Abstract
The significant morbidity and mortality associated with coronary artery disease has spurred the development of intravascular imaging devices to optimize the detection and assessment of coronary lesions and percutaneous coronary interventions. Intravascular ultrasound (IVUS) uses reflected ultrasound waves to quantitatively and qualitatively assess lesions; integrated backscatter and virtual histology IVUS more precisely characterizes plaque composition; angioscopy directly visualize thrombus and plaque; optical coherence tomography using near-infrared (NIR) light with very high spatial resolution provides more accurate images; and the recently introduced NIR spectroscopy identifies chemical components in coronary artery plaques based on differential light absorption in the NIR spectrum. This article reviews usefulness of these devices and hybrids thereof.
Keywords: Coronary artery disease, Diagnostic imaging, Percutaneous coronary intervention
Introduction
Despite considerable medical advances, coronary artery disease (CAD) remains one of the leading causes of death with a rising global incidence fueled by increasing proportions of elderly individuals and adoption of western-style diets and sedentary lifestyles.1) The last decades have witnessed meaningful progress in CAD diagnosis and treatment, including the introduction of intravascular imaging modalities to overcome the limited ability of traditional 2f-dimensional coronary angiography or "lumenography" to evaluate underlying vessel changes and hidden plaque burden, and its inability to identify plaque characteristics.2),3) This article reviews the role of intravascular imaging devices in pre-interventional analysis of lesion morphology and plaque composition; optimization of percutaneous coronary intervention (PCI); and neointimal stent coverage assessment at follow-up. Table 1 provides an overview of strengths and weaknesses of the intravascular imaging devices discussed below.
Table 1. Strengths and weaknesses of intravascular imaging devices.
| Characteristic | IVUS | VH IVUS | Angioscopy | OCT | NIRS |
|---|---|---|---|---|---|
| Axial resolution (µm) | 100 | 200 | 10-50 | 10-50 | NA |
| Assessment of lesion severity | ++ | ||||
| Identification of TCFA | ++ | ++ | +++ | + | |
| Identification of necrotic core | + | + | + | ++ | |
| Optimization of stent implantation | ++ | + | |||
| Evaluation of stent tissue coverage | + | + | ++ | ++ | |
| Assessment of stent failure | ++ | ++ |
IVUS: intravascular ultrasound, VH-IVUS: virtual histology-IVUS, OCT: optical coherence tomography, NIRS: near-infrared spectroscopy, TCFA: thin-cap fibroatheroma
Intravascular Ultrasound
Current intravascular ultrasound (IVUS), with 20-45 MHz frequ-ency and 100-200 µm axial resolution, is the most commonly used intravascular imaging modality pre-, during, and post-PCI. Grayscale IVUS allows the measurement of lumen, vessel, and plaque areas; qualitative assessment of preinterventional plaque composition; and guidance in stent use optimization.4) Preinterventional IVUS imaging identifies lesions at high risk for no-reflow or periprocedural myocardial infarction (MI). Attenuated plaque, defined as hypoechoic plaque with deep ultrasound attenuation without calcification or very dense fibrous plaque, is associated with a higher frequency of no reflow (26.7% vs. 4.6%, p<0.001) and deteriorated post-PCI coronary blood flow (8.0% vs. 2.8%, p=0.001) as compared with non-attenuated plaque.5) IVUS also allows the detection of calcified plaques which increase the risk of stent underexpansion (71% vs. 14%, p=0.007) and requires additional pre-stent implantation procedures, such as cutting balloon angioplasty or rotational atherectomy to obtain optimal stent expansion.6),7),8) IVUS-guided PCI in the pre-drug-eluting stent (DES) era significantly lowered restenosis and repeat revascularization rates by optimizing stent expansion, with a neutral effect on death and MI.9) Recent meta-analysis has shown that IVUS-guidance is associated with a lower risk of death, MI, repeat revascularization, and stent thrombosis after DES implantation.10),11) It seems that IVUS evaluations of stent underexpansion, malapposition, incomplete lesion coverage, and residual plaque contribute to reduce not only restenosis, but also thrombosis and improve clinical outcomes. However, it is still controversial whether routine IVUS guidance improves clinical outcomes in the DES era (Table 2). IVUS-guided optimization of stent deployment through the determination of stent size, length, and landing zone also improves clinical outcomes regardless of stent type.9),11),12),13)
Table 2. Odds ratio for major adverse cardiac events in IVUS-versus angiography-guided PCI.
| Study or subgroup | Year | MACE | IVUS | Angiography | Odds ratio | 95% CI | |||
|---|---|---|---|---|---|---|---|---|---|
| Event | Total | Event | Total | ||||||
| Pre-DES era | RESIST49) | 2000 | Death, MI, unstable angina or TLR at 18 months | 20 | 79 | 28 | 76 | 0.59 | 0.30-1.16 |
| SIPS50) | 2000 | Death, MI, TLR at 2 years | 30 | 121 | 55 | 148 | 0.75 | 0.30-1.16 | |
| OPTICUS51) | 2001 | Death, MI, CABG, RCR at 12 months | 52 | 273 | 49 | 275 | 1.21 | 0.77-1.90 | |
| TULIP52) | 2003 | Death, MI, TLR at 6 months | 4 | 73 | 14 | 71 | 0.40 | 0.17-0.93 | |
| Gaster et al.53) | 2003 | Death, MI, any revascularization (median 2.5 years) | 12 | 54 | 22 | 54 | 0.42 | 0.19-0.97 | |
| DIPOL54) | 2007 | Death, MI, RCR at 6 months | 6 | 83 | 13 | 80 | 0.42 | 0.16-1.13 | |
| AVID55) | 2009 | Death, MI, TLR, ST, CABG at 12 months | 68 | 369 | 70 | 375 | 0.98 | 0.68-1.42 | |
| DES era | Roy et al.56) | 2008 | Death, MI, TVR at 12 months | 128 | 884 | 143 | 884 | 0.88 | 0.68-1.14 |
| MAIN-COMPARE57) | 2009 | Death, MI, TVR at 3 years | 145 | 145 | 0.47 | 0.27-0.80 | |||
| HOME DES IVUS58) | 2010 | Death, MI, RCR at 18 months | 11 | 105 | 12 | 105 | 0.91 | 0.39-2.12 | |
| Claessen et al.59) | 2011 | Cardiac death, MI, TVR at 2 years | 85 | 631 | 148 | 873 | 0.81 | 0.61-1.08 | |
| Kim et al.60) | 2011 | Death, MI, TLR at 3 years | 53 | 487 | 59 | 487 | 0.73 | 0.44-1.19 | |
| Youn et al.61) | 2011 | Death, MI, TLR, TVR at 3 years | 16 | 125 | 39 | 216 | 0.66 | 0.35-1.25 | |
| EXCELLENT62) | 2013 | Cardiac death, MI, TLR at 12 months | 34 | 619 | 31 | 802 | 1.45 | 0.88-2.38 | |
| Ahn et al.63) | 2013 | Cardiac death, MI, TLR, ST at 2 years | 4 | 49 | 12 | 36 | 0.17 | 0.05-0.60 | |
| IRIS-DES64) | 2013 | Death, MI, TVR at 3 years | 54 | 1616 | 88 | 1628 | 0.60 | 0.43-0.86 | |
| Chen et al.65) | 2013 | Cardiac death, ST, MI, TLR, TVR at 12 months | 51 | 324 | 60 | 304 | 0.76 | 0.50-1.15 | |
| AVIO66) | 2013 | Death, MI, TVR at 2 years | 24 | 142 | 33 | 142 | 0.67 | 0.37-1.21 | |
| Hur et al.67) | 2013 | Death, MI, TVR, ST at 3 years | 2765 | 1816 | 0.85 | 0.71-1.03 | |||
| RESET68) | 2013 | Cardiac death, MI, TVR at 12 months | 12 | 269 | 20 | 274 | 0.59 | 0.28-1.24 | |
| ADAPT-DES69) | 2014 | Cardiac death, MI, ST at 12 months | 103 | 3349 | 238 | 5234 | 0.67 | 0.53-0.84 | |
IVUS: intravascular ultrasound, PCI: percutaneous coronary intervention, MACE: major adverse cardiac event, CI: confidence interval, DES: drug eluting stent, MI: myocardial infarction, TLR: target lesion revascularization, CABG: coronary artery bypass graft, RCR: repeat coronary revascularization, TVR: target vessel revascularization, ST: stent thrombosis
While it is difficult to differentiate lipid cores from fibrous tissue with grayscale IVUS, integrated backscatter IVUS more precisely defines tissue characteristics by presenting color-coded maps reflecting the structural and biochemical composition of atherosclerotic lesions.14),15) Virtual histology (VH) IVUS also allows a more accurate classification of plaque composition using a spectral analysis of radiofrequency data with electrocardiogram gating as follows: fibrous tissue (dark green), fibrofatty tissue (light green), necrotic core (red), and dense calcium (white).16) However, the current classification tree for analysis cannot clearly determine the presence of an intramural thrombus.17)
The potential value of VH IVUS-derived plaque types in the prediction of future adverse coronary events was evaluated in the Providing Regional Observations to Study Predictors of Events in the Coronary Tree (PROSPECT) study.18) The PROSPECT study was a natural history study of acute coronary syndrome (ACS) patients: all patients underwent PCI for a culprit lesion at baseline, followed by an angiogram and VH IVUS analyses of the three major coronary arteries. Clinical events occurring during the follow-up period were equally attributable to recurrence at the site of the culprit lesion and to nonculprit lesions. Although nonculprit lesions responsible for unanticipated events were frequently angiographically mild, most were characterized by a large plaque burden (≥70%), a small luminal area (≤4.0 mm2, or thin-cap fibroatheromas (TCFA; defined as a lesion fulfilling the following criteria in at least three consecutive frames: necrotic core ≥10% without evident overlying fibrous tissue, and percent atheroma volume ≥40%).
Plaque composition was also associated with the occurrence of distal embolization after PCI. Observational studies showed a clear relationship between the amount of necrotic core and distal embolization and the implied the need for adjunctive pharmacological or device-based interventions to reduce the incidence of distal embolizations.19),20),21)
Angioscopy
Angioscopy consists of a specially desinged fiberscope for coronary use and enables macroscopic pathological diagnosis of cardiovascular diseases from the inside.22) Angioscopy is a useful tool to detect thrombi and distinguish between the content of the thrombi (platelet- or fibrin-rich) based on their color (white or red). It can be also used to detect vulnerable plaque. A normal coronary artery appears as glistening white, whereas atherosclerotic plaque can be categorized as yellow or white.23) Thus, a higher yellow color intensity likely represents the lipid pool underneath a thin-fibrous cap. Currently, percutaneous angioscopy is mainly used to evalute the stabilization and regression of vulnerable plaques by medical, interventional, and surgical therapies. It is also useful in visualizing vulnerable stents: a lack of endothelization and stent malapposition can both be detected with this modality.24),25) However, the histological and molecular changes inside the plaque cannot be evaluated because angioscopic observation is limited to surface color and morphology. Also, quantitative assessment ofr color, distance, and volume is difficult.
Optical Coherence Tomography
Optical coherence tomography (OCT) is a catheter-based technology that provides high-resolution cross-sectional tissue images from backscattered infrared light with an axial resolution of 12-15 µm, 10 times higher that of IVUS.26) Despite providing in vivo images with better histological detail, the broad use of time-domain (TD) OCT has been hampered because it requires the inflation of a proximally placed balloon. The introduction of Fourier-domain (FD) OCT with a much faster frame rate and pullback speed than TD OCT has allowed for faster image acquisition with high-rate saline infusion and without proximal occlusion.27) Although the penetration of OCT is lower than that of IVUS, it affords a better evaluation of vulnerable plaque by providing a detailed image of the endoluminal borders, a higher detection rate of lipid core, measurement of fibrous cap, and macrophage detection. In comparative evaluation of culprit lesions in patients with acute MIs, OCT was more sensitive in detecting plaque rupture, plaque erosion, and TCFA (lipidic plaque with cap thickness <65 µm) than IVUS or coronary angioscopy.28) Because the resolution power of OCT is 10 times higher than that of IVUS, OCT is able to better delineate the lumen-vessel bo-undary. In the OPUS-CLASS study, both minimum lumen diameter and the area measured by IVUS were significantly greater than those measured by FD-OCT,29),30) underscoring that IVUS overestimates lumen area and has less reproducibility than FD-OCT, which provides accurate and reproducible quantitative measurements of coronary dimensions and more efficiently assesses functional stenosis severity, particularly in small vessels (Table 3).
Table 3. OCT-derived anatomical criteria for defining functional severity.
| Author | No. of lesions | Diagnosis | % DS | FFR | Functional significance | Cutoff | AUC | Diagnostic accuracy (%) |
|---|---|---|---|---|---|---|---|---|
| Shiono et al.70) | 62 | 58±17 | 0.72±0.14 | FFR <0.75 | MLD: 1.35 mm | MLD: 0.917 | MLD: 85.5 | |
| MLA: 1.91 mm2 | MLA: 0.904 | MLA: 85.4 | ||||||
| AS: 70% | AS: 0.940 | AS: 90.3 | ||||||
| Gonzalo et al.30) | 61 | Stable angina (39.3%) | 51±8 | 0.80±0.11 | FFR ≤0.80 | MLD: 1.34 mm | MLD: 0.73 | MLD: 73 |
| Asymptomatic control (25.0%) | MLA: 1.95 mm2 | MLA: 0.74 | MLA: 72 | |||||
| AS: 70% | AS: 0.61 | AS: 57 | ||||||
| Pyxaras et al.71) | 55 | Stable angina (78%) | 34±12 | 0.85±0.10 | FFR ≤0.80 | MLD: 1.59 mm | MLD: 0.80 | MLD: 79 |
| MLA: 2.88 mm2 | MLA: 0.78 | MLA: 72 | ||||||
| Pawlowski et al.72) | 71 | Stable angina (100%) | 50±8 (FFR<0.80) | 0.72±0.08 (FFR<0.80) | FFR <0.80 | MLD: 1.28 mm | MLD: 0.90 | MLD: 87 |
| 55±10 (FFR >0.80) | 0.92±0.09 (FFR>0.80) | MLA: 2.05 mm2 | MLA: 0.91 | MLA: 87 | ||||
| Reith et al.73) | 62 diabetic lesions | Stable angina (100%) | 52±9 | 0.79±0.13 | FFR ≤0.80 | MLD: 1.31 mm | MLD: 0.816 | MLD: 80.7 |
| MLA: 1.59 mm2 | MLA: 0.813 | MLA: 77.4 | ||||||
| AS: 70.6% | AS: 0.807 | AS: 72.4 |
OCT: optical coherence tomography, DS: diameter stenosis, FFR: fractional flow reserve, AUC: area under the curve, MLD: minimal lumen diameter, MLA: minimal lumen area, AS: area stenosis
In the evaluation of lesion morphology after stent implantation, OCT allows more frequent visualization than IVUS of stent features, including inadequate stent apposition (ISA), tissue protrusion, thrombus, and stent edge dissection.31) Gutiérrez-Chico et al.32) reported that maximal ISA distances <270 µm after stent implantation showed resolved ISA at follow-up, whereas maximal ISA distances ≥850 µm were associated with persistent ISA.32) A recent study by Im et al.33) demonstrated the following risk factors for specific conditions: 1) acute ISA: baseline diameter stenosis >70%, calcified lesion, and stent length >25 mm, 2) late-persistent ISA: acute ISA volume and acute ISA within stent edges, and 3) late-acquired ISA: plaque/thrombus prolapse after stent implantation.
Owing to its greater spatial resolution, OCT also effectively assesses neointimal healing and restenosis patterns within stented segments. Late in-stent restenosis (ISR occuring after 1 year) was documented by OCT to have heterogeneous neointima and a significantly higher incidence of lipid-rich neointima, TCFA-like neointima, microchannels within neointima, and neointimal disruption com-pared with early ISR.34) The latter atherosclerotic neointimal dege-nerative changes might underlie the clinical instabiliy of late ISR.35) Vergallo et al.36) reported that stents with greater neointimal hyperplasia were more frequently associated with features of vulnerability regardless of the stent type and time from implantation.
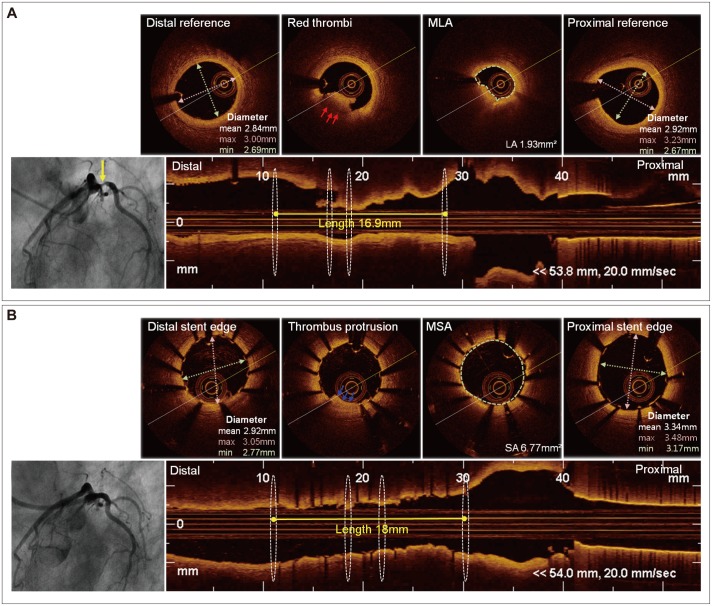
Optical coherence tomography also might be a useful tool in PCI optimization.37) In a study by Prati et al.38) angiographic plus OCT guidance compared with angiographic guidance alone led to additional interventions (repeat balloon inflation or additional stenting) in 34.7% of cases, in association with a significantly lower risk of car-diac death or MI during a 1-year follow-up period. On the other hand, Habara et al.39) reported that FD-OCT guidance was associated with smaller stent expansion and more frequent significant residual reference segment stenosis compared with IVUS guidance, suggesting a significant advantage for IVUS over OCT. In contrast, two university centers (Keimyung University and Ulsan University) evaluated clinical outcomes after a 1-year follow-up period after IVUS- or OCT-guided PCI, and the two strategies showed a similar incidence of MACE {cardiac death, MI, and target lesion revascularization; 3.9% vs. 4.0%, respectively, p=not significant (NS)} or definite/probable stent thrombosis (0.8% vs. 1.2%, respectively, p=NS), suggesting that OCT guidance is a possible alternative strategy for stent optimization (Fig. 1).40) OCT guidance can also guide intevention to avoid balloon angioplasty (POBA) and stenting in specific lesion subset. In a study by Cervinka et al.41) OCT showed clear differentiation between real lesions and thrombus formation in the setting of ST segment elevation MI and led to the avoidance of POBA and stenting. However, the OCT criteria to guide PCI and long-term clinical follow-up of OCT-guided PCI remain undefined; further studies are warranted to clarify the role of OCT in PCI guidance or optimization.
Fig. 1. OCT-guided PCI. A 63-year-old man was diagnosed with non-ST segment elevation myocardial infarction. A: baseline coronary angiogram showed significant stenosis of the proximal left circumflex artery. Pre-interventional OCT revealed a minimal lumen area of 1.93 mm2 and red thrombi (red arrow). The minimal lumen diameters at the proximal and distal reference segments were 2.69 mm and 2.67 mm, respectively, and the lesion length was 16.9 mm. B: a coronary angiogram after biolimus-eluting stent (2.75×18 mm) implantation. Post-interventional OCT showed A minimal stent area of 6.77 mm2 and a thrombic protrusion (blue arrow). OCT: optical coherence tomography, PCI: percutaneous coronary intervention, MLA: minimal lumen area, MSA: minimal stent area.
Even though OCT enables the accurate assessment of plaque types, stent strut positioning, and endothelization due to its better spatial resolution and faster data acquisition, its low axial penetration does not provide optimal visualization of the arterial wall. It also requires the injection of contrast during image acquisition, and therefore it cannot be performed in situations where there is no coronary flow, such as in complete occlusion.
Near-Infrared Spectroscopy
Near-infrared spectroscopy (NIRS) is based on light absorbance by organic molecules while scanning an artery through blood and during cardiac motion. The reflectance spectra from wavelengths between 400 nm and 2400 nm enable an analysis of the chemical composition of biological tissue. The goal of intracoronary NIRS is to provide a "chemogram" of the arterial wall to find lipid core plaque (LCP) and analyze it using a vulnerability index (Fig. 2). LCP is defined as a fibroatheroma >60 degrees in circumference extent, >200 µm thick, and with a fibrous cap having a mean thickness of <450 µm.42),43) LCP detection in human coronary autopsy specimens was validated by the SPECTroscopic Assessment of Coronary Lipid trial.44) Madder et al.43) evaluated the frequency of LCP according to clinical presentation. The target lesions responsible for ACS were frequently composed of LCP, and LCPs were often were found in remote, nontarget areas. As expected, both target and remote LCPs were more common in patients with ACS than in those with stable angina. Stent implantation in LCP-containing lesions can result in adverse angiographic and clinical outcomes, including no-reflow, distal embolization, and periprocedural MI. In addition to advanced atherosclerosis, NIRS helped to detect higher lipid content in early CAD with endothelial dysfunction, which may be closely associated with a rapid progression of coronary atherosclerosis and future cardiovascular events due to plaque rupture.45) Dixon et al.46) used NIRS to guide stent length selection and optimal lesion coverage. This finding suggested that the use of NIRS combined with conventional coronary angiography could avoid incomplete lesion coverage or possible placement of the stent ends within a LCP, which could result in adverse angiographic and clinical outcomes, including no-reflow, distal embolizations, and periprocedural MIs. Although information about the chemical composition may contribute to early identification of high risk plaque, a potential limitation may be its inability to assess the depth of a lipid core and its measurement of lipid volume has not been validated so far. Threfore, this weakness can be overcome by its use in combination with other imaging modalities such as IVUS or OCT.
Fig. 2. Angiographic and NIRS findings in acute STEMI. A 56-year-old patient with acute chest pain and inferior-posterior injury was referred for primary PCI (A). Angiography of the right coronary artery revealed complete occlusion (B). Aspiration yielded a thrombus characteristic of STEMI (C) and resulted in a TIMI flow grade 3 (D). NIRS performed after the TIMI flow grade 3 was established revealed a prominent, nearly circumferential lipid core plaque concentrated at the culprit site (E). NIRS: near-infrared spectroscopy, STEMI: ST segment elevation myocardial infarction, PCI: percutaneous coronary intervention, TIMI: Thrombolysis in Myocardial Infarction. Adopted from Madder RD, Goldstein JA, Madden SP, et al. JACC Cardiovasc Interv 2013;6:838-46.74).
Hybrid Intravascular Imaging
By combining different imaging modalities to overcome the innate limitations of each, hybrid imaging allows for a more detailed and comprehensive coronary artery evaluation. Hybrid coronary biplane angiography and IVUS provides information on vessel geometry through angiography and vessel wall pathology via IVUS.47) Three-dimensional models allow a comprehensive understanding of vessel geometry and plaque distribution and have been extensively used research tools to evaluate the association between local hemodynamic factors and plaque progression. The combination of angiography and OCT can provide detailed information regarding vessel geometry and plaque type, which may contribute to the identification of vulnerable or ruptured plaques and thus, the effect of blood flow on atherosclerotic evolution.
Hybrid IVUS and NIRS imaging provides insight on plaque composition and lesion architecture.48) The recently introduced true vessel characterization Imaging System (MC 7 system, InfraReDx, Burlington, MA, USA) allows the simultaneous assessment of plaque composition both in terms of its chemical and morphologic characteristics. It can be an alternative approach to assess the vulnerability of ostial coronary lesions not reachable by OCT.
The combination of IVUS and OCT may provide an optimal assessment of the volumetric and structural characteristics of a coronary artery. Combining both modalities allows for a more accurate detection of high-risk plaques and would be useful in planning and assessing the outcome of PCI. The correct stent diameter could be selected through the use of IVUS while a detailed evaluation of the final result and procedural complications could be obtained with OCT.
Conclusion
The ideal intravascular imaging device should enable both a rapid and an accurate assessment of the coronary arteries while providing sufficient information on local and systemic atherosclerosis-related factors. Grayscale IVUS is routinely used in daily practice. Although it provides information before, during, and after PCI, it has poor resolution and omits plaque composition. The introduction of OCT has addressed the resolution limitation, and various modalities including VH IVUS, OCT, angioscopy and NIRS are currently in use to provide information about plaque composition, such as plaque vulnerability. Hybrid intravascular imaging devices also provide better and more tailored data regarding PCI. More reliable and accurate imaging with further technological advances will allow for better understanding and treatment of CAD in the future.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Arnett EN, Isner JM, Redwood DR, et al. Coronary artery narrowing in coronary heart disease: comparison of cineangiographic and necropsy findings. Ann Intern Med. 1979;91:350–356. doi: 10.7326/0003-4819-91-3-350. [DOI] [PubMed] [Google Scholar]
- 3.White CW, Wright CB, Doty DB, et al. Does visual interpretation of the coronary arteriogram predict the physiologic importance of a coronary stenosis? N Engl J Med. 1984;310:819–824. doi: 10.1056/NEJM198403293101304. [DOI] [PubMed] [Google Scholar]
- 4.Hodgson JM, Graham SP, Savakus AD, et al. Clinical percutaneous imaging of coronary anatomy using an over-the-wire ultrasound catheter system. Int J Card Imaging. 1989;4:187–193. doi: 10.1007/BF01745149. [DOI] [PubMed] [Google Scholar]
- 5.Lee SY, Mintz GS, Kim SY, et al. Attenuated plaque detected by intravascular ultrasound: clinical, angiographic, and morphologic features and post-percutaneous coronary intervention complications in patients with acute coronary syndromes. JACC Cardiovasc Interv. 2009;2:65–72. doi: 10.1016/j.jcin.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Potkin BN, Keren G, Mintz GS, et al. Arterial responses to balloon coronary angioplasty: an intravascular ultrasound study. J Am Coll Cardiol. 1992;20:942–951. doi: 10.1016/0735-1097(92)90197-u. [DOI] [PubMed] [Google Scholar]
- 7.Tang Z, Bai J, Su SP, et al. Cutting-balloon angioplasty before drug-eluting stent implantation for the treatment of severely calcified coronary lesions. J Geriatr Cardiol. 2014;11:44–49. doi: 10.3969/j.issn.1671-5411.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mintz GS, Pichard AD, Popma JJ, Kent KM, Satler LF, Leon MB. Preliminary experience with adjunct directional coronary atherectomy after high-speed rotational atherectomy in the treatment of calcific coronary artery disease. Am J Cardiol. 1993;71:799–804. doi: 10.1016/0002-9149(93)90827-y. [DOI] [PubMed] [Google Scholar]
- 9.Parise H, Maehara A, Stone GW, Leon MB, Mintz GS. Meta-analysis of randomized studies comparing intravascular ultrasound versus angiographic guidance of percutaneous coronary intervention in pre-drug-eluting stent era. Am J Cardiol. 2011;107:374–382. doi: 10.1016/j.amjcard.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 10.Ahn JM, Kang SJ, Yoon SH, et al. Meta-analysis of outcomes after intravascular ultrasound-guided versus angiography-guided drug-eluting stent implantation in 26,503 patients enrolled in three randomized trials and 14 observational studies. Am J Cardiol. 2014;113:1338–1347. doi: 10.1016/j.amjcard.2013.12.043. [DOI] [PubMed] [Google Scholar]
- 11.Jang JS, Song YJ, Kang W, et al. Intravascular ultrasound-guided implantation of drug-eluting stents to improve outcome: a meta-analysis. JACC Cardiovasc Interv. 2014;7:233–243. doi: 10.1016/j.jcin.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 12.de Jaegere P, Mudra H, Figulla H, et al. Intravascular ultrasound-guided optimized stent deployment. Immediate and 6 months clinical and angiographic results from the Multicenter Ultrasound Stenting in Coronaries Study (MUSIC Study) Eur Heart J. 1998;19:1214–1223. doi: 10.1053/euhj.1998.1012. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Farooq V, Garcia-Garcia HM, et al. Comparison of intravascular ultrasound versus angiography-guided drug-eluting stent implantation: a meta-analysis of one randomised trial and ten observational studies involving 19,619 patients. EuroIntervention. 2012;8:855–865. doi: 10.4244/EIJV8I7A129. [DOI] [PubMed] [Google Scholar]
- 14.Kawasaki M, Takatsu H, Noda T, et al. Noninvasive quantitative tissue characterization and two-dimensional color-coded map of human atherosclerotic lesions using ultrasound integrated backscatter: comparison between histology and integrated backscatter images. J Am Coll Cardiol. 2001;38:486–492. doi: 10.1016/s0735-1097(01)01393-6. [DOI] [PubMed] [Google Scholar]
- 15.Kawasaki M, Takatsu H, Noda T, et al. In vivo quantitative tissue characterization of human coronary arterial plaques by use of integrated backscatter intravascular ultrasound and comparison with angioscopic findings. Circulation. 2002;105:2487–2492. doi: 10.1161/01.cir.0000017200.47342.10. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Granillo GA, Bruining N, Mc Fadden E, et al. Geometrical validation of intravascular ultrasound radiofrequency data analysis (Virtual Histology) acquired with a 30 MHz boston scientific corporation imaging catheter. Catheter Cardiovasc Interv. 2005;66:514–518. doi: 10.1002/ccd.20447. [DOI] [PubMed] [Google Scholar]
- 17.Nasu K, Tsuchikane E, Katoh O, et al. Impact of intramural thrombus in coronary arteries on the accuracy of tissue characterization by in vivo intravascular ultrasound radiofrequency data analysis. Am J Cardiol. 2008;101:1079–1083. doi: 10.1016/j.amjcard.2007.11.064. [DOI] [PubMed] [Google Scholar]
- 18.Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 19.Claessen BE, Maehara A, Fahy M, Xu K, Stone GW, Mintz GS. Plaque composition by intravascular ultrasound and distal embolization after percutaneous coronary intervention. JACC Cardiovasc Imaging. 2012;5(3 Suppl):S111–S118. doi: 10.1016/j.jcmg.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Wu X, Maehara A, Mintz GS, et al. Virtual histology intravascular ultrasound analysis of non-culprit attenuated plaques detected by grayscale intravascular ultrasound in patients with acute coronary syndromes. Am J Cardiol. 2010;105:48–53. doi: 10.1016/j.amjcard.2009.08.649. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Granillo GA, García-García HM, Mc Fadden EP, et al. In vivo intravascular ultrasound-derived thin-cap fibroatheroma detection using ultrasound radiofrequency data analysis. J Am Coll Cardiol. 2005;46:2038–2042. doi: 10.1016/j.jacc.2005.07.064. [DOI] [PubMed] [Google Scholar]
- 22.Uchida Y. Recent advances in coronary angioscopy. J Cardiol. 2011;57:18–30. doi: 10.1016/j.jjcc.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Takano M, Mizuno K. Coronary angioscopic evaluation for serial changes of luminal appearance after pharmacological and catheter interventions. Circ J. 2010;74:240–245. doi: 10.1253/circj.cj-09-0769. [DOI] [PubMed] [Google Scholar]
- 24.Kotani J, Awata M, Nanto S, et al. Incomplete neointimal coverage of sirolimus-eluting stents: angioscopic findings. J Am Coll Cardiol. 2006;47:2108–2111. doi: 10.1016/j.jacc.2005.11.092. [DOI] [PubMed] [Google Scholar]
- 25.Alfonso F. The "vulnerable" stent why so dreadful? J Am Coll Cardiol. 2008;51:2403–2406. doi: 10.1016/j.jacc.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 26.Yabushita H, Bouma BE, Houser SL, et al. Characterization of human atherosclerosis by optical coherence tomography. Circulation. 2002;106:1640–1645. doi: 10.1161/01.cir.0000029927.92825.f6. [DOI] [PubMed] [Google Scholar]
- 27.Takarada S, Imanishi T, Liu Y, et al. Advantage of next-generation frequency-domain optical coherence tomography compared with conventional time-domain system in the assessment of coronary lesion. Catheter Cardiovasc Interv. 2010;75:202–206. doi: 10.1002/ccd.22273. [DOI] [PubMed] [Google Scholar]
- 28.Kubo T, Imanishi T, Takarada S, et al. Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J Am Coll Cardiol. 2007;50:933–939. doi: 10.1016/j.jacc.2007.04.082. [DOI] [PubMed] [Google Scholar]
- 29.Kubo T, Akasaka T, Shite J, et al. OCT compared with IVUS in a coronary lesion assessment: the OPUS-CLASS study. JACC Cardiovasc Imaging. 2013;6:1095–1104. doi: 10.1016/j.jcmg.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalo N, Escaned J, Alfonso F, et al. Morphometric assessment of coronary stenosis relevance with optical coherence tomography: a comparison with fractional flow reserve and intravascular ultrasound. J Am Coll Cardiol. 2012;59:1080–1089. doi: 10.1016/j.jacc.2011.09.078. [DOI] [PubMed] [Google Scholar]
- 31.Kubo T, Imanishi T, Kitabata H, et al. Comparison of vascular response after sirolimus-eluting stent implantation between patients with unstable and stable angina pectoris: a serial optical coherence tomography study. JACC Cardiovasc Imaging. 2008;1:475–484. doi: 10.1016/j.jcmg.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Gutiérrez-Chico JL, Wykrzykowska J, Nüesch E, et al. Vascular tissue reaction to acute malapposition in human coronary arteries: sequential assessment with optical coherence tomography. Circ Cardiovasc Interv. 2012;5:20–29. S1–S8. doi: 10.1161/CIRCINTERVENTIONS.111.965301. [DOI] [PubMed] [Google Scholar]
- 33.Im E, Kim BK, Ko YG, et al. Incidences, predictors, and clinical outcomes of acute and late stent malapposition detected by optical coherence tomography after drug-eluting stent implantation. Circ Cardiovasc Interv. 2014;7:88–96. doi: 10.1161/CIRCINTERVENTIONS.113.000797. [DOI] [PubMed] [Google Scholar]
- 34.Ino Y, Kubo T, Kitabata H, et al. Difference in neointimal appearance between early and late restenosis after sirolimus-eluting stent implantation assessed by optical coherence tomography. Coron Artery Dis. 2013;24:95–101. doi: 10.1097/MCA.0b013e32835c872b. [DOI] [PubMed] [Google Scholar]
- 35.Kang SJ, Mintz GS, Akasaka T, et al. Optical coherence tomographic analysis of in-stent neoatherosclerosis after drug-eluting stent implantation. Circulation. 2011;123:2954–2963. doi: 10.1161/CIRCULATIONAHA.110.988436. [DOI] [PubMed] [Google Scholar]
- 36.Vergallo R, Yonetsu T, Uemura S, et al. Correlation between degree of neointimal hyperplasia and incidence and characteristics of neoatherosclerosis as assessed by optical coherence tomography. Am J Cardiol. 2013;112:1315–1321. doi: 10.1016/j.amjcard.2013.05.076. [DOI] [PubMed] [Google Scholar]
- 37.Rath PC, Reddy K, Agarwal MK, Purohit BV, Deb T, Reddy AM. Optical coherence tomography guided PCI - initial experience at Apollo Health City, Jubilee Hills, Hyderabad. Indian Heart J. 2014;66:31–37. doi: 10.1016/j.ihj.2013.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prati F, Di Vito L, Biondi-Zoccai G, et al. Angiography alone versus angiography plus optical coherence tomography to guide decision-making during percutaneous coronary intervention: the Centro per la Lotta contro l'Infarto-Optimisation of Percutaneous Coronary Intervention (CLI-OPCI) study. EuroIntervention. 2012;8:823–829. doi: 10.4244/EIJV8I7A125. [DOI] [PubMed] [Google Scholar]
- 39.Habara M, Nasu K, Terashima M, et al. Impact of frequency-domain optical coherence tomography guidance for optimal coronary stent implantation in comparison with intravascular ultrasound guidance. Circ Cardiovasc Interv. 2012;5:193–201. doi: 10.1161/CIRCINTERVENTIONS.111.965111. [DOI] [PubMed] [Google Scholar]
- 40.Kim IC, Hur SH, Cho YK, et al. Impact of optical coherence tomography-versus intravascular ultrasound-guided percutaneous coronary intervention on mid-term clinical outcomes. Eur Heart J. 2014;35(suppl 1):1141. [Google Scholar]
- 41.Cervinka P, Spaček R, Bystroň M, et al. Optical coherence tomography-guided primary percutaneous coronary intervention in ST-segment elevation myocardial infarction patients: a pilot study. Can J Cardiol. 2014;30:420–427. doi: 10.1016/j.cjca.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 42.Moreno PR, Lodder RA, Purushothaman KR, Charash WE, O'Connor WN, Muller JE. Detection of lipid pool, thin fibrous cap, and inflammatory cells in human aortic atherosclerotic plaques by near-infrared spectroscopy. Circulation. 2002;105:923–927. doi: 10.1161/hc0802.104291. [DOI] [PubMed] [Google Scholar]
- 43.Madder RD, Smith JL, Dixon SR, Goldstein JA. Composition of target lesions by near-infrared spectroscopy in patients with acute coronary syndrome versus stable angina. Circ Cardiovasc Interv. 2012;5:55–61. doi: 10.1161/CIRCINTERVENTIONS.111.963934. [DOI] [PubMed] [Google Scholar]
- 44.Waxman S, Dixon SR, L'Allier P, et al. In vivo validation of a catheter-based near-infrared spectroscopy system for detection of lipid core coronary plaques: initial results of the SPECTACL study. JACC Cardiovasc Imaging. 2009;2:858–868. doi: 10.1016/j.jcmg.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Choi BJ, Prasad A, Gulati R, et al. Coronary endothelial dysfunction in patients with early coronary artery disease is associated with the increase in intravascular lipid core plaque. Eur Heart J. 2013;34:2047–2054. doi: 10.1093/eurheartj/eht132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dixon SR, Grines CL, Munir A, et al. Analysis of target lesion length before coronary artery stenting using angiography and near-infrared spectroscopy versus angiography alone. Am J Cardiol. 2012;109:60–66. doi: 10.1016/j.amjcard.2011.07.068. [DOI] [PubMed] [Google Scholar]
- 47.Bourantas CV, Kourtis IC, Plissiti ME, et al. A method for 3D reconstruction of coronary arteries using biplane angiography and intravascular ultrasound images. Comput Med Imaging Graph. 2005;29:597–606. doi: 10.1016/j.compmedimag.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 48.Pu J, Mintz GS, Brilakis ES, et al. In vivo characterization of coronary plaques: novel findings from comparing greyscale and virtual histology intravascular ultrasound and near-infrared spectroscopy. Eur Heart J. 2012;33:372–383. doi: 10.1093/eurheartj/ehr387. [DOI] [PubMed] [Google Scholar]
- 49.Schiele F, Meneveau N, Seronde MF, et al. Medical costs of intravascular ultrasound optimization of stent deployment. Results of the multicenter randomized ‘REStenosis after Intravascular ultrasound STenting' (RESIST) study. Int J Cardiovasc Intervent. 2000;3:207–213. doi: 10.1080/14628840050515957. [DOI] [PubMed] [Google Scholar]
- 50.Frey AW, Hodgson JM, Müller C, Bestehorn HP, Roskamm H. Ultrasound-guided strategy for provisional stenting with focal balloon combination catheter: results from the randomized Strategy for Intracoronary Ultrasound-guided PTCA and Stenting (SIPS) trial. Circulation. 2000;102:2497–2502. doi: 10.1161/01.cir.102.20.2497. [DOI] [PubMed] [Google Scholar]
- 51.Mudra H, di Mario C, de Jaegere P, et al. Randomized comparison of coronary stent implantation under ultrasound or angiographic guidance to reduce stent restenosis (OPTICUS Study) Circulation. 2001;104:1343–1349. doi: 10.1161/hc3701.096064. [DOI] [PubMed] [Google Scholar]
- 52.Oemrawsingh PV, Mintz GS, Schalij MJ, et al. Intravascular ultrasound guidance improves angiographic and clinical outcome of stent implantation for long coronary artery stenoses: final results of a randomized comparison with angiographic guidance (TULIP Study) Circulation. 2003;107:62–67. doi: 10.1161/01.cir.0000043240.87526.3f. [DOI] [PubMed] [Google Scholar]
- 53.Gaster AL, Slothuus Skjoldborg U, Larsen J, et al. Continued improvement of clinical outcome and cost effectiveness following intravascular ultrasound guided PCI: insights from a prospective, randomised study. Heart. 2003;89:1043–1049. doi: 10.1136/heart.89.9.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gil RJ, Pawłowski T, Dudek D, et al. Comparison of angiographically guided direct stenting technique with direct stenting and optimal balloon angioplasty guided with intravascular ultrasound. The multicenter, randomized trial results. Am Heart J. 2007;154:669–675. doi: 10.1016/j.ahj.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 55.Russo RJ, Silva PD, Teirstein PS, et al. A randomized controlled trial of angiography versus intravascular ultrasound-directed bare-metal coronary stent placement (the AVID Trial) Circ Cardiovasc Interv. 2009;2:113–123. doi: 10.1161/CIRCINTERVENTIONS.108.778647. [DOI] [PubMed] [Google Scholar]
- 56.Roy P, Steinberg DH, Sushinsky SJ, et al. The potential clinical utility of intravascular ultrasound guidance in patients undergoing percutaneous coronary intervention with drug-eluting stents. Eur Heart J. 2008;29:1851–1857. doi: 10.1093/eurheartj/ehn249. [DOI] [PubMed] [Google Scholar]
- 57.Park SJ, Kim YH, Park DW, et al. Impact of intravascular ultrasound guidance on long-term mortality in stenting for unprotected left main coronary artery stenosis. Circ Cardiovasc Interv. 2009;2:167–177. doi: 10.1161/CIRCINTERVENTIONS.108.799494. [DOI] [PubMed] [Google Scholar]
- 58.Jakabcin J, Spacek R, Bystron M, et al. Long-term health outcome and mortality evaluation after invasive coronary treatment using drug eluting stents with or without the IVUS guidance. Randomized control trial. HOME DES IVUS. Catheter Cardiovasc Interv. 2010;75:578–583. doi: 10.1002/ccd.22244. [DOI] [PubMed] [Google Scholar]
- 59.Claessen BE, Mehran R, Mintz GS, et al. Impact of intravascular ultrasound imaging on early and late clinical outcomes following percutaneous coronary intervention with drug-eluting stents. JACC Cardiovasc Interv. 2011;4:974–981. doi: 10.1016/j.jcin.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 60.Kim JS, Hong MK, Ko YG, et al. Impact of intravascular ultrasound guidance on long-term clinical outcomes in patients treated with drug-eluting stent for bifurcation lesions: data from a Korean multicenter bifurcation registry. Am Heart J. 2011;161:180–187. doi: 10.1016/j.ahj.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 61.Youn YJ, Yoon J, Lee JW, et al. Intravascular ultrasound-guided primary percutaneous coronary intervention with drug-eluting stent implantation in patients with ST-segment elevation myocardial infarction. Clin Cardiol. 2011;34:706–713. doi: 10.1002/clc.20966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park KW, Kang SH, Yang HM, et al. Impact of intravascular ultrasound guidance in routine percutaneous coronary intervention for conventional lesions: data from the EXCELLENT trial. Int J Cardiol. 2013;167:721–726. doi: 10.1016/j.ijcard.2012.03.059. [DOI] [PubMed] [Google Scholar]
- 63.Ahn SG, Yoon J, Sung JK, et al. Intravascular ultrasound-guided percutaneous coronary intervention improves the clinical outcome in patients undergoing multiple overlapping drug-eluting stents implantation. Korean Circ J. 2013;43:231–238. doi: 10.4070/kcj.2013.43.4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahn JM, Han S, Park YK, et al. Differential prognostic effect of intravascular ultrasound use according to implanted stent length. Am J Cardiol. 2013;111:829–835. doi: 10.1016/j.amjcard.2012.11.054. [DOI] [PubMed] [Google Scholar]
- 65.Chen SL, Ye F, Zhang JJ, et al. Intravascular ultrasound-guided systematic two-stent techniques for coronary bifurcation lesions and reduced late stent thrombosis. Catheter Cardiovasc Interv. 2013;81:456–463. doi: 10.1002/ccd.24601. [DOI] [PubMed] [Google Scholar]
- 66.Chieffo A, Latib A, Caussin C, et al. A prospective, randomized trial of intravascular-ultrasound guided compared to angiography guided stent implantation in complex coronary lesions: the AVIO trial. Am Heart J. 2013;165:65–72. doi: 10.1016/j.ahj.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 67.Hur SH, Kang SJ, Kim YH, et al. Impact of intravascular ultrasound-guided percutaneous coronary intervention on long-term clinical outcomes in a real world population. Catheter Cardiovasc Interv. 2013;81:407–416. doi: 10.1002/ccd.23279. [DOI] [PubMed] [Google Scholar]
- 68.Kim JS, Kang TS, Mintz GS, et al. Randomized comparison of clinical outcomes between intravascular ultrasound and angiography-guided drug-eluting stent implantation for long coronary artery stenoses. JACC Cardiovasc Interv. 2013;6:369–376. doi: 10.1016/j.jcin.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 69.Witzenbichler B, Maehara A, Weisz G, et al. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents: the assessment of dual antiplatelet therapy with drug-eluting stents (ADAPT-DES) study. Circulation. 2014;129:463–470. doi: 10.1161/CIRCULATIONAHA.113.003942. [DOI] [PubMed] [Google Scholar]
- 70.Shiono Y, Kitabata H, Kubo T, et al. Optical coherence tomography-derived anatomical criteria for functionally significant coronary stenosis assessed by fractional flow reserve. Circ J. 2012;76:2218–2225. doi: 10.1253/circj.cj-12-0195. [DOI] [PubMed] [Google Scholar]
- 71.Pyxaras SA, Tu S, Barbato E, et al. Quantitative angiography and optical coherence tomography for the functional assessment of nonobstructive coronary stenoses: comparison with fractional flow reserve. Am Heart J. 2013;166:1010–1018. doi: 10.1016/j.ahj.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 72.Pawlowski T, Prati F, Kulawik T, Ficarra E, Bil J, Gil R. Optical coherence tomography criteria for defining functional severity of intermediate lesions: a comparative study with FFR. Int J Cardiovasc Imaging. 2013;29:1685–1691. doi: 10.1007/s10554-013-0283-x. [DOI] [PubMed] [Google Scholar]
- 73.Reith S, Battermann S, Jaskolka A, et al. Relationship between optical coherence tomography derived intraluminal and intramural criteria and haemodynamic relevance as determined by fractional flow reserve in intermediate coronary stenoses of patients with type 2 diabetes. Heart. 2013;99:700–707. doi: 10.1136/heartjnl-2013-303616. [DOI] [PubMed] [Google Scholar]
- 74.Madder RD, Goldstein JA, Madden SP, et al. Detection by near-infrared spectroscopy of large lipid core plaques at culprit sites in patients with acute ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2013;6:838–846. doi: 10.1016/j.jcin.2013.04.012. [DOI] [PubMed] [Google Scholar]