Sir,
Bloom syndrome is a rare disorder characterized by photosensitivity, growth deficiency, immunodeficiency, and increased susceptibility to neoplasms. Patients are prone to recurrent respiratory infections, owing to immunoglobulin abnormalities. We report the case of a child with Bloom syndrome with severe lung parenchymal destruction.
A 7-year-old girl presented with photosensitivity, persistent redness, and progressive scarring over the face since the age of one year. She had history of recurrent episodes of fever, cough, and respiratory distress for the past 4 years. The girl was full-term low birth weight baby, and her developmental milestones were normal, except for persistently poor gain in weight and height. History of first degree consanguinity was present in the parents. After a week of hospital stay, the patient developed high grade fever, cough with purulent sputum, and difficulty in breathing.
On examination, her weight (8.3 kg), height (91.5 cm), and head circumference (42 cm) were all below the fifth percentile. She had a small thin body frame with a narrow face, malar hypoplasia, and pointed nose. She had a dolichocephalic skull and a high arched palate. There was erythema, thin atrophic scarring, and numerous telangiectases over the face, especially the malar areas [Figure 1]. Respiratory system examination revealed tachypnea and intercostal and subcostal recession. Auscultation revealed decreased air entry in the left axillary, infrascapular, and inframammary areas with bronchial breath sounds in bilateral infraclavicular and the interscapular areas, suggesting consolidation. We considered the differential diagnosis of Ataxia telangiectasia, Russel-Silver syndrome, and Werner syndrome.
Figure 1.
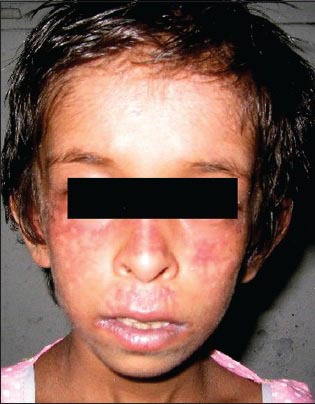
Child with characteristic facies with erythema, thin atrophic scarring, and numerous telangiectases over the face, especially the malar areas
Hematological investigations were normal, except for neutrophilic leukocytosis (18600 cells/cumm with 92% neutrophils). Urine and blood profile for porphyrins was negative. LE cell and Anti-Nuclear Antibody were negative. Levels of IgM and IgA were decreased, but IgG was normal. Sputum culture and sensitivity testing revealed growth of MRSA. A chest radiograph showed left opaque hemithorax with left mediastinal shift suggestive of collapsed lung [Figure 2a]. A contrast-enhanced computed tomography of the chest showed complete left volume loss with parenchymal destruction and areas of bronchiectatic changes indicating a destroyed lung [Figure 2b]. We thus concluded that recurrent lower respiratory tract infections since early childhood left behind bronchiectatic changes, which accumulated over time. The acute event was probably a mucus plug blocking the mouth of the left main bronchus leading to rapid complete destruction of the parenchyma.
Figure 2.
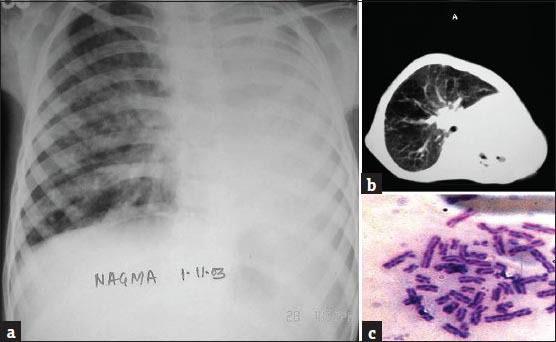
(a) Chest radiograph showing left opaque hemithorax with mediastinal shift to the left suggestive of collapse of the left lung, (b) CECT (Contrast-enhanced computed tomography) of the chest showing complete volume loss of left lung with areas of bronchiectasis. The left main bronchus cannot be visualized, and, (c) High frequency of seen on chromosomal analysis of patient's blood sample
A skin biopsy done from the face lesion showed a flattened, atrophic epidermis with dermoepidermal junctional edema, dermal vasodilatation, and moderate perivascular lymphocytic infiltrate suggestive of chronic actinic damage. Chromosomal analysis of the child's blood sample was done, which showed a very high frequency of sister chromatid exchange (SCE), with the mean SCE per cell being 70, (normal being 10 SCE per metaphase) [Figure 2c]. Multiple spontaneous chromosomal aberrations such as breaks, deletions, triradials, and quadriradials were also observed. Cytologic studies of her father's blood did not reveal any abnormality. On the basis of chromosomal analysis, the child was diagnosed as Bloom syndrome with severe lung destruction. She was started on intravenous antibiotics along with bronchodilators and low – flow oxygen in consultation with respiratory physicians. However, she spiraled into acute respiratory failure (Type II) and eventually succumbed to her pulmonary complications after 27 days.
Bloom syndrome (BS) is a rare autosomal-recessive disorder associated with immunodeficiency, genomic instability, and a predisposition to cancer caused by a mutation in the BLM gene on chromosome 15q26.1.[1] The protein encoded by the normal gene has DNA helicase activity and functions in the maintenance of genomic stability.[1] Cells from BS patients characteristically show an increased frequency of chromatid gaps, breaks, and rearrangements, most particularly quadriradials and increased sister chromatid exchanges (SCEs).[2] The latter two features are indicative of aberrant Homologous Recombination (a DNA repair pathway). Nicotera et al. suggested that the major biochemical defect in BS is chronic overproduction of the superoxide radical and inefficient removal of peroxide is responsible for the high rates of SCEs and chromosomal damage.[3]
Growth delay is usually the first manifestation. Other associated dermatological features and immunodeficiency are not present or recognizable at birth. Respiratory infection is the most important manifestation of immunodeficiency. Prolonged low levels of IgM have been reported in these patients, and this hypogammaglobulinemia is often causally associated with sinopulmonary infections.[4,5]
More than 170 case reports of Bloom syndrome have been published in literature to date. We did an extensive search of literature but were unable to find any other report of BS with pulmonary sequelae in a child. Nair et al.[6] have reported Bloom syndrome leading to serious pulmonary complications is of an adult Indian patient. They described a 24-year-old male with cystic bronchiectasis and emphysema with type II respiratory failure who eventually succumbed to his illness. The authors attempted genetic studies to confirm Bloom syndrome, but could not obtain cell growth, and thus termed him as probably having Bloom syndrome. To the best of our knowledge, this is the first report of Bloom syndrome with a destroyed lung in a child.
Acknowledgement
The authors wish to express their gratitude to Prof. R. N. K. Bamezai, Ph.D., F.N.A.Sc, F.I.M.S.A, F.A.M.S, F.N.A., Professor of Genetics and Director (Coordinator) National Center of Applied Human Genetics, School of Life Sciences, Jawaharlal Nehru University, New Delhi, for his invaluable help in chromosomal analysis of the patient.
References
- 1.Chabosseau P, Buhagiar-Labarchède G, Onclercq-Delic R, Lambert S, Debatisse M, Brison O, et al. Pyrimidine pool imbalance induced by BLM helicase deficiency contributes to genetic instability in Bloom syndrome. Nat Commun. 2011;2:368. doi: 10.1038/ncomms1363. [DOI] [PubMed] [Google Scholar]
- 2.Seki M, Nakagawa T, Seki T, Kato G, Tada S, Takahashi Y, et al. Bloom helicase and DNA topoisomerase III alpha are involved in the dissolution of sister chromatids. Mol Cell Biol. 2006;26:6299–307. doi: 10.1128/MCB.00702-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicotera TM, Notaro J, Notaro S, Schumer J, Sandberg AA. Elevated superoxide dismutase in Bloom's syndrome: A genetic condition of oxidative stress. Cancer Res. 1989;49:5239–43. [PubMed] [Google Scholar]
- 4.Kondo N, Motoyoshi F, Mori S, Kuwabara N, Orii T, German J. Long-term study of the immunodeficiency of Bloom's syndrome. Acta Paediatr. 1992;81:86–90. doi: 10.1111/j.1651-2227.1992.tb12088.x. [DOI] [PubMed] [Google Scholar]
- 5.German J. Bloom's syndrome. XX. The first 100 cancers. Cancer Genet Cytogenet. 1997;93:100–6. doi: 10.1016/s0165-4608(96)00336-6. [DOI] [PubMed] [Google Scholar]
- 6.Nair G, Lobo I, Jayalaksmi TK, Uppe A, Jindal S, Chandra A, et al. Bloom syndrome with lung involvement. Lung India. 2009;26:92–4. doi: 10.4103/0970-2113.53234. [DOI] [PMC free article] [PubMed] [Google Scholar]


