Abstract
Background:
Injury to the rotator cuff can cause irreversible changes to the structure and function of the associated muscles and bones. The temporal progression and pathomechanisms associated with these adaptations are unclear. The purpose of this study was to investigate the time course of structural muscle and osseous changes in a rat model of a massive rotator cuff tear.
Methods:
Supraspinatus and infraspinatus muscle architecture and biochemistry and humeral and scapular morphological parameters were measured three days, eight weeks, and sixteen weeks after dual tenotomy with and without chemical paralysis via botulinum toxin A (BTX).
Results:
Muscle mass and physiological cross-sectional area increased over time in the age-matched control animals, decreased over time in the tenotomy+BTX group, and remained nearly the same in the tenotomy-alone group. Tenotomy+BTX led to increased extracellular collagen in the muscle. Changes in scapular bone morphology were observed in both experimental groups, consistent with reductions in load transmission across the joint.
Conclusions:
These data suggest that tenotomy alone interferes with normal age-related muscle growth. The addition of chemical paralysis yielded profound structural changes to the muscle and bone, potentially leading to impaired muscle function, increased muscle stiffness, and decreased bone strength.
Clinical Relevance:
Structural musculoskeletal changes occur after tendon injury, and these changes are severely exacerbated with the addition of neuromuscular compromise.
Rotator cuff tears are a common degenerative condition found in approximately 30% of individuals over sixty years of age1 and resulting in pain and loss of functional range of motion in the shoulder2. While surgical treatment and repair of the tendon are possible, failure rates have been reported to be high as 20% to 94%, with an increasing prevalence of failure associated with increases in the size of the tear and the age of the patient3,4.
Muscle atrophy is associated with chronic, massive rotator cuff tears and has been documented with magnetic resonance imaging and computed tomography (CT) in humans and animal models5-7. Likewise, decreased muscle weight, volume, and/or fiber length have been observed in both animal models and human cadavers with rotator cuff injuries8,9. Previous work with a sheep model demonstrated a correlation between active force production and muscle atrophy following rotator cuff injury7. However, muscle mass, volume, and fiber length are overall poor indicators of muscle function. In contrast, architectural parameters such as physiological cross-sectional area have been previously shown to be good predictors of muscle force production10. Similarly, normalized fiber length (i.e., the number of sarcomeres in series) provides the best estimate of muscle excursion and velocity11,12. Shorter fibers, which become highly strained and result in larger forces at the repair site, have been implicated as one of the obstacles to the repair of massive rotator cuff tears13,14 and to the achievement of good tendon-to-bone healing15. Protein level adaptations, such as increased collagen content5 and adaptations in the intramyocellular protein titin16, may also influence muscle stiffness at the time of repair. Previous work with a rat model of a single rotator cuff tendon injury demonstrated transient changes in supraspinatus physiological cross-sectional area and sarcomere number17. This finding supports the concept that shorter fibers may lead to a stiffer muscle because higher sarcomere strains are needed to achieve anatomical repair. However, to our knowledge, there have been no quantitative measurements of collagen and titin in these muscles to support the idea of material property changes, which would further increase muscle stiffness after injury.
The contribution of suprascapular neuropathy or neurapraxia to muscle trophic changes associated with massive, retracted tears has not been clearly established. Alterations in nerve function may influence the clinical deterioration of cuff muscles and have been associated with massive rotator cuff tears18,19. Similarly, rotator cuff arthropathy in the setting of chronic, massive rotator cuff tears can lead to alterations in the osseous architecture of the shoulder (e.g., osteopenia, cartilage loss, proximal migration of the humeral head, abnormal bone wear, and osteophyte formation)20. These changes theoretically may be due to mechanical unloading and/or changes in trophic factor interactions among bone, muscle, and tendon. We are not aware of any animal studies assessing osseous architecture changes in the shoulder following a rotator cuff injury. Although some animal model data suggest that a combined tendon and nerve injury leads to more muscle changes than does a tendon injury alone21-24, to our knowledge no study has correlated changes in bone and muscle architecture to the severity of tendon and muscle injury.
In the current study, botulinum toxin A (BTX) was used in conjunction with tendon injury to mimic a chronic, massive rotator cuff injury leading to severe muscle atrophy. The objective of the study was to investigate the short-term (three-day) and long-term (eight and sixteen-week) muscle and bone adaptations that occur in a rodent model of a massive rotator cuff tear. Specifically, we focused on muscle structural parameters that are believed to influence active (contractile) and passive force generation. We hypothesized that injury to the rotator cuff would result in radial and longitudinal muscle atrophy, increased collagen content, and decreased scapular fossa depth and trabecular number and thickness. We also hypothesized that these changes would be exacerbated by additional BTX injury and chronicity. This information is clinically useful because a better understanding of the mechanisms by which rotator cuff muscles and the surrounding bone degenerate following injury may lead to therapeutic interventions that can one day improve clinical results following repair.
Materials and Methods
Animal Model and Surgical Methods
Fifty-five male Sprague-Dawley rats were used for this study. The animals were divided into three groups: bilateral dual tenotomy of the supraspinatus and infraspinatus tendons only (tenotomy-alone group), bilateral dual tenotomy of the supraspinatus and infraspinatus tendons with concomitant chemical denervation of the muscles induced with BTX (tenotomy+BTX group), and age-matched uninjured controls (control group). Following approval from the university’s Animal Studies Committee, the surgical procedures were performed after induction of anesthesia with isoflurane and a 1% oxygen carrier. Under sterile conditions, a 2-cm vertical incision was made over the scapulohumeral joint and the deltoid was detached from the cranial and lateral aspects of the acromion with use of electrocautery. The acromion was elevated with use of a 3-0 Vicryl (polyglactin) suture passed through the acromial notch to expose the underlying rotator cuff tendons. The supraspinatus tendon was exposed by supination of the forearm, and a number-11 blade was used to transect the supraspinatus tendon at its insertion on the humeral head. The forearm was then internally rotated 45° to expose the infraspinatus tendon, which was transected from the humeral head with use of a number-11 blade. Retraction of the tendons was confirmed visually by the surgeon (C.T.L.). In the tenotomy+BTX group, BTX diluted in sterile saline solution (∼9 U/kg) was injected into the supraspinatus muscle belly at the time of surgery. In the sixteen-week tenotomy+BTX group, a second injection of BTX was administered into the supraspinatus muscle belly at eight weeks postsurgery. No injections were performed in the tenotomy-alone group. The deltoid and trapezius muscles were then reattached with use of 3-0 Vicryl suture, and the skin was closed with staples. Postoperative animal care was administered by an animal care technician.
Animals were killed at three days, eight weeks, or sixteen weeks after injury (Table I). At the time of sacrifice, the supraspinatus and infraspinatus muscles from one shoulder were individually dissected, snap-frozen in liquid nitrogen, and stored at −80°C for biochemical analysis. In a subset of these animals, the contralateral shoulder was dissected en bloc and pinned in its anatomical orientations for evaluation of muscle architecture and bone morphology. All musculature except the supraspinatus and infraspinatus was then removed, and the shoulders were fixed in 4% paraformaldehyde overnight. Samples were then stored in 70% ethanol for further analysis of muscle architecture and bone morphology measurements. Because the osseous anatomy was disrupted on dissection (e.g., the acromion or scapular spine broke), eight shoulders were excluded from bone morphometric analyses (Table I).
TABLE I.
Number of Animals and Number of Shoulder Specimens Used in Each Analysis
| No. of Shoulder Specimens Used in Analysis |
||||
| Total No. of Animals | Biochemistry | Architecture | Bone Morphology | |
| 3 days | ||||
| Control | 6 | 6 | 6 | 6 |
| Tenotomy | 6 | 6 | 4 | 3 |
| Tenotomy+BTX | 5 | 5 | 4 | 4 |
| 8 weeks | ||||
| Control | 6 | 6 | 6 | 6 |
| Tenotomy | 6 | 6 | 5 | 5 |
| Tenotomy+BTX | 8 | 8 | 8 | 5 |
| 16 weeks | ||||
| Control | 6 | 6 | 6 | 4 |
| Tenotomy | 6 | 6 | 5 | 6 |
| Tenotomy+BTX | 6 | 6 | 6 | 4 |
Muscle Architecture
Specimens were sharply dissected from the scapulae to isolate the supraspinatus and infraspinatus muscles and were stored in phosphate-buffered saline solution. Muscle specimens were removed from the saline solution, gently blotted dry, and weighed. Muscle fiber sarcomere length, normalized muscle fiber length, and physiological cross-sectional area were measured as previously described for rat rotator cuff muscles17. Fiber length was normalized to a sarcomere length of 2.4 μm, which represents the optimum sarcomere length for rat muscle based on actin and myosin filament lengths25.
Bone Morphology
Following fixation, micro-CT (SkyScan 1076; SkyScan, Aartselaar, Belgium) was performed with a cone beam, 36-μm voxel resolution, 45-kV (177-μA) energy, standard resolution, and 300-msec integration time. Bone morphometric parameters, including total volume, bone volume fraction (bone volume divided by total volume), trabecular thickness, trabecular number, and trabecular spacing, were measured in the humeral head with use of Scanco Medical software (Brüttisellen, Switzerland). Bone architecture (scapular fossa depth) was measured with use of OsiriX 32-bit imaging software (open source version 5.5). Following imaging, specimens were stored in 70% ethanol at 4°C for further analysis.
Titin Molecular Weight Determination
Titin molecular weight was quantified with use of a previously developed method utilizing sodium dodecyl sulfate-vertical agarose gel electrophoresis (SDS-VAGE)26. Details of the method are available in the Appendix.
Collagen Content
The hydroxyproline content was measured with a modification of a previously published protocol27 to determine the collagen content (μg collagen/mg wet weight tissue) of the supraspinatus and infraspinatus muscles. The measured hydroxyproline content was used to calculate the collagen amount by using the constant 7.46, which corresponds to the average number of hydroxyproline residues in a collagen molecule28. Details of the methods are available in the Appendix.
Statistical Analysis
After the data were screened for normality and homogeneity of variances, two-way analysis of variance was used to compare groups for each dependent measure. Post-hoc Sidak tests were performed to identify specific group differences. Statistical analyses were performed with use of SPSS software 20.0 (IBM, Armonk, New York) and Prism 6.0b (GraphPad, La Jolla, California). Significance was set at p < 0.05, and all data are presented as the mean and standard deviation.
Source of Funding
The sources of funding for this study were National Institutes of Health (NIH) grants R01 AR057836, R24 HD050837, P30 AR057235, and T32 AR060712. The authors have no financial conflicts of interest related to this project.
Results
Muscle Architecture
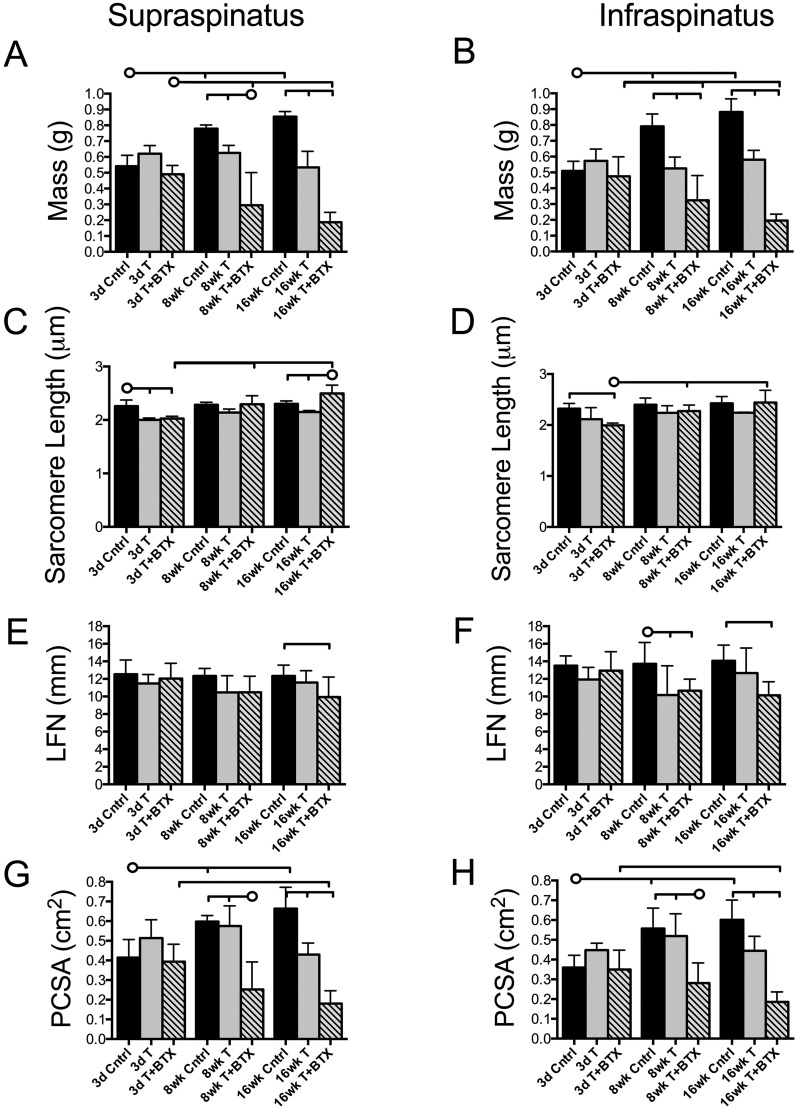
As expected, the control animals had significantly larger supraspinatus (p = 0.001) and infraspinatus (p < 0.001) muscle mass at eight weeks compared with the muscle mass at three days. The mass of both muscles was also increased at sixteen weeks, compared with the three-day value, in the control group (p < 0.001) (Figs. 1-A and 1-B). Tenotomy alone did not yield a significant reduction in supraspinatus or infraspinatus muscle mass over time, but these muscles were significantly smaller than the control muscles at sixteen weeks (p < 0.001). In contrast, the addition of BTX produced significant reductions in supraspinatus (p = 0.013) and infraspinatus (p = 0.034) muscle mass at eight weeks, as well as at sixteen weeks (p < 0.001), compared with the muscle mass at three days. Because of this active atrophy, the muscles in the tenotomy+BTX group had a significantly reduced mass compared with those in the controls (p < 0.001 for all comparisons) and compared with those in the tenotomy-alone group at eight weeks (p < 0.001 for the supraspinatus and p = 0.002 for the infraspinatus) and at sixteen weeks (p < 0.001).
Fig. 1.
Architectural measurements of the supraspinatus and infraspinatus muscles indicate that the mass and physiological cross-sectional area were progressively reduced in the tenotomy+BTX group. Muscle mass (Figs. 1-A and 1-B), sarcomere length (Figs. 1-C and 1-D), normalized fiber length (LFN) (Figs. 1-E and 1-F), and physiological cross-sectional area (PCSA) (Figs. 1-G and 1-H) are shown for each group at each time point. The horizontal lines without circles at the tops of the panels indicate significant differences (p < 0.05) between all groups with a vertical tick mark. The horizontal lines with circles indicate significant differences (p < 0.05) between the group identified with the circle and the groups identified with a vertical tick mark but no significant difference between the groups identified with the tick mark. Cntrl = control, T = tenotomy, BTX = botulinum toxin A, 3d = three-day, 8wk = eight-week, and 16wk = sixteen-week.
At three days, sarcomere length was significantly reduced, compared with the length in the control group, in the supraspinatus in both tenotomy groups (with and without BTX) (p < 0.01) and in the infraspinatus in the tenotomy+BTX group (p = 0.003) (Figs. 1-C and 1-D). These findings were consistent with muscle retraction. However, in both injury groups the sarcomere length recovered by eight weeks, and in the tenotomy+BTX group it slightly exceeded the control value at sixteen weeks. These data confirm the initial tenotomy-induced retraction of the muscles and suggest adaptation of longitudinal sarcomere lengths over time.
The normalized fiber length in both muscles remained constant over time in the control group. In the tenotomy+BTX group, the normalized fiber length was reduced in both the supraspinatus (p = 0.044) and the infraspinatus (p = 0.006), compared with the values in the control group, at sixteen weeks and in the infraspinatus at eight weeks (p = 0.025). The normalized muscle fiber length in the tenotomy-alone group became, on average, smaller than that in the control group over time, but the difference reached significance (p = 0.020) only in the infraspinatus muscle at eight weeks (Figs. 1-E and 1-F).
The physiological cross-sectional area followed a pattern similar to that of the muscle mass. In the control group, the physiological cross-sectional area was significantly increased at eight weeks in both the supraspinatus (p = 0.005) and the infraspinatus (p = 0.001) and at sixteen weeks in both muscles (p < 0.001) compared with the values at three days (Figs. 1-G and 1-H). Tenotomy alone did not yield significant reductions in the physiological cross-sectional area over time, but these values remained constant so the values for both the supraspinatus (p = 0.001) and the infraspinatus (p = 0.015) were significantly lower than the control values by sixteen weeks. However, the physiological cross-sectional areas of the supraspinatus and infraspinatus in the tenotomy+BTX group were significantly lower than the control or tenotomy-alone values at eight (p < 0.001) and sixteen (p < 0.001) weeks.
Bone Morphology
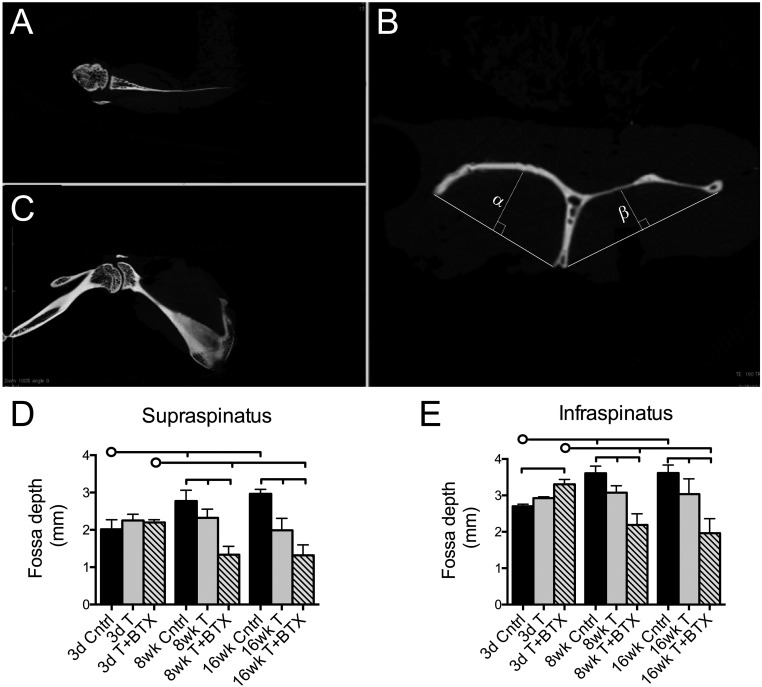
Bone architectural and morphometric parameters were evaluated with use of axial, sagittal, and coronal views of the scapula and humeral head (Figs. 2-A, 2-B, and 2-C). In the control animals, the supraspinatus and infraspinatus fossa depths were increased at eight weeks (p < 0.001) and sixteen weeks (p < 0.001) compared with the depths at three days (Figs. 2-D and 2-E). Tenotomy alone did not significantly decrease fossa depth over time; however, there were significant decreases, compared with the control values, in the fossa depths at eight weeks (p = 0.013 for the supraspinatus fossa and p = 0.005 for the infraspinatus fossa) and at sixteen weeks (p < 0.001 and p = 0.004, respectively) in the tenotomy-alone group. Scapulae from the tenotomy+BTX group demonstrated significant reductions in fossa depth at eight weeks and sixteen weeks compared with those in the control and tenotomy-alone groups (p < 0.001 for all comparisons). These data closely matched the changes in muscle mass.
Fig. 2.
Representative micro-CT images depicting axial (Fig. 2-A), sagittal (Fig. 2-B), and coronal (Fig. 2-C) views of the scapula. The sagittal oblique view (Fig. 2-B) was used to measure the infraspinatus fossa depth (α) and supraspinatus fossa depth (β). At eight and sixteen weeks, the supraspinatus and infraspinatus fossa depths were reduced, compared with the control values, in the tenotomy-alone and tenotomy+BTX groups and the fossa depths in the tenotomy+BTX group were significantly reduced compared with those in the tenotomy-alone group (Figs. 2-D and 2-E). The horizontal lines without circles at the tops of the panels indicate significant differences (p < 0.05) between all groups with a vertical tick mark. The horizontal lines with circles indicate significant differences (p < 0.05) between the group identified with the circle and the groups identified with a vertical tick mark but no significant difference between the groups identified with the tick mark. Cntrl = control, T = tenotomy, BTX = botulinum toxin A, 3d = three-day, 8wk = eight-week, and 16wk = sixteen-week.
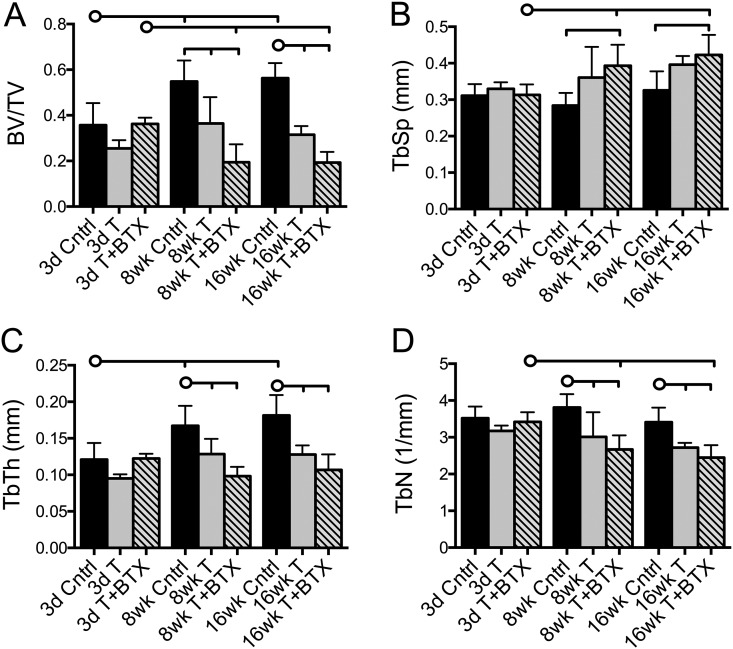
The humeral head bone volume fraction (bone volume divided by total volume) was significantly reduced, compared with the control value, in the tenotomy-alone group (p = 0.002) and tenotomy+BTX group (p < 0.001) at eight weeks and in both groups (p < 0.001) at sixteen weeks (Fig. 3-A). These changes tracked the changes in muscle mass, increasing over time in the control animals, remaining nearly constant over time in the tenotomy-alone group, and decreasing over time in the tenotomy+BTX group. Trabecular spacing was increased at eight weeks (p = 0.033) and sixteen weeks (p = 0.005), compared with the value at three days, only in the tenotomy+BTX group (Fig. 3-B). Tenotomy+BTX also led to significantly greater trabecular spacing compared with that in the control group at eight weeks (p = 0.001) and sixteen weeks (p = 0.006).
Fig. 3.
Humeral head measurements for bone volume fraction (bone volume [BV]/total volume [TV]) (Fig. 3-A), trabecular spacing (TbSp) (Fig. 3-B), trabecular thickness (TbTh) (Fig. 3-C), and trabecular number (TbN) (Fig. 3-D). The bone volume fraction was significantly reduced in the tenotomy+BTX group compared with the tenotomy-alone group at eight weeks, while trabecular spacing, trabecular thickness, and trabecular number were uniformly changed in both injury groups. The horizontal lines without circles at the tops of the panels indicate significant differences (p < 0.05) between all groups with a vertical tick mark. The horizontal lines with circles indicate significant differences (p < 0.05) between the group identified with the circle and the groups identified with a vertical tick mark but no significant difference between the groups identified with the tick mark. Cntrl = control, T = tenotomy, BTX = botulinum toxin A, 3d = three-day, 8wk = eight-week, and 16wk = sixteen-week.
In the control group, trabecular thickness increased over time, with a higher value at eight weeks (p = 0.001) and sixteen weeks (p < 0.001) than at three days, whereas trabecular thickness remained nearly constant over time in both injury groups (Fig. 3-C). There was a significant reduction in trabecular thickness, compared with the control value, at eight weeks in the tenotomy-alone group (p = 0.016) and the tenotomy+BTX group (p < 0.001) and at sixteen weeks in the tenotomy-alone group (p = 0.001) and the tenotomy+BTX group (p < 0.001), but the injury groups did not differ significantly from each other. The trabecular number was also significantly reduced, compared with the control value, at eight weeks and sixteen weeks in both the tenotomy-alone (p < 0.05) and the tenotomy+BTX (p < 0.001) group (Fig. 3-D).
Titin Molecular Weight Determination
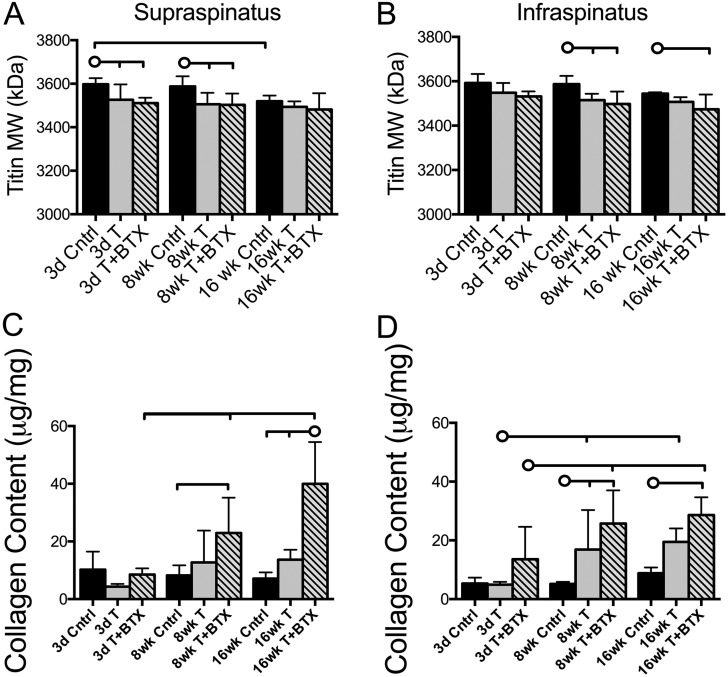
Titin molecular weight was significantly reduced in both the supraspinatus (p = 0.017) and the infraspinatus (p = 0.011) muscles at eight weeks in the tenotomy-alone group compared with the values in the control group (Figs. 4-A and 4-B). These reductions were also observed in the supraspinatus muscle at three days (p = 0.044). The addition of BTX had no further effect on titin molecular weight.
Fig. 4.
Titin molecular weight (MW) was decreased in the supraspinatus (Fig. 4-A) and infraspinatus (Fig. 4-B) muscles following tenotomy, compared with the control values, but with different time courses. The addition of nerve injury (tenotomy+BTX) did not yield any further change in titin. Collagen content was increased in the supraspinatus (Fig. 4-C) at eight weeks and sixteen weeks in the tenotomy+BTX group, compared with the control values, and similar changes were observed in the infraspinatus (Fig. 4-D). Tenotomy alone increased collagen content in the infraspinatus muscle at eight weeks, but this did not remain significant at the sixteen-week time point. The horizontal lines without circles at the tops of the panels indicate significant differences (p < 0.05) between all groups with a vertical tick mark. The horizontal lines with circles indicate significant differences (p < 0.05) between the group identified with the circle and the groups identified with a vertical tick mark but no significant difference between the groups identified with the tick mark. Cntrl = control, T = tenotomy, BTX = botulinum toxin A, 3d = three-day, 8wk = eight-week, and 16wk = sixteen-week.
Collagen Content
The most profound increases in muscle collagen content relative to the controls were observed in the tenotomy+BTX group at eight weeks (p = 0.005 for the supraspinatus and p < 0.001 for the infraspinatus) and sixteen weeks (p < 0.001 for both muscles) (Figs. 4-C and 4-D). Although there was some increase in the infraspinatus collagen content in the tenotomy-alone group compared with the control value at eight weeks (p = 0.034), this increase did not remain significant at the sixteen-week time point.
Discussion
The purpose of this study was to characterize adaptations in muscle and bone architecture as well as muscle biochemistry in response to a massive rotator cuff tear with and without muscle paralysis. Tenotomy with muscle paralysis in our rat model resulted in significant and progressive radial muscle atrophy (a decrease in physiological cross-sectional area) over sixteen weeks. Tenotomy with muscle paralysis induced substantially more severe muscle changes than tenotomy alone, a finding that is consistent with previous rodent studies21. In fact, tenotomy alone did not yield significant decreases in muscle dimensions over time. However, when compared with age-matched controls, the tenotomized muscles were significantly smaller, indicating that they had failed to grow normally over time. Tenotomy with muscle paralysis resulted in only mild longitudinal muscle atrophy (reductions in muscle fiber length), at the later time points, compared with the control values. This finding may be related to the fact that sarcomere lengths were only slightly reduced three days after the tenotomy, indicating that muscle retraction, although present, was mild. These findings agree with previous observations of only small decreases in muscle dimensions following single-muscle (supraspinatus) tenotomies in the rats17 and with observations in human cadaver shoulders with rotator cuff tears9. Taken together, these changes suggest severe impairment of muscle force production and more minor impairment of muscle excursion and velocity. These data do not support the idea that the high passive tensions observed during anatomical surgical reconstruction result from higher strains in muscles with shorter fibers. Future studies should be undertaken to investigate whether these muscle adaptations are reversible following tendon repair and/or rehabilitation.
Interestingly, changes in humeral head trabecular architecture were consistent with muscle unloading in both injury groups, while scapular fossa depth closely followed muscle architectural changes, which differed between the two injury groups. For example, decreased trabecular thickness and trabecular number were observed in both injury groups, suggesting that humeral head bone morphology is less robust when the supraspinatus and infraspinatus muscles are no longer connected to the humerus (and therefore no longer transmitting force to the humerus). In contrast, scapular fossa depths were more severely reduced in the tenotomy+BTX group compared with the tenotomy-alone group, and these differences tracked the changes measured in muscle mass and volume. A possible explanation for this differential osseous effect could be cross-talk between muscle and bone via paracrine factors leading to osseous adaptations that depend on the severity of the muscle degeneration29. This paracrine-mediated hypothesis warrants further experiments.
Also interestingly, small but distinct biochemical changes were observed in both injury groups and both muscles. Titin is a large intracellular structural protein in the sarcomere that has been implicated in determining the stiffness of single muscle fibers16. Decreases in molecular weight would be expected to increase stiffness at the single-fiber (cell) level16. These changes in titin molecular weight suggest that titin molecular weight may be regulated, at least in part, by the absence of mechanical loading or by changes in muscle fiber length, as would result from detachment of tendon from bone. Although it is appealing to speculate that muscle retraction would lead to shorter sarcomeres and therefore reduced titin length (molecular weight), our sarcomere data do not support this idea.
In contrast to tenotomy alone, the addition of muscle paralysis led to progressive increases in muscle collagen content, which suggests that proper innervation has a unique role in the prevention of rotator cuff muscle fibrosis. Increases in collagen content may also increase muscle stiffness5,8, but this simple correlation should be interpreted with caution, as the relationship between muscle collagen content and stiffness is weak30. Nevertheless, the sources of increased muscle stiffness after rotator cuff injury are a major focus of current work in this area, as increased muscle stiffness has been implicated in rotator cuff repairs that are difficult to perform in human patients. Rodent model systems may allow further exploration of the molecular mechanisms and sources of fibrosis, despite the fact that they do not exactly recapitulate the human condition.
The current study has several limitations. First, tenotomy in the animal model does not mimic the magnitude of muscle retraction observed in complete human rotator cuff tears, potentially mitigating longitudinal atrophy of the muscles. Second, the biochemical changes observed in the muscles imply passive stiffness changes at multiple size scales (titin indicates increased stiffness at the single-cell size scale and increased collagen content indicates increased stiffness at the muscle-fiber-bundle and whole-muscle size scales). However, we did not directly measure muscle passive mechanical changes implied by the biochemical findings. Third, the use of BTX in this model to exacerbate muscle atrophy may not directly recapitulate the unloading conditions seen in human patients. In contrast to neurotomy, which completely disrupts nerve structure and function and is used in some rotator cuff injury models21,31,32, injection of BTX temporarily disrupts cholinergic communication between the nerve and muscle (chemical denervation) while preserving noncholinergic communication. For this reason, it could be argued that neurotomy does not recapitulate the human condition as well as BTX, although neither injury is a compression neuropathy model. However, both models yield severe atrophy similar to what is observed in humans23,31. Finally, active mechanics were not tested in this study; thus, we are unable to quantify the physiological force-producing capacity of the muscle implied by the decreased physiological cross-sectional area.
In conclusion, these data suggest that the addition of muscle paralysis to massive tendon tears yields profound structural changes to the muscle and bone. These changes would presumably impair muscle active force-generating capacity, muscle stiffness, and bone strength. Further study is required to assess the mechanisms associated with the changes and to reconcile these findings with those observed in patients. The profound effects of advanced atrophy in this study highlight the importance of diagnosing and treating rotator cuff tears at risk for developing chronic degenerative changes before advanced changes occur. Further research is needed to understand the mechanisms of (1) aggressive muscle atrophy and (2) muscle-bone interactions under conditions of combined tendon and nerve injury.
Appendix
A detailed description of the methods used to determine titin molecular weight and collagen content is available with the online version of this article as a data supplement at jbjs.org.
Acknowledgments
Note: The authors acknowledge the technical support of Ki-Seok Lee, MD.
Footnotes
Investigation performed at the University of California San Diego, La Jolla, California, and Washington University, St. Louis, Missouri
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. In addition, one or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. No author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Lehman C, Cuomo F, Kummer FJ, Zuckerman JD. The incidence of full thickness rotator cuff tears in a large cadaveric population. Bull Hosp Jt Dis. 1995;54(1):30-1 Epub 1995 Jan 1. [PubMed] [Google Scholar]
- 2.Fuchs S, Chylarecki C, Langenbrinck A. Incidence and symptoms of clinically manifest rotator cuff lesions. Int J Sports Med. 1999April;20(3):201-5 Epub 1999 May 20. [DOI] [PubMed] [Google Scholar]
- 3.Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004February;86(2):219-24. [DOI] [PubMed] [Google Scholar]
- 4.Harryman DT 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991August;73(7):982-9 Epub 1991 Aug 1. [PubMed] [Google Scholar]
- 5.Gerber C, Meyer DC, Schneeberger AG, Hoppeler H, von Rechenberg B. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am. 2004September;86(9):1973-82 Epub 2004 Sep 3. [DOI] [PubMed] [Google Scholar]
- 6.Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994July;304:78-83 Epub 1994 Jul 1. [PubMed] [Google Scholar]
- 7.Meyer DC, Gerber C, Von Rechenberg B, Wirth SH, Farshad M. Amplitude and strength of muscle contraction are reduced in experimental tears of the rotator cuff. Am J Sports Med. 2011July;39(7):1456-61 Epub 2011 Feb 24. [DOI] [PubMed] [Google Scholar]
- 8.Safran O, Derwin KA, Powell K, Iannotti JP. Changes in rotator cuff muscle volume, fat content, and passive mechanics after chronic detachment in a canine model. J Bone Joint Surg Am. 2005December;87(12):2662-70 Epub 2005 Dec 3. [DOI] [PubMed] [Google Scholar]
- 9.Tomioka T, Minagawa H, Kijima H, Yamamoto N, Abe H, Maesani M, Kikuchi K, Abe H, Shimada Y, Itoi E. Sarcomere length of torn rotator cuff muscle. J Shoulder Elbow Surg. 2009Nov-Dec;18(6):955-9 Epub 2009 Jun 10. [DOI] [PubMed] [Google Scholar]
- 10.Powell PL, Roy RR, Kanim P, Bello MA, Edgerton VR. Predictability of skeletal muscle tension from architectural determinations in guinea pig hindlimbs. J Appl Physiol Respir Environ Exerc Physiol. 1984December;57(6):1715-21. [DOI] [PubMed] [Google Scholar]
- 11.Bodine SC, Roy RR, Meadows DA, Zernicke RF, Sacks RD, Fournier M, Edgerton VR. Architectural, histochemical, and contractile characteristics of a unique biarticular muscle: the cat semitendinosus. J Neurophysiol. 1982July;48(1):192-201. [DOI] [PubMed] [Google Scholar]
- 12.Winters TM, Takahashi M, Lieber RL, Ward SR. Whole muscle length-tension relationships are accurately modeled as scaled sarcomeres in rabbit hindlimb muscles. J Biomech. 2011January4;44(1):109-15 Epub 2010 Oct 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hersche O, Gerber C. Passive tension in the supraspinatus musculotendinous unit after long-standing rupture of its tendon: a preliminary report. J Shoulder Elbow Surg. 1998Jul-Aug;7(4):393-6 Epub 1998 Sep 30. [DOI] [PubMed] [Google Scholar]
- 14.Gimbel JA, Mehta S, Van Kleunen JP, Williams GR, Soslowsky LJ. The tension required at repair to reappose the supraspinatus tendon to bone rapidly increases after injury. Clin Orthop Relat Res. 2004September;426:258-65 Epub 2004 Sep 4. [DOI] [PubMed] [Google Scholar]
- 15.Gimbel JA, Van Kleunen JP, Lake SP, Williams GR, Soslowsky LJ. The role of repair tension on tendon to bone healing in an animal model of chronic rotator cuff tears. J Biomech. 2007;40(3):561-8 Epub 2006 Apr 4. [DOI] [PubMed] [Google Scholar]
- 16.Prado LG, Makarenko I, Andresen C, Krüger M, Opitz CA, Linke WA. Isoform diversity of giant proteins in relation to passive and active contractile properties of rabbit skeletal muscles. J Gen Physiol. 2005November;126(5):461-80 Epub 2005 Oct 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ward SR, Sarver JJ, Eng CM, Kwan A, Würgler-Hauri CC, Perry SM, Williams GR, Soslowsky LJ, Lieber RL. Plasticity of muscle architecture after supraspinatus tears. J Orthop Sports Phys Ther. 2010November;40(11):729-35 Epub 2010 Aug 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mallon WJ, Wilson RJ, Basamania CJ. The association of supraspinatus neuropathy with massive rotator cuff tears: a preliminary report. J Shoulder Elbow Surg. 2006Jul-Aug;15(4):395-8 Epub 2006 Jul 13. [DOI] [PubMed] [Google Scholar]
- 19.Costouros JG, Porramatikul M, Lie DT, Warner JJ. Reversal of supraspinatus neuropathy following arthroscopic repair of massive supraspinatus and infraspinatus rotator cuff tears. Arthroscopy. 2007November;23(11):1152-61 Epub 2007 Nov 8. [DOI] [PubMed] [Google Scholar]
- 20.Kannus P, Leppälä J, Lehto M, Sievänen H, Heinonen A, Järvinen M. A rotator cuff rupture produces permanent osteoporosis in the affected extremity, but not in those with whom shoulder function has returned to normal. J Bone Miner Res. 1995August;10(8):1263-71. [DOI] [PubMed] [Google Scholar]
- 21.Kim HM, Galatz LM, Lim C, Havlioglu N, Thomopoulos S. The effect of tear size and nerve injury on rotator cuff muscle fatty degeneration in a rodent animal model. J Shoulder Elbow Surg. 2012July;21(7):847-58 Epub 2011 Aug 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Laron D, Natsuhara K, Manzano G, Kim HT, Feeley BT. A mouse model of massive rotator cuff tears. J Bone Joint Surg Am. 2012April4;94(7):e41 Epub 2012 Apr 11. [DOI] [PubMed] [Google Scholar]
- 23.Killian ML, Lim CT, Thomopoulos S, Charlton N, Kim HM, Galatz LM. The effect of unloading on gene expression of healthy and injured rotator cuffs. J Orthop Res. 2013August;31(8):1240-8 Epub 2013 Mar 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samagh SP, Kramer EJ, Melkus G, Laron D, Bodendorfer BM, Natsuhara K, Kim HT, Liu X, Feeley BT. MRI quantification of fatty infiltration and muscle atrophy in a mouse model of rotator cuff tears. J Orthop Res. 2013March;31(3):421-6 Epub 2012 Sep 18. [DOI] [PubMed] [Google Scholar]
- 25.Schmutz S, Fuchs T, Regenfelder F, Steinmann P, Zumstein M, Fuchs B. Expression of atrophy mRNA relates to tendon tear size in supraspinatus muscle. Clin Orthop Relat Res. 2009February;467(2):457-64 Epub 2008 Oct 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warren CM, Krzesinski PR, Greaser ML. Vertical agarose gel electrophoresis and electroblotting of high-molecular-weight proteins. Electrophoresis. 2003June;24(11):1695-702. [DOI] [PubMed] [Google Scholar]
- 27.Edwards CA, O’Brien WD Jr. Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin Chim Acta. 1980June10;104(2):161-7. [DOI] [PubMed] [Google Scholar]
- 28.Neuman RE, Logan MA. The determination of collagen and elastin in tissues. J Biol Chem. 1950October;186(2):549-56. [PubMed] [Google Scholar]
- 29.Karsenty G, Ferron M. The contribution of bone to whole-organism physiology. Nature. 2012January19;481(7381):314-20 Epub 2012 Jan 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mannava S, Plate JF, Whitlock PW, Callahan MF, Seyler TM, Koman LA, Smith TL, Tuohy CJ. Evaluation of in vivo rotator cuff muscle function after acute and chronic detachment of the supraspinatus tendon: an experimental study in an animal model. J Bone Joint Surg Am. 2011September21;93(18):1702-11 Epub 2011 Sep 23. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Joshi SK, Samagh SP, Dang YX, Laron D, Lovett DH, Bodine SC, Kim HT, Feeley BT. Evaluation of Akt/mTOR activity in muscle atrophy after rotator cuff tears in a rat model. J Orthop Res. 2012September;30(9):1440-6 Epub 2012 Feb 29. [DOI] [PubMed] [Google Scholar]
- 32.Joshi SK, Kim HT, Feeley BT, Liu X. Differential ubiquitin-proteasome and autophagy signaling following rotator cuff tears and supraspinatus nerve injury. J Orthop Res. 2014January;32(1):138-44 Epub 2013 Sep 09. [DOI] [PMC free article] [PubMed] [Google Scholar]