Abstract
[1] The quadrupole mass spectrometer of the Sample Analysis at Mars (SAM) instrument on Curiosity rover has made the first high-precision measurement of the nonradiogenic argon isotope ratio in the atmosphere of Mars. The resulting value of 36Ar/38Ar = 4.2 ± 0.1 is highly significant for it provides excellent evidence that “Mars” meteorites are indeed of Martian origin, and it points to a significant loss of argon of at least 50% and perhaps as high as 85–95% from the atmosphere of Mars in the past 4 billion years. Taken together with the isotopic fractionations in N, C, H, and O measured by SAM, these results imply a substantial loss of atmosphere from Mars in the posthydrodynamic escape phase.
Keywords: planetary atmospheres, argon isotopes, solar system, Mars, habitability, atmospheric loss
1. Introduction
[2] A relatively high-precision direct measurement of the 36Ar/38Ar ratio in the Martian atmosphere has previously been postulated to be the most compelling datum to definitively tie the so-called “Martian meteorites” (shergottites, nakhlites, and chassignites, i.e., SNC) to Mars [e.g., Owen, 1992]. This is because previous estimates of the (supposed) Martian atmospheric 36Ar/38Ar values derived from trapped gases in these unique meteorites suggested a value near 4 [e.g., Wiens et al., 1986; Bogard et al., 2001], highly distinct from the relatively uniform 36Ar/38Ar values of 5.3–5.5 found in a wide variety of other solar system objects ranging from the Sun to Jupiter to Earth (see Table 1). The earliest analyses of shock glasses from shergottite EET79001 noted the presence of Ar trapped on ejection, with a 36Ar/38Ar value considerably less than the terrestrial value of 5.3. Wiens et al. [1986] deduced a Martian “atmospheric” ratio of 4.1 ± 0.2 from EETA79001. Swindle et al. [1986] derived a value of 3.60 ± 0.44. Bogard [1997] considered all shergottite data available up to that time and gave a range of 3.5–4.6 for 36Ar/38Ar in the meteorites and concluded that the Martian atmospheric ratio of 36Ar/38Ar in these meteorites is less than 3.9. However, deriving a precise value for Martian atmospheric 36Ar/38Ar from Martian meteorites is made difficult because of the presence of significant amounts of Ar produced by galactic cosmic ray reactions during transit from Mars to Earth. The most accurate determinations derive from the EET79001 impact glass, as EET79001 has a relatively low-exposure age of 0.6 Myr [Bogard et al., 2001].
Table 1.
Argon Isotope Ratio (36Ar/38Ar) in the Atmosphere of Mars Compared to the Mars Meteorites, Sun, Jupiter, and Earth
| Object | 36Ar/38Ar Ratio |
|---|---|
| Mars atmosphere (MSL/SAM 2013)a | 4.2 ± 0.1 |
| Mars atmosphere (Viking/GCMS 1976)bc | 4–7 |
| Mars meteoritesd | 3.5–4.6 |
| Sunef | 5.5 ± 0.01 |
| Jupiterg | 5.6 ± 0.25 |
| Earthh | 5.305 ± 0.008 |
This paper.
[3] Previous attempts to measure the argon isotopes in the atmosphere of Mars have met with limited success. Although radiogenic argon (40Ar) and the primordial argon isotopes (36Ar and 38Ar) were measured by the mass spectrometer on the Viking Lander [Biemann et al. 1976; Owen and Biemann, 1976], an accurate determination of the 36Ar/38Ar ratio could not be achieved because of large background levels in the mass 38 region and instrumental effects, and hence, only a range of 4–7 for the 36Ar/38Ar ratio was reported [Biemann et al., 1976].
[4] The quadrupole mass spectrometer (QMS) of the Sample Analysis at Mars (SAM) instrument on Curiosity rover has carried out several direct atmospheric composition measurements on Mars including argon [Mahaffy et al., 2013]. Although all argon isotopes were detected, the direct ingestion of Mars air could not yield a precise value for 36Ar/38Ar ratio due to insufficient signal to background ratio (or S/BG, defined as the total signal level divided by the background level) at m/z 36. Enrichment experiments were therefore conducted to enhance the signal both at m/z 36 and 38. The result is the first high-precision data on the value of 36Ar/38Ar ratio in the Martian atmosphere. These data provide definite proof that the “Martian” rocks came from Mars (section 4). Additionally, considering that argon must have been completely or nearly completely removed from the atmosphere of Mars during hydrogen-led hydrodynamic escape and early intense sputtering loss, the argon isotopes in the present atmosphere provide arguably the most stringent constraints on posthydrodynamic loss, especially since argon is chemically inert and it does not interact or exchange with the Martian surface or interior. The argon isotope fractionation is thus a key piece of the Mars habitability puzzle, which the Mars Science Laboratory (MSL) Mission is designed to address [Grotzinger et al., 2012]. This paper describes the argon isotope enrichment experiments (section 2) and their results (section 3) and significance in the context of Martian meteorites and atmospheric loss (section 4).
2. Measurement Technique—The Enrichment Experiments
[5] For optimal precision in measurements of noble gas abundances and isotope ratios, these species must be concentrated in the atmospheric sample through removal of active gases with components of SAM's gas-processing system [Mahaffy et al., 2012]. Three modes of the enrichment experiment have been devised to achieve this goal: dynamic mode, semistatic mode, and static mode. Results presented in this manuscript were obtained with dynamic and semistatic mode experiments, summarized below (the reader is referred to Mahaffy et al. [2012] for a detailed description of the enrichment experiment modes). For the dynamic mode enrichment experiment, the atmospheric sample in the SAM manifold is exposed to chemical scrubbers to remove H2O, CO2, and other chemically active gases while the QMS is continuously pumped by the wide range turbomolecular pump (WRP1). The process of ingestion of an atmospheric sample, followed by scrubbing, is repeated multiple times, gradually enriching the sample in noble gases. This results in increased density of the noble gases needed to achieve high signal-to-noise (S/N) and S/BG for the low-abundance isotopologues. Semistatic mode experiments follow the same procedure as described for dynamic mode but allow greater source pressures of noble gases by adding passive pumping by the getter in the QMS and only partially opening the high-conductance valve to the turbomolecular pump (WRP1). The higher pressure of noble gases inside the instrument thus gives enhanced signal over the dynamic enrichment mode. The first atmospheric enrichment experiment that was performed by SAM on Mars was a dynamic mode version of the noble gas enrichment experiment on sol 231 (Figure 1). A second, semistatic enrichment experiment was run on sol 341 (Figure 2). The 36Ar/38Ar ratio is stable across successive enrichment cycles at all m/z 36 count rates higher than ∼104 counts/s (lower panel of each figure), so there is no instrumental fractionation effect due to the enrichment process. Preflight and test bed experiments show that the SAM-QMS accurately reproduces the 36Ar/38Ar ratio in calibration gas samples.
Fig 1.
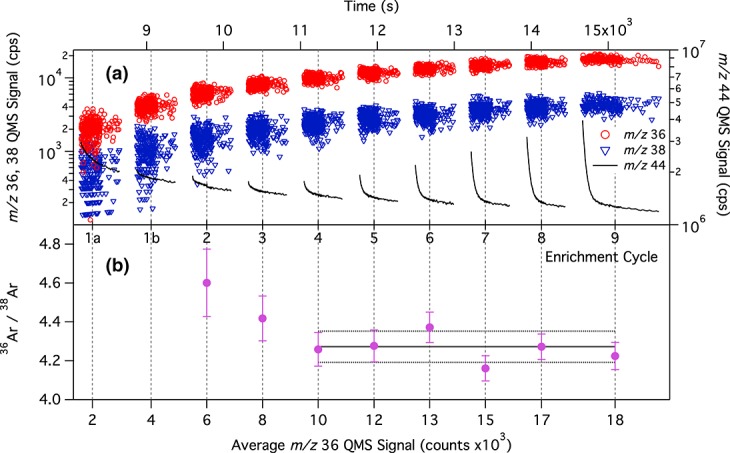
Results from the dynamic enrichment of argon 36 and 38 on Mars by the SAM instrument on MSL on sol 231. (a) The successive ingestion of samples and enrichment cycles of the Mars atmosphere increases the counts per second (cps) and S/N of the argon isotopologues (symbols, top left axis), while the major ion m/z 44 of atmospheric CO2 is scrubbed down to background levels (black trace, top right axis) via adsorption onto the SAM scrubbers. During a normal atmospheric ingestion, the m/z 44 peak would saturate the detector. The data shown for m/z 36 and 38 have been corrected for background signal as described in the text. Enrichment cycles 1a and 1b reference measurements of the same samples of atmosphere as transferred into the QMS through two different valves. Cycle 1a used a low-conductance valve; cycles 1b through 10 used the same higher-conductance valve. Cycle 1b is thus is the first true sample in this series. (b) The average ratio for each enrichment cycle is given as a function of argon 36 counts, with error bars representing the uncertainties introduced by scatter in the data and the multiple background subtraction methods used. All data from the final six enrichment cycles, where the 38Ar S/N > 3, are averaged to determine a 36Ar/38Ar ratio of 4.26 ± 0.08 for the dynamic enrichment run. Sol 0 is referenced to Curiosity's landing at Gale Crater (4.5895°S, 137.4417°E) on Mars at 15:03 local mean solar time or 05:17 UTC on 6 August 2012, in Mars Year 31.
Fig 2.
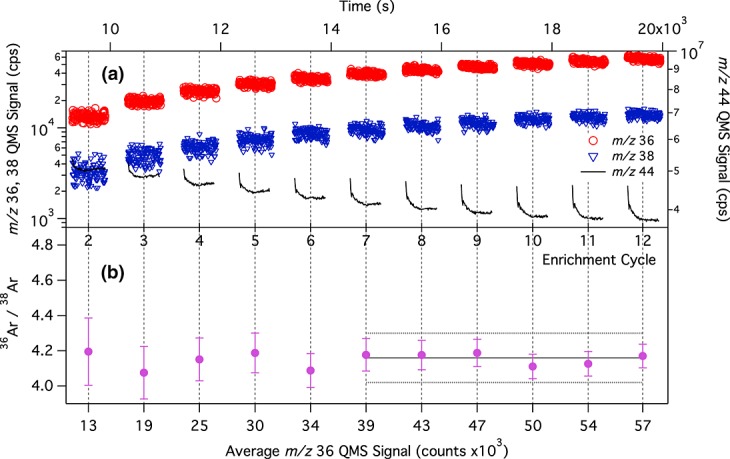
Same as Figure 1 but for the semistatic enrichment experiment on sol 341. All data from the final six enrichment cycles, where the 38Ar S/BG > 5, are averaged to determine a 36Ar/38Ar ratio of 4.16 ± 0.14 for the semistatic enrichment run. The uncertainty estimate includes statistical noise and the background correction.
3. Data Analysis and Results
[6] As discussed in Mahaffy et al. [2013], the removal of the contribution of the QMS and manifold background signal to the mass channels of interest must be carefully done for each experiment. In both enrichment experiments, background scans of the evacuated instrument and manifold were performed prior to the first atmospheric sample ingestion. However, unlike direct atmospheric measurements, these background scans spanned a small time window relative to the length of the enrichment experiment, making it difficult to characterize evolution of the background signal using the background scans alone. To model background evolution for the argon isotopes of interest (m/z 36 and 38), we used m/z 39 as a tracer mass to measure the exponential decay of background signals due to loss through continuous operation of WRP1 and the getters (m/z 39 contains actual signal from fragments of trace hydrocarbon species produced in the SAM system). The data at m/z 39 were normalized to match the signal levels of m/z 36 and 38 in these background scan intervals, and the normalized background signals were subtracted from the enriched sample data to derive the background-corrected signals. We estimate uncertainties in the 36Ar/38Ar ratio due to background corrections are 1.7% for sol 231 and 3.4% for sol 341, based on the difference in behavior of separate tracers at m/z 19 and 39.
[7] The ratio of 36Ar/38Ar was calculated at each time point, then averaged and binned per enrichment cycle as shown in Figure 1b for the dynamic experiment and Figure 2b for the semistatic experiment. In both cases, the ratio converges to a stable value of just over 4 as S/BG increases in later enrichment cycles. The two experiments give consistent measurements of the 36Ar/38Ar ratio: 4.26 ± 0.08 for sol 231 and 4.16 ± 0.14 for sol 341. The uncertainty in the reported 36Ar/38Ar ratio is the standard error of the mean of the ratio determined from each mass scan, combined with the uncertainty introduced through the background correction.
[8] Within the range of uncertainty, the 36Ar/38Ar ratios determined by the dynamic and semistatic enrichment experiments are in excellent agreement. We report a value of 4.2 ± 0.1 for the final 36Ar/38Ar ratio in the atmosphere of Mars, based on data from the two enrichment experiments.
4. Rocks from Mars and Loss of Atmosphere to Space
[9] The 36Ar/38Ar value of 4.2 ± 0.1 measured by the SAM-QMS is in excellent agreement with those inferred for the Mars atmosphere through analysis of the SNC meteorites and thus provides extremely strong evidence that these meteorites are in fact samples of the red planet. The atmospheric 36Ar/38Ar derived from EETA79001 [Wiens et al., 1986] is indeed nearly identical to that determined by SAM in situ from the surface of Mars.
[10] The argon isotope ratio is also an exceptionally good indicator of atmospheric loss to space. Planetesimals forming the terrestrial and the giant planets carried primordial argon with the 36Ar/38Ar value of 5.5 we find in the Sun (Table 1 and Figure 3). Because of its great mass, Jupiter retained all of its original volatiles over geologic time; thus, its 36Ar/38Ar remained unaltered and the Galileo probe indeed found it to be the same as in the Sun within the range of uncertainty. In contrast, fractionation has taken place on Mars (Table 1 and Figure 3) due to escape to space as a consequence of lower gravity and other effects such as solar wind interaction with the upper atmosphere [Jakosky et al., 1994; Luhmann et al., 1992]. The distinctively low 36Ar/38Ar value on Mars compared with other solar system objects reflects preferential loss of the lighter isotope of argon over time from the Martian atmosphere. In this way, this isotope ratio is similar to D/H, 14N/15N, 12C/13C, and 16O/18O (Table 2), all of which show significant enrichment of the heavier isotope due to atmospheric loss [e.g., Mahaffy et al., 2013; Webster et al., 2013; Wong et al., 2013; Owen et al., 1977; Nier and McElroy, 1977]. Taken together, the isotopic ratios of the different species allow a detailed picture of the history of the atmosphere to be constructed, including insights into the fraction of volatiles that have been lost from Mars over time. However, each isotope system has a complex and unique associated set of reservoirs (e.g., atmosphere, crust, planetary interior), geochemical processes (e.g., volcanic degassing, water-rock interaction), and loss mechanisms that contribute to its history, making this a difficult exercise in evolutionary modeling.
Fig 3.
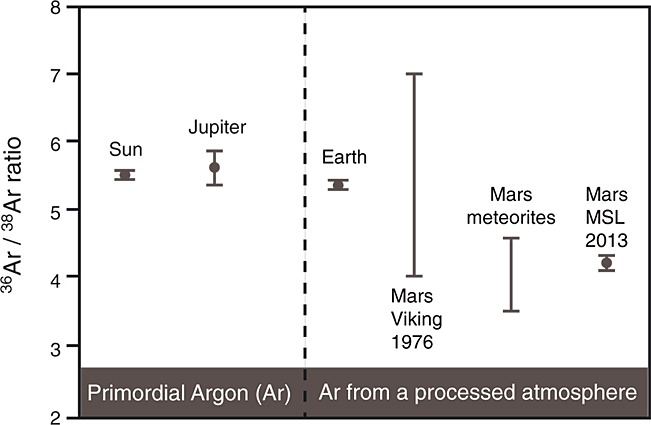
Comparison of the 36Ar/38Ar ratio measured in the atmosphere of Mars by Curiosity's SAM-QMS in 2013 with the Viking GCMS result in 1976, Mars meteorites, Earth, Jupiter and the Sun. See Table 1 for the values and references.
Table 2.
Isotope Fractionations in the Atmosphere of Mars Measured by the QMS and TLS Instruments of the SAM Suite on MSL
| Isotopes | Mars Value | SAM Instrument |
|---|---|---|
| 36Ar/38Ara | 4.2 ± 0.1 | QMS |
| 40Ar/36Arb | 1.9 (±0.3) ×103 | QMS |
| 14N/15Nc | 173 ±9 | QMS |
| δDd | 4950 ±1080 | TLS |
| δ13CVPDB | 45 ±12‰ | QMS |
| δ13CVPDB | 46 ±4‰ | TLS |
| δ18OSMOW | 48 ±5‰ | TLS |
This paper.
δ13C measured by the SAM Tunable Laser Spectrometer (TLS) and QMS in CO2 is relative to Vienna Peedee belemnite standard, where 13C/12C = 1.1237 × 10−2.
δ18O is relative to Standard Mean Ocean Water (SMOW) standard, where 18O/16O = 2.0052 × 10−3, and D/H is from H2O (D/HSMOW =1.5575 × 10−4).
[11] Because argon is a noble gas, in principle, it should be among the simpler systems to decipher. Despite Mars' relatively low-escape velocity, thermal escape from the exobase is negligible for argon due to its relatively large mass. On the other hand, because of the lack of global magnetic field and only a weak ionosphere induced field, solar wind interacts strongly with the upper atmosphere/ionosphere of Mars. As a consequence, solar wind-induced sputtering is a likely mechanism for loss leading to heavy isotope enrichment [Jakosky et al., 1994; Luhmann et al., 1992]. According to this mechanism, atmospheric ions such as O+ are picked up by the solar wind and accelerated antisunward as they move down the magnetotail. A fraction of these energetic ions or neutrals produced by their charge transfer impacts the exobase, thus providing sufficient energy of ∼1 keV to atmospheric species such as argon to escape by sputtering. As diffusive separation above the homopause results in the lighter isotope to be distributed to higher elevations than the heavier isotope, 36Ar is lost preferentially to space from the exobase, leading to an enrichment of the heavier isotope in the atmosphere. Modeling of early atmospheric processing prior to ∼4 Ga [e.g., Pepin, 1994] suggests that it was probably dominated by a combination of hydrodynamic escape, intense sputtering loss, and large-scale impact erosion which would have depleted atmospheric Ar to levels well below its current abundance. The current 36Ar/38Ar value of 4.2 ± 0.1 must then have been set by largely the balancing of atmospheric loss through solar wind erosion with the outgassing of mantle Ar with a solar 36Ar/38Ar ratio of 5.5 (e.g., trapped interior component of Chassigny with 36Ar/38Ar ≥5.26) [Mathew and Marti, 2001] since about 4 Ga. The specific history of the atmospheric 36Ar/38Ar value depends on the details of the rates of outgassing from volcanoes, additions or loss from impacts, and atmospheric erosion with time. Previous models [Jakosky et al., 1994; Pepin, 1994; Hutchins and Jakosky, 1996; Hutchins et al., 1997] indicate that loss of at least 50% of the original atmospheric argon is required and probably as much as 85–95% if other sources of chondritic 36Ar/38Ar contribute (e.g., late chondritic impacts or later-than-anticipated outgassing) to achieve the 36Ar/38Ar value determined by SAM and reported in this paper.
[12] The low 36Ar/38Ar ratio measured by SAM at Mars is not likely the result of spallogenic nuclear processes, which would require very low chlorine concentrations of <0.1wt.% in upper layers of rocks. Although the mean chlorine content of all surface rocks on Mars is unknown, chlorine has been found to be ubiquitous in every soil ever analyzed in situ (e.g., Clark et al. [1982] from Viking Landers) or from Mars orbit (Keller et al. [2007] from Mars Odyssey). Moreover, Cl concentrations are found to be relatively large, in the 0.3–1.2 wt.% range. If these large Cl abundances are representative also of global values in top layers of rocks, then, depending on the rate of diffusion of (spallogenically generated) argon out of rocks up to the exobase, the 36Ar/38Ar ratio in the Martian atmosphere would be larger, not smaller, than the solar value of 5.5, contrary to the value reported in this paper (4.2). This would imply even greater loss of argon from the atmosphere than discussed above.
5. Summary
[13] The 36Ar/38Ar ratio of 4.2 ± 0.1 determined by the SAM-QMS in the Martian atmosphere is the lowest 36Ar/38Ar yet measured on any object in the solar system, except certain SNCs. This measurement implies loss of atmosphere to space in the past 4 billion years. It also provides a definitive proof that SNCs came from Mars. The argon measurements provide one key element of the suite of measurements that can help unravel the history of loss of the Martian atmosphere. SAM atmospheric measurements are underway to (i) refine the precision of the measurement of the abundance and fractionation in the heavy noble gases, Kr and Xe, and (ii) compare the atmospheric isotope composition of C, O, and H in carbon dioxide and water with those in gases evolved from solid samples [Leshin et al., 2013] that may retain the isotopic signatures from the distant past. The surface-atmospheric measurements also provide ground truth for future upper atmospheric measurements such as those anticipated from the Mars Atmosphere and Volatile EvolutioN (MAVEN) mission where the spacecraft will only venture occasionally low enough to sample the well-mixed atmosphere. The combination of measurements of the current atmospheric isotopic composition and current atmospheric loss rates provided by data from instruments on Curiosity and MAVEN, respectively, may lead to improved models of conditions on Mars in the distant past that might have been more suitable habitats for microbial life.
Acknowledgments
[14] We thank John Grotzinger, Alexander Pavlov, Richard Becker, Andrew Steele, Paul Niles, and Susanne Schwenzer for comments on the manuscript and the MSL Team for successful operation of the mission. This research was supported by the NASA Mars Science Laboratory Project.
[15] The Editor thanks Roger Wiens and an anonymous reviewer for their assistance in evaluating this manuscript.
References
- Biemann K, Owen T, Rushneck DR, LaFleur AL. Howarth DW. The atmosphere of Mars near the surface: Isotope ratios and upper limits on noble gases. Science. 1976;194:76–78. doi: 10.1126/science.194.4260.76. [DOI] [PubMed] [Google Scholar]
- Bogard DD. A reappraisal of the Martian 36Ar/38Ar ratio. J. Geophys. Res. 1997;102:1653–1661. [Google Scholar]
- Bogard DD, Clayton RN, Marti K, Owen T. Turner G. Martian volatiles: Isotopic composition origin, and evolution. Chronol. Evol. Mars. 2001;96:425–458. [Google Scholar]
- Clark BC, Baird AK, Weldon RJ, Tsusaki DM, Schnabel L. Candelaria MP. Chemical composition of Martian fines. J. Geophys. Res. 1982;1982:10,059–10,067. [Google Scholar]
- Grotzinger JP. Mars science laboratory mission and science investigation. Space Sci. Rev. 2012;170 doi: 10.1007/s11214-012-9892-2. [Google Scholar]
- Hutchins KS. Jakosky BM. Evolution of Martian atmospheric argon: Implications for sources of volatiles. J. Geophys. Res. 1996;101:14,933–14,949. [Google Scholar]
- Hutchins KS, Jakosky BM. Luhmann JG. Impact of a paleomagnetic field on sputtering loss of Martian atmospheric argon and neon. J. Geophys. Res. 1997;102:9183–9189. [Google Scholar]
- Jakosky BM, Pepin RO, Johnson RE. Fox JL. Mars atmospheric loss and isotopic fractionation by solar-wind-induced sputtering and photochemical escape. Icarus. 1994;111:271–288. [Google Scholar]
- Keller JM. Equatorial and midlatitude distribution of chlorine measured by Mars Odyssey GRS. J. Geophys. Res. 2007;112 doi: 10.1029/2006JE002679. [Google Scholar]
- Lee JY, Marti K, Severinghaus JP, Kawamura K, Yoo HS, Lee JB. Kim JS. A redetermination of the isotopic abundances of atmospheric Ar. Geochim. Cosmochim. Acta. 2006;70:4507–4512. [Google Scholar]
- Leshin LA. Volatile, isotope and organic analysis of solid samples of Martian fines with the Mars curiosity rover. Science. 2013;341 doi: 10.1126/science.1238937. doi: 10.1126/science.1238937. [DOI] [PubMed] [Google Scholar]
- Luhmann JG, Johnson RE. Zhang MHG. Evolutionary impact of sputtering of the Martian atmosphere by O + pickup ions. Geophys. Res. Lett. 1992;19:2151–2154. [Google Scholar]
- Mahaffy PR, Niemann HB, Alpert A, Atreya SK, Demick J, Donahue TM, Harpold DN. Owen TC. Noble gas abundances and isotope ratios in the atmosphere of Jupiter from the Galileo Probe Mass Spectrometer. J. Geophys. Res. 2000;105:15,061–15,071. [Google Scholar]
- Mahaffy PR. The sample analysis at Mars investigation and instrument suite. Space Sci. Rev. 2012;170(1–4):401–478. doi: 10.1007/s11214-012-9879-z. [Google Scholar]
- Mahaffy PR, et al. Abundance and isotopic composition of gases in the Martian atmosphere: First results from the mars curiosity rover. Science. 2013;341(6143):263–266. doi: 10.1126/science.1237966. doi: 10.1126/science.1237966. [DOI] [PubMed] [Google Scholar]
- Mathew KJ. Marti K. Early evolution of Martian volatiles: Nitrogen and noble gas components in ALH84001 and Chassigny. J. Geophys. Res. 2001;106:1401–1422. [Google Scholar]
- Nier AO. McElroy MB. Composition and structure of Mars' upper atmosphere: Results from the neutral mass spectrometers on Viking 1 and 2. J. Geophys. Res. 1977;82:4341–4349. [Google Scholar]
- Owen T. In: The composition and early history of the atmosphere of Mars. Kieffer HH, et al., editors. Tucson, Ariz: Univ. of Arizona Press; 1992. pp. 818–834. [Google Scholar]
- Owen T. Biemann K. Composition of the atmosphere at the surface of Mars: Detection of argon-36 and preliminary analysis. Science. 1976;193:801–803. doi: 10.1126/science.193.4255.801. [DOI] [PubMed] [Google Scholar]
- Owen T, Biemann K, Rushneck DR, Biller JE, Howarth DW. Lafleur AL. The composition of the atmosphere at the surface of Mars. J. Geophys. Res. 1977;82:4635–4638. [Google Scholar]
- Pepin RO. Evolution of the Martian atmosphere. Icarus. 1994;111:289–304. [Google Scholar]
- Pepin RO, Schlutter DJ, Becker RH. Reisenfeld DB. Helium, neon, and argon composition of the solar wind as recorded in gold and other Genesis collector materials. Geochim. Cosmochim. Acta. 2012;89:62–80. [Google Scholar]
- Swindle TD, Carfee MW. Hohenberg CM. Xenon and other noble gases in shergottites. Geochim. Cosmochim. Acta. 1986;50:1001–1015. [Google Scholar]
- Vogel N, Heber VS, Baur H, Burnett DS. Wieler R. Argon, krypton, and xenon in the bulk solar wind as collected by the Genesis mission. Geochim. Cosmochim. Acta. 2011;75:3057–3071. [Google Scholar]
- Webster CR, et al. Isotope ratios of H, C, and O in Martian atmospheric carbon dioxide and water measured by the Curiosity rover. Science. 2013;341(6143):260–263. doi: 10.1126/science.1237961. [Google Scholar]
- Wiens RC, Becker RH. Pepin RO. The case for a Martian origin of the shergottites, II. Trapped and indigenous gas components in EETA 79001 glass. Earth Planet. Sci. Lett. 1986;77:149–158. [Google Scholar]
- Wong MH, et al. MSL/SAM measurements of nitrogen and argon isotopes in the Mars atmosphere. LPI Contrib. 2013;1719:1712. [Google Scholar]


