Abstract
Sturge-Weber syndrome is a neurocutaneous disorder with skin, eye, and brain involvement. Prior series suggest about 50% of patients have seizures/neurodeterioration. Low-dose (3-5 mg/kg/d) aspirin use in this population is controversial. This study further addresses the side effects and outcomes of low-dose aspirin usage in Sturge-Weber syndrome. Fifty-eight subjects on aspirin with brain involvement were analyzed in a retrospective chart review. Charts were evaluated for brain involvement, age at first seizure, and side effects. Subjects' clinical stability was compared using neurologic scores. The majority of subjects had neurologic scores reflecting reasonable seizure control (91%), none or mild hemiparesis (57%), no vision impairment (71%), and none or mild cognitive impairment (80%). Forty-nine reported no significant side effects, and 9 reported either allergic reaction or minimal to significant bleeding on aspirin. This cohort's clinical experience adds significant support for low-dose aspirin use to optimize neurodevelopmental outcome in Sturge-Weber syndrome with minimal side effects.
Keywords: Sturge-Weber syndrome, aspirin, neurocutaneous syndrome
Sturge-Weber syndrome is a rare neurocutaneous syndrome associated with vascular malformations of the face, eyes, and brain. Facial capillary malformations (port wine birthmarks) typically affect skin innervated by the ophthalmic division of the trigeminal nerve, whereas brain vascular malformations (leptomeningeal angiomatosis) occur mostly in the occipital and posterior parietal lobes.1 Patients commonly have ophthal-mologic and neurologic clinical features including glaucoma, visual field deficits, seizures, strokelike episodes, cognitive and behavioral issues, and headaches. Clinical profiles within the disorder vary: some patients are mildly affected, whereas others exhibit rapid progression with severe disability.
Disease progression has been linked to recurrent thrombosis and resulting venous stasis. Antiplatelet medications could reduce thrombosis and promote perfusion.2 Low-dose aspirin use has been reported in a few small series of subjects. One study by Maria showed a 65% reduction of strokelike episodes in 14 Sturge-Weber syndrome subjects on low-dose aspirin; another study by Udani reported decreased frequency of seizures and strokelike episodes coinciding with low-dose aspirin use in 6 Sturge-Weber syndrome patients.3,4 A recent online anonymous survey from our center reported decreased frequency of seizures and strokelike episodes in 34 Sturge-Weber syndrome subjects on low-dose aspirin. Side effects were seen in a small number of patients, specifically, increased bruising and gum bleeding.5 In addition, low-dose aspirin is used in pediatric patients as a preventive treatment in children with recurrent stroke.6
Overall, the use of low-dose aspirin as a treatment of Sturge-Weber syndrome is controversial; aspirin is not widely used in clinical practice. This study of the clinical experience at the Hunter Nelson Sturge-Weber Center follows up on the prior survey and summarizes data from a large number of Sturge-Weber syndrome patients treated with low-dose aspirin. We hypothesized that low-dose aspirin is safe and well tolerated in Sturge-Weber syndrome patients and that low-dose aspirin may improve or stabilize Sturge-Weber syndrome patients' seizures and neurologic outcomes.
Methods
The subjects were patients seen at the Kennedy Krieger Institute Hunter Nelson Sturge-Weber Center, a multidisciplinary clinic dedicated to the care and research of Sturge-Weber syndrome. The Johns Hopkins Institutional Review Board approved this research and all subjects signed informed consent. The Sturge-Weber Center research database contains records of subjects seen between January 2000 and August 2011. Sturge-Weber syndrome brain involvement was diagnosed based on unilateral or bilateral leptomeningeal enhancement on neuroimaging. Participants were on low-dose aspirin (3-5 mg/kg/d) therapy as part of their clinical treatment. Aspirin levels were not checked in patients. The dose was decreased or spaced out for those subjects with minor side effects in whom aspirin was not discontinued.
Charts for subjects under 18 years of age with confirmed Sturge-Weber syndrome brain involvement were evaluated for the following variables: gender, side of brain involvement (left, right, or bilateral), age of first seizure, age at which aspirin was started, and relevant side effects. Prospectively assigned Sturge-Weber syndrome neurologic scores were collected from each patient over multiple visits (Figure 1). This scoring system is based on disease severity with regard to seizures, cognition, hemiparesis, and vision, and has been clinically validated through correlation with MRI scores, perfusion deficits on neuroimaging, decreased power on quantitative EEG, and adaptive function testing.7-9
Figure 1.
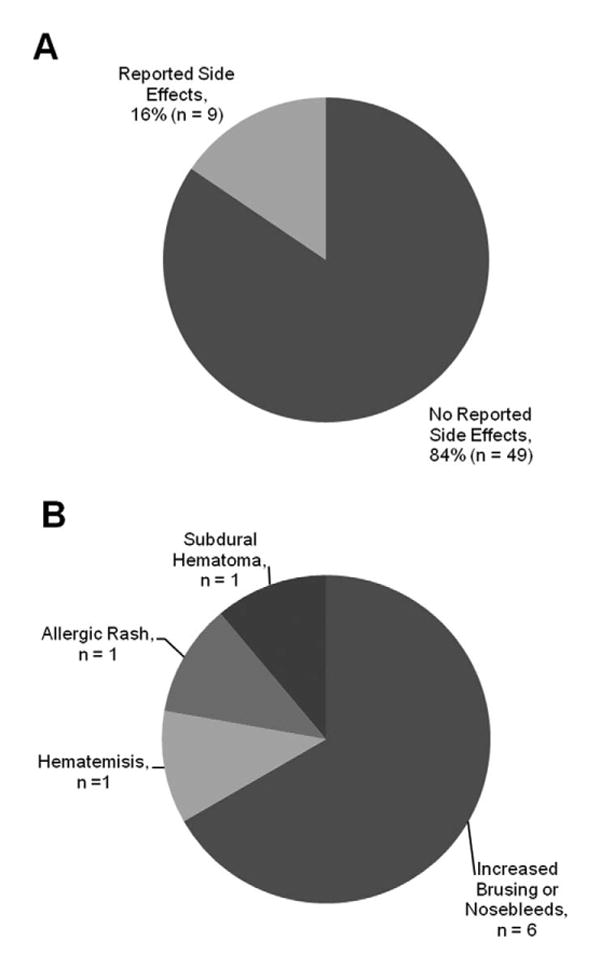
Aspirin side effects: (A) Overall incidence of side effects from aspirin seen in the Sturge-Weber syndrome cohort. (B) Frequency of each reported side effect.
Three subgroups from the Sturge-Weber syndrome database were compiled for analyses. Subgroup 1 (n = 58) was composed of all the subjects on whom information regarding side effects to aspirin therapy was available through follow-up visits at the center (n = 50) or e-mail/telephone follow-up (n = 8). Of these, 53 had epilepsy and 5 had no history of epilepsy; 4 of these 5 had a history of a visual gaze preference. This group was used to investigate the occurrence of side effects linked with low-dose aspirin use in Sturge-Weber syndrome.
Subgroup 2 (n = 35) was composed of the subjects with available magnetic resonance imaging (MRI) that had at least 2 visits to the Sturge-Weber Center during which they received prospective Sturge-Weber syndrome neurologic scores while on low-dose aspirin. Subjects from this subgroup were seen at the Center between July 2005 and August 2011. MRIs were reviewed by 2 researchers trained to look for atrophy and leptomeningeal enhancement based on a previously described scoring system.7 Both reviewers rated each MRI separately at 2 different times, then together once to reach consensus. Subjects from group 2 were classified as worsening, improving, or stable based on whether their neurologic scores on aspirin worsened (increased), improved (decreased), or remained stable (unchanged) between their first and last neurologic score as well as “good” and bad” based on a functional classification system of their neurologic scores. The group was used to determine the effects of aspirin on clinical symptoms longitudinally.
Subgroup 3 (n = 5) consisted of subjects who were started on low-dose aspirin prior to onset of their seizures. This group was used to evaluate the effects of starting low-dose aspirin prior to onset of seizures in young children with Sturge-Weber syndrome brain involvement. Additional demographic information for all 3 subgroups can be found in Table 1.
Table 1.
Sturge-Weber Syndrome Subgroups.
| Subgroup 1 | Subgroup 2 | Subgroup 3 | |
|---|---|---|---|
| Total subjects | 58 | 35 | 5 |
| Gender (male/female) | 34/24 | 20/15 | 3/2 |
| Median age in months at first seizure (range) | 5.5 (0-120) | 7 (0-120) | 5 (3-51) |
| Median age in months aspirin started (range) | 16 (1-144) | 13 (1-149) | 3(1-12) |
| Brain involvement (left/right/bilateral) | 27/21/10 | 16/14/5 | 2/3/0 |
All data were analyzed using Spearman correlations, chi-square analysis, Fisher exact tests, t tests, Mann-Whitney U, and Kruskal-Wallis as appropriate using SPSS (Statistical Package for Social Sciences) versions 18 and 19 (SPSS Inc, Chicago, Illinois). The significance level for all analyses was P < .05.
Results
Ninety-three Sturge-Weber syndrome subjects with diagnosed brain involvement were seen at the Hunter Nelson Sturge-Weber Center between January 2000 and August 2011. Of the 93 Sturge-Weber syndrome subjects with brain involvement seen during that period, 82 were either on aspirin at the time of their visit (n = 24) or were recommended to start aspirin (n = 58). Of those, 50 had at least 1 follow-up visit. The median number of follow-ups for those 50 subjects was 3.5 (mean 5.2 ± 4.84 visits) with a range from 1 to 18 follow-up visits. The median length of follow-up was 37.5 months (mean 37 ± 31 months) with a range from 1 to 153 months. An additional 8 subjects were seen only once at the Center but we obtained historical data on aspirin side effects prior to that visit or through subsequent emails or phone calls.
For the 35 Sturge-Weber syndrome subjects in subgroup 2, the median number of follow-up visits for those subjects was 4 (mean 6 ± 4.5 visits), with a range from 2 to 20 follow-up visits. The median length of follow-up was 47 months (mean 41.5 ± 25.1 months) with a range from 2 to 99 months.
Aspirin Side Effects and Complications
Of 58 Sturge-Weber syndrome subjects with brain involvement started on aspirin who have follow-up data (subgroup 1), 49 reported no significant side effects (Figure 1A). Of the 9 subjects reporting complications, 6 reported experiencing only minor side effects (increased nosebleeds or bruising). Three subjects reported serious side effects or had associated significant bleeding events that led to stopping the aspirin, including 1 instance each of allergic rash, hematemesis, and subdural hematoma (Figure 1B). The allergic rash resolved immediately on stopping the aspirin without further complications. The hematemesis occurred a week after starting the low-dose aspirin in an infant in the setting of severe hand-foot-and-mouth disease with upper GI lesions. The child required a transfusion but recovered completely and continued to do well with no onset of neurologic symptoms (seizures) until 5 years of age, at which time aspirin was restarted. The subdural hematoma occurred after minor trauma in a toddler. The low-dose aspirin was stopped and the subdural hematoma and symptoms (seizures and increased hemiparesis) resolved completely. Aspirin was stopped in 6 of the 9 subjects because of side effects, complications, or associated serious bleeding events.
MRI Results
Subjects that had at least 2 prospective neurologic scores assigned at the Sturge-Weber Center and available MRIs for analyses were selected for subgroup 2. MRIs were scored using criteria that allowed a minimum score of 8 (no asymmetry) and a maximum score of 32 (severe asymmetry). Subjects' brain injury scores ranged from 9 to 31 with a median total score of 14. Median scores were higher in the parietal lobes and on the left side (Table 2).
Table 2.
Median MRI Scores of Subgroup 2.a
| Sturge-Weber syndrome MRI scoring protocol | |||||
|---|---|---|---|---|---|
|
| |||||
| Frontal | Temporal | Parietal | Occipital | Total | |
| Left | 1 | 2 | 2 | 1 | 7 |
| Right | 1 | 1 | 2 | 1 | 6 |
| Total | 2 | 3 | 4 | 2 | 13 |
MRI scoring criteria: 1 = no asymmetry; 2 = mild asymmetry (atrophy or angiomatosis only); 3 = moderate asymmetry (angiomatosis and mild atrophy); 4 = severe asymmetry (angiomatosis and severe atrophy).
Follow-Up
The distributions of the final total neurologic scores and sub-scores in seizures, cognitive function, hemiparesis, and visual field cuts are seen in Figure 2. Figure 2A shows the distribution of final total neurologic scores for subgroup 2 (median 4; range 0-11), demonstrating that the majority of subjects' neurologic scores clustered at the mild end of the scale. For the total neurologic score, 4 of 5 subjects with bilateral brain involvement worsened, whereas only 13 of 30 subjects with unilateral brain involvement worsened. A roughly equal number of male and female subjects worsened in total neurologic score, but 9 male subjects improved, compared to only 3 female subjects. However, neither of these distributions was significantly different between the groups (Supplemental Table 1).
Figure 2.
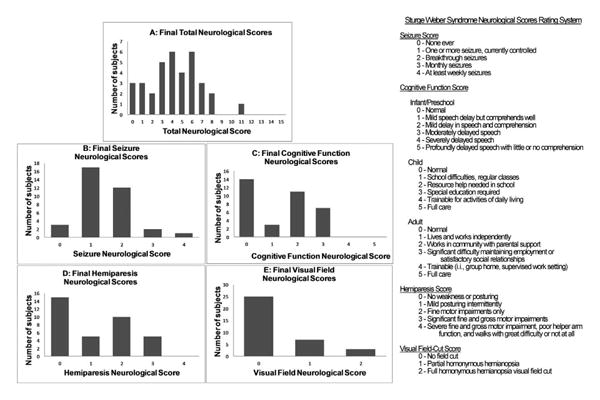
Final Sturge-Weber syndrome neurologic scores. (A) Frequency of Sturge-Weber syndrome subjects' Total Neurologic Scores across the entire range. (B) Frequency of Sturge-Weber syndrome subjects' Seizure Neurologic Scores across the entire range. (C) Frequency of Sturge-Weber syndrome subjects' Cognitive Function Neurologic Scores across the entire range. (D) Frequency of Sturge-Weber syndrome subjects' Hemiparesis Neurologic Scores across the entire range. (E) Frequency of Sturge-Weber syndrome subjects' Visual Field Neurologic Scores across the entire range.
Figure 2B shows the distribution of final seizure scores for subgroup 2 (median 1; range 0-4). Roughly one-third of subjects had improved seizures and two-thirds of subjects had stable or improved seizure control compared to their first visit (Supplemental Table 1). The seizure subscore was also split into “good” and “bad” outcomes as demonstrated in Table 3. Thirty-two of thirty-five subjects had a good seizure score between 0 and 2 (no seizures ever to breakthrough seizures in the past 6 months but not monthly) indicating that the majority of subjects had reasonably well-controlled seizures at their last visit. In comparing the frequency of “good” and “bad” seizure scores at the first and last neurologic score, there were fewer scores in the “bad” category at the last neurologic score (3) than there were at the first neurologic score (6). However, this result was not statistically significant by Fischer exact test (P = .477.) Of note, 15 of the 35 subjects were already on aspirin at the time of their first neurologic score.
Table 3.
Defining Outcomes.
| Score | Outcome | Definition | % with outcome |
|---|---|---|---|
| Seizure subscore | |||
| 0-2 | Good | No seizures to seizures within past 6 months, but not monthly | 91 |
| 3-4 | Bad | Monthly, weekly, or daily seizures | 9 |
| Cognitive function subscore | |||
| 0-2 | Good | Normal to mild impairment/some help required in school/work | 80 |
| 3-5 | Bad | Moderate impairment/special education to full care required | 20 |
| Hemiparesis subscore | |||
| 0-1 | Good | Normal to mild intermittent posturing | 57 |
| 2-4 | Bad | Fine motor impairment to severe motor impairment | 43 |
Figure 2C shows the distribution of final cognitive function scores for subgroup 2 (median 2; range 0-3); Twenty-eight of thirty-five subjects had a “good” cognitive score at last visit, suggesting that the majority of subjects did not display significant cognitive disability (mental retardation) (Table 3). For cognitive function subscore, there was a trend for significantly more subjects with bilateral involvement in the worsening (increasing cognitive function score) group compared to unilateral brain involvement (P = .09) (Supplemental Table 1). Slightly more than half of the subjects demonstrated stable or improving cognitive function scores at their last visit.
Figure 2D displays the distribution of final hemiparesis scores for subgroup 2 (median 1; range 0-3); twenty of thirty-five subjects had “good” hemiparesis scores, suggesting that most of the subjects had none to very mild evidence of hemiparesis at their last follow-up visit (Table 3). The distribution between males and females did not differ significantly. Figure 2E shows the distribution of final visual field cut scores for subgroup 2 (median 0; range 0-2).
Presymptomatic Aspirin Treatment Results
Out of the 82 patients seen at the Hunter Nelson Sturge-Weber Center on aspirin, 5 were started on low-dose aspirin before their first seizure, typically after other symptoms developed (early handedness, visual gaze preference, or strokelike episode). Potential risks/benefits were discussed with the parents before low-dose aspirin administration. Infants ≥4 kg in weight were started on one-fourth of an 81-mg baby aspirin tablet crushed or compounded for a dose of 3 to 5 mg/kg/d. The age of first seizure, age of final neurologic score, and the distribution of final neurologic scores and subscores between the 5 subjects started on low-dose aspirin presymptomatically and the 30 subjects in group 2 that were started on low-dose aspirin after seizure onset were not statistically significantly different by Mann-Whitney U analysis (Supplemental Table 2).
Discussion
In a group of 58 subjects, very few serious complications were reported, and low-dose aspirin (3-5 mg/kg/d) was well tolerated by almost all subjects. The majority of side-effects were minor (nosebleeds, bruising) and not every subject with complications found them severe enough to discontinue aspirin.
In 2 of the 3 cases with more significant events, low-dose aspirin was likely taken in association with the complications. For instance, with the allergic rash, the side-effect was directly attributable to low-dose aspirin. The rash immediately resolved when aspirin was discontinued. The subdural hematoma was caused by minor head trauma as a result of the subject falling from a chair; the child returned to baseline afterwards and the hematoma resolved completely. Subdural hematomas occur rarely in Sturge-Weber syndrome patients.10,11 The hematemesis was attributed to severe hand-foot-and-mouth disease with internal lesions instead of aspirin. The low-dose aspirin was stopped to prevent exacerbation of the hematemesis, but it was unlikely to be the cause. From these data and the literature, we conclude that the use of low-dose aspirin in young Sturge-Weber syndrome patients is generally safe, and any complications that occur can usually be quickly resolved by discontinuation of aspirin. As in other cases where low-dose aspirin is used for secondary stroke prevention, it is important for parents to be educated about the antiplatelet function of aspirin, and to report increased bruising, bleeding, or unexplained rashes and to inform other medical caregivers of the child's aspirin usage.
The spectrum of neurologic involvement reported with Sturge-Weber Syndrome varies widely.4 Final clinical neurologic scores indicated that most subjects displayed no visual field cuts or intermittent motor posturing/hemiparesis and had reasonably well-controlled seizures over the last 6 months. The cognitive function of most patients was mildly affected as well. Overall, a majority of Sturge-Weber syndrome patients on low-dose aspirin therapy were doing well both in terms of academics (attending regular schools with tutoring/assistance) and motor abilities (able to swim, ride a bike, or participate in dance/recreational sports). Their epilepsy was also sufficiently controlled and surgery was not pursued.
All 5 of the subjects with bilateral brain involvement had worsening cognitive function neurologic scores over time, despite being on low-dose aspirin. This is not unexpected, as subjects with bilateral brain involvement do have worsening cognitive function in previous studies.3 Worsening of cognitive function was also seen in a subset of the unilateral subjects; however, many subjects were infants when first seen and cognitive issues may become more apparent as children age. In addition, the cognitive function subscores reported here are more encouraging than in the literature. Many reports describe the number of Sturge-Weber syndrome patients suffering from intellectual disability (mental retardation) at 50% to 60%.1,12,13 In the data presented here, 80% of subgroup 2 subjects had cognitive function subscores labeled “good.” Although the cognitive function subscore of the neurologic score is not a direct measure of IQ, a prior study showed correlation between the total neurologic score and adaptive functioning of Sturge-Weber syndrome patients.9 Low-dose aspirin may contribute to improved cognitive outcomes; however, more research is needed.
Most (91%) of the subjects on low-dose aspirin had good final seizure scores (either no seizures in the last 6 months, or breakthrough seizures within the last 6 months but not monthly seizures). This was in an improvement from 83% at the first seizure score. This is a very high percentage compared to previous reports of Sturge-Weber syndrome patients with seizures not on low-dose aspirin. Pascual-Castroviejo reports 38% of patients achieved “satisfactory” seizure control without surgery (n = 47), and Kramer reports 50% of nonsurgical subjects with “high seizure frequency” (n = 10).14,15 This data provides further evidence that low-dose aspirin may be helpful in patients with Sturge-Weber Syndrome and epilepsy.
Twenty-four subjects were recommended to start on low-dose aspirin at their last visit to the Sturge-Weber Syndrome Center, but did not follow up at the clinic or were not able to be contacted for follow-up questions. We do not know if these subjects started aspirin therapy, if they had side effects or complications, or how they responded to the treatment. It is possible that they did not do well and did not return to this center. The majority of these subjects came from outside the mid-Atlantic region; the most likely reason for lack of follow-up is distance. Another limitation is referral bias. The Sturge-Weber Syndrome Center is a tertiary care center and subjects seen here likely have a higher disease severity than the general population. Sturge-Weber syndrome is a rare disease and our sample size is the largest series of subjects with Sturge-Weber syndrome treated with low-dose aspirin. We also did not look specifically at the effects of anticonvulsant regimes; all patients in the database are on combined therapy with 1 or more anticonvulsant. Typical anticonvulsants utilized include oxcarbazepine and levetiracetam at moderate to high dosage (40 and 55 mg/kg/d, respectively). The data presented here reflect not only the impact of low-dose aspirin but its combination with anticonvulsants tolerated and maximally effected for particular subjects. Finally, we did not check aspirin levels, and doing so in the future may further assist the management of subjects who demonstrate an inadequate clinical response.
This study summarizes the clinical experience of Sturge-Weber patients at the Hunter Nelson Sturge-Weber Center. Unlike the Internet-based questionnaire, this series encompassed clinical records for children with Sturge-Weber syndrome and information obtained by physicians rather than parents.5 Similar to that survey-based study, we found low-dose aspirin was well tolerated in Sturge-Weber syndrome patients as young as 1 month old. The effects of low-dose aspirin on seizure frequency and cognitive function are complicated by the natural progression of the disease as each subject ages; conclusions regarding these data are less clear. However, comparison of the follow-up data of the low-dose aspirin-treated population presented here to published descriptions of other Sturge-Weber syndrome populations suggests that those treated with a combination of low-dose aspirin and anticonvulsants may have better outcomes in terms of seizure frequency and cognitive disability than what has been reported. Further studies are needed to determine the mechanism of low-dose aspirin's effect and how to optimize its use in Sturge-Weber syndrome. In addition, investigation into the safety and efficacy of other antiplatelet agents (such as clopidogrel) might reveal whether these agents can be used instead of/in conjunction with low-dose aspirin in certain cases.
Supplementary Material
Acknowledgments
The work for this manuscript was done at the Kennedy Krieger Institute. Abstracts of this paper were presented at the Child Neurology Society Annual Meeting in 2011, the American Epilepsy Society Meeting in 2011, and the International Child Neurology Conference in 2012. Appreciation to Doris Lin for assistance with training MRI imaging ratings.
Financial Disclosure: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Brain Vascular Malformation Consortium (BVMC; U54NS065705) is a part of the National Institutes of Health (NIH) Rare Disease Clinical Research Network (RDCRN), supported through a collaboration between the NIH Office of Rare Diseases Research (ORDR) at the National Center for Advancing Translational Science (NCATS), and the National Institute of Neurological Disorders and Stroke (NINDS). Funding was also received from Hunter's Dream For A Cure.
Footnotes
Supplementary material for this article is available on the Journal of Child Neurology website at http://jcn.sagepub.com/supplemental.
Author Contributions: EIL and AKS performed chart review, data collection, and, along with AC, wrote the first draft of the manuscript. AC performed data collection and, together with AZ and EK, edited the manuscript. All authors took part in data analysis.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The Johns Hopkins Institutional Review Board approved this research and all subjects signed informed consent.
References
- 1.Thomas-Sohl KA, Vaslow DF, Maria BL. Sturge-Weber syndrome: a review. Pediatr Neurol. 2004;30:303–310. doi: 10.1016/j.pediatrneurol.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 2.Garcia J, Roach ES, McLean WT. Recurrent thrombotic deterioration in the Sturge-Weber syndrome. Childs Brain. 1981;8:427–433. doi: 10.1159/000120011. [DOI] [PubMed] [Google Scholar]
- 3.Maria BL, Neufeld JA, Rosainz LC, et al. Central nervous system structure and function in Sturge-Weber syndrome: evidence of neurologic and radiologic progression. J Child Neurol. 1998;13:606–618. doi: 10.1177/088307389801301204. [DOI] [PubMed] [Google Scholar]
- 4.Udani V, Pujar S, Munot P, et al. Natural history and magnetic resonance imaging follow-up in 9 Sturge-Weber syndrome patients and clinical correlation. J Child Neurol. 2007;22:479–483. doi: 10.1177/0883073807300526. [DOI] [PubMed] [Google Scholar]
- 5.Bay MJ, Kossoff EH, Lehmann CU, et al. Survey of aspirin use in Sturge-Weber syndrome. J Child Neurol. 2011;26:692–702. doi: 10.1177/0883073810388646. [DOI] [PubMed] [Google Scholar]
- 6.Roach ES, Golomb MR, Adams R, et al. Management of stroke in infants and children. Stroke. 2008;39:2644–2691. doi: 10.1161/STROKEAHA.108.189696. [DOI] [PubMed] [Google Scholar]
- 7.Lin DDM, Barker PB, Kraut MA, Comi A. Early characteristics of Sturge-Weber syndrome shown by perfusion MR imaging and proton MR spectroscopic imaging. Am J Neuroradiol. 2003;24:1912–1915. [PMC free article] [PubMed] [Google Scholar]
- 8.Hatfield LA, Crone NE, Kossoff EH, et al. Quantitative EEG asymmetry correlates with clinical severity in unilateral Sturge-Weber syndrome. Epilepsia. 2007;48:191–195. doi: 10.1111/j.1528-1167.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 9.Reesman J, Gray R, Suskauer SJ, et al. Hemiparesis is a clinical correlate of general adaptive dysfunction. J Child Neurol. 2009;24:701–708. doi: 10.1177/0883073808329529. [DOI] [PubMed] [Google Scholar]
- 10.Wyllie E, Comair YG, Kotagal P, et al. Epilepsy surgery in infants. Epilepsia. 1996;37:625–637. doi: 10.1111/j.1528-1157.1996.tb00626.x. [DOI] [PubMed] [Google Scholar]
- 11.Lopez J, Yeom KW, Comi A, Van Haren K. Case report of subdural hematoma in a patient with Sturge-Weber syndrome and literature review: questions and implications for therapy. J Child Neurol. doi: 10.1177/0883073812449514. published online ahead of print July 17, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Chapieski L, Friedman A, Lachar D. Psychological functioning in children and adolescents with Sturge-Weber syndrome. J Child Neurol. 2000;15:660–665. doi: 10.1177/088307380001501004. [DOI] [PubMed] [Google Scholar]
- 13.Jing Z. Sturge Weber syndrome: a case report and review of the literature. Chinese Med J. 2010;123:117–121. [PubMed] [Google Scholar]
- 14.Pascual-Castroviejo I, Pascual-Pascual S, Velazquez-Fragua R, Viano J. Sturge-Weber syndrome. Study of 55 patients. Can J Neurol Sci. 2008;35:301–307. doi: 10.1017/s0317167100008878. [DOI] [PubMed] [Google Scholar]
- 15.Kramer U, Kahana E, Shorer Z, Ben-Zeev B. Outcome of infants with unilateral Sturge-Weber syndrome and early onset seizures. Dev Med Child Neurol. 2000;42:756–759. doi: 10.1017/s0012162200001407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


