Abstract
Background and Objectives
We sought to evaluate the effect of the early use of ezetimibe/simvastatin (Vytorin®) on arterial healing and endothelialization after the implantation of a drug-eluting stent (DES) in a porcine model of coronary restenosis.
Materials and Methods
A total of 20 pigs (40 coronary arteries) were randomly allocated to a pretreatment or no treatment group. The pretreatment group (n=20) received oral ezetimibe/simvastatin (10/20 mg) daily for 7 days before stenting and the no pretreatment group (n=20) did not. All pigs were treated with ezetimibe/simvastatin (10/20 mg) daily after stenting for 4 weeks. Stenting was performed using a bare-metal stent (BMS, n=10) and three types of DES: biolimus A9-eluting stent (BES, n=10), zotarolimus-eluting stent (ZES, n=10), and everolimus-eluting stents (EES, n=10). Four weeks later, pigs underwent a follow-up coronary angiography and were sacrificed for histopathologic analysis.
Results
There were no significant differences between the pretreatment and no pretreatment groups in the internal elastic lamina area, lumen area, neointima area, stenotic area, injury score, fibrin score, and inflammation score. In both groups, the fibrin score was higher in pigs with DES than in BMS, particularly in ZES and EES. The inflammatory score was not different between DES and BMS.
Conclusion
In a porcine model of coronary restenosis, pretreatment with ezetimibe/simvastatin before DES implantation failed to improve arterial healing and endothelialization compared to treatment after stenting.
Keywords: Coronary restenosis, Drug-eluting stents, Ezetimibe, Hydroxymethylglutaryl-CoA reductase inhibitors
Introduction
Drug-eluting stents (DESs) are associated with delayed arterial healing and endothelialization compared to bare-metal stents (BMS). In addition to a lipid lowering effect, statins reduce vascular inflammatory reactions, improve endothelial function, and inhibit platelet aggregation and thrombus formation. The combination of ezetimibe and simvastatin {Vytorin®, MSD Pharma (Singapore) Pte Ltd., Singapore} was shown to be superior to statin monotherapy in reducing low density lipoprotein-cholesterol (LDL-C).1),2) Recent clinical research reported that statin pretreatment before percutaneous coronary intervention (PCI) was associated with a favorable clinical outcome.3) However, the effect of statin pretreatment on arterial healing and endothelialization after DES implantation is not well known. In the present study, we sought to evaluate whether pretreatment with ezetimibe/simvastatin improved delayed arterial healing and endothelialization after DES in a porcine model of coronary restenosis.
Materials and Methods
Animal study protocol
The present study was approved by the Ethics Committee of Ch-onnam National University Medical School and Chonnam National University Hospital (CNU IACUC-H-2012-1), and conformed to the Guidelines for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (Publication No. 85-23, revised 1996). The study animals were castrated male pigs weighing 20-25 kg. Aspirin 100 mg and clopidogrel 75 mg were given daily for 5 days before the procedure. On the procedure day, pigs were anesthetized with zolazepam and tiletamine (2.5 mg/kg; Zoletil50 ®, Virbac, Caros, France), xylazine (3 mg/kg; Rompun®, Bayer AG, Leverkusen, Germany), and azaperone (6 mg/kg; Stresnil®, Janssen-Cilag, Neuss, Germany). Continuous supplemental oxygen was supplied through an oxygen mask. After a subcutaneous injection of 2% lidocaine, the left carotid artery was surgically exposed, and a 7 Fr sheath was inserted. Continuous hemodynamic and surface electrocardiographic monitoring was maintained throughout the procedure. After intravenous administration of heparin (bolus of 5000 units), the target coronary artery was engaged using a standard 7 Fr guide catheter and baseline angiograms of both coronary arteries were performed using non-ionic contrast agent in two orthogonal views.
Stent-induced stenosis
A total of 20 pigs (40 coronary arteries) were divided into 2 groups according to pretreatment with ezetimibe/simvastatin before stent implantation. Stenting was randomly performed in the proximal portion of the left anterior descending coronary artery and left circumflex coronary artery. Pretreatment group (n=20) received oral ezetimibe/simvastatin 10/20 mg daily for 7 days before stenting and were maintained on the same dose after the stenting for the next 4 weeks. The no pretreatment group (n=20) did not receive ezetimibe/simvastatin 10/20 mg prior to the stenting but did receive it daily after stenting for 4 weeks. Stenting was performed using a BMS (Coroflex Blue®, B. Braun Vascular Systems, Berlin, Germany; 3.0×19 mm, n=10) and three types of DES: biolimus A9-eluting stent (BES, BioMatrix®, Biosensors Interventional Technologies Pte Ltd., Singapore; 3.0×18 mm, n=10), zotarolimus-eluting stent (ZES, Endeavor Resolute®, Medtronic CardioVascular, Minneapolis, MN, USA; 3.0×18 mm, n=10), and everolimus-eluting stents (EES, Promus Element®, Boston Scientific, Natick, MA, USA, 3.0×18 mm, n=10). The stent was deployed by inflating the balloon to nominal pressure at the injury site with the resulting stent-to-artery ratio of 1.3 to 1. A repeat coronary angiogram was obtained immediately after stent implantation. All pigs received 100 mg of aspirin and 75 mg of clopidogrel orally per day throughout the study period. Four weeks later, the pigs underwent a repeat angiography and the same orthogonal views were obtained. They were euthanized with an intracoronary injection of potassium chloride (15%, 20 mL). The hearts were extracted, and the coronary arteries were pressure-perfusion fixed at 70 mm Hg in 10% neutral buffered formalin overnight. The arteries were sectioned, and processed for histopathologic analysis.
Histopathologic examination
Histopathologic evaluation was conducted by an experienced cardiovascular pathologist. The specimens were embedded in methyl methacrylate and sections were cut with a low-speed diamond wafer mounted to a Buehler Isomet saw (Buehler Ltd., Lake Bluff, IL, USA). The stent wires were left intact in the cross sections to minimize potential artifacts from their removal. Fifty to 100 µm sections were obtained approximately 1 mm apart and stained with hematoxylin-eosin and Carstair's stain (Figs. 1 and 2). Measurements of the histopathologic sections were performed using a calibrated microscope, digital video imaging system, and microcomputer program (Visus 2000 Visual Image Analysis System, IMT Tech, San Diego, CA, USA). Borders were manually traced for the lumen area, the area circumscribed by the internal elastic lamina (IEL), and the innermost border of the external elastic lamina area. Morphometric analysis of the neointimal area for a given vessel was calculated as the measured IEL area minus the lumen area. The measurements were made on 5 cross sections from the proximal and distal ends and 3 midpoints of each stented segment. Histopathologic stenosis was calculated as 100×{1-(lesion lumen area/lesion IEL area)} and fibrin was identified on hematoxylin and eosin and Carstair's histochemical stained sections.
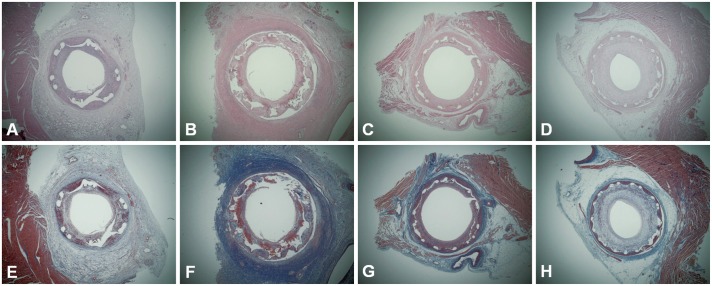
Fig. 1. The hematoxylin-eosin stain (A-D) and Carstair's fibrin stain (E-H) (magnitude×20) by stent type in swine pretreated with ezetimibe/simvastatin. A and E: BES, B and F: ZES, C and G: EES, D and H: BMS. Greater fibrin deposition and inflammation around the stent struts are shown in DES, particularly in ZES and EES. BES: biolimus A9-eluting stent, BMS: bare-metal stent, DES: drug-eluting stent, EES: everolimus-eluting stent, ZES: zotarolimus-eluting stent.
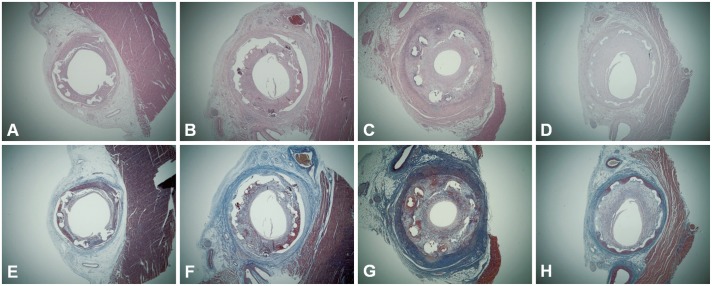
Fig. 2. The hematoxylin-eosin stain (A-D) and Carstair's fibrin stain (E-H) (magnitude×20) by stent type in swine without ezetimibe/simvastatin pretreatment. A and E: BES, B and F: ZES, C and G: EES, D and H: BMS. Greater fibrin deposition and inflammation around the stent struts are observed in DES, compared to BMS. EES shows the most severe inflammatory cell infiltration. BES: biolimus A9-eluting stent, BMS: bare-metal stent, DES: drug-eluting stent, EES: everolimus-eluting stent, ZES: zotarolimus-eluting stent.
Evaluation of arterial injury
Arterial injuries at each strut site were determined by the anatomic structures penetrated by each strut. A numeric value was assigned, as previously described by Schwartz et al.:4) 0=no injury; 1=break in the internal elastic membrane; 2=perforation of the media; and 3=perforation of the external elastic membrane to the adventitia. The average injury score for each segment was calculated by dividing the sum of the injury scores by the total number of struts in the examined section.
Calculation of inflammation and fibrin scores
Inflammation score for each individual strut was defined as follows: 0=no inflammatory cells surrounding the strut; 1=light, non-circumferential lymphohistiocytic infiltrate surrounding the strut; 2=localized, moderate-to-dense cellular aggregate surrounding the strut non-circumferentially; and 3=circumferential dense lymphohistiocytic cell infiltration of the strut. The inflammation score for each cross section was calculated by dividing the sum of the individual inflammation scores by the total number of struts in the examined section. Inflammatory cell counts were normalized to the injury score in the neointima. The number of uncovered struts (defined as no coverage by any tissue, except the thrombus or fibrin-platelet complex) was recorded. Arterial healing was assessed by fibrin deposition and ordinal data for fibrin were collected on each stent section using a scale of 0-3 as previously reported.5)
Statistical analysis
Statistical analysis was done using the Statistical Package for the Social Sciences (SPSS) for Windows (version 17.0; SPSS Inc., Chicago, IL, USA). Continuous variables were presented as the median (interquartile range) and comparisons were made by Mann-Whitney U test. A value of p<0.05 was considered statistically significant.
Results
There were no significant differences between pretreatment and no pretreatment groups in the IEL area {4.69 mm2 (4.20-5.01) vs. 4.56 mm2 (4.20-4.77), p=0.289}, lumen area {2.47 mm2 (1.62-3.02) vs. 2.21 mm2 (1.63-2.57), p=0.355}, neointima area {2.47 mm2 (1.57-3.23) vs. 2.37 mm2 (1.47-2.92), p=0.820}, stenotic area {50.1% (36.1-68.3) vs. 51.8% (34.9-64.5), p=1.000}, injury scores {1.25 (1.08-1.49) vs. 1.16 (1.09-1.30), p=0.297}, fibrin score {1.75 (1.45-2.00) vs. 1.74 (1.29-2.00), p=0.738}, and inflammation scores {1.08 (1.02-1.76) vs. 1.09 (1.02-2.44), p=0.659} (Table 1). When compared to according to stent type, no significant differences were observed between the pretreated and not pretreated groups in the IEL, lumen area, neointima area, stenotic area, injury score, fibrin score, and inflammation score (Table 2). The fibrin score was higher in DES than in BMS in both the pretreatment and no pretreatment groups {1.94 (1.64-2.00) vs. 1.07 (0.60-1.64), p=0.005 and 2.00 (1.57-2.00) vs. 0.44 (0.00-1.33), p=0.001, respectively} (Table 3). Greater fibrin deposition and inflammation around the stent struts were observed in DES, particularly in ZES and EES, compared to BMS (Figs. 1 and 2). The inflammation score was, however, not statistically different between DES and BMS {1.08 (1.00-2.27) vs.1.06 (1.03-1.15), p=0.759 and 1.35 (1.01-2.94) vs. 1.06 (1.01-1.13), p=0.349, respectively} (Table 3).
Table 1. Coronary artery morphometric measurements according to pretreatment with ezetimibe/simvastatin.
| Pretreatment (n=20) | No pretreatment (n=20 | p | |
|---|---|---|---|
| IEL area (mm2) | 4.69 (4.20-5.01) | 4.56 (4.20-4.77) | 0.289 |
| Lumen area (mm2) | 2.47 (1.62-3.02) | 2.21 (1.63-2.57) | 0.355 |
| Neointima area (mm2) | 2.47 (1.57-3.23) | 2.37 (1.47-2.92) | 0.820 |
| Area stenosis (%) | 50.1 (36.1-68.3) | 51.8 (34.9-64.5) | 1.000 |
| Injury score | 1.25 (1.08-1.49) | 1.16 (1.09-1.30) | 0.297 |
| Fibrin score | 1.75 (1.45-2.00) | 1.74 (1.29-2.00) | 0.738 |
| Inflammation score | 1.08 (1.02-1.76) | 1.09 (1.02-2.44) | 0.659 |
IEL: internal elastic lamina
Table 2. Coronary artery morphometric measurements according to pretreatment with ezetimibe/simvastatin by stent type.
| BES | ZES | EES | DES | BMS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pretreatment (n=5) | No pretreatment (n=5) | p | Pretreatment (n=5) | No pretreatment (n=5) | p | Pretreatment (n=5) | No pretreatment (n=5) | p | Pretreatment (n=15) | No pretreatment (n=15) | p | Pretreatment (n=5) | No pretreatment (n=5) | p | |
| IEL area (mm2) | 4.36 (4.01-4.75) | 4.18 (3.98-4.39) | 0.690 | 5.47 (4.83-5.54) | 4.77 (4.68-5.17) | 0.151 | 4.31 (3.88-4.91) | 4.57 (4.13-4.75) | 0.841 | 4.61 (4.09-5.21) | 4.57 (4.18-4.77) | 0.539 | 4.77 (4.30-4.94) | 4.49 (3.75-4.68) | 0.421 |
| Lumen area (mm2) | 2.83 (1.64-3.17) | 2.47 (2.17-3.15) | 0.917 | 2.44 (1.44-3.16) | 2.32 (1.22-3.17) | 0.917 | 2.77 (1.55-4.42) | 1.56 (0.42-1.68) | 0.056 | 2.77 (1.63-3.29) | 1.91 (1.56-2.60) | 0.174 | 2.07 (1.52-2.56) | 2.31 (2.06-2.83) | 0.548 |
| Neointima area (mm2) | 1.65 (0.98-2.90) | 1.38 (1.15-2.03) | 0.917 | 3.10 (1.98-3.75) | 2.45 (1.99-3.46) | 0.841 | 1.87 (1.25-3.70) | 3.35 (2.62-3.98) | 0.222 | 1.93 (1.53-3.58) | 2.42 (1.75-3.35) | 0.624 | 2.69 (2.16-3.00) | 1.89 (1.21-2.46) | 0.056 |
| Area stenosis (%) | 37.3 (23.5-62.7) | 34.3 (27.8-48.6) | 0.917 | 55.8 (38.1-72.8) | 51.6 (39.2-74.7) | 0.917 | 50.1 (27.2-84.2) | 68.5 (60.4-90.1) | 0.421 | 48.1 (31.8-69.9) | 54.9 (34.3-68.5) | 0.567 | 56.8 (46.0-66.4) | 44.8 (32.7-53.4) | 0.151 |
| Injury score | 1.18 (1.07-1.44) | 1.16 (1.08-1.33) | 0.905 | 1.32 (1.13-1.46) | 1.12 (1.02-1.17) | 0.111 | 1.43 (1.20-1.71) | 1.50 (1.25-2.14) | 0.548 | 1.30 (1.13-1.52) | 1.18 (1.08-1.46) | 0.511 | 1.18 (1.00-1.34) | 1.08 (1.02-1.16) | 0.421 |
| Fibrin score | 1.56 (1.47-1.71) | 1.95 (1.54-2.00) | 0.151 | 2.00 (1.97-2.45) | 2.00 (1.74-2.14) | 0.421 | 2.00 (1.68-2.17) | 2.00 (0.92-2.25) | 0.841 | 1.94 (1.64-2.00) | 2.00 (1.57-2.00) | 0.935 | 1.07 (0.60-1.64) | 0.44 (0.00-1.33) | 0.310 |
| Inflammation score | 1.08 (1.01-2.26) | 1.02 (1.01-1.49) | 0.421 | 1.60 (1.01-2.10) | 1.35 (1.02-2.71) | 0.690 | 1.02 (1.00-2.55) | 2.94 (1.72-2.97) | 0.151 | 1.08 (1.00-2.27) | 1.35 (1.01-2.94) | 0.436 | 1.06 (1.03-1.15) | 1.06 (1.01-1.13) | 0.841 |
BES: biolimus A9-eluting stent, BMS: bare-metal stent, DES: drug-eluting stent, EES: everolimus-eluting stent, IEL: internal elastic lamina, ZES: zotarolimus-eluting stent
Table 3. Coronary artery morphometric measurements compared between DES and BMS.
| Pretreatment | No pretreatment | |||||
|---|---|---|---|---|---|---|
| DES (n=15) | BMS (n=5) | p | DES (n=15) | BMS (n=5) | p | |
| IEL area (mm2) | 4.61 (4.09-5.21) | 4.77 (4.30-4.94) | 0.965 | 4.57 (4.28-4.77) | 4.49 (3.75-4.68) | 0.672 |
| Lumen area (mm2) | 2.77 (1.63-3.29) | 2.07 (1.52-2.56) | 0.306 | 1.90 (1.56-2.60) | 2.31 (2.06-2.83) | 0.445 |
| Neointima area (mm2) | 1.93 (1.53-3.58) | 2.69 (2.16-3.00) | 0.612 | 2.42 (1.75-3.35) | 1.89 (1.21-2.46) | 0.306 |
| Area stenosis (%) | 48.1 (31.8-69.9) | 56.8 (46.0-66.4) | 0.553 | 54.9 (34.3-68.5) | 44.8 (32.7-53.4) | 0.266 |
| Injury score | 1.30 (1.13-1.52) | 1.18 (1.00-1.34) | 0.219 | 1.18 (1.08-1.46) | 1.08 (1.02-1.16) | 0.056 |
| Fibrin score | 1.94 (1.64-2.00) | 1.07 (0.60-1.64) | 0.005 | 2.00 (1.57-2.00) | 0.44 (0.00-1.33) | 0.001 |
| Inflammation score | 1.08 (1.00-2.27) | 1.06 (1.03-1.15) | 0.759 | 1.35 (1.01-2.94) | 1.06 (1.01-1.13) | 0.349 |
DES: drug-eluting stent, BMS: bare-metal stent, IEL: internal elastic lamina
Discussion
In the present study using a porcine model of coronary restenosis, pretreatment with ezetimibe/simvastatin before stenting did not significantly inhibit neointimal hyperplasia and an inflammatory reaction compared to the conventional use of ezetimibe/simvastatin after stenting. The outcome was consistent in both DES and BMS.
Previous studies with the 1st-generation DES demonstrated that polymer-based sirolimus-eluting stents (SES, Cypher®, Cordis Corp., Miami Lakes, FL, USA) and paclitaxel-eluting stents (PES, Taxus®, Boston Scientific Corp., Natick, MA, USA) significantly reduced neointimal hyperplasia by inhibiting proliferation and migration of smooth muscle cells, but delayed arterial healing with persistent fibrin deposition and incomplete reendothelialization.6),7) However, there is a paucity of data on the efficacy of statin pretreatment after implantation of recently introduced 2nd-generation DES such as ZES-, EES-, and biolimus-eluting stents.
Statins, in addition to their lipid-lowering properties, are known to reduce vascular inflammation,8),9) improve endothelial function,10) and inhibit platelet aggregation and thrombus formation.11) Anti-inflammatory effects, in particular, are thought to play a major role in reducing cardiovascular events in patients with acute coronary syndrome, and has been proven in many clinical trials.8),12),13),14) Inflammatory reactions are an essential component in the initiation and progression of atherosclerosis.15),16) Recent research conducted in animals revealed that inflammatory reactions surrounding stent struts after DES implantation were associated with neointimal hyperplasia.17) Statin pretreatment before PCI was associated with favorable clinical outcome.3) The exact mechanisms of the effect of preemptive statin usage are not certain, but appear to be related to the pleiotropic effects of statins rather than their lipid-lowering properties.
Ezetimibe reduces plasma cholesterol levels by inhibiting its intestinal absorption without affecting the absorption of triglycerides or lipid-soluble vitamins.18) The combination of ezetimibe and simvastatin (Vytorin®) was shown to be superior to statin monotherapy in reducing LDL-C.1),2),19)
The effect of ezetimibe on atherosclerosis has been evaluated in a number of animal stuides.20),21),22),23),24) The proven effect of lowering LDL-C and non-high density lipoprotein-cholesterol (HDL-C) is presumed to be the key mechanism for inhibition of atherosclerosis by ezetimibe.25) Additionally, it has been suggested that efflux of cholesterol from macrophage foam cells by HDL-mediated reverse cholesterol transport plays a role in plaque stabilization and regression.26) In addition, ezetimibe was shown to inhibit intimal hyperplasia by reducing cell proliferation and enhancing apoptosis in a rabbit autologous vein graft by restoring acetylcholine-induced endothelial intracellular Ca2+ increase and endothelium-dependent nitric oxide-mediated relaxation.27) In a porcine coronary restenosis model, combined therapy with ezetimibe and simvastatin inhibited neointimal hyperplasia after implantation of SES and PES but did not reduce inflammatory infiltration and arterial healing.28) In a rabbit model of atherosclerosis induced by balloon de-endothelialization, ezetimibe reduced atherosclerotic lesion size and plaque inflammation when combined with simvastatin.24)
In the present study, all the pigs received ezetimibe/simvastatin after stenting until the end of the study period. Therefore, pretreatment with ezetimibe/simvastatin during the preceding 7 days may not have shown significant difference compared to the no pretreatment group. When comparing between type of DES regardless of pretreatment, BES had a lower area of stenosis and inflammatory score than ZES and EES, which is almost equivalent to those of BMS, except for fibrin score. These findings are, in general, consistent with recent studies using porcine coronary restenosis models.29),30) Park et al.29) reported that BES appeared to reliably reduce the inflammatory response at overlapping segments as well as non-overlapping segments. Lim et al.30) observed that BES showed better histopathological characteristics than ZES and EES one month after stenting in the porcine coronary restenosis model. BES was more effective in inhibiting neointimal hyperplasia compared to ZES and EES, while BES and ZES were more effective in reducing the inflammatory reaction compared to EES. Currently, there is insufficient data to explain this finding, but the more favorable outcome with BES may be partly related to the use of a bioabsorbable polylactic acid polymer unlike ZES and EES, which uses permanent polymer-carrier-based platforms that may be associated with inflammation, late thrombosis, and restenosis. This needs to be elucidated in a future study with longer-term follow-up.
There are several limitations in the present study. First, stenting was performed in normal, non-atherosclerotic porcine coronary arteries with oversized stents for neointimal proliferation, which is different from human clinical scenarios with preexisting atherosclerosis and stent sizes matched to the reference vessel. Second, we did not examine the inflammatory response shortly after DES implantation when there is not much neointimal formation, which may have been valuable in the histopatholgic assessment of the group pretreated with ezetimibe/simvastatin. Finally, we did not conduct longer-term follow-up experiments to examine late neointimal inflammatory reaction and arterial healing.
In conclusion, a porcine model of coronary restenosis failed to demonstrate that pretreatment with ezetimibe/simvastatin before DES implantation improved arterial healing and endothelialization compared to treatment with ezetimibe/simvastatin after stenting.
Acknowledgments
This study was supported by a grant (No. 201303-11) from the Korea Society of Cardiology (2011).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Davidson MH, McGarry T, Bettis R, et al. Ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia. J Am Coll Cardiol. 2002;40:2125–2134. doi: 10.1016/s0735-1097(02)02610-4. [DOI] [PubMed] [Google Scholar]
- 2.Ballantyne CM, Houri J, Notarbartolo A, et al. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation. 2003;107:2409–2415. doi: 10.1161/01.CIR.0000068312.21969.C8. [DOI] [PubMed] [Google Scholar]
- 3.Patti G, Pasceri V, Colonna G, et al. Atorvastatin pretreatment improves outcomes in patients with acute coronary syndromes undergoing early percutaneous coronary intervention: results of the ARMYDA-ACS randomized trial. J Am Coll Cardiol. 2007;49:1272–1278. doi: 10.1016/j.jacc.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz RS, Huber KC, Murphy JG, et al. Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol. 1992;19:267–274. doi: 10.1016/0735-1097(92)90476-4. [DOI] [PubMed] [Google Scholar]
- 5.Kolodgie FD, John M, Khurana C, et al. Sustained reduction of in-stent neointimal growth with the use of a novel systemic nanoparticle paclitaxel. Circulation. 2002;106:1195–1198. doi: 10.1161/01.cir.0000032141.31476.15. [DOI] [PubMed] [Google Scholar]
- 6.Finn AV, Kolodgie FD, Harnek J, et al. Differential response of delayed healing and persistent inflammation at sites of overlapping sirolimus- or paclitaxel-eluting stents. Circulation. 2005;112:270–278. doi: 10.1161/CIRCULATIONAHA.104.508937. [DOI] [PubMed] [Google Scholar]
- 7.Drachman DE, Edelman ER, Seifert P, et al. Neointimal thickening after stent delivery of paclitaxel: change in composition and arrest of growth over six months. J Am Coll Cardiol. 2000;36:2325–2332. doi: 10.1016/s0735-1097(00)01020-2. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Rifai N, Pfeffer MA, et al. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1998;98:839–844. doi: 10.1161/01.cir.98.9.839. [DOI] [PubMed] [Google Scholar]
- 9.Bustos C, Hernández-Presa MA, Ortego M, et al. HMG-CoA reductase inhibition by atorvastatin reduces neointimal inflammation in a rabbit model of atherosclerosis. J Am Coll Cardiol. 1998;32:2057–2064. doi: 10.1016/s0735-1097(98)00487-2. [DOI] [PubMed] [Google Scholar]
- 10.Dupuis J, Tardif JC, Cernacek P, Théroux P. Cholesterol reduction rapidly improves endothelial function after acute coronary syndromes. The RECIFE (reduction of cholesterol in ischemia and function of the endothelium) trial. Circulation. 1999;99:3227–3233. doi: 10.1161/01.cir.99.25.3227. [DOI] [PubMed] [Google Scholar]
- 11.Lacoste L, Lam JY, Hung J, Letchacovski G, Solymoss CB, Waters D. Hyperlipidemia and coronary disease. Correction of the increased thrombogenic potential with cholesterol reduction. Circulation. 1995;92:3172–3177. doi: 10.1161/01.cir.92.11.3172. [DOI] [PubMed] [Google Scholar]
- 12.Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1999;100:230–235. doi: 10.1161/01.cir.100.3.230. [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM, Rifai N, Lowenthal SP. Rapid reduction in C-reactive protein with cerivastatin among 785 patients with primary hypercholesterolemia. Circulation. 2001;103:1191–1193. doi: 10.1161/01.cir.103.9.1191. [DOI] [PubMed] [Google Scholar]
- 14.Albert MA, Danielson E, Rifai N, Ridker PM PRINCE Investigators. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001;286:64–70. doi: 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- 15.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 16.van der Wal AC, Becker AE, van der Loos CM, Das PK. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation. 1994;89:36–44. doi: 10.1161/01.cir.89.1.36. [DOI] [PubMed] [Google Scholar]
- 17.Hong YJ, Jeong MH, Lee SR, et al. Anti-inflammatory effect of abciximab-coated stent in a porcine coronary restenosis model. J Korean Med Sci. 2007;22:802–809. doi: 10.3346/jkms.2007.22.5.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Heek M, Farley C, Compton DS, Hoos L, Davis HR. Ezetimibe selectively inhibits intestinal cholesterol absorption in rodents in the presence and absence of exocrine pancreatic function. Br J Pharmacol. 2001;134:409–417. doi: 10.1038/sj.bjp.0704260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerzner B, Corbelli J, Sharp S, et al. Efficacy and safety of ezetimibe coadministered with lovastatin in primary hypercholesterolemia. Am J Cardiol. 2003;91:418–424. doi: 10.1016/s0002-9149(02)03236-8. [DOI] [PubMed] [Google Scholar]
- 20.Davis HR, Jr, Compton DS, Hoos L, Tetzloff G. Ezetimibe, a potent cholesterol absorption inhibitor, inhibits the development of atherosclerosis in ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2001;21:2032–2038. doi: 10.1161/hq1201.100260. [DOI] [PubMed] [Google Scholar]
- 21.Nakagami H, Osako MK, Takami Y, et al. Vascular protective effects of ezetimibe in ApoE-deficient mice. Atherosclerosis. 2009;203:51–58. doi: 10.1016/j.atherosclerosis.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 22.Dietrich T, Hucko T, Bourayou R, et al. High resolution magnetic resonance imaging in atherosclerotic mice treated with ezetimibe. Int J Cardiovasc Imaging. 2009;25:827–836. doi: 10.1007/s10554-009-9487-5. [DOI] [PubMed] [Google Scholar]
- 23.Graf K, Dietrich T, Tachezy M, et al. Monitoring therapeutical intervention with ezetimibe using targeted near-infrared fluorescence imaging in experimental atherosclerosis. Mol Imaging. 2008;7:68–76. [PubMed] [Google Scholar]
- 24.Gómez-Garre D, Muñoz-Pacheco P, González-Rubio ML, Aragoncillo P, Granados R, Fernández-Cruz A. Ezetimibe reduces plaque inflammation in a rabbit model of atherosclerosis and inhibits monocyte migration in addition to its lipid-lowering effect. Br J Pharmacol. 2009;156:1218–1227. doi: 10.1111/j.1476-5381.2008.00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis HR, Jr, Lowe RS, Neff DR. Effects of ezetimibe on atherosclerosis in preclinical models. Atherosclerosis. 2011;215:266–278. doi: 10.1016/j.atherosclerosis.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Briand F, Naik SU, Fuki I, et al. Both the peroxisome proliferator-activated receptor delta agonist, GW0742, and ezetimibe promote reverse cholesterol transport in mice by reducing intestinal reabsorption of HDL-derived cholesterol. Clin Transl Sci. 2009;2:127–133. doi: 10.1111/j.1752-8062.2009.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maekawa T, Komori K, Morisaki K, Itoh T. Ezetimibe reduces intimal hyperplasia in rabbit jugular vein graft. J Vasc Surg. 2012;56:1689–1697. doi: 10.1016/j.jvs.2012.05.071. [DOI] [PubMed] [Google Scholar]
- 28.Cho JS, Jeong MH, Sim DS, et al. Effects of combined therapy with ezetimibe plus simvastatin after drug-eluting stent implantation in a porcine coronary restenosis model. J Korean Med Sci. 2010;25:716–722. doi: 10.3346/jkms.2010.25.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park KH, Jeong MH, Kim JM, et al. The impact of triple anti-platelet therapy for endothelialization and inflammatory response at overlapping bioabsorbable polymer coated drug-eluting stents in a porcine coronary model. Int J Cardiol. 2013;168:1853–1858. doi: 10.1016/j.ijcard.2012.12.070. [DOI] [PubMed] [Google Scholar]
- 30.Lim KS, Jeong MH, Bae IH, et al. Histopathological comparison among biolimus, zotarolimus and everolimus-eluting stents in porcine coronary restenosis model. Korean Circ J. 2013;43:744–751. doi: 10.4070/kcj.2013.43.11.744. [DOI] [PMC free article] [PubMed] [Google Scholar]