Abstract
Object
Intracranial surgery causes cortical injury from incisions, hemorrhage, retraction, and electrocautery. The term “surgical brain injury” (SBI) has been developed to categorize this injury inherent to the procedure. Neuroinflammation plays a significant role in SBI. Traditional antiinflammatory therapies are often limited by their immunosuppressive side effects and poor CNS penetration. This study uses mucosal tolerance to develop an immune system that is tolerant to brain myelin basic protein (MBP) so that inflammation can be suppressed in a timely and site-specific manner following surgical disruption of the blood-brain barrier.
Methods
A standard SBI model using CD57 mice was used. Nasopharyngeal mucosa was exposed to vehicle, ovalbumin, or MBP to develop mucosal tolerance to these antigens. Immunological tolerance to MBP was confirmed in vivo through hypersensitivity testing. Neurological scores, cerebral edema, and interleukin (IL)–1β and transforming growth factor (TGF)–β1 cytokine levels were measured 48 hours postoperatively.
Results
Hypersensitivity testing confirmed the development of immune tolerance to MBP. Myelin basic protein– tolerant mice demonstrated reduced neurological injury, less cerebral edema, decreased levels of IL-1β, and increased levels of TGFβ1 following SBI.
Conclusions
Developing preoperative immunological tolerance to brain antigens through mucosal tolerance provides neuroprotection, reduces brain edema, and modulates neuroinflammation following SBI.
Keywords: surgical brain injury, mucosal tolerance, neuroprotection, traumatic brain injury, mouse
Nearly all intracranial procedures involve an element of cortical incision, retraction, electrocautery, and vascular or microvascular occlusion. In addition, cranial procedures involve inherent surgical risks of intraoperative hypotension, blood loss, and consequential cerebral ischemia. These inherent risks have been acknowledged by neurosurgeons through the development of minimally invasive surgical techniques as well as perioperative neuroprotective therapies. The adaptation of tools such as the endoscope, functional mapping, and the use of stereotactic localization for the purposes of making smaller, more precisely located craniotomies, clearly delineates a recognition of the inherent invasiveness and risk to normal brain tissues involved in cranial procedures.23,26,37 Pharmacological approaches to perioperative neuroprotection, such as the use of osmotic agents and burst suppression, are frequently used with the goal of minimizing complications and improving outcome.32,35 The advancement of therapies in perioperative neuroprotection for cranial surgery has been a recent topic of discussion and experimental investigation.18,19 These investigations have led to the adoption of the term “surgical brain injury” (SBI), which encompasses the damage to the CNS that is inherent to the intracranial procedure itself. Clinically, SBI cannot be easily demarcated from the underlying brain pathology, which makes it difficult, if not impossible, to study SBI as an independent outcome variable. However, animal models have been developed to quantify the impact of SBI on brain physiology as well as neurological outcome.18,19 This experiment investigates the potential role for modification of the CNS inflammatory response as a neuroprotective strategy against SBI using an experimental rodent model.
Early investigations involving the CNS and the immune system led to the belief that the brain was an immunologically privileged organ, neither susceptible to nor capable of eliciting an inflammatory response.2,28 Although the CNS exhibits some characteristics of immune privilege, it has since been established that inflammation is a common part of CNS physiology and represents a common pathological reaction to almost every neurological disease.7,12,16,41,45 Initially considered to only have a pathological role following injury, cerebral inflammation is now known to be a complex system of checks and balances.
Activated leukocytes arrive at the site of CNS injury and release prostaglandins, free radicals, complement cascade factors, and proinflammatory cytokines, which in turn mobilize additional immune and glial cells.25,39,44 This inflammatory upregulation is necessary to mount an adequate response, but its extent is also limited by inherent antiinflammatory mediators that suppress both humoral and cellular immune activation.22,48 Ideally a perfect balance between proinflammatory processes and regulation would result in the eradication of the insult without any side effects. However, this is often not the case, and inflammatory processes frequently exacerbate brain injury and worsen neurological outcomes.15,22 Consequently, immunosuppressive agents have been investigated as a means of neuroprotection.1,36,47
There is a strong inflammatory reaction surrounding the corticotomy in the SBI model that is characterized by increased neutrophil activation, increased levels of inflammatory cytokines, cell death, and disruption of the BBB.16–19 In our experiment we attempt to limit the damage caused by neuroinflammation following SBI by inducing mucosal tolerance to the CNS antigen MBP. Mucosal tolerance is a method of inducing immune tolerance to a specific antigen through chronic exposure of that antigen to the mucosal surfaces of the subject.42 Tolerance occurs after repetitive low-dose exposure of the antigen to mucosal surfaces (typically oral or nasopharynx surfaces in experimental models). Reexposure of the same or similar antigens to the immune system of tolerant subjects results in a modified immune response that is characterized by specific T-regulatory lymphocytes.42 This specific T-cell population secretes cytokines, such as TGFβ1, which suppress the cell-mediated immune response at the site of antigen exposure or injury.8,9,13,20,21,29,42 Mucosal tolerance to MBP has been previously shown to improve outcomes after ischemic stroke.5,6,14 We investigate the role of mucosal tolerance to MBP as a potential neuroprotectant in a rodent model of SBI.
Methods
Experimental Animals
This protocol was approved by the Institutional Animal Care and Use Committee at Loma Linda University. Male CD57 mice weighing 20–25 g were used (Harlan Corporation). Animals were housed in a climate-controlled environment with strict day/night light cycles. Seventy-six animals were subjected to tolerization regimens of vehicle (PBS), ovalbumin, or MBP prior to craniotomy. Divided groups were: sham + PBS (12 mice), sham + ovalbumin (12 mice), sham + MBP (12 mice), SBI + PBS (12 mice), SBI + ovalbumin (14 mice), and SBI + MBP (14 mice). Body weight was measured immediately before, 24 hours after, and 48 hours after surgery. Six randomly selected animals from each group were then killed at 48 hours and their brains were used for molecular analysis; the remaining animals were used to determine brain water content.
Development of Mucosal Tolerance
Before sham surgery or SBI craniotomy, 50 μg of bovine MBP (50 μg/10 μl of vehicle; Sigma Aldrich), 50 μg of ovalbumin (50 μg/10 μl of vehicle; Sigma Aldrich), or 10 μl of vehicle (PBS) was instilled into the nasopharynx (5 μl in each nostril) every other day for 5 treatments over 10 days as previously described.14 Animals were briefly sedated for the nasal application with isoflurane anesthetic 0.5%–5% in 70% medical air with 30% O2. Surgery was performed 24 hours after the last treatment. Myelin basic protein was chosen as the antigen for the development of mucosal tolerance because it was previously shown to improve outcome in experimental models of stroke in dosages similar to those used in this experiment. 5,6,14
Experimental Craniotomy
A standardized SBI model was used, as described in previous reports.16,17 Briefly, prior to surgery, general anesthesia was induced with 80 mg/kg ketamine intraperitoneally and 10 mg/kg xylazine intraperitoneally. Spontaneous ventilation without airway management was permitted by this anesthetic combination. After induction of general anesthesia, mice were placed prone in a Benchmark stereotactic frame under a surgical operating microscope. Scalp fur and skin were cleaned and prepared in a sterile manner. A No. 11 blade was used to incise the skin down to the skull through a single sagittal incision. The periosteum was reflected to expose the right frontal skull. Using the bregma as a landmark, a small square of skull (approximately 4 × 4 mm) was thinned and removed with a bone drill. A durotomy was performed, and the entire right frontal lobe anterior to the bregma was excised by sharp dissection and electrocautery. The resection was carried down to the skull base. Preliminary studies were conducted to demonstrate the consistency of the size of resection based on the weight of the removed specimen. Once the brain tissue was excised, intraoperative packing and saline irrigation along with brief (approximately 1 second) bipolar electrocautery application to the cut surfaces was used to control bleeding. Hemostasis was confirmed by close observation after removal of packing. After hemostasis was assured, the skin was closed with 5-0 silk suture (Ethicon, Inc.). Sham surgery included general anesthesia, skin incision, and craniotomy but no durotomy. A heating pad was used to maintain the body temperature at 36.0° ± 0.5°C during surgery and as the animal recovered from anesthesia. Animals were labeled in a manner that did not reveal their treatment to the surgeon, and the surgeon was unaware of the treatments received by the animals prior to and during surgery. Treatments were randomly assigned to the animals by an independent lab assistant and recorded on a spreadsheet.
Neurological Scoring
A modified Garcia scoring system performed by an independent researcher blinded to the experimental conditions was used to assess neurological function in the mice 24 and 48 hours after surgery as previously described. 16 Briefly, sensorimotor testing was graded on a scale from 0 to 3 in 7 areas: spontaneous activity, side stroking response, vibrissae response, limb symmetry when suspended by the tail, lateral turning when suspended by the tail, symmetry of walking on the forelimbs when partially suspended by the tail, and climbing ability/ response. Neurological scores were assigned as follows: 0 = complete deficit, 1 = definite deficit with some function, 2 = mild deficit or decreased response, and 3 = no evidence of deficit/symmetrical responses. The maximum score on this scale is 21 points, representing normal neurological function.
Brain Water Content
Brain water content of the cerebral hemispheres, cerebellum, and brainstem was examined by an investigator blinded to treatment to assess brain edema at 48 hours as described previously.16,17 Briefly, mice were killed under anesthesia (isoflurane anesthetic 5% in 70% medical air with 30% O2) 48 hours after SBI, and brains were removed and separated into 4 hemispheric subsections, cerebellum, and brainstem. Samples were immediately weighed on a high-precision balance. Brain samples were then dried for 48 hours at 105°C and then weighed again. Brain water content for each hemisphere was calculated using the formula: ([wet weight − dry weight]/wet weight) × 100%.
Brain Cytokine Levels
Standard Western blotting protocol17 using the antibodies anti-TGFβ1 (Cell Signaling) and IL-1β (Santa Cruz Biotechnology, Inc.) was performed at 48 hours on brain tissue from the hemisphere ipsilateral to the cortical resection. Briefly, animals were killed under anesthesia (isoflurane anesthetic 5% in 70% medical air with 30% O2) 48 hours after SBI, and brains were immediately removed and stored at −80°C until analysis (6 mice for each group). Protein extraction from the hemisphere ipsilateral to craniotomy was obtained by gently homogenizing in radioimmunoprecipitation assay lysis buffer (Santa Cruz Biotechnology, Inc.) and further centrifuged at 14,000 g at 4°C for 30 minutes. The supernatant was used as whole-cell protein extract and the protein concentration was determined using a detergent-compatible assay (DC Protein Assay, Bio-Rad Laboratories, Inc.). Equal amounts of protein (50 μg) were loaded on a sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel. Protein was electrophoresed and transferred to a nitrocellulose membrane; the membrane was then blocked and incubated with 1 of 2 primary antibodies, anti-TGFβ1 (Cell Signaling) or IL-1β (Santa Cruz Biotechnology, Inc.), overnight at 4°C. Nitrocellulose membranes were then incubated with secondary antibodies (Santa Cruz Biotechnology, Inc.) for 1 hour at room temperature. Immunoblots were then probed with an ECL Plus chemiluminescence reagent kit (Amersham Biosciences) and exposed to radiographs. The data were analyzed using ImageJ 1.41 software (NIH).
Delayed Hypersensitivity Testing
Eighteen additional animals were divided into groups of 6 and subjected to 1 of the 3 tolerization regimens. These animals were used for in vivo confirmation of tolerance to MBP by measuring delayed-type hypersensitivity to MBP as previously described.6 Briefly, naive animals (those not undergoing sham or SBI surgery) underwent tolerization to PBS (6 mice), MBP (6 mice), or ovalbumin (6 mice) in the same manner as the surgical groups. Twenty-four hours after tolerization, animals were immunized with bovine MBP (50 μg of MBP in 50 μl of PBS mixed with 50 μl of complete Freund’s adjuvant) by injection into the subcutaneous tissues of the left thigh. Animals were then rechallenged with bovine MBP (50 μg of MBP in 50 μl of PBS) by injection into the dorsal surface of the right foot 15 days later. Change in footpad thickness was measured 48 hours later with a torque-sensitive digital microcaliper (Mitutoyo) at a resolution of 0.0001 ± 2 μm.
Statistical Analysis
Data were expressed as means ± SEMs. One-way ANOVA was used to compare differences among groups and expressed as means ± SEMs using SigmaStat for Windows version 3.5. Data were found to be significant at a p value < 0.05. The Holm-Sidak test for pairwise comparisons was completed following 1-way ANOVA.
Results
Physiological Parameters
Seventy-six mice were subjected to a tolerization regimen with MBP, ovalbumin, or treatment with vehicle (PBS) prior to craniotomy. There were no significant differences in temperature, and none of the animals died among the experimental groups. Sham surgery did not result in a significant reduction in postoperative body weight. There were no differences in the body weights among the sham groups (sham + PBS, sham + ovalbumin, and sham + MBP). The mice in the SBI groups lost weight at both 24 and 48 hours following SBI compared with those in the sham groups and preoperative measurements. No significant differences in weight existed among the mice in the SBI groups postoperatively. The differences in the mean values among the groups were statistically significant (p < 0.001). All pairwise multiple comparison procedures were made using the Holm-Sidak method and significance was set at p < 0.05.
Delayed-Type Hypersensitivity
The increase in footpad thicknesses of MBP-tolerant mice was significantly less than those of PBS- or ovalbumin-treated mice following the immune challenge with MBP via injection into the footpad (Fig. 1). This measurement represents the severity of the inflammatory reaction against MBP.
Fig. 1.
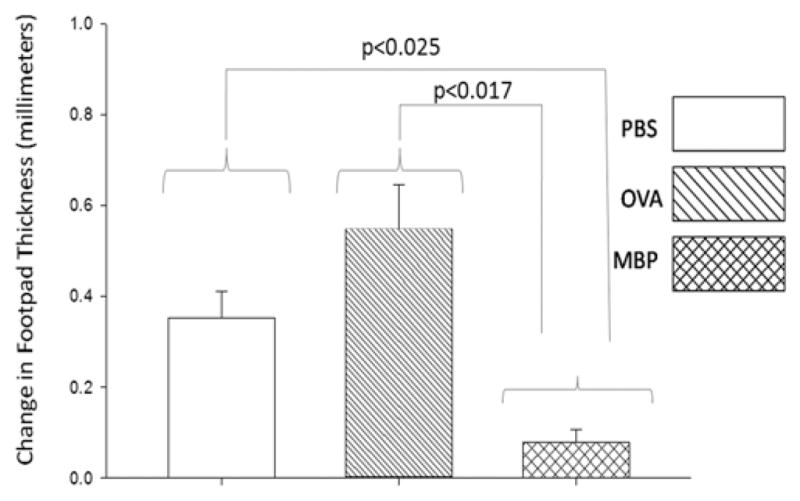
Graph of hypersensitivity to MBP. Measurement of footpad thickness shows that MBP-tolerant mice developed a much smaller inflammatory reaction, than PBS- or ovalbumin (OVA)–treated mice, when MBP was injected subcutaneously into the footpad following MBP immunization. The Holm-Sidak test for pairwise comparisons was performed and the p values were less than the critical levels indicated in the figure (statistically significant). Six mice per group. Error bars represent the SEM.
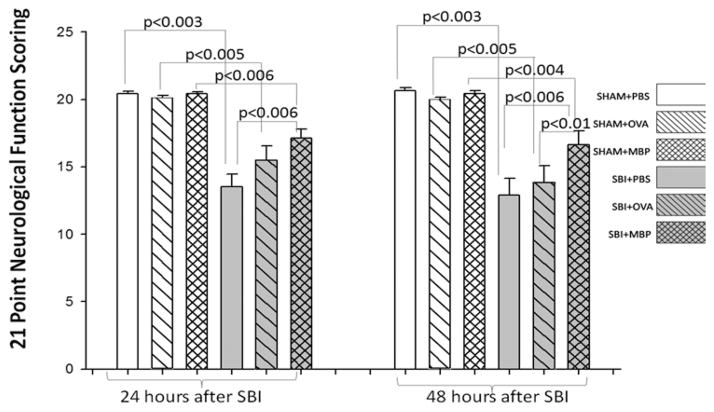
Neurological Scores
There were no significant differences in neurological score among any of the sham groups (Fig. 2). All mice receiving SBI demonstrated a significant reduction in neurological score compared with their corresponding sham groups at 24 and 48 hours. Animals tolerant to MBP prior to craniotomy demonstrated improved neurological function compared with PBS-treated groups 24 hours after SBI. Forty-eight hours after SBI, animals tolerant to MBP demonstrated improved neurological function compared with both PBS- and ovalbumin-treated groups. This appearance of a statistical difference between the ovalbumin and MBP groups appears to be the result of a further decline in function at 48 hours for the ovalbumin group (Fig. 2); it is likely related to the progression of cerebral edema, which peaked at 48–72 hours in this model.
Fig. 2.
Graph comparing postoperative neurological scores among 6 groups of mice (12 mice per group except SBI + OVA and SBI + MBP [14 mice per group]). Surgical brain injury resulted in significant loss of neurological function 24 and 48 hours after surgery. The MBP-tolerant groups show a significant preservation of neurological function 24 and 48 hours after SBI. The Holm-Sidak test for pairwise comparisons was performed and the p values were less than the critical levels indicated in the figure (statistically significant).
Brain Water Content
Brain water content was not significantly different among any of the sham groups (Fig. 3). Experimental craniotomy significantly increased brain water content in the right frontal lobe surrounding the resection site 48 hours after surgery compared with sham in all groups. Post-SBI brain water content of the right frontal lobe in animals tolerant to MBP was significantly reduced in comparison with vehicle- or ovalbumin-treated groups. The remaining brain regions did not show any significant increases in brain water content following craniotomy (data not shown).
Fig. 3.
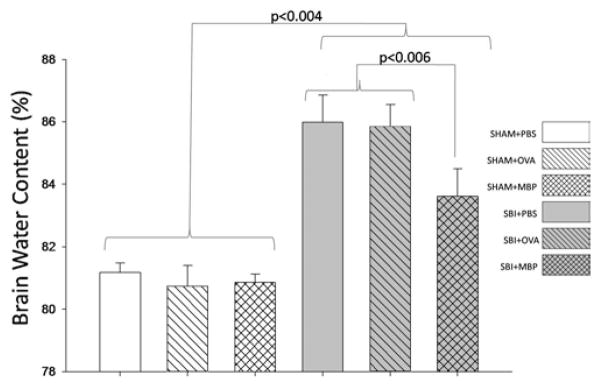
Graph comparing brain water content 48 hours after SBI among 6 groups of mice (6 mice per group for all groups except SBI + OVA and SBI + MBP [8 mice per group]). Brain water content was unchanged among treatments in the sham groups. Surgical brain injury caused significant increases in brain water content among all treatment groups. The postoperative increase in brain water content was significantly reduced in MBP-tolerant mice compared with vehicle (PBS) and ovalbumin groups. The Holm-Sidak test for pairwise comparisons was performed and the p values were less than the critical levels indicated in the figure (statistically significant).
Expression of IL-1β and TGFβ1
Western blot analysis of the right hemisphere showed that IL-1β levels in sham animals were not affected by treatment (Fig. 4 left). Surgical brain injury increased the expression of IL-1β in PBS- and ovalbumin-treated groups (p < 0.001, 1-way ANOVA), while MBP-tolerant animals did not demonstrate a significant increase in the expression of this proinflammatory cytokine.
Fig. 4.
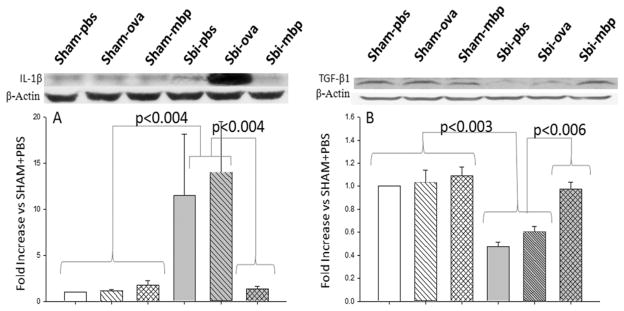
Graph showing the effects of mucosal tolerance on cerebral cytokine levels following SBI among 6 groups of mice (6 mice per group). Left: Levels of IL-1β were equal among all sham groups. Levels were significantly increased in the SBI + PBS and SBI + OVA groups. The MBP-tolerant mice did not demonstrate an increase in IL-1β after SBI. Levels of IL-1β were significantly higher in the SBI + PBS and SBI + OVA groups compared with the SBI + MBP group. Right: Levels of TGFβ1 were equal among all sham groups. Levels of TGFβ1 were reduced by approximately 50% in the SBI + PBS and SBI + OVA groups. There was no reduction in TGFβ1 in MBP-tolerant mice after SBI. Post-SBI levels of TGFβ1 were significantly higher in the MBP-tolerant mice than in the SBI + PBS or SBI + OVA groups. Expression levels of each protein using Western blots are expressed as a ratio of β-actin levels for normalization. The Holm-Sidak test for pairwise comparisons was performed and the p values were less than the critical levels indicated in the figure (statistically significant).
The levels of TGFβ1 in sham animals were not affected by treatment (Fig. 4 right). Craniotomy decreased the expression of TGFβ1 in PBS- and ovalbumin-treated groups by approximately 50%, whereas preoperative levels were preserved with MBP tolerance. The expression of TGFβ1 was significantly elevated in the MBP-treated groups compared with PBS- or ovalbumin-treated groups.
Statistical Power
The power of all tests performed with an alpha level of 0.05 was > 0.800, except for the testing of IL-1β expression, which had a power of 0.542.
Discussion
Inflammatory mediators likely play a significant role in the traumatic (cortical incision) and ischemic (brain retraction) injury associated with SBI. Previous experiments in SBI models have found significant cerebral edema and inflammatory pathology surrounding the corticotomy. 16,17 Additionally, experiments in traumatic and ischemic brain injury have found that delayed deterioration is associated with an elevation of proinflammatory mediators.3 Our experiment has demonstrated that tolerance to MBP attenuates SBI, as animals in the treatment group had less postoperative edema, and improved postoperative neurological function (Figs. 2 and 3). This neuroprotection is associated with the maintenance of inherent levels of the immunosuppressant cytokine TGFβ1 (Fig. 4 right) and inhibition of the proinflammatory cytokine IL-1β (Fig. 4 left).
Mucosal tolerance to MBP resulted in significant reductions in postoperative IL-1β (Fig. 4 left), which is a proinflammatory cytokine that orchestrates systemic inflammatory responses, and is a major contributor to neuroinflammation. 3 It is expressed at relatively low levels in the brain under physiological conditions and regulates many important physiological functions within the CNS.3 However, under pathophysiological conditions there is a marked elevation in the level of IL-1β and its receptor. 3,10,33,38,46 Given the significant changes in IL-1β expression following brain injury, it has been a target of numerous investigations and is one of the most studied proinflammatory cytokines today.40 Therefore, we selected this cytokine as a means of quantifying the degree of inflammation following SBI in our experiment. Microglia are the earliest major source of IL-1β after experimental CNS injury, infection, or inflammation, whereas neurons, astrocytes, and oligodendrocytes are likely secondary sources of IL-1β.3 The proinflammatory cytokine IL-1β augments T-cell responses to mitogens, leads to the recruitment of leukocytes through adhesion molecule expression, and amplifies other proinflammatory cytokines.3 The experimental inhibition of IL-1β has provided further evidence of its importance in orchestrating neuroinflammatory responses. Yamasaki et al.46 reported that intraventricular injection of anti–IL-1β antibodies to rats reduces ischemic brain damage. Additionally, mice lacking the gene for the enzyme caspase-1, which is required to activate IL-1β, exhibited reduced brain damage in several models of ischemia.3 Increases of IL-1β levels are known risk factors for poor outcome after stroke or brain injury, and high circulating levels of inflammatory markers are predictive of poor clinical outcome in stroke patients.3 Our data supports these findings by demonstrating that the attenuation of postoperative IL-1β is associated with improved surgical outcomes.
Transforming growth factor–β1 is a pleiotropic cytokine, and in the CNS it has been shown to promote the survival of neurons and inhibit microglial and astrocyte proliferation.27 It is recognized as the hallmark cytokine secreted by T-regulatory lymphocytes, following the induction of mucosal tolerance. These lymphocytes, through the action of their cytokines such as TGFβ1, markedly inhibit local proinflammatory cells. Given the prominence of TGFβ1 secretion by T-regulatory lymphocytes, we selected this cytokine as a marker for the effectiveness of our mucosal tolerance treatments. The use of this cytokine has correlated well with the induction of mucosal tolerance in other experiments.5,6 Transforming growth factor–β1 is expressed in the normal adult brain by parenchymal microglial cells, exerting a trophic antiinflammatory effect in the adult CNS.27 Transforming growth factor–β1 is likely a significant contributor to the partial immunological privilege of the brain, which is demonstrated by the brain’s increased tolerance of foreign tissue grafts and resilience to developing inflammatory reactions in comparison with other body tissues.28 We demonstrated a baseline expression of TGFβ1 in sham animals, which was significantly reduced following SBI (Fig. 4 right). However, treatment with mucosal tolerance to MBP preserved endogenous levels of TGF-β1 (Fig. 4 right), and was associated with decreased cerebral edema (Fig. 3) and improved neurological score (Fig. 2). We believe that TGFβ1 provides these neuroprotective effects through its antiinflammatory properties.27 Our findings are consistent with other experiments in that endogenous levels of TGFβ1 are present in the cortex at detectable levels.24 Experiments by Becker et al.5,6 and Gee et al.14 in ischemic stroke models also support our findings. Becker et al.6 found that mucosal tolerance to MBP before middle cerebral artery occlusion reduced infarct size. Gee et al.14 demonstrated that lymphocytes collected 1 month after middle cerebral artery occlusion in animals that had undergone MBP immune tolerance expressed higher levels of TGFβ1. The elevation of TGFβ1 was associated with improved neurological outcome and reduced brain atrophy following ischemic stroke.
Our experiment provides evidence for a unique antiinflammatory therapy against SBI that potentially avoids many of the pitfalls of other antiinflammatory approaches. Previous trials and experiments testing systemically administered antiinflammatory therapies for brain injury have been met with variable success. The use of steroids in traumatic brain injury has proven to be detrimental to outcome.34 Steroid use in spinal cord injury is still highly debated, but it is no longer a standard of care in many hospitals.30 In ischemic stroke, antiinflammatory treatments have been shown to improve outcome in animal models,5,14 whereas human trials have failed to show clinical benefit.11 Systemic antiinflammatory agents are frequently limited by side effects such as infection, pneumonia, or sepsis.11,30 Mucosal tolerance to brain antigens can overcome these disadvantages by providing antiinflammatory therapy in a timely and site-specific fashion. Through mucosal tolerance it is possible to modulate the immune system’s interaction with specific antigens as opposed to suppressing the entire system. We believe that further investigation into its protective mechanisms is warranted given the findings in this study as well as other studies in models of ischemic stroke and multiple sclerosis. 4–6,31 Our data have shown that the treatment regimen changes the animal’s systemic immune response to MBP through the mitigated delayed hypersensitivity reaction (Fig. 1). In addition, the cytokine profile of the neuroinflammatory reaction was significantly changed, with increases in TGFβ1 and decreases in IL-1β (Fig. 4). This change in cytokine profile is suggestive of changes in the T lymphocyte subtype that is dominating the inflammatory reaction, as TGFβ1 is characteristic of T-regulatory lymphocytes’ reactions. The presence of T-regulatory lymphocytes is associated with neuroprotection through their local inhibition of cell-mediated immunity.5,6,29,49 The association between the altered cytokine profiles, reduced cerebral edema, and improved neurological outcomes suggests that these cytokines may be responsible for the clinical improvement of the animals. Our experiment also demonstrated clinical safety, because the animals did not demonstrate toxicity to the treatment. The animals treated with MBP had no deleterious changes in weight or neurological function following treatment. In the future, long-term neurological outcomes and histological examination of brains to determine side effects will be required. However, the efficacy of mucosal tolerance to MBP is currently undergoing testing in humans for the treatment of rheumatoid arthritis and multiple sclerosis, and no toxicities or autoimmune reactions have been found.38,43
Conclusions
These findings provide evidence for the efficacy and safety of mucosal tolerance to MBP, and open the door for further investigation as a potential perioperative neuroprotectant. The ability to regulate inflammatory reactions following neurological surgery allows for the possibility of less cerebral edema, quicker recoveries, and potential reductions in postoperative neurological deficits. It may also allow for the brain to be tolerant of longer ischemia times, through the reduction of reperfusion injuries and their associated inflammation.
Abbreviations used in this paper
- BBB
blood-brain barrier
- IL
interleukin
- MBP
myelin basic protein
- PBS
phosphate-buffered saline
- SBI
surgical brain injury
- TGF
transforming growth factor
Footnotes
Disclosure
This study was supported by a grant (No. NS053407) awarded by NIH to Dr. Zhang.
Author contributions to the study and manuscript preparation include the following. Conception and design: Ayer, Colohan, Zhang. Acquisition of data: Ayer, Jafarian, Chen. Analysis and interpretation of data: Ayer. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Zhang. Administrative/technical/material support: Applegate. Study supervision: Zhang.
References
- 1.Allahtavakoli M, Moloudi R, Arababadi MK, Shamsizadeh A, Javanmardi K. Delayed post ischemic treatment with Rosiglitazone attenuates infarct volume, neurological deficits and neutrophilia after embolic stroke in rat. Brain Res. 2009;1271:121– 127. doi: 10.1016/j.brainres.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 2.Barker CF, Billingham RE. Immunologically privileged sites. Adv Immunol. 1977;25:1–54. [PubMed] [Google Scholar]
- 3.Basu A, Krady JK, Levison SW. Interleukin-1: a master regulator of neuroinflammation. J Neurosci Res. 2004;78:151–156. doi: 10.1002/jnr.20266. [DOI] [PubMed] [Google Scholar]
- 4.Becker K, Kindrick D, Relton J, Harlan J, Winn R. Antibody to the alpha4 integrin decreases infarct size in transient focal cerebral ischemia in rats. Stroke. 2001;32:206–211. doi: 10.1161/01.str.32.1.206. [DOI] [PubMed] [Google Scholar]
- 5.Becker KJ, Kindrick DL, Lester MP, Shea C, Ye ZC. Sensitization to brain antigens after stroke is augmented by lipopolysaccharide. J Cereb Blood Flow Metab. 2005;25:1634–1644. doi: 10.1038/sj.jcbfm.9600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker KJ, McCarron RM, Ruetzler C, Laban O, Sternberg E, Flanders KC, et al. Immunologic tolerance to myelin basic protein decreases stroke size after transient focal cerebral ischemia. Proc Natl Acad Sci U S A. 1997;94:10873–10878. doi: 10.1073/pnas.94.20.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC. CNS immune privilege: hiding in plain sight. Immunol Rev. 2006;213:48–65. doi: 10.1111/j.1600-065X.2006.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Inobe J, Kuchroo VK, Baron JL, Janeway CA, Jr, Weiner HL. Oral tolerance in myelin basic protein T-cell receptor transgenic mice: suppression of autoimmune encephalomyelitis and dose-dependent induction of regulatory cells. Proc Natl Acad Sci U S A. 1996;93:388–391. doi: 10.1073/pnas.93.1.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 10.Clark SR, McMahon CJ, Gueorguieva I, Rowland M, Scarth S, Georgiou R, et al. Interleukin-1 receptor antagonist penetrates human brain at experimentally therapeutic concentrations. J Cereb Blood Flow Metab. 2008;28:387–394. doi: 10.1038/sj.jcbfm.9600537. [DOI] [PubMed] [Google Scholar]
- 11.Enlimomab Acute Stroke Trial Investigators. Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology. 2001;57:1428–1434. doi: 10.1212/wnl.57.8.1428. [DOI] [PubMed] [Google Scholar]
- 12.Esiri MM. The interplay between inflammation and neurodegeneration in CNS disease. J Neuroimmunol. 2007;184:4–16. doi: 10.1016/j.jneuroim.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Fukaura H, Kent SC, Pietrusewicz MJ, Khoury SJ, Weiner HL, Hafler DA. Induction of circulating myelin basic protein and proteolipid protein-specific transforming growth factor-beta1- secreting Th3 T cells by oral administration of myelin in multiple sclerosis patients. J Clin Invest. 1996;98:70–77. doi: 10.1172/JCI118779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gee JM, Kalil A, Thullbery M, Becker KJ. Induction of immunologic tolerance to myelin basic protein prevents central nervous system autoimmunity and improves outcome after stroke. Stroke. 2008;39:1575–1582. doi: 10.1161/STROKEAHA.107.501486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hendriks JJ, Teunissen CE, de Vries HE, Dijkstra CD. Macrophages and neurodegeneration. Brain Res Brain Res Rev. 2005;48:185–195. doi: 10.1016/j.brainresrev.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Hyong A, Jadhav V, Lee S, Tong W, Rowe J, Zhang JH, et al. Rosiglitazone, a PPAR gamma agonist, attenuates inflammation after surgical brain injury in rodents. Brain Res. 2008;1215:218–224. doi: 10.1016/j.brainres.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jadhav V, Matchett G, Hsu FP, Zhang JH. Inhibition of Src tyrosine kinase and effect on outcomes in a new in vivo model of surgically induced brain injury. J Neurosurg. 2007;106:680–686. doi: 10.3171/jns.2007.106.4.680. [DOI] [PubMed] [Google Scholar]
- 18.Jadhav V, Solaroglu I, Obenaus A, Zhang JH. Neuroprotection against surgically induced brain injury. Surg Neurol. 2007;67:15–20. doi: 10.1016/j.surneu.2006.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jadhav V, Zhang JH. Surgical brain injury: prevention is better than cure. Front Biosci. 2008;13:3793–3797. doi: 10.2741/2968. [DOI] [PubMed] [Google Scholar]
- 20.Khoury SJ, Hancock WW, Weiner HL. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor beta, interleukin 4, and prostaglandin E expression in the brain. J Exp Med. 1992;176:1355–1364. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khoury SJ, Lider O, al-Sabbagh A, Weiner HL. Suppression of experimental autoimmune encephalomyelitis by oral administration of myelin basic protein. III. Synergistic effect of lipopolysaccharide. Cell Immunol. 1990;131:302–310. doi: 10.1016/0008-8749(90)90256-q. [DOI] [PubMed] [Google Scholar]
- 22.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 23.Li KW, Nelson C, Suk I, Jallo GI. Neuroendoscopy: past, present, and future. Neurosurg Focus. 2005;19(6):E1. doi: 10.3171/foc.2005.19.6.2. [DOI] [PubMed] [Google Scholar]
- 24.Lindholm D, Castrén E, Kiefer R, Zafra F, Thoenen H. Transforming growth factor-beta 1 in the rat brain: increase after injury and inhibition of astrocyte proliferation. J Cell Biol. 1992;117:395–400. doi: 10.1083/jcb.117.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147 (Suppl 1):S232–S240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maciunas RJ. Computer-assisted neurosurgery. Clin Neurosurg. 2006;53:267–271. [PubMed] [Google Scholar]
- 27.Makwana M, Jones LL, Cuthill D, Heuer H, Bohatschek M, Hristova M, et al. Endogenous transforming growth factor beta 1 suppresses inflammation and promotes survival in adult CNS. J Neurosci. 2007;27:11201–11213. doi: 10.1523/JNEUROSCI.2255-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medawar PB. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]
- 29.Miller A, Lider O, Roberts AB, Sporn MB, Weiner HL. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor beta after antigen- specific triggering. Proc Natl Acad Sci U S A. 1992;89:421– 425. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nesathurai S. Steroids and spinal cord injury: revisiting the NASCIS 2 and NASCIS 3 trials. J Trauma. 1998;45:1088–1093. doi: 10.1097/00005373-199812000-00021. [DOI] [PubMed] [Google Scholar]
- 31.Peron JP, Yang K, Chen ML, Brandao WN, Basso AS, Commodaro AG, et al. Oral tolerance reduces Th17 cells as well as the overall inflammation in the central nervous system of EAE mice. J Neuroimmunol. 2010;227:10–17. doi: 10.1016/j.jneuroim.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Raivich G, Bohatschek M, Kloss CU, Werner A, Jones LL, Kreutzberg GW. Neuroglial activation repertoire in the injured brain: graded response, molecular mechanisms and cues to physiological function. Brain Res Brain Res Rev. 1999;30:77–105. doi: 10.1016/s0165-0173(99)00007-7. [DOI] [PubMed] [Google Scholar]
- 33.Relton JK, Rothwell NJ. Interleukin-1 receptor antagonist inhibits ischaemic and excitotoxic neuronal damage in the rat. Brain Res Bull. 1992;29:243–246. doi: 10.1016/0361-9230(92)90033-t. [DOI] [PubMed] [Google Scholar]
- 34.Roberts I, Yates D, Sandercock P, Farrell B, Wasserberg J, Lomas G, et al. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial. Lancet. 2004;364:1321–1328. doi: 10.1016/S0140-6736(04)17188-2. [DOI] [PubMed] [Google Scholar]
- 35.Rozet I, Tontisirin N, Muangman S, Vavilala MS, Souter MJ, Lee LA, et al. Effect of equiosmolar solutions of mannitol versus hypertonic saline on intraoperative brain relaxation and electrolyte balance. Anesthesiology. 2007;107:697–704. doi: 10.1097/01.anes.0000286980.92759.94. [DOI] [PubMed] [Google Scholar]
- 36.Solaroglu I, Cahill J, Tsubokawa T, Beskonakli E, Zhang JH. Granulocyte colony-stimulating factor protects the brain against experimental stroke via inhibition of apoptosis and inflammation. Neurol Res. 2009;31:167–172. doi: 10.1179/174313209X393582. [DOI] [PubMed] [Google Scholar]
- 37.Tharin S, Golby A. Functional brain mapping and its applications to neurosurgery. Neurosurgery. 2007;60 (4 Suppl 2):185–202. doi: 10.1227/01.NEU.0000255386.95464.52. [DOI] [PubMed] [Google Scholar]
- 38.Trentham DE, Dynesius-Trentham RA, Orav EJ, Combitchi D, Lorenzo C, Sewell KL, et al. Effects of oral administration of type II collagen on rheumatoid arthritis. Science. 1993;261:1727– 1730. doi: 10.1126/science.8378772. [DOI] [PubMed] [Google Scholar]
- 39.Vecil GG, Larsen PH, Corley SM, Herx LM, Besson A, Goodyer CG, et al. Interleukin-1 is a key regulator of matrix metalloproteinase-9 expression in human neurons in culture and following mouse brain trauma in vivo. J Neurosci Res. 2000;61:212–224. doi: 10.1002/1097-4547(20000715)61:2<212::AID-JNR12>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 40.Vitkovic L, Bockaert J, Jacque C. “Inflammatory” cytokines: neuromodulators in normal brain? J Neurochem. 2000;74:457–471. doi: 10.1046/j.1471-4159.2000.740457.x. [DOI] [PubMed] [Google Scholar]
- 41.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiner HL. Oral tolerance: immune mechanisms and the generation of Th3-type TGF-beta-secreting regulatory cells. Microbes Infect. 2001;3:947–954. doi: 10.1016/s1286-4579(01)01456-3. [DOI] [PubMed] [Google Scholar]
- 43.Weiner HL, Mackin GA, Matsui M, Orav EJ, Khoury SJ, Dawson DM, et al. Double-blind pilot trial of oral tolerization with myelin antigens in multiple sclerosis. Science. 1993;259:1321– 1324. doi: 10.1126/science.7680493. [DOI] [PubMed] [Google Scholar]
- 44.Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99:4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 45.Williams AJ, Wei HH, Dave JR, Tortella FC. Acute and delayed neuroinflammatory response following experimental penetrating ballistic brain injury in the rat. J Neuroinflammation. 2007;4:17. doi: 10.1186/1742-2094-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamasaki Y, Matsuura N, Shozuhara H, Onodera H, Itoyama Y, Kogure K. Interleukin-1 as a pathogenetic mediator of ischemic brain damage in rats. Stroke. 1995;26:676–681. doi: 10.1161/01.str.26.4.676. [DOI] [PubMed] [Google Scholar]
- 47.Yi JH, Park SW, Brooks N, Lang BT, Vemuganti R. PPARgamma agonist rosiglitazone is neuroprotective after traumatic brain injury via anti-inflammatory and anti-oxidative mechanisms. Brain Res. 2008;1244:164–172. doi: 10.1016/j.brainres.2008.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yong VW, Marks S. The interplay between the immune and central nervous systems in neuronal injury. Neurology. 2010;74 (Suppl 1):S9–S16. doi: 10.1212/WNL.0b013e3181c97d04. [DOI] [PubMed] [Google Scholar]
- 49.Ziebell JM, Morganti-Kossmann MC. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics. 2010;7:22–30. doi: 10.1016/j.nurt.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]