.Donckier JE, Michel L, Delos M, Havaux X, Van Beneden R. Interrelated overexpression of endothelial and inducible nitric oxide synhases, endothelin-1 and angiogenic factors in human papillary thyroid carcinoma. Clin Endocrinol. 2006;64:703–10. doi: 10.1111/j.1365-2265.2006.02535.x. [DOI] [PubMed] [Google Scholar]
- 17.Shirasuna K, Nitta A, Sineengard J, Thimizu T, Bpllwein H, Mayamoto A. Vascular and immune regulation of corpus luteum development, maintenance, and regression in the cow. Domest Anim Endocrinol. 2012;43:198–211. doi: 10.1016/j.domaniend.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Rossi GP, Pitter G. Genetic variation in the endothelin system. Do polymorphisms affect the therapeutic strategies? Ann N Y Acad Sci. 2006;1069:34–50. doi: 10.1196/annals.1351.004. [DOI] [PubMed] [Google Scholar]
- 19.Salani D, Di Castro V, Nicrotra MR, Rosano L, Tecce R, Vanuti A, et al. Role of endothelin-1 in neovascularisation of ovarian carcinoma. Am J Pathol. 2000;157:1537–47. doi: 10.1016/S0002-9440(10)64791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salani D, Taraboletti G, Rosano L, Di Castro V, Bosotti P, Giavazzi R, et al. Endothelin-1 induces an angiogenic phenotype in cultured endothelial cells and stimulats neovascularisation in vivo. Am J Pathol. 2000;157:1703–11. doi: 10.1016/S0002-9440(10)64807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ali H, Loizidou M, Dashwood M, Savage F, Sheard C, Taylor I. Stimmulation of colorectal cancer cell line growh by ET-1 and its inhibition by ET (A) antagonists. Gut. 2000;47:685–8. doi: 10.1136/gut.47.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicoloff BJ, Nestle FO. Recent insights in the immunopharmacogenesis of psoriasis provide new therapeutic opportunities. J Clin Invest. 2004;113:1664–75. doi: 10.1172/JCI22147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonifati C, Mussi A, Carducci M, Pitarello A, D’Auria L, Venuti A, et al. Endothelin-1 levels are increased in sera and lesional skin extracts of psoriatic patients and correlate with disease severity. Acta Dermatol Venereol. 1998;78:22–6. doi: 10.1080/00015559850135779. [DOI] [PubMed] [Google Scholar]
- 24.Trevisan G, Stinco G, Giasante C, Fiotti N, Vidimari P, Kokelj F. Psoriasis and endothelins. Acta Derma Venerol. 1994;186:139–40. [PubMed] [Google Scholar]
- 25.Barden AE, Herbison C, Beilin LJ, Michael CA, Walters BN, Van Bockxmeer FM. Association between the endothelin-1 gene Lys198Asn polymorphism blood pressure and plasma endothelin levels in normal and preeclamptic pregnancy. J Hypertens. 2001;19:1775–82. doi: 10.1097/00004872-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Montasser ME, Shimmin LC, Gu D, Chen J, Gu C, Kelly TN, et al. Blood pressure response to potassium supplementation is associated with genetic variation in endothelin1 (EDN1) and interactions with E selectin (SELE) in Rural Chinese. J Hypertens. 2010;28:748–55. doi: 10.1097/HJH.0b013e3283355672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sonenberg N. mRNA translation: İnfluence of 5’ and 3’ untranslated regions. Curr Opin Genet Dev. 1994;4:310–5. doi: 10.1016/s0959-437x(05)80059-0. [DOI] [PubMed] [Google Scholar]
- 28.Menter A, Gottlieb A, Feldman SR, Van Voorhees AS, Leonardi CL, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 1. Overwiew of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826–50. doi: 10.1016/j.jaad.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 29.Ljosaa TM, Stubhaug A, Mork C, Moum T, Wahl AK. Improvement in psoriasis area and severity index score predicts improvement in skin pain over time in patients with psoriasis. Acta Derm Venereol. 2013;93:330–4. doi: 10.2340/00015555-1456. [DOI] [PubMed] [Google Scholar]
- 30.Coimbra S, Oliveira H, Reis F, Belo L, Rocha S, Quintanilha A, et al. C-reactive protein and leukocyte activation in psoriasis vulgaris according to severity and therapy. J Eur Acad Dermatol Venereol. 2010;24:789–96. doi: 10.1111/j.1468-3083.2009.03527.x. [DOI] [PubMed] [Google Scholar]


