Abstract
Background and Aims Rock pools are small, geologically stable freshwater ecosystems that are both hydrologically and biologically isolated. They harbour high levels of plant endemism and experience environmental unpredictability driven by the presence of water over variable temporal scales. This study examined the hypothesis that the sediment seed bank in monsoon tropical freshwater rock pools would persist through one or more periods of desiccation, with seed dormancy regulating germination timing in response to rock pool inundation and drying events.
Methods Seeds were collected from seven dominant rock pool species, and germination biology and seed dormancy were assessed under laboratory conditions in response to light, temperature and germination stimulators (gibberellic acid, karrikinolide and ethylene). Field surveys of seedling emergence from freshwater rock pools in the Kimberley region of Western Australia were undertaken, and sediment samples were collected from 41 vegetated rock pools. Seedling emergence and seed bank persistence in response to multiple wetting and drying cycles were determined.
Key Results The sediment seed bank of individual rock pools was large (13 824 ± 307 to 218 320 ± 42 412 seeds m−2 for the five species investigated) and spatially variable. Seedling density for these same species in the field ranged from 13 696 to 87 232 seedlings m−2. Seeds of rock pool taxa were physiologically dormant, with germination promoted by after-ripening and exposure to ethylene or karrikinolide. Patterns of seedling emergence varied between species and were finely tuned to seasonal temperature and moisture conditions, with the proportions of emergent seedlings differing between species through multiple inundation events. A viable seed bank persisted after ten consecutive laboratory inundation events, and seeds retained viability in dry sediments for at least 3 years.
Conclusions The persistent seed bank in freshwater rock pools is likely to provide resilience to plant communities against environmental stochasticity. Since rock pool communities are often comprised of highly specialized endemic and range-restricted species, sediment seed banks may represent significant drivers of species persistence and diversification in these ecosystems.
Keywords: Ephemeral freshwater wetland, ethylene, hydrophytes, sandstone rock pools, seed dormancy, seed germination, seedling emergence patterns, sediment seed bank
INTRODUCTION
Ephemeral wetlands, such as rock pools, are highly stressful habitats for biota due to extreme and variable environmental conditions on both a diurnal and a seasonal time scale (Heilmeier et al., 2005). These conditions continuously test the tolerance limits of organisms inhabiting rock pools, and only a few taxa are able to persist in them long term (Brendonck et al., 2010; Jocque et al., 2010). While individual rock pool communities generally have low species diversity, they harbour a high proportion of specialist and endemic taxa that contribute to regional biodiversity (Pinder et al., 2000; Tuckett et al., 2010; Cross, 2014). The availability of water is regarded as the most ecologically limiting factor in highly ephemeral habitats (Deil, 2005), particularly in arid tropical regions such as northern Australia. In this environment, unpredictable and often seasonally variable rainfall results in a hydroregime almost exclusively reliant upon the balance between precipitation and evaporation (Vanschoenwinkel et al., 2009; Aponte et al., 2010; Brendonck et al., 2010). Individual rock pools are often shallow (1–10 cm depth), small in volume (around 100–500 L) and have a limited catchment zone (Porembski and Barthlott, 2000; Jocque et al., 2010; Cross, 2014). Rock pools are biologically isolated at local, meta-community and regional scales (Deil, 2005), and they probably represent some of the oldest and most geologically stable freshwater ecosystems on the planet (Deil, 2005; Jocque et al., 2010; Tapper et al. 2014). Their antiquity, coupled with the dominance of annual taxa in rock pool communities, suggests that they represent a model ecosystem for examining the role of the sediment seed bank as a driver of community composition and resilience.
The cues responsible for seed dormancy alleviation are not well understood for many aquatic plants (Tuckett et al., 2010; Baskin and Baskin, 2014). However, the persistence of dormant wetland plant seeds through numerous inundation events interspersed with drought events suggests that the cues for dormancy loss are complex and diverse across the resident seed bank (Baskin et al., 2000; Deil, 2005; Brock et al., 2003). Therefore, in regions where rainfall is unpredictable in timing and/or amount, wetland flora may possess syndromes of seed dormancy and germination requirements driven principally by ecohydrological factors. These syndromes are likely to be pronounced in areas such as the Australian monsoon tropics, where highly seasonal rainfall results in wetland communities that undergo an annual shift from periodic inundation to months of continued desiccation (Finlayson, 2005; Bowman et al., 2010).
The duration and periodicity of flooding are known to regulate seedling emergence in freshwater taxa from different wetland communities (Brock 2011; Carta et al., 2013; Ge et al., 2013). Drought events are a disturbance phase during which annual-dominated aquatic communities must survive and then recover (Brock et al., 2003; Heilmeier et al., 2005). They facilitate the persistence of species with short life histories that can establish rapidly from a dormant propagule bank when conditions are suitable (Deil, 2005). The development of large and long-lived seed banks appears to be a common trait of species inhabiting seasonal and ephemeral wetlands globally (Leck and Brock, 2000; James et al., 2007; Aponte et al., 2010), particularly in semi-arid environments and regions experiencing unpredictable or irregular precipitation (Brock, 2011; Cross et al., 2014). Studies suggest that the sediment seed banks of ephemeral and seasonal wetlands can survive long dry periods, retain significant species diversity for longer than 10 years without wetting, and are not exhausted by single or successive wetting events (Leck and Brock, 2000; Brock et al., 2003; Brock, 2011). A large seed bank consisting of seeds of different ages and in various stages of dormancy may result in plant communities that are resilient to unpredictable environmental conditions and seasonal reproductive failure (Deil, 2005; Merritt et al., 2007; Baskin and Baskin, 2014). This resilience may be enhanced by seeds in the sediment seed bank becoming responsive to germination cues such as sediment moisture and ethylene gas after periods of warm dry conditions (i.e. after- ripening) during seasonal drought and undergoing seasonal dormancy cycling as germination conditions change over time (Merritt et al., 2007; Cross et al., 2014). After-ripening has been reported as an effective dormancy-breaking treatment for the seeds of ephemeral wetland plants (Schütz, 1997; Carta et al., 2013) and is well documented in several Australian families with representatives in the monsoon tropics (Merritt et al., 2007; Tuckett et al., 2010).
This study investigated the seed biology and seed bank ecology of wetland plants inhabiting sandstone freshwater rock pools in the Kimberley region of northern Western Australia. Our purpose was to determine the processes governing ecological resilience and intra- and interseasonal persistence in these communities. We hypothesized that seed banks are a significant driver of species persistence in ephemeral freshwater rock pools, with seeds strongly responsive to ecohydrological cues and possessing dormancy characteristics that confer resilience of the rock pool community to environmental stochasticity. To test this hypothesis, the study compared (1) the morphology, germination biology and dormancy type in seeds of seven freshwater rock pool species; (2) the seedling emergence patterns of seeds in the sediment seed bank in response to light, inundation and repeated drought events; and (3) the persistence of seeds in the sediment seed bank over several years of dry storage and in response to seasonal inundation and drought events.
MATERIALS AND METHODS
Study site and rock pool habitat characteristics
The study site is located in the catchment of the Morgan River, on the north-west tip of the Gardner Plateau in the monsoon tropical North Kimberley region of northern Western Australia (14°47'46''S, 126°31'27''E; Fig. 1). Rainfall in the North Kimberley is highly seasonal, with around 95 % falling during the November to April summer wet season (approx. 1200 mm annually; McKenzie et al., 2009). However, the timing of onset and intensity of the wet season is unpredictable (Garnett and Williamson, 2010), and the amount of rainfall is consistently exceeded by evapotranspiration rates (>2000 mm annually; Luke et al., 1989). Thus, the hydroregime is dynamic and transient, with rock pools drying rapidly after rainfall events and experiencing an unpredictable hydroperiod in both timing and duration. The study site has an average of 80 rainfall days per year and a range of 36–130 d (based on 35 years of data; Australian Bureau of Meteorology, http://www.bom.gov.au/climate/data/). During the wet season, there is an average of 7·4 periods lasting longer than 5 d between rainfall events (minimum two, maximum 15), with a mean duration of 10·4 d.
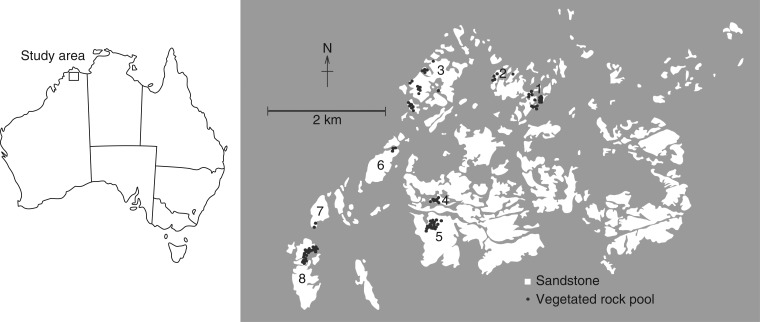
Fig. 1.
Location of vegetated rock pools on exposed sandstone pavement within the study area. Eight major clusters of vegetated rock pools were present (annotated), with 1–4 located to the north and 5–8 located to the south of the Morgan River. Grey areas indicate the surrounding matrix of savannah woodland.
More than 3000 rock pools occur within the study site, including approx. 200 with vegetation, on approx. 10 km2 of discontinuous sandstone pavement (Fig. 1). The majority of rock pool communities display monodominance, and harbour only a single species. Rock pools consist of geologically weathered depressions of varying size and depth in the sandstone bedrock, and they are distributed almost exclusively on flat or gently sloping sandstone pavement (Supplementary Data Fig. S1). An overview of the physical characteristics of rock pools at the study site (i.e. surface area, sediment depth and pool depth) is presented by Cross (2014) and Cross et al. (2014). The majority of sampled rock pools are shallow (27·9 ± 1·9 mm), small (surface area <1 m2), of limited volume (100–500 L) and have a thin layer of nutrient-poor loamy sand or sandy loam sediment (12·5 ± 0·5 mm depth). The mineral composition of rock pool sediments is presented in Supplementary Data Table S1. The number of seedlings (or sporophytes for Isoetes) in all rock pools was surveyed in March and April 2012, using ten 12·5 × 12·5 cm quadrats randomly placed along two perpendicular transects spanning the width of each rock pool in each of 111 vegetated pools, and seedling density was estimated for each species present.
To determine the seasonal temperatures of rock pool sediments, temperature probes (Hobo U23 Pro v2, Onset, Bourne, USA) were buried under 5 mm of sediment at the centre of two pools. The first rock pool (A) had a surface area of 3·2 m2, a depth of 25 mm and a sediment depth of 15 mm, and the second one (B) had a surface area of 4·8 m2, a depth of 43 mm and a sediment depth of 13 mm. Temperature was recorded from March 2012 to April 2013 (Fig. 2). The water temperature of flooded rock pools was assessed at midday during floristic surveys in various pools using a D-54 Water Quality Meter (Horiba, Fukuoka, Japan).
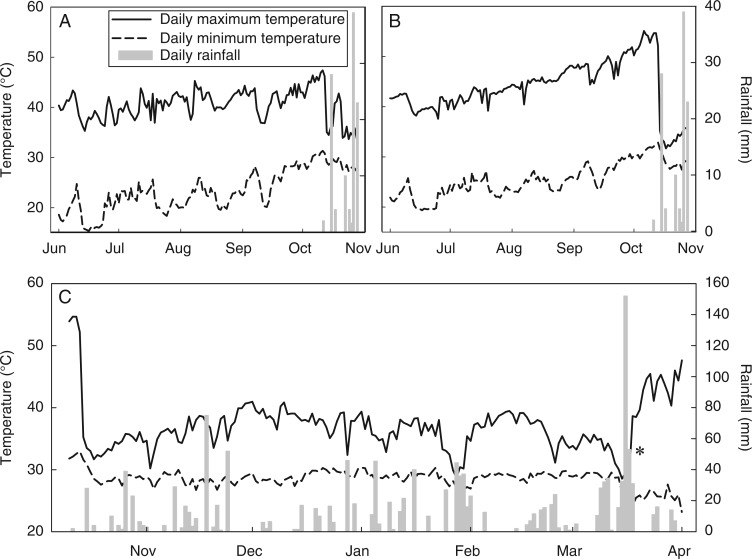
Fig. 2.
Annual temperature profiles for the top 10 mm of sediment in Kimberley sandstone rock pools, with daily rainfall data overlaid (Australian Bureau of Meteorology; http://www.bom.gov.au/climate/data/). June–November dry season temperatures in 2012 are presented for both a shallow pool (3·2 m2 surface area with a depth of 25 mm and a sediment depth of 15 mm; A) and a deeper pool (4·8 m2 surface area with a depth of 43 mm and a sediment depth of 13 mm; B), and October–April wet season temperatures for the deeper pool (C). Temperature probes were washed out of the rock pool on 16 March 2012 during a flood event.
Sediment collection
Dry sediments from 27 vegetated rock pools were collected at the end of the dry season in early October 2011, shortly before the onset of the summer wet growing season. Sites were selected to represent the full range of variation in the size, depth and plant association of the rock pool habitats. Ten dry sediment cores (10 cm3) were collected from the top 1 cm of the sediment within each rock pool and combined to provide one composite sample per pool. Samples were stored dry in paper bags for 3–7 d at ambient temperature (approx. 30 °C) before they were transported to the laboratory at Kings Park and Botanic Garden, Perth, Western Australia. Prior to use in experimental trials, all sediment samples were sieved (2·86 mm gauge) to remove large debris and stony material.
Seed bank ecology and composition
Species composition, seed density, interannual persistence, and persistence through drying events of rock pool sediment seed banks were determined using seedling emergence assays. The resilience of the seed bank, defined here as the capacity for seedling recruitment after disturbance events, was assessed using variable water regimes and wetting/drying events (Brock et al., 1994; Boedeltje et al., 2002; Brock, 2011). To assess species composition and the timing of seedling emergence from the sediment seed bank, dry sediment from all 27 rock pools was irrigated to field capacity with deionized (DI) water and incubated at constant 35 °C at a 12 h photoperiod (30 W cool white fluorescent tubes with a photon flux density at seed level of approx. 50 μmol m−2 s−1). The germination conditions employed are conducive to maximum seedling emergence from Kimberley rock pool sediments (Cross, 2014; Cross et al., 2014). Ten replicates were used for each site, with each replicate consisting of 50 g of sediment spread across a 10 × 10 cm square plastic Petri dish sealed with plastic cling wrap after irrigation with DI water. Emergence was scored after 6, 12 and 24 h, and then daily for 30 d. At the end of this period, sediments were dried in dishes at 40 °C for 7 d. Then, the sediment was rehydrated and scored again for seedling emergence after 6, 12 and 24 h, and then daily for 29 d. This process was repeated twice, giving three inundation events.
Light response of seeds in the seed bank
To determine the light response of seeds in the sediment seed bank to consecutive wetting and drying cycles, dry sediment from eight vegetated rock pools consisting of the dominant taxa Eriocaulon sp. Morgan River, Myriophyllum callitrichoides and Myriophyllum sp. Harding Range was irrigated to field capacity and incubated at constant 35 °C as described above. There were four 25 g replicate sediment samples per rock pool. In addition, four replicates for each rock pool were irrigated in darkness, wrapped in aluminium foil and incubated at 35 °C. Emergence was scored weekly for 4 weeks in the light/dark cycle treatments but only after 4 weeks in dark treatments to avoid exposing the seeds to light during incubation. After 4 weeks, sediments were dried as previously described before they were rehydrated and dark treatments re-wrapped with aluminium foil. Then, seedling emergence was monitored and scored as previously described. This latter process was repeated four times for a total of five inundation events.
Persistence of the seed bank through wetting/drying cycles
To examine the effect of water depth on seedling emergence and the persistence of the sediment seed bank through repeated wetting/drying cycles, sediment was collected from 12 vegetated rock pools consisting of Eriocaulon sp. Morgan River, Microcarpaea minima, M. callitrichoides, Myriophyllum sp. Harding Range and Portulaca sp. rock pools. Four 50 g replicates of sediment from each rock pool were spread across the bottom of 100 mm round plastic containers and irrigated to field capacity, sealed with airtight plastic lids and incubated in a 12 h photoperiod at constant 35 °C as previously described. In addition, for each rock pool, four replicates were irrigated to twice field capacity (i.e. waterlogged, sensu Cross et al., 2014) and emergence scored weekly for 4 weeks. At the end of this period, sediments were dried and then rehydrated and scored for seedling emergence as previously described. This process was repeated nine times for a total of ten inundation events.
To determine whether the seed bank persisted over multiple years, sediment from six vegetated rock pools consisting of Eriocaulon sp. Morgan River, M. minima, M. callitrichoides, Myriophyllum sp. Harding Range and Portulaca sp. rock pools was spread in 12·5 × 12·5 cm square Petri dishes, which were then sealed with plastic cling wrap and incubated dry at 35 °C as previously described. Four 50 g replicates of sediment were used for each rock pool. After 11 months of incubation, sediments were irrigated to field capacity and seedling emergence scored weekly for 4 weeks. Then, sediments were dried as previously described and incubated for an additional 11 months at 35 °C before being rehydrated and scored for emergence as described above. This process was repeated twice for a total of three inundation events over a 3 year period. Twelve control replicates for each rock pool were incubated dry at 35 °C for the duration of the experiment, with four of them irrigated to field capacity and scored for emergence at each wetting cycle. This procedure provided sediment that had been stored continuously dry under identical conditions for 1, 2 and 3 years.
Seed and embryo characteristics
Mature seeds (brown and fruits dehiscing) of seven annual rock pool species were collected from numerous rock pools in the study site in March and April 2011 and April 2012 (Table 1). These species represent those that are dominant in rock pools in the study site (Cross, 2014; Cross et al., 2014). Seed collections for each species were pooled and cleaned manually by gently rubbing material over 2 mm to 250 μm gauge steel sieves with a handheld rubber stopper. Seeds were separated from chaff using a vacuum aspirator and stored in a controlled environment room at 15 °C and 15 % relative humidity prior to use in experiments. Seed quality was determined for three replicates of 100 seeds of each species via X-ray analysis (MX-20 digital X-ray cabinet, Faxitron, Tucson, AZ, USA). Seeds were scored as filled if the endosperm was fully developed, not shrunken or retracted from the testa, and showed no sign of internal damage.
Table 1.
Seed and embryo characteristics for selected rock pool flora in the North Kimberley, Western Australia
| Family | Species | Life form | Seed fill (% ± s.e.) | Seed size |
Embryo type | E:S | TSW (g) | Buoyancy (B) | |
|---|---|---|---|---|---|---|---|---|---|
| Length (mm ± s.e.) | Width (mm ± s.e.) | ||||||||
| Eriocaulaceae | Eriocaulon scullionii G.J.Leach | Dwarf ephemeral rosulate macrophyte | 100·0 ± 0·0 | 0·21 ± 0·02 | 0·21 ± 0·01 | Basal | 0·20 ± 0·01 | 0·044 | 0·33 |
| Eriocaulon sp. Morgan River (A.T.Cross 62) | Dwarf ephemeral rosulate macrophyte | 100·0 ± 0·0 | 0·34 ± 0·03 | 0·30 ± 0·02 | Basal | 0·20 ± 0·02 | 0·037 | 0·73 | |
| Haloragaceae | Myriophyllum callitrichoides subsp. striatum Orchard | Dwarf ephemeral epihydate | 97·0 ± 0·6 | 2·40 ± 0·07 | 0·78 ± 0·03 | Linear | 0·74 ± 0·05 | 0·108 | 0·69 |
| Myriophyllum sp. Harding Range (M.D.Barrett and R.L.Barrett MDB 1825) | Dwarf ephemeral epihydate | 87·0 ± 0·3 | 2·29 ± 0·06 | 0·92 ± 0·05 | Linear | 0·74 ± 0·05 | 0·097 | 0·68 | |
| Phrymaceae | Microcarpaea minima (Retz.) Merr. | Dwarf ephemeral submerged herb | 100·0 ± 0·0 | 0·39 ± 0·02 | 0·24 ± 0·02 | Basal | 0·71 ± 0·04 | 0·008 | 0·64 |
| Portulacaceae | Portulaca bicolor F.Muell. | Dwarf ephemeral tenagophyte | 98·0 ± 0·3 | 0·88 ± 0·04 | 0·79 ± 0·03 | Folded | 0·97 ± 0·03 | 0·146 | 0·31 |
| Portulaca sp. rock pools (K.A.Menkhorst 310) | Dwarf ephemeral tenagophyte | 89·0 ± 4·1 | 0·84 ± 0·05 | 0·78 ± 0·04 | Folded | 0·94 ± 0·03 | 0·161 | 0·33 | |
Life form classifications from Cook (2004); embryo types derived from Martin (1946) and Baskin and Baskin (2007).
E:S: seed:embryo length ratio; TSW, average weight of 1000 seeds; B, buoyancy value determined from seed flotation experiments, ranging from 0 (all seeds sank immediately) to 1 (all seeds remained floating after 48 h).
To assess the water permeability of the seed coat, three 0·05 g replicates of dry, freshly collected seeds from each of the seven species were placed into small nylon mesh bags. Each bag was weighed, filled with seeds and placed in a Petri dish lined with filter paper irrigated with DI water. The bags of seeds were weighed at time 0, and after 2, 15 and 30 min and 1, 1·5, 2, 4, 6, 24, 48 and 72 h of imbibition, after they had been gently patted dry on paper towels before each measurement. Percentage water uptake of seeds was determined gravimetrically based on the fresh weight of non-imbibed seeds after subtracting the weight of the bags, with the percentage increase in seed mass calculated by [(W1 − Wd)/Wd] × 100, where W1 and Wd are the mass of imbibed and dry seeds, respectively (Turner et al., 2006, 2009).
Seed buoyancy was assessed using methods adapted from Coops and van der Velde (1995), Guja et al. (2010) and van den Broek et al. (2005). For each species, three replicates of 100 seeds were placed into 850 mL plastic containers (110 mm diameter × 110 mm height) containing 700 mL of DI water. Containers were incubated under ambient conditions (22–26 °C, approx. 50 % relative humidity). The water level was kept constant and the water surface agitated gently at each recording period by gently shaking the containers. The number of seeds that remained floating was recorded at time 0 and after 2, 15 and 30 min and 1, 2, 6, 12, 24, 36 and 48 h. Seed buoyancy (B) is the sum of the floating fraction at each successive interval of time divided by the time elapsed during the interval,
where Fn is number of seeds floating after n min and Ftotal is the total number of seeds. Thus, values of B range from 0 (all seeds sank immediately) to 1 (all seeds remained floating after 48 h).
We determined whether embryo growth occurs inside seeds prior to radicle emergence, and thus if the seeds have morphological/morphophysiological dormancy (Baskin and Baskin, 2014). One hundred seeds of each species were placed in 90 mm Petri dishes on 0·7 % (w/v) water agar containing 2·89 mm gibberellic acid (GA3; Sigma Aldrich Chemicals, St. Louis, MO, USA) sealed with plastic cling wrap and incubated at 30 °C on a 12 h photoperiod. Prior to incubation and each week for 8 weeks, ten seeds were dissected and seed and embryo lengths measured under a dissecting microscope equipped with an ocular micrometer to determine the embryo length to seed length (E:S) ratio.
Germination biology
To assess the seed germination response of rock pool taxa to temperature, light and chemical germination stimuli, freshly collected seeds of each species were plated onto water agar controls and on water agar containing 2·89 mm GA3 or 0·67 μm karrikinolide (KAR1; Flematti et al., 2004), or water agar after exposure to 50 nmol ethylene gas (C2H4) for 24 h. Four replicates of 25 seeds for each treatment were placed in incubators at constant 10, 15, 20, 25, 30 or 35 °C on a 12 h photoperiod and in constant darkness (plated in darkness and wrapped in aluminium foil). For all experiments, germination (radicle >1 mm) was scored weekly for 8 weeks of incubation in light/dark treatments but only after 8 weeks in the dark treatments. Upon completion of the experiment, all non-germinated seeds were cut-tested to determine viability (Turner et al. 2006). Seeds with a firm, white endosperm and embryo were judged to be viable. Germination percentages are based on the number of viable seeds.
To determine the effect of dry after-ripening on alleviation of dormancy, fresh seeds of each species were enclosed in a polycarbonate electrical enclosure box (28 × 28 × 14 cm; NHP Fibox, Richmond, Australia) above a non-saturated solution of LiCl (364 g L−1), creating a relative humidity of 50 % (Hay et al., 2008), and incubated at constant 35 °C (Tuckett et al., 2010). After 1, 3 and 6 months, four replicates of 25 seeds were placed on water agar, water agar containing 0·67 μm KAR1 or water agar after exposure to 50 nmol ethylene gas (C2H4) for 24 h. Methods of seed exposure to ethylene followed Cross et al. (2014). Germination was scored weekly for 8 weeks of incubation.
Statistical analyses
Poisson loglinear regression (SPSS Statistics 21, IBM, New York, USA) with backward stepwise selection based on Wald tests was used to test the main effects and two-way interactions of fixed factors (flooding depth, light exposure, temperature, inundation event, duration of dry storage and site) on the number of seedlings that emerged from the sediment seed bank. Binary logistic regression was used to assess the main and interaction effects of light, temperature, GA3, KAR1, ethylene and after-ripening on seed germination. One-way analysis of variance (ANOVA) was used to test the effect of rock pool size and depth on daily temperature maxima and minima, difference in diurnal variation between seasons and difference in seedling emergence for each species between individual inundation events, and pairwise differences between treatments. Preliminary analyses of all data were conducted to test the assumptions of normality (Kolmogorov–Smirnov test), linearity and homoscedasticity (Levene’s test). Where necessary, data were log10 transformed to help meet the assumption of normality and equal variance. All statistical tests were conducted using the 95 % confidence interval (CI), with significance determined by P < 0·05. Data are presented as mean ± 1 s.e. of the raw data unless stated otherwise.
RESULTS
Rock pool habitat characteristics
Rock pool sediments in the study site are subjected to high temperatures year-round, with daily maxima generally remaining above 30 °C and daily minima rarely falling below 20 °C (Fig. 2). Individual rock pools exhibited markedly different temperature profiles during the June–November dry season. The daily maximum and minimum temperatures for pool A (mean 40·6 ± 0·2 °C, range 33·7–47·4 °C; and mean 23·4 ± 4·1 °C, range 15·3–31·4 °C, respectively) were significantly lower (P < 0·001) than those of pool B (mean 43·8 ± 0·4 °C, range 31·7–55·1 °C; and 25·5 ± 0·3 °C, range 19·4–33·0 °C, respectively). Surface water temperature during the wet season was generally between 40 and 45 °C (mean 42·3 °C) in both pools, with occasional measurements of up to 55 °C in shallow waters during days of high ambient temperature (>40 °C).
Seed bank ecology and composition
Field surveys of seedling emergence in vegetated rock pools suggest a high spatial variability of seed bank size between individual rock pools and within and between species (Table 2). Seedlings emerged rapidly from sediments maintained at field capacity, with >75 % of all emergence occurring within 72 h of initial wetting. Eriocaulon sp. Morgan River was the most common species throughout in situ rock pool communities (in 70 % of all quadrats) and was the only species present in as many as 48 rock pools. Myriophyllum sp. Harding Range and M. callitrichoides are generally co-dominant with Eriocaulon sp. Morgan River, and they rarely occurred in monospecific stands. When these species were present, their average seedling density was significantly higher than that of any of the other taxa (P < 0·001). An exception is M. minima, which had the highest average seedling density but was present in only two rock pools.
Table 2.
Numbers of emergent seedlings observed in North Kimberley rock pools (in situ seedling survey), and recorded from field capacity sediments incubated at constant 35 °C and exposed to 12/12 h light/dark conditions (ex situ incubated sediments)
| Family | Species |
In situ seedling survey: seedlings per quadrat |
Ex situ incubated sediments: seedling emergence |
|||||
|---|---|---|---|---|---|---|---|---|
| % quadrats | Mean ± s.e. | Maximum | % samples | Total | Mean ± s.e. | Maximum | ||
| Centrolepidaceae | Centrolepis exserta (R.Br.) Roem. & Schult. | 4 ·3 | 1 ·4 ± 0 ·1 | 3 | 2 ·0 | 17 | 2 ·8 ± 0 ·8 | 6 |
| Eriocaulaceae | Eriocaulon scullionii G.J.Leach | 3 ·0 | 16 ·2 ± 2 ·4 | 64 | 3 ·7 | 447 | 20 ·3 ± 3 ·0 | 49 |
| Eriocaulon sp. Morgan River (A.T.Cross 62) | 70 ·9 | 218 ·1 ± 8 ·8 | 1363 | 82 ·8 | 95,566 | 203 ·8 ± 13 ·6 | 2317 | |
| Haloragaceae | Myriophyllum callitrichoides subsp. striatum Orchard | 13 ·7 | 159 ·4 ± 18 ·0 | 1102 | 7 ·6 | 5042 | 105 ·1 ± 28 ·2 | 831 |
| Myriophyllum sp. Harding Range (M.D.Barrett and R.L.Barrett MDB 1825) | 16 ·3 | 138 ·4 ± 12 ·9 | 906 | 7 ·3 | 3505 | 77 ·9 ± 21 ·1 | 593 | |
| Isoetaceae | Isoetes coromandelina subsp. macrotuberculata C.R.Marsden* | 0 ·5 | 1 ·2 ± 0 ·1 | 2 | 0 ·3 | 1 | 1 ·0 ± 1 ·0 | 1 |
| Phrymaceae | Microcarpaea minima (Retz.) Merr. | 1 ·5 | 470 ·7 ± 53 ·6 | 987 | 2 ·9 | 9319 | 776 ·6 ± 152 ·6 | 1703 |
| Poaceae | Micraira brevis subsp. Theda (M.D.Barrett 1505) | 2 ·5 | 0 ·0 ± 0 ·0 | 0 | 0 ·0 | 0 | 0 ·0 ± 0 ·0 | 0 |
| Micraira lazaridis L.G.Clark, Wendel & Craven | 7 ·0 | 0 ·0 ± 0 ·0 | 0 | 0 ·0 | 0 | 0 ·0 ± 0 ·0 | 0 | |
| Portulacaceae | Portulaca bicolor F.Muell. | 1 ·8 | 1 ·8 ± 0 ·1 | 3 | 2 ·1 | 241 | 12 ·0 ± 2 ·1 | 31 |
| Portulaca sp. rock pools (K.A.Menkhorst 310) | 6 ·0 | 14 ·8 ± 3 ·3 | 214 | 8 ·9 | 1323 | 11 ·6 ± 2 ·9 | 219 | |
In situ surveys involved placing five quadrats randomly along two perpendicular transects in each of 11 rock pools (total vegetated quadrats: n = 1066). Sediments from 27 randomly selected rock pools were tested in incubator trials, with ten replicates per pool (n = 270).
% quadrats, species occurrence in quadrats as a percentage of total quadrats; % samples, seedling emergence from incubated sediment replicates as a percentage of total replicates.
Mean ± s.e. and maximum values of seedling density in situ and seedling emergence from incubated sediments are presented.
*Seedling count indicates the number of sporophytes per quadrat for Isoetes coromandelina subsp. macrotuberculata.
Rock pools contained a large seed bank, and seedlings of all species recorded in field surveys emerged from incubated sediments. Incubated sediments yielded a total of 116 807 seedlings of eight species, and there was marked variability in the number of seedlings between replicates and species (Table 2). Eriocaulon sp. Morgan River was the most common and abundant species, accounting for about 80 % of all seedlings, followed by M. minima (7·9 %), M. callitrichoides (4·3 %) and Myriophyllum sp. Harding Range (3·0 %). Additionally, there were 1363 seedlings (1·2 % of all emergents) of the terrestrial Triodia aff. bynoei (C.E.Hubb.) Lazarides (Poaceae), a sandstone species commonly observed in close proximity to rock pools at the study site. The number of seedlings that emerged from incubated sediment did not differ significantly from that of those observed in situ for any species except M. minima (P = 0·02).
Light response of seeds in the seed bank
Seedling emergence of Eriocaulon sp. Morgan River in constant darkness was reduced by >90 % compared with sediments on a light/dark cycle (11·2 ± 3·5 vs. 132·4 ± 21·1; P < 0·001). However, the interaction effect between light and wetting cycle was not significant (P = 0·305). No seeds of any other species tested germinated in darkness.
Persistence of the seed bank through wetting/drying cycles
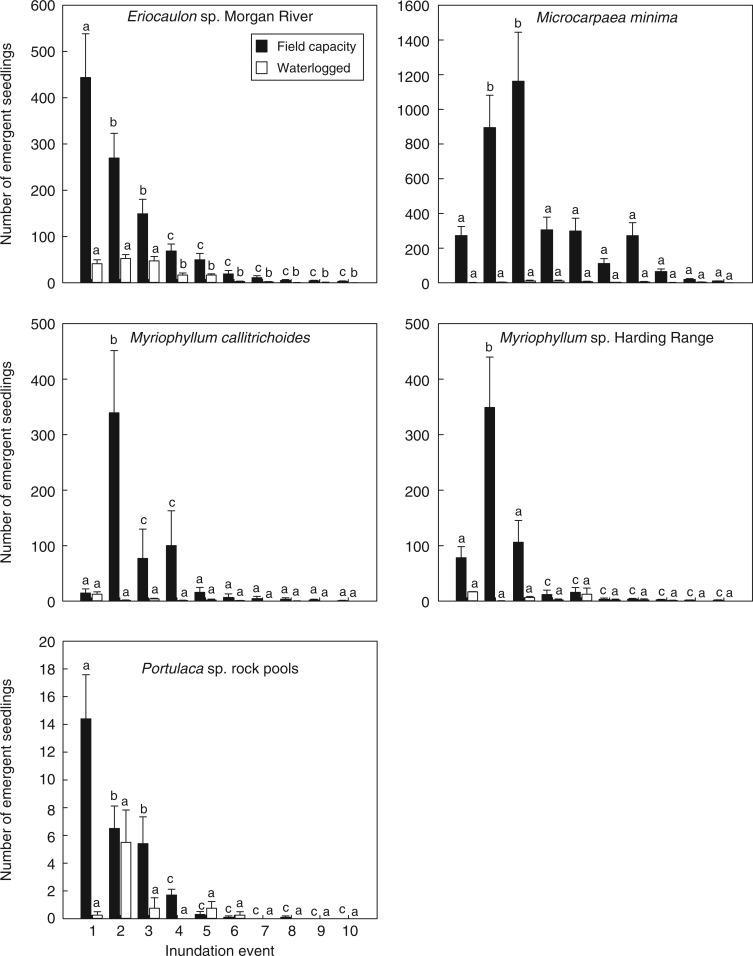
Seedlings of Eriocaulon sp. Morgan River, M. callitrichoides, Myriophylum sp. Harding Range and M. minima emerged in all ten consecutive wetting cycles and those of Portulaca sp. rock pools over six consecutive cycles (Fig. 3). The main effect of wetting cycle on emergence was highly significant for all species (P < 0·001), with different species displaying variable emergence patterns over repeated wetting events. The main effect of flooding depth was also highly significant for all species (P < 0·001), with a 75–95 % reduction for all species in inundated treatments compared with sediments maintained at field capacity (Fig. 3). The interaction between flooding depth and inundation event was also significant for all species (P < 0·001). Seedling emergence for Eriocaulon sp. Morgan River and Portulaca sp. rock pools declined from the first to the tenth inundation event, whereas the highest emergence was in either the second or third inundation event for M. callitrichoides, Myriophyllum sp. Harding Range and M. minima (Fig. 3).
Fig. 3.
Seedling emergence (mean ± s.e.) from the sediment seed bank of five rock pool species over ten consecutive inundation events, with sediments irrigated to either field capacity (hashed columns) or waterlogged (white columns) and incubated for 4 weeks at 35 °C on a 12/12 h light/dark cycle. Annotated lettering indicates the within-treatment significance of seedling emergence between inundation events.
Total seed bank size for each species over ten inundation events was 218 320± 42 412 seeds m−2 for M. minima, 65 520± 12 53 seeds m−2 for Eriocaulon sp. Morgan River, 36 064± 13 271 seeds m−2 for M. callitrichoides, 36 874± 6581 seeds m−2 for Myriophyllum sp. Harding Range and 13 824± 307 seeds m−2 for Portulaca sp. rock pools.
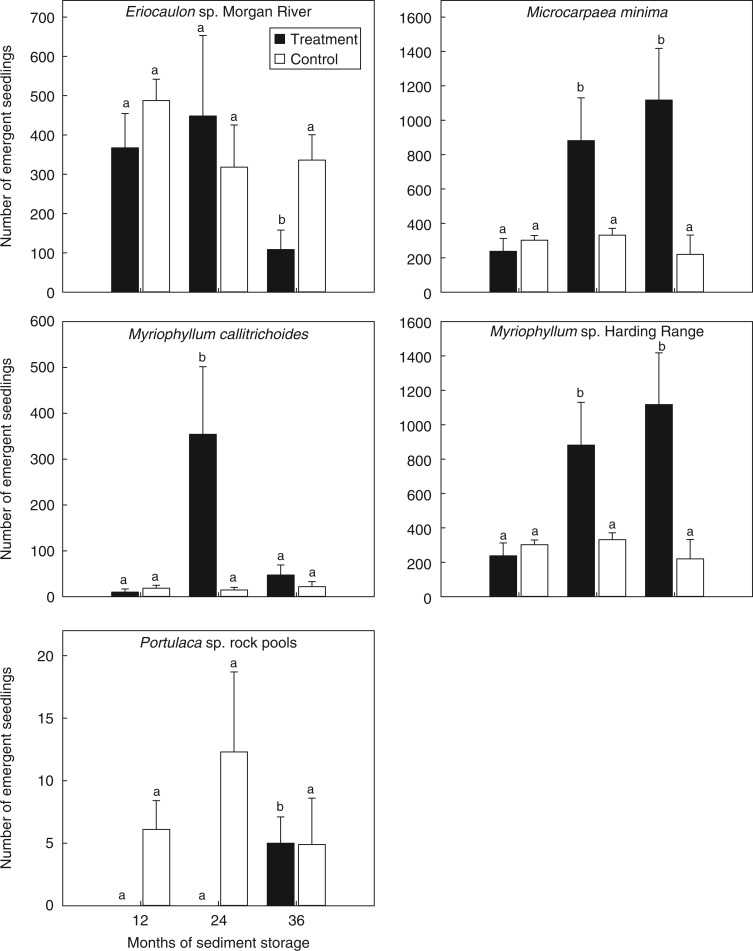
Seeds of Eriocaulon sp. Morgan River, M. callitrichoides, Myriophyum sp. Harding Range, M. minima and Portulaca sp. rock pools retained viability in the sediment seed bank over 3 years in both continuous dry storage and annual inundation events (Fig. 4). Seedling emergence in Portulaca sp. rock pools was recorded in a single sample after 36 months of dry storage. Based on the number of seeds that germinated from the seed bank, no apparent seed decline was evident under laboratory storage conditions over a 3 year period for any of the species tested (P > 0·05).
Fig. 4.
Seedling emergence (mean ± s.e.) from the sediment seed bank of five rock pool species in response to annual inundation over 3 years of dry storage (Treatment), and in control sediments stored dry at 35 °C with inundation events occurring after 12, 24 and 36 months (Control). Sediments were maintained dry at 35 °C during storage, and incubated at 35 °C during inundation events. Annotated lettering indicates the within-treatment significance of seedling emergence between inundation events.
Seed and embryo characteristics
Seeds of all species tested were small (0·2–2·4 mm), lightweight (0·04–0·16 mg), readily imbibed water and had high seed fill (>85 %) (Table 1). Seed buoyancy was variable between species (Table 1), with the highest percentages of seeds remaining floating after 48 h observed for Eriocaulon sp. Morgan River (B = 0·73). No significant embryo growth was recorded in any species prior to germination (P > 0·05 for all species) (Table 1).
Germination biology
Seeds of all seven species tested were dormant at maturity, with limited or no germination in control treatments at any of the six temperatures (Table 3). No germination was observed in any treatment at any temperature for Eriocaulon scullionii or M. minima or in dark-incubated treatments for all other taxa. Most seeds (>90 %) germinated within 3–7 d, with germination occurring only at or above 25 °C for all species tested. Although exposure of seeds to GA3 or KAR1 at higher temperatures stimulated germination in all species except Eriocaulon sp. Morgan River (Table 3), germination never exceeded 50 % in any species. There were significant interactions between KAR1 and temperature and between GA3 and temperature for all species (P < 0·001 in all cases).
Table 3.
Germination of freshly collected seed of Kimberley sandstone rock pool taxa, incubated at various constant temperatures on a 12 h photoperiod
| Species | 25 °C |
30 °C |
35 °C |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | G | K | E | C | G | K | E | C | G | K | E | |
| Eriocaulon sp. Morgan River | 8 ± 3 | 12 ± 2 | 10 ± 2 | 2 ± 1 | 1 ± 1 | 1 ± 1 | 4 ± 2 | 0 | 1 ± 1 | 1 ± 1 | 1 ± 1 | 0 |
| Myriophyllum callitrichoides subsp. striatum | 0 | 0 | 0 | 1 ± 1 | 0 | 2 ± 2 | 2 ± 1 | 9 ± 4* | 0 | 37 ± 7** | 40 ± 9** | 43 ± 6** |
| Myriophyllum sp. Harding Range | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 ± 2* | 1 ± 1 | 1 ± 1 | 0 | 11 ± 2* |
| Portulaca bicolor | 0 | 0 | 0 | 0 | 0 | 11 ± 3** | 5 ± 1* | 30 ± 4** | 0 | 4 ± 2* | 0 | 13 ± 3** |
| Portulaca sp. rock pools | 0 | 0 | 22 ± 10** | 20 ± 6** | 1 ± 1 | 28 ± 9** | 30 ± 9** | 13 ± 3** | 0 | 17 ± 3** | 24 ± 5** | 21 ± 6** |
No germination was observed below 25 °C in any species, and these temperatures have been omitted. Treatments included water agar control (C), water agar containing 2·89 mm GA3 (G) or 0·67 μm KAR1 (K), or water agar after exposure to 50 nmol ethylene gas (E), with four replicates of 25 filled seeds for each treatment.
Asterisks for each species indicate significant difference from controls at each temperature: *P < 0·05, **P < 0·001.
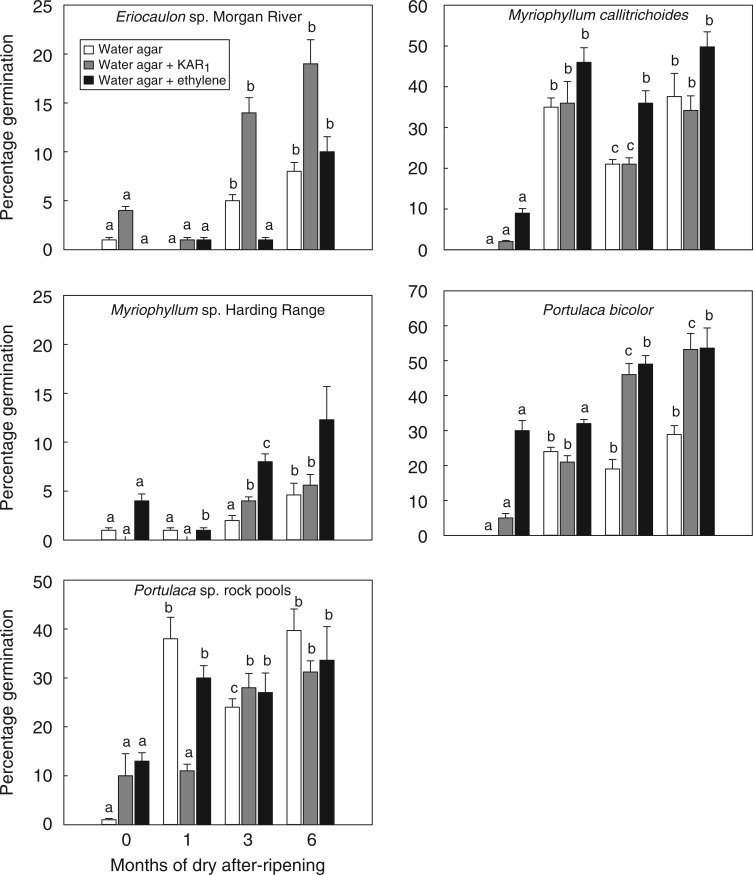
Germination of seeds incubated on water agar increased markedly for all species during a 6 month period of after-ripening (Fig. 5). Although dormancy was not broken in all seeds of any species following after-ripening (i.e. germination never achieved 100 % of viable seed), the main effect of dry after-ripening on germination percentage was significant for all species tested: P < 0·001 for M. callitrichoides, Portulaca bicolor and Portulaca sp. rock pools; P = 0·003 for Eriocaulon sp. Morgan River; and P = 0·021 for Myriophyllum sp. Harding Range. There was significant interaction between after-ripening and exposure to ethylene post-after-ripening for all species: P < 0·001 for Portulaca sp. rock pools; P = 0·002 for M. callitrichoides and P. bicolor; P = 0·008 for Myriophyllum sp. Harding Range; and P = 0·02 for Eriocaulon sp. Morgan River. KAR1 was an effective germination stimulant, with a significant interaction between after-ripening and post-after-ripening exposure to KAR1 for Eriocaulon sp. Morgan River (P = 0·02), Myriophyllum sp. Harding Range (P = 0·01), M. callitrichoides (P = 0·045), P. bicolor (P = 0·002) and Portulaca sp. rock pools (P < 0·001). There were few differences between species in rate of after-ripening, with a significant response after 1 month in M. callitrichoides, P. bicolor and Portulaca sp. rock pools, after 3 months in Eriocaulon sp. Morgan River, and after 6 months in Myriophyllum sp. Harding Range.
Fig. 5.
Germination percentage (mean ± s.e.) of the seeds of rock pool species in response to up to 6 months of dry after-ripening (35 °C and 50 % relative humidity). Seeds of each species were plated either on water agar (white columns), water agar with KAR1 (hashed columns), or water agar after exposure to ethylene gas (black columns). Annotated lettering indicates the within-treatment significance of germination percentage during after-ripening.
DISCUSSION
Here we present the first large-scale study of seed and seed bank ecology in ephemeral monsoon tropical rock pools. The data suggest that the persistent sediment seed bank in rock pools in the Australian monsoon rropics provides a community-wide resilience to environmental stochasticity (unpredictable and transient water availability). Although spatially variable, the seed bank is similar in species composition and abundance to that of above-ground floristic diversity (Table 2), with all annual taxa well represented. This similarity differs from what has been found in other such studies, where the seed bank in ephemeral wetlands is poorly reflective of above-ground plant diversity (e.g. Leck and Brock, 2000; Carta et al. 2013), and it may reflect the high levels of endemism and specialization of plants in these insular rock pool communities. Eriocaulon sp. Morgan River, M. callitrichoides, Myriophyllum sp. Harding Range and Portulaca sp. rock pools are strict rock pool specialists, and all taxa recorded from Kimberley rock pools are either locally or regionally endemic (Cowie et al., 2000; Cross, 2014). Only a portion of the seeds in the seed bank are cued to germinate by any single inundation event, and the seed bank is likely to be comprised of a mix of seeds of different ages and in various stages of dormancy. On a seasonal scale, the seed bank of freshwater rock pools in the Kimberley probably buffers rock pool plant species against local extinction by false starts to the wet season (rainfall events before the true onset of monsoonal conditions) and periods of inundation too short for reproductive success.
For all rock pool species, a high proportion (92–100 %) of freshly matured seeds were dormant, with limited germination of non-treated seeds on water agar at any of the six temperatures tested. Seeds of all seven species required light for germination, and the positive response of seeds of most species to GA3 and KAR1, together with fully developed embryos, suggests they have physiological dormancy, which is the most common kind of seed dormancy in aquatic plants (Tuckett et al., 2010; Baskin and Baskin, 2014). Dormancy at maturity and a light requirement for germination have been reported for the seeds of annual wetland taxa from numerous seasonal or ephemeral wetland habitats in the USA (Baskin et al., 1993, 2000, 2004; Kettenring and Galatowitsch, 2007), ephemeral wetland depressions in the Mediterranean Basin (Carta et al., 2013), flood-meadows in Central Europe and Germany (Schütz, 1997; Hölzel and Otte, 2004), and vernal pools in south-western Australia (Tuckett et al., 2010). For all seven species investigated in our study, seed germination percentage increased significantly over a 6 month period of dry after-ripening (Fig. 5). The need for after-ripening is a common mechanism of seed dormancy alleviation in seasonal wetland species (Schütz, 1997; Baskin et al., 2000, 2004; Carta et al., 2013). The germination percentage of after-ripened seeds in M. callitrichoides, Myriophyllum sp. Harding Range and P. bicolor was increased further by exposure to ethylene gas (Fig. 5) and in Eriocaulon sp. Morgan River by exposure to KAR1. Ethylene is produced rapidly in rock pool sediments by microbes following inundation (Cross et al., 2014). The response to KAR1 is unusual for seeds in a wetland ecosystem, since the molecule is predominantly associated with fire responses of seeds in the soil seed bank. However, the biogenic production of KAR1 through wetting/drying cycles leading to enhanced oxidation of organic matter may represent a potential source of the molecule in freshwater rock pools (Dixon et al., 2009).
The timing and amount of seasonal rainfall appear to be major factors governing ecology in rock pool habitats, with resident species exhibiting complex germination patterns in relation to seasonal temperature and moisture cues. A level of persistence through inundation cycles is common in the sediment seed bank of ephemeral habitats, with only a small proportion of seeds emerging after each wetting event (Leck and Brock, 2000; Deil, 2005). The presence of seeds with various degrees of dormancy in the seed bank of Kimberley rock pools is likely to provide plant communities with significant resilience to unpredictable rainfall (Deil, 2005; Long et al., 2014). A portion of the seed bank is able to respond rapidly to suitable environmental cues and exploit unpredictable rainfall events, while the other portion(s) remain dormant through these periods, thus facilitating community persistence if reproductive success is not attained in response to the initial or subsequent inundation events. Hydrological modelling suggests that small rock pools can be filled by precipitation events of approx. 10 mm of rainfall (Altermatt et al., 2009; Cross, 2014), and thus episodes of low amounts of rainfall may provide suitable germination conditions for rock pool taxa, despite them being insufficient to provide similar conditions in larger freshwater habitats.
The seed bank in Kimberley rock pools is not exhausted by up to ten consecutive wetting and drying cycles, and different patterns of emergence were observed for different species in response to variation in the number of wetting and drying events (Fig. 3). Seedling emergence patterns suggest that dormancy alleviation in highly ephemeral habitats is likely to involve a complex interaction between multiple environmental factors. Plant diversity and community composition in ephemeral habitats is strongly influenced by the duration, extent and timing of inundation events (Casanova and Brock, 2000; Deil, 2005; James et al., 2007), and it is evident from our study that seeds of individual species in the seed bank respond differently to different combinations of cues. Seedling emergence strategies in rock pool taxa may be linked to the typical characteristics of rock pools inhabited by each species. For example, the ubiquitous Eriocaulon sp. Morgan River does not appear to have a preference for rock pools with particular hydrogeological characteristics (Cross, 2014), and a large proportion of the seeds of this species in the seed bank emerged in the first wetting cycle. Seedlings of this taxon develop rapidly to reproductive maturity in 3–4 weeks (Cross, 2014), and therefore the seed bank may be replenished by exploiting short periods of inundation. Additionally, the sheer size of the sediment seed bank in Eriocaulon sp. Morgan River (approx. 50 000− 80 000 seeds m−2) may buffer populations against reproductive failure in response to any given inundation event. In contrast, M. callitrichoides and Myriophyllum sp. Harding Range occur in deeper rock pools (Cross, 2014), where water may persist for longer periods during dry spells in the wet season. These species take >4 weeks to reach reproductive maturity (Cross, 2014), and thus germination may be delayed until the second or third inundation event, which ensures that seedlings do not emerge during brief periods of early wet season inundation.
Seedling emergence from rock pool sediments occurs rapidly after inundation events and appears to be synchronous with the production of biogenic ethylene from sediments (Cross et al., 2014). The duration and periodicity of flooding are crucial in regulating seedling emergence in ephemeral wetlands (Liu et al., 2005; Capon, 2007; Aponte et al., 2010; Brock, 2011). In our study, seedling emergence from waterlogged rock pool sediments was significantly lower than that from sediments maintained at field capacity (Fig. 3), a relationship also observed in intermittently inundated freshwater wetlands in other tropical (Deil, 2005) and sub-tropical regions (Johnson, 2004; Liu et al., 2005). This emergence strategy appears to be common in ephemeral habitats experiencing an unpredictable hydroregime and is believed to favour the rapid establishment of short-lived annual taxa (Deil, 2005; Alvarez et al., 2012; Cross et al., 2014).
Rock pool plants in northern Australia exhibit a syndrome of dry-phase adaptation that is convergent with that of species in other parts of the world. Eight of the nine species that emerged from sediments in incubator trials are dwarf ephemeral therophytes. Short-lived annual herbs that rely on the sediment seed bank for their continued existence at a site are a common component of the flora of ephemeral wetlands in many regions, including Brazil (Tabosa et al., 2012), North America (Bonis et al., 1995; Bliss and Zedler, 1998), Chile (Alvarez et al., 2012), the Mediterranean region (Aponte et al., 2010; Carta et al., 2013), Africa (Brock and Rogers, 1998) and Australia (Leck and Brock, 2000; Brock et al., 2003; Warwick and Brock, 2003; Tuckett et al., 2010). The ninth species emerging from sediments in our study, Isoetes coromandelina subsp. macrotuberculata, is a geophyte that resprouts from corms in the sediment following drought periods (Cowie et al., 2000). All species recorded in field surveys emerged from the sediment diaspore bank, with the exception of the perennials Micraira brevis subsp. Theda and M. lazaridis. These species are capable of resurrection of vegetative parts following drought periods (R. L. Barrett and M. D. Barrett, pers. comm.), and no seedlings of either of these taxa were observed during vegetation surveys or in sediment incubation studies. Limited recruitment from seeds appears characteristic of perennial rock pool vegetative-resurrection species (Heilmeier et al., 2005).
Results from our study suggest that the ecology of rock pool communities in the monsoon tropics is driven by the timing and amount of seasonal rainfall, with recruitment occurring rapidly in response to inundation of the sediment. However, the seed bank is not exhausted by single or even multiple wetting events, and thus it confers a level of resilience to plant communities in the unpredictable monsoonal climate. To elucidate further the ecological importance of the sediment seed bank in ephemeral rock pools, future studies should determine if diaspores are dispersed between pools at various scales and thus verify whether rock pools constitute a meta-community; determine the degree of local adaptation of rock pool taxa in relation to putative hydrogeological drivers; examine whether genetic differentiation is expressed within and between populations disjunct at local, regional and continental scales; and explore the role of hydrogeological characteristics in delineating species occurrence and range margins.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the folowing. Figure S1: typical freshwater rock pool habitat on sandstone pavement in the North Kimberley, Western Australia. Table S1: sediment chemistry and physical characteristics for sandstone rock pools in the Kimberley region of north Western Australia
ACKNOWLEDGEMENTS
We thank Celia Mitchell, Mark Warrington and Katherine Chuk for assistance in the field between 2010 and 2012. The Myers family and Dunkeld Pastoral supported fieldwork on Theda Station between 2011 and 2013, and Cecelia Myers and staff at Theda and Doongan Stations are particularly thanked for their hospitality and support. Critical review of the manuscript by Professor Alison Powell and two anonymous reviewers is gratefully acknowledged. This work was supported by an Australian Postgraduate Award to A.T.C. from the Commonwealth of Australia, a research grant to A.T.C. from the Friends of Kings Park, and a personal donation from John Crone.
LITERATURE CITED
- Altermatt F, Pajunen VI, Ebert D. 2009. Desiccation of rock pool habitats and its influence on population persistence in a Daphnia metacommunity. PLOS One 4: doi:10.1371/journal.pone.0004703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez M, San Martin J, Deil U. 2012. Nanism and ephemerism as reasons for a hidden abundance in vernal pool plants: the example of Lepuropetalon spathulatum in Chile. Feddes Repertorium 123: 55–66. [Google Scholar]
- Aponte C, Kazakis G, Ghosn D, Papanastasis VP. 2010. Characteristics of the soil seed bank in Mediterranean temporary ponds and its role in ecosystem dynamics. Wetlands Ecology and Management 18: 243–253. [Google Scholar]
- Baskin CC, Baskin JM, Chester EW. 1993. Seed germination ecophysiology of four summer annual mudflat species of Cyperaceae. Aquatic Botany 45: 41–52. [Google Scholar]
- Baskin CC, Baskin JM. 2014. Seeds. Ecology, biogeography, and evolution of dormancy and germination, 2nd edn San Diego: Academic Press. [Google Scholar]
- Baskin CC, Baskin JM, Chester EW. 2000. Effect of flooding on the annual dormancy cycle and on germination of seeds of the summer annual Schoenoplectus pershianus (Cyperaceae). Aquatic Botany 67: 109–116. [Google Scholar]
- Baskin CC, Baskin JM, Chester EW. 2004. Seed germination ecology of the summer annual Cyperus squarrosus in an unpredictable mudflat habitat. Acta Oecologica 26: 9–14. [Google Scholar]
- Bliss SA, Zedler PH. 1998. The germination process in vernal pools: sensitivity to environmental conditions and effects on community structure. Oecologia 113: 67–73. [DOI] [PubMed] [Google Scholar]
- Boedeltje G, ter Heerdt GNJ, Bakker JP. 2002. Applying the seedling-emergence method under waterlogged conditions to detect the seed bank of aquatic plants in submerged sediments. Aquatic Botany 72: 121–128. [Google Scholar]
- Bonis A, Lepart J, Gillas P. 1995. Seed bank dynamics and coexistence of annual macrophytes in a temporary and variable habitat. Oikos 74: 81–92. [Google Scholar]
- Bowman DMJS, Brown GK, Braby MF, et al. 2010. Biogeography of the Australian monsoon tropics. Journal of Biogeography 37: 201–216. [Google Scholar]
- Brendonck L, Jocque M, Hulsmans A, Vanschoenwinkel B. 2010. Pools ‘on the rocks’: freshwater rock pools as model system in ecological and evolutionary research. Limnetica 29: 25–40. [Google Scholar]
- Brock MA. 2011. Persistence of seed banks in Australian temporary wetlands. Freshwater Biology 56: 1312–1327. [Google Scholar]
- Brock MA, Rogers KH. 1998. The regeneration potential of the seed bank of an ephemeral floodplain in South Africa. Aquatic Botany 61: 123–135. [Google Scholar]
- Brock MA, Theodore K, O’Donnell L. 1994. Seed bank methods for Australian wetlands. Australian Journal of Marine and Freshwater Research 45: 483–493. [Google Scholar]
- Brock MA, Nielsen DL, Shiel RJ, Green JD, Langley JD. 2003. Drought and aquatic community resilience: the role of eggs and seeds in sediments of temporary wetlands. Freshwater Biology 48: 1207–1218. [Google Scholar]
- van den Broek T, van Diggelen R, Bobbink R. 2005. Variation in seed buoyancy of species in wetland ecosystems with different flooding dynamics. Journal of Vegetation Science 16: 579–586. [Google Scholar]
- Capon SJ. 2007. Effects of flooding on seedling emergence from the soil seed bank of a large desert floodplain. Wetlands 27: 904–914. [Google Scholar]
- Carta A, Bedini G, Muller JV, Probert RJ. 2013. Comparative seed dormancy and germination of eight annual species of ephemeral wetland vegetation in a Mediterranean climate. Plant Ecology 214: 339–349. [Google Scholar]
- Casanova MT, Brock MA. 2000. How do depth, duration and frequency of flooding influence the establishment of wetland communities? Plant Ecology 147: 237–250. [Google Scholar]
- Coops H, van der Velde G. 1995. Seed dispersal, germination and seedling growth of six helophyte species in relation to water-level zonation. Freshwater Biology 34: 13–20. [Google Scholar]
- Cowie ID, Short PS, Osterkamp Madsen M. 2000. Floodplain Flora. A flora of the coastal floodplains of the Northern Territory, Australia. Canberra: Australian Biological Resources Study. [Google Scholar]
- Cross AT. 2014. Between a rock and a hard place: community structure, seasonal ecology, and local adaptation in ephemeral arid-tropical freshwater rock pools. PhD thesis, the University of Western Australia. Perth. [Google Scholar]
- Cross AT, Cawthray GR, Merritt DJ, Turner SR, Renton M, Dixon KW. 2014. Biogenic ethylene promotes seedling emergence from the sediment seed bank in an ephemeral tropical rock pool habitat. Plant and Soil 380: 73–87. [Google Scholar]
- Deil U. 2005. A review on habitats, plant traits and vegetation of ephemeral wetlands – a global perspective. Phytocoenologia 35: 533–705. [Google Scholar]
- Dixon KW, Merritt DJ, Flematti GR, Ghisalberti EL. 2009. Karrikinolide – a phytoreactive compound derived from smoke with applications in horticulture, ecological restoration and agriculture. Acta Horticulturae 813: 155–170. [Google Scholar]
- Finlayson CM. 2005. Plant ecology of Australia’s tropical floodplain wetlands: a review. Annals of Botany 96: 541–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flematti G, Ghisalberti E, Dixon K, Trengove R. 2004. A compound from smoke that promotes seed germination. Science 305: 977. [DOI] [PubMed] [Google Scholar]
- Garnett ST, Williamson G. 2010. Spatial and temporal variation in precipitation at the start of the rainy season in tropical Australia. Rangeland Journal 32: 215–226. [Google Scholar]
- Ge XL, Liu J, Wang RQ. 2013. Effects of flooding on the germination of seed banks in the Nansi Lake wetlands, China. Journal of Freshwater Ecology 28: 225–237. [Google Scholar]
- Guja LK, Merritt DJ, Dixon KW. 2010. Buoyancy, salt tolerance and germination of coastal seeds: implications for oceanic hydrochorous dispersal. Functional Plant Biology 37: 1175–1186. [Google Scholar]
- Hay F, Adams J, Manger K, Probert R. 2008. The use of non-saturated lithium chloride solutions for experimental control of seed water content. Seed Science and Technology 36: 737–746. [Google Scholar]
- Heilmeier H, Durka W, Woitke M, Hartung W. 2005. Ephemeral pools as stressful and isolated habitats for the endemic aquatic resurrection plant Chamaegigas intrepidus. Phytocoenologia 35: 449–468. [Google Scholar]
- Holzel N, Otte A. 2004. Ecological significance of seed germination characteristics in flood-meadow species. Flora 199: 12–24. [Google Scholar]
- James CS, Capon SJ, White MG, Rayburg SC, Thomas MC. 2007. Spatial variability of the soil seed bank in a heterogenous ephemeral wetland system in semi-arid Australia. Plant Ecology 190: 205–217. [Google Scholar]
- Jocque M, Vanschoenwinkel B, Brendonck L. 2010. Freshwater rock pools: a review of habitat characteristics, faunal diversity and conservation value. Freshwater Biology 55: 1587–1602. [Google Scholar]
- Johnson S. 2004. Effects of water level and phosphorus enrichment on seedling emergence from marsh seed banks collected from northern Belize. Aquatic Botany 79: 311–323. [Google Scholar]
- Kettenring KM, Galatowitsch SM. 2007. Temperature requirements for dormancy break and seed germination vary greatly among 14 wetland Carex species. Aquatic Botany 87: 209–220. [Google Scholar]
- Leck MA, Brock MA. 2000. Ecological and evolutionary trends in wetlands: evidence from seeds and seed banks in New South Wales, Australia and New Jersey, USA. Plant Species Biology 15: 97–112. [Google Scholar]
- Liu G, Zhou J, Li W, Cheng Y. 2005. The seed bank in a subtropical freshwater marsh: implications for wetland restoration. Aquatic Botany 81: 1–11. [Google Scholar]
- Long RL, Gorecki MJ, Renton M, et al. 2014. The ecophysiology of seed persistence: a mechanistic view of the journey to germination or demise. Biological Reviews of the Cambridge Philosophical Society (in press). [DOI] [PubMed] [Google Scholar]
- Luke GJ, Burke KL, O’Brien TM. 2003. Evaporation data for Western Australia. Resource Management Technical Report No. 65. Perth: Department of Agriculture Western Australia. [Google Scholar]
- McKenzie NL, Start AN, Burbidge AA, Kenneally KF, Burrows ND. 2009. Protecting the Kimberley: a synthesis of scientific knowledge to support conservation management in the Kimberley region of Western Australia . Perth: Department of Environment and Conservation. [Google Scholar]
- Merritt D, Turner S, Clarke S, Dixon K. 2007. Seed dormancy and germination stimulation syndromes for Australian temperate species. Australian Journal of Botany 55: 336–344. [Google Scholar]
- Pinder AM, Halse SA, Shiel RJ, McRae JM. 2000. Granite outcrop pools in south Western Australia: loci of diversification and refugia for aquatic invertebrates. Journal of the Royal Society of Western Australia 83: 149–161. [Google Scholar]
- Porembski S, Barthlott W. 2000. Inselbergs. Biotic diversity of isolated outcrops in tropical and temperate regions. Ecological Studies, Vol. 146. Berlin: Springer. [Google Scholar]
- Schütz W. 1997. Primary dormancy and annual dormancy cycles in seeds of six temperate wetland sedges. Aquatic Botany 59: 75–85. [Google Scholar]
- Tabosa AB, Matias LQ, Martins FR. 2012. Live fast and die young: the aquatic macrophyte dynamics in a temporary pool in the Brazillian semiarid region. Aquatic Botany 102: 71–78. [Google Scholar]
- Tapper SL, Byrne M, Yates CJ, et al. 2014. Isolated with persistence or dynamically connected? Genetic patterns in a common granite outcrop endemic. Diversity and Distributions 20: 987–1001. [Google Scholar]
- Tuckett RE, Merritt DJ, Hay FR, Hopper SD, Dixon KW. 2010. Dormancy, germination and seed bank storage: a study in support of ex situ conservation of macrophytes of southwest Australian temporary pools. Freshwater Biology 55: 1118–1129. [Google Scholar]
- Turner SR, Merritt D, Ridley EC, et al. 2006. Ecophysiology of seed dormancy in the Australian endemic species Acanthocarpus preissii (Dasypogonaceae). Annals of Botany 98: 1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner SR, Commander LE, Baskin JM, Baskin CC, Dixon KW. 2009. Germination behaviour of Astroloma xerophyllum (Ericaceae), a species with woody indehiscent endocarps. Botanical Journal of the Linnaean Society 160: 299–311. [Google Scholar]
- Vanschoenwinkel B, Hulsmans A, De Roek E, De Vries C, Seaman M, Brendonck L. 2009. Community structure in temporary freshwater pools: disentangling the effects of habitat size and hydro-regime. Freshwater Biology 54: 1487–1500. [Google Scholar]
- Warwick NWM, Brock MA. 2003. Plant reproduction in temporary wetlands: the effects of seasonal timing, depth, and duration of flooding. Aquatic Botany 77: 153–167. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.