Abstract
Acoustic complexity of a stimulus has been shown to modulate the electromagnetic N1 (latency ~110 ms) and P2 (latency ~190 ms) auditory evoked responses. We compared the relative sensitivity of electroencephalography (EEG) and magnetoencephalography (MEG) to these neural correlates of sensation. Simultaneous EEG and MEG were recorded while participants listened to three variants of a piano tone. The piano stimuli differed in their number of harmonics: the fundamental frequency (f0), only,or f0 and the first two or eight harmonics. The root mean square (RMS) of the amplitude of P2 but not N1 increased with spectral complexity of the piano tones in EEG and MEG. The RMS increase for P2 was more prominent in EEG than MEG, suggesting important radial sources contributing to the P2 only in EEG. Source analysis revealing contributions from radial and tangential sources was conducted to test this hypothesis. Source waveforms revealed a significant increase in the P2 radial source amplitude in EEG with increased spectral complexity of piano tones. The P2 of the tangential source waveforms also increased in amplitude with increased spectral complexity in EEG and MEG. The P2 auditory evoked response is thus represented by both tangential (gyri) and radial (sulci) activities. The radial contribution is expressed preferentially in EEG, highlighting the importance of combining EEG with MEG where complex source configurations are suspected.
Keywords: EEG, MEG, Auditory cortex, Source analysis, Spectral complexity
Introduction
There is a growing interest in combining various imaging modalities to investigate perceptual and cognitive functions in humans. For instance, in theory the combination of EEG (electroencephalography) and MEG (magnetoencephalography) (for detailed review see [1, 2]), which measure electrical and magnetic signals respectively, should provide complementary information that can help identify the neural correlates underlying a particular brain function [3]. However, EEG and MEG are seldom used simultaneously or compared directly in the same subjects.
There are several similarities and differences between EEG and MEG that have been discussed thoroughly in prior work [4–8]. The differences are briefly highlighted here. Because the EEG signal is highly dependent on volume conduction, the various head tissues (brain, cerebrospinal fluid, skull and scalp) will cause smearing of the EEG signal at tissue boundaries. MEG signal on the other hand is less affected by volume conduction, especially for isotropic media where the contribution from secondary volume currents diminishes completely [1]. Therefore, MEG has a better signal-to-noise ratio than EEG and, in general, better source localization accuracy [7, 9]. Another important difference between the two imaging modalities is that radial sources (i.e. from gyri) do not contribute much to the MEG signal, whereas these sources sum with other activities and are recorded in EEG [2]. This can be advantageous or disadvantageous depending on the objective of the measurement. For example, if we are mainly interested in tangential sources (i.e. from sulci), the MEG signal will reveal these sources uncontaminated by radial sources, hence increasing source localization accuracy. However, at the same time, the interpretation of MEG data would be limited to sulci and may miss important contributions from gyri to the particular process under study. There is accumulating evidence indicating that several auditory tasks generate activity in the superior temporal plane and lateral surface of the superior temporal gyrus that can only be modeled with both tangential and radial sources [10–12].
Recently, Shahin et al. [13] measured EEG and MEG simultaneously while participants were presented with three variants of a C4 piano tone and a pure tone of the C4 fundamental frequency (f0). They found increased amplitude of the scalp measured P2 (~200 ms) but not the N1 (~100 ms) auditory evoked response (AER) with increased spectral complexity of piano tones. This P2 enhancement was more pronounced in EEG compared to MEG, suggesting contributions from radial sources that are not detected by MEG but sum up at the scalp with tangential sources in EEG. The current study examined whether the combination of EEG and MEG can provide meaningful differences that add to our understanding of the neural sources underlying the N1 and P2 waves. Specifically, we aimed to clarify whether the enhanced P2 auditory evoked response in EEG compared to MEG reported for spectrally complex piano sounds by Shahin et al. [13] can be accounted for by the presence of radial sources detected in EEG. Previous studies have shown imaging modality effects (differences and similarities between EEG and MEG) when investigating the N1 and P2 responses [3, 14–16]. Neukirch et al. [3] showed that the N1 in EEG increased in amplitude and localized deeper as intensity of sounds increased, while the N1 in MEG localized more lateral with increased intensity of sounds and increased in amplitude initially and tended to plateau for the highest intensities. They hypothesized that secondary current sources generated by deep sources led to the effect of higher intensities on the N1. In another study, Siedenberg et al. [15] showed that activities of the N1 and P2 were highly reproducible in both imaging modalities but MEG was less effective than EEG in revealing the later N2 and P3 components. The N2 and P3 differ from the N1 and P2 activity in that they may reflect endogenous cognitive processes (N2: [17], P3: [18]) likely attributed to a distributed brain activity.
In the present study we modeled N1 and P2 responses evoked by piano sounds of increasing spectral complexity and measured simultaneously by EEG and MEG with one regional source [19] in each hemisphere. A regional source in MEG contains two orthogonal vectors which are both tangential to the scalp (T1 and T2, Fig. 1A). A regional source in EEG contains the same tangential vectors as in MEG and an additional radial vector (R, Fig. 1A). Each of the above vectors is usually represented by a trace depicting its amplitude as a function of time termed “source waveform”. We hypothesized that the P2 of the tangential sources will exhibit comparable enhancement in EEG and MEG with increased spectral complexity of tones while the P2 of the radial source, detected in EEG only, will also exhibit enhancement with increased spectral complexity. The summation of the radial and tangential activities at the scalp will render larger differences of P2 amplitudes occurring in EEG than MEG as spectral complexity is increased. The data of Shahin et al. [13] were used, which were subjected to further analysis and new results reported here.
Fig. 1.
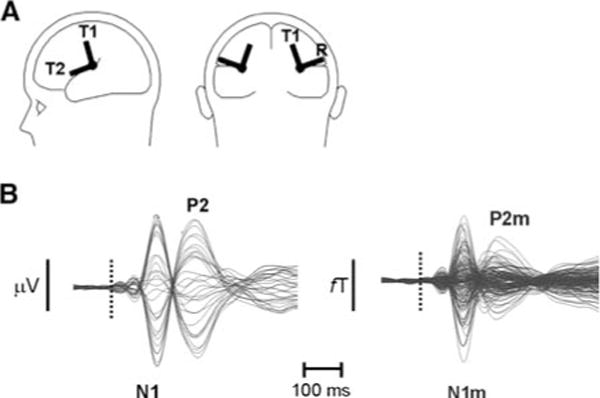
(A) Depiction of regional sources. Each regional source consists of two or three dipoles, one radial (R, EEG only) and two tangential (T1 and T2, EEG & MEG). (B) Example of auditory evoked potential (overlay of 32 traces) and field (overlay of 151 traces) for one subject, depicting the N1/P2 and N1m/P2m, respectively. Dotted line depicts the sound onset
Methods
Participants
Sixteen right-handed volunteers (age 28 ± 6 years; 4 females) with normal hearing participated in the study. Participants gave written informed consent in accordance with the guidelines of the Research Ethics Committee of Baycrest.
Stimuli
The stimuli consisted of four tones (500 ms in duration): (1) a C4 piano tone containing the fundamental frequency (f0 = 262 Hz) and eight harmonics (termed “piano”) or (2) f0 and the first two harmonics (termed “piano2”) or (3) f0 with no harmonics (termed “piano0”) and (4) a pure sine tone with only f0 (termed “pure”). Stimuli were presented binaurally through plastic tubes at 60 dB SL above each participant’s audiometric threshold, while participants watched a silent movie. Eight runs (2 for each tone type), each containing 60 stimuli of the same tone type were presented in a fixed order for all subjects. Tones were presented with a variable interstimulus interval between 3 s and 4 s offset to onset.
Recording and Analyses
Auditory evoked potentials and fields were recorded simultaneously using a CTF System EEG amplifier (32 Ag/AgCl Easy Cap, 10–20 system) and 151-channel whole head MEG system (VSM MedTech, British Columbia, Canada). EEG channels were referenced to Cz and grounded at the collar bone. EEG and MEG were sampled at 312.5 Hz, DC to 100-Hz.
Continuous EEG and MEG files for each subject were loaded into Brain Electrical Source Analysis (BESA) software (version 5.1, MEGIS Gräfelfing—Germany), digitally filtered between 0.1 Hz and 20 Hz, and divided into epochs of 600 ms duration including a 100-ms pre-stimulus baseline interval. Trials containing amplitude shifts in any channel greater than ±200 μV in EEG were rejected. The same trials were rejected in MEG. Accepted trials (mean 86%, range 75–98%) were re-referenced to an average reference (EEG only) and averaged according to stimulus type within each run. Figure 1B shows an example of average waveforms in EEG (left) and MEG (right) for one subject depicting the evoked N1(m) and P2(m). Two subsequent analyses were performed on these averages, root mean square (RMS) and source analysis (SA).
Root Mean Square (RMS)
Root mean square waveforms were calculated for each subject’s run in Matlab (v. 6.0). The N1(m) peak amplitude was defined as the RMS maximum during the interval 80–130 ms after stimulus onset. The P2(m) peak was determined as the RMS maximum during the interval 170 and 220 ms after stimulus onset. The N1(m) and P2(m) RMS values were averaged across runs of the same tone type. In a subsequent step the N1(m) and P2(m) RMS values of music tones were normalized to the RMS of pure tone. Normalization was calculated as the difference between the amplitude of the component for the specific stimulus type minus the amplitude of the component for pure tone divided by the amplitude of the component for pure tone [e.g. (RMS P2 piano—RMS P2 pure)/RMS P2 pure]. This standardization was done to filter out the effect of the fundamental frequency (pure tone had the same fundamental as the other tones) from the final result.
Source Analysis
First, source locations of the N1(m) and P2(m) in EEG and MEG were obtained by way of the inverse solution [20, 21], as implemented in BESA. A spherical head model is used. Two regional sources (one for each hemisphere) were used to fit separately the N1(m) and P2(m) waves for each individual’s run average waveforms. Source fits were constrained to the window of ±16 ms around the N1(m) or P2(m) peak. The average goodness of fit exceeded 80% for both EEG and MEG data. Second, the x–y–z-coordinates obtained from the individual fits were averaged across tone types and participants to construct separate models for each EEG and MEG component (N1, N1m, P2, P2m). Third, the EEG and MEG models were then applied back (as spatial filters) onto the averaged EEG and MEG data for each subject to obtain the corresponding N1(m) and P2(m) source waveforms. The orientations of the tangential components of the regional sources were held constant across subjects and for EEG and MEG fits. The radial component in EEG was held constant across subjects.
The source waveforms were averaged across the two runs for each stimulus type. The N1(m) and P2(m) peak amplitudes were defined for the same time windows as in the RMS analysis reported above. The two tangential components for N1(m) and P2(m) were collapsed into one value by calculating their vector norm (sqrt {T12 + T22}). In a subsequent step the N1(m) and P2(m) dipole moments of music tones were normalized to the dipole moments of pure tone (similar to the RMS normalization discussed above).
Statistical Analyses
All tests were two-tailed (α = 0.05) and corrected for sphericity violations (Greenhouse-Geisser) where appropriate.
Root mean square amplitudes were evaluated separately for EEG and MEG by an ANOVA (Statistica Version 6.0; Statsoft Inc, Tulsa, OK) with stimulus type (piano0, piano2, piano) as the only factor.
Source locations for the N1(m) and P2(m) were evaluated separately using ANOVAs with modality (EEG/MEG), tone type and coordinate (x, y, z) as factors. Effects of spectral complexity on the N1(m) and P2(m) dipole moments (vector norm of N1(m) and P2(m)) were evaluated separately for EEG and MEG by ANOVAs with factors stimulus type, hemisphere, and—for EEG only—component (Tangential/Radial). For the EEG data, a second ANOVA of the N1 and P2 dipole moments was done for the radial and tangential (vector norm of T1 and T2) components separately to test whether results were significant even when segregating the two components.
Results
Source Locations
The mean N1(m) or P2(m) sources were very close in location when measured either by EEG or MEG and did not differ between tone types for each component. All sources localized to the supratemporal plane as shown in Fig. 2A. Average Talairach coordinates for N1(m) and P2(m) in mm (x (Medial–Lateral) = 38, y (Anterior–Posterior) = −15, z (Inferior–Superior) = 14) were consistent with sources generated in or near the primary auditory cortex [10, 11, 22]. There was no main effect of tone type on the location of the N1(m) or P2(m). However, N1(m) sources localized more anterior (F(1, 15) = 4.7; P < 0.05) and lateral (F(1, 15) = 4.5; P < 0.05) in the right hemisphere compared to the left hemisphere. Also the N1 localized more medial than its magnetic counterpart the N1m (F(1, 15) = 8.1; P = 0.02). Figure 2B shows the between-subject mean standard deviation values (collapsing over tones and hemispheres) for source locations in the x (medial–lateral), y (anterior–posterior) and z (inferior–superior) coordinates. There was on average less between-subject-variability of source locations in MEG than EEG.
Fig. 2.
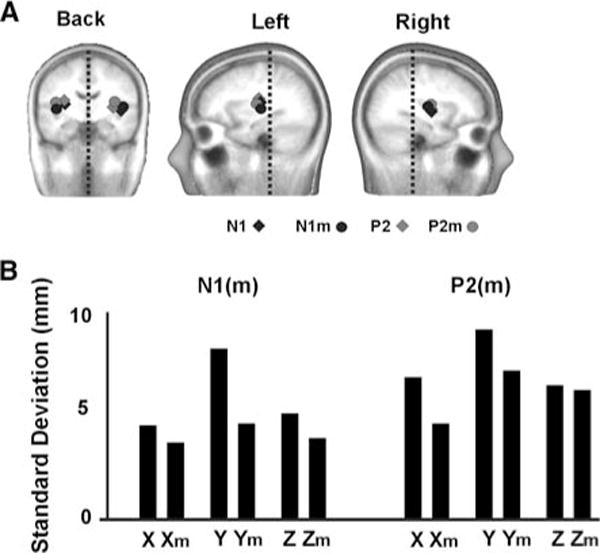
(A) Source locations for the N1, N1m, P2, P2m auditory evoked responses. Dotted lines are drawn for symmetry reference. (B) Between subject mean standard deviation values (collapsing over tones and hemispheres) for source locations in the x (medial–lateral), y (anterior–posterior) and z (inferior–posterior). m (as in Xm) denotes the magnetic value
Root Mean Square (RMS)
Figure 3A shows the N1(m) (mean latency ~109 ms) and P2(m) (mean latency ~196 ms) peak RMS amplitudes evoked by the piano0, piano2 and piano tones. A main effect of stimulus type showed that P2 responses increased in amplitude with increased spectral complexity of tones in EEG and MEG. However, this effect was much greater in EEG (F(2, 30) = 26.0, P < 0.0001) than MEG (F(2, 30) = 4.4, P < 0.03). When the above contrasts were based on normalized RMS values, the P2 still increased in amplitude with increased spectral complexity of tones for EEG (F(2, 30) = 18.3, P < 0.0001) and approached but did not reach significance for MEG (F(2, 30) = 2.3, P < 0.15). N1(m) did not show significance with increased tone spectral complexity.
Fig. 3.
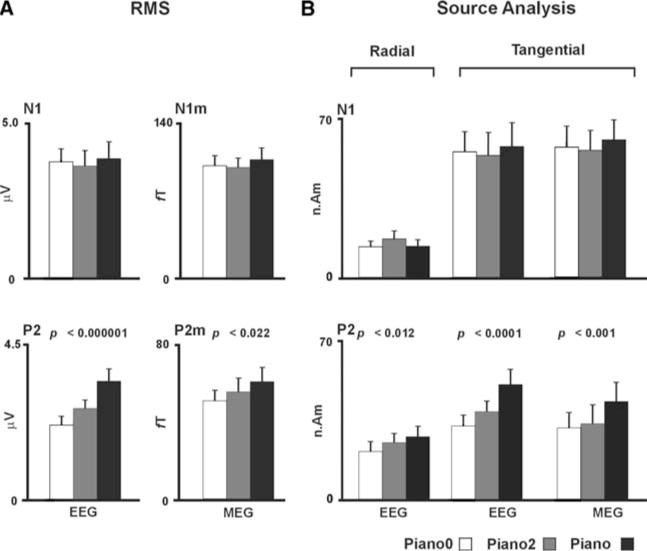
(A) Grand mean (n = 16) of N1 (top) and P2 (bottom) RMS values and error bars (one standard error) for piano0, piano2 and piano tones in EEG and MEG. (B) The grand mean (n = 16) of the N1 (top) and P2 (bottom) dipole moments for the radial (EEG only) and tangential components and error bars (one standard error) for piano0, piano2 and piano tones in EEG and MEG
Dipole Moments
Figure 3B shows the magnitude of the dipole moments for the radial and tangential components of the N1(m) and P2(m) response. Main effect of stimulus type showed that P2(m) dipole moments for the radial (EEG only) and tangential sources increased in amplitude with increased spectral complexity (P2: F(2, 30) = 32.5, P < 0.0001; P2m: F(2, 30) = 9.24, P < 0.001). There was a component main effect due to larger tangential sources compared to radial sources for the P2 (F(1, 15) = 17.3, P < 0.001), and also an interaction between component and tone (F(2, 30) = 7.6, P < 0.003) attributed to a greater enhancement of P2 of the piano tone compared to P2 of piano2 or piano0 occurring for the tangential compared to the radial component (P < 0.001 or better; LSD test). There was a hemisphere main effect for the P2m, where larger P2m dipole moments occurred in the left than the right hemisphere (F(1, 15) = 8.1, P < 0.01). Effect of stimulus type was significant even when the EEG radial and tangential P2 components were evaluated separately (P = 0.012 or better). When contrasts were based on normalized dipole moments, the P2 still increased in amplitude with increased spectral complexity of tones for EEG (P < 0.012 or better) and approached but did not reach significance for MEG (P < 0.1). N1(m) dipoles did not show significance with increased spectral complexity. However, an effect of component occurred (F(1, 15) = 57.0, P < 0.0001) for the N1 in the EEG contrast, which was attributed to larger N1 dipole moments occurring for the tangential compared to the radial components.
Discussion
The current results reveal that the greater P2 enhancement as a function of spectral complexity observed in EEG compared to MEG is partly due to increased activity of the radial sources, which are not detected by MEG. Previous studies investigating the N1(m) and P2(m) did not reveal a radial contribution [13, 23] related to the spectral complexity of sounds.
The N1 and P2 dipoles localized to the supratemporal plane, consistent with generators in the primary and non-primary auditory cortices [10, 11, 16, 22, 24–26]. It should be noted that the N1 and P2 activities are likely generated by several cortical sources and were not confined to one point as reported here. For example, a recent study by Yvert et al. [27], where distributed source model was used to localize the N1 activity applied to intracranial recordings, suggested a more distributed activity for the N1 wave, which spanned Heschl’s gyrus and sulcus, planum temporale, and superior temporal gyrus. Discrete modeling of these activities (used here) may reflect the location of the center of mass of all generators, biased toward the predominant contributor. Source analysis of the current data generally showed consistency between the two imaging modalities. EEG and MEG both revealed hemispheric asymmetries for auditory activities. The N1 and N1m occurring in the right hemisphere exhibited more anterior and lateral locations than that of the left hemisphere, reflecting documented asymmetries of an anterior shift of the right compared to the left auditory cortex in anatomical [28], MR [29] and MEG [30] studies and a lateral shift of the right compared to the left auditory cortex in anatomical [28], and MEG [30] findings. Also, in general MEG showed slightly less between-subject-variability of source locations. This observation should be interpreted with caution, given that the sources were not co-registered on the anatomy of individual subjects. Increased subject variability of source locations in EEG compared to MEG may reflect the reduced signal-to-noise ratio of the auditory signal in EEG due to volume conduction or overlap with additional radial and/or deep sources.
The question of how different components (radial or tangential) of the N1(m) and P2(m) waves vary with task or type of stimuli is noteworthy because both of these waves have been reported to reflect perceptual significance and are influenced by learning [12, 13, 23, 31–34]. For example, the N1 and P2 morphologies have been shown to reflect the stages of brain development [35, 36] where the N1 and P2 appear to develop after age 4–5, continue to increase to about age 10–12, and decrease thereafter until they plateau at age 18–19. Shahin et al. [36] reported that the N1 response at age 4–5 years is best accounted for by a tangential dipole, while the P2 response is mainly radial and lateral to the N1 source at this particular age, suggesting activity in the lateral portion of the auditory cortex early in life. As the brain matures, the orientation of the sources underlying the P2 wave appears to change, with the tangential component being the major contributor to the scalp recorded P2 wave in young and older adults. These observations suggest that tangential and radial components of the N1 and P2 waves may follow different developmental trajectories and express distinctive functional properties, including sensitivity to remodeling by acoustic experience or the enhanced sensitivity of P2 radial and tangential sources to the spectral complexity of sounds documented here. Although we did not attempt to localize the tangential and radial activities separately (in regional source the radial and tangential components are bound together), it is likely that the tangential activities reflect sources originating in or near Heschl’s gyrus. The tangential component may index the initial processing of acoustical cues such as sound onset and pitch. In contrast, the radial sources may represent activity arising from the gyri. That is, increased sound complexity may recruit wider areas of the auditory cortex that extend to the lateral portion of the superior temporal gyrus. This interpretation is in line with neurophysiological studies in animal data showing that the lateral belt of the non-primary auditory cortex responds preferentially to complex sounds—such as animal calls—compared to pure tones which are preferentially processed in the core regions of the auditory cortex [37, 38].
Differences intrinsic to EEG and MEG are informative with respect to the circumstances under which one method may be preferable to another. The issue of localization accuracy [7, 8, 39, 40] of the two methods is particularly important for specific clinical applications, such as localizing epileptic discharges prior to surgery. However, the intention is also to achieve an understanding of how brain processes—perceptual or cognitive—may vary with a specific stimulus or task while maintaining as accurate spatial information as possible. Our results indicate that when auditory stimuli become more complex, such as in music perception, information pertaining to the P2 auditory evoked response is better revealed when using EEG. Therefore, our findings suggest that MEG should be combined with EEG when investigating tasks relating to complex sounds.
Acknowledgments
This research was supported by grants from the Canadian Institutes of Health Research, the Natural Sciences and Engineering Research Council of Canada, and the International Foundation for Music Research. We wish to thank Dr. Christo Pantev, Dr. Bernhard Ross, Dr. Laurel Trainor and Kristina Backer for helpful suggestions.
Contributor Information
Antoine J. Shahin, Email: ajshahin@ucdavis.edu, UC Davis Center for Mind and Brain, 267 Cousteau Place, Davis, CA 95618, USA Rotman Research Institute of Baycrest, Toronto, ON, Canada.
Larry E. Roberts, Department of Psychology, Neuroscience, and Behavior, McMaster University, Hamilton, ON, Canada
Lee M. Miller, UC Davis Center for Mind and Brain, 267 Cousteau Place, Davis, CA 95618, USA
Kelly L. McDonald, Rotman Research Institute of Baycrest, Toronto, ON, Canada
Claude Alain, Rotman Research Institute of Baycrest, Toronto, ON, Canada; Department of Psychology, University of Toronto, Toronto, ON, Canada.
References
- 1.Hämäläinen M, Hari R, Ilmoniemi RJ, Kunuutila J, Lounasmaa OV. Magnetoencephalography: theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev Modern Phys. 1993;65:413–97. [Google Scholar]
- 2.Nunez PL, Srinivasan R. Electric fields of the brain: the neurophysics of EEG. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 3.Neukirch M, Hegerl U, Kotitz R, Dorn H, Gallinat J, Herrmann WM. Comparison of the amplitude/intensity function of the auditory evoked N1m and N1 components. Neuropsychobiology. 2002;45:41–8. doi: 10.1159/000048672. [DOI] [PubMed] [Google Scholar]
- 4.Cuffin BN, Cohen D. Comparison of the magnetoencephalogram and electroencephalogram. Electroencephalogr Clin Neurophysiol. 1979;47:132–46. doi: 10.1016/0013-4694(79)90215-3. [DOI] [PubMed] [Google Scholar]
- 5.Cohen D, Cuffin BN. Demonstration of useful differences between magnetoencephalogram and electroencephalogram. Electroencephalogr Clin Neurophysiol. 1983;56:38–51. doi: 10.1016/0013-4694(83)90005-6. [DOI] [PubMed] [Google Scholar]
- 6.Phillips JW, Leahy RM, Mosher JC, Timsari B. Imaging neural activity using MEG and EEG. IEEE Eng Med Biol Mag. 1997;16:34–42. doi: 10.1109/51.585515. [DOI] [PubMed] [Google Scholar]
- 7.Barkley GL. Controversies in neurophysiology. MEG is superior to EEG in localization of interictal epileptiform activity. Pro Clin Neurophysiol. 2004;115:1001–9. doi: 10.1016/j.clinph.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Baumgartner C. Controversies in clinical neurophysiology. MEG is superior to EEG in the localization of interictal epileptiform activity. Con Clin Neurophysiol. 2004;115:1010–20. doi: 10.1016/j.clinph.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Leahy RM, Mosher JC, Spencer ME, Huang MX, Lewine JD. A study of dipole localization accuracy for MEG and EEG using a human skull phantom. Electroencephalogr Clin Neurophysiol. 1998;107:159–73. doi: 10.1016/s0013-4694(98)00057-1. [DOI] [PubMed] [Google Scholar]
- 10.Scherg M, Vajsar J, Picton TW. A source analysis of the late human auditory evoked potentials. J Cogn Neurosci. 1989;1:336–55. doi: 10.1162/jocn.1989.1.4.336. [DOI] [PubMed] [Google Scholar]
- 11.Picton TW, Alain C, Woods DL, John MS, Scherg M, Valdes-Sosa P, Bosch-Bayard J, Trujillo NJ. Intracerebral sources of human auditory evoked potentials. Audiol Neurootol. 1999;4:64–79. doi: 10.1159/000013823. [DOI] [PubMed] [Google Scholar]
- 12.Shahin A, Bosnyak DJ, Trainor LJ, Roberts LE. Enhancement of neuroplastic P2 and N1c auditory evoked potentials in skilled musicians. J Neurosci. 2003;23:5545–52. doi: 10.1523/JNEUROSCI.23-13-05545.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shahin A, Roberts LE, Pantev C, Trainor LJ, Ross B. Modulation of P2 auditory-evoked responses by the spectral complexity of musical sounds. Neuroreport. 2005;16:1781–5. doi: 10.1097/01.wnr.0000185017.29316.63. [DOI] [PubMed] [Google Scholar]
- 14.Pantev C, Bertrand O, Eulitz C, Verkindt C, Hampson S, Schuierer G, Elbert T. Specific tonotopic organizations of different areas of the human auditory cortex revealed by simultaneous magnetic and electric recordings. Electroencephalogr Clin Neurophysiol. 1995;94:26–40. doi: 10.1016/0013-4694(94)00209-4. [DOI] [PubMed] [Google Scholar]
- 15.Siedenberg R, Goodin DS, Aminoff MJ, Rowley HA, Roberts TP. Comparison of late components in simultaneously recorded event-related electrical potentials and event-related magnetic fields. Electroencephalogr Clin Neurophysiol. 1996;99:191–7. doi: 10.1016/0013-4694(96)95215-3. [DOI] [PubMed] [Google Scholar]
- 16.Huotilainen M, Winkler I, Alho K, Escera C, Virtanen J, Ilmoniemi RJ, Jääskeläinen IP, Pekkonen E, Näätänen R. Combined mapping of human auditory EEG and MEG responses. Electroencephalogr Clin Neurophysiol. 1998;108:370–9. doi: 10.1016/s0168-5597(98)00017-3. [DOI] [PubMed] [Google Scholar]
- 17.Surwillo WW. Cortical evoked potentials in monozygotic twins and unrelated subjects: comparisons of exogenous and endogenous components. Behav Genet. 1980;10:201–9. doi: 10.1007/BF01066270. [DOI] [PubMed] [Google Scholar]
- 18.Picton TW. The P300 wave of the human event-related potential. J Clin Neurophysiol. 1992;9:456–79. doi: 10.1097/00004691-199210000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Scherg M. Fundamentals of dipole source potential analysis. In: Grandori F, Hoke M, Romani GL, editors. Auditory evoked magnetic fields and electric potentials. Advances in audiology. Vol. 6. Basel: Karger; 1990. pp. 40–69. [Google Scholar]
- 20.Helmholz H. Ueber einiger gezetze der vertailung elektrischer strome in korperlichen leiter mit anwendungauf die thierishch electrischeb versuche. Pogg Ann Physik Chemie. 1853;33:353–77. [Google Scholar]
- 21.Oosterom AV. History and evaluation of methods for solving the inverse problem. J Clin Neurophysiol. 1991;8:371–80. doi: 10.1097/00004691-199110000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Lütkenhöner B, Steinstrater O. High-precision neuromagnetic study of the functional organization of the human auditory cortex. Audiol Neurootol. 1998;3:191–213. doi: 10.1159/000013790. [DOI] [PubMed] [Google Scholar]
- 23.Seither-Preisler A, Krumbholz K, Lütkenhöner B. Sensitivity of the neuromagnetic N100m deflection to spectral bandwidth: a function of the auditory periphery? Audiol Neurootol. 2003;8:322–37. doi: 10.1159/000073517. [DOI] [PubMed] [Google Scholar]
- 24.Hari R, Pelizzone M, Mäkelä JP, Hallstrom J, Leinonen L, Lounasmaa OV. Neuromagnetic responses of the human auditory cortex to on- and offsets of noise bursts. Audiology. 1987;26:31–43. doi: 10.3109/00206098709078405. [DOI] [PubMed] [Google Scholar]
- 25.Pantev C, Eulitz C, Hampson S, Ross B, Roberts LE. The auditory evoked “off” response: sources and comparison with the “on” and the “sustained” responses. Ear Hear. 1996;17:255–65. doi: 10.1097/00003446-199606000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Engelien A, Schulz M, Ross B, Arolt V, Pantev C. A combined functional in vivo measure for primary and secondary auditory cortices. Hear Res. 2000;148:153–60. doi: 10.1016/s0378-5955(00)00148-9. [DOI] [PubMed] [Google Scholar]
- 27.Yvert B, Fischer C, Bertrand O, Pernier J. Localization of human supratemporal auditory areas form intracerebral auditory evoked potentials using distributed source models. Neuroimage. 2005;28:140–53. doi: 10.1016/j.neuroimage.2005.05.056. [DOI] [PubMed] [Google Scholar]
- 28.Rademacher J, Morosan P, Schormann T, Schleicher A, Werner C, Freund HJ, Zilles K. Probabilistic mapping and volume measurement of human primary auditory cortex. NeuroImage. 2001;13:669–83. doi: 10.1006/nimg.2000.0714. [DOI] [PubMed] [Google Scholar]
- 29.Penhune VB, Zatorre RJ, MacDonald JD, Evans AC. Inter-hemispheric anatomical differences in human primary auditory cortex: probabilistic mapping and volume measurement from magnetic resonance scans. Cereb Cortex. 1996;6:661–72. doi: 10.1093/cercor/6.5.661. [DOI] [PubMed] [Google Scholar]
- 30.Mäkelä JP, Hämäläinen M, Hari R, McEvoy L. Whole-head mapping of middle-latency auditory evoked magnetic fields. Electroencephalogr Clin Neurophysiol. 1994;92:414–21. doi: 10.1016/0168-5597(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 31.Menning H, Roberts LE, Pantev C. Plastic changes in the auditory cortex induced by intensive frequency discrimination training. Neuroreport. 2000;11:817–22. doi: 10.1097/00001756-200003200-00032. [DOI] [PubMed] [Google Scholar]
- 32.Tremblay K, Kraus N, McGee T, Ponton C, Otis B. Central auditory plasticity: changes in the N1–P2 complex after speech-sound training. Ear Hear. 2001;22:79–90. doi: 10.1097/00003446-200104000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Reinke KS, He Y, Wang C, Alain C. Perceptual learning modulates sensory evoked response during vowel segregation. Brain Res Cogn Brain Res. 2003;17:781–91. doi: 10.1016/s0926-6410(03)00202-7. [DOI] [PubMed] [Google Scholar]
- 34.Bosnyak DJ, Eaton RA, Roberts LE. Distributed auditory cortical representations are modified when non-musicians are trained at pitch discrimination with 40 Hz amplitude modulated tones. Cereb Cortex. 2004;14:1088–99. doi: 10.1093/cercor/bhh068. [DOI] [PubMed] [Google Scholar]
- 35.Ponton CW, Eggermont JJ, Kwong B, Don M. Maturation of human central auditory system activity: evidence from multichannel evoked potentials. Clin Neurophysiol. 2000;111:220–36. doi: 10.1016/s1388-2457(99)00236-9. [DOI] [PubMed] [Google Scholar]
- 36.Shahin A, Roberts LE, Trainor LJ. Enhancement of auditory cortical development by musical experience in children. Neuroreport. 2004;15:1917–21. doi: 10.1097/00001756-200408260-00017. [DOI] [PubMed] [Google Scholar]
- 37.Rauschecker JP, Tian B, Hauser M. Processing of complex sounds in the macaque nonprimary auditory cortex. Science. 1995;268:111–4. doi: 10.1126/science.7701330. [DOI] [PubMed] [Google Scholar]
- 38.Tian B, Reser D, Durham A, Kustov A, Rauschecker JP. Functional specialization in rhesus monkey auditory cortex. Science. 2001;292:290–3. doi: 10.1126/science.1058911. [DOI] [PubMed] [Google Scholar]
- 39.Cohen D, Cuffin BN, Yunokuchi K, Maniewski R, Purcell C, Cosgrove GR, Ives J, Kennedy JG, Schomer DL. MEG versus EEG localization test using implanted sources in the human brain. Ann Neurol. 1990;28:811–7. doi: 10.1002/ana.410280613. [DOI] [PubMed] [Google Scholar]
- 40.Crease RP. Images of conflict: MEG vs. EEG. Science. 1991;253:374–5. doi: 10.1126/science.1862336. [DOI] [PubMed] [Google Scholar]


