Abstract
Background and Objectives
This study was aimed at assessing left ventricular torsion (LVtor) mechanics using speckle tracking echocardiography (STE), establishing normal reference values of principal LVtor parameters, and analyzing the age-related changes in normal children.
Subjects and Methods
Eighty children (aged 3 months to 15 years) with normal cardiac function and rhythm were recruited. LVtor parameters including rotations, twist and untwist, torsion, and their rate indices were measured using STE. Age and heart rate related changes of the parameters were analyzed.
Results
Speckle tracking echocardiography analyses for LVtor parameters had excellent reliability in 64 of 80 subjects (80%) (intraclass correlation coefficients; 0.93-0.97). Early systolic twist (EST) motions (-8.4--0.1°) were observed in all subjects during an early 20±7% of systolic time intervals. The peak systolic twist and torsion were 17.0±6.5° and 2.9±1.3°/cm, respectively. The peak twist velocity was recorded at 51±13% of systolic time and the peak untwist velocity at 13.8±11.5% of diastolic time intervals. Multivariate analysis showed that heart rate change was an independent predictor of changes in torsion parameters; significantly decreasing LV length-normalized apical and basal rotation, torsion, and twist and untwist rate with increasing age. Isovolumetric recoil rate was independent of change in age and heart rate.
Conclusion
Left ventricle showed unique torsion mechanics in children with EST, torsion, and untwists. Heart rate was an independent predictor of the change in torsion parameters with aging.
Keywords: Ventricular function, Speckle tracking echocardiography, Torsion, Rotation, Child
Introduction
Left ventricular (LV) myocardial contraction and relaxation causes a twist and untwist motion due to the helical arrangement of myocardial fibers in the subendocardium and subepicardium of the LV.1),2) As viewed from the apex, the LV apex makes a counterclockwise rotation while the base of the LV makes a clockwise rotation during ventricular systole, and vice versa during ventricular diastole, resulting in LV twist or torsion and untwist motion during the cardiac cycle.2) Understanding of the three-dimensional (3D) LV deformation movements has provided a new insight into the ventricular functions that were previously unavailable with conventional analysis of LV contraction of the long and short axis.2,3,4)
Various techniques such as implanted radiopaque markers, biplane cine angiography, sonomicrometry, optical devices, magnetic resonance imaging (MRI), and echocardiography using tissue Doppler imaging have been used to explore LV twist motion in experimental and clinical settings, with limitations to clinical applicability.2),5,6,7)
Recent improvements in ultrasound technique using two-dimensional speckle tracking echocardiography (2D STE), a novel imaging technique for quantifying complex 3D cardiac motions, has the advantage of angle independency for easier measurement of LV twist and untwist movements. This technique was validated with sonomicrometry and tagged MRI.8,9,10,11,12) Several reports on LV torsion (LVtor) mechanics using STE and the age-related changes in normal children and adolescents have significant inter-study discrepancies.13,14,15,16)
Accordingly, we assessed the feasibility of 2D STE in measurement of LVtor mechanics, determined normal reference values of principal parameters of LVtor, and analyzed the age-related changes in normal healthy children.
Subjects and Methods
Study population
A total of 80 healthy children, aged 3 months to 15 years, were included in the study. They were referred to the cardiac outpatient clinic for echocardiography due to heart murmur, nonspecific chest pain, palpitation, dizziness or syncope, or history of a sibling with congenital heart disease. Comprehensive echocardiography according to our protocol confirmed normal cardiac structure and function, except for patent foramen ovale or trivial degree of tricuspid regurgitation. All subjects had normal sinus rhythm. The body weight and height of all subjects were recorded and the body surface area was derived. The Institutional Review Board of Seoul St. Mary's Hospital approved this study.
Echocardiographic assessment
All echocardiographic assessment and STE image acquisition were done by one designated pediatric cardiologist, using the Vivid 7 ultrasound machine (GE Medical Systems, Horten, Norway). Key images acquired for at least 3 cardiac cycles were saved to customized EchoPAC software (version 7.1; GE Medical Systems, Horten, Norway) for off-line analysis. All echocardiographic indices or images were obtained during a dominant heart rate ±5 beats per minutes in each subject for the optimization of time intervals between images during the cardiac cycle. Each time interval of events (events timing) was normalized by each cardiac cycle length to adjust to heart rate, thereby expressed as a percent (%) of systolic time interval for systolic events or a percent (%) of diastolic time for diastolic events. The average value of 3 cardiac cycles was used for analyses.
The routine echocardiographic examination included LV ejection fraction (LV EF) from standard M-mode assessment of the mid-LV parasternal long axis view, and mitral inflow and mitral septal tissue Doppler data from the apical 4-chamber view. For determining time interval during cardiac cycle, total cycle lengths were recorded and isovolumetric contraction time (IVCT), systolic time, isovolumetric relaxation time (IVRT), and diastolic time were derived from mitral inflow (mitral valve opening and mitral valve closure) and aortic outflow {aortic valve opening and aortic valve closure (AVC)} Doppler studies. Each time interval was normalized by cycle length to adjust to heart rate as mentioned above.
Two-dimensional speckle tracking echocardiography for left ventricular torsion
Two short-axis plain images (basal and apical) were obtained with appropriate echo probes matched to each subjects' body size at a frame rate range of 80-100 frame/sec to assess LVtor motion. Basal plain images were obtained at the level of mitral valve leaflets, as close as possible to the mitral valve annulus if all 6 basal myocardial segments were well defined. The apical plain images were obtained as close as possible to the LV apex, if circular LV apical lumen and myocardium were well defined. LV length was defined as the length between LV apical endocardium and the mitral valve annular line. It was measured from the apical 4-chamber image at end-diastole (at the onset of Q-wave on electrocardiogram). An independent physician trained to use the EchoPAC software but not involved in image acquisition, conducted the off-line STE analyses for LVtor movement, as described previously.15),17)
Terminology and measurements
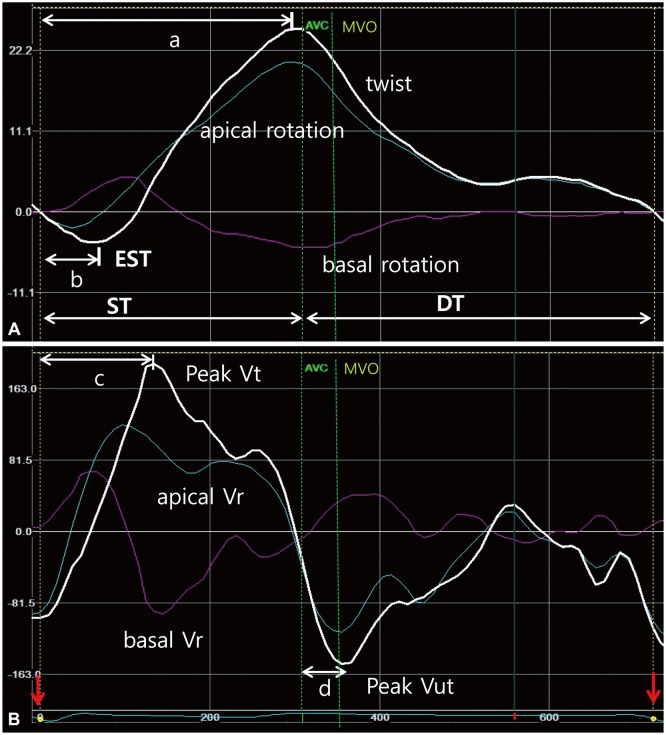
Rotation is a clockwise or counterclockwise movement around the long axis of the heart. As seen from the apex, counterclockwise rotation is expressed as a positive value and clockwise rotation is expressed as a negative one. LV twist was defined by the difference between apical rotation and basal rotation and was calculated as the difference between the counterclockwise apical rotation curve (positive) and clockwise basal rotation curve (negative) for each isochronal point from a discrete time derivative of the twist-time curve (Fig. 1A). Early systolic twist (EST) was defined as a transient LV twist during early systole including IVCT; and systolic twist (ST) was defined as a dominant LV twist during mid- and late systole (Fig. 1A). LVtor was defined as a value of LV ST normalized by LV longitudinal length {LVtor (degree/cm)=LV ST/LV length}. Time to peak EST, time to peak ST; time to peak twist velocity (Vt) during systole, and time to peak untwist velocity (Vut) were defined as in Fig. 1, and expressed as the percent (%) of each systolic time (from the onset of Q-wave to AVC), or diastolic time (from AVC to the end of cardiac cycle) to adjust for heart rate in each subject (Fig. 1). Isovolumic untwisting recoil was defined as a percent of LV untwisting during IVRT (=LV untwist during IVRT/peak LV twist×100, %), and isovolumic untwisting recoil rate as isovolumic untwisting recoil/IVRT (%/ms).
Fig. 1. Representative curves of left ventricular (LV) torsion movement. A: LV apical and basal rotation, and twist curves. Positive values express counterclockwise rotation or twist as seen from the apex. During early systole including isovolumetric contraction time, LV twists in opposite direction (early systolic twist, EST) of a dominant LV systolic twist during mid- or late systole. B: LV basal and apical rational velocity (Vr), and peak twist (Vt) and untwist (Vut) velocity curves. Peak twist velocity is observed at around a half of systolic time, whereas peak untwist velocity is seen during early diastolic period. Red arrows indicate the reference point of starting cardiac cycle (Q-wave). AVC: aortic valve closure, MVO: mitral valve opening, ST: systolic time, DT: diastolic time, a: time to peak systolic twist, b: time to peak EST, c: time to peak Vt during systole, d: time to peak Vut during diastole.
Reproducibility
Twenty-five subjects were randomly selected to assess intra- and interobserver variability for LVtor measurements. Two independent observers reanalyzed the data sets of apical rotation, basal rotation, and peak ST.
Statistical analysis
Data were analyzed using commercially available software, PASW Statistics (version 18 for windows; IBM, Armonk, NY, USA). Quantitative data were expressed as the mean±standard deviation. The associations between age, heart rate and each echocardiographic parameter were analyzed by Bivariate correlations with Pearson's correlation coefficients; age and heart rate were entered as covariates to predict changes in each torsion parameter in the multivariate regression analysis. The intra- and interobserver variability was evaluated using the reliability analysis providing intraclass correlation coefficients. A p<0.05 was considered statistically significant.
Results
Feasibility and reproducibility of speckle tracking echocardiography analysis for left ventricular torsion
A total of 80 children with good echocardiographic images for speckle tracking were included in the STE analyses for LVtor parameters. Among them, good speckle tracking and reliable measurements of torsion parameters were possible in 64 subjects (80%), who finally comprised of the study population for analysis. Demographic and routine standard echocardiography data were summarized in Table 1.
Table 1. Demographic and conventional echocardiography data.
| Variables | Value, mean±SD |
|---|---|
| Demographic data | |
| Male:female | 42:22 |
| Age (year) | 6.8±4.2 |
| Body weight (kg) | 27±16 |
| Body surface area (m2) | 0.9±0.4 |
| Heart rate (beats/min) | 91±17 |
| 2-dimensional echocardiography | |
| End-diastolic LV length (L) (cm) | 6.0±1.2 |
| End-diastolic LV diameter (D) (cm) | 3.2±0.7 |
| LV geometric index: D/L | 0.55±0.06 |
| LV ejection fraction (%) | 66±6 |
| Pulse-wave Doppler | |
| Early diastolic peak velocity (E) (m/s) | 1.09±0.16 |
| Late diastolic peak velocity (A) (m/s) | 0.59±0.14 |
| E/A ratio | 1.94±0.47 |
| MV E deceleration time (ms) | 139±20 |
| LV myocardial performance index | 0.24±0.07 |
| Tissue Doppler imaging (TDI) | |
| MV septal annular systolic velocity (S') (m/s) | 0.07±0.01 |
| MV septal annular early diastolic velocity (E') (m/s) | 0.13±0.02 |
| MV septal annular late diastolic velocity (A') (m/s) | 0.06±0.02 |
| E'/A' ratio | 2.26±0.72 |
| LV myocardial performance index by TDI | 0.42±0.08 |
| E/E' ratio | 8.58±2.18 |
LV: left ventricle, MV: mitral valve
The intra- and interobserver variability for LV basal rotation, apical rotation, and peak LV ST were assessed using reliability analysis. The results showing intraclass correlation coefficients >0.9 in all analyses with robust statistical significance were summarized in Table 2.
Table 2. Intraobserver and interobserver variability test (n=25).
| Intraclass correlation coefficients | 95% CI | p | |
|---|---|---|---|
| Intraobserver variability | |||
| Basal rotation | 0.96 | 0.92-0.98 | <0.001 |
| Apical rotation | 0.95 | 0.89-0.98 | <0.001 |
| Peak systolic twist | 0.97 | 0.94-0.99 | <0.001 |
| Interobserver variability | |||
| Basal rotation | 0.95 | 0.88-0.98 | <0.001 |
| Apical rotation | 0.93 | 0.85-0.97 | <0.001 |
| Peak systolic twist | 0.96 | 0.92-0.98 | <0.001 |
CI: confidence interval
Left ventricular early systolic events
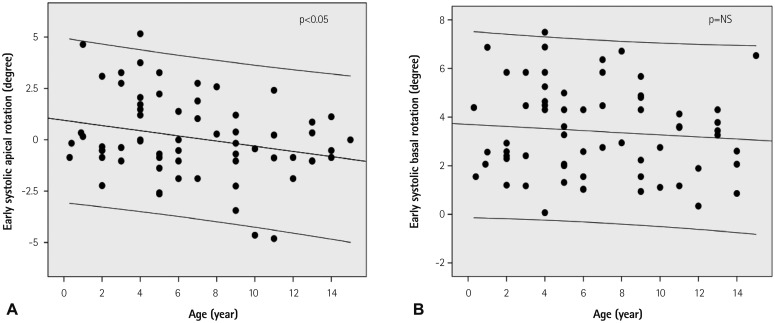
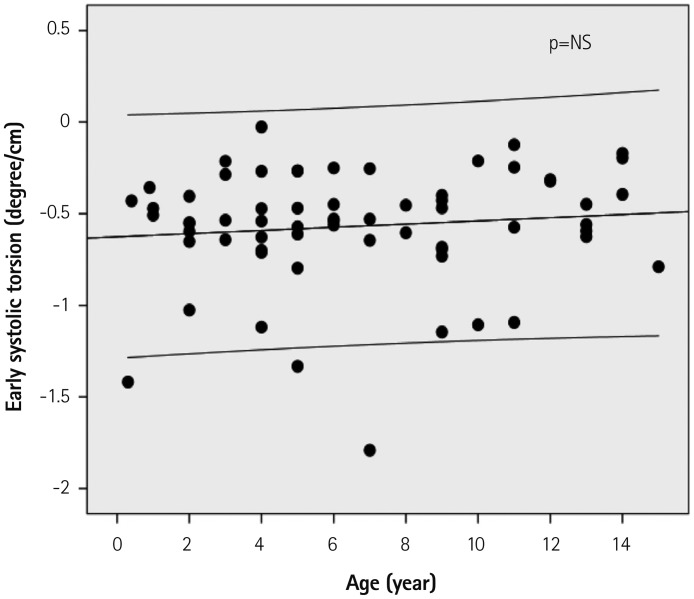
During the early systolic period including IVCT, transient basal counterclockwise rotations (range, 0.1-7.5°) were observed in all subjects, whereas apical rotations during that period were inconsistent (range, -4.8-5.16°), showing clockwise (n=34, 53%), counterclockwise (n=27, 42%) or no rotations (n=3, 5%) (Table 3, Fig. 2). The apical segment rotation motion changed more clockwise with increasing age (p<0.05) (Fig. 2A), whereas the basal segment motion did not change significantly with aging (Fig. 2B). As a result of the rotational movements of the LV basal and apical segments during the early systolic period, LV showed an EST (-3.3±1.7°, range; -8.4--0.1°) and EStor (-0.6±0.3°/cm, range; -1.8--0.3°/cm) in all subjects (Figs. 1 and 3, Table 3). The LV EST or EStor were not correlated with increasing age (Fig. 3). The time interval from the onset of LV contraction to the peak LV EST was 19.1±-6.9% of the total systolic time (Fig. 1), which did not change with increasing age.
Table 3. LV torsion data from speckle tracking echocardiography.
| Variables | Value, mean±SD (range) |
|---|---|
| Early systolic twist | |
| Basal rotation (deg) | 3.4±1.9 (0.1-7.5) |
| Apical rotation (deg) | 0.1±2.0 (-4.8-6.2) |
| Peak early systolic twist (deg) | -3.3±1.7 (-8.4--0.1) |
| Peak early systolic torsion (deg/cm) | -0.6±0.3 (-1.8--0.3) |
| Time to peak early systolic torsion (% ST) | 20±7 (3-33) |
| Systolic twist | |
| Basal rotation (deg) | -5.0±2.6 |
| Apical rotation (deg) | 13.9±5.0 |
| Peak basal rotation velocity (deg/s) | -82.8±25.9 |
| Peak apical rotation velocity (deg/s) | 101.3±37.6 |
| Peak systolic twist (deg) | 17.0±6.5 |
| Peak systolic torsion (deg/cm) | 2.9±1.3 |
| Peak systolic twist velocity (deg/s) | 142±48 |
| Peak torsion velocity, (deg/s)/cm | 24.9±11.0 |
| Time to peak twist (% ST) | 92±5 |
| Time to peak twist velocity (% ST) | 51±13 |
| Diastolic untwist | |
| LV untwisting during IVRT (deg) | -3.4±2.5 |
| LV untwisting velocity during IVRT (deg/s) | -98±66 |
| Isovolumic untwisting recoil (%)* | 20±15 |
| Isovolumic untwisting recoil rate (%/ms)† | 0.55±0.38 |
| Peak untwisting velocity (deg/s) | -158.0±61.4 |
| Peak untwisting velocity-N, (deg/s)/cm | -27.6±12.8 |
| Time to peak untwisting velocity (% DT) | 13.8±11.5 |
*LV untwisting during IVRT/peak LV twist×100, †Isovolumic untwisting recoil/ IVRT. LV: left ventricular, ST: systolic time, N: normalized by left ventricular length, IVRT: isovolumic relaxation time, DT: diastolic time
Fig. 2. Regression plots of early systolic apical (A) and basal rotation (B) with 95% confidence intervals. The apical segment showed clockwise (n=34), counterclockwise (n=27), or no rotation (n=3) during early systole, which changed more clockwise with increasing age (A). In contrast, the basal segment showed early systolic counterclockwise rotation in all subject, which did not change significantly with aging (B). NS: not significant.
Fig. 3. Regression plot of early systolic torsion (EStor) with 95% confidence interval. All showed negative values of Estor, which were in opposite direction of the dominant left ventricular systolic twist (positive value) during mid- and late systole. NS: not significant.
Left ventricular mid- and late systolic events
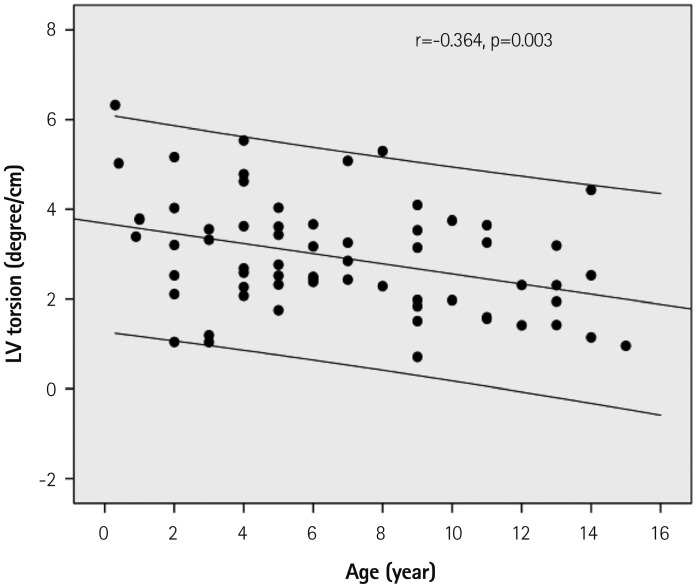
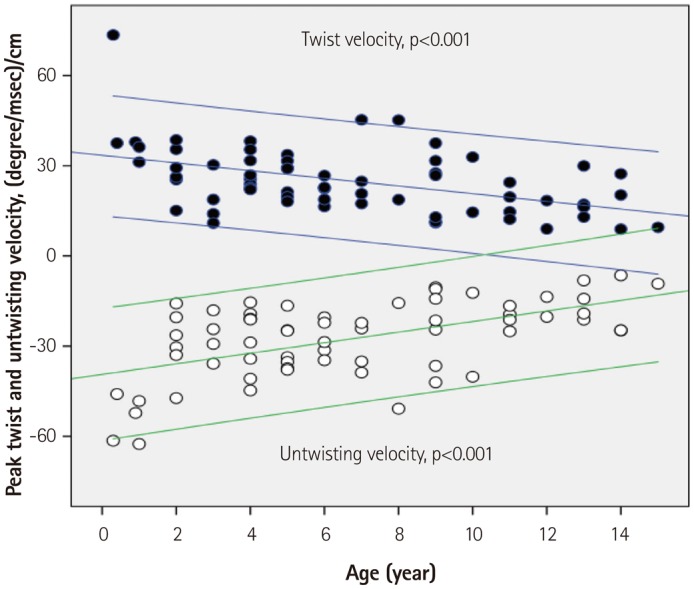
All subjects showed clockwise basal rotation (range; -12.9--1.4°) and counterclockwise apical rotation (range; 3.1-28.7°) during mid- and late systole (Figs. 1 and 4, Table 3). The rotation angle from baseline was significantly greater in the apical segment than in basal segment (13.9±5.0° vs. 5.0±2.6°, p<0.001). Peak apical counterclockwise rotation velocity was faster than peak basal clockwise rotation velocity (101.3±37.6° vs. 82.8±25.9°, p<0.001). When normalized by LV length, both apical and basal rotation decreased with aging (Fig. 4). LV ST began at 20±7% of systolic time (after the EST) and ended at 92±5% of systolic time (Fig. 1, Table 3). The peak ST was not correlated with increasing age, but LVtor decreased with aging (Fig. 5). The peak LV twist velocity was recorded at 51±13% of systolic time, and decreased with increasing age when normalized by LV length (Fig. 6).
Fig. 4. Regression plots of apical and basal rotation normalized by left ventricular length with 95% confidence interval. An age related decrease was observed.
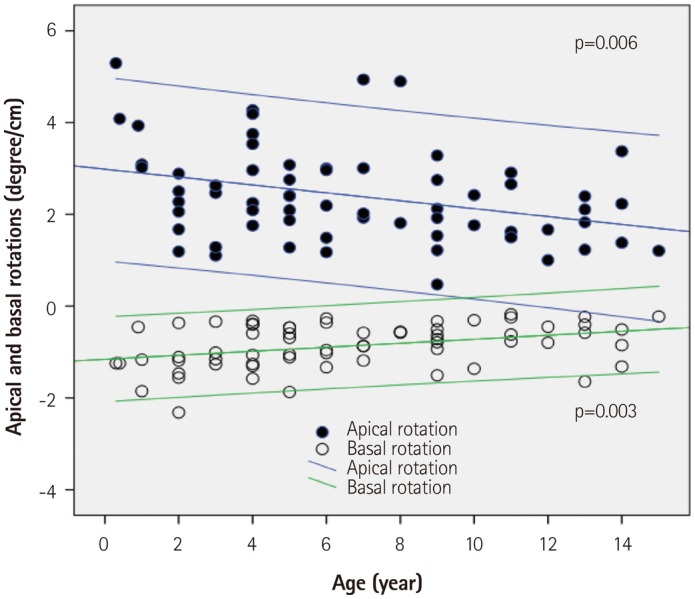
Fig. 5. Regression plot of the peak left ventricular (LV) torsion with 95% confidence interval, which decreased with increasing age.
Fig. 6. Regression plots of peak twist and untwisting velocities with 95% confidence intervals. When normalized by left ventricular length, peak twist and untwisting velocities decreased with increasing age.
Left ventricular diastolic events
Given that LV ST motion ended at 92±5% of systolic time, LV untwist motion began before the AVC and reached peak untwisting velocity at 13.8±11.5% of diastolic time (Fig. 1, Table 3). Peak untwisting velocity decreased with increasing age when normalized by LV length (Fig. 6). Isovolumic untwisting recoil (LV untwist during IVRT) was 20±15%, and the isovolumic untwisting recoil rate was 0.55±0.38%/ms, which was unchanged with increasing age.
Correlations and age related changes
The peak LV ST showed good correlations with systolic basal and apical rotation, peak basal and apical rotation velocity, peak ST velocity, and peak untwisting velocity (Table 4). EST, isovolumic untwisting recoil, or recoil rate was not correlated with the peak LV twist.
Table 4. Correlations between peak LV systolic twist and other variables.
| Variables | Peak LV twist | |
|---|---|---|
| Pearson's correlation | p | |
| Early systolic twist | 0.122 | NS |
| Systolic basal rotation | -0.492 | <0.001 |
| Systolic apical rotation | 0.860 | <0.001 |
| Peak basal rotation velocity | -0.341 | 0.006 |
| Peak apical rotation velocity | 0.549 | <0.001 |
| Peak systolic twist velocity | 0.805 | <0.001 |
| Peak untwisting velocity | -0.685 | <0.001 |
| Isovolumic untwisting recoil | 0.178 | NS |
| Isovolumic untwisting recoil rate | 0.125 | NS |
LV: left ventricular, NS: not significant
Table 5 shows correlations of torsion parameters with age and heart rate. Most torsion parameters including rotations, twist, torsion, and velocity parameters showed significant correlation with age and heart rate. However, multivariate analysis showed that the only heart rate was an independent predictor of the torsion parameters (Table 5). Parameters during IVRT including LV untwisting velocity during IVRT, isovolumic untwisting recoil, isovolumic untwisting recoil rate did not show any significant correlations with age and heart rate. Time to peak untwisting velocity showed good correlation with heart rate, but other event timings were constant throughout aging and not correlated with heart rate.
Table 5. Correlations of torsion parameters with age and heart rate.
| Variables | Univariate p* | Multivariate p† | ||
|---|---|---|---|---|
| Age | Heart rate | Age | Heart rate | |
| Torsion parameters | ||||
| Peak basal rotation (deg/cm) | 0.003 | <0.001 | NS | 0.03 |
| Peak apical rotation (deg/cm) | 0.006 | <0.001 | NS | 0.012 |
| Peak basal rotation velocity (deg/s) | 0.016 | <0.001 | NS | <0.001 |
| Peak apical rotation velocity (deg/s) | NS | 0.005 | NS | 0.001 |
| Peak systolic torsion (deg/cm) | 0.003 | <0.001 | NS | 0.008 |
| Peak systolic twist velocity (deg/s) | NS | 0.001 | NS | 0.001 |
| Peak torsion velocity, (deg/s)/cm | <0.001 | <0.001 | NS | <0.001 |
| Peak untwisting velocity (deg/s) | 0.028 | 0.001 | NS | <0.05 |
| Peak untwisting velocity-N, (deg/s)/cm | <0.001 | <0.001 | NS | <0.001 |
| LV untwisting velocity during IVRT (deg/s) | NS | NS | NS | NS |
| Isovolumic untwisting recoil (%) | NS | NS | NS | NS |
| Isovolumic untwisting recoil rate (%/ms) | NS | NS | NS | NS |
| Events timing | ||||
| Time to peak early systolic torsion (% ST) | NS | NS | NS | NS |
| Time to peak twist (% ST) | NS | NS | NS | NS |
| Time to peak twist velocity (% ST) | NS | NS | NS | NS |
| Time to peak twist (% ST) | NS | NS | NS | NS |
| Time to peak twist velocity (% ST) | NS | NS | NS | NS |
| Time to peak untwisting velocity (% DT) | NS | 0.002 | NS | 0.001 |
*Bivariate correlation analysis, †Multivariate linear regression analysis. N: normalized by left ventricular length, IVRT: isovolumic relaxation time, ST: systolic time, DT: diastolic time, LV: left ventricular
Discussion
We assessed LVtor deformation using 2D STE in healthy infants and children (aged 3 months to 15 years) and described their characteristics in detail. The major findings were as follows: during early systolic periods (about 20% of systolic time) including IVCT, a transient early systolic negative twist or torsion with a direction opposite to the dominant late ST was observed in all subjects; during mid- and late systole, basal clockwise rotation and apical counterclockwise rotation was observed in all subjects, resulting in positive LV twist, which decreased with aging when normalized to LV length; the peak LV twist occurred around 90% of systolic time with peak twist velocity around 50% of systolic time; LV untwist began late systole with 20% untwisting during IVRT, and showed peak untwisting velocity during early diastolic time; and the peak twist and untwisting velocity decreased with aging when normalized by LV length. The feasibility and reproducibility were acceptable.
Feasibility and reliability
It was possible to get good quality STE data sets of basal and apical slices for reliable measurements of torsion parameters in 80% of subjects. There are no previous reports on the feasibility of STE for measuring torsion parameters in only children. Previous reports in adults showed relatively low feasibility (35-75%), which might be a challenging issue in STE measures of torsion mechanics in adults.17,18,19) One study including children and young adults aged 3 to 40 years (53 children <16 years) reported that complete data sets could be obtained in only 66% of controls.13) Reliable measurements can be obtained from good quality of 2D images with a good echo window, adequate frame rates, and without dropouts of ultrasound data throughout the cardiac cycle. It is more challenging to obtain good quality 2D images in adults than in children, because of large body size and relatively poor echo window. Our data indicated that measurements of torsion parameters using STE is feasible in children. Additionally, intra- and interobserver reliability were excellent with high intraclass correlation coefficients. These findings suggest that STE is a good imaging modality for assessing complex LV deformation in children.
Early systolic events
During the early systolic period (the first ~20% of systolic time), transient clockwise basal rotation in all subjects, and changing apical rotation pattern from counterclockwise to clockwise apical rotation with aging were observed. Hence an 'early systolic LV twist' that was opposite in direction to the dominant LV ST during late systole was observed in all subjects. Some reports have addressed early systolic rotation patterns by using different imaging techniques with inter-study discrepancies.8),13),17),19) The reason for this discrepancy remains unclear and has been attributed to the difference in temporal resolution between imaging techniques.2),8),11) A similar pattern of early systolic rotation and twist (~-2°) was confirmed only by STE in an earlier study using MRI and STE in adults.8) Another study in adults by van Dalen et al.19) also confirmed the early systolic rotation patterns, in which the magnitude of basal rotation decreased with aging. Takahashi et al.13) reported early systolic counterclockwise basal rotation (-4.5°) in children, but did not address apical rotation in their study.
The mechanism of the EST motion is explained by a time delay in an electrical activation, thereby leading to a physiological asynchrony of mechanical shortening between the subendocardial and subepicardial regions.1),2),13) Because of the sequential transmural spread of electrical activation from the subendocardial to subepicardial region, the shortening of inner subendocardial fibers with right-handed helix is accompanied by stretching of the outer subepicardial fibers with left-handed helix during IVCT.1),2),20),21)
We firstly confirmed that the LV during childhood, although not fully matured, also showed a unique pattern of rotation and twist during the early systolic period.
Left ventricular mid- and late systolic events
We also demonstrated a uniform LV counter clockwise apical and clockwise basal rotation, and a 'positive' LV twist or torsion during mid- and late systole as previously reported. However, the magnitude of rotations, twist, and torsion, and age related changing patterns of these parameters were quite different from previous studies. 7),13,14,15) Takahashi et al.13) reported normal values of torsion parameters in 111 volunteers including 53 children (3-16 years), and showed mean peak LV twist of around 11°, and mean peak LVtor around 1.65°/cm, which were lower in magnitude compared to our study (peak ST; 17.0±6.5°, peak systolic torsion; 2.9±1.3°/cm). The magnitude of basal rotation was similar, but apical rotation of their study was smaller, with a smaller peak LV twist, as compared to our study. Zhang et al.15) also reported normal data in children with much smaller magnitudes of rotation and twist values than our results. The reason for these discrepancies between studies is not clear and may be a major limitation to STE for LVtor movement measures. Furthermore, these reported data may not be useful as reference values in clinical practice.
Because the rotation angle in a cylinder is proportional to shaft length, the farther the slice from the equator level, the greater rotation angle recorded even in the same heart.22) Additionally, since the equator level of LV twist is around one-third of LV length from the LV base, the magnitude of LV apical rotation is greater than that of basal rotation.23) One possible explanation for the inter-study discrepancies is difference in image acquisition sites for STE analysis, especially the position of LV apical slice sampling. Parisi et al.24) compared LVtor measurements between 2D and 3D STE in 30 healthy subjects, and demonstrated that the 2D LV apical level acquisition, even when recorded in a standard manner, determines variability of measurements; the twist value was higher when the apical rotation was measured from an apical section closer to the apex. We made every effort to get adequate images in terms of sampling position of as close as possible to the LV base and apex for each image slice, which may explain the higher values of LV twist motions. Thus, current anatomical markers for image acquisition adopted from the previous studies need to be redefined to overcome the pitfalls of this imaging technique.
Diastolic events
Diastolic untwist begins immediately after the peak ST, actually before closure of the aortic valve, and reaches its peak velocity during early diastolic period. A significant amount of untwisting occurs in the early diastolic period, especially IVRT (about 20% in this study). These findings were consistent with the previously reported results.13,14,15),25) Myocardial relaxation is dependent on the rates of cross-bridge deactivation within the sarcomere and is associated with the release of restoring forces stored in the ventricular wall during systole.25) Rapid LV untwisting during early diastole decrease in LV pressure rapidly during IVRT and enables the LV to suck blood effectively during mitral valve opening. Dong et al.25) reported that the isovolumic untwisting recoil rate was closely correlated (r= -0.86, p<0.0001) with the relaxation time constant (τ), independent of loading conditions, in an animal study using MRI tagging. They suggested that this noninvasive parameter could be a good tool for preload independent assessment of LV relaxation.25) We also derived the isovolumic untwisting recoil rate (0.55±0.38%/ms) using STE, which was constant throughout aging, and might be a handy tool to assess LV diastolic function in various clinical settings.
Correlations and age related change
The peak LV basal and apical rotation, and twist did not change with increasing age, but decreased significantly with aging when normalized by LV length. The LV peak twist and untwisting velocity normalized by LV length decreased significantly with aging. Previous reports on the changing patterns during childhood also showed significant inter-study discrepancies of unexplained reasons.7),13,14,15) We speculated that differences in the position of imaging acquisition for each apical and basal slice may partially explain inter-study discrepancies.
The mechanism of age related changes in torsion mechanics have been attributed to changing or maturation patterns of calcium transport into the sarcoplasmic reticulum, alterations in connective tissue and relative proportions of 2 titin isoforms (N2B and N2BA), which are responsible for elastic recoil within the sarcomere, and change in relative contributions of subendocardial and subepicarcial myocardium to age-related LVtor mechanics.7),13),15) However, while reasonable to speculate, it is difficult to directly prove the associations between the physiologic or histopathologic changes in LV and aged-related LVtor mechanics. We clearly and firstly demonstrated by multivariate analysis that the change in heart rate is responsible for changes in LVtor mechanics, especially velocity profiles during childhood.
Event timings may increase with aging along with decrease in heart rates.13),14),19) However, all event timings including time to peak twist, torsion, peak ST velocity, and peak diastolic untwisting velocity were constant with childhood ages when standardized with cardiac cycle length (systolic and diastolic time intervals). Given that heart rate change is significant during childhood, especially during infancy, it should be incorporated into the analyses of LV mechanics. Interestingly, isovolumic untwisting recoil and isovolumic untwisting recoil rate were independent of changes in age and heart rate. This finding was consistent with the result by Dong et al.,25) and may provide a novel parameter of assessing LV relaxation.
Clinical implications
Clinical application of torsion parameters is still limited but emerging. Cheung et al.26) studied LVtor mechanics in 36 childhood cancer survivors, and found that impairment of LVtor mechanics is evident in children after anthracycline therapy, even in those with "normal" LV EFs. Another recent study on 14 children with genotype-positive, phenotype-negative hypertrophic cardiomyopathy (HCM) showed that children with genetically proven HCM had increased LV rotation and twist, as compared to normal controls, before the development of hypertrophy.27) The authors suggested that apical rotation by STE may be a clinically useful early marker of HCM before the onset of hypertrophy.27) Additionally, Khoo et al.28) reported that patients with normal LV EFs and cardiovascular magnetic resonance features of myocarditis had altered LVtor parameters when compared to normal controls. These results collectively suggest that the novel echocardiographic torsion parameters may improve early detection of patients with important "subclinical" LV dysfunction in various clinical settings.28)
Limitations
Since this study was done in children up to 15 years of age, some subjects needed medications for sedation such as chloral hydrate, which might have influenced the LV function, heart rate, or blood pressure, thereby leading to change in LVtor mechanics. The other important limitation of this study was associated with the fundamental drawback of 2D STE, in which the levels of image acquisition for the basal and apical rotation could not yet be clearly defined. However, all efforts were made to get good quality images, and to get imaging slices as far as possible from the equator level of LV twist that may provide more accurate parameters of LVtor mechanics. Thus, current anatomical markers for image acquisition as described in the previous studies may be redefined to overcome the pitfalls of this imaging technique.
Conclusions
We assessed and described LVtor mechanics in detail with the determination of normal reference values of LVtor parameters in children aged 3 months to 15 years. Although not fully matured, LV in infants and children also showed unique torsion mechanics, including early systolic negative twist during IVRT, systolic positive twist, and early diastolic untwist. Age related changes of rotation, torsion, and velocity parameters were evident and closely related to changes in heart rate with increasing age.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Sengupta PP, Korinek J, Belohlavek M, et al. Left ventricular structure and function: basic science for cardiac imaging. J Am Coll Cardiol. 2006;48:1988–2001. doi: 10.1016/j.jacc.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 2.Sengupta PP, Tajik AJ, Chandrasekaran K, Khandheria BK. Twist mechanics of the left ventricle: principles and application. JACC Cardiovasc Imaging. 2008;1:366–376. doi: 10.1016/j.jcmg.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen PM, Le Grice IJ, Smaill BH, Hunter PJ. Mathematical model of geometry and fibrous structure of the heart. Am J Physiol. 1991;260(4 Pt 2):H1365–H1378. doi: 10.1152/ajpheart.1991.260.4.H1365. [DOI] [PubMed] [Google Scholar]
- 4.Vendelin M, Bovendeerd PH, Engelbrecht J, Arts T. Optimizing ventricular fibers: uniform strain or stress, but not ATP consumption, leads to high efficiency. Am J Physiol Heart Circ Physiol. 2002;283:H1072–H1081. doi: 10.1152/ajpheart.00874.2001. [DOI] [PubMed] [Google Scholar]
- 5.Young AA, Cowan BR. Evaluation of left ventricular torsion by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012;14:49. doi: 10.1186/1532-429X-14-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Notomi Y, Setser RM, Shiota T, et al. Assessment of left ventricular torsional deformation by Doppler tissue imaging: validation study with tagged magnetic resonance imaging. Circulation. 2005;111:1141–1147. doi: 10.1161/01.CIR.0000157151.10971.98. [DOI] [PubMed] [Google Scholar]
- 7.Notomi Y, Srinath G, Shiota T, et al. Maturational and adaptive modulation of left ventricular torsional biomechanics: Doppler tissue imaging observation from infancy to adulthood. Circulation. 2006;113:2534–2541. doi: 10.1161/CIRCULATIONAHA.105.537639. [DOI] [PubMed] [Google Scholar]
- 8.Helle-Valle T, Crosby J, Edvardsen T, et al. New noninvasive method for assessment of left ventricular rotation: speckle tracking echocardiography. Circulation. 2005;112:3149–3156. doi: 10.1161/CIRCULATIONAHA.104.531558. [DOI] [PubMed] [Google Scholar]
- 9.Amundsen BH, Helle-Valle T, Edvardsen T, et al. Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol. 2006;47:789–793. doi: 10.1016/j.jacc.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 10.Urbano Moral JA, Arias Godinez JA, Maron MS, et al. Left ventricular twist mechanics in hypertrophic cardiomyopathy assessed by three-dimensional speckle tracking echocardiography. Am J Cardiol. 2011;108:1788–1795. doi: 10.1016/j.amjcard.2011.07.047. [DOI] [PubMed] [Google Scholar]
- 11.Leitman M, Lysyansky P, Sidenko S, et al. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr. 2004;17:1021–1029. doi: 10.1016/j.echo.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 12.Blessberger H, Binder T. NON-invasive imaging: two dimensional speckle tracking echocardiography: basic principles. Heart. 2010;96:716–722. doi: 10.1136/hrt.2007.141002. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi K, Al Naami G, Thompson R, Inage A, Mackie AS, Smallhorn JF. Normal rotational, torsion and untwisting data in children, adolescents and young adults. J Am Soc Echocardiogr. 2010;23:286–293. doi: 10.1016/j.echo.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 14.Al-Naami GH. Torsion of young hearts: a speckle tracking study of normal infants, children, and adolescents. Eur J Echocardiogr. 2010;11:853–862. doi: 10.1093/ejechocard/jeq078. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Zhou QC, Pu DR, Zou L, Tan Y. Differences in left ventricular twist related to age: speckle tracking echocardiographic data for healthy volunteers from neonate to age 70 years. Echocardiography. 2010;27:1205–1210. doi: 10.1111/j.1540-8175.2010.01226.x. [DOI] [PubMed] [Google Scholar]
- 16.Kaku K, Takeuchi M, Tsang W, et al. Age-related normal range of left ventricular strain and torsion using three-dimensional speckle-tracking echocardiography. J Am Soc Echocardiogr. 2014;27:55–64. doi: 10.1016/j.echo.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Kim HK, Sohn DW, Lee SE, et al. Assessment of left ventricular rotation and torsion with two-dimensional speckle tracking echocardiography. J Am Soc Echocardiogr. 2007;20:45–53. doi: 10.1016/j.echo.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Takeuchi M, Nakai H, Kokumai M, Nishikage T, Otani S, Lang RM. Age-related changes in left ventricular twist assessed by two-dimensional speckle-tracking imaging. J Am Soc Echocardiogr. 2006;19:1077–1084. doi: 10.1016/j.echo.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 19.van Dalen BM, Soliman OI, Vletter WB, ten Cate FJ, Geleijnse ML. Age-related changes in the biomechanics of left ventricular twist measured by speckle tracking echocardiography. Am J Physiol Heart Circ Physiol. 2008;295:H1705–H1711. doi: 10.1152/ajpheart.00513.2008. [DOI] [PubMed] [Google Scholar]
- 20.Sengupta PP, Khandheria BK, Korinek J, Wang J, Belohlavek M. Biphasic tissue Doppler waveforms during isovolumic phases are associated with asynchronous deformation of subendocardial and subepicardial layers. J Appl Physiol (1985) 2005;99:1104–1111. doi: 10.1152/japplphysiol.00191.2005. [DOI] [PubMed] [Google Scholar]
- 21.Ashikaga H, Coppola BA, Hopenfeld B, Leifer ES, McVeigh ER, Omens JH. Transmural dispersion of myofiber mechanics: implications for electrical heterogeneity in vivo. J Am Coll Cardiol. 2007;49:909–916. doi: 10.1016/j.jacc.2006.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taber LA, Yang M, Podszus WW. Mechanics of ventricular torsion. J Biomech. 1996;29:745–752. doi: 10.1016/0021-9290(95)00129-8. [DOI] [PubMed] [Google Scholar]
- 23.Beyar R, Yin FC, Hausknecht M, Weisfeldt ML, Kass DA. Dependence of left ventricular twist-radial shortening relations on cardiac cycle phase. Am J Physiol. 1989;257(4 Pt 2):H1119–H1126. doi: 10.1152/ajpheart.1989.257.4.H1119. [DOI] [PubMed] [Google Scholar]
- 24.Parisi V, Losi MA, Contaldi C, et al. Speckle-tracking analysis based on 2D echocardiography does not reliably measure left ventricular torsion. Clin Physiol Funct Imaging. 2013;33:117–121. doi: 10.1111/cpf.12002. [DOI] [PubMed] [Google Scholar]
- 25.Dong SJ, Hees PS, Siu CO, Weiss JL, Shapiro EP. MRI assessment of LV relaxation by untwisting rate: a new isovolumic phase measure of tau. Am J Physiol Heart Circ Physiol. 2001;281:H2002–H2009. doi: 10.1152/ajpheart.2001.281.5.H2002. [DOI] [PubMed] [Google Scholar]
- 26.Cheung YF, Li SN, Chan GC, Wong SJ, Ha SY. Left ventricular twisting and untwisting motion in childhood cancer survivors. Echocardiography. 2011;28:738–745. doi: 10.1111/j.1540-8175.2011.01429.x. [DOI] [PubMed] [Google Scholar]
- 27.Forsey J, Benson L, Rozenblyum E, Friedberg MK, Mertens L. Early changes in apical rotation in genotype positive children with hypertrophic cardiomyopathy mutations without hypertrophic changes on two-dimensional imaging. J Am Soc Echocardiogr. 2014;27:215–221. doi: 10.1016/j.echo.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Khoo NS, Smallhorn JF, Atallah J, Kaneko S, Mackie AS, Paterson I. Altered left ventricular tissue velocities, deformation and twist in children and young adults with acute myocarditis and normal ejection fraction. J Am Soc Echocardiogr. 2012;25:294–303. doi: 10.1016/j.echo.2011.10.010. [DOI] [PubMed] [Google Scholar]