Abstract
Background
Hepatitis B virus (HBV) infection, the predominant cause of hepatocellular carcinoma (HCC) worldwide, disproportionately affects Asian Americans. Limited data exists on the variability and characteristics of infection that determine disease progression risk within US Asian ethnic subgroups.
Methods
Retrospective analyses were conducted on a large, community-based HBV screening and treatment program in New York City (NYC). From 2005-2008 the program enrolled 7,272 Asian-born individuals. Determinants of HBV seroprevalence were calculated and risk factors for HCC progression were compared across Asian subgroups.
Results
Among newly tested individuals, 13% were HBV positive. Seroprevalence varied significantly with age, gender, education, birthplace, and family history of infection. Chinese-born individuals, particularly from the Fujian province, had the highest seroprevalence (23.2% and 33.1%, respectively). Clinical and virologic characteristics placed HBV-infected individuals at significant risk for HCC. Significant differences in HCC risk existed among Asian subgroups in bivariate analysis, including age, gender, HBV viral load and HBeAg status. Differences in HBV genotype and family history of HCC may further HCC risk among subgroups.
Conclusions
Asian immigrants in NYC have a high prevalence of HBV infection and are at significant risk of disease progression and HCC. Although heterogeneity in HBV seroprevalence was found by Asian subgroups, HCC risk among infected individuals was primarily explained by age and gender differences. Country and province of birth, age, and gender may further explain seroprevalence differences.
Impact
Findings provide estimates of HBV burden in Asian ethnic subgroups and identify high risk groups to target for screening and treatment that can prevent HCC.
Keywords: hepatitis B virus, epidemiology, liver cancer, Asian Americans, health disparities
Introduction
Hepatocellular carcinoma (HCC) is the most frequent type of primary liver cancer worldwide, accounting for about 80% of liver cancers and approximately 500,000 deaths annually (1, 2). Hepatitis B (HBV) is the leading underlying cause of HCC, and a strong correlation is demonstrated between the prevalence of hepatitis B surface antigen (HBsAg) and HCC (3, 4). Approximately 5% of the world population (300-400 million) is chronically infected with HBV; 75% of whom are Asian (5, 6). More than two-thirds of new, worldwide liver cancer cases occur in Asian countries (7), and in China alone, an estimated 383,203 deaths in 2012 was attributed to liver cancer (8).
In the United States (US), the number of newly diagnosed cases of primary liver cancer each year is less than one-tenth that of China; in 2013, there were an estimated 30,640 new cases of liver cancer and 21,670 deaths in the US (9). The burden of disease is greatest among Asian Americans, for whom the incidence of liver cancer is 2 times greater than in non-Hispanic whites (10, 11). Liver cancer incidence and mortality rates are especially high among Asian American men, most notably Vietnamese men (12). In a recent study of age-adjusted cancer mortality rates among Asian Americans in New York City (NYC) (5), Korean males had the highest liver cancer mortality rate compared to all racial/ethnic groups, and rates among Chinese men were 2.5 times higher than rates for non-Hispanic white men (5). Liver cancer was the second and third cause of cancer deaths in NYC Chinese and Korean men, respectively. The difference in rates are related to the prevalence of HBV, but could include other potential factors that contribute to the development of HCC (6), such as aflatoxin exposure (13, 14), alcohol consumption (15), family history of HCC (16, 17), and HBV genotype (18, 19).
While less than 0.5% of the US population tests positive for HBsAg, the prevalence of chronic HBV infection among Asian Americans is estimated to be between 9 and 15%, and may be as high as 25% in select subgroups of recent immigrants (20-26). Asian Americans, the fastest growing racial/ethnic group in the US, are largely foreign-born (27) and account for 60% of persons in the US with chronic HBV (28). However, Asian Americans represent over 30 ethnic subgroups characterized by tremendous diversity, including differences in socioeconomic status (SES), access to resources, migration patterns, and immigration histories. While recent studies have increasingly focused on the epidemiology of HBV infection among Asian Americans in the aggregate (29, 30), research on infection disaggregated by subgroup has lagged, and few studies exist on the specific virologic characteristics and progression of disease in Asian subgroups in the US, particularly among those identified by community screening programs (30-32).
The majority of published literature on the progression of HBV infection comes from studies conducted in HBV-endemic countries, clinical patient trials, or hepatology patient clinical data. These studies have shown that the major determinant of disease progression and HCC in chronically infected persons is the level of HBV viral load (VL) in the blood. High levels of plasma HBV VL have been shown to be strong predictors for the risk of HCC (33). Hepatitis B e antigen (HBeAg), a HBV protein found in the blood when HBV is replicating at high rates, has also been associated with a 60-100-fold increase in the risk of HCC (34). More recently, studies have used a combination of these risk factors, including HBV VL, age, gender, HBeAg status, and HBV genotype to more accurately model HCC risk (18). Moreover, antiviral therapy is indicated for a subgroup of HBV patients and has been shown to reduce progression of fibrosis, reduce or even reverse cirrhosis (35, 36), and mitigate risk of HCC (37). Various guidelines have been developed by American Association for the Study of Liver Diseases (AASLD) (38), the European Association for the Study of the Liver (EASL) (39, 40), and the Asian Pacific Association for the Study of the Liver (APASL) (41) for initiating antiviral therapy in individuals at greatest risk of progression and sequalae. Limited information, however, is available on the actual burden of disease and the risks of progression and complications among the larger population of HBV-infected Asians, or by Asian subgroups, in the US. Accurate statistics on HBV infected individuals are important in assessing and targeting public health needs and estimating future health care costs related to HBV infection.
In this study, we attempt to address these gaps in knowledge by analyzing retrospective data from a community-based screening, care, and treatment program, the Asian American Hepatitis B Program (AAHBP), implemented from 2004 to 2008 in NYC, home to the biggest Asian American population (42). AAHBP, the largest screening program of Asian Americans and the original prototype of linkage-to-care approaches in the US (43), was unique in that it collected detailed demographic data on participants and provided no-cost evaluation and treatment to HBsAg positive individuals. In this study, we analyze the prevalence of chronic HBV infection by Asian ethnic subgroup, the predictors of HBsAg positivity, and the clinical and virologic characteristics of HBV-infected individuals in order to develop a more complete and nuanced understanding of the burden of disease and risk of progression and HCC outcome in this population.
Materials and Methods
Study Population
AAHBP was a city-wide collaboration of community-based organizations, academic institutions, community health centers, and the NYC Department of Health and Mental Hygiene (DOHMH) that provided no-cost HBV screening, vaccination, and treatment (43, 44). The program adopted a comprehensive strategy to increase HBV awareness, provide community-based outreach and education, and conduct free HBV screening and vaccination, which included follow-up medical care and treatment to HBV-positive individuals. The study was approved by the Institutional Review Boards of New York University School of Medicine and the DOHMH.
Between January 2005 and June 2008, individuals were offered HBV serologic testing at education and screening events or by drop-in at 12 participating health care centers and community-based organizations serving Asian Americans throughout NYC. Comprehensive post-screening clinical evaluation, care, and treatment were provided to HBV-infected individuals. Demographic and epidemiologic information was collected at the time of screening and clinical information was collected for HBsAg-positive individuals returning for follow-up visits. Positive individuals were evaluated at the AAHBP Hepatitis Clinics at Bellevue Hospital Center, Gouverneur Healthcare Services or Charles B Wang Community Health Center. Additionally, HBsAg positive individuals referred to AAHBP clinics by the DOHMH were included in the analysis of the clinical results for HBsAg positive individuals.
A total of 7,272 individuals aged 18 and over, who were born in an Asian country and had confirmed HBsAg results participated in AAHBP. Among these individuals, 4,301 (59.1%) had not previously received or did not know if they had received a HBV screening.
Laboratory Tests
Blood samples were tested for HBsAg and hepatitis B surface antibody (HBsAb) using ELISA tests (Immulite 2000, DPC, Los Angeles, CA) within 72 hours of collection. Individuals testing positive for HBsAg were further evaluated by physical exam and additional laboratory investigations were performed, including hepatitis B e-antigen (HBeAg), a marker of active viral disease, alanine aminotransferase (ALT), a liver enzyme marker, and HBV VL, a measure of how much virus is present. The upper limit of normal ALT levels was defined as 30 U/L for men and 20 U/L for women per the AASLD Practice Guidelines (45).
Statistical Analysis
Prevalence estimates and 95% confidence intervals (CIs) were calculated for newly screened individuals as previously described (46). Adjusted logistic regression was performed using demographic and risk factors that were statistically significant in bivariate analysis at (α<0.05); odds ratios (ORs) and 95% CIs are presented. Separate analyses were performed on all newly screened Chinese and Korean participants. Clinical characteristics for all HBsAg positive individuals, regardless of prior screening, were assessed for all individuals receiving detailed clinical testing. Clinical characteristics were stratified by country of birth and province of birth where applicable. HBsAg+ subjects’ initial intake ALT and HBV VL were used in an analysis looking at participants who might meet previously described guidelines for antiviral therapy (37, 39, 40, 45). All guidelines recommend treating patients with ALT > 2 times the upper limit of normal (2xULN) and HBV VL ≥100,000 copies/ml. Additional analyses were performed as needed to explain clinical characteristics of infected individuals. All analyses were conducted using SPSS version 20.0 (Armonk, NY: IBM Corp).
Results
HBV Seroprevalence
Among 4,301 newly screened, Asian-born individuals, approximately 49% were born in China, 36% in Korea, 9% in Malaysia, 1% in the Philippines, 1% in Taiwan, 2% in South Asia (India, Pakistan, or Bangladesh), 1% in Vietnam, and 1% in another Asian country (Table 1). A total of 574 individuals (13.3%) tested positive for HBsAg.
Table 1.
Prevalence and predictors of HBsAg+ among all newly screened foreign-born Asians, n=4,301
| N (%) | Prevalence (95% CI) | ORa (95% CI) | p-value | |
|---|---|---|---|---|
| Gender | ||||
| Male | 2139 (49.7) | 18.8 (17.1, 20.5) | 2.4 (1.9, 2.9) | <0.001 |
| Female | 2162 (50.3) | 7.9 (6.8, 9.0) | 1.0 | |
| Age | ||||
| 18-29 | 762 (17.7) | 24.7 (21.6, 27.8) | 5.0 (2.4, 10.5) | <0.001 |
| 30-39 | 874 (20.3) | 19.8 (17.2, 22.4) | 4.0 (2.0, 8.3) | <0.001 |
| 40-49 | 990 (23.0) | 11.6 (9.6, 13.6) | 2.8 (1.3, 5.7) | 0.006 |
| 50-59 | 955 (22.2) | 7.4 (5.7, 9.1) | 1.9 (0.9, 3.9) | 0.095 |
| 60-69 | 505 (11.8) | 2.8 (1.4, 4.2) | 0.9 (0.4, 2.1) | 0.767 |
| 70+ | 215 (5.0) | 6.0 (2.8, 9.2) | 1.0 | |
| Country of Birth | ||||
| China | 2088 (48.5) | 23.2 (21.4, 25.0) | 3.5 (2.5, 4.9) | <0.001 |
| Korea | 1551 (36.1) | 3.8 (2.9, 4.8) | 1.0 | |
| Malaysia | 381 (8.9) | 3.1 (1.4, 4.8) | 0.5 (0.3, 1.0) | 0.044 |
| Philippines | 53 (1.2) | 3.8 (-1.4, 9.0) | 1.5 (0.3, 6.2) | 0.614 |
| Taiwan | 60 (1.4) | 13.3 (4.7, 21.9) | 4.4 (2.0, 10.0) | <0.001 |
| South Asia | 69 (1.6) | 7.2 (1.1, 13.3) | 1.2 (0.4, 3.6) | 0.711 |
| Vietnam | 40 (0.9) | 5.0 (-1.8, 11.8) | 1.2 (0.3, 5.0) | 0.839 |
| Other Asian country | 59 (1.4) | 1.7 (-2.4, 5.8) | 0.4 (0.1, 2.8) | 0.339 |
| Years in US | ||||
| Less than 5 | 714 (16.6) | 18.2 (15.4, 21.0) | 1.1 (0.8, 1.4) | 0.676 |
| 5 to 10 | 1413 (32.9) | 15.2 (13.3, 17.1) | 0.9 (0.7, 1.1) | 0.202 |
| More than 10 | 1841 (42.8) | 10.5 (9.1, 11.9) | 1.0 | |
| Unknown | 333 (7.7) | 10.5 (7.2, 13.8) | 0.8 (0.5, 1.3) | 0.357 |
| Education | ||||
| Elementary school | 533 (12.8) | 16.5 (13.4, 19.7) | 2.4 (1.6, 3.5) | <0.001 |
| Junior high school | 1047 (25.1) | 21.0 (18.5, 23.5) | 2.0 (1.4, 2.7) | <0.001 |
| High school/vocational school | 1427 (34.2) | 13.6 (11.8, 15.4) | 1.7 (1.2, 2.3) | 0.002 |
| College | 1164 (27.9) | 6.5 (5.1, 7.9) | 1.0 | |
| Family History of HBV | ||||
| No | 2625 (61.3) | 9.9 (8.8, 11.0) | 1.0 | |
| Yes | 561 (13.1) | 26.9 (23.2, 30.6) | 2.3 (1.8, 3.0) | <0.001 |
| Not sure | 1095 (25.6) | 14.5 (12.4, 16.6) | 1.3 (1.0, 1.6) | 0.037 |
Adjusted for all other variables in the model
Infection rates were highest among Chinese (23.2%) and Taiwanese (13.3%), and lowest among Koreans (3.8%) and Malaysians (3.1%). Males were significantly more likely to be HBsAg positive compared to females (18.8% vs. 7.9%). Male gender, younger age, birthplace in China, Malaysia, and Taiwan, lower education, fewer years lived in the US, and a family history of HBV were significantly related to HBsAg positivity in bivariate analysis. Once placed into adjusted logistic regression, years lived in the US was no longer significant. Newly screened, Asian-born individuals testing positive for HBsAg were significantly more likely to be male, younger in age, Chinese-born, to have attained lower levels of education, and to have a family history of HBV.
Among the subset of newly screened Chinese-born individuals (Table 2), approximately 53.5% were born in the Fujian province; the prevalence of HBsAg positivity was significantly higher among individuals born in the Fujian province compared to those born in other Chinese provinces (33.1% vs. 11.7%). Further differences by province are shown in Figure 1. Male gender, younger age, birthplace in the Fujian province, fewer years lived in the US, lower education, and a family history of HBV were significantly related to HBsAg positivity in bivariate analysis. Once placed into adjusted logistic regression, years lived in the US was no longer significant. Newly screened, Chinese-born individuals testing positive for HBsAg were significantly more likely to be male, in a younger age group, born in the Fujian province, to have attained lower levels of education, and to have a family history of HBV.
Table 2.
Prevalence and predictors of HBsAg+ among all newly screened Chinese, n=2,088
| N (%) | Prevalence (95% CI) | ORa (95% CI) | p-value | |
|---|---|---|---|---|
| Gender | ||||
| Male | 1180 (43.5) | 29.7 (27.1, 32.3) | 2.3 (1.8, 3.0) | <0.001 |
| Female | 908 (56.5) | 14.9 (12.6, 17.2) | 1.0 | |
| Age | ||||
| 18-29 | 511 (24.5) | 34.2 (30.1, 38.3) | 4.7 (1.6, 14.0) | 0.005 |
| 30-39 | 516 (24.7) | 31.4 (27.4, 35.4) | 4.5 (1.6, 13.1) | 0.005 |
| 40-49 | 494 (23.7) | 18.2 (14.8, 21.6) | 3.1 (1.1, 8.8) | 0.039 |
| 50-59 | 366 (17.5) | 11.2 (8.0, 14.4) | 1.9 (0.6, 5.6) | 0.244 |
| 60-69 | 113 (5.4) | 7.1 (2.4, 11.8) | 1.2 (0.3, 4.5) | 0.735 |
| 70+ | 88 (4.2) | 10.2 (3.9, 16.5) | 1.0 | |
| Chinese Province | ||||
| Fujian | 1117 (54.9) | 33.1 (30.3, 35.9) | 2.0 (1.5, 2.8) | <0.001 |
| Other province | 916 (45.1) | 11.7 (9.6, 13.8) | 1.0 | |
| Years in US | ||||
| Less than 5 | 438 (21.0) | 26.9 (22.8, 31.1) | 1.1 (0.8, 1.6) | 0.540 |
| 5 to 10 | 790 (37.8) | 24.4 (21.4, 27.4) | 0.9 (0.7, 1.2) | 0.655 |
| More than 10 | 724 (34.7) | 20.6 (17.7, 23.6) | 1.0 | |
| Unknown | 136 (6.5) | 18.4 (11.9, 24.9) | 0.7 (0.4, 1.2) | 0.235 |
| Education | ||||
| Elementary school | 327 (16.0) | 24.2 (19.6, 28.8) | 1.9 (1.1, 3.2) | 0.017 |
| Junior high school | 751 (36.8) | 27.7 (24.5, 30.9) | 1.6 (1.0, 2.6) | 0.038 |
| High school/vocational school | 702 (34.5) | 22.8 (19.7, 25.9) | 1.7 (1.1, 2.7) | 0.024 |
| College | 259 (12.7) | 12.2 (8.2, 16.2) | 1.0 | |
| Family History of HBV | ||||
| No | 1151 (55.4) | 18.7 (16.5, 21.0) | 1.0 | |
| Yes | 396 (19.0) | 32.1 (27.5, 36.7) | 1.8 (1.4, 2.4) | <0.001 |
| Not sure | 532 (25.6) | 26.3 (22.6, 30.0) | 1.2 (1.0, 1.6) | 0.102 |
Adjusted for all other variables in the model
Figure 1.
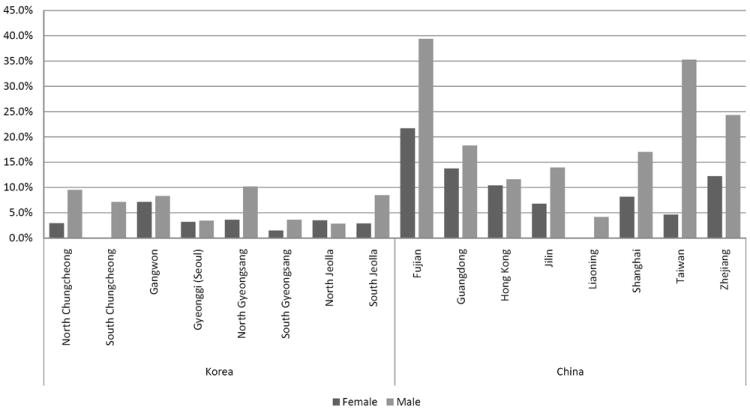
HBsAg+ by Chinese and Korean provinces among newly screened adults
Among the subset of newly screened individuals born in Korea (Table 3), males were significantly more likely than females to be HBsAg positive (4.9% vs. 2.9%), and individuals with a family history of HBV were more likely to be HBsAg positive compared to individuals without a family history of HBV (11.5% vs. 3.2%). Once placed into adjusted logistic regression, male gender and family history of HBV remained significant for newly screened, Korean-born individuals testing positive for HBsAg.
Table 3.
Prevalence and predictors of HBsAg+ among all newly screened Koreans, n=1,551
| N (%) | Prevalence (95% CI) | ORa (95% CI) | p-value | |
|---|---|---|---|---|
| Gender | ||||
| Male | 690 (44.5) | 4.9 (3.3, 6.5) | 2.0 (1.1, 3.5) | 0.018 |
| Female | 861 (55.5) | 2.9 (1.8, 4.0) | 1.0 | |
| Age | ||||
| 18-29 | 192 (12.4) | 4.2 (1.4, 7.0) | 1.1 (0.3, 4.3) | 0.839 |
| 30-39 | 207 (13.3) | 3.9 (1.3, 6.5) | 0.9 (0.2, 3.3) | 0.831 |
| 40-49 | 315 (20.3) | 4.8 (2.4, 7.2) | 1.4 (0.4, 4.4) | 0.615 |
| 50-59 | 412 (26.6) | 4.9 (2.8, 7.0) | 1.0 (0.3, 3.1) | 0.939 |
| 60-69 | 320 (20.6) | 1.2 (0.0, 2.4) | 0.3 (0.1, 1.1) | 0.066 |
| 70+ | 105 (6.8) | 3.8 (0.1, 7.5) | 1.0 | |
| Korean Province | ||||
| Gyeonggi (Seoul) | 725 (49.8) | 3.3 (2.0, 4.6) | 0.7 (0.4, 1.2) | 0.208 |
| Not Gyeonggi | 730 (50.2) | 4.2 (2.7, 5.7) | 1.0 | |
| Years in US | ||||
| Less than 5 | 175 (11.3) | 4.6 (1.5, 7.7) | 1.2 (0.5, 2.7) | 0.740 |
| 5 to 10 | 327 (21.1) | 3.4 (1.4, 5.4) | 0.7 (0.4, 1.6) | 0.451 |
| More than 10 | 898 (57.9) | 3.7 (2.5, 4.9) | 1.0 | |
| Unknown | 151 (9.7) | 4.6 (1.3, 7.9) | 1.4 (0.6, 3.6) | 0.438 |
| Education | ||||
| Elementary school | 90 (6.0) | 3.3 (-0.4, 7.0) | 1.1 (0.3, 4.2) | 0.855 |
| Junior high school | 109 (7.3) | 1.8 (-0.7, 4.3) | 0.6 (0.1, 2.8) | 0.539 |
| High school/vocational school | 547 (36.7) | 4.0 (2.4, 5.6) | 1.2 (0.6, 2.1) | 0.635 |
| College | 747 (50.0) | 4.3 (2.9, 5.8) | 1.0 | |
| Family History of HBV | ||||
| No | 1039 (67.4) | 3.2 (2.1, 4.3) | 1.0 | |
| Yes | 113 (7.3) | 11.5 (5.6, 17.4) | 4.7 (2.3, 9.8) | <0.001 |
| Not sure | 390 (25.3) | 3.1 (1.4, 4.8) | 1.0 (0.5, 2.0) | 0.963 |
Adjusted for all other variables in the model
Characteristics of HBV-Infected Subjects
Clinical characteristics and HCC development among HBV-infected
All individuals were asymptomatic when initially evaluated, with no overt evidence of liver disease. Among the 1,620 individuals found to be HBsAg positive regardless of previous screening, 1,227 (75.7%) had further tests performed at AAHBP clinics (Table 4). Overall, 32% of participants were HBeAg positive. More than 70% of participants had a detectable HBV VL (≥300 copies/mL), signifying active viral replication, and 24% had HBV VL ≥ 1,000,000 copies/mL associated with a high risk of HCC. Approximately 18% of participants had ALT > 2xULN.
Table 4.
Characteristics of hepatitis B infected participants
| All Asian Countries (n=1,227) | Korea (n=135) | Malaysia (n=37) | China (n=1,029) | p-value | Fujian Province (n=708) | Non-Fujian Province (n=233) | p-value Fujian vs. non-Fujian | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Gender | 0.043 | <0.001 | ||||||
| Male | 747 (60.8) | 73 (54.1) | 18 (48.6) | 645 (62.7) | 500 (70.6) | 128 (54.9) | ||
| Female | 481 (39.2) | 62 (45.9) | 19 (51.4) | 384 (37.3) | 208 (29.4) | 105 (45.1) | ||
| Age | <0.001 | <0.001 | ||||||
| Mean (SD) | 35.4 (11.0) | 46.2 (11.8) | 44.2 (11.0) | 33.6 (9.8) | 32.2 (9.1) | 40.2 (8.6) | ||
| Years in US | <0.001 | 0.206 | ||||||
| Mean (SD) | 8.4 (6.5) | 14.3 (8.7) | 10.0 (5.5) | 7.4 (5.7) | 7.7 (5.2) | 8.3 (6.8) | ||
| ALTa | 0.403 | 0.143 | ||||||
| Median | 27.0 | 26.5 | 31.0 | 27.0 | 27.0 | 26.0 | ||
| ≤ ULN | 555 (45.7) | 63 (47.0) | 11 (30.6) | 468 (46.0) | 333 (47.5) | 114 (49.8) | ||
| 1-2 ULN | 436 (35.9) | 48 (35.8) | 18 (50.0) | 362 (35.6) | 239 (34.1) | 83 (36.2) | ||
| > 2xULN | 224 (18.4) | 23 (17.2) | 7 (19.4) | 188 (18.5) | 129 (18.4) | 32 (14.0) | ||
| HbeAg | <0.001 | 0.002 | ||||||
| Positive | 360 (31.6) | 25 (19.8) | 3 (8.6) | 322 (33.9) | 239 (36.5) | 56 (26.0) | ||
| Negative | 778 (68.4) | 101 (80.2) | 32 (91.4) | 630 (66.1) | 416 (63.5) | 159 (74.0) | ||
| HBV VL (copies/mL) | 0.010 | 0.139 | ||||||
| Median | 3055.0 | 1310.0 | 4966.0 | 3210.0 | 3268.0 | 2900.0 | ||
| <300 (Undetectable) | 330 (27.5) | 44 (33.1) | 3 (8.1) | 278 (27.7) | 181 (26.2) | 63 (29.7) | ||
| 300-9,999 | 382 (31.8) | 45 (33.8) | 18 (48.6) | 312 (31.1) | 220 (31.8) | 70 (33.0) | ||
| 10,000-99,999 | 131 (10.9) | 14 (10.5) | 7 (18.9) | 103 (10.3) | 69 (10.0) | 18 (8.5) | ||
| 100,000-999,999 | 68 (5.7) | 3 (2.3) | 4 (10.8) | 57 (5.7) | 36 (5.1) | 18 (8.5) | ||
| ≥1,000,000 | 289 (24.1) | 27 (20.3) | 5 (13.5) | 253 (25.2) | 186 (26.9) | 43 (20.3) | ||
Females - > 20 is elevated, Males - > 30 is elevated
During the course of follow-up, 7 individuals developed HCC. Six were from China and one was from Korea. Six of the individuals were male. The median age was 50, and 5 out of the 6 Chinese individuals were born in the Fujian province. Twenty-two individuals were diagnosed with cirrhosis on the basis of liver biopsy or MRI.
Estimation of HCC Risk (Surrogate Markers of HCC Risk)
Using known major predictors of HCC including HBeAg, HBV VL, genotype, family history of HCC
Among infected individuals, Chinese were most likely to be younger and male, while Koreans were older and had lived longer in the US (see Table 4). Among Chinese, individuals from the Fujian province were more likely to be younger and male compared to individuals from outside of the Fujian province. Chinese were significantly more likely to be HBeAg positive than Koreans and Malaysians, and Chinese from the Fujian province were significantly more likely to be HBeAg positive than individuals born in another Chinese province. The distribution of HBV VL differed significantly by Asian subgroup, and median HBV VL was higher among Chinese and Malaysians compared to Koreans. Initial comparisons of HBV VL and HBeAg stratified by age bracket suggested differences between Chinese and Koreans; however, logistic regression analyses (not shown) indicated that younger age and male gender were the major determinants of differences in higher HBV VL among Koreans and Chinese. Younger age was the major determinant of differences in positive HBeAg and higher ALT among Koreans and Chinese as well as higher HBV VL and positive HBeAg among Chinese.
Genotype data was available on patients at one clinical site. Among 105 Chinese, 42% were genotype B and 57% were genotype C; among 16 Koreans, 100% were genotype C; and among 4 Malaysians, 50% were genotype B and 50% were genotype C. Family history of HCC was obtained on around 50% of participants; descriptive information is provided on 862 Chinese, 124 Koreans, and 36 Malaysians. Approximately 12.3% of Chinese, 25% of Koreans, and 22.2% of Malaysians reported a family history of HCC.
Using specific guidelines for starting antiviral treatment as an approximate indicator of progression and HCC risk
Using calculations based on established guidelines (37, 39, 40, 45), approximately 11% of HBV-infected persons meet criteria for treatment, with higher rates for Fujianese Chinese (11.7%) and Korean (12.0%) individuals (Table 5). Many individuals with lower HBV VL and ALT might also be treated, especially when considering additional risk factors such as age, gender, family history of HCC, viral mutations, and genotype. Individuals with HBV VL between 10,000 and 100,000 copies/mL and ALT 2xULN, an additional 7.9%, might also be treated (per APASL guidelines), although in this case, proportionately more Koreans (8.8%) and Malaysians (20.6%) than Chinese (7.0%) would be treated.
Table 5.
Grouping by APASL treatment guidelines (among HBsAg positive individuals with HBV DNA, ALT, and HBeAg)
| All Asian Countries (n=1,113) |
Korea (n=125) |
Gyeonggi Province (n=53) |
Non- Gyeonggi Province (n=56) |
Malaysia (n=34) |
China (n=927) |
Fujian Province (n=641) |
Non-Fujian Province (n=206) |
|
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| HBV DNA ≥ 100,000 and ALT >2xULN | 124 (11.2) | 15 (12.0) | 6 (11.3) | 7 (12.5) | 2 (5.9) | 105 (11.3) | 75 (11.7) | 16 (7.8) |
| HBeAg- and VL between 10,000 - 99,999 and ALT >2xULN | 88 (7.9) | 11 (8.8) | 8 (15.1) | 2 (3.6) | 7 (20.6) | 65 (7.0) | 43 (6.7) | 13 (6.3) |
| HBV DNA <10,000 | 654 (58.9) | 823 (65.6) | 32 (60.4) | 40 (71.4) | 19 (55.9) | 541 (76.1) | 367 (57.3) | 128 (62.1) |
Discussion
We found that the overall seroprevalence of chronic HBV infection among Asian immigrants participating in a community-based screening program in NYC was very high; 13.4% of newly-tested individuals had chronic HBV infection. Male gender, younger age, lower education, and family history of HBV infection were associated with higher HBV prevalence. The most important determinant of prevalence was country of birth; this was especially true for Chinese (23.1%) compared to most other groups. Among Chinese, there was further diversity in the seroprevalence rates related to geographic location; the highest seroprevalence was seen among individuals from Fujian province.
HBV infection in Asian immigrants in NYC closely mirrors the epidemiology of HBV in Asia (47). The prevalence in Chinese immigrants grouped by province/region of birth is similar to that found in China with the exception of the Fujian province (7, 8, 10). The prevalence of chronic HBV in Fujianese immigrants in our study was more than twice the rate (17.1%) reported for the Fujian province in the China National Survey, the province with the highest HBV infection prevalence in China (7, 11, 25, 26). Higher infection rates among the Fujianese in our sample may be due to specific migration-related factors that overlap with HBV risk factors. For example, it has been estimated that 80% of Fujianese immigrants to the US reside in NYC – numbering between 300,000-500,000 – with the majority migrating from the rural eastern costal region around the capital of Fuzhou (48); these are regions found to have higher HBV infection rates (49). Putian County in Fujian was found to have the highest infection rate (27.5%) (11), similar to that found among Fujianese immigrants in our study. A recent study conducted in Fujian province identified individuals who are 15-24 years of age, male, with lower educational attainment, and demographic characteristics that correspond with those of Fujianese participants in our study, were more likely to immigrant internationally from Fujian (50).
The large variation in HBsAg seroprevalence reported in studies conducted across the US (51) may be a reflection of differences in migration patterns of Asian-born immigrants in that region. For example, the prevalence rates in Chinese immigrants in the northeast where Fujianese immigrants have predominantly settled are generally higher than prevalence rates from western regions in the US that are predominantly settled by Chinese immigrants from Hong Kong, where HBV seroprevalence is much lower (48). Consequently, the burden of HBV and its complications is expected to be higher in areas populated by Asians emigrating from countries and specific geographic areas with higher HBV seroprevalence.
The true burden of disease, however, requires more than just knowledge of seroprevalence rates. Individual risk of liver disease and HCC are important co-factors. The clinical and virologic data obtained on HBV-infected subjects in this study indicate that a large proportion of Asian Americans with chronic HBV infection have a high risk of developing HCC. Furthermore, this risk is not homogeneous: the clinical data presented indicate significant heterogeneity in the virologic and host factors that are highly associated with risk of HCC. The interpretation of these differences is complex. In general, Fujianese immigrants have higher HBV VLs and a greater proportion of HBeAg positivity than Koreans or Chinese from other provinces. We found that 22 individuals were diagnosed with cirrhosis on the basis of liver biopsy or MRI. This likely underestimates the true prevalence, as liver biopsies were only performed for clinical indications such as elevated AFP, persistently elevated ALT, or abnormal ultrasound or MRI.
In our analysis, however, these findings mostly reflect differences in the age and gender distributions among these groups, with Fujianese immigrants being significantly younger and male, correlating with HBeAg positivity and higher HBV VL. Therefore, in ascribing risk and comparing subjects in one study to another, it is important to take age and gender into consideration. We observed two important risk factors that were significantly different by subgroup: genotype and family history of HCC (16). Koreans had a much higher proportion of genotype C, which is associated with a higher risk of HCC (52, 53). This is consistent with data from Korea where nearly 100% of HBV-infected patients are infected with genotype C2 (54). Similarly, the distribution of genotypes for Chinese individuals is consistent with data from China (55). Additionally, nearly twice as many Koreans reported family members with HCC. Genotype and family history may explain why NYC Koreans have a higher risk of HCC (5). Factors such as alcohol intake, genetic predisposition and aflatoxin may also affect risks differences between groups. Fujianese immigrants appear more likely to develop HCC at an early age (56) for reasons that are still unclear, but are likely related to some of those factors. Risk stratification using a comprehensive scoring system that accounts for age, gender, HBV VL, HBeAg status, and family history of HCC and genotype (18) may potentially provide a better way to calculate disease burden in specific subgroups and more effectively target individuals at a greater risk for HCC and for whom treatments may bring the greatest benefit. Treatment is at the present time the only way to significantly reduce the risk of HCC and other complications of HBV. Screening for HCC among those at highest risk using ultrasound and serology are also important in allowing for treatment initiation at an early stage of malignancy resulting in better outcomes.
The present analysis is the first large in-depth study of HBV infection in Asian Americans in NYC. Potential limitations should be considered in the interpretation of the study findings. The screening program may have attracted participants with known chronic HBV infection seeking treatment. However, our estimate of HBV seroprevalence within the community is conservative and is based on the group of newly screened participants, defined as those who reported no prior HBV screening (46). The results were comparable to those reported in different Asian countries and specific Chinese regions. In addition, findings may be specific to NYC’s immigrant communities and may not be generalizable to other Asian populations in the US. As presented earlier, 80% of all Fujianese immigrants to the US reside in NYC; thus, contextual factors including migration history and patterns need to be considered. Estimates of the HBV prevalence among Asian Americans should take into consideration both the country and geographic area of birth among immigrant populations.
We believe that our findings are an accurate reflection of the current disproportionate burden of chronic HBV infection among Asian immigrants living in NYC. For example, the DOHMH conducted an investigation of a randomly selected representative sample of newly reported HBV cases from 2008-2009. Findings indicate that 61% of HBV patients were male, 67% Asian (56% indicated their birth country as China) and the median age was 38 years (57). However, HBV prevalence is not static, and changes expected in the epidemiology of HBV in Asia will eventually have a profound effect on the prevalence of HBV in new Asian immigrants. We expect to see a decline in HBV infection rates among younger Asian immigrants from countries such as Korea and China where national newborn hepatitis B vaccination programs have only recently been implemented (58-60).
Although mass HBV vaccination programs will eventually lead to a decrease in the number of imported cases of chronic HBV, the absolute number of cases will likely continue to rise for some time due to current immigration patterns. Many Asian adults born prior to the institution of universal vaccination will continue to migrate to the US, and at the same time a large reservoir of persons chronically infected remains undetected in the US. HBV infection and its sequelae will continue to be a serious US public health challenge for decades to come. For example, despite ongoing public health efforts from 1990 to 2008, liver cancer incidence among Asian Americans have increased (61). It will become increasingly important to detect asymptomatic cases of HBV and provide early evaluation and treatment to prevent chronic liver diseases, hepatic failure and HCC. The results of this study may have practical implications for the prevention and management of the disease in other Asian communities in the US (57). Despite high HBV prevalence, screening rates for HBV among Asian Americans continue to be insufficient. Nearly two-thirds of foreign-born HBV-infected Asian Americans are unaware of their infection status (20, 25) and between 40 and 65% of Asian Americans have not been screened for HBV (62). Community-based screening programs to address this burden of HBV across Asian populations in the US that are culturally tailored and targeted within specific high risk groups are needed. Along with screening and early detection of HBV infection, however, further evaluation and active monitoring is absolutely essential to reducing the risk of death from HCC to ensure needed antiviral treatment and active monitoring of liver cancer is provided (63-66).
In summary, Asian immigrants in NYC have a high rate of chronic HBV infection and a substantial ongoing risk for liver cancer. HBV seroprevalence varies considerably by ethnic subgroup and geographic place of birth, whereas the risk of disease progression and HCC among infected individuals appears relatively uniform except where there are large differences in HBV genotype. While Fujianese immigrants have the highest HBV seroprevalence, the risk of liver cancer is higher in HBV-infected individuals born in Korea because of the very high rate of genotype C infection. More studies are needed to accurately ascertain and predict HCC risk in order to better inform public health strategies that target high risk subgroups for screening, and HBV disease management. Early antiviral treatment and liver cancer monitoring for those at risk are absolutely key to reducing the burden of disease in individuals already infected.
Acknowledgments
Asian American Hepatitis B Program (AAHBP) was funded by the City Council of New York from 2004-2008. The following is a partial list of AAHBP partners and contributors and their affiliated organizations during this time period: American Cancer Society: Ming-Der Chang, PhD, Arlene Chin, Seongho Kim, Kira Kim; Bellevue Hospital: William Bateman, MD, Chris Cho, Edith Davis, Scott Fuller, Dan Chen, PhD, Janice Charles, Thomas Jasper, Robert Boyd, Larry Martin, Victor Infante, Sindy Yiu, Yvonne Ho; Charles B. Wang Community Health Center: Thomas Tsang, MD, Alan Tso, MD, Christina Lee, Elsie Lee, MD, Betty Cheng, CSW, Janice Magno, MPH, Christine Cheng, MD, Catherine Lee, MD; Korean Community Services: Jinny Park, MPH, Kay Chun, MD, Linda Lee, MA Gouverneur Healthcare Services: Pearl Korenblit, MD, Lily Yiu, L Chiang, PA, S Shi, MD, Angela Tu, MD, Maria De La Croz; NY Downtown Hospital: William Wang, Waiwah Chung, RN, Charles Ho, Eric Poon, MD; NYU School of Medicine: Kejia Wan, MPH, John Nolan, Paige Baker, Rona Luo, Jenny Bute, Gemma Rochford, MingXia Zhan, Ruchel Ramos, MPA, Henrietta Ho-Asjoe, MPS, Nadia Islam, PhD, Alex Sherman, MD, Hillel Tobias, MD, Helene Lupatkin, MD, Michael Phillips, Florence Pua, MD, Andy O. Miller, MD, Chun T. Wong, MD, Gerald Villanueva, MD, and Mariano Rey, MD.
We gratefully acknowledge the support of the NYC Department of Health and Mental Health (Dr. Stephen Friedman, Dr. Jane Zucker, Dr. Isaac Weisfuse, Kevin Mahoney) and the help of Dr. XiaoFeng Liang, the Chinese Center for Disease Control and Prevention, and Dr. Yan YanKai, Fujian Province CDC of China in providing HBV epidemiological information for China.
Financial support: New York City Office of the City Council (to H Pollack, C Trinh-Shevrin and S Wang), National Institute for Minority Health and Health Disparities P60 MD000538 (to H Pollack, S Kwon, L Wyatt, C Trinh-Shevrin), Centers for Disease Control and Prevention REACH U.S. Program U58 DP001022 (to H Pollack, S Kwon, S Wang, L Wyatt, C Trinh-Shevrin), Centers for Disease Control and Prevention National REACH Program U58 DP004685 (to S Kwon, L Wyatt, C Trinh-Shevrin).
Footnotes
Potential conflicts of interest: None
References
- 1.Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15(Suppl 4):5–13. doi: 10.1634/theoncologist.2010-S4-05. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. USA: American Cancer Society; Apr 16, 2014. What is liver cancer? Available from: http://www.cancer.org/cancer/livercancer/detailedguide/liver-cancer-what-is-liver-cancer. [Google Scholar]
- 3.Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981;2:1129–33. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 5.Huang V, Li W, Tsai J, Begier E. Cancer Mortality among Asians and Pacific Islanders in New York City, 2001-2010. J Cancer Epidemiol. 2013;2013 doi: 10.1155/2013/986408. 986408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–73 e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfizer. The Burden of Cancer in Asia. USA: Pfizer Medical Division; 2008; Apr 16, 2014. Available from: http://www.pfizer.com/files/products/cancer_in_asia.pdf. [Google Scholar]
- 8.International Agency for Research on Cancer. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. France: International Agency for Research on Cancer; 2012; May 1, 2014. Available from: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx. [Google Scholar]
- 9.National Cancer Institute. Cancer Facts & Figures 2013. Atlanta: American Cancer Society; 2013; Apr 16, 2014. Available from: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf. [Google Scholar]
- 10.National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Bethesda, Maryland: National Cancer Institute; 2014. Apr 16, Available from: http://seer.cancer.gov/faststats/ [Google Scholar]
- 11.Miller BA, Chu KC, Hankey BF, Ries LA. Cancer incidence and mortality patterns among specific Asian and Pacific Islander populations in the U.S. Cancer Causes Control. 2008;19:227–56. doi: 10.1007/s10552-007-9088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller BA, Kolonel L, Bernstein L, Young JL, Swanson GM, West DW, et al. Racial/ethnic cancer patterns in the United States 1988--1992. Bethesda: National Cancer Institute; 1996. [Google Scholar]
- 13.Ross RK, Yuan JM, Yu MC, Wogan GN, Qian GS, Tu JT, et al. Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma. Lancet. 1992;339:943–6. doi: 10.1016/0140-6736(92)91528-g. [DOI] [PubMed] [Google Scholar]
- 14.Wang LY, Hatch M, Chen CJ, Levin B, You SL, Lu SN, et al. Aflatoxin exposure and risk of hepatocellular carcinoma in Taiwan. Int J Cancer. 1996;67:620–5. doi: 10.1002/(SICI)1097-0215(19960904)67:5<620::AID-IJC5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 15.Donato F, Tagger A, Gelatti U, Parrinello G, Boffetta P, Albertini A, et al. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol. 2002;155:323–31. doi: 10.1093/aje/155.4.323. [DOI] [PubMed] [Google Scholar]
- 16.Yu MW, Chang HC, Liaw YF, Lin SM, Lee SD, Liu CJ, et al. Familial risk of hepatocellular carcinoma among chronic hepatitis B carriers and their relatives. J Natl Cancer Inst. 2000;92:1159–64. doi: 10.1093/jnci/92.14.1159. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, Wu Q-J, Xie L, Chow W-H, Rothman N, Li H-L, et al. Prospective cohort studies of association between family history of liver cancer and risk of liver cancer. Int Journal Cancer. 2014;135:1605–14. doi: 10.1002/ijc.28792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee MH, Yang HI, Liu J, Batrla-Utermann R, Jen CL, Iloeje UH, et al. Prediction models of long-term cirrhosis and hepatocellular carcinoma risk in chronic hepatitis B patients: risk scores integrating host and virus profiles. Hepatology. 2013;58:546–54. doi: 10.1002/hep.26385. [DOI] [PubMed] [Google Scholar]
- 19.Yu MW, Yeh SH, Chen PJ, Liaw YF, Lin CL, Liu CJ, et al. Hepatitis B virus genotype and DNA level and hepatocellular carcinoma: a prospective study in men. J Natl Cancer Inst. 2005;97:265–72. doi: 10.1093/jnci/dji043. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen TT, Taylor V, Chen MS, Jr, Bastani R, Maxwell AE, McPhee SJ. Hepatitis B awareness, knowledge, and screening among Asian Americans. J Cancer Educ. 2007;22:266–72. doi: 10.1007/BF03174128. [DOI] [PubMed] [Google Scholar]
- 21.Ha NB, Trinh HN, Nguyen TT, Leduc TS, Bui C, Ha NB, et al. Prevalence, risk factors, and disease knowledge of chronic hepatitis B infection in Vietnamese Americans in California. J Cancer Educ. 2013;28:319–24. doi: 10.1007/s13187-013-0466-0. [DOI] [PubMed] [Google Scholar]
- 22.Wasley A, Kruszon-Moran D, Kuhnert W, Simard EP, Finelli L, McQuillan G, et al. The prevalence of hepatitis B virus infection in the United States in the era of vaccination. J Infect Dis. 2010;202:192–201. doi: 10.1086/653622. [DOI] [PubMed] [Google Scholar]
- 23.Hwang JP, Mohseni M, Gor BJ, Wen S, Guerrero H, Vierling JM. Hepatitis B and hepatitis C prevalence and treatment referral among Asian Americans undergoing community-based hepatitis screening. Am J Public Health. 2010;100(Suppl 1):S118–24. doi: 10.2105/AJPH.2009.162776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kallman JB, Tran S, Arsalla A, Haddad D, Stepanova M, Fang Y, et al. Vietnamese community screening for hepatitis B virus and hepatitis C virus. J Viral Hepat. 2011;18:70–6. doi: 10.1111/j.1365-2893.2010.01278.x. [DOI] [PubMed] [Google Scholar]
- 25.Lin SY, Chang ET, So SK. Why we should routinely screen Asian American adults for hepatitis B: a cross-sectional study of Asians in California. Hepatology. 2007;46:1034–40. doi: 10.1002/hep.21784. [DOI] [PubMed] [Google Scholar]
- 26.Sheikh MY, Mouanoutoua M, Walvick MD, Khang L, Singh J, Stoltz S, et al. Prevalence of hepatitis B virus (HBV) infection among Hmong immigrants in the San Joaquin Valley. J Community Health. 2011;36:42–6. doi: 10.1007/s10900-010-9283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.United States Census Bureau. Asians Fastest-Growing Race or Ethnic Group in 2012, Census Bureau Reports. Washington, D.C.: United States Census Bureau; 2013. [April 16, 2014] Available from: http://www.census.gov/newsroom/releases/archives/population/cb13-112.html. [Google Scholar]
- 28.Weinbaum CM, Williams I, Mast EE, Wang SA, Finelli L, Wasley A, et al. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57:1–20. [PubMed] [Google Scholar]
- 29.Mellen JS, Xia VW, Hashemzadeh M, Imagawa D, Jamal M, Hoefs J, et al. The clinical presentation of chronic hepatitis B virus infection in Asian Americans: a single center retrospective study. J Clin Gastroenterol. 2010;44:364–70. doi: 10.1097/MCG.0b013e3181b5c7a8. [DOI] [PubMed] [Google Scholar]
- 30.Xu JJ, Tien C, Chang M, Rhee J, Tien A, Bae HS, et al. Demographic and serological characteristics of Asian Americans with hepatitis B infection diagnosed at community screenings. J Viral Hepat. 2013;20:575–81. doi: 10.1111/jvh.12073. [DOI] [PubMed] [Google Scholar]
- 31.Sarkar M, Shvachko VA, Ready JB, Pauly MP, Terrault NA, Peters MG, et al. Characteristics and Management of Patients with Chronic Hepatitis B in an Integrated Care Setting. Dig Dis Sci. 2014 doi: 10.1007/s10620-014-3142-2. Epub 2014 Apr 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Misra R, Jiobu K, Zhang J, Liu Q, Li F, Kirkpatrick R, et al. Racial disparities in hepatitis B infection in Ohio: screening and immunization are critical for early clinical management. J Investig Med. 2013;61:1121–8. doi: 10.2310/JIM.0b013e3182a70f10. [DOI] [PubMed] [Google Scholar]
- 33.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 34.Yang HI, Lu SN, Liaw YF, You SL, Sun CA, Wang LY, et al. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002;347:168–74. doi: 10.1056/NEJMoa013215. [DOI] [PubMed] [Google Scholar]
- 35.Pan CQ, Trinh H, Yao A, Bae H, Lou L, Chan S, et al. Efficacy and safety of tenofovir disoproxil fumarate in asian-americans with chronic hepatitis B in community settings. PLoS One. 2014;9:e89789. doi: 10.1371/journal.pone.0089789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu CY, Lin JT, Ho HJ, Su CW, Lee TY, Wang SY, et al. Association of Nucleos(t)ide analogue therapy with reduced risk of hepatocellular carcinoma in patients with chronic hepatitis B-A nationwide cohort study. Gastroenterology. 2014;147:143–51 e5. doi: 10.1053/j.gastro.2014.03.048. [DOI] [PubMed] [Google Scholar]
- 37.Liaw YF. Hepatitis B virus replication and liver disease progression: the impact of antiviral therapy. Antivir Ther. 2006;11:669–79. [PubMed] [Google Scholar]
- 38.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–2. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 39.European Association For The Study Of The L. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227–42. doi: 10.1016/j.jhep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 40.European Association For The Study Of The L. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–85. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 41.Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatology international. 2008;2:263–83. doi: 10.1007/s12072-008-9080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.US Census Bureau. The Asian Population: 2010. Washington, D.C.: U.S Census Bureau; 2012. [April 16, 2014] Available from: http://www.census.gov/prod/cen2010/briefs/c2010br-11.pdf. [Google Scholar]
- 43.Pollack H, Wang S, Wyatt L, Peng CH, Wan K, Trinh-Shevrin C, et al. A comprehensive screening and treatment model for reducing disparities in hepatitis B. Health Aff (Millwood) 2011;30:1974–83. doi: 10.1377/hlthaff.2011.0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trinh-Shevrin C, Pollack HJ, Tsang T, Park J, Ramos MR, Islam N, et al. The Asian American hepatitis B program: building a coalition to address hepatitis B health disparities. Prog Community Health Partnersh. 2011;5:261–71. doi: 10.1353/cpr.2011.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee JK, Shim JH, Lee HC, Lee SH, Kim KM, Lim YS, et al. Estimation of the healthy upper limits for serum alanine aminotransferase in Asian populations with normal liver histology. Hepatology. 2010;51:1577–83. doi: 10.1002/hep.23505. [DOI] [PubMed] [Google Scholar]
- 46.Centers for Disease Control and Prevention. Screening for chronic hepatitis B among Asian/Pacific Islander populations--New York City, 2005. MMWR Morb Mortal Wkly Rep. 2006;55:505–9. [PubMed] [Google Scholar]
- 47.Kowdley KV, Wang CC, Welch S, Roberts H, Brosgart CL. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology. 2012;56:422–33. doi: 10.1002/hep.24804. [DOI] [PubMed] [Google Scholar]
- 48.Zhao X. The New Chinese America: Class, Economy, and Social Hierarchy. New Jersey: Rutgers University Press; 2010. [Google Scholar]
- 49.Chen Y, Wang X, Shang P. The study of tendency of hepatitis B virus surface antigen in Chinese population. Chin J ExpClin Infect Dis. 2007;1 [Google Scholar]
- 50.Liang Z, Chunyu MD. Migration within China and from China to the USA: The effects of migration networks, selectivity, and the rural political economy in Fujian Province. Pop Stud-J Demog. 2013;67:209–23. doi: 10.1080/00324728.2012.756116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rein DB, Lesesne SB, Leese PJ, Weinbaum CM. Community-based hepatitis B screening programs in the United States in 2008. J Viral Hepat. 2010;17:28–33. doi: 10.1111/j.1365-2893.2009.01165.x. [DOI] [PubMed] [Google Scholar]
- 52.Wong GL, Chan HL, Yiu KK, Lai JW, Chan VK, Cheung KK, et al. Meta-analysis: The association of hepatitis B virus genotypes and hepatocellular carcinoma. Aliment Pharmacol Ther. 2013;37:517–26. doi: 10.1111/apt.12207. [DOI] [PubMed] [Google Scholar]
- 53.Shi YH. Correlation between Hepatitis B Virus Genotypes and Clinical Outcomes. Jpn J Infect Dis. 2012;65:476–82. doi: 10.7883/yoken.65.476. [DOI] [PubMed] [Google Scholar]
- 54.Kim H, Jee YM, Song BC, Shin JW, Yang SH, Mun HS, et al. Molecular epidemiology of hepatitis B virus (HBV) genotypes and serotypes in patients with chronic HBV infection in Korea. Intervirology. 2007;50:52–7. doi: 10.1159/000096313. [DOI] [PubMed] [Google Scholar]
- 55.Zeng G, Wang Z, Wen S, Jiang J, Wang L, Cheng J, et al. Geographic distribution, virologic and clinical characteristics of hepatitis B virus genotypes in China. J Viral Hepat. 2005;12:609–17. doi: 10.1111/j.1365-2893.2005.00657.x. [DOI] [PubMed] [Google Scholar]
- 56.Wan DW, Tzimas D, Smith JA, Kim S, Araujo J, David R, et al. Risk factors for early-onset and late-onset hepatocellular carcinoma in Asian immigrants with hepatitis B in the United States. Am J Gastroenterol. 2011;106:1994–2000. doi: 10.1038/ajg.2011.302. [DOI] [PubMed] [Google Scholar]
- 57.Centers for Disease Control and Prevention. Surveillance for chronic hepatitis B virus infection - New York City, June 2008-November 2009. MMWR Morb Mortal Wkly Rep. 2012;61:6–9. [PubMed] [Google Scholar]
- 58.Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, et al. Evaluation of the impact of hepatitis B vaccination among children born during 1992-2005 in China. J Infect Dis. 2009;200:39–47. doi: 10.1086/599332. [DOI] [PubMed] [Google Scholar]
- 59.Park NH, Chung YH, Lee HS. Impacts of vaccination on hepatitis B viral infections in Korea over a 25-year period. Intervirology. 2010;53:20–8. doi: 10.1159/000252780. [DOI] [PubMed] [Google Scholar]
- 60.Tong MJ, Pan CQ, Hann HW, Kowdley KV, Han SH, Min AD, et al. The management of chronic hepatitis B in Asian Americans. Dig Dis Sci. 2011;56:3143–62. doi: 10.1007/s10620-011-1841-5. [DOI] [PubMed] [Google Scholar]
- 61.Gomez SL, Noone AM, Lichtensztajn DY, Scoppa S, Gibson JT, Liu L, et al. Cancer incidence trends among Asian American populations in the United States, 1990-2008. J Natl Cancer Inst. 2013;105:1096–110. doi: 10.1093/jnci/djt157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu KQ, Pan CQ, Goodwin D. Barriers to screening for hepatitis B virus infection in Asian Americans. Dig Dis Sci. 2011;56:3163–71. doi: 10.1007/s10620-011-1840-6. [DOI] [PubMed] [Google Scholar]
- 63.Lim SG, Mohammed R, Yuen MF, Kao JH. Prevention of hepatocellular carcinoma in hepatitis B virus infection. J Gastroenterol Hepatol. 2009;24:1352–7. doi: 10.1111/j.1440-1746.2009.05985.x. [DOI] [PubMed] [Google Scholar]
- 64.Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98–107. doi: 10.1002/hep.26180. [DOI] [PubMed] [Google Scholar]
- 65.Liaw YF. Impact of therapy on the outcome of chronic hepatitis B. Liver international : official journal of the International Association for the Study of the Liver. 2013;33(Suppl 1):111–5. doi: 10.1111/liv.12057. [DOI] [PubMed] [Google Scholar]
- 66.Eun JR, Lee HJ, Kim TN, Lee KS. Risk assessment for the development of hepatocellular carcinoma: according to on-treatment viral response during long-term lamivudine therapy in hepatitis B virus-related liver disease. J Hepatol. 2010;53:118–25. doi: 10.1016/j.jhep.2010.02.026. [DOI] [PubMed] [Google Scholar]


