Abstract
Background and Aims The inverse relationship between atmospheric CO2 partial pressure (pCO2) and stomatal frequency in many species of plants has been widely used to estimate palaeoatmospheric CO2 (palaeo-CO2) levels; however, the results obtained have been quite variable. This study attempts to find a potential new proxy for palaeo-CO2 levels by analysing stomatal frequency in Quercus guyavifolia (Q. guajavifolia, Fagaceae), an extant dominant species of sclerophyllous forests in the Himalayas with abundant fossil relatives.
Methods Stomatal frequency was analysed for extant samples of Q. guyavifolia collected from17 field sites at altitudes ranging between 2493 and 4497 m. Herbarium specimens collected between 1926 and 2011 were also examined. Correlations of pCO2–stomatal frequency were determined using samples from both sources, and these were then applied to Q. preguyavaefolia fossils in order to estimate palaeo-CO2 concentrations for two late-Pliocene floras in south-western China.
Key Results In contrast to the negative correlations detected for most other species that have been studied, a positive correlation between pCO2 and stomatal frequency was determined in Q. guyavifolia sampled from both extant field collections and historical herbarium specimens. Palaeo-CO2 concentrations were estimated to be approx. 180–240 ppm in the late Pliocene, which is consistent with most other previous estimates.
Conclusions A new positive relationship between pCO2 and stomatal frequency in Q. guyavifolia is presented, which can be applied to the fossils closely related to this species that are widely distributed in the late-Cenozoic strata in order to estimate palaeo-CO2 concentrations. The results show that it is valid to use a positive relationship to estimate palaeo-CO2 concentrations, and the study adds to the variety of stomatal density/index relationships that available for estimating pCO2. The physiological mechanisms underlying this positive response are unclear, however, and require further research.
Keywords: Stomatal density, stomatal index, atmospheric CO2 concentration, palaeo-CO2 reconstruction, altitudinal gradient, historical specimen, climate change, oak, Quercus guyavifolia, Q. guajavifolia
INTRODUCTION
Palaeoatmospheric CO2 (palaeo-CO2) concentration estimates provide important palaeoenvironmental information in geological time and a baseline reference to understand future climatic change. Atmospheric CO2 concentration has been hypothesized to be a primary determinant of global climate change; periods of low atmospheric CO2 concentrations witnessed major glaciations, whereas those with higher CO2 concentrations had warmer conditions (Retallack, 2001; Kürschner et al., 2008; Lunt et al., 2008; Lacis et al., 2010; Smith et al., 2010). This CO2–temperature relationship is conspicuous during the Quaternary and has also been confirmed for other time periods: for example, the Paleocene–Eocene thermal maximum (PETM) was a brief but intense interval of global warming associated with elevated atmospheric CO2 concentration (Zachos et al., 2005). In addition, CO2 levels play a crucial role in affecting the ecology and physiology of plants.
To understand the relationship of CO2, climate change and ecological function of CO2, many attempts have been made to estimate palaeo-CO2 throughout the Phanerozoic (Pagani et al., 1999; Pearson and Palmer, 2000; Berner and Kothavala, 2001; Berner, 2006; Tripati et al., 2009; Seki et al., 2010). A comparatively reliable method is to measure the CO2 composition of air locked in glacial ice (Petit et al., 1999; Lüthi et al., 2008). However, this method can only be applied for the past 800 000 years because of the absence of older glacial ice (Lüthi et al., 2008). Pre-ice core CO2 concentration estimations rely on numerous independent palaeobotanical and geochemical proxies and biogeochemical models derived from palaeobotanical and geochemical proxies, such as geochemical models (Berner and Kothavala, 2001; Berner, 2006), the δ13C in palaeosols (Ekart et al., 1999; Myers et al., 2012), δ13C of the organic remains of phytoplankton (Seki et al., 2010), the δ11B and B/Ca ratio of marine carbonate (Pearson and Palmer, 2000; Tripati et al., 2009; Seki et al., 2010) and stomatal frequency [expressed as stomatal density (SD) or stomatal index (SI)] in fossil leaves (e.g. Royer et al., 2001; Kürschner et al., 2008). Among these approaches, the δ13C in palaeosols method and the stomatal frequency method are terrestrial-based proxies, and the others are marine-based proxies. However, estimates of the palaeo-CO2 concentration made using these different approaches are quite variable (Royer et al., 2001a; Beerling and Royer, 2011). Thus, more research efforts focused on a single time period using different proxies is required. This is especially true for the mid-Miocene climatic optimum [18–15 million years ago (Ma)] and the middle to late Pliocene (3·6–2·6 Ma) (Beerling and Royer, 2011) because both of them were globally warm and relatively recent and may be comparable with the Earth’s immediate future with increasing greenhouse gases. Fortunately, there are abundant fossils from these two time periods (van der Burgh et al., 1993; Kürschner et al., 1996; Kürschner et al., 2008; Retallack, 2009; Stults et al., 2011) and we also found many oak fossils, providing an ideal opportunity to study the CO2–temperature relationship during these warm climate intervals by estimating the palaeo-CO2 concentration using the stomatal frequency method.
Generally, the stomatal frequency method is based on the inverse correlation between atmospheric CO2 partial pressure (pCO2) and leaf stomatal frequency which is species specific and observed in many C3 plants (Woodward, 1987; Kürschner et al., 2001; Royer, 2001; Beerling and Royer, 2002a; Kouwenberg et al., 2003). The method has been widely used to estimate palaeo-CO2 levels by applying the correlation to closely related plant fossils. Numerous genera and species have been used, such as Ginkgo (Retallack, 2001; Royer et al., 2001b; Beerling and Royer, 2002a; Retallack, 2009; Smith et al., 2010), Metasequoia (Royer et al., 2001b; Doria et al., 2011), other conifers (Passalia, 2009; Steinthorsdottir and Vajda, 2013), cycads (McElwain et al., 1999; Haworth et al., 2011b), Quercus (van der Burgh et al., 1993; Kürschner et al., 1996), Lauraceae (McElwain, 1998; Greenwood et al., 2003; Kürschner et al., 2008) and Betula (Finsinger and Wagner-Cremer, 2009). The precision of identifying nearest living relatives (NLRs) of fossil species and the accuracy of the correlation between stomatal frequency and pCO2 can have profound effects on the estimates because the relationship is species specific. Royer (2001) summarized the stomatal frequency of 176 previously published C3 plant species and showed that a majority of the species had inverse correlations; some had no significant relationship; only ≤12 % had a positive correlation; and species from the same genus may have inconsistent relationships (McElwain et al., 1995; Rundgren and Beerling, 1999; Eide and Birks, 2004; Finsinger and Wagner-Cremer, 2009; Haworth et al., 2010b). Almost all the previous studies have been based on an inverse relationship between atmospheric pCO2 and stomatal frequency to estimate palaeo-CO2 levels. Recently, a positive relationship between atmospheric pCO2 and stomatal frequency in Typha orientalis was used to estimate palaeo-CO2 levels during the Plio-Pleistocene transition (Bai et al., 2014). These studies indicate that it is essential to establish the specific stomatal frequency–pCO2 relationship of a fossil’s NLR before using the relationship to estimate palaeo-CO2 levels. To achieve this, many studies have used three primary approaches to investigate the response of stomatal frequency to CO2 variation: (1) study leaves collected over an extended period of time because atmospheric CO2 concentration has increased from approx. 280 to 390 ppm over the last 150 years; (2) study leaves from different locations along an altitudinal gradient because atmospheric pCO2 declines as barometric pressure decreases with increasing altitude; and (3) greenhouse experiments in which stomatal frequency can be counted in response to elevated CO2 concentrations. The first and third approaches have been widely used (Woodward, 1987; van der Burgh et al., 1993; Royer et al., 2001b; Greenwood et al., 2003; Kouwenberg et al., 2003; Haworth et al., 2011a), although the first approach may be somewhat constrained by the availability of historical specimens. The third approach misrepresents the potential for proportional population changes within a gene pool, and it does not consider taxonomic differences in plant generation times (Royer, 2001), and thus may fail to reflect long-term, genetic responses to slow changes in the environment of plants (Woodward, 1988; Beerling and Chaloner, 1993; McElwain and Chaloner, 1995). The second method has been used less frequently (McElwain, 2004; Kouwenberg et al., 2007) because of the difficulty of collecting one species over a long altitudinal gradient.
Oak plants of Quercus guyavifolia H. Lév. [= Q. pannosa Hand.-Mazz. (Flora of China), Q. guajavifolia H. Lév. (Flora of China, Volume 4, page 375)] is a dominant species in the sclerophyllous forests along the steep altitudinal gradients in the Qinghai-Tibet Plateau and Hengduan Mountains. There are also abundant Q. preguyavaefolia Tao (Zhou, 1992) fossils in the late-Cenozoic strata of this region (Zhou, 1999). For example, the Longmen flora (Su et al., 2013) and the Fudong flora (Tao, 1986; Huang et al., 2013) in south-western China, both from the Sanying Formation of the Pliocene, are dominated by Q. preguyavaefolia fossils; the Namling flora in Tibet from the Wulong Formation (middle Miocene, 15 Ma) is the earliest recorded occurrence of Q. preguyavaefolia fossils (Li and Guo, 1976; Spicer et al., 2003). Together, these fossils provide ideal material to estimate the atmospheric CO2 concentration history of the late Cenozoic and to study the CO2–temperature relationship during warm climate intervals in the mid-Miocene climatic optimum and the middle to late Pliocene. In this study, we chose Q. guyavifolia (the NLR of Q. preguyavaefolia fossils, Fig. 2) in order to (1) determine how the stomatal frequency of Q. guyavifolia responds to decreasing pCO2 (increasing altitude) and to generate calibration curves of stomatal frequency vs. atmospheric pCO2; (2) test whether samples collected along an altitudinal gradient provide results consistent with results from historical herbarium specimens; (3) estimate the late-Pliocene atmospheric CO2 concentration using two contemporaneous Q. preguyavaefolia fossils; and (4) compare CO2 levels estimated using the stomatal frequency of Q. guyavifolia with previous estimates. This is the first study to use both extant field collections from along an altitudinal gradient and historical herbarium specimens to establish a specific stomatal frequency–pCO2 relationship.
Fig. 2.
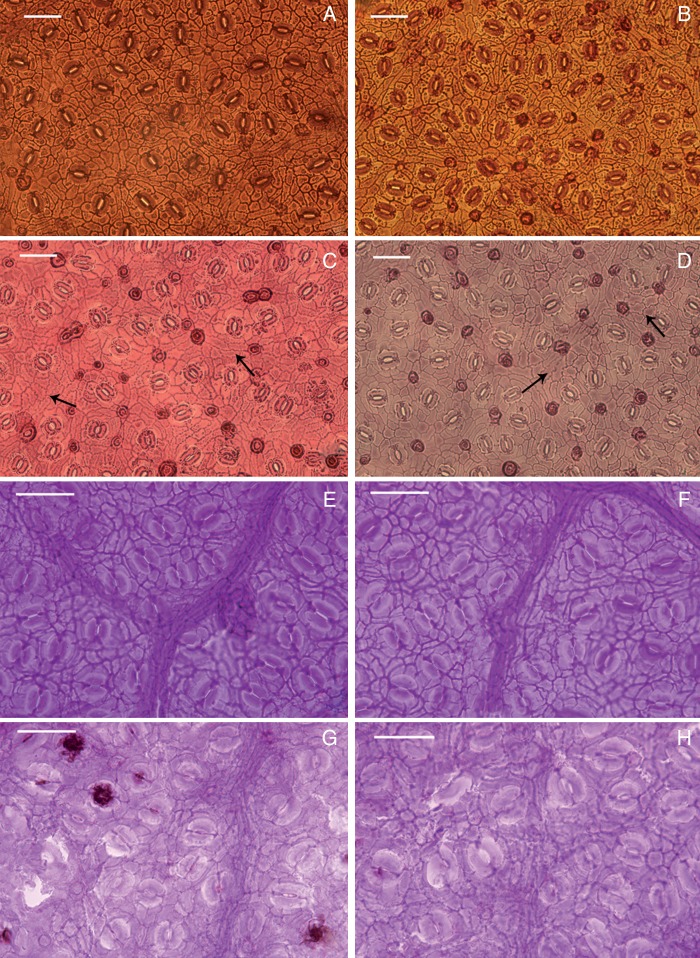
Comparisons of leaf morphology of extant Quercus guyavifolia and fossil Q. preguyavaefolia. (A, B) Branches of extant Q. guyavifolia. (C, D) Cleared leaves of extant Q. guyavifolia. (E, F) and (G, H) are leaf fossils from the Hunshuitang flora and the Qingfucun flora respectively. Scale bars = 1 cm.
MATERIALS AND METHODS
Collection of extant altitudinal material, historical herbarium specimens and fossil samples
Extant sun and shade leaves of Quercus guyavifolia were collected from five individuals at each of 17 sites at elevations ranging from 2493 to 4497 m (the altitudinal range of Q. guyavifolia distributions is approx. 2500–4500 m), i.e. pCO2 22·695–29·134 Pa (Supplementary Data Table S1; Fig. 1). Sun and shade leaves were collected because light intensity affects these two types of leaves differently and has a positive effect on the stomatal frequency (Kürschner, 1997; Royer, 2001; Lake et al., 2002; McElwain, 2004; Kouwenberg et al., 2007). Sun leaves were collected from outer branches; shade leaves were collected from within and beneath canopies. To account for the high natural population variability in stomatal frequency (Poole and Kürschner, 1999; Beerling and Royer, 2002a), four sun and four shade leaves were collected from each of five Q. guyavifolia trees at each site.
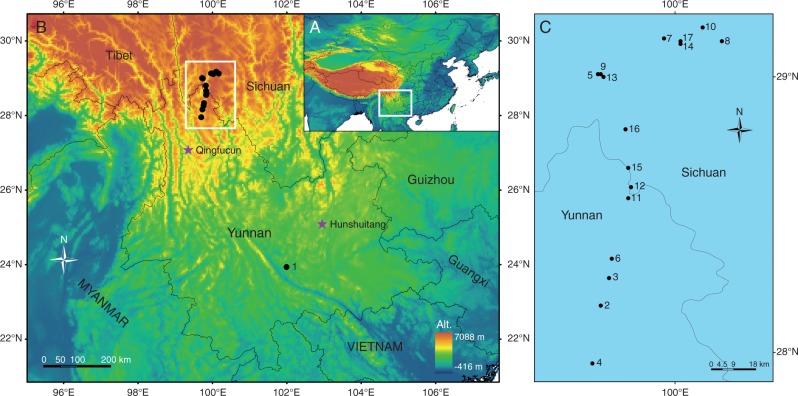
Fig. 1.
The locations of 17 sites (black points) where extant field samples of leaf materials of Quercus guyavifolia were collected and two sites (purple stars) where fossil materials of Q. preguyavaefolia were collected. (A) The study area. (B) Locations where extant and fossil leaf materials were collected. (C) Detailed location map showing 16 of the 17 collection sites in the boundary (grey line) region between Yunnan and Sichuan Provinces.
Herbarium samples of Q. guyavifolia from the Herbarium of the Kunming Institute of Botany, Chinese Academy of Sciences (KUN) were examined and they spanned the time period 1926–1995. Specimens from similar altitudes (range 2800–3300 m) were chosen (Supplementary Data Table S2) so as to limit stomatal frequency variation that might be caused by factors associated with changing altitude. Sample HS006 collected in the field extends the time period to 2011, i.e. pCO2 21·207–26·539 Pa (Table S2).
Fossil leaves of Q. preguyavaefolia (Fig. 2E–H) were collected from two different late-Pliocene floras – the Hunshuitang and the Qingfucun floras (Fig. 1). The Hunshuitang flora collection site is located 25 km north-east of Kunming, central Yunnan Province, south-western China (25°06'N, 102°57'E, altitude 2102 m). The Hunshuitang flora belongs to the Ciying Formation from the late Pliocene (Yunnan Bureau of Geology and Mineral Resources, 1978, 1990; Jiang et al., 2003). The Qingfucun flora, located in Weixi county, north-western Yunnan (27°05'N, 99°21'E, altitude 2476 m), is from the Sanying Formation, late Pliocene (3·6 Ma; Li et al., 2013). We analysed five fossil specimens of Q. preguyavaefolia from the Hunshuitang flora and four from the Qingfucun flora.
Stomatal analysis of extant samples collected along an altitudinal gradient
Mature leaves (1–2 years of leaf growth, mostly 2 years) were chosen for cuticle preparation. Leaf fragments of Q. guyavifolia were macerated using a 1:1 solution of glacial acetic acid and 30 % H2O2 at 80°C for approx. 3 h after the thick trichomes were removed. The lower cuticles without mesophyll tissue were stained using safranin O solution and then mounted in glycerine on glass slides (Stace, 1965; Poole and Kürschner, 1999). Cuticle images were taken using a light microscope (Leica DM 1000) attached to a Leica DFC 295 camera. The size of the images for stomatal and epidermal cell counts was 468 × 351 µm2 (approx. 0·1643 mm2). In addition, cleared leaves of Q. guyavifolia (Fig. 2C, D) were made following the protocol of Hickey and Wolfe (1975) to compare the leaf morphology of extant and fossil material.
Stomatal density (SD) is measured as the number of stomata per mm2 of leaf. Stomatal index (SI) is the proportion of stomata to the total number of epidermal cells and measured as:
| (1) |
where ED is epidermal cell density.
Stomatal and epidermal cells were counted using the software package ImageJ version 1.42q (http://rsb.info.nih.gov/ij). Samples came from five individuals at each site; four leaves were taken from each individual; three microscope fields were counted per leaf. The leaves of Q. guyavifolia are hypostomatous (He et al., 1994) so the images of stomatal and epidermal cell counts were all made on the abaxial surface. This resulted in 60 counts (5 individuals × 4 leaves × 3 counts) for each site. Stomatal and epidermal cells were counted in intercostal areas and restricted to the mid-lamina region to minimize variability (Poole et al., 1996). The SD and SI for each site were calculated as the mean of 60 counts per site.
Stomatal analysis of historical herbarium material
The experimental protocols for cuticle preparation of herbarium samples were the same as for extant field material. Three mature leaves were collected from each historical herbarium specimen; five microscope fields were counted per leaf, thus 15 counts were made for each specimen.
Stomatal analysis of fossil material
Fossil leaf fragments were treated successively with 20 % HCl, 40 % HF and 20 % HCl again to remove calcareous and siliceous materials, and then macerated using 3·5 % NaClO solution for 10 min to 1 h until they became white or translucent. After removing the mesophyll tissue, the lower cuticles were stained using safranin O and mounted in glycerine on glass slides (Ye, 1981; Kerp, 1990; Leng, 2000). Cuticular images were taken using a light microscope (Leica DM 750) linked to a Leica DFC 295 camera. The size of the images was 298 × 223 µm2 (approx. 0·0665 mm2). Five to ten cuticular images were counted for each fossil; a separate mean of the counts was calculated for the two fossil sites. The cuticles examined in fossil leaves were from the same part of the leaf as those for the extant leaves (intercostal area near mid-lamina). All cuticular slides were deposited at the Laboratory of Environmental Change and Its Impact on Plants Group in the Kunming Institute of Botany, Chinese Academy of Sciences.
Calibration curves
Calibration curves of stomatal frequency vs. pCO2 for sun and shade leaves were constructed based on extant samples. Atmospheric pCO2 used in the calibration curves were calculated from the elevation range using eqn (2) (Beerling and Royer, 2002a, derived from Jones, 1992):
| (2) |
where p1 and p2 are the CO2 partial pressures (Pa) at sea level and at the site, respectively; R is the gas constant (8·3144 Pa m3 mol−1 K−1); T is the mean annual temperature (K) of the range in elevation; MA is the molecular weight of air (0·028964 kg mol−1); g is the acceleration due to gravity (9·8 m s−2); and elev (p2) is the elevation (m) of the site. As altitude increases, pCO2 decreases from 29·134 to 22·695 Pa (Supplementary Data Table S1). The calculated atmospheric pCO2 should have no significant difference from the field atmospheric pCO2 (McElwain, 2004).
Calibration curves were constructed using linear regression analysis, using R version 2.14.1 (http://www.R-project.org). To determine if sun and shade leaves should be analysed separately or combined, differences in slopes and y-intercepts of their constructed curves were tested by analysis of covariance using SPSS Statistics version 18.0 (http://www.spss.com.cn).
Calibration curves of stomatal frequency vs. pCO2 were also constructed using historical herbarium materials. Historical levels of atmospheric CO2 at sea level were obtained from Etheridge et al. (1996) and from the CO2 Now website (http://co2now.org/). Using this information, atmospheric pCO2 at the sites was calculated using eqn (2).
Palaeo-CO2 estimate
The stomatal frequency of the fossil material was analysed and applied to the calibration curves prepared using the extant field materials and historical herbarium samples, respectively, to estimate palaeo-CO2 levels during the late Pliocene. Envelopes of uncertainty were obtained after propagating uncertainties in the calibration function and fossil leaf measurements. Due to differences between sun and shade leaves, it was necessary to construct morphotype-specific calibration curves for each type of leaf. Sun leaves are characterized by straight to rounded epidermal cell walls (Fig. 3A, B) whereas shade leaves show a pronounced undulation of the epidermal cell walls (Fig. 3C, D) (Kürschner, 1997), and on this basis fossils from the Hunshuitang flora (Figs 2E, F and 3E, F) and the Qingfucun flora (Figs 2G, H and 3G, H) were all assessed to be sun leaves. Sun leaves of historical herbarium samples were also chosen according to their epidermal features. This necessarily determined that the calibration curve for CO2 estimation was prepared exclusively using sun leaves of Q. guyavifolia; the calibration curve generated using shade leaves will be used in future work for Q. preguyavaefolia fossils from different sites. We then compared our atmospheric CO2 concentration estimates from the Hunshuitang and Qingfucun floras with other published results of atmospheric CO2 concentration during the late Pliocene (van der Burgh et al., 1993; Kürschner et al., 1996; Pearson and Palmer, 2000; Tripati et al., 2009; Seki et al., 2010).
Fig. 3.
Images of the cuticle of sun (A, B) and shade (C, D) leaves of extant Quercus guyavifolia and Q. preguyavaefolia fossils from the Hunshuitang (E, F) and Qingfucun (G, H) floras. Scale bars = 50 µm. Black arrows indicate the undulant epidermal cell walls in shade leaves.
RESULTS
Stomatal frequency of extant Quercus guyavifolia and calibration curves
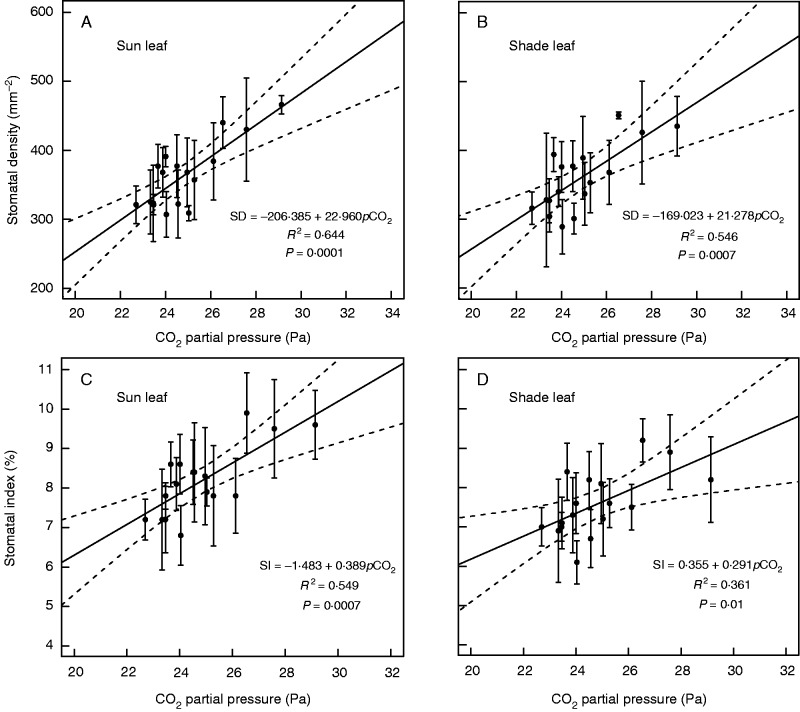
The calibration curves show a significant (P ≤ 0·01) positive linear relationship between stomatal frequency and atmospheric pCO2 for both sun and shade leaves in extant field samples of Quercus guyavifolia collected along an altitudinal gradient (Supplementary Data Table S1; Fig. 4).
Fig. 4.
Relationship between stomatal frequency (A, B, stomatal density; and C, D stomatal index) and CO2 partial pressure of Quercus guyavifolia sun (A, C) and shade (B, D) leaves. Error bars are ±1 s.d. The solid line indicates the best fit in classical regression analysis. Dashed lines are 95 % confidence limits.
The slopes of the calibration curves constructed by sun and shade leaves, respectively, are not different (P > 0·05). The SD and SI of shade leaves were 1·4 and 7·8 %, respectively, lower than those of sun leaves. There was a significant difference in intercept of SI–pCO2 curves between sun and shade leaves (P < 0·05), but not for SD–pCO2 curves (P > 0·05), indicating that the SI of shade leaves was lower than that of sun leaves, but the SD was not.
Stomatal frequency of historical herbarium materials and calibration curves
There was no significant relationship between SD and atmospheric pCO2 for historical herbarium materials, but a significant (P < 0·05) positive linear relationship between SI and pCO2 was found (Fig. 5).
Fig. 5.
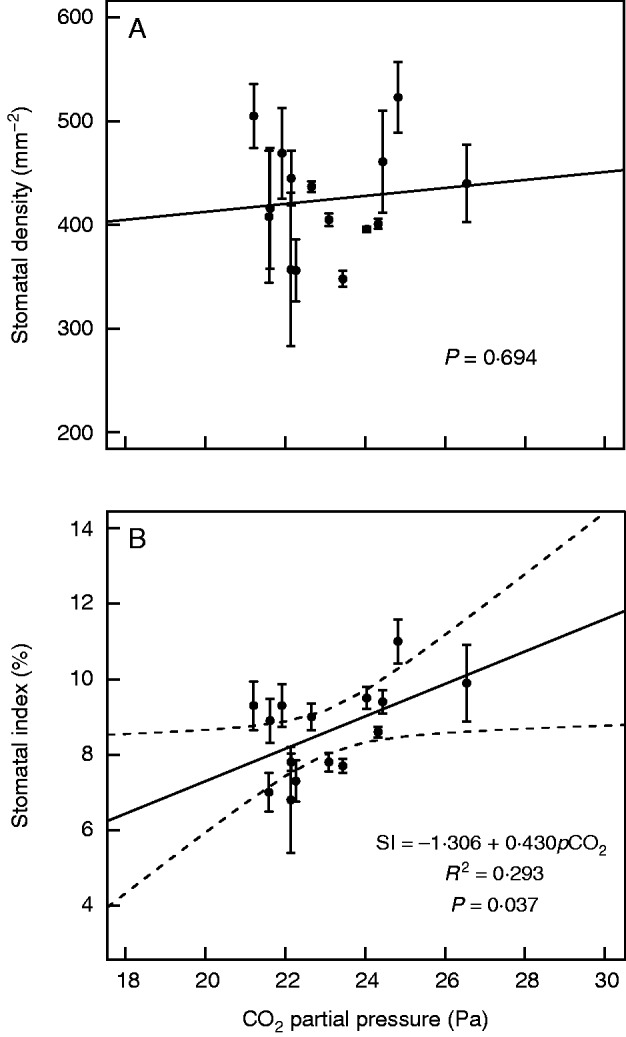
Relationship between stomatal frequency (A, stomatal density and B, stomatal index) and CO2 partial pressure of Quercus guyavifolia historical herbarium specimens. Error bars are ±1 s.d. The solid line indicates the best fit in classical regression analysis. Dashed lines are 95 % confidence limits.
Palaeo-CO2 estimate of the late Pliocene
The relationship between SI and atmospheric pCO2 for both extant specimens collected along an altitudinal gradient and historical herbarium materials were compared (Fig. 6). Results from both sources showed a significant positive linear relationship between SI and pCO2 (Fig. 6).
Fig. 6.
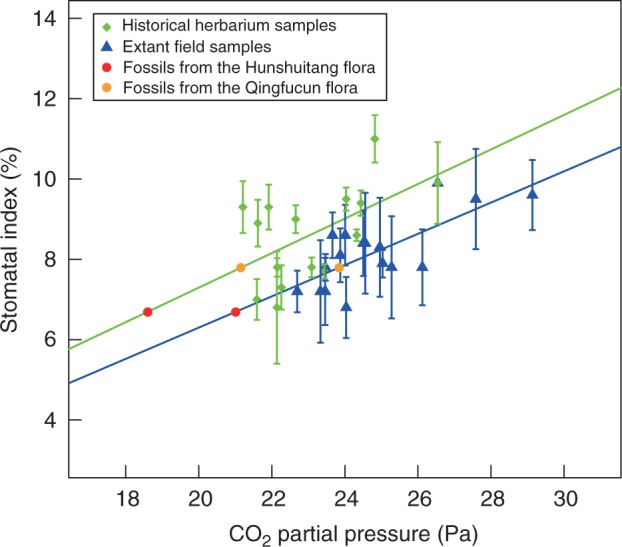
Correlation curves constructed using the SI of extant field collections along an altitudinal gradient and historical herbarium samples (see key), and comparison of estimated palaeo-pCO2 values (fossils from the Hunshuitang and Qingfucun flora, as indicated).
Fossils from the Hunshuitang flora had a mean SD of 516 ± 91 mm−2, and a mean SI of 6·69 ± 0·76 % (Table 1). Using the SD and SI of fossil material, the palaeo-CO2 concentration was estimated using the equations in Fig. 4A and 4C, respectively, which were both derived from extant sun leaves (Table 1). The palaeo-CO2 concentration was also estimated using the equation in Fig. 5B which was derived from sun leaves of historical specimens (Table 1; Fig. 6).
Table 1.
Estimates of CO2 partial pressure (pCO2) derived by using the calibration curves of stomatal frequency and pCO2 in Quercus guyavifolia sun leaves from both extant field samples (Fig. 4A, C) and historical herbarium samples (Fig. 5B)
| Sample | Age | No. of fossils | Total image counts | SF (mean ± s.d.) | pCO2-E (mean ± s.e.) | pCO2-H (mean ± s.e.) |
|---|---|---|---|---|---|---|
| Hunshuitang flora | Late Pliocene | 5 | 46 | SD: 516 ± 91 mm−2 | 31·46 ± 2·79 Pa (310·51 ± 27·51 ppm) | – |
| SI: 6·69 ± 0·76 % | 21·01 ± 3·47 Pa (207·36 ± 34·25 ppm) | 18·60 ± 5·53 Pa (183·52 ± 54·63 ppm) | ||||
| Qingfucun flora | Late Pliocene, 3·6 Ma | 4 | 34 | SD: 496 ± 24 mm−2 | 30·59 ± 1·57 Pa (301·92 ± 15·47 ppm) | – |
| SI: 7·79 ± 0·1 % | 23·84 ± 2·57 Pa (235·26 ± 25·37 ppm) | 21·15 ± 3·84 Pa (208·77 ± 37·87 ppm) |
SF, stomatal frequency; pCO2-E, estimates of CO2 partial pressure from extant field samples; pCO2-H, estimates of CO2 partial pressure from historical herbarium samples.
Fossils from the Qingfucun flora had a mean SD of 496 ± 24 mm−2, and a mean SI of 7·79 ± 0·1 % (Table 1). Palaeo-CO2 levels of the late Pliocene were estimated by applying the equations in Fig. 4A and 4C to the SD and SI, respectively, of the fossils (Table 1). The palaeo-CO2 was also estimated using the equation in Fig. 5B to compare with the estimates from the extant field samples (Table 1; Fig. 6).
DISCUSSION
Positive relationship between stomatal frequency and pCO2 in Quercus guyavifolia
We found a positive relationship between stomatal frequency and atmospheric pCO2 in Q. guyavifolia sun and shade leaves collected along an altitudinal gradient, which is consistent with the pattern from the historical herbarium samples. It is an unusual and interesting phenomenon because most other species show an inverse relationship (Woodward, 1987; van der Burgh et al., 1993; Woodward and Kelly, 1995; Beerling and Royer, 2002b; Royer, 2003; Kouwenberg et al., 2007; Franks and Beerling, 2009). However, several studies also report that in some species stomatal frequency increases with atmospheric CO2 concentration (Ferris and Taylor, 1994; Royer, 2001). In particular, some Quercus species such as Q. rubra and Q. robur grown in climate-controlled greenhouses show an increase in SD at elevated CO2 (Dixon et al., 1995; Atkinson et al., 1997). Although greenhouse results are not necessarily reliable, these results indicate to a certain extent that a positive relationship between stomatal frequency and atmospheric pCO2 in other species of Quercus may be not unexpected. Recently, this positive correlation between SI and atmospheric pCO2 in historical herbarium specimens of Typha orientalis has been used to estimate atmospheric CO2 during the Plio-Pleistocene transition (Bai et al., 2014). This is supportive evidence of the reliability of the positive relationship used to estimate palaeo-CO2.
The positive relationship detected between stomatal frequency and atmospheric pCO2 in Q. guyavifolia was observed from both the altitudinal collection and the historical herbarium samples. These two independently derived results showed the same pattern. The altitudinal samples were collected in Yunnan and Sichuan provinces; the historical samples were collected from similar altitudes in northern and north-western Yunnan province. To test if this unusual, positive relationship between pCO2 and stomatal frequency is affected by other environmental factors, the relationships between stomatal frequency and other climatic factors (mean annual temperature, mean annual precipitation, annual mean relative humidity) were tested by simple linear regression analysis. The result showed that only atmospheric pCO2 significantly correlates to stomatal frequency of Q. guyavifolia (Figs 4 and 5) rather than the mean annual temperature (P > 0·05 for both the altitudinal collection and the historical herbarium samples), mean annual precipitation (P > 0·05 for both the altitudinal collection and the historical herbarium samples) and annual mean relative humidity (P > 0·05 for both the altitudinal collection and the historical herbarium samples), confirming that the positive relationship between stomatal frequency and pCO2 in Q. guyavifolia is determined by atmospheric pCO2. Together with evidence from other studies (Dixon et al., 1995; Atkinson et al., 1997; Bai et al., 2014), we propose that the positive relationship between stomatal frequency and pCO2 in Q. guyavifolia is reliable and this relationship can be used as a basis to estimate palaeo-CO2 levels.
The physiological mechanism underlying the positive stomatal response to pCO2 is probably complicated. Stomata play a central role in the uptake of photosynthetic CO2 and water loss from the leaf. Both physiological (stomatal aperture change) and morphological (SD change) strategies can be used by plants to regulate gas exchange (Haworth et al., 2013). The positive stomatal response to pCO2 may be contributed by multiple factors. One of the factors is possibly leaf nitrogen content. Previous studies found that elevated CO2 concentration can increase leaf nitrogen content in jack pine and white birch seedlings (Zhang and Dang, 2005). Plants of Q. pannosa (= Q. guyavifolia) and Q. aquifolioides grown at lower altitudes (higher atmospheric pCO2) have higher leaf nitrogen content (or higher nitrogen allocation in the photosynthetic system), stomatal conductance, photosynthetic rate and carboxylation rate (Zhang et al., 2005; Feng et al., 2013). High leaf nitrogen content significantly increases carboxylation capacity (Rogers et al., 1998; Pérez et al., 2011) and consequently results in a decrease in the ratio of the intercellular to atmospheric CO2 concentration and an increase in δ13C values (Sasakawa et al., 1989; Cordell et al., 1999), and therefore an increase of SD (Qiang et al., 2003).
Differences in stomatal frequency between sun and shade leaves
Our results confirm that distinguishing sun leaves from shade leaves is necessary when using the stomatal method to estimate palaeo-CO2 levels (Kürschner, 1997). Stomatal frequency of both sun and shade leaves in Q. guyavifolia decreased with decreasing pCO2. However, SI of shade leaves was lower than that of sun leaves although the SD was not different between sun and shade leaves. This corroborates results from previous studies which showed that stomatal frequency of sun leaves is higher than that of shade leaves (Kürschner, 1997; Wagner, 1998; Royer, 2001; Kouwenberg et al., 2007) resulting from the positive effect of light intensity on stomatal frequency (Lake et al., 2001, 2002; Kouwenberg et al., 2007). Kürschner (1997) reported that the SD of Q. petraea sun leaves was about 45 % higher than that of shade leaves, and up to 60 % higher in fossil leaves of Q. pseudocastanea. Kouwenberg et al. (2007) also observed a higher SD and SI in sun leaves compared with shade leaves in both Q. kelloggii (up to 38 % higher) and Nothofagus solandri.
Comparison of stomatal density and stomatal index
The SD and SI gave different estimates for palaeo-CO2. The SI is a more precise proxy for palaeo-CO2 estimation than SD because SI removes the effect of other environmental factors such as temperature, water stress and humidity on the size and/or spacing of epidermal cells, which will result in higher or lower SDs (Salisbury, 1927; Kürschner et al., 1996; Kürschner, 1997; Royer, 2001; Sun et al., 2003; McElwain, 2005; Kouwenberg et al., 2007; Haworth et al., 2010a). Here we showed that the SI of historical herbarium samples had a significant positive response to atmospheric pCO2 but SD did not, confirming that SD is more variable than SI and therefore not as reliable as SI for palaeo-pCO2 estimation. However, in practice, many fossil leaves are not well preserved and epidermal cells are difficult to identify. Thus SI analysis is impossible and SD becomes the sole option but may give rise to error. Fortunately, our fossils were well preserved and SI could be accurately calculated. Therefore, our results are probably more precise than those derived only by SD. We also used SD for palaeo-pCO2 calculation in order to compare with the results derived from SI (Table 1). Our comparison of palaeo-pCO2 levels estimated from SD and SI provides an example of overpredicting pCO2 levels using SD.
Late-Pliocene atmospheric CO2 levels
As a test of the applicability and reliability of the positive relationship of stomatal frequency and pCO2 in Q. guyavifolia as a proxy for palaeo-pCO2 levels, palaeo-pCO2 was estimated using Q. preguyavaefolia fossils. Our results derived using the SI calibration data set indicate late-Pliocene atmospheric CO2 levels of approx. 210 and 240 ppm derived using extant field collections and approx. 180 and 210 ppm derived from herbarium samples, which are consistent with most other estimates. Beerling and Royer (2011) compiled 370 estimates of Cenozoic atmospheric CO2 levels obtained using different protocols; they show that since the Miocene most atmospheric CO2 estimates were lower than present-day levels of approx. 390 ppm. We compared our palaeo-CO2 estimates during the late Pliocene with those based on stomatal frequency of other species (terrestrial-based proxy) and other proxies such as alkenone and boron (marine-based proxies) for the same time period (Fig. 7). All of the estimates indicate palaeo-CO2 levels between approx. 190 and approx. 430 ppm for the late Pliocene, with the majority indicating that atmospheric CO2 levels in the late Pliocene were lower than at present. However, even atmospheric CO2 estimates derived from the same source (terrestrial-based or marine-based proxies) are still quite different. Therefore, atmospheric CO2 estimates to date have not been sufficient to account for the warm climate interval during the late Pliocene. More research is required to obtain more precise estimates of palaeo-CO2 during this period.
Fig. 7.
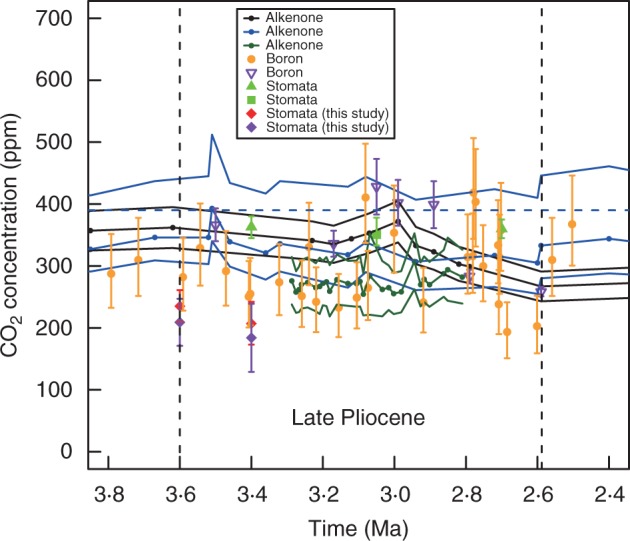
Estimates of palaeo-CO2 concentration during the late Pliocene derived using different methods. The palaeo-CO2 estimates derived from alkenone (see key; black lines above and below represent uncertainties) and boron (purple) are cited from Seki et al. (2010) modified by Beerling and Royer (2011); alkenone (blue lines above and below represent uncertainties; Zhang et al., 2013); alkenone (dark green lines above and below represent uncertainties; Badger et al., 2013); boron data (orange; Bartoli et al., 2011); stomata data (van der Burgh et al., 1993; Kürschner et al., 1996); stomata data (Stults et al., 2011); stomatal data (red) and stomatal data (purple) are palaeo-CO2 levels estimated from field collections along an altitudinal gradient and from historical herbarium samples respectively (this study). Error bars represent uncertainties (see Materials and Methods). One of our fossils is 3·6 Ma, and the other is unknown (sometime during the late Pliocene). The period between the two vertical dashed lines is the late Pliocene (3·6–2·588 Ma) (International Chronostratigraphic Chart; version 2013). The blue horizontal dashed line indicates recent levels of atmospheric CO2 concentration (390 ppm).
In addition, our comparatively low atmospheric CO2 values may be related to elevations in the fossil sites. It is generally accepted that the Qinghai-Tibet Plateau reached its current height in the late Miocene and retained it (Spicer et al., 2003); previous studies have also showed that western Yunnan had approached its highest altitude before the late Pliocene (Sun et al., 2011). The palaeoaltitude of the Xianfeng flora, about 60 km away from the Hunshuitang locality, was about 1936 m in the late Miocene (Jacques et al., 2014). This means that the altitudes of our fossil sites in the late Pliocene were probably similar to present-day altitudes (approx. 2000–2500 m). If the palaeoaltitudes of the two sites are taken into account, atmospheric CO2 levels during the late Pliocene should be approx. 270 and 320 ppm derived by extant field collections and approx. 240 and 280 ppm derived by herbarium samples. Thus, these results are very similar and correspond well to other estimates, confirming that the positive relationship between stomatal frequency and atmospheric pCO2 in Q. guyavifolia is reliable as a proxy for estimating palaeo-CO2 levels.
Actually, a correction for pCO2 at the elevation is necessary to obtain more accurate results. Clearly, when attempting to reconstruct palaeo-pCO2 using the stomatal frequency of fossils, the pCO2 estimates will be influenced by the elevation at which the now-fossilized plants were growing. Thus, to obtain more precise pCO2 estimates it is essential to apply a correction factor for pCO2 related to altitude. This should also be done for samples collected along an altitudinal range (McElwain, 2004) and for historical herbarium samples (Greenwood et al., 2003) when constructing the stomatal frequency–pCO2 curve. Unfortunately, few previous studies have made this correction on either extant specimens, herbarium specimens or fossils. Therefore, we suggest that future studies should incorporate a correction factor related to altitude for all specimens and samples.
Recently, there has been much debate about whether the linear relationship between stomatal frequency and pCO2 continues when the ambient CO2 concentration rises beyond approx. 500 ppm, because the stomatal frequency of some plant species may lose sensitivity at these high levels (Woodward and Bazzaz, 1988; Kürschner et al., 1997; Beerling and Royer, 2002a, b; Beerling et al., 2009; Haworth et al., 2011a). Nevertheless, the pCO2 estimate reported in this study, and the stomatal frequency–pCO2 training sets are applicable because atmospheric CO2 levels during the Pliocene remained below 500 ppm according to our results and those of previous estimates (Fig. 7).
Conclusions
We have shown a significant positive relationship between atmospheric pCO2 and stomatal frequency in Q. guyavifolia that can be used as a proxy to estimate late-Cenozoic palaeo-CO2 concentrations. This is the first study in which both field samples collected along an altitudinal gradient and historical herbarium samples of a single species have been used to estimate palaeo-CO2 concentration. In addition, a positive relationship between stomatal frequency and pCO2 has seldom been used to estimate palaeo-CO2 concentration. Our estimated palaeo-CO2 concentration provides new independent data for late-Cenozoic CO2 estimates derived using vascular land plants. There are, however, three sources of variation. First, sun and shade leaves give different results of stomatal frequency, confirming that it is necessary to analyse sun and shade leaves separately when using the stomatal method to estimate palaeo-CO2. Secondly, the estimates derived from SD and SI using sun leaves were also different, so more precise estimates will be possible when we find and analyse fossils that have well-preserved shade leaves in addition to sun leaves. Thirdly, our research concluded that atmospheric CO2 levels in the late Pliocene were approx. 180–240 ppm. Although these results are consistent with other studies reporting CO2 levels lower than modern atmospheric concentrations, when more appropriate fossil material becomes available then much more accurate estimates will be possible.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxford journals.org and consist of the following. Table S1: location, altitude, pCO2, stomatal density and stomatal index of Q. guyavifolia sun and shade leaves where extant field samples were collected. Table S2: collection time, location, altitude, pCO2, stomatal density and stomatal index of Q. guyavifolia sun leaves from historical herbarium specimens.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China [grant no. 41030212 to Z.K.Z.] and the National Basic Research Program of China, 973 Program [grant no. 2012CB821901 to Z.K.Z.]. We are grateful to the Herbarium of Kunming Institute of Botany, Chinese Academy of Sciences (KUN) for providing herbarium samples, Shi-Bao Zhang for providing a physiological explanation of our results, Guo-Feng Li, Hong-Xin Liu, Qian Hu and Fang-Ming Zhang for their assistance in collecting extant field samples, Xi-Xi Zhang from Yunnan Agricultural University for cuticle counts, and He Xu from Xishuangbanna Tropical Botanical Garden for preparing cleared leaves of Quercus guyavifolia. We are also grateful to the referees who provided constructive suggestions and comments.
LITERATURE CITED
- Atkinson CJ, Taylor JM, Wilkins D, Besford RT. 1997. Effects of elevated CO2 on chloroplast components, gas exchange and growth of oak and cherry. Tree Physiology 17: 319–325. [DOI] [PubMed] [Google Scholar]
- Badger MPS, Schmidt DN, Mackensen A, Pancost RD. 2013. High-resolution alkenone palaeobarometry indicates relatively stable pCO2 during the Pliocene (3·3–2·8 Ma). Philosophical Transactions of the Royal Society A: Mathematical Physical and Engineering Sciences 371: 20130094. [DOI] [PubMed] [Google Scholar]
- Bai YJ, Chen LQ, Ranhotra PS, Wang Q, Wang YF, Li CS. 2015. Reconstructing atmospheric CO2 during the Plio-Pleistocene transition by fossil Typha. Global Change Biology 21: 874–881. [DOI] [PubMed] [Google Scholar]
- Bartoli G, Hönisch B, Zeebe RE. 2011. Atmospheric CO2 decline during the Pliocene intensification of Northern Hemisphere glaciations. Paleoceanography 26: PA4213. [Google Scholar]
- Beerling DJ, Chaloner WG. 1993. Evolutionary responses of stomatal density to global CO2 change. Biological Journal of the Linnean Society 48:343–353. [Google Scholar]
- Beerling DJ, Royer DL. 2002a. Fossil plants as indicators of the phanerozoic global carbon cycle. Annual Review of Earth and Planetary Sciences 30: 527–556. [Google Scholar]
- Beerling DJ, Royer DL. 2002b. Reading a CO2 signal from fossil stomata. New Phytologist 153: 387–397. [DOI] [PubMed] [Google Scholar]
- Beerling DJ, Royer DL. 2011. Convergent Cenozoic CO2 history. Nature Geoscience 4: 418–420. [Google Scholar]
- Beerling DJ, Fox A, Anderson CW. 2009. Quantitative uncertainty analyses of ancient atmospheric CO2 estimates from fossil leaves. American Journal of Science 309: 775–787. [Google Scholar]
- Berner RA. 2006. GEOCARBSULF: a combined model for Phanerozoic atmospheric O2 and CO2. Geochimica et Cosmochimica Acta 70: 5653–5664. [Google Scholar]
- Berner RA, Kothavala Z. 2001. GEOCARB III: a revised model of atmospheric CO2 over phanerozoic time. American Journal of Science 301: 182–204. [Google Scholar]
- van der Burgh J, Visscher H, Dilcher DL, Kürschner WM. 1993. Paleoatmospheric signatures in Neogene fossil leaves. Science 260: 1788–1790. [DOI] [PubMed] [Google Scholar]
- Cordell S, Goldstein G, Meinzer FC, Handley LL. 1999. Allocation of nitrogen and carbon in leaves of Metrosideros polymorpha regulates carboxylation capacity and δ13C along an altitudinal gradient. Functional Ecology 13: 811–818. [Google Scholar]
- Dixon M, Le Thiec D, Garrec JP. 1995. The growth and gas exchange response of soil-planted Norway spruce [Picea abies (L.) Karst.] and red oak (Quercus rubra L.) exposed to elevated CO2 and to naturally occurring drought. New Phytologist 129: 265–273. [DOI] [PubMed] [Google Scholar]
- Doria G, Royer DL, Wolfe AP, Fox A, Westgate JA, Beerling DJ. 2011. Declining atmospheric CO2 during the late Middle Eocene climate transition. American Journal of Science 311: 63–75. [Google Scholar]
- Eide W, Birks HH. 2004. Stomatal frequency of Betula pubescens and Pinus sylvestris shows no proportional relationship with atmospheric CO2 concentration. Nordic Journal of Botany 24: 327–339. [Google Scholar]
- Ekart DD, Cerling TE, Montañez IP, Tabor NJ. 1999. A 400 million year carbon isotope record of pedogenic carbonate: implications for paleoatmospheric carbon dioxide. American Journal of Science 299: 805–827. [Google Scholar]
- Etheridge DM, Steele LP, Langenfelds RL, Francey RJ, Barnola JM, Morgan VI. 1996. Natural and anthropogenic changes in atmospheric CO2 over the last 1000 years from air in Antarctic ice and firn. Journal of Geophysical Research 101: 4115–4128. [Google Scholar]
- Feng QH, Centritto M, Cheng RM, Liu SR, Shi ZM. 2013. Leaf functional trait responses of Quercus aquifolioides to high elevations. International Journal of Agriculture and Biology 15: 69–75. [Google Scholar]
- Ferris R, Taylor G. 1994. Stomatal characteristics of four native herbs following exposure to elevated CO2. Annals of Botany 73: 447–453. [Google Scholar]
- Finsinger W, Wagner-Cremer F. 2009. Stomatal-based inference models for reconstruction of atmospheric CO2 concentration: a method assessment using a calibration and validation approach. The Holocene 19: 757–764. [Google Scholar]
- Franks PJ, Beerling DJ. 2009. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proceedings of the National Academy of Sciences, USA 106: 10343–10347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood DR, Scarr MJ, Christophel DC. 2003. Leaf stomatal frequency in the Australian tropical rainforest tree Neolitsea dealbata (Lauraceae) as a proxy measure of atmospheric pCO2. Palaeogeography, Palaeoclimatology, Palaeoecology 196: 375–393. [Google Scholar]
- Haworth M, Gallagher A, Elliott-Kingston C, Raschi A, Marandola D, McElwain JC. 2010a. Stomatal index responses of Agrostis canina to CO2 and sulphur dioxide: implications for palaeo-[CO2] using the stomatal proxy. New Phytologist 188: 845–855. [DOI] [PubMed] [Google Scholar]
- Haworth M, Heath J, McElwain JC. 2010b. Differences in the response sensitivity of stomatal index to atmospheric CO2 among four genera of Cupressaceae conifers. Annals of Botany 105: 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth M, Elliott-Kingston C, McElwain JC. 2011a. The stomatal CO2 proxy does not saturate at high atmospheric CO2 concentrations: evidence from stomatal index responses of Araucariaceae conifers. Oecologia 167: 11–19. [DOI] [PubMed] [Google Scholar]
- Haworth M, Fitzgerald A, McElwain JC. 2011b. Cycads show no stomatal-density and index response to elevated carbon dioxide and subambient oxygen. Australian Journal of Botany 59: 630–639. [Google Scholar]
- Haworth M, Elliott-Kingston C, McElwain JC. 2013. Co-ordination of physiological and morphological responses of stomata to elevated [CO2] in vascular plants. Oecologia 171: 71–82. [DOI] [PubMed] [Google Scholar]
- He JS, Chen WL, Wang XL. 1994. Morphological and anatomical features of Quercus section suber and its adaptation to the ecological environment. Acta Phytoecologica Sinica 18: 219–227 (in Chinese with English abstract). [Google Scholar]
- Hickey LJ, Wolfe JA. 1975. The bases of angiosperm phylogeny: vegetative morphology. Annals of the Missouri Botanical Garden 62: 538–589. [Google Scholar]
- Huang YJ, Liu YS, (Christopher), Jacques FMB, Su T, Xing YW, Zhou ZK. 2013. First discovery of Cucubalus (Caryophyllaceae) fossil, and its biogeographical and ecological implications. Review of Palaeobotany and Palynology 190: 41–47. [Google Scholar]
- Jacques FMB, Su T, Spicer RA, Xing YW, Huang YJ, Zhou ZK. 2014. Late Miocene southwestern Chinese floristic diversity shaped by the southeastern uplift of the Tibetan Plateau. Palaeogeography, Palaeoclimatology, Palaeoecology 411: 208–215. [Google Scholar]
- Jiang CS, Zhou RQ, Hu YX. 2003. Features of geological structure for Kunming basin. Journal of Seismological Research 26: 67–74 (in Chinese with English abstract). [Google Scholar]
- Jones HG. 1992. Plants and microclimate: a quantitative approach to environmental plant physiology. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Kerp H. 1990. The study of fossil gymnosperms by means of cuticular analysis. Palaios 5: 548–569. [Google Scholar]
- Kouwenberg LLR, McElwain JC, Kürschner WM, et al. 2003. Stomatal frequency adjustment of four conifer species to historical changes in atmospheric CO2. American Journal of Botany 90: 610–619. [DOI] [PubMed] [Google Scholar]
- Kouwenberg LLR, Kürschner WM, McElwain JC. 2007. Stomatal frequency change over altitudinal gradients: prospects for paleoaltimetry. Paleoaltimetry: Geochemical and Thermodynamic Approaches 66: 215–241. [Google Scholar]
- Kürschner WM. 1997. The anatomical diversity of recent and fossil leaves of the durmast oak (Quercus petraea Lieblein/Q. pseudocastanea Goeppert) – implications for their use as biosensors of palaeoatmospheric CO2 levels. Review of Palaeobotany and Palynology 96: 1–30. [Google Scholar]
- Kürschner WM, van der Burgh J, Visscher H, Dilcher DL. 1996. Oak leaves as biosensors of late Neogene and early Pleistocene paleoatmospheric CO2 concentrations. Marine Micropaleontology 27: 299–312. [Google Scholar]
- Kürschner WM, Wagner F, Visscher EH, Visscher H. 1997. Predicting the response of leaf stomatal frequency to a future CO2-enriched atmosphere: constraints from historical observations. Geologische Rundschau 86: 512–517. [Google Scholar]
- Kürschner WM, Wagner F, Dilcher DL, Visscher H. 2001. Using fossil leaves for the reconstruction of Cenozoic paleoatmospheric CO2 concentrations. In: Gerhard LC, Harrison WE, Hanson BM, eds. Geological perspectives of global climate change . Tulsa, OK: The American Association of Petroleum Geologists, 169–189. [Google Scholar]
- Kürschner WM, Kvacek Z, Dilcher DL. 2008. The impact of Miocene atmospheric carbon dioxide fluctuations on climate and the evolution of terrestrial ecosystems. Proceedings of the National Academy of Sciences, USA 105: 449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüthi D, Le Floch M, Bereiter B, et al. 2008. High-resolution carbon dioxide concentration record 650,000–800,000 years before present. Nature 453: 379–382. [DOI] [PubMed] [Google Scholar]
- Lacis AA, Schmidt GA, Rind D, Ruedy RA. 2010. Atmospheric CO2: principal control knob governing earth’s temperature. Science 330: 356–359. [DOI] [PubMed] [Google Scholar]
- Lake JA, Quick WP, Beerling DJ, Woodward FI. 2001. Plant development: signals from mature to new leaves. Nature 411: 154–154. [DOI] [PubMed] [Google Scholar]
- Lake JA, Woodward FI, Quick WP. 2002. Long-distance CO2 signalling in plants. Journal of Experimental Botany 53: 183–193. [DOI] [PubMed] [Google Scholar]
- Leng Q. 2000. An effective method of observing fine venation from compressed angiosperm fossil leaves. Acta Palaeontologica Sinica 39: 157–158 (in Chinese with English abstract). [Google Scholar]
- Li HM, Guo SX. 1976. The Miocene flora from Namling of Xizang. Acta Palaeontologica Sinica 15: 7–20 (in Chinese with English abstract). [Google Scholar]
- Li SH, Deng CL, Yao HT, et al. 2013. Magnetostratigraphy of the Dali Basin in Yunnan and implications for late Neogene rotation of the southeast margin of the Tibetan Plateau. Journal of Geophysical Research: Solid Earth 118: 791–807. [Google Scholar]
- Lunt DJ, Foster GL, Haywood AM, Stone EJ. 2008. Late Pliocene Greenland glaciation controlled by a decline in atmospheric CO2 levels. Nature 454: 1102–1105. [DOI] [PubMed] [Google Scholar]
- McElwain JC. 1998. Do fossil plants signal palaeoatmospheric carbon dioxide concentration in the geological past? Philosophical Transactions of the Royal Society of London B: Biological Sciences 353: 83–96. [Google Scholar]
- McElwain JC. 2004. Climate-independent paleoaltimetry using stomatal density in fossil leaves as a proxy for CO2 partial pressure. Geology 32: 1017–1020. [Google Scholar]
- McElwain JC. 2005. Climate-independent paleoaltimetry using stomatal density in fossil leaves as a proxy for CO2 partial pressure: comment and reply. Geology 33: e83–e83. [Google Scholar]
- McElwain JC, Chaloner WG. 1995. Stomatal density and index of fossil plants track atmospheric carbon dioxide in the Palaeozoic. Annals of Botany 76: 389–395. [Google Scholar]
- McElwain JC, Mitchell FJG, Jones MB. 1995. Relationship of stomatal density and index of Salix cinerea to atmospheric carbon dioxide concentrations in the Holocene. The Holocene 5: 216–219. [Google Scholar]
- McElwain JC, Beerling DJ, Woodward FI. 1999. Fossil plants and global warming at the Triassic–Jurassic boundary. Science 285: 1386–1390. [DOI] [PubMed] [Google Scholar]
- Myers TS, Tabor NJ, Jacobs LL, Mateus O. 2012. Estimating soil pCO2 using paleosol carbonates: implications for the relationship between primary productivity and faunal richness in ancient terrestrial ecosystems. Paleobiology 38: 585–604. [Google Scholar]
- Pagani M, Freeman KH, Arthur MA. 1999. Late Miocene atmospheric CO2 concentrations and the expansion of C4 grasses. Science 285: 876–879. [DOI] [PubMed] [Google Scholar]
- Passalia MG. 2009. Cretaceous pCO2 estimation from stomatal frequency analysis of gymnosperm leaves of Patagonia, Argentina. Palaeogeography, Palaeoclimatology, Palaeoecology 273: 17–24. [Google Scholar]
- Pearson PN, Palmer MR. 2000. Atmospheric carbon dioxide concentrations over the past 60 million years. Nature 406: 695–699. [DOI] [PubMed] [Google Scholar]
- Pérez P, Alonso A, Zita G, Morcuende R, Martínez-Carrasco R. 2011. Down-regulation of Rubisco activity under combined increases of CO2 and temperature minimized by changes in Rubisco kcat in wheat. Plant Growth Regulation 65: 439–447. [Google Scholar]
- Petit JR, Jouzel J, Raynaud D, et al. 1999. Climate and atmospheric history of the past 420,000 years from the Vostok ice core, Antarctica. Nature 399: 429–436. [Google Scholar]
- Poole I, Kürschner WM. 1999. Stomatal density and index: the practice. In: Jones TP, Rowe NP, eds. Fossil plants and spores: modern techniques . London: Geological Society, 257–260. [Google Scholar]
- Poole I, Weyers JDB, Lawson T, Raven JA. 1996. Variations in stomatal density and index: implications for palaeoclimatic reconstructions. Plant, Cell and Environment 19: 705–712. [Google Scholar]
- Qiang WY, Wang XL, Chen T, et al. 2003. Variations of stomatal density and carbon isotope values of Picea crassifolia at different altitudes in the Qilian Mountains. Trees – Structure and Function 17: 258–262. [Google Scholar]
- Retallack GJ. 2001. A 300-million-year record of atmospheric carbon dioxide from fossil plant cuticles. Nature 411: 287–290. [DOI] [PubMed] [Google Scholar]
- Retallack GJ. 2009. Greenhouse crises of the past 300 million years. Geological Society of America Bulletin 121: 1441–1455. [Google Scholar]
- Rogers A, Fischer BU, Bryant J, et al. 1998. Acclimation of photosynthesis to elevated CO2 under low-nitrogen nutrition is affected by the capacity for assimilate utilization. Perennial ryegrass under free-air CO2 enrichment. Plant Physiology 118: 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer DL. 2001. Stomatal density and stomatal index as indicators of paleoatmospheric CO2 concentration. Review of Palaeobotany and Palynology 114: 1–28. [DOI] [PubMed] [Google Scholar]
- Royer DL. 2003. Estimating latest cretaceous and tertiary atmospheric CO2 from stomatal indices. Geological Society of America Special Paper 369: 79–93. [Google Scholar]
- Royer DL, Berner RA, Beerling DJ. 2001a. Phanerozoic atmospheric CO2 change: evaluating geochemical and paleobiological approaches. Earth-Science Reviews 54: 349–392. [Google Scholar]
- Royer DL, Wing SL, Beerling DJ, et al. 2001b. Paleobotanical evidence for near present-day levels of atmospheric CO2 during part of the Tertiary. Science 292: 2310–2313. [DOI] [PubMed] [Google Scholar]
- Rundgren M, Beerling DJ. 1999. A Holocene CO2 record from the stomatal index of subfossil Salix herbacea L. leaves from northern Sweden. The Holocene 9: 509–513. [Google Scholar]
- Salisbury EJ. 1927. On the causes and ecological significance of stomatal frequency, with special reference to the woodland flora. Philosophical Transactions of the Royal Society B: Containing Papers of a Biological Character 216: 1–65. [Google Scholar]
- Sasakawa H, Sugiharto B, O’Leary MH, Sugiyama T. 1989. δ13C values in maize leaf correlate with phosphoenolpyruvate carboxylase levels. Plant Physiology 90: 582–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki O, Foster GL, Schmidt DN, Mackensen A, Kawamura K, Pancost RD. 2010. Alkenone and boron-based Pliocene pCO2 records. Earth and Planetary Science Letters 292: 201–211. [Google Scholar]
- Smith RY, Greenwood DR, Basinger JF. 2010. Estimating paleoatmospheric pCO2 during the Early Eocene Climatic Optimum from stomatal frequency of Ginkgo, Okanagan Highlands, British Columbia, Canada. Palaeogeography, Palaeoclimatology, Palaeoecology 293: 120–131. [Google Scholar]
- Spicer RA, Harris NBW, Widdowson M, et al. 2003. Constant elevation of southern Tibet over the past 15 million years. Nature 421: 622–624. [DOI] [PubMed] [Google Scholar]
- Stace CA. 1965. Cuticular studies as an aid to plant taxonomy. Bulletin of the British Museum (Natural History), Botany Series 4: 3–78. [Google Scholar]
- Steinthorsdottir M, Vajda V. 2013. Early Jurassic (late Pliensbachian) CO2 concentrations based on stomatal analysis of fossil conifer leaves from eastern Australia. Gondwana Research (in press). [Google Scholar]
- Stults DZ, Wagner-Cremer F, Axsmith BJ. 2011. Atmospheric paleo-CO2 estimates based on Taxodium distichum (Cupressaceae) fossils from the Miocene and Pliocene of Eastern North America. Palaeogeography, Palaeoclimatology, Palaeoecology 309: 327–332. [Google Scholar]
- Su T, Jacques FMB, Spicer RA, et al. 2013. Post-Pliocene establishment of the present monsoonal climate in SW China: evidence from the late Pliocene Longmen megaflora. Climate of the Past 9: 1911–1920. [Google Scholar]
- Sun BN, Dilcher DL, Beerling DJ, Zhang CJ, Yan DF, Kowalski E. 2003. Variation in Ginkgo biloba L. leaf characters across a climatic gradient in China. Proceedings of the National Academy of Sciences, USA 100: 7141–7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun BN, Wu JY, Liu YS, (Christopher), et al. 2011. Reconstructing Neogene vegetation and climates to infer tectonic uplift in western Yunnan, China. Palaeogeography, Palaeoclimatology, Palaeoecology 304: 328–336. [Google Scholar]
- Tao JR. 1986. Neogene flora of Lanping and its significance in middle watershed of Selween–Mekong–Yantze Rivers. Beijing, China: Science and Technology Publishing House (in Chinese). [Google Scholar]
- Tripati AK, Roberts CD, Eagle RA. 2009. Coupling of CO2 and ice sheet stability over major climate transitions of the last 20 million years. Science 326: 1394–1397. [DOI] [PubMed] [Google Scholar]
- Wagner F. 1998. The influence of environment on the stomatal frequency in birch . PhD Thesis, Utrecht University, Utrecht, The Netherlands. [Google Scholar]
- Woodward FI. 1987. Stomatal numbers are sensitive to increases in CO2 from pre-industrial levels. Nature 327: 617–618. [Google Scholar]
- Woodward FI. 1988. The responses of stomata to changes in atmospheric levels of CO2. Plants Today 1: 132–135. [Google Scholar]
- Woodward FI, Bazzaz FA. 1988. The responses of stomatal density to CO2 partial pressure. Journal of Experimental Botany 39: 1771–1781. [Google Scholar]
- Woodward FI, Kelly CK. 1995. The influence of CO2 concentration on stomatal density. New Phytologist 131: 311–327. [Google Scholar]
- Ye MN. 1981. On the preparation methods of fossil cuticle. In: Palaeontological Society of China, ed. Selected Papers of the 12th Annual Conference of the Palaeontological Society of China. Beijing, China: Science Press, 170–179 (in Chinese). [Google Scholar]
- Yunnan Bureau of Geology and Mineral Resources. 1978. Regional stratigraphic table of SW China: Yunnan Volume. Beijing, China: Geological Publishing House (in Chinese). [Google Scholar]
- Yunnan Bureau of Geology and Mineral Resources. 1990. Regional geology of Yunnan Province, Geological Memoirs. Beijing, China: Geological Publishing House (in Chinese). [Google Scholar]
- Zachos JC, Röhl U, Schellenberg SA, et al. 2005. Rapid acidification of the ocean during the Paleocene–Eocene thermal maximum. Science 308: 1611–1615. [DOI] [PubMed] [Google Scholar]
- Zhang SB, Zhou ZK, Hu H, Xu K, Yan N, Li SY. 2005. Photosynthetic performances of Quercus pannosa vary with altitude in the Hengduan Mountains, southwest China. Forest Ecology and Management 212: 291–301. [Google Scholar]
- Zhang SR, Dang QL. 2005. Effects of soil temperature and elevated atmospheric CO2 concentration on gas exchange, in vivo carboxylation and chlorophyll fluorescence in jack pine and white birch seedlings. Tree Physiology 25: 523–531. [DOI] [PubMed] [Google Scholar]
- Zhang YG, Pagani M, Liu ZH, Bohaty SM, DeConto R. 2013. A 40-million-year history of atmospheric CO2. Philosophical Transactions of the Royal Society A: Mathematical Physical and Engineering Sciences 371: 20130096. [DOI] [PubMed] [Google Scholar]
- Zhou ZK. 1992. A taxonomical revision of fossil evergreen sclerophyllous oaks from China. Acta Botanica Sinica 34: 954–961 (in Chinese with English abstract). [Google Scholar]
- Zhou ZK. 1999. Fossils of the Fagaceae and their implications in systematics and biogeography. Acta Phytotaxonomica Sinica 37: 369–385 (in Chinese with English abstract). [Google Scholar]