Abstract
Background and Aims Myrcia section Aulomyrcia includes ∼120 species that are endemic to the Neotropics and disjunctly distributed in the moist Amazon and Atlantic coastal forests of Brazil. This paper presents the first comprehensive phylogenetic study of this group and this phylogeny is used as a basis to evaluate recent classification systems and to test alternative hypotheses associated with the history of this clade.
Methods Fifty-three taxa were sampled out of the 120 species currently recognized, plus 40 outgroup taxa, for one nuclear marker (ribosomal internal transcribed spacer) and four plastid markers (psbA-trnH, trnL-trnF, trnQ-rpS16 and ndhF). The relationships were reconstructed based on Bayesian and maximum likelihood analyses. Additionally, a likelihood approach, ‘geographic state speciation and extinction’, was used to estimate region- dependent rates of speciation, extinction and dispersal, comparing historically climatic stable areas (refugia) and unstable areas.
Key Results Maximum likelihood and Bayesian inferences indicate that Myrcia and Marlierea are polyphyletic, and the internal groupings recovered are characterized by combinations of morphological characters. Phylogenetic relationships support a link between Amazonian and north-eastern species and between north-eastern and south-eastern species. Lower extinction rates within glacial refugia suggest that these areas were important in maintaining diversity in the Atlantic forest biodiversity hotspot.
Conclusions This study provides a robust phylogenetic framework to address important ecological questions for Myrcia s.l. within an evolutionary context, and supports the need to unite taxonomically the two traditional genera Myrcia and Marlierea in an expanded Myrcia s.l. Furthermore, this study offers valuable insights into the diversification of plant species in the highly impacted Atlantic forest of South America; evidence is presented that the lowest extinction rates are found inside refugia and that range expansion from unstable areas contributes to the highest levels of plant diversity in the Bahian refugium.
Keywords: Aulomyrcia, Atlantic forest, diversification rates, diversity patterns, extinction, GeoSSE, Marlierea, Myrcia, Myrtaceae, speciation
INTRODUCTION
‘The distinction between Myrcia and Marlierea is a somewhat nebulous one, and it is probable that Marlierea comprises a phylogenetically diverse assemblage of species which have been arbitrarily assigned to the genus because of the character of the irregularly splitting calyx.’ (McVaugh, 1956, page 166).
Myrcia s.l. (sensu Lucas et al., 2007) comprises 753 species (Govaerts et al., 2014); representing the second-largest Neotropical genus of Myrtaceae after Eugenia (1038 species; Govaerts et al., 2014). Taxonomic classification in Myrcia has traditionally emphasized characters of the calyx and mode of opening of the flower (McVaugh, 1956; Berg, 1855–1856); as described, molecular studies show that these traits do not group species naturally (Lucas et al., 2007, 2011).
The most recent phylogenetic analysis of Myrcia s.l. (Lucas et al., 2011) recovered nine major clades that have been treated as nine informal groups. Myrcia section Aulomyrcia (Lucas et al., unpubl. res.) represents one of these groupings. This section occurs primarily in rainforests from two of the most threatened biomes in the world, with ∼80 species in Amazonia and related forests of Central and northern South America and ∼40 species in the Atlantic coastal rainforests of Brazil (Lucas et al., unpubl. res.). According to Lucas et al. (2011), representatives of this clade are characterized by a closed calyx and a combination of flower, inflorescence and venation characters (Supplementary Data Fig. S1), such as: (1) inflorescence axes emerging from a single terminal whorl and primary axes irregularly or asymmetrically branched; (2) calyx lobes free to partially fused, irregularly tearing vertically through the hypanthium; (3) hypanthium extended into a tube flared beyond the ovary, but inconspicuous after deep tearing; and (4) a (usually) bilocular ovary, with two ovules per locule.
As currently circumscribed, Myrcia s.l. encompasses four genera that were recognized during most of the 20th century: Calyptranthes, Gomidesia, Myrcia and Marlierea. However, earlier studies (Lucas et al., 2011) have shown that the first two genera are monophyletic and nested in the paraphyletic Myrcia and Marlierea, indicating that the current circumscription of these genera needs to be re-evaluated. McVaugh (1956) suspected the artificiality of Marlierea but maintained the genus in his studies on Tropical American Myrtaceae based on the presence of persistent bracts and the abortion of the primary axis of the inflorescence.
The importance of Myrcia s.l. as a structural and ecological component of the Atlantic rainforest of Brazil makes this group a good proxy for the diversity of other important angiosperm groups in the Atlantic rainforest (Murray-Smith et al., 2009), one of the biodiversity hotspots (Myers et al., 2000). Within this hotspot there are areas of higher angiosperm diversity and areas of elevated Myrcia diversity (Murray-Smith et al., 2009) that may correspond to refugia (Carnaval and Moritz, 2008). Refugia (Haffer, 1969) are thought to act as ‘museums’ for the maintenance of biodiversity due to their expected lower extinction rates and/or higher speciation rates, when compared with unstable areas, resulting in a positive net diversification balance. Refugia can act as ‘safe havens’ (Keppel et al., 2012) where biota would be protected for a long time, contributing important pieces of information for a better understanding of the evolutionary processes that may have driven modern species diversity.
This study aimed to test the monophyly of Myrcia section Aulomyrcia and further test its phylogenetic placement in the genus and to examine phylogenetic relationships within this section. This phylogenetic framework was then used as a basis to make inferences on the evolutionary history of Myrcia section Aulomyrcia, especially concerning speciation and extinction dynamics along the Atlantic forest.
MATERIAL AND METHODS
Taxonomic and molecular sampling
We sampled 93 taxa of tribe Myrteae, including 83 Myrcia s.l., of which 55 were from Myrcia section Aulomyrcia, encompassing as much as possible of the morphological and geographical variation observed in the group (Appendix; Supplementary Data Fig. S1). Approximately 40 % of species in section Aulomyrcia (Lucas et al., unpubl. res.) were represented. Representatives of all four traditionally recognized genera of Myrcia s.l. (Calyptranthes, Gomidesia, Myrcia and Marlierea), representative of all nine clades and subclades of section Aulomyrcia of Lucas et al. (2011), were included. Ten additional genera from Myrteae were also studied. Myrtus communis was used as the outgroup taxon in all analyses, following Lucas et al. (2011). To encompass morphological and geographical variation of widely distributed species, multiple individuals were included for those taxa. For example, we included phenotypes from the morphological range of Myrcia racemosa; specimens from Bahia have significantly thicker leaves and inflorescences than those from São Paulo. Species names and nomenclature used in this work follow Sobral et al. (2014). The internal transcribed spacer (ITS) of the ribosomal nuclear region and four plastid markers (psbA-trnH, trnL-trnF, trnQ-rps16 and ndhF) were used (Supplementary Data Table S1), leading to a total of 461 sequence accessions (Appendix), 64 % of which were generated for this study and 36 % were obtained from other studies (Lucas et al., 2007, 2011; MF Santos, USP, São Paulo, Brazil, unpubl. res.; C. Wilson, RBG Kew, UK, unpubl. res.).
DNA sequencing
Total DNA was extracted mainly from 0·3 g of silica-gel-dried leaf material (in some cases from 0·2 g when using herbarium specimens) using a modified version 2× cetyltrimethyl ammonium bromide (CTAB) protocol (Doyle and Doyle, 1987). Total DNA was further purified for long-term storage in the RBG Kew DNA and Tissue Collections by equilibrium centrifugation in caesium chloride–ethidium bromide gradients (1·55 g ml–1) followed by butanol extraction of ethidium bromide and dialysis to remove caesium chloride. Amplification and purification of target DNA regions were executed according to the protocols outlined in Lucas et al. (2007, 2011) using internal primers for some regions when necessary (Appendix). PCR conditions are listed in Supplementary Data Table S2. Sequencing was performed according to Lucas et al. (2007). DNA sequences were assembled using MUSCLE (Edgar, 2004) and edited in GENEIOUS 7.0.2 with subsequent manual adjustments when necessary. Any doubtful base calls and all phylogenetically informative base changes were compared with the general consensus and individually checked. A paralogous copy of ITS was identified in Myrcia riodocensis and this sequence was excluded. All sequences have been deposited in GenBank and the DNA samples are deposited in the RBG Kew DNA and Tissue Collections (Appendix). Due to the inheritance of the plastid genome as a single linked unit, the resulting four plastid DNA matrices were combined into a single dataset of 3477 bp. The molecular datasets, nuclear and combined plastid regions, were then analysed independently and simultaneously.
Phylogenetic analyses
Phylogenetic reconstruction using maximum likelihood (ML) and Bayesian inference was performed on all datasets. For the combined dataset, one partition was applied for the nuclear region and another for all four plastid regions combined. The best nucleotide substitution model estimated for each partition according to the Akaike information criterion (AIC) using JModeltest 2 (Darriba et al., 2012) was GTR + gamma + invariable sites.
Maximum likelihood analyses were performed with RAxML v7.6.3 (Stamatakis, 2006) using the rapid bootstrap algorithm with 1000 replicates, combined with a search of the best- scoring ML tree under default parameters. Bayesian analyses were performed using MrBayes, version 3.2.1 (Ronquist et al., 2012). Two independent analyses, each of four chains, were conducted with 5 000 000 generations of Monte Carlo Markov chains (MCMCs) and a sampling frequency of 1000. Results were examined with Tracer version 1.5 (Drummond and Rambaut, 2007) to ensure that the analyses reached convergence and that the effective sample size of each parameter was >200. A consensus tree with posterior probabilities (PPs) was generated using the ‘sumt’ option in MrBayes with the default burn-in of 10 % (500 trees in our case). The consensus tree and PPs were visualized with FigTree 1.4 (Rambaut, 2012).
To estimate the temporal evolution of Myrcia s.l. we used the Bayesian inference approach implemented in the package BEAST version 1.8.0 (Drummond et al., 2012) using the combined data set and applying the same partitions and models as previously described for the MrBayes analyses (see above). An uncorrelated relaxed molecular clock assuming a lognormal distribution of rates and a Yule speciation model were applied. Four runs of 30 000 000 generations were performed, sampling one tree every 1000th generation. Parameter convergence was confirmed by examining their posterior distributions in TRACER version 1.5 (Drummond and Rambaut, 2007). The MCMC sampling was considered sufficient when the effective sampling size of each parameter was >200, as assessed using TRACER version 1.5. All analyses were performed on the CIPRES portal (Miller et al., 2010). A maximum clade credibility tree with median branch lengths and a 95 % highest posterior density interval on nodes was built using TreeAnnotator version 1.8 (Drummond et al., 2012) based on the remaining set of trees after burn-in (for each run a burn-in period of 3 000 000 generations was applied). Calibration was performed using the fossil Paleomyrtinaea princetonensis from the Palaeocene (Crane et al., 1990; Pigg et al., 1993) to the early Eocene (Manchester, 1999) of North America, comprising well-preserved fruits and seeds, which is probably related to Psidium and/or Mosiera (Pigg et al., 1993). The most recent common ancestor of Myrteae and the outgroup taxa (crown node of Myrteae) was constrained using a lognormal distribution with a median of 55·8 Mya (corresponding to the lower bound of the Eocene), lower quartile (2·5 %) of 54·94 Mya and superior quartile of (97·5 %) 61·9 Mya, achieved using an offset value of 54·8 Mya.
Diversification analyses
We used a likelihood approach, geographic state speciation and extinction (GeoSSE) (Goldberg et al., 2011), as implemented in the R package ‘diversitree’ (FitzJohn, 2012), to estimate region-dependent rates of speciation, extinction and dispersion from the calibrated phylogenetic trees generated with BEAST. We removed outgroup taxa and pruned the tree to only include species occurring in the Atlantic forest, each represented by one accession to avoid potential bias from over-representation of intraspecific variation. The resulting tree contained 31 terminals (∼60 % of Myrcia section Aulomyrcia species known for this biome, plus nine undetermined species that will be taxonomically treated in other studies). The majority of the 40 % of species of Aulomyrcia not sampled here are species known only from the type, some of which may eventually be synonymized with currently described species (Lucas et al., unpubl. res.). However, we evaluated the possible bias of incomplete molecular sampling in GeoSSE, and taking this into account using the ‘sampling.f’ function of ‘diversitree’ package did not qualitatively change the results, although there was an increase in support of the estimated rates, thus reinforcing the interpretations proposed here based on the assumption of complete sampling. The results from the incomplete molecular sample are available from the first author upon request.
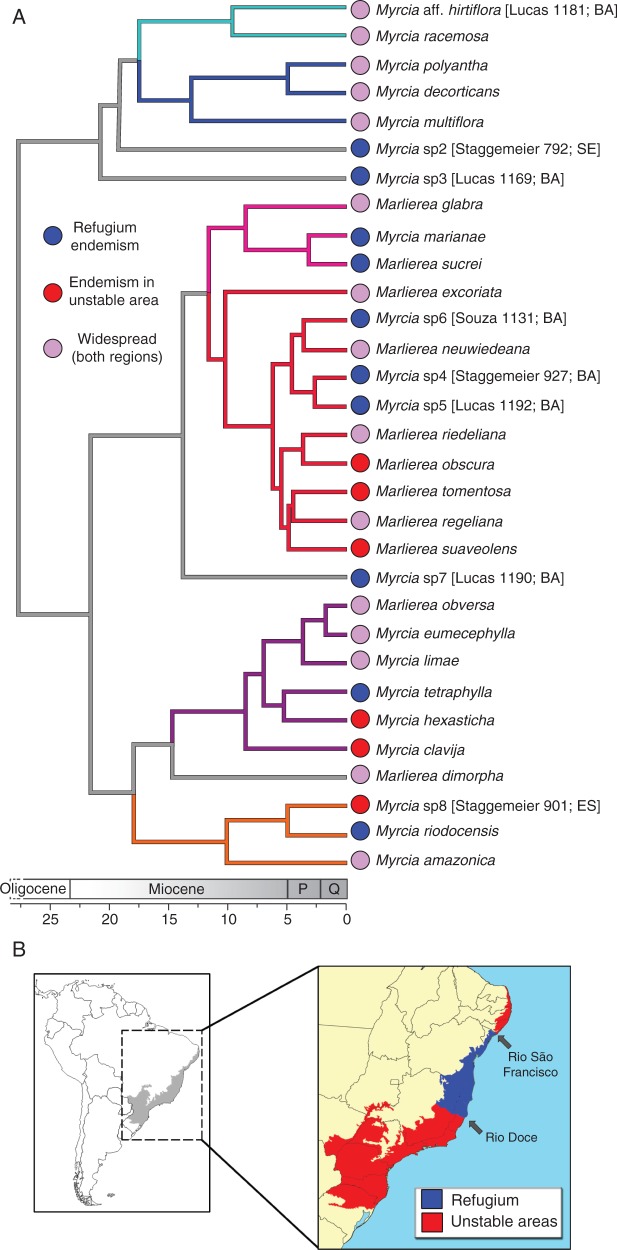
Occurrence records were obtained for each species from herbarium collection records from the CRIA database (speciesLink, 2014) and filtered to remove clearly erroneous localities and identifications as recommended by Giovanni et al. (2012). Each species was scored (see below) in accordance with forest refugium hypothesis proposed for the Atlantic forest by Carnaval and Moritz (2008). These authors identified refugia as historically stable areas where forest presence was inferred by hindcasting the distribution of the biome using ecological niche modelling including two Atlantic forest definitions, one narrower and other broader. Stable areas were defined if a given place belonged to the biome in past projections using two methods (BIOCLIM and MAXENT) and two time frames (6 and 21 ka). Using this approach, Carnaval and Moritz (2008) inferred two refugia areas in the Atlantic forest: a large area in the central corridor (Bahia) along the Brazilian coast, extending from the Rio Doce northward to the Rio São Francisco (Fig. 1), and a geographically restricted and smaller area in the state of Pernambuco. The Bahian refugium hypothesis was more robust, being inferred from all analyses, whereas the Pernambuco region was returned in incongruent positions related to the definition (broader or narrower) of Atlantic forest that was applied. In addition, the Bahian refugium encompasses a larger area, more suitable for macroecological analyses; for these reasons we used the Bahian refugium only in our GeoSSE analyses.
Fig. 1.
Chronogram showing phylogenetic relationships, divergence times and geographical distribution of the 31 species of section Aulomyrcia (A) found in the Atlantic Forest (B; grey area). The Bahia refugium (blue; see text) and unstable areas (red), as proposed by Carnaval and Moritz (2008). Branches for subclades are coloured to identify the informal clades described here (Subclades are detailed in Fig. 2 and 3)
In the GeoSSE model each species is coded for three states (Fig. 1): endemic to a refugium (‘A’), endemic to unstable areas (‘B’) or widespread, occurring in both regions (‘AB’). In this two-region model, range expansions are considered transitions from A or B to AB, occurring with per-lineage dispersal rates dA and dB, respectively. The reverse process, range contraction from AB to A or B, occurs with per-lineage extinction rates xB and xA, respectively. Extinction from a region is independent of presence in the other regions, so global extinction of species from states A and B also occurs at per-lineage rates xA and xB, respectively. Lineage extinction in the GeoSSE model thus depends on range size and location, because more events are required for extinction of an AB species. The method does not allow instantaneous transitions between A and B or immediate global extinction of AB because each of these requires two events. All model rates are constant over time and across lineages. Speciation occurs within each region (sA and sB) and when a widespread species produces two new species, one in each different region (sAB). We consider sAB not to be negligible in our scenario because study species with small ranges occur in the transition of refugium and unstable areas; these species might therefore give rise two new species, one in A and other in B as a result of a single cladogenetic event.
We constructed two likelihood functions representing different evolutionary models for the relationship between occurrence and diversification rate using the ‘subplex’ algorithm to search the likelihood space (R package ‘diversitree’; FitzJohn, 2012). We estimated the full model (all parameters were allowed to vary) and a model with free extinction (sA ∼ sB ∼ sAB; dA ∼ dB). The parameters were estimated by ML and we used the AIC ML to compare adjustment between the models. The best model is the one with the lowest AIC score but models scoring no more than 2 units higher still have ‘substantial’ support (Burnham and Anderson, 2002). All tests were repeated for each of the 1000 phylogenetic trees sampled randomly from the Bayesian posterior distribution produced by BEAST to represent variation among dated trees (uncertainty of phylogenetic hypothesis and calibration) that could yield wide confidence intervals for clade ages.
In accordance with the refugium hypothesis (Haffer, 1969) we would expect to find the highest diversification balance within refugia due to lower extinction rates in these areas (xA < xB). In contrast we would expect lower net diversification and higher extinction rates in historically unstable regions.
RESULTS
Phylogenetic relationships
The ITS partition presented greater variability (18·3 % potentially informative characters) than the four combined plastid regions (6·5 ± 1·6 %; Table 1). Analyses conducted for each partition (nuclear and plastid) returned topologies inspected by eye and found to be congruent for major clades. The combined dataset provided greater resolution and higher levels of support; our results and discussion therefore focus on the combined results. The phylogenetic reconstructions obtained with ML and Bayesian results were highly congruent. The ML tree is available as Supplementary Data Fig. S2 and the Bayesian results are used here for further discussion.
Table 1.
Sequence statistics summary for ITS and the combined plastid region (CP) used to infer phylogenetic relationships in Myrcia section Aulomyrcia
| CP (%) | ITS (%) | |
|---|---|---|
| Number of taxa | 93 | 90 |
| Aligned length (bp) | 3477 | 748 |
| Variable characters | 567 | 229 |
| Potentially parsimony-informative characters (%) | 226 (6·5) | 137 (18·3) |
| Mean GC content (%) | 25·0 | 50·1 |
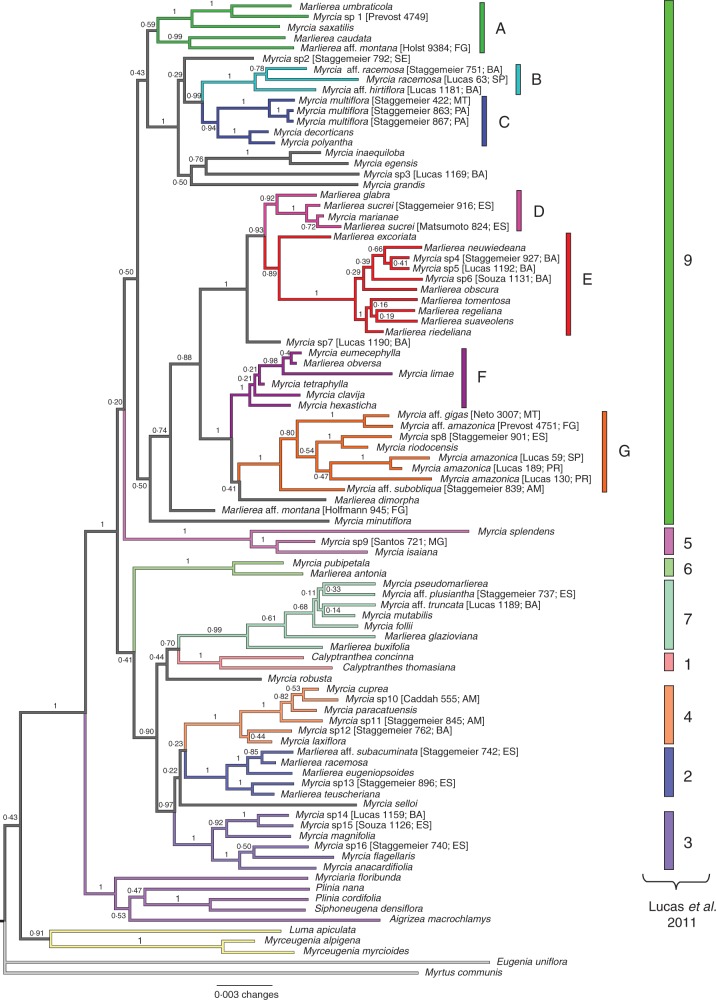
Myrcia section Aulomyrcia emerges as a monophyletic group, but with low support (PPBEAST, 0·88; PPBAYES, 0·50). Inside Myrcia section Aulomyrcia, seven subclades were identified (Figs 2 and 3) that correspond entirely or with minor exceptions to consistent combinations of morphological characters (Fig. 4). Usually these characters, taken individually, are not informative to place a species in a particular subclade, but when they are taken altogether, the seven subclades become diagnosable morphologically (Fig. 4). The other sections identified in Lucas et al. (2011) were well supported here (PPBEAST, 1·0; PPBAYES, 0·91) except for clade 8, as only one species was included and its position was unstable between analyses. The relationships among clades were not always well supported.
Fig. 2.
Phylogenetic hypothesis for Myrcia section Aulomyrcia, maximum credibility tree and branch lengths generated from the Bayesian inference based on a combined dataset of nuclear and plastid DNA markers (ITS, psbA-trnH, trnL-trnF, trnQ-rpS16, ndhF). Clades from Lucas et al. (2011) are indicated by the numbers 1–9 on the right of the tree. Subclades in section Aulomyrcia are coloured to identify informal clades as defined here. Values on branches are posterior probabilities.
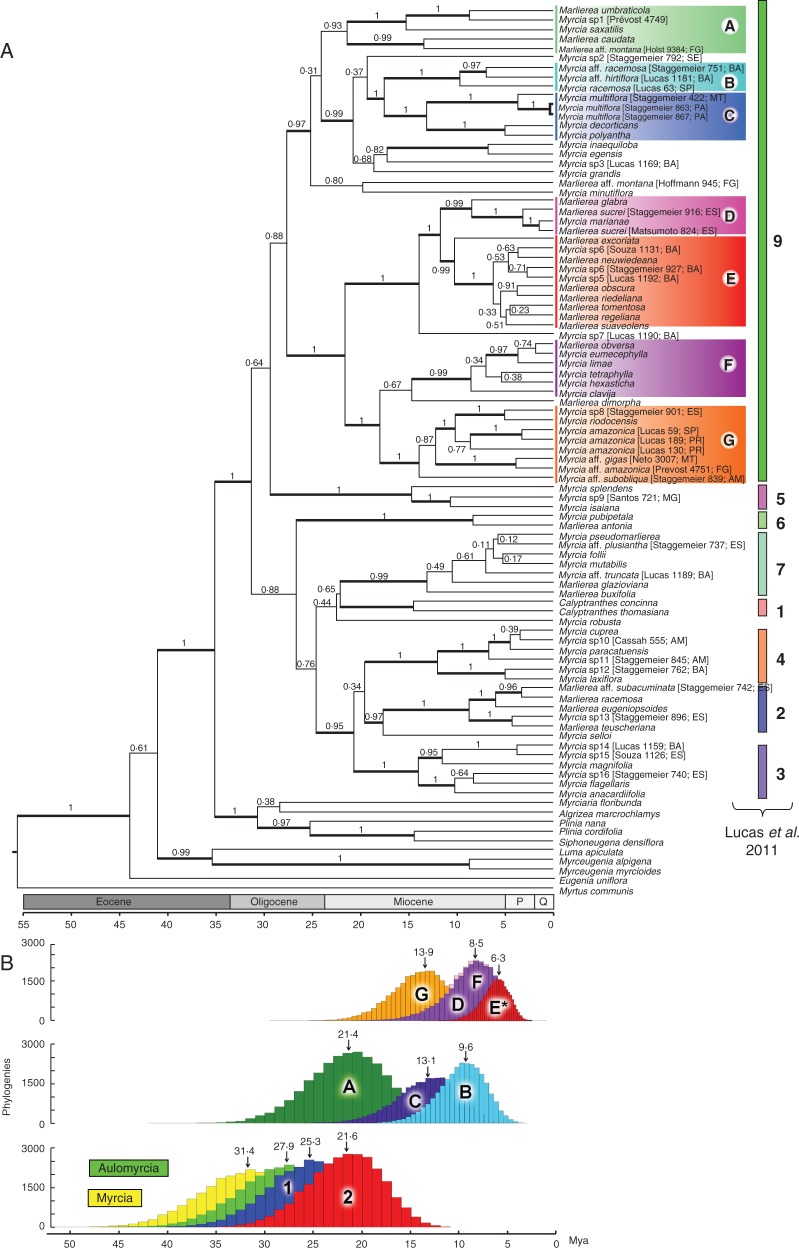
Fig. 3.
Maximum credibility clade tree (A) with divergence time estimates and posterior probabilities obtained from the BEAST analyses of Myrcia section Aulomyrcia based on combined analyses of nuclear (ITS) and plastid (psbA-trnH, trnL-trnF, trnQ-rpS16, ndhF) regions and using Paleomyrtinaea princetonensis as a calibration point on the root of the tree (i.e. crown node of tribe Myrteae; 55·8 Mya). Posterior probability values are indicated on branches; branches with values ≥ 0·95 are thicker. P, Pliocene; Q, Quaternary. Histograms illustrate uncertainty around the age estimates of the crown node of selected clades produced by the 27 000 post-burn-in trees obtained from BEAST (B).
Fig. 4.
Combinations of morphological traits supporting the results of the phylogenetic analyses based on molecular data in Myrcia section Aulomyrcia. Branch colours identify informal clades within section Aulomyrcia as defined here. Shape of calyx in the bud: Myrcia multiflora (I), Marlierea obversa (II), Marlierea glabra (III); midveins: Myrcia marianae (IV), Myrcia aff. subobliqua (V), Marlierea obversa (VI), Myrcia inaequiloba (VII); corky petioles in Marlierea umbraticola (VIII); grey dense pubescence in the mature fruits of Myrcia racemosa (IX); free calyx lobes in immature fruits of Myrcia polyantha (X); triangular panicles in Myrcia decorticans (XI); long bracts in Myrcia marianae (XII); buds of Marlierea neuwiedeana (XIII); old flower illustrating irregularly splitting calyx lobes in Marlierea excoriata (XIV); verticillate arrangement in the leaves in Myrcia tetraphylla (XV); stout inflorescence in Myrcia aff. subobliqua (XVI); pyramidal inflorescence in Myrcia amazonica (XVII).
The first group in section Aulomyrcia includes subclades A, B and C plus seven species in the BEAST analysis (or five species in Bayesian analysis) (PPBEAST, 0·97; PPBAYES, 0·43; Group 1, Fig. 3). The two additional species, Marlierea aff. montana (Hoffman 945) and Myrcia minutiflora, emerge with a second group composed of subclades D, E, F and G (PPBEAST, 0·99; PPBAYES, 0·5; Group 2, Fig. 3) after Bayesian analysis.
Species from subclade A are from the Amazon region and are divided into two further species groups: Marlierea umbraticola, Myrcia sp1 (Prévost 4749) and Myrcia saxatilis emerge as sister to Marliera caudata plus Marlierea aff. montana (Holst 9384). Subclade B is composed of Myrcia racemosa plus Myrcia aff. hirtiflora. Subclade C includes Myrcia multiflora, the type species of Myrcia section Aulomyrcia [Myrcia multiflora] plus Myrcia decorticans and Myrcia polyantha.
The second group is composed mainly of species from the Atlantic rainforest. Subclades D and E comprise species currently described as Marlierea due to their wholly or partially fused calices, morphological traits of this traditional genus (sensu Cambessèdes, 1829). Marlierea sucrei emerges in subclade D with Marlierea glabra and Myrcia marianae, a recently described (Staggemeier and Lucas, 2014) species from Una, Bahia. Subclade G includes multiple accessions of Myrcia amazonica from northern and southern Brazil, a further Amazonian species, Myrcia aff. subobliqua and two species from Espírito Santo (Myrcia riodocensis and Staggemeier 901). In summary, subclades B, C and G are composed exclusively of Myrcia sensu DC., whereas D and E are exclusively comprised of Marlierea sensu Cambessèdes; clades A and F contain species currently described as both taxa.
Dating
Trees obtained from the BEAST analyses agree well with those from the Bayesian and ML phylogenetic hypotheses. The mean age for Myrcia s.l. is 31·4 million years ago (Mya) with 95 % confidence limits (95 % CI) of 22·3–41·0 Mya (Late Miocene to Early Eocene; Fig. 3B). The average age of Myrcia section Aulomyrcia (Lucas et al., unpubl. res.) is 27·9 Mya (95 % CI 19·3–36·3 Mya; Late Miocene to Mid Oligocene; Fig. 3B). Section Aulomyrcia species endemic to the Amazonian and Guiana Shields (subclade A) are older (21·4 Mya, 95 % CI 14·0–29·0 Mya) than Atlantic species (Fig. 3B). Subclade E (excluding Marlierea excoriata) exhibits short branch lengths and younger age estimates; there is no congruence inside this subclade between the Bayesian and BEAST analyses, probably due to low phylogenetic signal within the subclade due to recent speciation 6·2 Mya (95 % CI 3·7–9·3 Mya; Mid Pleistocene to Early Miocene).
Diversification
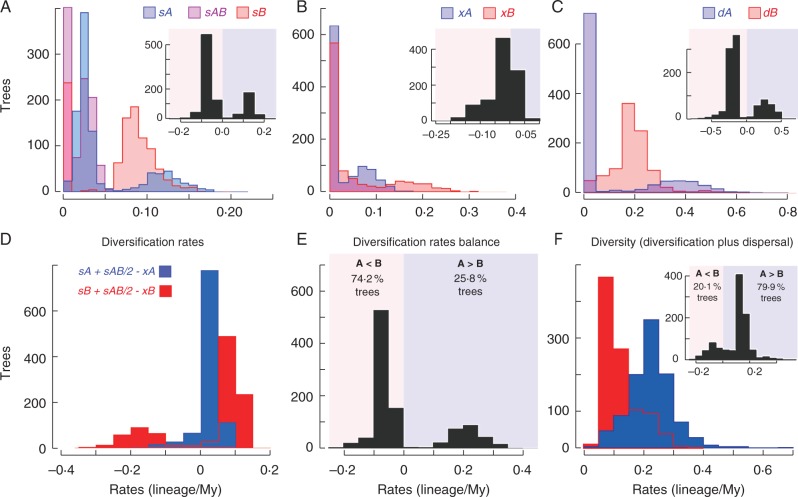
Inside refugium, lineage evolution rates are lower than in unstable areas, a pattern supported in >70 % of trees examined (Fig. 5A–C). Speciation in refugium is three times lower than in unstable areas (median sA, 0·03; Fig. 5A), whereas extinction is 2·2 times lower (xA, 0·0049 × 10–3; Fig. 5B). Results suggest that dispersal from refugium to unstable areas is rare (>1 × 10–4; Fig. 5C), supported by 68·3 % of trees sampled.
Fig. 5.
Frequency histograms of estimated speciation (A), extinction (B) and dispersal (C) rates (events per lineage per million years) in the full model for Myrcia section Aulomyrcia in the Bahian refugium (blue bars; A) versus the unstable areas (red bars; B). Rates of speciation, extinction and dispersal were combined to calculate diversification rates (speciation minus extinction rates; D) and their balance (E); and net increased diversity (diversification plus dispersal rates; F). Inset histograms show the differences in rates between areas (rates A minus B).
Moreover, within refugium net diversification is lower than elsewhere (median A, 0·04; median B, 0·08; Fig. 5D), supported in 74·2 % of trees (Fig. 5E). However, when all rates (diversification plus dispersal) are taken into account, the diversity balance is positive and higher inside refugium; ∼80 % of trees support the highest diversity in refugium (Fig. 5F). Dispersal events from unstable areas to refugium are common (median dB, 0·18; Fig. 5C).
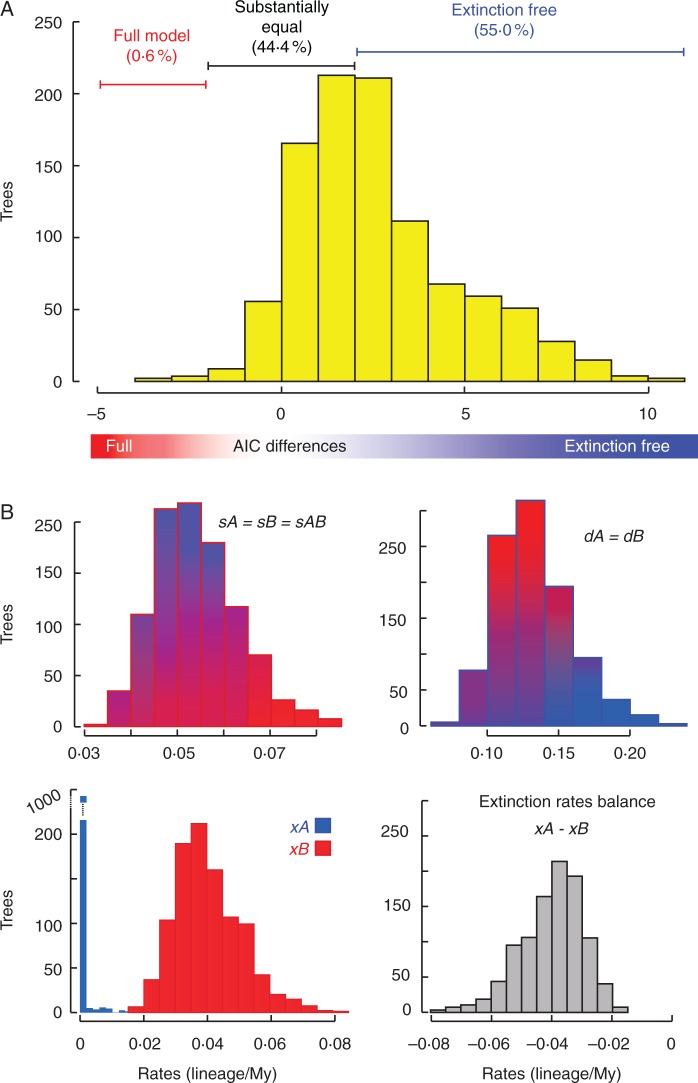
Considering our initial hypothesis under the simplest model, holding dispersal and speciation constant and allowing only extinction to vary, 92·9 % of the trees had lower AIC than for the full model. In addition, the extinction-free model was substantially better (|ΔAIC| > 2) in 55 % of trees (Fig. 6A). Extinction rates inside refugium tend to zero, whereas in other regions they are higher (xB, 0·04 ± 0·01; Fig. 6B), corroborating our initial hypothesis that the highest extinction rates are found in climatically unstable areas; this pattern was strongly supported (100 % of trees; Fig. 6B).
Fig. 6.
(A) GeoSSE results for AIC differences between full and free extinction models. (B) Speciation, dispersal and extinction rates in free extinction model.
DISCUSSION
In this study we reconstruct phylogenetic relationships within Myrcia section Aulomyrcia (Lucas et al., unpubl. res.) based on 53 taxa from this section plus 40 outgroups and using one nuclear and four plastid DNA regions. Our results provide the first comprehensive understanding of phylogenetic relationships in Myrcia section Aulomyrcia, further supporting the need to unite taxonomically the two traditional genera Myrcia and Marlierea in an expanded Myrcia s.l. The morphological distinction of Myrcia and Marlierea described by McVaugh (1956) is that the former has a calyx with five (rarely four) distinct lobes, whereas Marlierea Cambessèdes has a closed or barely open bud in which the calyx splits irregularly at anthesis. This distinction was suspected to be false as some species in each genus have partially fused calyx lobes. Results presented here demonstrate molecular affinity between species until now believed to be in separate genera, confirming the suspicions (e.g. McVaugh 1956, 1968) that these traits cannot be relied on to support such a division.
Our results also supported ideas put forward by McVaugh (1956) regarding the distribution of species of Myrtaceae in South America and their relationship in tropical forests. McVaugh (1956) emphasized the importance of studying species occurring in the Amazon and Atlantic forests to understand the distribution of the family better because species composition from the north-eastern states of Brazil are often distinct from those of southern Brazil and usually distinct from those of the Amazon, but may show affinities to both. In our phylogenetic study, two main arrangements were identified: Amazonian plus north-eastern Atlantic species in subclades A–C (including the widespread species Myrcia multiflora and the widely distributed Atlantic forest species Myrcia racemosa); and north-eastern plus south-eastern species in subclades D–G except for two Amazonian species emerging in subclade G, one of which is the widely distributed Myrcia amazonica.
Myrtaceae date back to the Cretaceous (86 Mya; Sytsma et al., 2004) and our results suggest that Myrcia s.l. probably first evolved at the transition between the Eocene and Oligocene (Fig. 3). Unfortunately, the fossil record of Myrteae is poor as fleshy fruits are rarely well preserved compared with dry fruits from other members of the family (e.g. tribe Eucalypteae). Only a single reliable fossil was available for the calibration procedure and as a result the scenario outlined here to explain the origin and biogeographical history of Myrcia section Aulomyrcia should be treated with caution. The lack of an extensive fossil record and the different calibration node might explain in part the discrepancies between these results and those obtained by Biffin et al. (2010) (a mid-Miocene origin for Myrcia), rather than differences in sampling density for Myrteae. However, dates for subclade A were older (Fig. 3B) than those for other clades (around 8 million of years of divergence), suggesting an Amazonian origin for Myrcia section Aulomyrcia. The highest diversity in this region (twice that in the Atlantic forest) would confirm this origin if speciation rates are assumed to be equivalent throughout the phylogenetic tree.
The emergence of Amazonian and southern species in subclade G supports suggestions of a link between the Amazonian and Atlantic forests. The first-diverging species in subclade G is Myrcia aff. subobliqua, an Amazonian species sister to seven species which can be assigned to two groups. Myrcia gigas and Myrcia amazonica aff. amazonica (Prévost 4751 from French Guyana), also from the Amazon, compose the first group, which emerges as a sister to a group including species from Espírito Santo plus three accessions of Myrcia amazonica from southern Brazil (São Paulo and Paraná). These relationships indicate that the link between these two tropical forest biomes probably took place via the cerrado biome, supported by the presence of the widely distributed species Myrcia amazonica in this clade, which occurs in cerrado but not in caatinga.
It is remarkable that Myrcia inaequiloba, Myrcia egensis, Myrcia grandis (all Amazonian species) and another probably new species from Bahia (Lucas 1169) emerge in a weakly supported subclade that is sister to a further arrangement formed of subclades B and C because these species were presumed, based on shared morphology, to be more closely related to species found in subclade A, comprising only Amazonian species. In subclades B and C, the widespread Myrcia multiflora and the widely distributed Atlantic forest species Myrcia racemosa are sister to four north-eastern species (Myrcia aff. hirtiflora, Staggemeier 792, M. decorticans and M. polyantha) supporting McVaugh’s suggestion that Amazonian species are more closely related to north-eastern rather than south-eastern species of the Atlantic forest. More recently, Batalha-Filho et al. (2013) discussed connections between the Amazonian and Atlantic forests in birds and suggested two distinct connections between these two forests, through the southern portions of cerrado (Mato Grosso and Mato Grosso do Sul) and in the north linking to the north-eastern forests through the caatinga.
There are few Marlierea species in the first group of clades and they have only slightly fused calyx lobes. The majority of species with closed calices occur in the second group of clades, apparently a younger group. The occurrence of completely closed calices throughout clades D and E, with incompletely fused lobes a feature of clade F (and therefore species described as a mix of Myrcia or Marlierea) and free calyx lobes in clade G, suggests that the closure of the calyx was a more recent, secondary event and that the ancestor of Myrcia section Aulomyrcia had an open calyx. The path of calyx closure in the second group of clades is unclear; a hypothesis for future testing is that the ancestor of the second group had free calyx lobes, then full or partial calyx closure occurred independently in clades D + E and in clade F, respectively. Alternatively, the common ancestor of the second group may have had a completely or incompletely fused calyx and the free calyx lobes of clade G results from a subsequent loss of the closed bud.
In recent years, increased investigation of Myrcia s.l. molecular data and morphology has meant that consistently diagnosable species groups are becoming the norm rather than the exception (Lucas et al., 2007, 2011). However, the morphological and molecular data are not always congruent, as shown in some cases, such as in subclade D, where Myrcia marianae, a new species represented by Staggemeier 764 (Staggemeier and Lucas, 2014) emerges between two accessions of Marlierea sucrei (Figs 2 and 3). This new species does not share many morphological traits with Marlierea sucrei, but on a molecular basis they are indistinguishable (Staggemeier and Lucas, 2014). These results demonstrate that the five molecular markers employed here are not sufficiently divergent to separate all morphologically distinct species within this group.
Lucas et al. (2011) found that Marlierea sucrei emerges with six species originally described in Marlierea subsection Clausae sensu Legrand (1962). However, we found Marlierea sucrei to be related to the new species Myrcia marianae (Staggemeier 764) and Marlierea glabra, whereas the other six species from Lucas et al. (2011) are grouped with four more from subclade E. Clades D and E are defined by completely closed buds (Supplementary Data Fig. S1 and Fig. 4) and, except for Marlierea neuwiediana and Marlierea riedeliana, all species were previously assigned to Marlierea. subsection Clausae. Clade E exhibits short branch lengths, very low internal support (Figs 2 and 3) and appears to be the youngest clade in section Aulomyrcia (Fig. 3B).
Marlierea dimorpha emerges with low support as sister to subclade G in the MrBayes analysis (Fig. 2), but as sister to subclade F in the BEAST analysis (Fig. 3). This species resembles those of subclade F (presence of bracts, terminal inflorescence, big flower buds and leaves with a raised midvein).
Myrcia micropetala, included in Lucas et al. (2011), was not included in our analysis as we were unable to amplify every DNA marker; however, in an exploratory analysis with ITS, trnL-trnF and psbA-trnH (not presented here) we found a well-supported arrangement between Myrcia micropetala and Myrcia aff. hirtiflora emerging as sister to Myrcia racemosa. Morphological comparison of material from all taxa included in subclade B demonstrates a striking morphological gradient from thinner forms of leaves and inflorescences in the south of the Atlantic forest to thicker in the north. The type specimen of Myrcia racemosa is from Rio de Janeiro; from there to southern Brazil this species has thin, sometimes membranaceous leaves that become thicker in the more northern part of its distribution (i.e. Bahia). In the geographical region where the distributions of Myrcia racemosa and Myrcia hirtiflora overlap (Bahia) the distinction based on morphology can sometimes be laborious. A similar situation is observed with Myrcia hirtiflora and Myrcia micropetala, where the latter species is encountered with small leaves and inflorescences. Molecular similarity among species in the well supported subclade B is reflected by strong morphological similarities that have produced a wealth of misidentified collections for these three species in many Brazilian and international herbaria. However, although a continuum of certain characters exists between these three species, they are clearly distinguishable entities based on a combination of other traits such as venation and hairs.
Misidentified collections are also common for Myrcia decorticans. Some taxonomists identified many specimens that are morphologically matched with Prévost 4749 as Myrcia decorticans, in line with McVaugh (1969). However, the type for this species is described from Bahia (Martius s.n.) and matches Staggemeier 799 from north-eastern Brazil. The specimen Prévost 4749 could instead be assigned to Marlierea gleasonii; a thorough review of this complex is required.
Lucas et al. (2011) note that clade 8 (Myrcia tomentosa, Myrcia selloi, Myrcia laruoteana in their sample) shares morphological features such as asymmetrical panicles, a glabrous staminal disc and the hypanthium somewhat extended beyond the ovary, with species from clade 9. These species were at some time treated by authors as Aulomyrcia. As noted above, the analysis presented here includes only a single sample of Myrcia selloi but its position is unstable and it repeatedly emerges elsewhere than clade 9; apparently these shared characteristics have evolved more than once in Myrcia s.l. The species of clade 8 consistently exhibit morphological characters quite different from species in clade 9 (e.g. completely free calyx lobes acutely reflexed in the fruit, buds constricted beneath the ovary, and flowering and vegetative branchlets emerging from a single point). It is therefore clear that these groups of species have independent origins.
Elsewhere in Myrcia s.l. the feature of the closed calyx occurs in clades other than Myrcia section Aulomyrcia. A partially closed calyx is also found in the clade containing species of or matching the diagnosis of Eugeniopsis (clade 2; Lucas et al. 2011), a genus now in the synonymy of Marlierea, and in a further clade of species previously described in both Myrcia and Marlierea (clade 7; Lucas et al., 2011). Calyptranthes spp. also have a completely fused calyx (clade 1; Lucas et al., 2011). These clades also demonstrate consistent morphologies otherwise quite separate from the species of Myrcia section Aulomyrcia. In terms of the clades of Lucas et al. (2011), clade 1 and clade 7 have markedly symposial branching, strongly cymose inflorescences, long, internally glabrous turbinate buds with a dehiscent calyptra or short calyx lobes partially tearing horizontally from the rim of the hypanthium, respectively. Clade 2 has partially fused buds that tear longitudinally at anthesis, symmetrical panicles and characteristic lenticels covering the bark and often the underside of the leaves. The clear multiple origins of the closed bud throughout Myrcia s.l. must now be explained by further study of the anatomy of various closed calyx groups alongside the environmental implications of bud closure. Such results, with those presented here, will allow confirmation or otherwise of hypotheses of the timing and significance of calyx closure.
Diversification
The Carnaval–Moritz hypothesis (Carnaval and Moritz, 2008) appears realistic for a representation of biome dynamics when compared with other refugial scenarios proposed for the Atlantic forest (e.g. Thomé et al., 2010; Tonini et al., 2013). The Carnaval–Moritz hypothesis was the only one that modelled the occurrence of the entire forest; others were taxon- specific to fauna (especially anurans) and may be linked with biological requirements of these species rather than the dynamics of the biome as a whole.
Evidence suggests that diversification rates differ in species of Myrcia section Aulomyrcia, with the lowest rates occurring within refugium. However, the tendency of range expansion in unstable areas resulted in multiple species colonizing adjacent refugia. Consequently, range expansion contributes to higher plant diversity in the Bahian refugium. Results indicate that this area acts as a biodiversity museum and centre of species accumulation, maintaining the high diversity of Myrcia section Aulomyrcia in the central corridor of the Atlantic forest. This analysis indicates a positive balance of diversity based on diversification rates and dispersal, in climatically more predictable areas, i.e. refugia. Reasons for higher speciation rates in unstable areas remain to be explored in future studies.
Concluding remarks
This study provides a basic phylogenetic background with which to address ecological and evolutionary questions based on the hyper-diverse clade of Myrcia s.l., an ecologically important Neotropical genus (∼750 species). The inclusion of more species and regions of genome will be important in understanding relationships between the species of Myrcia section Aulomyrcia better. For example, the inclusion in future studies of further species predicted to emerge in clade F (e.g. Marlierea verticillaris and Myrcia insularis) will help clarify the placement of Marlierea dimorpha.
Despite advances presented here to improve understanding of relationships inside Myrcia s.l., much work remains to be done. Amazonian species remain poorly investigated and the relationships between Amazonian and Atlantic forest species still need attention. Insights into these would improve understanding of the early evolution of Myrcia and species disjunctions between some of the most threatened biomes on Earth.
Our GeoSSE analysis was based on a single section of Myrcia s.l. with good representation from the Atlantic forest, but this dataset does not include the complete diversity in the genus. Despite uncertainty in some of the species relationships, the patterns recovered allow conclusions to be drawn about the diversification pattern of species in an area of angiosperm endemism located in a relatively poorly known and highly threatened area of the Atlantic rainforest of north-eastern Brazil. More specifically, significantly lower rates of extinction and accumulation of species inside refugia corroborate the importance of these processes in maintaining diversity in a region that is well known for its great biological richness. Studies evaluating similar questions in an evolutionary context in other taxonomic groups (e.g. birds, mammals, reptiles and other angiosperm families) are greatly needed so that the mechanisms associated with the origin, maintenance and evolution of biodiversity can be deciphered. Finally, based on the ecological importance of this group in Neotropical forests, we expect that the results presented here will provide a basis for further taxonomic work (e.g. Lucas et al., unpubl. res.) and ecological studies investigating drivers of speciation of large genera in tropical forest communities.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Fig. S1: images of species of Myrcia section Aulomyrcia. Fig. S2: maximum likelihood tree. Table S1: primers used for PCR. Table S2: PCR conditions.
ACKNOWLEDGEMENTS
We are grateful to Thomas Both, Laszlo Csiba, Dion Devey, Jim Clarkson, Laura Martinez, J. Floriano Pastore and Maria José G. de Andrade for help in the laboratory; Carol Proença, Jair Q. de Faria Jr, Marcelo C. Souza, Marcos Sobral, M. Anália D. de Souza, Matheus F. Santos and Priscila O. Rosa for Myrtaceae identifications; André Cardoso, Beatriz Simara and M. Anália D. de Souza for logistical support; Matheus F. Santos for discussions about Myrcia sensu lato; and colleagues from Laboratório de Ecologia Teórica e Síntese at UFG for discussions about the GeoSSE analyses. We are especially grateful to Lucia Lohmann, Marcos Sobral, Luis Mauricio Bini and Fabrício Villalobos for reviewing earlier versions of this manuscript, and Ana Carnaval for discussion about Atlantic forest refugia. This work was supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico – Brasil) through edital UNIVERSAL (grant 473468/2010-7) and a sandwich fellowship to V.G.S. to develop part of this research at RBG Kew, UK (236687/2012-3); the Bentham-Moxon Trust supported laboratory costs; and V.G.S. received a PhD fellowship in Brazil from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior).
Appendix
Vouchers for DNA samples used in the phylogenetic analysis, with GenBank numbers (two letters plus six numbers) or the RBG Kew's DNA and Tissue Collections numbers (five numbers)
| Species | Collector | Voucher | ITS | psbA-trnH | trnL-trnF | trnQ-rpS16a | ndhF |
|---|---|---|---|---|---|---|---|
| Algrizea macrochlamys (DC.) Proença & NicLugh. | Giulietti, A.M. 1648 | K | AM234126 | AM489809 | JN091320 | KP722283 | 16833 |
| Calyptranthes concinna DC. | Lucas, E. 74 | K, ESA | KP722378 | AM489817 | KP722334 | KP722231 | KP722454 |
| Calyptranthes thomasiana O.Berg | Pollard, B.J. 1195 | K | AM234106 | AM489820 | JN091325 | KP722211 | KP722434 |
| Eugenia uniflora L. | Lucas, E. 207 | K | AM234088 | AM489828 | KP722326a | KP722202 | KP722418 |
| Luma apiculata (DC.) Burret | Lucas, E. 208 | K | AM234101 | AM489843 | KP722331 | KP722209 | KP722433 |
| Marlierea aff. montana (Aubl.) Amshoff | Hoffmann, B. 945 | US | 43531 | 43531 | 43531 | – | 43531 |
| Marlierea aff. montana (Aubl.) Amshoff | Holst, B. 9384 | – | KP722377 | KP722285a | KP722333a | KP722229 | KP722453 |
| Marlierea aff. subacuminata Kiaersk. | Staggemeier, V.G. 742 | K, UB | KP722397 | KP722305 | KP722355 | KP722252 | KP722475 |
| Marlierea antonia (O.Berg) D.Legrand | Santos, M.F. 840 | SPF, K | 43245 | 43245 | 43245 | KP722277 | 43245 |
| Marlierea buxifolia Amshoff | Clarke, H.D. 5707 | US | – | 43536 | – | – | 43536 |
| Marlierea caudata McVaugh | Zappi, D. 1506 | K | 42097 | 42097 | 42097 | KP722232 | KP722455 |
| Marlierea dimorpha O.Berg | Folli, D.; 6649 | K | KP722416a | KP722324 | KP722374 | KP722271 | KP722494 |
| Marlierea eugeniopsoides (D.Legrand & Kausel) D.Legrand | Lucas, E. 61 | K | AM234107 | AM489845 | JN091327 | KP722205 | KP722429 |
| Marlierea excoriata Mart. | Matsumoto, K. 825 | UEC | JN091203 | JN091394 | JN091328 | KP722226 | KP722449 |
| Marlierea glabra Cambess. | Staggemeier, V.G. 935 | UB, K, RB, IAN, UFG, HUFSJ | KP722391 | KP722299 | KP722349 | KP722245 | KP722469 |
| Marlierea glazioviana Kiaersk. | Matsumoto, K. 799 | UEC | JN091204 | JN091395 | JN091329 | KP722275 | KP722451 |
| Marlierea neuwiedeana (O.Berg) Nied. | Staggemeier, V.G. 793 | UB, K, UFG, RB | KP722402 | KP722310 | KP722360 | KP698774 | KP722480 |
| Marlierea obscura O.Berg | Matsumoto, K. 836 | UEC | JN091205 | JN091396 | JN091330 | KP722228 | KP722452 |
| Marlierea obversa D.Legrand | Matsumoto, K. 820 | UEC | JN091206 | JN091397 | JN091331 | KP722227 | KP722450 |
| Marlierea regeliana O.Berg | Matsumoto, K. 814 | UEC | JN091208 | JN091399 | JN091333 | KP722225 | KP722448 |
| Marlierea riedeliana (O.Berg) D.Legrand | Lucas, E. 88 | K | AM234109 | AM489847 | KP722330 | KP722208 | KP722432 |
| Marlierea suaveolens Cambess. | Lucas, E. 85 | K | AM234108 | AM489846 | KP722329 | KP722207 | KP722431 |
| Marlierea subacuminata Kiaersk. | Lucas, E. 225 | K | JN091207 | JN091398 | JN091332 | KP722218 | KP722443 |
| Marlierea sucrei G.M.Barroso & Peixoto | Matsumoto, K. 824 | UEC | JN091209 | JN091400 | JN091335 | KP722222 | KP722445 |
| Marlierea sucrei G.M.Barroso & Peixoto | Staggemeier, V.G. 916 | UB, K | KP722388 | KP722295 | KP722345 | KP722242 | KP722465 |
| Marlierea teuscheriana (O. Berg.) D. Legrand | Lucas, E. 633 | K | 43712 | 43712 | 43712 | KP722280 | 43712 |
| Marlierea tomentosa Cambess. | Matsumoto, K. 798 | UEC | JN091210 | JN091401 | JN091336 | KP722224 | KP722447 |
| Marlierea umbraticola (Kunth) O.Berg | Souza, M.A.D. sn | INPA | KP722392 | KP722300 | KP722350 | KP722246 | KP722470 |
| Myrceugenia alpigena (DC.) Landrum | Lucas, E. 167 | K | AM234098 | AM489854 | KP722376 | JN661090 | KP722441 |
| Myrceugenia myrcioides (Cambess.) O.Berg | Lucas, E. 82 | K | AM234097 | AM489853 | 16821 | KP722281 | 16821 |
| Myrcia aff. amazonica DC. | Prévost, M.F. 4751 | K | JN091214a | JN091405 | JN091339 | KP722215b | KP722439 |
| Myrcia aff. amazonica DC. | Neto, L.A.; 3007 | INPA | KP722417 | KP722325 | KP722375 | KP722272 | KP722495 |
| Myrcia aff. hirtiflora DC | Lucas, E. 1181 | K | KP722409 | KP722317 | KP722367 | KP722264 | KP722487 |
| Myrcia aff. plusiantha Kiaersk. | Staggemeier, V.G. 737 | K, UB, UFG, RB, IAN | KP722395 | KP722303 | KP722353a | KP722250 | KP722473 |
| Myrcia aff. subobliqua (Benth.) Nied. | Staggemeier, V.G. 839 | K, UB, UFG, RB, IAN, INPA | KP722396 | KP722304 | KP722354 | KP722251 | KP722474 |
| Myrcia aff. truncata Sobral | Lucas, E. 1189 | K | KP722412 | KP722320 | KP722370 | KP722267 | KP722490 |
| Myrcia amazonica DC. | Lucas, E. 130 | K | JN091215 | JN091406 | JN091340 | – | – |
| Myrcia amazonica DC. | Lucas, E. 59 | K | JN091213 | JN091404 | JN091338 | KP722240 | KP722422 |
| Myrcia amazonica DC. [as detergens] | Lucas, E. 189 | K | JN091212 | JN091403 | JN091337 | KP722213 | KP722437 |
| Myrcia anacardiifolia Gardner | Nadruz, M. 999 | K | JN091212 | JN091407 | JN091341 | KP722210 | KP722419 |
| Myrcia clavija Sobral | Lucas, E. 244 | K | JN091220 | JN091411 | KP722332 | KP722217 | KP722442 |
| Myrcia cuprea (O.Berg) Kiaersk. | Staggemeier, V.G. 862 | K, UB | KP722394a | KP722302 | KP722352a | KP722248 | KP722472 |
| Myrcia decorticans DC | Staggemeier, V.G. 799 | UB,K | KP722383 | KP722290 | KP722339a | KP722237 | KP722460 |
| Myrcia egensis (O.Berg) McVaugh | Araújo, M.H.T. 311 | SPF, INPA | 43272 | 43272 | 43272 | – | 43272 |
| Myrcia eumecephylla (O.Berg) Nied. | Matsumoto, K. 803 | UEC | JN091223 | JN091414 | JN091349 | KP722223 | KP722446 |
| Myrcia flagellaris (D.Legrand) Mattos | Lucas, E. 83 | K | AM234113 | AM489836 | JN091350 | KP722206 | KP722430 |
| Myrcia follii G.M.Barroso & Peixoto | Staggemeier, V.G. 907 | UB, K, CVRD, UFG, RB | KP722384 | KP722291 | KP722340a | KP722238 | KP722461 |
| Myrcia grandis McVaugh | Staggemeier, V.G. 850 | UB, K, UFG, RB, INPA | KP722385 | KP722292a | KP722341a | KP698772 | KP722462 |
| Myrcia hexasticha Kiaersk. | Lucas, E. 194 | K | JN091227 | JN091418 | JN091354 | KP722214 | KP722438 |
| Myrcia inaequiloba (DC.) Lemée | Lucas, E. 105 | K | JN091228 | JN091419 | JN091355 | KP722204 | KP722428 |
| Myrcia isaiana G.M.Barroso & Peixoto | Lucas, E. 60 | K | JN091229 | JN091420 | JN091356 | KP722249 | KP722423 |
| Myrcia laxiflora Cambess. | Meirelles, J. 307 | RB | KP722403a | KP722311 | KP722361a,b | KP722257 | KP722481 |
| Myrcia limae G.M.Barroso & Peixoto | Cordeiro, M.J. 310 | RB | 43210a | KP722284 | – | – | – |
| Myrcia magnifolia (O.Berg) Kiaersk. | Lucas, E. 1182 | K | KP722411 | KP722319 | KP722369 | KP722266 | KP722489 |
| Myrcia marianae Staggemeier & Lucas | Staggemeier, V.G. 764 | UB, K, UFG, RB, SPF, IAN, HRCB | KP722381 | KP722288 | KP722337 | KP722235 | KP722458 |
| Myrcia minutiflora Sagot | Sasaki, D. 2394 | K | KP722399 | KP722307 | KP722357a | KP722254 | KP722477 |
| Myrcia multiflora (Lam.) DC. | Staggemeier, V.G. 422 | UB, IAN, HUFSJ | KP722379a | KP722286 | KP722335a | KP722233 | KP722456 |
| Myrcia multiflora (Lam.) DC. | Staggemeier, V.G. 863 | UB, K | KP722386a | KP722293 | KP722342a | KP722239 | KP722463 |
| Myrcia multiflora (Lam.) DC. | Staggemeier, V.G. 867 | UB | KP722387 | KP722294 | KP722343 | KP698771 | KP722464 |
| Myrcia mutabilis (O.Berg) N.Silveira | Mazine, F. 1052 | ESA | JN091233 | JN091424 | KP722344 | KP722241 | KP722435 |
| Myrcia paracatuensis Kiaersk. | Mello-Silva, R. 1713 | K | AM234118 | AM489859 | KP722328a | KP722230 | KP722421 |
| Myrcia polyantha DC | Staggemeier, V.G. 797 | UB, K | KP722400 | KP722308a | KP722358 | KP722255 | KP722478 |
| Myrcia pseudomarlierea Sobral | Souza, M.C. 1139 | RB | KP722404 | KP722312 | KP722362a | KP722258 | KP722482 |
| Myrcia pubipetala Miq. | Lucas, E. 86 | K | AM234114 | AM489855 | JN091364 | KP722273 | KP722426 |
| Myrcia racemosa (O.Berg) Kiaersk. | Lucas, E. 63 | K | AM234120 | AM489861 | JN091366 | KP722259 | KP722424 |
| Myrcia racemosa (O.Berg) Kiaersk. | Staggemeier, V.G. 751 | UB | KP722380 | KP722287 | KP722336a | KP722234 | KP722457 |
| Myrcia riodocensis G.M.Barroso & Peixoto | Staggemeier, V.G. 917 | UB, K, UFG, RB, CVRD | – | KP722296 | KP722346 | KP722243 | KP722466 |
| Myrcia robusta Sobral | Lucas, E. 727 | K | 36229 | 36229 | 36229 | – | 36229 |
| Myrcia saxatilis (Amshoff) McVaugh | Lucas, E. 98 | K | AM234119 | AM489860 | JN091370 | KP722203 | KP722427 |
| Myrcia selloi (Spreng.) N.Silveira | Lucas, E. 110 | K | JN091240 | JN091431 | JN091371 | KP722212 | KP722436 |
| Myrcia sp1 | Prévost, M.F. 4749 | K | JN091221 | JN091412 | JN091347 | KP722216b | KP722440 |
| Myrcia sp2 | Staggemeier, V.G. 792 | UB, K, UFG | KP722382 | KP722289a | KP722338a | KP722236 | KP722459 |
| Myrcia sp3 | Lucas, E. 1169 | K | KP722410 | KP722318 | KP722368 | KP722265 | KP722488 |
| Myrcia sp4 | Staggemeier, V.G. 927 | UB, K, UFG, IAN | KP722390a | KP722298 | KP722348 | KP722244 | KP722468 |
| Myrcia sp5 | Lucas, E. 1192 | K | KP722414 | KP722322 | KP722372 | KP722269 | KP722492 |
| Myrcia sp6 | Souza, M.C. 1131 | RB | KP722405 | KP722313 | KP722363a | KP722260 | KP722483 |
| Myrcia sp7 | Lucas, E. 1190 | K | KP722413 | KP722321 | KP722371 | KP722268 | KP722491 |
| Myrcia sp8 | Staggemeier, V.G. 901 | UB, K, UFG | KP722401 | KP722309 | KP722359a | KP722256 | KP722479 |
| Myrcia sp9 | Santos, M.F. 721 | SPF, K | 43267 | 43267 | 43267 | KP722279 | 43267 |
| Myrcia sp10 | Caddah, M.K. 555 | SPF, INPA | – | 43260 | 43260 | KP722278 | – |
| Myrcia sp11 | Staggemeier, V.G. 845 | UB, K, UFG, IAN | KP722398 | KP722306 | KP722356 | KP722253 | KP722476 |
| Myrcia sp12 | Staggemeier, V.G. 762 | K, UB, UFG | KP722393 | KP722301 | KP722351 | KP722247 | KP722471 |
| Myrcia sp13 | Staggemeier, V.G. 896 | UB, K, UFG, IAN | KP722407 | KP722315 | KP722365a | KP722262 | KP722485 |
| Myrcia sp14 | Lucas, E. 1159 | K | KP722415 | KP722323 | KP722373 | KP722270 | KP722493 |
| Myrcia sp15 | Souza, M.C. 1126 | RB | KP722406a | KP722314 | KP722364a | KP722261 | KP722484 |
| Myrcia sp16 | Staggemeier, V.G. 740 | UB | KP722408 | KP722316 | KP722366 | KP722263 | KP722486 |
| Myrcia splendens (Sw.) DC. | Lucas, E. 73 | K | AM234122 | AM489863 | JN091374 | KP722274 | KP722425 |
| Myrcia tetraphylla Sobral | Staggemeier, V.G. 926 | UB, K, UFG, HUFSJ, RB, R, CVRD | KP722389 | KP722297 | KP722347 | KP698773 | KP722467 |
| Myrciaria floribunda (H.West ex Willd.) O.Berg | Mazine, F. 796 | K | AM234094 | AM489870 | 16827 | KP722282 | 16827 |
| Myrtus communis L. | Lucas, E. 211 | K | AM234149 | AM489872 | KP722327 | KP722221 | KP722420 |
| Plinia cordifolia (D.Legrand) Sobral | Mazine, F. 957 | K | AM489411 | AM489570 | 20679 | KP722219 | 20679 |
| Plinia nana Sobral | Mazine, F. 662 | K | 35640 | 35640 | 35640a | KP722276 | 35640 |
| Siphoneugena densiflora O.Berg | Mazine, F. 1050 | K, ESA | AM489412 | AM489571 | JN091389 | KP722220b | KP722444 |
aFor these accessions we used internal primers (see details Table S1). For psbA-trnH we were occasionally unable to amplify the trnH region; we therefore designed an internal primer to complement the forward psbA primer (Table S1) and overlapped these two strands. The trnL intron and trnL–F spacers were generally amplified in one reaction using primers c and f, but in some cases, the intron (primers c and d) and the intergenic spacer (primers e and f) were amplified separately. Two internal primers for region trnQ-rpS16 were used.
bFor these regions we obtained just partial sequences.
Abbreviations – Institution/State: CVRD - Companhia Vale do Rio Doce/ES; EAFM - Instituto Federal de Educação, Ciência e Tecnologia do Amazonas/AM; ESA - Universidade de São Paulo - ESALQ/SP; HRCB - Herbário Rio Clarense/SP; HUFSJ - Universidade Federal de São João del Rei/MG; IAC - Instituto Agronômico de Campinas/SP; IAN - Instituto Agronômico do Norte - Embrapa Oriental/AM; INPA - Instituto Nacional de Pesquisas Amazônicas/AM; K - Royal Botanic Gardens - Kew/UK; R - Museu Nacional/RJ; RB - Jardim Botânico do Rio de Janeiro/RJ; SP - Instituto de Botânica/SP; SPF - Universidade de São Paulo/SP; UB - Universidade de Brasília/UB; UEC - Universidade Estadual de Campinas/SP; UFG - Universidade Federal de Goiás/GO.
LITERATURE CITED
- Batalha-Filho H, Fjeldså J, Fabre P-H, Miyaki CY. 2013. Connections between the Atlantic and the Amazonian forest avifaunas represent distinct historical events. Journal of Ornithology 154: 41–50. [Google Scholar]
- Berg O. 1855–1856. Revisio Myrtacearum Americae. Linnaea 27: 1–472. [Google Scholar]
- Biffin E, Lucas EJ, Craven LA, Costa IR, Harrington MG, Crisp MD. 2010. Evolution of exceptional species richness among lineages of fleshy-fruited Myrtaceae. Annals of Botany 106: 79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. 2002. Model selection and multi-model inference: a practical information-theoretic approach. Springer. [Google Scholar]
- Cambessèdes A. 1829. Marlierea. In: Saint-Hillaire A, Jussieu A, Cambessedes J, eds. Flora Brasiliae Meridionalis. Paris: Belin Bibliopolam. [Google Scholar]
- De Candolle AP. 1828. Prodromus systematis naturalis regni vegetabilis. Paris: Treuttel & Würts. [Google Scholar]
- Carnaval AC, Moritz C. 2008. Historical climate modelling predicts patterns of current biodiversity in the Brazilian Atlantic forest. Journal of Biogeography 35: 1187–1201. [Google Scholar]
- Crane PR, Manchester SR, Dilcher DL. 1990. A preliminary survey of fossil leaves and well-preserved reproductive structures from the Sentinel Butte Formation (Paleocene) near Almont, North Dakota. Fieldiana Geology 20: 1–64. [Google Scholar]
- Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9: 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. 1987. A rapid isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin 19: 11–15. [Google Scholar]
- Drummond A, Rambaut A. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolutionary Biology 7: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond AJ, Suchard MA, Xie D, Rambaut A. 2012. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Molecular Biology and Evolution 29: 1969–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzJohn RG. 2012. Diversitree: comparative phylogenetic analyses of diversification in R. Methods in Ecology and Evolution 3: 1084–1092. [Google Scholar]
- Giovanni R, Bernacci LC, Siqueira MF, Rocha FS. 2012. The real task of selecting records for ecological niche modelling. Natureza e Conservação 10: 139–144. [Google Scholar]
- Goldberg EE, Lancaster LT, Ree RH. 2011. Phylogenetic inference of reciprocal effects between geographic range evolution and diversification. Systematic Biology 60: 451–465. [DOI] [PubMed] [Google Scholar]
- Govaerts R, Sobral M, Ashton P, et al. 2014. World checklist of Myrtaceae. Royal Botanic Gardens, Kew. http://apps.kew.org/wcsp/ (5 April 2014). [Google Scholar]
- Haffer J. 1969. Speciation in Amazonian forest birds. Science 165: 131–137. [DOI] [PubMed] [Google Scholar]
- Keppel G, Van Niel KP, Wardell-Johnson GW, et al. 2012. Refugia: identifying and understanding safe havens for biodiversity under climate change. Global Ecology and Biogeography 21: 393–404. [Google Scholar]
- Legrand CD. 1962. Sinopsis de las Especies de Marlierea del Brasil. Comunicaciones Botanicas del Museo de Historia Natural de Montevideo 3: 1–39. [Google Scholar]
- Lucas EJ, Harris SA, Mazine FF, et al. 2007. Suprageneric phylogenetics of Myrteae, the generically richest tribe in Myrtaceae (Myrtales). Taxon 56: 1105–1128. [Google Scholar]
- Lucas EJ, Matsumoto K, Harris SA, Nic Lughadha EM, Benardini B, Chase MW. 2011. Phylogenetics, morphology, and evolution of large genus Myrcia s.l (Myrtaceae). International Journal of Plant Sciences 172: 915–934. [Google Scholar]
- Manchester S. 1999. Biogeographical relationships of North American Tertiary floras. Annals of the Missouri Botanical Garden 86: 472–522. [Google Scholar]
- McVaugh R. 1956. Tropical American Myrtaceae: notes on generic concepts and descriptions of previously unrecognized species. Fieldiana Botany 29: 145–228. [Google Scholar]
- McVaugh R. 1968. The genera of American Myrtaceae: an interim report. Taxon 17: 354–418. [Google Scholar]
- McVaugh R. 1969. Botany of the Guayana Highland – part III. Myrtaceae. Memoirs of the New York Botanical Garden 18: 79. [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE) 14 November 2010. New Orleans, LA: 1–8. [Google Scholar]
- Murray-Smith C, Brummitt NA, Oliveira-Filho AT, et al. 2009. Plant diversity hotspots in the Atlantic coastal forests of Brazil. Conservation Biology 23: 151–163. [DOI] [PubMed] [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, Fonseca GABd, Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853–858. [DOI] [PubMed] [Google Scholar]
- Pigg KB, Stockey RA, Maxwell SL. 1993. Paleomyrtinaea, a new genus of permineralized myrtaceous fruits and seeds from the Eocene of British Columbia and Paleocene of North Dakota. Canadian Journal of Botany 71: 1–9. [Google Scholar]
- Rambaut A. 2012. FigTree. http://tree.bio.ed.ac.uk/software/figtree/ (October 2013). [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, et al. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobral M, Proença C, Souza M, Mazine F, Lucas E. 2014. Myrtaceae. In: Lista de Espécies da Flora do Brasil. Rio de Janeiro, Brazil: Jardim Botânico do Rio de Janeiro. [Google Scholar]
- speciesLink. 2014. Rede speciesLink is available on: http://www.splink.org.br. ALCB, ASE, BCTw, BHCB, BOTU, CEPEC, CESJ, CGMS, CRI, CVRD, EAC, EAFM, ESA, F, FLOR, FUEL, FURB, G, HAS, HCF, HEPH, HERBAM, HJ, HMC, HPL, HSJRP, HST, HUCO, HUCPE, HUEFS, HUEG, HUEM, HUESB, HUFU, HUMC, HUTO, HVASF, IAC, ICN, INPA, IRAI, JOI, JPB, MAC, MBM, MBML, MFS, MOBOT, MOSS, MPUC, NY, P, PEUFR, R, RB, SinBiota, SP, SPF, SPSF, TEPB, UB, UEC, UESC, UFACPZ, UFG, UFP, UFRN, UPCB, VIES (12 January, 2014).
- Staggemeier VG, Lucas E. 2014. Morphological diagnosis of a new species in Myrcia sensu lato (Myrtaceae) from Bahia, Brazil, with molecular highlights. Phytotaxa, 181: 229–237. [Google Scholar]
- Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- Sytsma KJ, Litt A, Zjhra ML, et al. 2004. Clades, clocks, and continents: historical and biogeographical analysis of Myrtaceae, Vochysiaceae, and relatives in the Southern Hemisphere. International Journal of Plant Sciences 165: S85–S105. [Google Scholar]
- Thomé MTC, Zamudio KR, Giovanelli JGR, Haddad CFB, Baldissera FA, Jr, Alexandrino J. 2010. Phylogeography of endemic toads and post-Pliocene persistence of the Brazilian Atlantic Forest. Molecular Phylogenetics and Evolution 55: 1018–1031. [DOI] [PubMed] [Google Scholar]
- Tonini JFR, Costa LP, Carnaval AC. 2013. Phylogeographic structure is strong in the Atlantic Forest; predictive power of correlative paleodistribution models, not always. Journal of Zoological Systematics and Evolutionary Research 51: 114–121. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.