Abstract
Background
Diabetes mellitus in pregnancy causes defects in infant heart, includingthe outflow tracts (OFTs). Development of the aorta and pulmonary artery, which are derived from the common OFT in the embryo, is regulated by the transforming growth factor β (TGFβ) and Wnt families, and can be perturbed by hyperglycemia-generated intracellular stress conditions. However, the underlying cellular and molecular mechanisms remain to be delineated.
Methods
Female mice were induced diabetic with streptozotocin. Embryonic and fetal OFTs were examined morphologically and histologically. Cell proliferation was assessed using BrdU-incorporation assay. Oxidative and endoplasmic reticulum (ER) stresses and TGFβ factors were detected using immunohistochemistry. The expression of genes in the Wnt signaling system was assessed using real-time RT-PCR array. The role of Activin-A in cell proliferation was addressed by treating embryos cultured in high glucose with Activin-A.
Results
Maternal diabetes caused complex abnormalities in the OFT, including aortic and pulmonary stenosis and persistent truncus arteriosus. The development of the endocardial cushions was suppressed, manifested with insufficient cellularization of the tissues. Cell proliferation was significantly decreased under oxidative and ER stress conditions. The expression of genes in the Wnt signaling was significantly altered. Activin-A and Smad3 were found to be expressed in the OFT. Treatment with Activin-A rescued cell proliferation in the endocardial cushions.
Conclusions
Maternal diabetes generates oxidative and ER stress conditions, suppresses TGFβ and Wnt signaling, inhibits cell proliferation and cellularization of the endocardial cushions, leading to OFT septal defects. Activin-A plays a role in hyperglycemia-suppressed proliferation of the endocardial cells.
Keywords: Diabetic embryopathy, cardiac defects, outflow tract, TGFβ, Wnt
INTRODUCTION
In diabetic embryopathy, a diabetic complication during early pregnancy that causes structural birth defects in infants, abnormalities are commonly present in the central nervous and cardiovascular systems (Correa et al., 2008; Mills, 1982). In the cardiovascular system, more complex defects are seen in the outflow structures than in other parts of the heart (Corrigan et al., 2009; Ferencz et al., 1990).
The outflow structures of a mature heart, consisting of the aorta and pulmonary artery, are derived from a single segment of the heart tube, known as the outflow tract (OFT), in the embryo (Neeb et al., 2013). The common OFT is divided into the two great arteries, which connect to the left and right ventricles, respectively. Septation of these great vessels is achieved by fusion of non-muscular tissues, known as the endocardial cushions, within the OFT tube (Anderson et al., 2003; Webb et al., 2003). Most anomalies in the outflow regions are associated with OFT septation, including persistent truncus arteriosus, interrupted aortic arch, aortic stenosis, pulmonary stenosis, and double outlet of right or ventricle (Correa et al., 2008; Ferencz et al., 1990; Neeb et al., 2013; Wren et al., 2003).
The development of the endocardial cushions begins with a single layer of endocardial cells and acellular matrix, known as cardiac jelly, between the endocardium and myocardium. The endocardial cells differentiate into mesenchymal cells, a process known as epithelial-mesenchymal transformation, and migrate into the cardiac jelly to cellularize the structures. Both endocardial and mesenchymal cells are active in mitosis to increase the number of cells in the endocardial cushions (Farrell and Kirby, 2001; Person et al., 2005).
Maternal diabetes disturbs metabolic homeostasis in the cells of the embryo and, in turn, affects the development of the endocardial cushions. The adverse intracellular conditions, including oxidative and endoplasmic reticulum stresses (Morgan et al., 2008b; Zhao, 2012), may disrupt the signaling of the transforming growth factor β (TGFβ) and Wnt families in the developing heart (Cohen et al., 2008; Gessert and Kuhl, 2010; Kruithof et al., 2012; Nakajima et al., 2000).
The TGFβ family, consisting of TGFβs, Bone morphogenetic proteins, Inhibins, and Activins, exerts activities via their transmembrane receptors and intracellular effectors, including Smad transcription factors (Massague, 1998; Massague and Chen, 2000). It has been shown that the TGFβ system is involved in heart malformation in diabetic embryopathy, particular, Activin-A, which is a homodimer of Inhibin βA.
Wnt factors also transduce signals through receptors and intracellular effectors, leading to regulation of gene expression (Nusse, 2005; van Amerongen and Nusse, 2009). Wnt signaling can be inhibited by endogenous inhibitors (Cruciat and Niehrs, 2013). Gene knockout experiments have clearly demonstrated that factors in the Wnt signaling system play important roles in OFT development (Cohen et al., 2008; Gessert and Kuhl, 2010). However, their involvement in OFT malformation in diabetic embryopathy remains to be addressed.
MATERIALS AND METHODS
Generation of diabetic mice
Use of animals was approved by the Institutional Animal Care and Use Committee of University of Maryland Baltimore. Eight-week old female mice (C57BL; Jackson Laboratory, Bar Harbor, ME) were injected intravenously with streptozotocin (STZ; Sigma-Aldrich, St. Louis, MO) in 0.1 M citrate buffer (pH 4.5) at a 65 mg/kg dosage to eliminate the insulin-producing β cells in the pancreas (Yang et al., 2008; Zhao, 2010). When blood glucose levels, monitored daily, reached ≥250 mg/dl or 14 mM (seven days after STZ administration), the mice were categorized as diabetes. Before mating with normal male mice, the glucose levels were restored to the normal range (4.5–8.5 mM) by subcutaneous implantation of LinBit insulin pellets (LinShin Canada, Canada). Male and female mice were paired in the afternoon. The presence of the vaginal plug in the next morning was designated as embryonic (E) day 0.5. At E5.5, insulin implants were removed to make the female mice hyperglycemic again (~ 20 mM glucose), which usually occurs at E7.5. A group of mice with insulin pellets retained were used as controls. At desired developmental stages (E10.5, E11.5, and E15.5), the mice were euthanized and the embryos were collected for examination.
Whole embryo culture
Embryos of non-diabetic mice at E9.5 were dissected in Hank’s saline with the visceral yolk sac left intact. A small incision was made on the yolk sac membrane to allow Activin-A to reach to the embryo more efficiently. Embryos (4/bottle) were cultured in 5 ml of 75% rat serum in minimum essential medium (Life Science, Grand Island, NY) at 38°C in 30 rev/min rotation in the roller bottle system (Sturm and Tam, 1993). Embryos were treated with D-glucose (22 mM) with or without activin-A (50 ng/ml; R&D Systems, Minneapolis, MN) for 24 hours. Embryos cultured in 8 mM glucose were used as euglycemic control.
Great artery labeling
The ventral thoracic wall of E15.5 fetus was opened to expose the heart. Evans Blue dye was injected into the heart through the apexes of the ventricles to label the great arteries. Photographs of the heart were taken using a CCD camera on a Zeiss dissecting microscope.
Histology and immunohistochemistry
The embryos were fixed in 4% paraformaldehyde (PFA) in phosphate buffered saline (PBS, pH 7.4) at 4°C for 16 hours, followed by dehydration through a series of concentrations of ethanol and embedding in paraffin wax through mediation steps of xylene. Tissue sections at 6 μm thickness were cut using a microtome.
Tissue sections were dewaxed in xylene and rehydrated through a reverse ethanol concentration series to water. For histological preparation, the sections were stained with hematoxylin and eosin (Junqueira and Carneiro, 2003).
For immunohistochemistry, the sections were boiled in a citrate buffer (10 mM Citrate Acid, pH 6.0) for 5 minutes to unmask antigens, equilibrated in PBS (pH 7.4), incubated with primary antibodies recognizing 4-hydroxynonenal (4-HNE; Millipore, Temecula, CA), 3-nitrotyrosine (3-NT, Cell Signaling Technology, Danvers, MA), protein disulfide isomerase (PDI; Cell Signaling Technology), phosphorylated (p-) calnexin (Abcam, Cambridge, MA), Inhibin βA (EMD Biosciences, Gibbstown, NJ), and p-Smad3 (Cell Signaling Technology) (Zhao, 2012).
Signals were detected using secondary antibodies conjugated with alkaline phosphatase (AP; Santa Cruz Biotechnology, Santa Cruz, CA) and its substrates (nitro-blue tetrazolium and 5-bromo-4-chloro-3’-indolyphosphate). Tissue sections were counterstained with fast red.
Cell proliferation assay
Cell proliferation was assessed using by BrdU (5’-bromo-2’-deoxyuridine) incorporation assay (Gratzner, 1982). For in vivo labeling, pregnant mice were given single intraperitoneal injection of BrdU (50 mg/kg in saline, Sigma-Aldrich, St. Louis, MO) (Zhao, 2010). For in vitro labeling, BrdU was added to the embryo cultures at 30 μg/ml (Zhao, 2013). After two hours of labeling, the embryos were quickly dissected out of the uteri or harvested from cultures and fixed in 4% PFA for histological preparation.
Tissue sections were incubated with 20 μg/ml proteinase K in PBS for 15 minutes at room temperature, followed by 4 N HCl for 20 minutes and neutralization with 100 mM Tris-HCl (pH 8.5), 140 mM NaCl. The sections were incubated with anti-BrdU antibody (Sigma-Aldrich) for 1 hour at room temperature.
The signals for BrdU were detected using secondary antibodies conjugated with fluorescein or AP. The sections were counterstained with DAPI (4’,6-diamidino-2-phenylindole, dihydrochloride) or fast red. The ratio of BrdU-positive/total nuclei in unit area was calculated and subject to analysis.
Real-time RT-PCR array
Malformed hearts in the diabetic groups and normal hearts in the non-diabetic control groups at E10.5 were isolated in cold PBS. Total RNA was extracted from individual hearts using RNeasy kit (Qiagen, Valencia, CA), following the manufacturer’s instructions. RNA samples from each group were used to synthesize cDNA using RT (reverse transcription) First Strand kit (Qiagen) after a removal of genomic DNA contamination with DNase I. Real-time polymerase chain reaction (PCR) of mouse Wnt arrays (PAMM-043) was performed using Real-Time PCR kit (Qiagen) following the manufacturer’s instructions on an Eppendoff thermal cycler. Data were analyzed using the Qiagen online program to identify genes with significant differences (p<0.05) in expression level between diabetic and control groups.
Statistical analysis
The numbers of cells in the endocardial cushions and ratios of BrdU-positive cells in total number of cells were summarized as Mean ± SD. T-texts were performed to determine the differences between control and diabetic groups, with p<0.05 considered as statistically significant.
RESULTS
OFT malformations in the embryonic hearts of diabetic mice
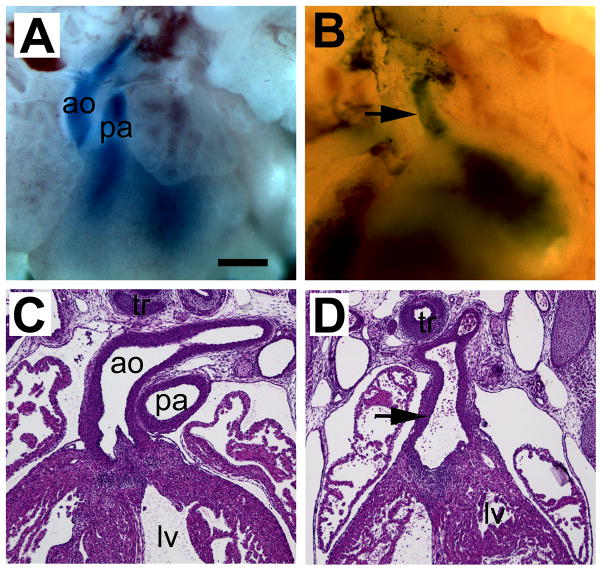
The OFTs of the hearts were examined at E15.5 when septation is complete. In the control (CON) group, 17 fetuses from 3 litters were examined for OFT abnormalities. All showed separated aorta and pulmonary artery extruding from the hearts (Fig. 1A). In the diabetic (DM) group, 6 out of 26 fetuses (23%) from 5 litters showed OFT abnormalities, four hearts exhibited characteristics of aortic and pulmonary stenosis (data not shown). Two hearts had single outflow trunks connecting to both ventricles (Fig. 1B).
Fig. 1.
OFT defects in diabetic embryopathy. (A,B) Whole mount preparation of hearts at E15.5. The great arteries are labeled with blue dye. (C,D) Frontal sections of hearts at E15.5. (A,C) Non-diabetic controls. (B,D) Diabetic group. Arrows indicated single outflow trunk. ao, aorta; lv, left ventricle; pa, pulmonary artery; tr, trachea. Scale bar = 100 μm in A,B; 50 μm in C,D.
In histological examination, the aorta and pulmonary artery were clearly discernible in the CON group (Fig. 1C). However, in the DM group, a single outflow trunk was present connecting to both ventricles (Fig. 1D), resembling persistent truncus arteriosus.
Oxidative and ER stress in the OFT
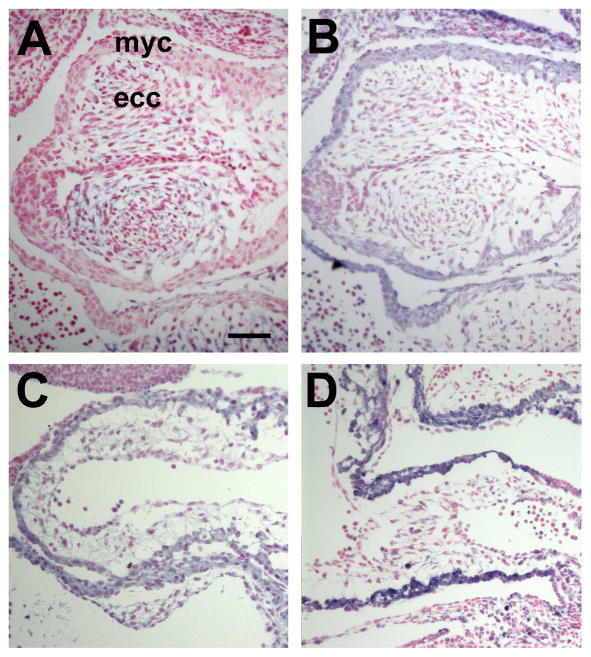
Previous studies have shown elevated levels of oxidative and ER stress markers in the embryonic hearts of DM group (Zhao, 2012). To determine that these stress conditions are present in the OFT, oxidative stress markers, 4-HNE (product of lipoperoxidation) and 3-NT (product of protein oxidation/nitration), and ER stress markers, PDI and p-calnexin, were examined using immunohistochemistry. In four E10.5 and E11.5 embryos of the DM group examined, all markers were localized in the OFT. 3-NT was restricted to the endocardial cushions (Fig. 2A). 4-HNE, PDI, and p-calnexin were in both the myocardium and endocardial cushions, with higher levels in the myocardium (Figs. 2B,C,D).
Fig. 2.
Oxidative and ER stress in the OFT of embryos of diabetic pregnancies. Immunohistochemistry of oxidative and ER stress markers (blue color). Tissue sections were counterstained wit fast red. (A) 3-NT, E11.5. (B) 4-HNE, E11.5. (C) PDI, E10.5. (D) p-calnexin, E10.5. ecc, endocardial cushion; myc, myocardium. Scale bar = 20 μm in all images.
Suppressed cellularization and cell proliferation in OFT endocardial cushions
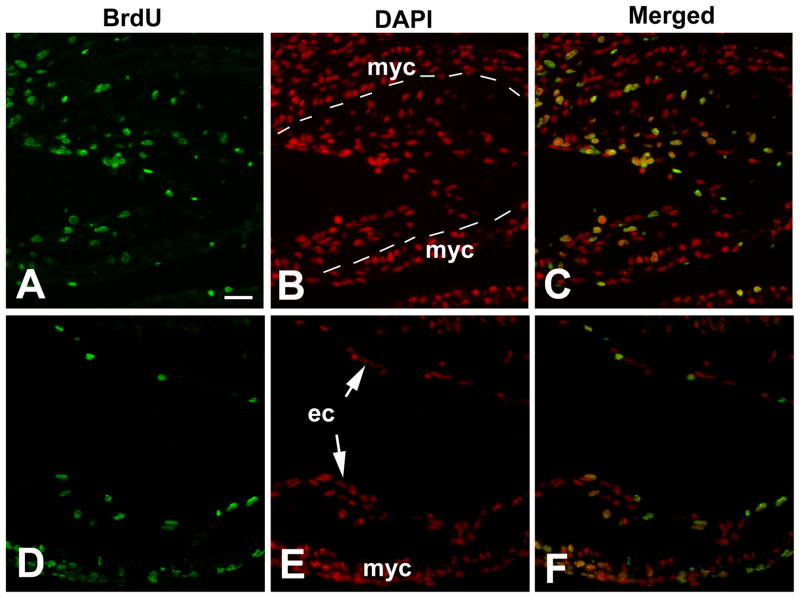
The development of the endocardial cushions requires cellularization of the cardiac jelly (Person et al., 2005). Compared with those in the CON group (Fig. 3B; 30.25 ± 9.29/unit area, n = 4 hearts), the endocardial cushions in the DM group contained fewer mesenchymal cells (Fig 3E; 12.5 ± 3.42/unit area, n = 4 hearts, p<0.05).
Fig. 3.
Cell proliferation in the OFT in diabetic embryopathy. BrdU incorporation assay of embryos at E10.5. (A,B,C) Non-diabetic controls. (D,E,F) Diabetic group. (A,D) BrdU. (B,E) DAPI. (C,F) Merged images. Broken lines mark the boundaries between the myocardium (myc) and endocardial cushion. ec, endocardium. Scale bar = 50 μm in all images.
To investigate the cellular mechanisms, cell proliferation was assessed using BrdU incorporation assay. Compared with that in the CON group (35.85% ± 10.11; Fig. 3A; n = 4), the rate of cell mitosis in the endocardial cushions was significantly lower in the DM group (17.93% ± 5.63, n=4, p<0.05; Fig. 3D).
Expression of genes in the TGFβ and Wnt signaling in the hearts
The TGFβ and Wnt families play important roles in heart development (Cohen et al., 2008; Kruithof et al., 2012). To address the question of whether Inhibin βA and its effector, Smad3, are present in the OFT, localization of these proteins was examined in normal E10.5 hearts (n = 4) using immunohistochemistry. Inhibin βA was localized in the myocardium (Fig. 4A). Reciprocally, Smad3, in an active (phosphorylated) form, was localized in the nuclei of the endocardial cells (Fig. 4B).
Fig. 4.
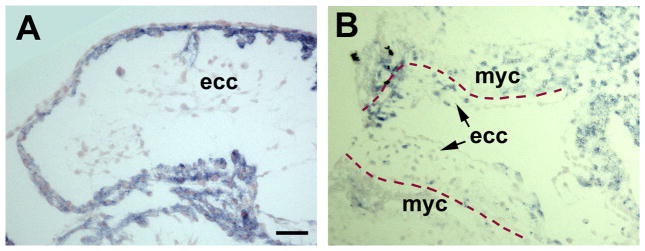
Expression of TGFβ factors in the OFT. Immunohistochemistry of Inhibin βA (A) and p-Smad3 (B) in the hearts of normal E10.5 embryos. Broken lines mark the boundaries between the endocardial cushion (ecc) and myocardium (myc). Scale bar = 20 μm in all images.
Gene knockout experiments have shown that deletion of genes in the Wnt signaling largely produce abnormalities in the OFT structures (Cohen et al., 2008). To address the question of whether the Wnt signaling system is involved in hyperglycemia-induced heart malformations, the expression of 84 genes in the Wnt signaling system in the hearts of E10.5 embryos from the DM group was examined using real-time RT-PCR, and compared with that in the CON group. Twenty four genes were shown to be significantly either up- or down-regulated by maternal diabetes (Table 1).
Table 1.
Expression of genes in the Wnt signaling in the embryonic hearts of diabetic mice
| Gene symbol | Refseq | Description | Fold change | P value |
|---|---|---|---|---|
| Aes | NM_010347 | Amino-terminal enhancer of split | 5.746 | 0.027 |
| Apc | NM_007462 | Adenomatosis polyposis coli | −0.030 | 0.040 |
| Ccnd3 | NM_007632 | Cyclin D3 | −0.056 | 0.021 |
| Csnk2a1 | NM_007788 | Casein kinase 2, α1 polypeptide | 10.643 | 0.050 |
| Ctnnb1 | NM_007614 | Catenin (cadherin associated protein), β1 | −0.123 | 0.043 |
| Dixdc1 | NM_178118 | DIX domain containing 1 | −0.138 | 0.034 |
| Dvl2 | NM_007888 | Dishevelled 2, dsh homolog (Drosophila) | 3.747 | 0.045 |
| Fbxw11 | NM_134015 | F-box and WD-40 domain protein 11 | 4.800 | 0.020 |
| Fosl1 | NM_010235 | Fos-like antigen 1 | −0.044 | 0.038 |
| Fshb | NM_008045 | Follicle stimulating hormone β | −0.070 | 0.048 |
| Fzd4 | NM_008055 | Frizzled homolog 4 (Drosophila) | −0.037 | 0.034 |
| Fzd8 | NM_008058 | Frizzled homolog 8 (Drosophila) | −0.047 | 0.046 |
| Gsk3b | NM_019827 | Glycogen synthase kinase 3β | −0.194 | 0.041 |
| Lrp5 | NM_008513 | Low density lipoprotein receptor-related protein 5 | −0.033 | 0.050 |
| Nlk | NM_008702 | Nemo like kinase | −0.181 | 0.042 |
| Porcn | NM_023638 | Porcupine homolog (Drosophila) | −0.066 | 0.037 |
| Ppp2r5d | NM_009358 | Protein phosphatase 2, regulatory subunit B, δ isoform | −0.035 | 0.034 |
| Sfrp1 | NM_013834 | Secreted frizzled-related protein 1 | −0.161 | 0.041 |
| T | NM_009309 | Brachyury | −0.032 | 0.045 |
| Tcf7l1 | NM_009332 | Transcription factor 7-like 1 (HMG box) | −0.253 | 0.045 |
| Wif1 | NM_011915 | Wnt inhibitory factor 1 | 4.684 | 0.042 |
| Wnt2 | NM_023653 | Wingless-related MMTV integration site 2 | −0.213 | 0.047 |
| Wnt6 | NM_009526 | Wingless-related MMTV integration site 6 | −0.124 | 0.034 |
| Wnt8a | NM_009290 | Wingless-related MMTV integration site 8A | −0.234 | 0.050 |
Real-time RT-PCR assay of total RNA samples (n = 4 in each group) from E10.5 hearts. Positive and negative numbers in Fold change indicate increases and decreases in the DM group, respectively, compared with the CON group.
Effect of Activin-A on cell proliferation in the OFT
Activin-A and Smad3 are expressed in the heart (Zhao, 2013). To address the question of whether this signaling pathway plays a role in hyperglycemia-induced OFT malformation, mouse embryos cultured in high glucose were treated with Activin-A (50 ng/ml). In the high glucose group, lower rate of BrdU-labeling was observed in the endocardial cushions (Fig. 5B), compared with that in the euglycemic controls (Fig. 5A). Treatment with Activin-A significantly rescued cell proliferation in the OFT of the embryos cultured in high glucose (22.28% ± 5.09, n = 4; Fig. 5C), compared with the high glucose group (11.59% ± 6.19, n = 4, p<0.05, Fig. 5B).
Fig. 5.
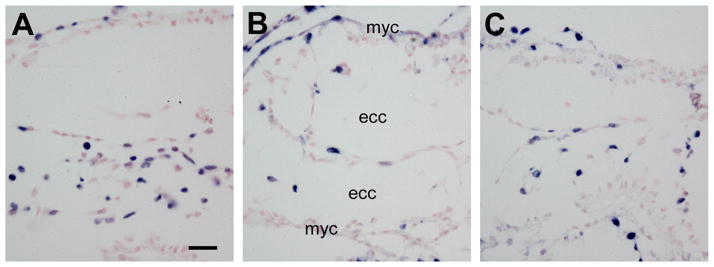
Effect of Activin-A on cell proliferation in the OFT of embryos cultured in high glucose. BrdU incorporation assay (blue) of E9.5 embryos cultured for 24 hours. (A) Euglycemic control. (B) High glucose. (C) High glucose + Activin-A. ecc, endocardial cushion; myc, myocardium. Scale bar = 20 μm in all images.
DISCUSSION
In infants of diabetic pregnancies, defects are most common in the cardiovascular system (Correa et al., 2008; Ferencz et al., 1990). A large number of abnormalities are seen in the OFT regions, including persistent truncus arteriosus, great artery (aortic and pulmonary) stenosis and atresia, and disrupted aortic arch. Malformations in the OFT are also associated with other structures of the heart, resulting in complex defects, such as Tetralogy of Fallot. Many of the defects have been recapitulated in diabetic animal models (Moazzen et al., 2014; Molin et al., 2004; Morgan et al., 2008b; Roest et al., 2009; Siman et al., 2000), including persistent truncus arteriosus, great artery stenosis in the present study, allowing investigations into the underlying mechanisms.
Persistent truncus arteriosus is caused by failure in septation of the OFT via fusion of the endocardial cushions (Neeb et al., 2013). In diabetic embryopathy, this type of anomaly is found to be associated with hypoplasia of the endocardial cushions. Such inadequate tissue growth results in failure of endocardial cushion fusion to form the septum. The underdevelopment of the endocardial cushions is a result of insufficient cellularization of the cardiac jelly during the critical period of endocardial cushion development. Cells to populate the OFT endocardial cushions come from two major sources, namely the endocardium via epithelial-mesenchymal transformation (EMT) and neural crest in the neural tube (Brown and Baldwin, 2006; Person et al., 2005).
Neural crest cells play a pivotal role in OFT development and are associated with hyperglycemia-induced OFT malformations (Gitler et al., 2002; Morgan et al., 2008a; Morgan et al., 2008b). However, the mechanisms by which neural crest cells regulate OFT development are still unknown. Neural crest cells are present in relatively small numbers and occupy restricted regions in OFT endocardial cushions, unlikely to significantly contribute to the cellularization of the cushions (Brown and Baldwin, 2006; Stoller and Epstein, 2005). Neural crest cells do not migrate into the AV cushions, which are important structures for AV septation and associated with diabetes-induced ventricular septal defects (Zhao, 2010; 2012). The observations in the present study suggest that the endocardial cells contribute considerably to the growth of the OFT cushions, similar as seen in the AV cushions. Taken these observations together, it is suggested that hyperglycemia mainly affects endocardial cells in the endocardial cushions of the OFT. It is worth noting that, in addition to the defects in the conotruncal regions, abnormalities are also seen in the aortic arch (Molin et al., 2004; Siman et al., 2000). These anomalies are formed due to abnormal cushion remodeling during late stages of cardiogenesis, in which neural crest are also involved (Brown and Baldwin, 2006; Molin et al., 2004).
In the endocardial cushions of the OFT and AV channel, endocardial cells undergo EMT and migration to populate the tissues (Person et al., 2005). It has been shown that cell EMT and migration are suppressed by hyperglycemia in the AV cushions (Zhao, 2012; 2013). Similar effects are also implicated in the OFT endocardial cushions. The endocardial cells are also actively dividing to proliferate. Maternal hyperglycemia suppresses endocardial mitosis in both OFT and AV cushions (Zhao, 2010), suggesting that post-migration proliferation is essential for endocardial cushion development and vulnerable to hyperglycemic insult.
These cellular activities in the developing heart can be perturbed by changes in intracellular metabolism, which lead to stress conditions in the cells of the embryo (Morgan et al., 2008b; Zhao, 2012). Oxidative stress has been observed in the OFT of the heart (Morgan et al., 2008b). Further, treatments with antioxidants can reduce malformations in this structure (Moazzen et al., 2014; Morgan et al., 2008b). In addition, hyperglycemia-induced ER stress is also detected in the OFT (present study). It has been shown that ER stress is present in AV endocardial cushions and shown to inhibit EMT and migration (Zhao, 2012). The observations suggest that the endocardial cushions in different regions of the heart are under similar cellular stress conditions when exposed to hyperglycemia.
The development of the endocardial cushions is regulated by the signaling of the Wnt and TGFβ families (Cohen et al., 2008; Nakajima et al., 2000; Person et al., 2005). Gene knockout experiments have shown that many genes in the Wnt signaling system are associated with abnormalities in the OFT, suggesting that the Wnt system is important for OFT development (Cohen et al., 2008; Neeb et al., 2013).
Real-time RT-PCR examination reveals that 24 out of 84 genes in the Wnt signaling system are affected by maternal diabetes, indicating that they are involved in diabetes-induced cardiac malformations in the embryos. Maternal hyperglycemia mainly downregulates the genes that positively mediate the Wnt signaling. For example, Wnts (Wnt2, 6, and 8a) are decreased, along with their receptors (fzd4 and 8) and effector (β-catenin). In contrast, Wnt negative regulators, wif1 (Wnt inhibitory factor 1) and aes (amino-terminal enhancer of split) are dramatically increased. One of the components in the β-catenin destructive complex, casein kinase 2, is significantly upregulated, although other members of the complex, apc (adenomatosis polyposis coli) and gsk3β are slightly decreased.
Alteration of Wnt expression has been shown to be associated with maternal diabetes-induced caudal dysplasia (Pavlinkova et al., 2008). Similar changes in genes in the canonical pathway are observed, including downregulation of Wnts, fzds, β-catenin, apc, and gsk3β, suggesting similar regulation of Wnt expression by hyperglycemia in different embryonic tissues. These investigations also suggest that maternal diabetes suppresses Wnt signaling, at least, at the gene expression level. Further work is aimed delineate other mechanisms in protein expression, modification, and function.
Perturbation of TGFβ signaling by maternal diabetes has been observed in the heart (Kumar et al., 2007; Zhao, 2010). Among the TGFβ factors, the inhibin βA gene is the most affected. The homodimer of Inhibin βA, known as Activin A, has been shown to play a role in cardiogenesis (Moore et al., 1998; Sugi and Lough, 1995). The present study demonstrates that Activin-A can promote cell proliferation in OFT endocardial cushions under high glucose conditions.
The OFTs exhibit the most complex defects in the heart in diabetic embryopathy. These abnormalities are associated with septation of the embryonic OFT. The present study demonstrates that the septal defects are caused by decreases in cell proliferation and cellularization of the endocardial cushions. The perturbed cellular activity is associated with increases in oxidative and ER stresses and alteration in TGFβ and Wnt signaling.
Acknowledgments
The author thanks Hua Li for technical assistance. Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD076245.
Footnotes
The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
References
- Anderson RH, Webb S, Brown NA, Lamers W, Moorman A. Development of the heart: (3) formation of the ventricular outflow tracts, arterial valves, and intrapericardial arterial trunks. Heart. 2003;89(9):1110–1118. doi: 10.1136/heart.89.9.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CB, Baldwin HS. Neural crest contribution to the cardiovascular system. Adv Exp Med Biol. 2006;589:134–154. doi: 10.1007/978-0-387-46954-6_8. [DOI] [PubMed] [Google Scholar]
- Cohen ED, Tian Y, Morrisey EE. Wnt signaling: an essential regulator of cardiovascular differentiation, morphogenesis and progenitor self-renewal. Development. 2008;135(5):789–798. doi: 10.1242/dev.016865. [DOI] [PubMed] [Google Scholar]
- Correa A, Gilboa SM, Besser LM, Botto LD, Moore CA, Hobbs CA, Cleves MA, Riehle-Colarusso TJ, Waller DK, Reece EA. Diabetes mellitus and birth defects. Am J Obstet Gynecol. 2008;199(3):237.e231–239. doi: 10.1016/j.ajog.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan N, Brazil DP, McAuliffe F. Fetal cardiac effects of maternal hyperglycemia during pregnancy. Birth Defects Res A Clin Mol Teratol. 2009;85(6):523–530. doi: 10.1002/bdra.20567. [DOI] [PubMed] [Google Scholar]
- Cruciat CM, Niehrs C. Secreted and transmembrane wnt inhibitors and activators. Cold Spring Harb Perspect Biol. 2013;5(3):a015081. doi: 10.1101/cshperspect.a015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MJ, Kirby ML. Cell biology of cardiac development. Int Rev Cytol. 2001;202:99–158. doi: 10.1016/s0074-7696(01)02004-6. [DOI] [PubMed] [Google Scholar]
- Ferencz C, Rubin JD, McCarter RJ, Clark EB. Maternal diabetes and cardiovascular malformations: predominance of double outlet right ventricle and truncus arteriosus. Teratology. 1990;41(3):319–326. doi: 10.1002/tera.1420410309. [DOI] [PubMed] [Google Scholar]
- Gessert S, Kuhl M. The multiple phases and faces of wnt signaling during cardiac differentiation and development. Circ Res. 2010;107(2):186–199. doi: 10.1161/CIRCRESAHA.110.221531. [DOI] [PubMed] [Google Scholar]
- Gitler AD, Brown CB, Kochilas L, Li J, Epstein JA. Neural crest migration and mouse models of congenital heart disease. Cold Spring Harb Symp Quant Biol. 2002;67:57–62. doi: 10.1101/sqb.2002.67.57. [DOI] [PubMed] [Google Scholar]
- Junqueira LC, Carneiro J. Basic Histology. 10. New York: McGraw-Hill; 2003. [Google Scholar]
- Kruithof BP, Duim SN, Moerkamp AT, Goumans MJ. TGFbeta and BMP signaling in cardiac cushion formation: lessons from mice and chicken. Differentiation. 2012;84(1):89–102. doi: 10.1016/j.diff.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Kumar SD, Dheen ST, Tay SS. Maternal diabetes induces congenital heart defects in mice by altering the expression of genes involved in cardiovascular development. Cardiovasc Diabetol. 2007;6:34. doi: 10.1186/1475-2840-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Massague J, Chen YG. Controlling TGF-β signaling. Genes Dev. 2000;14(6):627–644. [PubMed] [Google Scholar]
- Mills JL. Malformations in infants of diabetic mothers. Teratology. 1982;25(3):385–394. doi: 10.1002/tera.1420250316. [DOI] [PubMed] [Google Scholar]
- Moazzen H, Lu X, Ma NL, Velenosi TJ, Urquhart BL, Wisse LJ, Gittenberger-de Groot AC, Feng Q. N-Acetylcysteine prevents congenital heart defects induced by pregestational diabetes. Cardiovasc Diabetol. 2014;13(1):46. doi: 10.1186/1475-2840-13-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molin DG, Roest PA, Nordstrand H, Wisse LJ, Poelmann RE, Eriksson UJ, Gittenberger-De Groot AC. Disturbed morphogenesis of cardiac outflow tract and increased rate of aortic arch anomalies in the offspring of diabetic rats. Birth Defects Res A Clin Mol Teratol. 2004;70(12):927–938. doi: 10.1002/bdra.20101. [DOI] [PubMed] [Google Scholar]
- Moore CS, Mjaatvedt CH, Gearhart JD. Expression and function of activin beta A during mouse cardiac cushion tissue formation. Dev Dyn. 1998;212(4):548–562. doi: 10.1002/(SICI)1097-0177(199808)212:4<548::AID-AJA8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Morgan SC, Lee HY, Relaix F, Sandell LL, Levorse JM, Loeken MR. Cardiac outflow tract septation failure in Pax3-deficient embryos is due to p53-dependent regulation of migrating cardiac neural crest. Mech Dev. 2008a;125(9–10):757–767. doi: 10.1016/j.mod.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan SC, Relaix F, Sandell LL, Loeken MR. Oxidative stress during diabetic pregnancy disrupts cardiac neural crest migration and causes outflow tract defects. Birth Defects Res A Clin Mol Teratol. 2008b;82(6):453–463. doi: 10.1002/bdra.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Yamagishi T, Hokari S, Nakamura H. Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: roles of transforming growth factor (TGF)-β and bone morphogenetic protein (BMP) Anat Rec. 2000;258(2):119–127. doi: 10.1002/(SICI)1097-0185(20000201)258:2<119::AID-AR1>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Neeb Z, Lajiness JD, Bolanis E, Conway SJ. Cardiac outflow tract anomalies. Wiley Interdiscip Rev Dev Biol. 2013;2(4):499–530. doi: 10.1002/wdev.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15(1):28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- Pavlinkova G, Salbaum JM, Kappen C. Wnt signaling in caudal dysgenesis and diabetic embryopathy. Birth defects research Part A, Clinical and molecular teratology. 2008;82(10):710–719. doi: 10.1002/bdra.20495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person AD, Klewer SE, Runyan RB. Cell biology of cardiac cushion development. Int Rev Cytol. 2005;243:287–335. doi: 10.1016/S0074-7696(05)43005-3. [DOI] [PubMed] [Google Scholar]
- Roest PA, Molin DG, Schalkwijk CG, van Iperen L, Wentzel P, Eriksson UJ, Gittenberger-de Groot AC. Specific local cardiovascular changes of Nepsilon-(carboxymethyl)lysine, vascular endothelial growth factor, and Smad2 in the developing embryos coincide with maternal diabetes-induced congenital heart defects. Diabetes. 2009;58(5):1222–1228. doi: 10.2337/db07-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siman CM, Gittenberger-De Groot AC, Wisse B, Eriksson UJ. Malformations in offspring of diabetic rats: morphometric analysis of neural crest-derived organs and effects of maternal vitamin E treatment. Teratology. 2000;61(5):355–367. doi: 10.1002/(SICI)1096-9926(200005)61:5<355::AID-TERA7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Stoller JZ, Epstein JA. Cardiac neural crest. Semin Cell Dev Biol. 2005;16(6):704–715. doi: 10.1016/j.semcdb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Sturm K, Tam PP. Isolation and culture of whole postimplantation embryos and germ layer derivatives. Methods Enzymol. 1993;225:164–190. doi: 10.1016/0076-6879(93)25013-r. [DOI] [PubMed] [Google Scholar]
- Sugi Y, Lough J. Activin-A and FGF-2 mimic the inductive effects of anterior endoderm on terminal cardiac myogenesis in vitro. Dev Biol. 1995;168(2):567–574. doi: 10.1006/dbio.1995.1102. [DOI] [PubMed] [Google Scholar]
- van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136(19):3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- Webb S, Qayyum SR, Anderson RH, Lamers WH, Richardson MK. Septation and separation within the outflow tract of the developing heart. J Anat. 2003;202(4):327–342. doi: 10.1046/j.1469-7580.2003.00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren C, Birrell G, Hawthorne G. Cardiovascular malformations in infants of diabetic mothers. Heart. 2003;89(10):1217–1220. doi: 10.1136/heart.89.10.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Zhao Z, Reece EA. Activation of oxidative stress signaling that is implicated in apoptosis with a mouse model of diabetic embryopathy. Am J Obstet Gynecol. 2008;198(1):130.e131–137. doi: 10.1016/j.ajog.2007.06.070. [DOI] [PubMed] [Google Scholar]
- Zhao Z. Cardiac malformations and alteration of TGFβ signaling system in diabetic embryopathy. Birth Defects Res B Dev Reprod Toxicol. 2010;89(2):97–105. doi: 10.1002/bdrb.20225. [DOI] [PubMed] [Google Scholar]
- Zhao Z. Endoplasmic reticulum stress in maternal diabetes-induced cardiac malformations during critical cardiogenesis period. Birth Defects Res B Dev Reprod Toxicol. 2012;95(1):1–6. doi: 10.1002/bdrb.20330. [DOI] [PubMed] [Google Scholar]
- Zhao Z. Activin-A in diabetes-induced cardiac malformations in embryos. Birth Defects Res B Dev Reprod Toxicol. 2013;98(3):260–267. doi: 10.1002/bdrb.21060. [DOI] [PubMed] [Google Scholar]