Abstract
Reductions in C4 levels may predispose individuals to infection with encapsulated bacteria as well as autoimmunity. In this study, we examined the role C4 has in protection against Streptococcus pneumoniae-induced autoimmunity. Mild respiratory infection with serotype 19F pneumococci selectively induced systemic anti-dsDNA IgA production in naïve C4-/- mice, but not C3-/- or wild type mice. Systemic challenge with virulent serotype 3 pneumococci also induced anti-dsDNA IgA production in immune C4-/- mice. Remarkably, pneumococcal polysaccharide (PPS) vaccination alone induced C4-/- mice to produce increased anti-dsDNA IgA levels that were maintained in some mice for months. These effects were most pronounced in female C4-/- mice. Importantly, immunization-induced increases in anti-dsDNA IgA levels were strongly associated with increased IgA deposition in kidneys. Cross-reactivity between pneumococcal antigens and dsDNA played a partial role in the induction of anti-dsDNA IgA, but a major role for PPS-associated TLR2 agonists was also revealed. Administration of the TLR2/4 antagonist, OxPAPC, at the time of PPS immunization completely blocked the production of anti-dsDNA IgA in C4-/- mice without suppressing PPS-specific Ab production. The TLR2 agonist, Pam3Csk4, similarly induced anti-dsDNA IgA production in C4-/- mice, which OxPAPC also prevented. LPS, a TLR4 agonist, had no effect. Pam3Csk4, but not LPS, also induced dsDNA-specific IgA production by C4-/- splenic IgA+ B cells in vitro, indicating TLR2 agonists can stimulate autoAb production via B cell-intrinsic mechanisms. Collectively, our results show an important role for C4 in suppressing autoAb production elicited by cross-reactive antigens and TLR2 agonists associated with S. pneumoniae.
Introduction
The role environmental triggers play in promoting autoimmunity remains elusive due in part to the complex nature of autoimmune disease. Nonetheless, mounting evidence increasingly supports a role for bacterial and viral infections in initiating and/or exacerbating autoimmunity (1-4). Pathogens may influence autoimmunity through antigen-specific mechanisms that involve crossreactivity between pathogen and host antigens (molecular mimicry) or superantigen activation of lymphocytes. Alternatively, pathogens can trigger nonspecific (bystander) activation of the immune system, ultimately resulting in enhanced inflammation, activation of autoreactive lymphocytes, and tissue damage (4). Identifying the mechanistic basis for pathogen-elicited autoimmunity is key for improving therapies for autoimmune diseases.
Interestingly, genetic deficiencies in classical complement components predispose humans to both bacterial infections and autoimmune diseases. C4 is an essential component of the classical complement cascade leading to generation of the C3 convertase. Complete deficiency in C4 (ie., deficiency in both C4A and C4B isoforms found in humans) manifests in recurrent infections as well as systemic autoimmune diseases characterized by autoantibody production, most commonly systemic lupus erythematosus (SLE) (5, 6). Deficiency in either C4A or C4B isoforms has similarly been associated with an increased risk of infection, autoimmune rheumatic diseases, including SLE, Henoch-Schonlein purpura, juvenile idiopathic arthritis, rheumatoid arthritis, and increased disease progression in IgA nephropathy (5, 7-9). The age of disease onset and severity varies considerably for C4 deficiency and this underscores the importance additional genetic and/or environmental factors play in disease development and pathogenesis. As infection may contribute to autoimmunity through mechanisms involving molecular mimicry or bystander activation, it is plausible to hypothesize that in complete or partial C4 deficiencies exposure to infectious agents may promote autoimmunity. Determining the role C4 plays in maintaining the balance between the protective immune response to pathogens and tolerance to self antigens is critical to understanding how C4 levels (which may plummet during flares) influence autoimmune disease development and progression.
Similar to data derived from humans, studies in mice provide evidence for the essential role of C4 in regulating tolerance, autoimmunity, and resistance to infection. C4-deficiency exacerbates autoimmunity in autoimmune-susceptible lpr mice (10, 11). Consistent with lupus-like disease, C4-/- mice also produce anti-dsDNA IgM, IgG and IgA Abs with high penetrance in aged female mice, with ~50% of aged female C4−/− mice demonstrating glomerular immune complex deposition in the absence of measurable renal disease (12, 13). Anti-DNA autoreactivity is also evident in heterozygous C4+/- mice (12, 13). In the context of protection against pathogens, C4 is essential for Ab- or lectin-initiated classical pathway activation, as well as the Ab-independent SignR1/C1q pathway of complement activation (14). C4-/- mice are therefore susceptible to extracellular pathogens, including Group B Streptococcus (15) and systemic and intranasal S. pneumoniae infection (14, 16, 17).
The role C4 plays in preventing bacterial infections and immune-complex mediated autoimmunity is clear (10-13, 18-20). However, whether microbes can promote the development of autoimmunity in the context of C4 deficiency remains to be explored. In the current study, we examined the extent to which C4 is required for protection against pneumococcal infections and for suppressing S. pneumoniae-induced autoantibody production. Remarkably, C4, but not C3, deficiency resulted in significantly increased production of dsDNA-reactive IgA following both systemic and respiratory pneumoccocal infections. Moreover, immunization with pneumococcal polysaccharides was sufficient to induce dsDNA-reactive IgA production in C4-/- mice. This effect was due to a combination of molecular mimicry by pneumococcal cell wall-associated phosphorylcholine and TLR2 agonists. The results of this study may have potential implications for S. pneumoniae-related induction or exacerbation of dsDNA-specific IgA production in C4-deficient or C4lo individuals.
Materials and Methods
Mice
C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME) and bred in-house. C3-/- and C4-/- mice were as described (15) and were purchased from Jackson and for some studies were backcrossed 2 additional times onto the C57BL/6 background. Mice used in experiments were age- and sex-matched. Mice were housed under specific pathogen free conditions, except for infection challenge experiments. All studies and procedures were approved by the Wake Forest University Animal Care and Use Committee.
Immunizations and Infections
Mice were immunized i.p. or s.c. with vaccine-grade Pneumovax23 (Merck, Whitehouse Station, NJ) containing 1 μg of each of 23 PPS or 40 μg PPS3 only (ATCC, Manassas, VA), unless indicated otherwise. Proteinase K treatment or DNASE1 treatment of PPS was carried out by incubating 100 μg of PPS3 in PBS containing 5 mM CaCl2 and 1 mM MgCl2 with 0.5 ug of proteinase K (Sigma) or 10 units of DNASE1 (Promega) respectively at 37°C for 1 hour. Reactions were inactivated using 0.5 mM EDTA (proteinase K only) and heating at 65°C for 15 minutes. PPS was dialyzed against PBS and then filter-sterilized prior to immunizations. For OxPAPC (Invivogen) treatment, mice were injected with 40 μg OxPAPC i.p. on days 0, 1, 2 and 3, with Pneumovax (1 μg) or Pam3Csk4 Vaccigrade (0.1 μg; Invivogen) administered on day 0. LPS (10 μg; E. coli O111:B4, Sigma cat #3012) was administered i.p.
S. pneumoniae infections were carried out using WU2 and EF3030 strains as previously described (21). For i.p. infection, mice received 104 CFU WU2 strain S. pneumoniae in 200 μl PBS. For i.t. inoculations, mice were anesthetized using isofluorane and then administered 107 CFU EF3030 in 40 μl PBS. Mice were monitored every 12 hrs and humanely sacrificed upon demonstrating signs of morbidity. To determine lung CFU, whole lungs were homogenized in 1 ml PBS and serial dilutions were plated on TSA-II 5% sheep red blood agar plates (BBL) coated with 4 μg/ml gentamicin and incubated overnight at 37°C.
ELISAs, Crithidia lucilliae immunofluorescence, and flow cytometry
Pneumococcal antigen and DNA-specific ELISAs were performed as previously described (21, 22). For PPS3-specific ELISAs, serum samples were diluted in TBS containing 1% BSA (TBS-BSA) and incubated with 10 μg/ml cell wall polysaccharide (CWPS; Statens Serum Institut, Denmark) to adsorb non-capsular polysaccharide Abs. PPS3-specific Ab levels were determined by adding diluted serum samples to Maxisorb plates that had been pre-coated with 5 μg/ml PPS3 in PBS overnight at room temperature and then blocked with TBS-BSA. For PC-specific ELISAs, serum samples were diluted in TBS-BSA and incubated with plates coated with 5 μg/ml PC2-BSA (Biosearch). For DNA-specific ELISAs, serum samples were diluted in TBS-BSA and incubated with plates coated with 2 μg/ml calf thymus DNA (Sigma) as previously described (22). For dsDNA-specific ELISAs assessing cross-reactive (blocking) antigens, sera was preincubated 1hr at room temperature with 5 to 25 μg/ml dsDNA, CWPS, PPS3, or PC2-BSA prior to applying diluted sera to dsDNA-coated ELISA plates. AP-conjugated polyclonal goat anti-mouse Ig isotype Abs (Southern Biotechnology Associates) and pNPP (Sigma) were used to detect antigen-specific Ab. ELISA values are reported as relative absorbance units (mean OD405nm reading for serum samples minus mean OD405nm reading from wells with serum omitted). Sera were analyzed for native DNA reactivity using Kallestad Crithidia luciliae subsrate slides (BioRad). The presence of anti-dsDNA antibody was tested using serum samples at a 1:10 dilution and detecting kinetoplast reactivity with FITC-conjugated goat anti-mouse Ig and -IgA (Southern Biotechnology). The samples were visualized using a fluorescence microscope. Serum reactivity (1:10 dilution) against heat-killed EF3030 (107 CFU/sample) was determined using goat anti-mouse IgA-FITC (Southern Biotechnology) with detection by flow cytometry, as previously described (23).
IgA+ B cell subsets were detected using flow cytometry. Peritoneal cavity and spleen cells were isolated and stained as previously described (24), using the fluorochrome-labeled Abs against the following antigens: IgA (Southern biotechnology), CD5 (Biolegend), CD11b (eBioscience), CD19 (BD Biosciences), and B220 (Biolegend). Samples were analyzed using a CantoII instrument (BD Biosciences).
Kidney pathology
Proteinuria was measured semiquantitatively using Uristix (Bayer). For immunohistology, kidneys were frozen in OCT medium. Five micrometer cryostat sections were processed for immunofluorescence microscopy using acetone fixation followed by blocking in PBS containing 4% normal calf serum. Sections were stained with goat anti-mouse IgA- or Ig-FITC (Southern Biotechnology Associates). Goat Ig served as a control. Sections were mounted using Vectashield containing DAPI (Vector Labs). Antibody deposition was evaluated using a Nikon TE300 Fluorescent Microscope in conjunction with ImageJ analysis (NIH). Quantification of immunofluorescence intensity (≥10 glomeruli/section) was calculated as the mean integrated intensity per glomerular area minus the background staining intensity. Periodic-Schiff staining was performed on 3 μm sections cut from formalin-fixed, paraffin-embedded kidneys. Sections were scored blindly by a board-certified pathologist. Glomerular mesangial nuclei counts were performed by counting nuclei in 10 random glomerular corpuscles per section. For glomerular damage, glomerular basement membrane thickening and mesangial expansion was evaluated using a 0-4 scoring system [0 = inapparent, 1 = <50% of glomeruli, segmental; 2 = >50% of glomeruli, segmental; 3 = <50% of glomeruli, predominantly diffuse; and 4 = >50% of glomeruli, predominantly diffuse]) with at least 20 random glomeruli assessed per section.
In vitro B cell activation
IgA+ spleen B cells were isolated using biotinylated anti-mouse IgA (Biolegend) in conjunction with anti-biotin magnetic microbeads (Miltenyi Biotech), according to manufacturer’s instructions. IgA+ and IgA- spleen cells were cultured in duplicate at 2 ×105 cell/well in a 96-well plate for 6 days in complete media alone or in the presence of LPS (20 μg/ml) or Pam3Csk4 (0.5 μg/ml). Supernatants were harvested, diluted 3-fold in PBS, and dsDNA-specific IgA levels were assessed by ELISA.
To assess the effects of OxPAPC on B cell activation, splenocytes were cultured for 2 days with LPS (20 μg/ml), Pam3Csk4 (0.5 μg/ml), or goat anti-mouse IgM F(ab’)2 (5 μg/ml; Jackson Immunoresearch) plus anti-CD40 (0.1 μg/ml, HM40-3; BD Biosciences), in the presence or absence of OxPAPC (20 μg/ml). Cells were stained with fluorochrome-labeled mAbs against mouse CD86 (eBioscience) and CD19 (BD Biosciences) and analyzed using a Canto II flow cytometer.
Statistical analysis
Data are shown as means ± SEM. Differences between sample means were assessed using Student’s t test. Differences in survival curves were assessed using the Log Rank test.
Results
Pneumococcal infection induces dsDNA autoAb (IgA) production in C4-/- mice
C4-/-, but not C3-/-, mice spontaneously produce autoAbs against dsDNA and other self-antigens (12, 13). We therefore assessed whether respiratory pneumococcal infection induced or exacerbated anti-dsDNA Ab production in complement-deficient mice. To do this, we utilized EF3030, a mild colonizing serotype 19F strain, for intratracheal (i.t.) challenge in naïve mice. All C4-/- and wild type mice survived following i.t. challenge with 107 CFU EF3030, whereas ~53% of C3-/- mice succumbed to infection (n=15 C3-/- mice; n=11 wild type mice, p=0.007; data not shown). Lung bacterial counts were not detected in wild type and most C4-/- and surviving C3-/- mice 14 days post EF3030 infection (data not shown). As shown in Fig. 1A, naïve female C4-/- mice, but not C3-/- mice, had significantly higher levels of dsDNA-specific IgM and IgA levels than wild type mice. Fourteen days following i.t. EF3030 infection, dsDNA-specific serum IgM and IgG levels were slightly (although not significantly) increased in wild type, C4-/-, and surviving C3-/- mice. Strikingly, anti-dsDNA IgA levels were significantly increased in C4-/- mice following infection, whereas levels were unchanged in wild type and C3-/- mice. An infection-induced increase in dsDNA-specific IgA was observed in nearly all C4-/- mice. Thus, mild respiratory pneumococcal infection with a serotype 19F pneumococcal strain induces dsDNA-specific IgA in C4-/- mice.
Figure 1. Pneumococcal infection induces dsDNA autoAb (IgA) production in C4-/- mice.
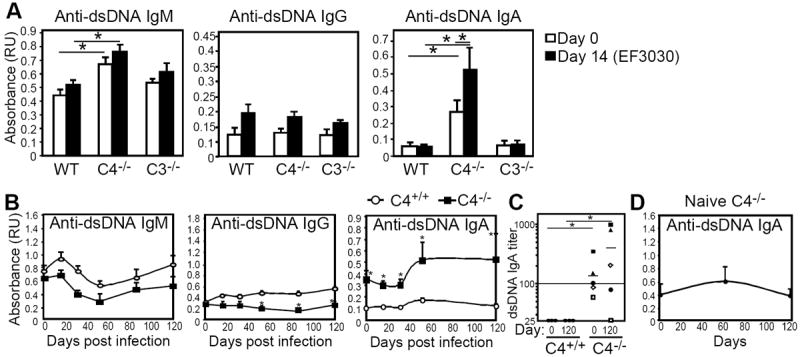
A) Mean (± SEM) dsDNA-specific serum IgM, IgG, and IgA levels in WT (n=6), C4-/-(n=7), and C3-/- (n=3) mice on day 0 and 14 following i.t. challenge with 107 CFU EF3030 as assessed by ELISA. Results are representative of 2 experiments. B) Mean ± (SEM) serum dsDNA-specific IgM, IgG, and IgA levels in Pneumovax-immune C4+/+ and C4-/- littermates over a 120-day period following i.p. challenge with 104 CFU WU2. C) DsDNA-specific serum IgA titers in C4+/+ and C4-/- littermates on day 0 and 120 days post-infection. Symbols represent titers of individual mice. Horizontal lines indicate mean values. D) DsDNA-specific serum IgA levels (mean ± SEM) in aged-matched naïve C4-/- mice over a 120-day period. In A-C, asterisks (*) indicate significant differences between the indicated groups (p<0.05).
The increase in anti-dsDNA IgA in C4-/- mice following respiratory infection could be due to infection at a mucosal site, where IgA production predominates. We therefore assessed whether systemic pneumococcal infection with the highly virulent serotype 3 strain, WU2, would similarly induce increased autoAb production in complement-deficient mice. C4-/- mice have increased susceptibility to WU2 (data not shown), but Pneumovax immunization elicits normal Ab production against serotype 3 capsular polysaccharide (PPS-3) in C4-/- mice (25) and was able to provide significant protection against systemic lethal WU2 challenge (104 CFU; 10 times the LD50 for naïve mice) in both wild type and C4-/- mice, but not C3-/- mice (data not shown). We therefore assessed infection-induced autoAb production in mice that had been previously immunized. Following systemic challenge, Pneumovax-immunized C4-/- mice showed striking increases in dsDNA-specific IgA levels that were maintained in some mice up to 120 days post infection (Fig. 1B-C). Increases in dsDNA-specific IgA endpoint titers paralleled changes observed in relative absorbance values (Fig. 1C). In contrast, infected C4+/+ littermates and naïve age-matched C4-/- mice monitored over the same period did not show changes in dsDNA-specific IgA levels (Fig. 1B and 1D). Moreover, infection was associated with only a slight modulation of dsDNA-specific IgM levels and had no effect on IgG levels relative to d0 values (Fig. 1B). Finally, serum samples (4 out of 5) from C4-/- mice collected 120 days post infection also showed reactivity with native dsDNA using the Crithidia luciliae immunofluorescence assay, whereas serum from wild type mice showed no reactivity (data not shown). Thus, serotype 19F pneumococcal respiratory infection, as well as serotype 3 systemic infection following Pneumovax immunization, significantly increased anti-dsDNA IgA levels in C4-/- mice.
PPS immunization induces anti-dsDNA Ab in C4-/- mice, with the highest levels observed in female mice
Increases in dsDNA-specific IgA in immune C4-/- mice following systemic infection (Fig. 1B-C) could have been attributed to immunization and/or infection. Therefore, we assessed the extent to which Pneumovax (PPS) immunization alone influenced anti-dsDNA Ig levels in C4-/- mice. As shown in Fig. 2A, as little as 23 μg Pneumovax (containing ~1 μg each of 23 different PPS) administered i.p. significantly increased dsDNA-reactive IgM and IgA, but not IgG, levels in C4-/- mice but not wild type mice. Immunization-induced increases in dsDNA-specific IgM were generally transient in C4-/- mice, with the exception of one mouse which maintained very high levels over a 120-day period (Fig. 2A-B and data not shown). However, immunization-induced increases in dsDNA-specific IgA levels remained above pre-immune levels for most C4-/- mice (Fig. 2C). Anti-dsDNA IgA endpoint titer analysis showed that relative to day 0 titers, day 120 post-immunization titers were increased 2-3-fold in three C4-/- mice, ~10-fold in two mice, and over 100-fold in one mouse (Fig. 2C). As expected, dsDNA-specific IgM and IgA levels remained low and stable in immune wild type mice over the 120-day period (Fig. 2B-C), as was the case for naïve C4-/- mice (Fig. 1D). Consistent with these ELISA data, sera (day 14 and day 120 post-immunization) from the majority (~60-70%) of immune C4-/-, but not wild type mice, exhibited total Ig, as well as IgA, reactivity with native dsDNA using the Crithidia luciliae immunofluorescence assay (data not shown). Importantly, the effect of increased anti-dsDNA IgA levels following Pneumovax immunization in C4-/- mice was observed following s.c. immunization (Fig. 2D) in addition to i.p. immunization (Fig. 2A-B), indicating that Pneumovax induces dsDNA-specific IgA production in C4-/- mice regardless of the administration route.
Figure 2. Pneumovax or PPS-3 immunization induces anti-dsDNA Ab in C4-/- mice, with the highest levels observed in female mice.
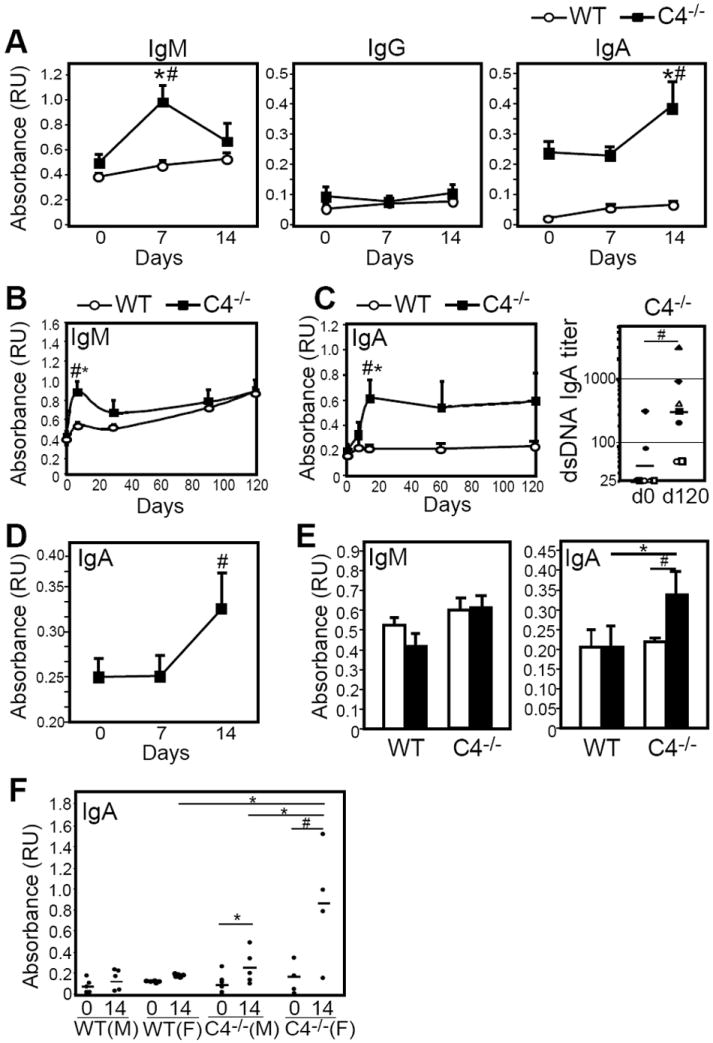
A) Serum dsDNA-specific IgM, IgG, and IgA levels in WT (n=7) and C4-/- (n=7) female mice following i.p. immunization with Pneumovax. B-C) Mean (± SEM) serum dsDNA-specific IgM (B) and IgA (C) levels in WT and C4-/- female mice over a 120-day period following i.p. Pneumovax immunization. Day 0 and d120 endpoint titers are shown for C4-/- mice in C (right panel). D) Mean serum dsDNA-specific IgA levels in C4-/- mice following s.c. immunization with Pneumovax. E) Mean serum dsDNA-specific IgM and IgA levels in WT and C4-/- male mice on day 0 and 14 following i.p. immunization with 40 μg PPS3 (n=3 mice/group). F) Mean serum dsDNA-specific IgA levels in WT and C4-/- male (M) and female (F) mice on day 0 and 14 following i.p. immunization with 40 μg PPS3 (filled circles indicate individual mice). In A-F, asterisks (*) indicate significant differences between WT and C4-/- values, while pound signs (#) indicate significant differences between pre-immune and post-immune values for the same group (p<0.05).
In contrast to female C4-/- mice, 2-3 month old naïve male C4-/- mice had anti-dsDNA IgM and IgA levels that were comparable to wild type mice (Fig. 2E). However, immunization of male C4-/- mice with 40 μg of purified PPS3 alone elicited significant increases in dsDNA-specific IgA levels, but had no effect in wild type male mice (Fig. 2E). Nonetheless, a comparison of dsDNA-specific IgA levels in wild type and C4-/- male and female mice demonstrated that female C4-/- mice produced the greatest amount of dsDNA-specific IgA following PPS3 immunization (Fig. 2F). Although variation was often observed in the levels of dsDNA-specific IgA produced by C4-/- female mice following infection or PPS3/Pneumovax immunization (Figs. 1-2), the finding that C4-/- female mice were more sensitive to immunization-induced autoAb production is consistent with the increased anti-nuclear Ab levels observed in aged (10 month-old) naive female C4-/- mice relative to male mice (12).
Increased anti-dsDNA IgA levels are strongly associated with increased IgA deposition in kidneys of C4-/- mice
We also assessed other indicators of autoimmunity, including proteinuria and Ab deposition in kidneys of female C4-/- and wild type mice 5-6 months following Pneumovax immunization. Average proteinuria scores 5 months post immunization did not significantly differ among immune C4-/- mice (59 ± 12 mg/dl), immune-infected C4-/- mice (42 ± 7 mg/dl), immune wild type (36 ± 5 mg/dl), and age-matched naive C4-/- (42 ± 7 mg/dl) and wild type mice (30 ± 0 mg/dl). IgA, as well as total Ig deposition, was increased in kidneys of female C4-/- mice (~7-8 months of age) relative to age-matched wild type mice, regardless of whether they were immunized with Pneumovax (Px) alone or immunized and challenged with WU2 (Px + infx) 5 months prior to analysis (Fig. 3A-B). This is consistent with previous reports of increased Ig deposition in kidneys of aging naïve C4-/- mice (12, 13). However, there was a significant increase in the intensity of glomerular IgA fluorescence in C4-/- mice that had been immunized 5 months prior with Pneumovax (regardless of WU2 challenge) relative to age-matched naïve C4-/- mice (Fig. 3B). In contrast, we did not detect significant differences in glomerular total Ig fluorescence between immune and naïve C4-/- mice. This suggests that although Pneumovax immunization/pneumococcal infection was associated with a selective increase in IgA deposition, deposition of other isotypes was less likely affected.
Figure 3. IgA deposition in kidneys of C4-/- mice is positively correlated with dsDNA-specific IgA serum levels.
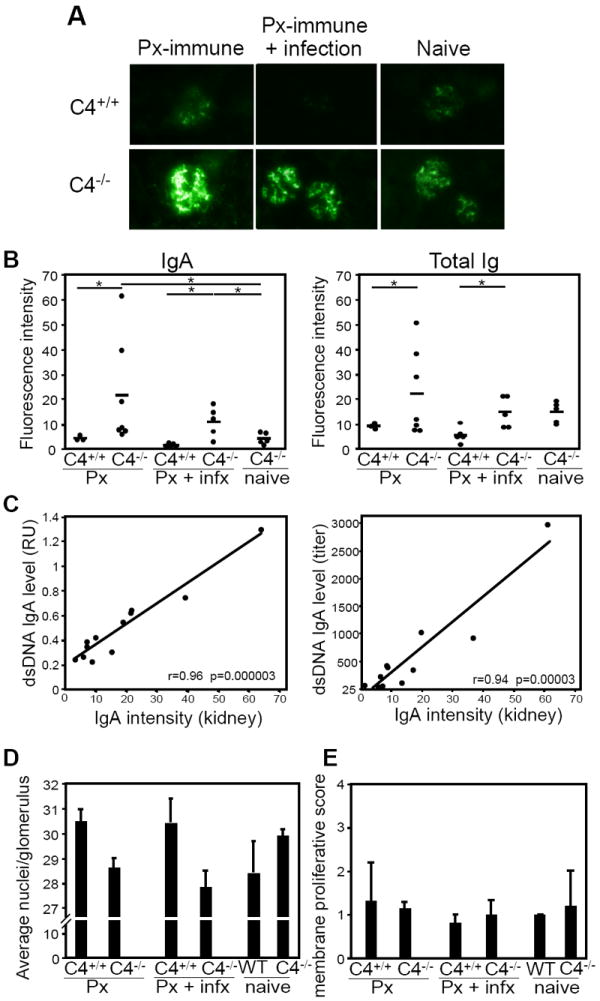
A) Representative glomerular IgA staining in kidneys harvested from wild type and C4-/- mice 5 months post-Pneumovax (Px) immunization alone (left panels) or 5 months post-Pneumovax (Px) immunization with i.p. WU2 infection challenge on d14 (middle panels). IgA staining of aged (9-10 month-old) naïve wild type and C4-/-mice is shown (right panels). FITC-conjugated goat anti-mouse IgA was used for detection (400X magnification). B) Quantitation of mean IgA and Ig fluorescence (mean fluorescence intensity) in kidney sections of C4+/+ and C4-/- mice 5 months post Pneumovax immunization (Px), 5 months post Pneumovax immunization with i.p. WU2 infection on d14 (Px + infx), and age-matched naïve C4-/- mice. Fluorescence intensity of glomerular Ig staining was quantified using ImageJ software and expressed as density per unit area (with the average fluorescence measured from 10 or more glomeruli). Values for individual mice are shown. Mean fluorescent intensity is indicated as horizontal bars. Significant differences in means are indicated (*p<0.05). C) Graphs depict the association (pairwise comparison) between the mean fluorescence intensity signals of IgA staining in kidneys (as shown in A and quantitated in B) and the levels of dsDNA-specific serum IgA 5 months post immunization measured as absorbance values obtained at a 1:100 dilution (left panel) or endpoint titers (right panel). Pearson correlation coefficients are indicated (r values) along with significance (p values). D-E) Glomerular cellularity (D; glomerular mesangial nuclei counts were performed by counting nuclei in 10 random glomerular corpuscles and expressed as average nuclei per glomerulus) and mesangial proliferation (E; glomerular basement membrane thickening or mesangial expansion using a 0-4 scoring system [0 = inapparent, 1 = <50% of glomeruli, segmental; 2 = >50% of glomeruli, segmental; 3 = <50% of glomeruli, predominantly diffuse; and 4 = >50% of glomeruli, predominantly diffuse]) was determined by analysis of periodic-Schiff stained 3 μM thick formalin-fixed paraffin-embedded kidney sections (at least 20 random glomeruli were assessed per section). Age-matched wild type and C4-/- groups are as described for (B).
Finally, we assessed whether there was an association between dsDNA-specific serum IgA levels in Pneumovax-immunized C4-/- mice and the intensity of IgA deposition measured in kidneys. Figure 3C shows that in C4-/- mice, a strong and highly significant positive correlation exists between the glomerular IgA fluorescence that was detected and the level of dsDNA-specific serum IgA measured as either relative absorbance units (r=0.96, p=0.000003) or reciprocal titer (r=0.94, p=0.00003, respectively). Nonetheless, despite the presence of IgA within kidneys, histological analysis of glomerular cellularity and mesangial proliferation did not reveal signs of kidney damage in these mice (Fig. 3D-E). Thus, Pneumovax immunization was associated with increased dsDNA-specific IgA levels and IgA deposition in kidneys of C4-/- mice, but was not associated with measurable kidney damage. Notably, systemic pneumococcal challenge following Pneumovax immunization did not appear to enhance anti-dsDNA IgA levels or IgA deposition in kidneys of C4-/- mice over that observed with immunization alone.
Increased dsDNA-specific IgA levels in C4-/- mice following PPS immunization are partially due to increased levels of dsDNA-specific Abs that cross-react with PC
Although 40 μg PPS3 induced anti-dsDNA IgA production in C4-/- mice, it did not induce significant PPS3-specific IgA production (Fig. 4A), which is consistent with the previously described phenomenon of high dose PPS tolerance (26). Pneumovax (1 μg PPS3)-induced PPS3-specific IgA production was not significantly different between C4-/- and wild type mice (Fig. 4B). PPS3-specific ELISAs were assessed using cell wall polysaccharide (CWPS) as a blocking agent. We therefore also examined IgA responses against a dominant cell wall contaminant, phosphorylcholine (PC). As shown in Fig. 4A-B, naïve C4-/- mice exhibited higher PC-specific IgA levels relative to wild type mice, but PPS3 as well as Pneumovax induced PC-specific IgA production in both wild type and C4-/- mice.
Figure 4. Increased dsDNA-specific IgA levels in C4-/- mice following PPS immunization is partially due to PC-crossreactive Ab.
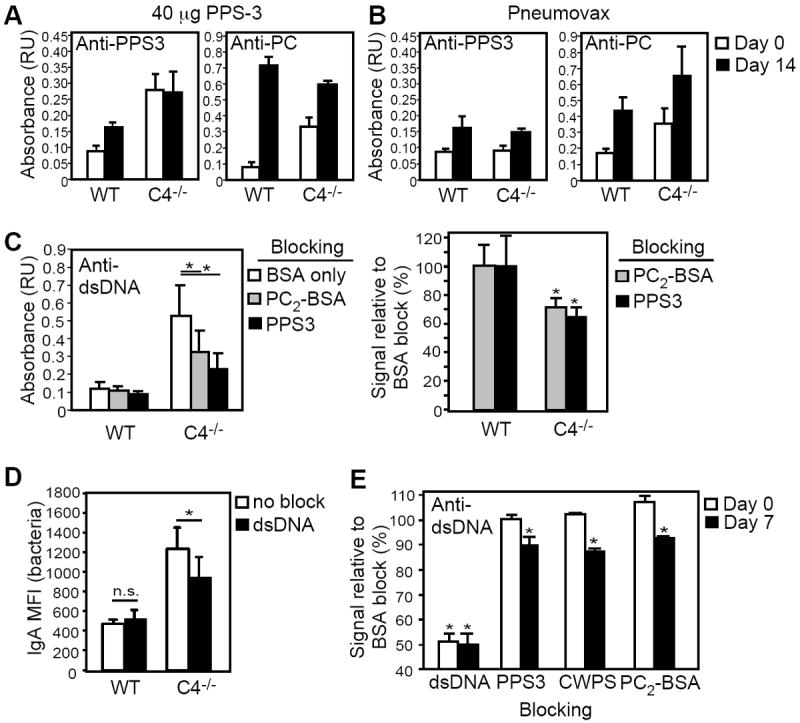
A-B) PPS3- and PC-specific IgA levels in WT (n=7) and C4-/- (n=5) mice on day 0 (white bars) and day 14 (black bars) following i.p. immunization with 23 μg Pneumovax or 40 μg PPS3. C) Inhibition of dsDNA IgA binding by preincubation of immune sera (day 14 post 40 μg PPS3) with 5 μg/ml PC2-BSA or PPS3. dsDNA-specific IgA levels measured by ELISA are shown for WT and C4-/- sera in the left panel (n=6-9 mice/group). The percent of signal obtained for blocking conditions relative to BSA only-blocking (BSA) conditions are graphed in the right panel. D) Reactivity of serum IgA from Pneumovax-immune (d14) WT (n=4) and C4-/- (n=6) mice with heat-killed EF3030 in the presence and absence of dsDNA blocking, as assessed by flow cytometry. Differences in mean fluorescence intensities (MFI) of blocked and non-blocked samples were determined by paired t-test. E) Inhibition of anti-dsDNA IgA binding by preincubation of naïve and immune C4-/- sera (day 7 post Pneumovax) with 25 μg/ml dsDNA, PPS3, CWPS, and PC2-BSA. The percent of signal obtained for blocking conditions relative to BSA only-blocking (BSA) conditions are shown. For A-D, mean (± SEM) values are shown. Asterisks (*) in C-E indicate significant differences between values for non-blocking vs. blocking conditions (p<0.05).
Anti-dsDNA Abs have been hypothesized to arise by molecular mimicry by pathogen-derived antigens, including the pneumococcal antigens, phosphorylcholine and pneumococcal choline kinase (27-31). Therefore, we assessed whether anti-dsDNA IgA Abs in C4-/- mice were cross-reactive with pneumococcal polysaccharides or associated contaminants (eg., CWPS and/or PC). Relative to BSA control blocking conditions, PPS3 preparations significantly inhibited the dsDNA reactivity of high dose PPS3-elicited IgA Abs from C4-/- mice (~35% reduction in binding), but had no effect on dsDNA reactivity of sera from wild type mice (Fig. 4C). Since increased PPS3-specific IgA Abs were not detected in immune C4-/- sera (Fig. 4A), we postulated that PPS3 blocking of dsDNA-specific Abs might be attributable to a contaminating pneumococcal Ag rather than PPS. Indeed, blocking C4-/-, but not wild type, immune sera with BSA that was weakly haptenated with PC (PC2-BSA) significantly inhibited (~30% decrease) dsDNA-specific IgA binding relative to BSA-only blocking (Fig. 4C). Thus, at least one-third of the dsDNA-reactive IgA in C4-/- mice immunized with high dose PPS3 is cross-reactive with PC.
We assessed whether vaccine-grade Pneumovax-elicited anti-dsDNA IgA Abs in C4-/- mice were similarly crossreactive with pneumococcal antigens. Free dsDNA partially inhibited IgA from immunized C4-/- (25 ± 7% reduction in MFI, p<0.05 vs. WT), but not WT mice from binding to S. pneumoniae strain EF3030 (Fig. 4D). As expected, free dsDNA significantly inhibited d0 and d7 Pneumovax-immune sera from binding to plate-bound dsDNA relative to BSA-only blocking (~50% inhibition; Fig. 4E). Naïve C4-/- IgA binding to dsDNA was not inhibited by PPS3, CWPS, or PC2BSA. However, dsDNA-specific IgA in immune C4-/- sera was significantly inhibited by PPS3, CWPS, and PC2-BSA (~10-15% inhibition; Fig. 4E). The less effective blocking of Pneumovax-elicited dsDNA IgA binding (versus high dose PPS3-elicited IgA; Fig. 4C) by pneumococcal cell wall antigens may reflect a different level of cell wall contamination products in the Pneumovax vaccine lot versus the PPS3-only preparation. Nonetheless, with both Pneumovax and PPS3-only immunization, a portion (~10-30%) of the dsDNA-specific IgA elicited in C4-/- mice is cross-reactive with PC.
TLR2/4 agonists associated with pneumococcal polysaccharides induce dsDNA-specific IgA production in C4-/- mice
Since molecular mimicry by PC or other pneumococcal antigens could not completely explain the increased dsDNA-specific IgA levels in C4-/- mice, we considered the potential for pneumococcal cell wall-associated TLR agonists (eg., pneumolysin, lipotechoic acid, lipoproteins) to promote IgA production by dsDNA-reactive B cells. Proteinase K treatment had no effect on PPS3 immunization-induced increases in anti-dsDNA IgA levels in C4-/- mice (Fig. 5A), nor PPS3-specific IgM or IgG responses (Fig. 5B), indicating that pneumolysin (a TLR4 agonist sensitive to proteinase K, ref.(32)) was unlikely to play a role. Similarly, DNase treatment of PPS had no effect on increased dsDNA-specific IgA levels in C4-/- mice (data not shown). To further investigate the potential for pneumococcal associated-TLR2/4 agonists to induce anti-dsDNA IgA production, we utilized a TLR2/4 antagonist, OxPAPC, which has been shown to selectively suppress TLR2/4-mediated activation of macrophages relative to other TLR in vitro, and suppress Pam3Csk4-mediated inflammatory responses in vivo (33). We found that OxPAPC significantly suppressed blasting and CD86 upregulation in B cells activated by LPS (TLR4) and Pam3Csk4 (TLR2), but was not effective in significantly reducing activation elicited by BCR and CD40 costimulation (Fig. 5C). Remarkably, administration of OxPAPC to C4-/- mice at the time of Pneumovax immunization completely blocked increases in anti-dsDNA IgA levels (Fig. 5D), but nonetheless allowed productive PPS3-specific IgM, IgG and IgA responses (Fig. 5E). Moreover, when these same mice were immunized again 28 days later with Pneumovax in the absence of OxPAPC, significant increases in dsDNA-specific IgA levels were then observed (Fig. 5D), demonstrating they had the potential to produce anti-dsDNA in the absence of the TLR2/4 inhibitor.
Figure 5. TLR2/4 agonists associated with pneumococcal polysaccharides induce dsDNA-specific IgA production in C4-/- mice.
A-B) Mean dsDNA-specific serum IgA levels (A) and PPS3-specific IgM and IgG levels (B) in C4-/- mice (n=7) following immunization with 1 μg PPS3 (open circles) or proteinase K-treated PPS3 (filled squares). C) The TLR2/4 antagonist, OxPAPC suppresses B cell activation. Splenocytes from wild type mice (n=3) were cultured for 2 days with LPS (20 μg/ml), Pam3Csk4 (0.5 μg/ml), or anti-IgM (5 μg/ml + anti-CD40 (0.1 μg/ml) in the absence or presence of OxPAPC (20 μg/ml). Mean blasting (FSC) and CD86 upregulation (CD86 mean fluorescence intensity) of CD19+ B cells are graphed, with significant differences between untreated and OxPAPC-treated samples indicated by asterisks (*, p<0.05). D) Mean dsDNA-specific serum IgA levels in C4-/- mice following treatment with 40 μg OxPAPC on days 0, 1, 2 and 3 and 1 μg Pneumovax administered on days 0 and 28. E) PPS3-specific IgM, IgG, and IgA levels in C4-/- mice (n=10) receiving 1 μg Pneumovax and OxPAPC. F) Mean serum dsDNA-specific IgA levels in C4-/- and wild type mice (n=5/group) following administration of 0.1 μg Pam3Csk4 i.p. G) Mean serum dsDNA-specific IgA levels in C4-/- mice (n=4-5/group) following administration of 0.1 μg Pam3Csk4 i.p. on day 0 and treatment with 40 μg OxPAPC on days 0, 1, 2 and 3. H) Mean serum dsDNA-specific IgM and IgA levels in C4-/- and wild type mice (n=5/group) following administration of 10 μg LPS i.p. In A-H, pound signs (#) indicate significant differences (p<0.05) between values for immune and naive sera and asterisks (*) indicate significant differences (p<0.05) between genotypes or treatment groups.
Administration of a small quantity (0.1 μg) of the TLR1/2 agonist, Pam3CSK4, to C4-/- mice significantly increased dsDNA-specific IgA, but not IgM or IgG, levels and had no effect in wild type mice (Fig. 5F and data not shown). However, administration of OxPAPC completely prevented Pam3CSK4-induced dsDNA IgA production in C4-/- mice (Fig. 5G). LPS did not have a measurable effect on anti-dsDNA IgA production in C4-/- or wild type mice, but induced moderate and transient increases in dsDNA-reactive IgM (Fig. 5H). From this data, we conclude that TLR2 agonists associated with pneumococcal polysaccharide vaccine preparations may promote dsDNA-specific IgA production in autoimmune-prone C4-/- mice.
TLR2 agonists directly induce dsDNA-specific IgA production by IgA+ B cells from C4-/- mice
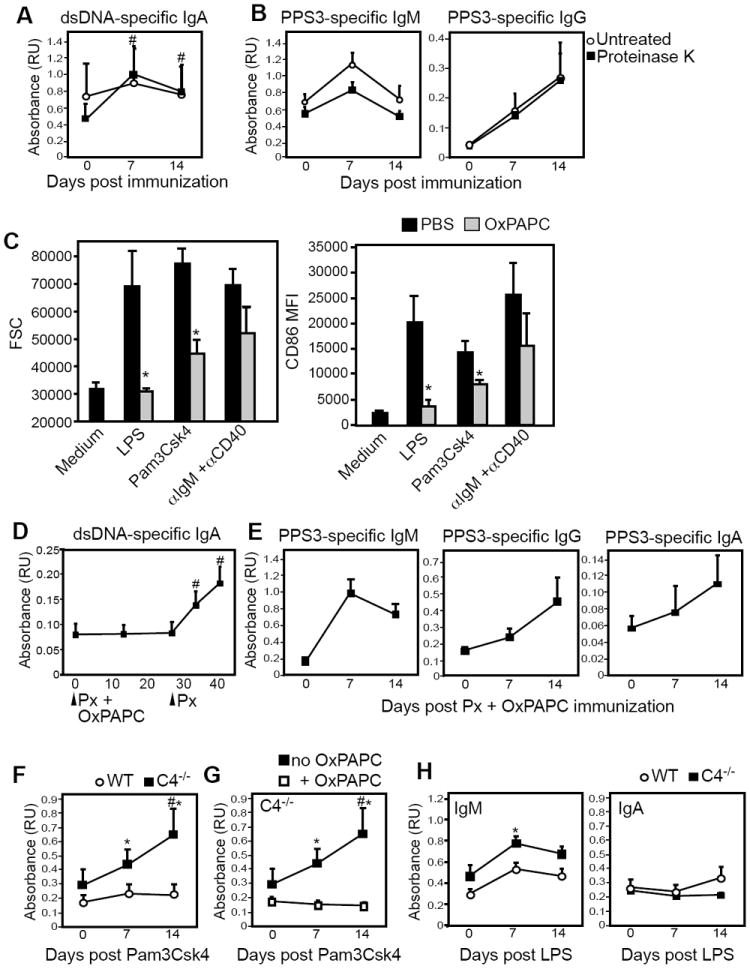
We next investigated whether TLR agonists could act directly on B cells from C4-/- mice to induce dsDNA-specific IgA production. To assess whether TLR2 or TLR4 agonists could promote anti-dsDNA IgA production by preexisting IgA+ cells or IgA- B cells, we stimulated IgA+ and IgA--sorted splenocytes from wild type and C4-/- mice with LPS or Pam3Csk4 and examined anti-dsDNA IgA production. As shown in Figure 6, Pam3Csk4, but not LPS, significantly increased anti-dsDNA IgA levels produced by IgA+ B cells from C4-/- mice over levels measured for Pam3Csk4-activated wild type B cells (p<0.05) and C4-/- B cells cultured in media alone (p<0.05, pairwise t-test). Increased anti-dsDNA IgA production was not observed in activated IgA- cells (Fig. 6), or purified B cell cultures from WT or C4-/- mice (data not shown), suggesting that Pam3Csk4- or LPS-derived signals are not sufficient to induce measurable IgA isotype switching and subsequent IgA production in cultured dsDNA-specific IgA- B cells. From these data, we conclude that the TLR2 agonist, Pam3Csk4, can directly induce increased IgA secretion by dsDNA-specific IgA+ B cells.
Figure 6. TLR2 agonists directly induce dsDNA-specific IgA production by IgA+ B cells from C4-/- mice.
IgA+ and IgA- splenocytes from wild type and C4-/- mice (n=4 mice/genotype) were cultured for 6 days in complete media alone or in the presence of LPS (20 μg/ml) or Pam3Csk4 (0.5 μg/ml). Supernatants were harvested, diluted 3-fold in PBS, and dsDNA-specific IgA levels were assessed by ELISA. Mean dsDNA-specific IgA values are shown. Asterisks (*) indicate increases in anti-dsDNA IgA levels were significantly greater than than that observed for wild type mice and pound signs (#) indicate significant differences (p<0.05) between treatment conditions.
IgA+ B-1a cells are expanded in C4-/- mice
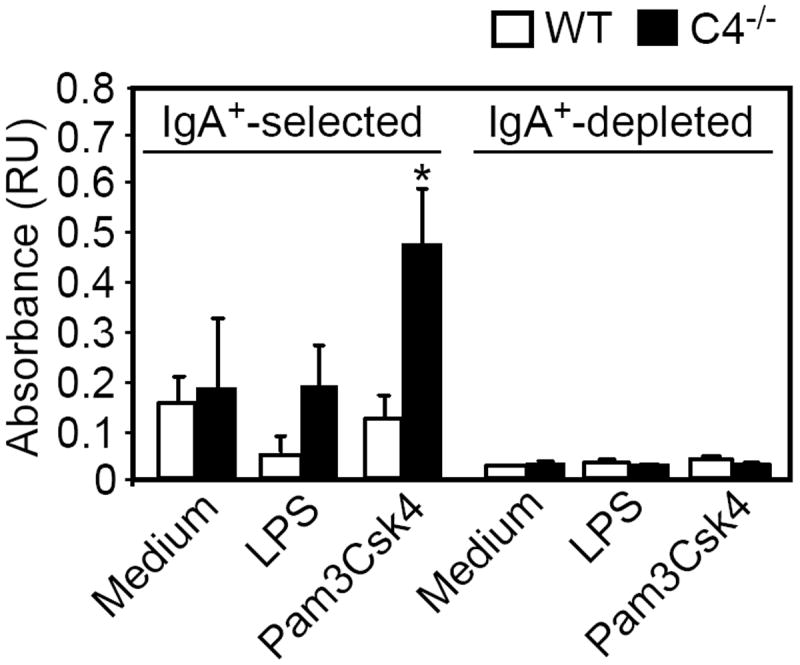
The induction of dsDNA-specific IgA production in C4-/- splenic IgA+ cells was an intriguing result, as conventional splenic B cells have been reported to produce little IgA following TLR2 stimulation (34, 35). In contrast, B-1a cells are known to be highly responsive to Pam3Csk4 (34, 35) and produce dsDNA Abs (36, 37). We therefore assessed the frequency as well as the phenotype of IgA+ B cells in C4-/- mice. Splenic IgA+ cell frequencies were increased in C4-/- mice (1.4-fold), albeit not significantly (Fig. 7A). However, peritoneal IgA+ cells were significantly increased (~2-fold) in C4-/- mice. Notably, total peritoneal cavity and spleen cell yields did not differ between wild type and C4-/- mice [WT spleen: (9.55 ± 1.2) × 107 vs. C4-/- spleen: (9.25± 0.4) × 107 and WT peritoneal cavity: (1.25 ± 0.28) × 107 vs. C4-/- peritoneal cavity: (1.4 ± 0.2) × 107]. Phenotypic analysis of IgA+ B cells revealed that IgA+ B-1a cells (CD19+CD5+CD11b+ in peritoneal cavity and CD19+CD5+ in spleen), but not CD5- IgA+ B cells, were significantly increased in C4-/- mice (Fig. 7B). The expanded IgA+ B-1a cell population, known to harbor dsDNA-reactive B cells, may therefore be a likely source of TLR2-elicited dsDNA-specific IgA production in C4-/- mice.
Figure 7. C4-/- mice have increased frequencies of peritioneal cavity and splenic IgA+ B-1a cells.
A) IgA+ B cell frequencies in peritoneal cavities and spleens of wild type and C4-/- mice as assessed by flow cytometry. B) The expression of CD5 and CD11b on peritoneal and splenic IgA+ B cells (CD19+B220+/lo) was examined for wild type mice and C4-/- mice by flow cytometry. The mean (±SEM) frequencies of total IgA+ cells (A) and splenic and peritoneal cavity CD5-IgA+ B cells and splenic CD5+IgA+ (B-1a) B cells and peritoneal cavity CD5+CD11b+IgA+ (B-1a) B cells (B) are shown, with significant differences between wild type and C4-/- mice indicated by asterisks (*, p<0.05). Pooled results obtained using 7-8 mice per genotype in 2 separate experiments.
Discussion
The association between C4 deficiency (even partial deficiency) and autoimmunity is well-established (5-13). Similarly, there is an association between C4 deficiency and increased susceptibility to infections, including those caused by S. pneumoniae (14, 16, 17), for which our study provides further evidence. Our study demonstrates there is a clear link between infection and autoimmunity in the context of C4-deficiency and presents several major findings. First, mild S. pneumoniae serotype 19F respiratory infection and even pneumococcal polysaccharide immunization elicit significant increases in dsDNA-specific IgA production in C4-/- mice. Second, the pneumococcal cell wall contaminants, phosphorylcholine (via molecular mimicry) and to a larger part, TLR2 agonists, are responsible for promoting dsDNA-reactive IgA production following pneumococcal polysaccharide immunization in C4-/- mice. Our data suggests that TLR2 agonists can directly promote increased dsDNA-specific IgA production by dsDNA-specific IgA+ B cells in C4-/- mice - a finding that may directly relate to expansion of the IgA+ B-1a cell pool that occurs in the absence of C4. Collectively, our results indicate a cause-effect relationship between increased exposure to bacteria or bacterial products and increased autoAb production in the context of C4 deficiency.
Remarkably, mild pneumococal respiratory infection, as well as intraperitoneal and subcutaneous PPS immunization, induced the production of dsDNA-specific IgA in C4-/-, but not C3-/-, or wild type mice. C4-/- mice have a predisposition to developing increased frequencies of dsDNA-specific B cells at a young age, likely due to impaired clearance of DNA-containing immune complexes and/or altered negative selection (12, 13). Increased production of dsDNA-reactive IgA may have been elicited in these mice, in part, as a result of pneumococcal cell wall-associated PC engaging and activating Ab secretion in B cells with dual PC/dsDNA-reactivity (28). Indeed, PC-BSA and cell wall polysaccharide partially inhibited dsDNA-reactive IgA binding in ELISAs and conversely, dsDNA partially inhibited IgA from C4-/- mice, but not wild type mice, from binding to whole bacteria. Together, these results suggest a fraction of the dsDNA-specific IgA elicited by S. pneumoniae in C4-/- mice is cross-reactive with PC or other pneumococcal antigens. PC-reactive B cells in these mice may alternatively acquire mutations that enable binding of dsDNA following pneumococcal exposure, as has been observed in some studies (30, 31). Further work is necessary to distinguish between these possibilities and to determine whether S. pneumoniae-induced PC/dsDNA-specific IgA production in C4-/- mice occurs independently of T cell help.
Our data suggests that induction of dsDNA-reactive IgA production in C4-/- mice may largely depend on the TLR agonists associated with the pneumococcal cell wall contaminants that are known to be present within PPS (including the Pneumovax vaccine) preparations (38). The major TLR agonists expressed by S. pneumoniae include peptidoglycan, lipotechoic acid, and lipoproteins (TLR2 agonists), and pneumolysin (TLR4) (32, 39, 40). OxPAPC, an oxidized phospholipid previously shown to specifically suppress TLR2- and TLR4-mediated activation of macrophages (33)(and herein, TLR2/4-mediated in vitro B cell activation), completely suppressed Pneumovax- and Pam3Csk4-mediated increases in anti-dsDNA serum IgA levels. It remains possible that OxPAPC could function via other mechanisms to limit dsDNA-specific IgA production, although the fact that the PPS3-specific Ab response was not affected by OxPAPC suggests some level of specificity for Ab-producing B cells with dsDNA reactivity. Taken together, our results are more supportive of a role for pneumococcal TLR2, as opposed to TLR4, agonists in mediating dsDNA-specific IgA production. First, the inability to eliminate Pneumovax-induced anti-dsDNA IgA production using proteinase K treatment suggests pneumolysin is unlikely to be playing a role. Second, Pam3Csk4, but not LPS, induced anti-dsDNA IgA production in both C4-/- mice and in C4-/- IgA+ B cell cultures. Not surprisingly, a role for TLR2 in autoimmunity is emerging, including research demonstrating pathogen-independent roles for TLR2 in promoting anti-dsDNA Ab production in a mouse lupus model (41) and in SLE patient-derived PBMCs responding to HMGB1-containing dsDNA immune complexes (42). Additional work supports that TLR2 tolerance induction (via chronic administration of Pam3Csk4 or similar agonists) suppresses the development of diabetes in autoimmune-prone NOD mice (43, 44). Our results add to our understanding of TLR2 modulation of autoimmunity by demonstrating that pathogens and/or vaccines bearing TLR2 agonists may induce autoAb production in autoimmune-prone C4-/- mice.
The mechanism(s) by which pneumococcal TLR agonists and Pam3Csk4 induce anti-dsDNA IgA production in C4-/- mice is not yet clear. However, our in vitro data demonstrate that TLR2 agonists can directly stimulate dsDNA-reactive IgA+, but not IgA-, B cells to secrete IgA. Follicular and marginal zone B cells produce very little IgA in response to TLR agonists, whereas B-1 cells produce very high levels of IgA in response to TLR agonists, including Pam3Csk4 (34, 35). Moreover, B-1a cells are a major source of dsDNA Ab and can initiate autoimmunity, including lupus (36, 37) and diabetes (45). That C4-/- mice have a significantly expanded IgA+ B-1a cell population raises the possibility that this dysregulated B cell population may indeed be contributing to S. pneumoniae-induced dsDNA-specific IgA production. Importantly, our data does not exclude the possibility that a TLR2-responsive non-B cell population, such as dendritic cells, could support IgA production by autoreactive B cells. Future studies assessing the effects of C4 levels on B-1a cell and IgA regulation, along with the role that distinct B cell subsets and non-B cell populations play in anti-dsDNA IgA responses, are clearly warranted.
Increased anti-dsDNA IgA levels were maintained for months in immunized C4-/- mice and were strongly correlated with increased IgA deposition in kidneys. Nonetheless, we did not observe changes in kidney pathology or proteinuria associated with this increased IgA deposition. This may be due to the autoimmune-resistant B6 genetic background, the requirement for C4 to initiate complement-mediated deposition (as we were unable to detect C3 deposition) and hence pathology, and/or the general lack of pathogenicity of the anti-DNA IgA Abs elicited following immunization. Dual-reactive PC/dsDNA-specific mouse IgG2b and IgM mAb clones have been shown to elicit protection against pneumococcal infection but also elicit kidney pathology in BALB/c mice (28, 46). The effects of an anti-dsDNA Ab in eliciting kidney damage depend on the fine specifity of an Ab for dsDNA (28, 47, 48) as well as the Ab isotype. For example, while dsDNA-specific IgG is most often pathogenic (47, 48), anti-dsDNA IgM may be protective against nephritis in some cases (49, 50). Indeed, anti-PC IgM is positively associated with low disease activity in lupus patients (51). The effects of anti-dsDNA/PC IgA are less clear. Studies have revealed that anti-dsDNA IgA levels are significantly higher in lupus patients exhibiting more active disease (52-54). Moreover, treatment of mice with an anti-PC IgA Ab was shown to elicit severe glomerulonephritis and proteinuria when pneumococcal cell wall was coadministered (55), supporting at least the role for IgA-pneumococcal antigen immune complexes in causing renal damage in mice. However, further studies are required to determine the extent to which anti-dsDNA IgA mAbs elicited by pneumococcal antigens influence kidney damage in both nonautoimmune and autoimmune settings.
The implications that our study has for humans are not yet clear. Work from Diamond and colleagues clearly support a link between pneumococcal polysaccharide immunization in humans and the production of anti-pneumococcal Abs bearing the same idiotype associated with anti-DNA Abs in normal individuals (56), although increases in DNA-binding were not observed in these subjects. In contrast, lupus patients may produce Abs that cross-react with both bacterial antigen and DNA (27). It is nonetheless important to stress that several studies have shown that Pneumovax does not increase total dsDNA-specific serum Ig levels or disease activity in lupus patients (57, 58). However, whether changes in dsDNA-specific IgA levels occur as a result of Pneumovax immunization in autoimmune patients, especially those with low C4 levels, is not yet clear, as this has not specifically been measured in previous studies. It is interesting that patients with complete or partial (C4B) deficiencies are prone to infections with encapsulated bacteria, including Streptococcus pneumoniae (19), and that C4B deficiency is associated with significantly increased IgA nephropathy (59). Thus, our findings demonstrating a link between C4 deficiency, increased pneumococcal infection, and autoreactive IgA production and IgA kidney deposition in mice model some observations made for human patients. Future studies aimed at deciphering the mechanisms by which C4 regulates IgA production by both autoreactive and normal B cells, along with studies assessing the effects of anti-dsDNA IgA, may ultimately lead to more effective therapies for autoimmunity.
Acknowledgments
The authors thank Ryan Egan for his assistance with experiments.
This work was supported by a Mid-Atlantic Arthritis Foundation grant and by Wake Forest School of Medicine startup funds.
Footnotes
Conflict of interest
The authors have no conflicting financial interests.
References
- 1.Ercolini AM, Miller SD. The role of infections in autoimmune disease. Clin Exp Immunol. 2009;155:1–15. doi: 10.1111/j.1365-2249.2008.03834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Getts DR, Chastain EM, Terry RL, Miller SD. Virus infection, antiviral immunity, and autoimmunity. Immunol Rev. 2013;255:197–209. doi: 10.1111/imr.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barzilai O, Ram M, Shoenfeld Y. Viral infection can induce the production of autoantibodies. Curr Opin Rheumatol. 2007;19:636–643. doi: 10.1097/BOR.0b013e3282f0ad25. [DOI] [PubMed] [Google Scholar]
- 4.Sfriso P, Ghirardello A, Botsios C, Tonon M, Zen M, Bassi N, Bassetto F, Doria A. Infections and autoimmunity: the multifaceted relationship. J Leukoc Biol. 2010;87:385–395. doi: 10.1189/jlb.0709517. [DOI] [PubMed] [Google Scholar]
- 5.Hauptmann G, Tappeiner G, Schifferli JA. Inherited deficiency of the fourth component of human complement. Immunodefic Rev. 1988;1:3–22. [PubMed] [Google Scholar]
- 6.Yang Y, Chung EK, Zhou B, Lhotta K, Hebert LA, Birmingham DJ, Rovin BH, Yu CY. The intricate role of complement component C4 in human systemic lupus erythematosus. Curr Dir Autoimmun. 2004;7:98–132. doi: 10.1159/000075689. [DOI] [PubMed] [Google Scholar]
- 7.McLean RH, Wyatt RJ, Julian BA. Complement phenotypes in glomerulonephritis: increased frequency of homozygous null C4 phenotypes in IgA nephropathy and Henoch-Schonlein purpura. Kidney Int. 1984;26:855–860. doi: 10.1038/ki.1984.228. [DOI] [PubMed] [Google Scholar]
- 8.Gilliam BE, Wolff AE, Moore TL. Partial C4 deficiency in juvenile idiopathic arthritis patients. J Clin Rheumatol. 2007;13:256–260. doi: 10.1097/RHU.0b013e318156b9e3. [DOI] [PubMed] [Google Scholar]
- 9.Wopenka U, Thysell H, Sjoholm AG, Truedsson L. C4 phenotypes in IgA nephropathy: disease progression associated with C4A deficiency but not with C4 isotype concentrations. Clin Nephrol. 1996;45:141–145. [PubMed] [Google Scholar]
- 10.Prodeus AP, Goerg S, Shen LM, Pozdnyakova OO, Chu L, Alicot EM, Goodnow CC, Carroll MC. A critical role for complement in maintenance of self-tolerance. Immunity. 1998;9:721–731. doi: 10.1016/s1074-7613(00)80669-x. [DOI] [PubMed] [Google Scholar]
- 11.Einav S, Pozdnyakova OO, Ma M, Carroll MC. Complement C4 is protective for lupus disease independent of C3. J Immunol. 2002;168:1036–1041. doi: 10.4049/jimmunol.168.3.1036. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, Koralov SB, Kelsoe G. Complement C4 inhibits systemic autoimmunity through a mechanism independent of complement receptors CR1 and CR2. J Exp Med. 2000;192:1339–1351. doi: 10.1084/jem.192.9.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paul E, Pozdnyakova OO, Mitchell E, Carroll MC. Anti-DNA autoreactivity in C4-deficient mice. Eur J Immunol. 2002;32:2672–2679. doi: 10.1002/1521-4141(200209)32:9<2672::AID-IMMU2672>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 14.Kang YS, Do Y, Lee HK, Park SH, Cheong C, Lynch RM, Loeffler JM, Steinman RM, Park CG. A dominant complement fixation pathway for pneumococcal polysaccharides initiated by SIGN-R1 interacting with C1q. Cell. 2006;125:47–58. doi: 10.1016/j.cell.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 15.Wessels MR, Butko P, Ma M, Warren HB, Lage A, Carroll MC. Studies of group B streptococcal infection in mice deficient in complement C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc Natl Acad Sci USA. 1995;92:11490–11494. doi: 10.1073/pnas.92.25.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown JS, Hussell T, Gilliland SM, Holden DW, Paton JC, Ehrenstein MR, Walport MJ, Botto M. The classical pathway is the dominant complement pathway required for innate immunity to Streptococcus pneumoniae infection in mice. Proc Natl Acad Sci USA. 2002;99:16969–16974. doi: 10.1073/pnas.012669199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mold C, Rodic-Polic B, Du Clos TW. Protection from Streptococcus pneumoniae infection by C-reactive protein and natural antibody requires complement but not Fc gamma receptors. J Immunol. 2002;168:6375–6381. doi: 10.4049/jimmunol.168.12.6375. [DOI] [PubMed] [Google Scholar]
- 18.Rowe PC, McLean RH, Wood RA, Leggiadro RJ, Winkelstein JA. Association of homozygous C4B deficiency with bacterial meningitis. The Journal of infectious diseases. 1989;160:448–451. doi: 10.1093/infdis/160.3.448. [DOI] [PubMed] [Google Scholar]
- 19.Bishof NA, Welch TR, Beischel LS. C4B deficiency: a risk factor for bacteremia with encapsulated organisms. J Infect Dis. 1990;162:248–250. doi: 10.1093/infdis/162.1.248. [DOI] [PubMed] [Google Scholar]
- 20.Jaatinen T, Lahti M, Ruuskanen O, Kinos R, Truedsson L, Lahesmaa R, Lokki ML. Total C4B deficiency due to gene deletion and gene conversion in a patient with severe infections. Clin Diagn Lab Immunol. 2003;10:195–201. doi: 10.1128/CDLI.10.2.195-201.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haas KM, Blevins MW, High KP, Pang B, Swords WE, Yammani RD. Aging Promotes B-1b Cell Responses to Native, but Not Protein-Conjugated, Pneumococcal Polysaccharides: Implications for Vaccine Protection in Older Adults. J Infect Dis. 2014;209:87–97. doi: 10.1093/infdis/jit442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haas KM, Watanabe R, Matsushita T, Nakashima H, Ishiura N, Okochi H, Fujimoto M, Tedder TF. Protective and pathogenic roles for B cells during systemic autoimmunity in NZB/W F1 mice. J Immunol. 2010;184:4789–4800. doi: 10.4049/jimmunol.0902391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Haas KM. Programmed cell death 1 suppresses B-1b cell expansion and long-lived IgG production in response to T cell-independent type 2 antigens. J Immunol. 2011;187:5183–5195. doi: 10.4049/jimmunol.1101990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haas KM, Poe JC, Tedder TF. CD21/35 promotes protective immunity to Streptococcus pneumoniae through a complement-independent but CD19-dependent pathway that regulates PD-1 expression. J Immunol. 2009;183:3661–3671. doi: 10.4049/jimmunol.0901218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin JZ, Bell MK, Thorbecke GJ. Effect of various adjuvants on the antibody response of mice to pneumococcal polysaccharides. J Biol Response Mod. 1989;8:190–205. [PubMed] [Google Scholar]
- 27.Kowal C, Weinstein A, Diamond B. Molecular mimicry between bacterial and self antigen in a patient with systemic lupus erythematosus. Eur J Immunol. 1999;29:1901–1911. doi: 10.1002/(SICI)1521-4141(199906)29:06<1901::AID-IMMU1901>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 28.Putterman C, Limpanasithikul W, Edelman M, Diamond B. The double edged sword of the immune response: mutational analysis of a murine anti-pneumococcal, anti-DNA antibody. J Clin Invest. 1996;97:2251–2259. doi: 10.1172/JCI118666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chowdhry IA, Kowal C, Hardin J, Zhou Z, Diamond B. Autoantibodies that bind glomeruli: cross-reactivity with bacterial antigen. Arthritis Rheum. 2005;52:2403–2410. doi: 10.1002/art.21143. [DOI] [PubMed] [Google Scholar]
- 30.Ray SK, Putterman C, Diamond B. Pathogenic autoantibodies are routinely generated during the response to foreign antigen: a paradigm for autoimmune disease. Proc Natl Acad Sci USA. 1996;93:2019–2024. doi: 10.1073/pnas.93.5.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diamond B, Scharff MD. Somatic mutation of the T15 heavy chain gives rise to an antibody with autoantibody specificity. Proc Natl Acad Sci USA. 1984;81:5841–5844. doi: 10.1073/pnas.81.18.5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Price KE, Camilli A. Pneumolysin localizes to the cell wall of Streptococcus pneumoniae. J Bacteriol. 2009;191:2163–2168. doi: 10.1128/JB.01489-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erridge C, Kennedy S, Spickett CM, Webb DJ. Oxidized phospholipid inhibition of toll-like receptor (TLR) signaling is restricted to TLR2 and TLR4: roles for CD14, LPS-binding protein, and MD2 as targets for specificity of inhibition. J Biol Chem. 2008;283:24748–24759. doi: 10.1074/jbc.M800352200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roy B, Brennecke AM, Agarwal S, Krey M, Duber S, Weiss S. An intrinsic propensity of murine peritoneal B1b cells to switch to IgA in presence of TGF-beta and retinoic acid. PLoS One. 2013;8:e82121. doi: 10.1371/journal.pone.0082121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Genestier L, Taillardet M, Mondiere P, Gheit H, Bella C, Defrance T. TLR agonists selectively promote terminal plasma cell differentiation of B cell subsets specialized in thymus-independent responses. J Immunol. 2007;178:7779–7786. doi: 10.4049/jimmunol.178.12.7779. [DOI] [PubMed] [Google Scholar]
- 36.Zhong X, Lau S, Bai C, Degauque N, Holodick NE, Steven SJ, Tumang J, Gao W, Rothstein TL. A novel subpopulation of B-1 cells is enriched with autoreactivity in normal and lupus-prone mice. Arthritis Rheum. 2009;60:3734–3743. doi: 10.1002/art.25015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Enghard P, Humrich JY, Chu VT, Grussie E, Hiepe F, Burmester GR, Radbruch A, Berek C, Riemekasten G. Class switching and consecutive loss of dsDNA-reactive B1a B cells from the peritoneal cavity during murine lupus development. Eur J Immunol. 2010;40:1809–1818. doi: 10.1002/eji.200940050. [DOI] [PubMed] [Google Scholar]
- 38.Sen G, Khan AQ, Chen Q, Snapper CM. In vivo humoral immune responses to isolated pneumococcal polysaccharides are dependent on the presence of associated TLR ligands. J Immunol. 2005;175:3084–3091. doi: 10.4049/jimmunol.175.5.3084. [DOI] [PubMed] [Google Scholar]
- 39.Schroder NW, Morath S, Alexander C, Hamann L, Hartung T, Zahringer U, Gobel UB, Weber JR, Schumann RR. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J Biol Chem. 2003;278:15587–15594. doi: 10.1074/jbc.M212829200. [DOI] [PubMed] [Google Scholar]
- 40.Tomlinson G, Chimalapati S, Pollard T, Lapp T, Cohen J, Camberlein E, Stafford S, Periselneris J, Aldridge C, Vollmer W, Picard C, Casanova JL, Noursadeghi M, Brown J. TLR-Mediated Inflammatory Responses to Streptococcus pneumoniae Are Highly Dependent on Surface Expression of Bacterial Lipoproteins. J Immunol. 2014;193:3736–3745. doi: 10.4049/jimmunol.1401413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lartigue A, Colliou N, Calbo S, Francois A, Jacquot S, Arnoult C, Tron F, Gilbert D, Musette P. Critical role of TLR2 and TLR4 in autoantibody production and glomerulonephritis in lpr mutation-induced mouse lupus. J Immunol. 2009;183:6207–6216. doi: 10.4049/jimmunol.0803219. [DOI] [PubMed] [Google Scholar]
- 42.Wen Z, Xu L, Chen X, Xu W, Yin Z, Gao X, Xiong S. Autoantibody induction by DNA-containing immune complexes requires HMGB1 with the TLR2/microRNA-155 pathway. J Immunol. 2013;190:5411–5422. doi: 10.4049/jimmunol.1203301. [DOI] [PubMed] [Google Scholar]
- 43.Kim DH, Lee JC, Kim S, Oh SH, Lee MK, Kim KW, Lee MS. Inhibition of autoimmune diabetes by TLR2 tolerance. J Immunol. 2011;187:5211–5220. doi: 10.4049/jimmunol.1001388. [DOI] [PubMed] [Google Scholar]
- 44.Filippi CM, Ehrhardt K, Estes EA, Larsson P, Oldham JE, von Herrath MG. TLR2 signaling improves immunoregulation to prevent type 1 diabetes. Eur J Immunol. 2011;41:1399–1409. doi: 10.1002/eji.200939841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diana J, Simoni Y, Furio L, Beaudoin L, Agerberth B, Barrat F, Lehuen A. Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat Med. 2013;19:65–73. doi: 10.1038/nm.3042. [DOI] [PubMed] [Google Scholar]
- 46.Limpanasithikul W, Ray S, Diamond B. Cross-reactive antibodies have both protective and pathogenic potential. J Immunol. 1995;155:967–973. [PubMed] [Google Scholar]
- 47.Ehrenstein MR, Katz DR, Griffiths MH, Papadaki L, Winkler TH, Kalden JR, Isenberg DA. Human IgG anti-DNA antibodies deposit in kidneys and induce proteinuria in SCID mice. Kidney Int. 1995;48:705–711. doi: 10.1038/ki.1995.341. [DOI] [PubMed] [Google Scholar]
- 48.Ohnishi K, Ebling FM, Mitchell B, Singh RR, Hahn BH, Tsao BP. Comparison of pathogenic and non-pathogenic murine antibodies to DNA: antigen binding and structural characteristics. Int Immunol. 1994;6:817–830. doi: 10.1093/intimm/6.6.817. [DOI] [PubMed] [Google Scholar]
- 49.Witte T, Hartung K, Sachse C, Matthias T, Fricke M, Deicher H, Kalden JR, Lakomek HJ, Peter HH, Schmidt RE. IgM anti-dsDNA antibodies in systemic lupus erythematosus: negative association with nephritis. SLE Study Group. Rheumatol Int. 1998;18:85–91. doi: 10.1007/s002960050063. [DOI] [PubMed] [Google Scholar]
- 50.Werwitzke S, Trick D, Kamino K, Matthias T, Kniesch K, Schlegelberger B, Schmidt RE, Witte T. Inhibition of lupus disease by anti-double-stranded DNA antibodies of the IgM isotype in the (NZB × NZW)F1 mouse. Arthritis Rheum. 2005;52:3629–3638. doi: 10.1002/art.21379. [DOI] [PubMed] [Google Scholar]
- 51.Gronwall C, Akhter E, Oh C, Burlingame RW, Petri M, Silverman GJ. IgM autoantibodies to distinct apoptosis-associated antigens correlate with protection from cardiovascular events and renal disease in patients with SLE. Clin Immunol. 2012;142:390–398. doi: 10.1016/j.clim.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Villalta D, Bizzaro N, Bassi N, Zen M, Gatto M, Ghirardello A, Iaccarino L, Punzi L, Doria A. Anti-dsDNA antibody isotypes in systemic lupus erythematosus: IgA in addition to IgG anti-dsDNA help to identify glomerulonephritis and active disease. PLoS One. 2013;8:e71458. doi: 10.1371/journal.pone.0071458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Witte T, Hartung K, Matthias T, Sachse C, Fricke M, Deicher H, Kalden JR, Lakomek HJ, Peter HH, Schmidt RE. Association of IgA anti-dsDNA antibodies with vasculitis and disease activity in systemic lupus erythematosus. SLE Study Group. Rheumatol Int. 1998;18:63–69. doi: 10.1007/s002960050059. [DOI] [PubMed] [Google Scholar]
- 54.Miltenburg AM, Roos A, Slegtenhorst L, Daha MR, Breedveld FC. IgA anti-dsDNA antibodies in systemic lupus erythematosus: occurrence, incidence and association with clinical and laboratory variables of disease activity. J Rheumatol. 1993;20:53–58. [PubMed] [Google Scholar]
- 55.Montinaro V, Esparza AR, Cavallo T, Rifai A. Antigen as mediator of glomerular injury in experimental IgA nephropathy. Lab Invest. 1991;64:508–519. [PubMed] [Google Scholar]
- 56.Grayzel A, Solomon A, Aranow C, Diamond B. Antibodies elicited by pneumococcal antigens bear an anti-DNA--associated idiotype. J Clin Invest. 1991;87:842–846. doi: 10.1172/JCI115088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Elkayam O, Paran D, Caspi D, Litinsky I, Yaron M, Charboneau D, Rubins JB. Immunogenicity and safety of pneumococcal vaccination in patients with rheumatoid arthritis or systemic lupus erythematosus. Clin Infect Dis. 2002;34:147–153. doi: 10.1086/338043. [DOI] [PubMed] [Google Scholar]
- 58.Klippel JH, Karsh J, Stahl NI, Decker JL, Steinberg AD, Schiffman G. A controlled study of pneumococcal polysaccharide vaccine in systemic lupus erythematosus. Arthritis Rheum. 1979;22:1321–1325. doi: 10.1002/art.1780221201. [DOI] [PubMed] [Google Scholar]
- 59.Welch TR, Berry A, Beischel LS. C4 isotype deficiency in IgA nephropathy. Pediatr Nephrol. 1987;1:136–139. doi: 10.1007/BF00849283. [DOI] [PubMed] [Google Scholar]


