Abstract
Objective
Hydrogen gas has been demonstrated to neutralize free radicals and reduce oxidative stress recently. Our objective was to determine the therapeutic effect of H2 inhalation and its antioxidative activity on early brain injury after subarachnoid hemorrhage.
Design
Controlled in vivo laboratory study.
Setting
Animal research laboratory.
Subjects
One hundred thirty-seven adult male Sprague-Dawley rats weighing 280–350 g.
Interventions
Subarachnoid hemorrhage was induced by endovascular perforation method in rats. Subarachnoid hemorrhage rats were treated with 2.9% hydrogen gas inhaled for 2 hrs after perforation. At 24 and 72 hrs, mortality, body weight, neurologic deficits, and brain water content were assessed. Blood–brain barrier permeability and apoptosis were also measured at 24 hrs. To investigate the antioxidative activity of hydrogen gas, the expression of malondialdehyde, nitrotyrosine, and 8-hydroxyguanosine, which are oxidative markers of lipid, protein, and DNA damage, respectively, were measured at 24 hrs.
Measurements and Main Results
Hydrogen gas significantly alleviated brain edema and blood–brain barrier disruption, reduced apoptosis, and improved neurologic function at 24 hrs but not 72 hrs after subarachnoid hemorrhage. These effects were associated with the amelioration of oxidative injury of lipid, protein, and DNA.
Conclusions
Hydrogen gas could exert its neuroprotective effect against early brain injury after subarachnoid hemorrhage by its antioxidative activity.
Keywords: free radicals, hydrogen, oxidative stress, subarachnoid hemorrhage
Aneurysmal subarachnoid hemorrhage (SAH) has a devastating rate of mortality and morbidity (1). Studies have suggested that early brain injury, which is a pathophysiological event occurring before cerebral vasospasm, contributes to the mortality after SAH (2, 3). One of the key factors involved in the pathogenesis of early brain injury is oxidative stress (4), which is caused by free radicals, including excess production of reactive oxygen species and reactive nitrogen species. Therefore, an antioxidative strategy by using free radical scavengers has attracted attention in the treatment of SAH.
Antioxidative activity of hydrogen gas (H2) has been demonstrated recently. A study by Ohsawa et al (5) reported that inhaled H2 (1% to 4% concentration) markedly decreased oxidative stress and protected brain against ischemia/reperfusion injury and stroke in rats. It was also demonstrated that 2% H2 or hydrogen-rich water reduced oxidative stress and inflammation in the intestine (6) and kidney (7) after organ transplants. Mechanistically these were the result of the scavenging of free radicals by H2. In the present study, we tested if inhalation of hydrogen gas could reduce early brain injury through neutralizing free radicals after SAH.
MATERIALS AND METHODS
Animals
One hundred thirty-seven adult male Sprague-Dawley rats (Harlan, Indianapolis, IN), weighing from 280 to 350 g were used in this study. Animals were randomly divided into three groups: sham-operated, SAH, and SAH treated with 2.9% hydrogen for 2 hrs. All animal protocols were approved by the Institutional Animal Care and Use Committee at Loma Linda University.
Induction of SAH and Hydrogen Administration
The endovascular perforation model of SAH in rats was performed as described previously (8, 9) with small modification. Briefly, general anesthesia was induced with 4% isoflurane in a 70%/30% mixture of medical air and oxygen. After endotracheal intubation was performed, rats were ventilated with a rodent ventilator (Kent Scientific, Torrington, CT). Isoflurane was deceased to 2.5% to maintain anesthesia during surgery. A heating pad was used to maintain the rectal temperature at 36.0°C ± 0.5°C. The left external carotid artery was ligated and cut with a 3- to 4-mm stump remaining. A sharpened 4-0 suture was then cannulated into the left internal carotid artery from the external carotid artery stump to perforate the vessel wall at the bifurcation of the anterior cerebral artery and middle cerebral artery. Sham-operated rats underwent identical procedures except perforation. All animals were kept ventilating until they recovered from anesthesia.
For the gaseous treatment, 2.9% hydrogen premixed with 20% oxygen and balanced nitrogen was purchased from the manufacturer (Gilmore Liquid Air, South El Monte, CA). Inhalation of hydrogen gas started 1 hr after SAH. Rats were placed in a transparent chamber that had an inlet connected with 2.9% hydrogen gas and an outlet to ventilating hood. At the beginning of treatment, flow rate of mixed gas was 8 L/min for 5 mins, which was followed by a maintenance flow rate of 6 L/min. A handheld hydrogen detector (H2scan, Valencia, CA) was used to confirm the concentration of hydrogen gas intermittently throughout the treatment. The treatment lasted 2 hrs. Then animals were housed individually with free access to food and water in animal facility.
Assessment of SAH Grade
SAH grade was indicated by a previously published grade scale (10). Scores ranging from 0 to 18 represented the severity of SAH. Sham-operated rats got a score of 0. This assessment was performed by a blinded observer.
Neurobehavioral Test
At 24 and 72 hrs after SAH, a neurobehavioral test was performed in all rats with the modified Garcia’s method (11). An 18-point system was used to evaluate animals’ neurologic deficits from six aspects, including spontaneous activity, symmetry in the movement of all four limbs, forepaw outstretching, climbing, body proprioception, and response to vibrissae touch. Fewer scores indicated more deficits. This test was performed in a blinded manner to avoid bias.
Brain Water Content
After decapitation under deep anesthesia (5% isoflurane), the rat brain was quickly removed and immediately divided into two hemispheres, cerebellum and brain stem, and weighed respectively (wet weight). After dehydrating in a 1058C oven for 72 hrs, brain samples were weighed again (dry weight). Brain water content was calculated according to the following formula: [(wet weight − dry weight)/wet weight] × 100% (12).
Blood–Brain Barrier Permeability
Evans blue extravasation was measured as previously described (8). Briefly, Evans blue dye (2%; 5 mL/kg) was injected into the left femoral vein at 23 hrs after SAH. After an intermission of 1 hr, animals were euthanized by intracardial perfusion. Evans blue concentration in each brain sample was determined by spectrophotometer (Genesis 10uv Scanning; Thermo Electron Corporation, Madison, WI) at 615 nm.
Measurement of Lipid Peroxidation
Lipid peroxidation was estimated by measurement of malondialdehyde (MDA) concentration in right hemispheres at 24 hrs after SAH with a commercially available kit (LPO-586; Oxis International, Beverly Hills, CA). The measurement was performed according to the manufacturer’s instructions. Results were expressed as pmol/mg protein.
Western Blot
Protein extract from left hemispheres was performed as previously described (13). Protein concentration was determined by DC assay (Bio-Rad, Hercules, CA). Western blot analysis was performed as previously described (8). In brief, equal amounts of protein samples (30 μg) from different samples were loaded on 10% sodium dodecyl sulfate–polyacrylaminde gels. Proteins were then electrophoresed and transferred to a nitrocellulose membrane. After incubation with primary antibody (mouse antinitrotyrosine, 1:500, Abcam, Cambridge, MA; ab61392) at 4°C overnight, the membrane was probed with corresponding second antibody for 1 hr at room temperature. Immunoblot was then visualized by the ECL kit (GE Healthcare, Piscataway, NJ). Blot bands were finally quantified by densitometry with Image J software (Image J 1.42q; National Institutes of Health, Bethesda, MD). Beta-actin (1:2000; Santa Cruz Biotechnology, Santa Cruz, CA) was also blotted on the same membrane as loading controls.
Morphologic Assessment
Ten-micrometer coronal brain sections were cut as previously described in a cryostat (Leica CM3050S, Buffalo Grove, IL) (14). Sections from each animal were divided into several subsets for Nissl staining and immunofluorescence respectively.
For Nissl staining, sections were dried at 37°C for 30 mins and dehydrated in 0.1% cresyl violet for 5 mins. After rinsing with water, sections were dehydrated in increasing concentrations of ethanol and cleared in xylenes and then mounted with permount, coverslipped, and observed under a light microscope (13).
Double fluorescent labeling was performed as described previously (15). The following primary antibodies were used: goat anti-8 hydroxyguanosine (8-OHG) diluted 1:200 antibody (Abcam, ab10802) paired with mouse antineuronal nuclei diluted 1:200 antibody (Millipore, Billerica, MA; MAB377), rabbit antiglial fibrillary acidic protein diluted 1:50 antibody (Santa Cruz; sc-9065), and rabbit anti-CD34 diluted 1:100 antibody (Abbiotec, San Diego, CA; 250591), respectively. Sections were observed under a fluorescent microscope (Olympus, Tokyo, Japan). The In situ Cell Death Detection Kit (Roche Inc., Mannheim, Germany) was used for terminal deoxynucleotide transferase-mediated dUTP nick-end labeling staining according to the manufacturer’s instructions.
Statistics
For statistical analysis of the mortality, Fisher’s exact test was used in two groups’ comparisons. Rest data were presented as means ± SEM. Statistical differences between the various groups were assessed by one-way analysis of variance followed by Tukey’s test. A probability level of p < .05 was considered statistically significant.
RESULTS
Mortality and Exclusion
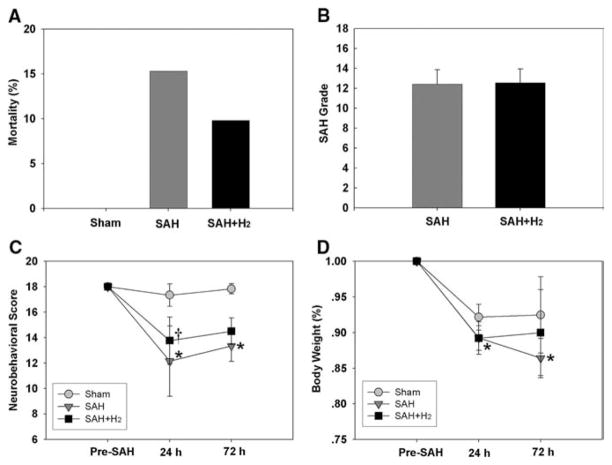
No significant change within physiological parameters (body temperature, blood gases) occurred in this study. Fourteen rats died before the scheduled euthanasia time totally. None of them was sham-operated animal (zero of 27 rats). The mortality in the SAH group was 15.3% (nine of 59 rats) and 9.8% (five of 51 rats) in the SAH + H2 group (Fig. 1A, p > .05, SAH vs. SAH+ H2). Most of them died within 3 hrs after SAH.
Figure 1.
A, Mortality was 15.3% and 9.8% in the subarachnoid hemorrhage (SAH) group and SAH + hydrogen (H2) group (p > .05, Fisher’s exact test). B, Similar severity of SAH bleeding was obtained in the SAH group (n = 27) and SAH + H2 group (n = 31, p > .05). C, Hydrogen gas (SAH + H2) alleviated neurologic dysfunction (p < .05 vs. SAH) at 24 hrs, whereas the improvement of neurobehavioral score at 72 hrs was not significant (p > .05 vs. SAH). D, This graph shows the body weight loss after SAH. No significant improvement was detected between the SAH group and SAH + H2 group at either 24 (p > .05) or 72 hrs (p > .05). At 24 hrs, sham: n = 27, SAH: n = 27, SAH + H2: n = 31; at 72 hrs, n = 6 in all groups. *p < .05 vs. sham, †p < .05 vs. SAH.
Considering brain injury in the endovascular perforation model is variable and is related to the severity of bleeding volume (10), SAH rats were divided into three categories according to the severity of bleeding: mild (SAH grade from 0 to 8), moderate (SAH grade from 9 to 14), and severe (SAH grade from 15 to 18). Similar to previous studies (9, 10), our study showed that the brain edema and neurobehavioral deficit in the mild group had no significant difference compared with the sham-operated group. Twenty-six mild SAH rats were then excluded from this study. Twelve severe SAH rats were excluded because of high mortality and severe neurologic dysfunction. Therefore, only moderate SAH rats were included for the following studies.
The average SAH grades had no significant difference among SAH groups (12.4 ± 1.4 in SAH group, n = 27, vs. 12.6 ± 1.4 in SAH + H2 group, n = 31, p > .05; Fig. 1B). This indicated similar bleeding severity between the SAH group and SAH + H2 group.
Neurobehavioral Test and Body Weight Loss
Compared with sham animals, SAH significantly impaired neurologic function at both 24 (p < .05) and 72 hrs (p < .05) after SAH. Hydrogen-treated rats presented with improved neurobehavioral score compared with SAH rats at 24 (p < .05), but not at 72 hrs (p > .05; Fig. 1C). Hydrogen inhalation failed to prevent body weight loss at either 24 (p > .05) or 72 hrs (p > .05; Fig. 1D) (At 24 hrs, sham: n = 27, SAH: n = 27, SAH + H2: n = 31; at 72 hrs, n = 6 in all groups.)
Brain Water Content and Blood–Brain Barrier Permeability
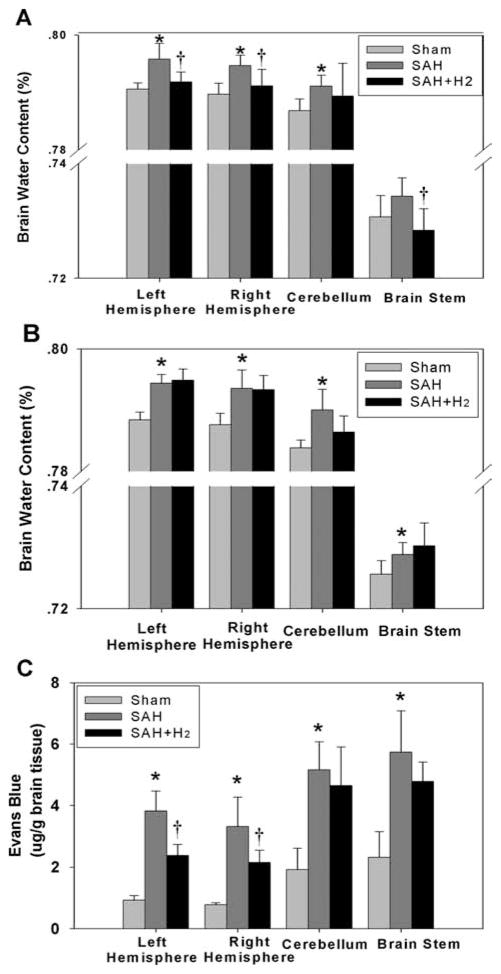
At 24 hrs, hydrogen treatment significantly attenuated bilateral brain edema (p < .05, sham: n = 6, SAH: n = 6, SAH + H2: n = 10; Fig. 2A). However, a single administration of hydrogen did not show a persistent effect and failed to attenuate brain edema at 72 hrs (p > .05, n = 6; Fig. 2B). Similarly, Evans blue extravasation was significantly decreased in bilateral cerebral hemispheres at 24 hrs in the SAH + H2 group (p < .05, n = 6; Fig. 2C).
Figure 2.
Hydrogen treatment decreased brain water content significantly in the right hemisphere, left hemisphere, and brain stem at 24 hrs after subarachnoid hemorrhage (SAH) (A) (p < .05, sham: n = 6, SAH: n = 6, SAH + hydrogen (H2): n = 10) but not at 72 hrs (B) in any brain regions (p > .05, n = 6) after SAH. C, Extravasated Evans blue in all brain regions was observed in the SAH group (p < .05) and H2 treatment decreased Evans blue extravasation in both hemispheres (p < .05), but not in the cerebellum and brain stem (p > .05) at 24 hrs after SAH (n = 6). *p < .05 vs. sham, †p < .05 vs. SAH.
Nissl Staining
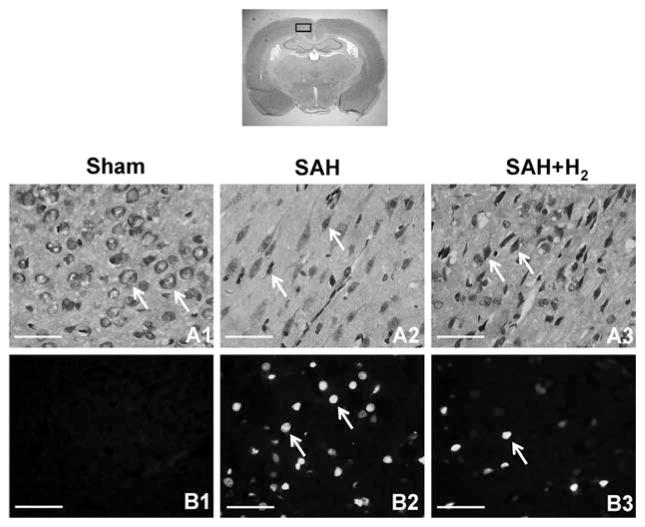
Figure 3A1–A3 showed Nissl staining in the ipsilateral cortex at 24 hrs after SAH (n = 3). Evident damage was seen in the SAH group with a decrease of cell number, abnormal shape, and dark staining resulting from condensation of cytoplasm and karyoplasms. More stain-positive cells and better cellular shape were detected in the SAH + H2 group compared with SAH animals.
Figure 3.
Nissl staining was performed on brain sections obtained from the sham, subarachnoid hemorrhage (SAH), and hydrogen (H2) treatment groups. Neurons in the cerebral cortex at 24 hrs after SAH (n = 3) are shown in upper panels. Intact neurons could be seen in sham animals. Arrows indicate normal neurons (A1). Numerous foci of neuronal damage were present in the cerebral cortex in SAH animals. Arrows indicate deformed neurons (A2). Hydrogen treatment prevented the appearance of injured neurons and more positive stained cells and better cellular shape were observed (A3). The bottom panels show the results of terminal deoxynucleotide transferase-mediated dUTP nick-end labeling staining after 24 hrs after SAH (n = 3). No positive staining was detected in the sham group (B1). SAH rats presented massive terminal deoxynucleotide transferase-mediated dUTP nick-end labeling-positive cells in the cerebral cortex (B2), whereas evident reduction of terminal deoxynucleotide transferase-mediated dUTP nick-end labeling-positive cells could be seen in the SAH + hydrogen group (B3). Arrows indicate cells that are positive. Scale bars represent 100 μm.
Terminal Deoxynucleotide Transferase-Mediated dUTP Nick-End Labeling Staining
Apoptosis in ipsilateral cortex was labeled by terminal deoxynucleotide transferase-mediated dUTP nick-end labeling staining. Figure 3B1–B3 showed terminal deoxynucleotide transferase-mediated dUTP nick-end labeling-positive cells were markedly increased after 24 hrs in SAH rats (n = 3). Hydrogen gas reduced the number of terminal deoxynucleotide transferase-mediated dUTP nick-end labeling-positive cells.
Oxidative Injury of Lipid, Protein, and DNA
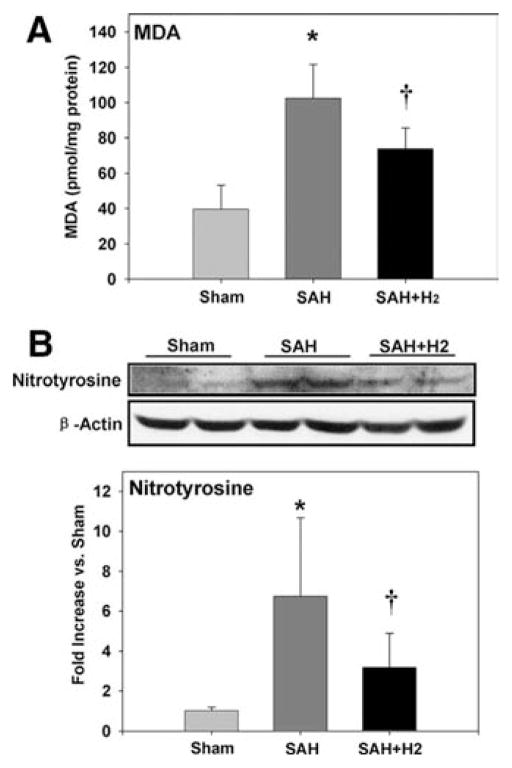
MDA is a product of lipid peroxidation. Figure 4A showed brain concentration of MDA at 24 hrs after SAH (n = 6). The SAH group was associated with an increased concentration of MDA (p < .05). Hydrogen treatment significantly suppressed the production of MDA (p < .05).
Figure 4.
A, subarachnoid hemorrhage (SAH) increased malondialdehyde (MDA) concentration in right hemispheres (p < .05), whereas hydrogen (H2) treatment significantly suppressed the production of malondialdehyde (p < .05) at 24 hrs after SAH (n = 6). B, SAH increased the expression of nitrotyrosine at 24 hrs after SAH, and this increase was alleviated by hydrogen treatment (n = 6, p < .05). *p < .05 vs. sham, †p < .05 vs. SAH.
Nitrotyrosine is a marker of nitric oxide-dependent oxidative damage. Figure 4B showed the expression of nitrotyrosine at 24 hrs after SAH (n = 6). The ratio of nitrotyrosine content increased by 675% after SAH when compared with the sham group, and 318% elevation was detected in the SAH + H2 group (p < .05 vs. SAH).
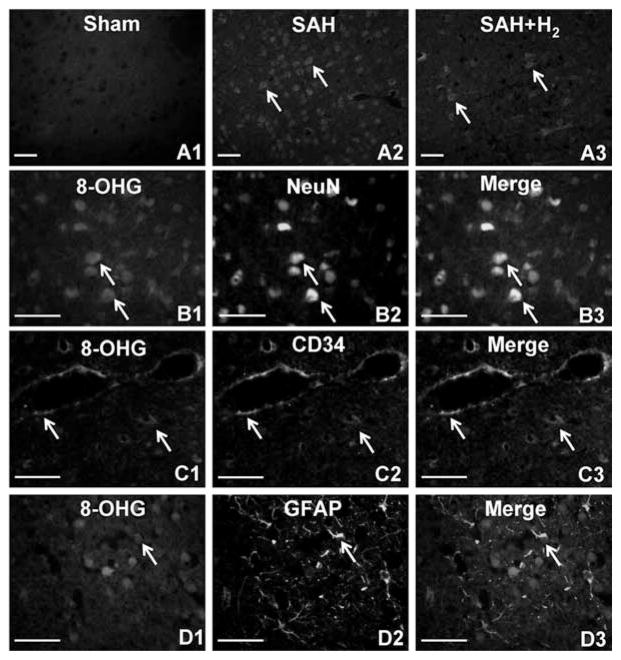
8-OHG, which is a modified base that occurs in DNA as a result of attack by free radicals, is a sensitive marker of oxidative damage of DNA. Figure 5A1–A3 showed immunofluorescent stain of 8-OHG at 24 hrs after SAH (n = 3). Strong immunostaining of 8-OHG was detected in SAH but not in hydrogen-treated animals. For double fluorescent labeling (n = 3), the 8-OHG-positive cells in the SAH group were labeled by Texas red (Figs. 5B1, C1, D1); neuronal nuclei-, CD34-, and glial fibrillary acidic protein-positive cells that represented neurons, vascular endothelial cells, and astrocytes, respectively, were labeled by fluorescein isothiocyanate (Figs. 5B2, C2, D2). Merged pictures (Figs. 5B3, C3, D3) showed that 8-OHG mainly colocalized in neurons and endothelial cells and rarely in astrocytes.
Figure 5.
A1–A3, 8 hydroxyguanosine (8-OHG) immunostaining in the cerebral cortex (n = 3) at 24 hrs after subarachnoid hemorrhage (SAH). No immunoreactivity of 8-OHG was observed in the sham group (A1). Stronger oxidative injures were detected in SAH rats (A2) compared with those given hydrogen (H2) treatment (A3). The bottom three panels show the double fluorescent labeling to display the localization of 8-OHG in SAH rats (n = 3). 8-OHG-positive cells (B1, C1, D1); neuronal nuclei (NeuN), CD34, and glial fibrillary acidic protein-positive (GFAP)-positive cells that represented neurons (B2), endothelial cells (C2), and astrocytes (D2), respectively. Colocalization of 8-OHG with neurons (B3) and endothelial cells (C3) were revealed by merged pictures. However, 8-OHG rarely displayed in astrocytes (D3). Arrows indicate cells that are positive. Scale bars represent 100 μm.
DISCUSSION
Although hydrogen gas can be explosive in concentration >5%, it is neither explosive nor dangerous in low concentration. In the present study, we observed that hydrogen gas inhalation applied at 1 hr after SAH ameliorated oxidative stress, including lipid peroxidation, DNA, and protein damage, reduced apoptosis, preserved blood–brain barrier integrity, alleviated brain edema, and improved neurologic deficits. However, a one-time application of hydrogen produced a transient neuroprotective effect at 24 hrs and this beneficial effect did not extend into 72 hrs after SAH.
The term early brain injury was recently coined and referred to the acute injuries to the whole brain within the first 72 hrs after the aneurysmal rupture. Mitochondrial dysfunction after SAH leads to oxidative stress, which plays a major role in early brain injury by overpowering the natural defense system of superoxide dismutase, catalase, and glutathione peroxidase (16). After an aneurysm rupture, excessive reactive oxygen species and reactive nitrogen species including hydroxyl radical, super-oxide anion, hydrogen peroxide, nitric oxide, and peroxynitrite, are produced consequently (4). In the present study, we found marked increase of the expression of MDA, nitrotyrosine, and 8-OHG, which are oxidative markers of lipid, protein, and DNA damage, respectively, after SAH in this animal model. These results indicated that oxidative stress was involved in early brain injury after SAH. Lipid peroxidation, protein inactivation, and DNA damage, which result in cellular dysfunction and/or apoptosis (17), occur mostly in endothelial cells and neurons. Injury to endothelial cells is associated with blood–brain barrier disruption, which in turn contributes to brain edema and cell death (18). In experimental SAH, brain edema and cellular apoptosis are considered as two major components of early brain injury and are responsible for poor outcomes (2). Consistent with previous publications, we observed neuronal apoptosis, blood–brain barrier disruption, and brain edema in this animal model and these pathologic changes may be responsible for the deficits in neurologic function we observed. Additional, we observed that brain edema and blood–brain barrier disruption occurred bilaterally regardless of the perforation side.
The new observation of this study is that inhalation of hydrogen gas at 1 hr after induction of SAH reduced oxidative stress, prevented brain edema, and improved neurologic function. Although this is the first time the neuroprotective action of hydrogen gas is shown in an experimental SAH rat model, the mechanisms of hydrogen gas-induced neuroprotection were reported previously by others and by us. A study by Ohsawa et al (5) has demonstrated that hydrogen gas exerted protective effects in a focal cerebral ischemia/reperfusion rat model by selectively reducing cytotoxic oxygen radicals. We observed similar results in this SAH-induced early brain injury rat model, which represents a combined insult of ischemia and hemorrhage. Furthermore, by using double immunofluorescence staining at 24 hrs after SAH, we observed a colocalization of 8-OHG with neuronal nuclei and CD34 but not with glial fibrillary acidic protein, indicating oxidative stress occurred mostly in neurons and vascular endothelial cells, but not in astrocytes. Therefore, it seems that the therapeutic effect of hydrogen gas is mainly associated with the protection of neurons and endothelial cells, the key injury cell types of early brain injury (19).
This new observation is also consistent with some of our recent publications that hydrogen gas reduced not only brain infarction, but also decreased hyperglycemia-enhanced hemorrhagic transformation after middle cerebral artery occlusion (20). Besides studies in the brain (5, 20, 21), hydrogen treatment was also reported recently in cardiac ischemia/reperfusion injury (22, 23) and inflammation after organ transplantation (6, 7). All of these observations shared a similar antioxidative activity of hydrogen gas or hydrogen saline. These studies demonstrated that hydrogen has broad applications for injuries involve ischemia/reperfusion. In the meantime, we also noticed that many antioxidants had failed to demonstrate beneficial effects in clinical trials. These failures could be attributed to many factors, including inadequate assessment and consideration of dose–response relationships, therapeutic window, optimum dosing, treatment duration, and perhaps insufficient sample size (24). However, hydrogen is different from most free radical scavengers that hydrogen selectively scavenges hydroxyl radicals, which is the most harmful radical and there is no endogenous or external scavenging agents for hydroxyl radicals (25). Therefore, it is worthwhile to study hydrogen for SAH. Hydrogen has showed satisfying therapeutic effects in diverse animal models of brain and other organ injuries (25) and has been applied extensively in deep divers (26).
A limitation of this study is that a single application of hydrogen gas for 2 hrs failed to achieve neuroprotective effect at 72 hrs after SAH in this animal model. This, however, comes without surprise because brain injury is a dynamic and continued event of ischemia/reperfusion, and reperfusion often generates excessive reactive oxygen species (20). In addition to ischemia and reperfusion, free radical generations from blood clots are features of SAH pathophysiology (4). SAH is different from ischemia/reperfusion injury occurring in focal cerebral ischemia, in which free radicals are mainly produced during the reperfusion period. The production of free radicals after SAH continues and contributes to the delayed pathogenesis of delayed cerebral vasospasm (27). Because hydrogen gas directly removes hydroxyl radicals, once hydrogen application is stopped, excessive oxygen species will continue tissue damage as we had observed at 72 hrs after SAH. The results from this study indicate that multiple and prolonged use of hydrogen therapy may be warranted for future SAH brain injury studies, and cerebral vasospasm after SAH may be alleviated by hydrogen treatment as well.
CONCLUSIONS
Hydrogen gas is neuroprotective during early brain injury period after SAH possibly by its antioxidative activity. Multiple administrations of hydrogen gas may have clinical potentials for the management of SAH.
Acknowledgments
This study was partially supported by grants (NS053407) from the National Institutes of Health to Dr. Zhang.
Footnotes
The authors have not disclosed any potential conflicts of interest.
References
- 1.Suarez JI, Tarr RW, Selman WR. Aneurysmal subarachnoid hemorrhage. N Engl J Med. 2006;354:387–396. doi: 10.1056/NEJMra052732. [DOI] [PubMed] [Google Scholar]
- 2.Cahill J, Calvert JW, Zhang JH. Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2006;26:1341–1353. doi: 10.1038/sj.jcbfm.9600283. [DOI] [PubMed] [Google Scholar]
- 3.Kusaka G, Ishikawa M, Nanda A, et al. Signaling pathways for early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2004;24:916–925. doi: 10.1097/01.WCB.0000125886.48838.7E. [DOI] [PubMed] [Google Scholar]
- 4.Ayer RE, Zhang JH. Oxidative stress in subarachnoid haemorrhage: Significance in acute brain injury and vasospasm. Acta Neurochir Suppl. 2008;104:33–41. doi: 10.1007/978-3-211-75718-5_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 6.Buchholz BM, Kaczorowski DJ, Sugimoto R, et al. Hydrogen inhalation ameliorates oxidative stress in transplantation induced intestinal graft injury. Am J Transplant. 2008;8:2015–2024. doi: 10.1111/j.1600-6143.2008.02359.x. [DOI] [PubMed] [Google Scholar]
- 7.Cardinal JS, Zhan J, Wang Y, et al. Oral hydrogen water prevents chronic allograft nephropathy in rats. Kidney Int. 2010;77:101–109. doi: 10.1038/ki.2009.421. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki H, Ayer R, Sugawara T, et al. Protective effects of recombinant osteopontin on early brain injury after subarachnoid hemorrhage in rats. Crit Care Med. 2010;38:612–618. doi: 10.1097/CCM.0b013e3181c027ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugawara T, Ayer R, Jadhav V, et al. Simvastatin attenuation of cerebral vasospasm after subarachnoid hemorrhage in rats via increased phosphorylation of Akt and endothelial nitric oxide synthase. J Neurosci Res. 2008;86:3635–3643. doi: 10.1002/jnr.21807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugawara T, Ayer R, Jadhav V, et al. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods. 2008;167:327–334. doi: 10.1016/j.jneumeth.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia JH, Wagner S, Liu KF, et al. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- 12.Xi G, Hua Y, Keep RF, et al. Brain edema after intracerebral hemorrhage: The effects of systemic complement depletion. Acta Neurochir Suppl. 2002;81:253–256. doi: 10.1007/978-3-7091-6738-0_66. [DOI] [PubMed] [Google Scholar]
- 13.Ostrowski RP, Colohan AR, Zhang JH. Mechanisms of hyperbaric oxygen-induced neuroprotection in a rat model of subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2005;25:554–571. doi: 10.1038/sj.jcbfm.9600048. [DOI] [PubMed] [Google Scholar]
- 14.Chen C, Ostrowski RP, Zhou C, et al. Suppression of hypoxia-inducible factor-1alpha and its downstream genes reduces acute hyperglycemia-enhanced hemorrhagic transformation in a rat model of cerebral ischemia. J Neurosci Res. 2010;88:2046–2055. doi: 10.1002/jnr.22361. [DOI] [PubMed] [Google Scholar]
- 15.Tsubokawa T, Solaroglu I, Yatsushige H, et al. Cathepsin and calpain inhibitor E64d attenuates matrix metalloproteinase-9 activity after focal cerebral ischemia in rats. Stroke. 2006;37:1888–1894. doi: 10.1161/01.STR.0000227259.15506.24. [DOI] [PubMed] [Google Scholar]
- 16.George JF, Agarwal A. Hydrogen: Another gas with therapeutic potential. Kidney Int. 2010;77:85–87. doi: 10.1038/ki.2009.432. [DOI] [PubMed] [Google Scholar]
- 17.Facchinetti F, Dawson VL, Dawson TM. Free radicals as mediators of neuronal injury. Cell Mol Neurobiol. 1998;18:667–682. doi: 10.1023/A:1020685903186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiss N, Miller F, Cazaubon S, et al. The blood–brain barrier in brain homeostasis and neurological diseases. Biochim Biophys Acta. 2009;1788:842–857. doi: 10.1016/j.bbamem.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 19.Ostrowski RP, Colohan AR, Zhang JH. Molecular mechanisms of early brain injury after subarachnoid hemorrhage. Neurol Res. 2006;28:399–414. doi: 10.1179/016164106X115008. [DOI] [PubMed] [Google Scholar]
- 20.Chen CH, Manaenko A, Zhan Y, et al. Hydrogen gas reduced acute hyperglycemia-enhanced hemorrhagic transformation in a focal ischemia rat model. Neuroscience. 2010;169:402–414. doi: 10.1016/j.neuroscience.2010.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji X, Liu W, Xie K, et al. Beneficial effects of hydrogen gas in a rat model of traumatic brain injury via reducing oxidative stress. Brain Res. 2011;1354:196–205. doi: 10.1016/j.brainres.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 22.Hayashida K, Sano M, Ohsawa I, et al. Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem Biophys Res Commun. 2008;373:30–35. doi: 10.1016/j.bbrc.2008.05.165. [DOI] [PubMed] [Google Scholar]
- 23.Sun Q, Kang Z, Cai J, et al. Hydrogen-rich saline protects myocardium against ischemia/reperfusion injury in rats. Exp Biol Med (Maywood) 2009;234:1212–1219. doi: 10.3181/0812-RM-349. [DOI] [PubMed] [Google Scholar]
- 24.Kreiter KT, Mayer SA, Howard G, et al. Sample size estimates for clinical trials of vasospasm in subarachnoid hemorrhage. Stroke. 2009;40:2362–2367. doi: 10.1161/STROKEAHA.109.547331. [DOI] [PubMed] [Google Scholar]
- 25.Huang CS, Kawamura T, Toyoda Y, et al. Recent advances in hydrogen research as a therapeutic medical gas. Free Radic Res. 2010;44:971–982. doi: 10.3109/10715762.2010.500328. [DOI] [PubMed] [Google Scholar]
- 26.Fontanari P, Badier M, Guillot C, et al. Changes in maximal performance of inspiratory and skeletal muscles during and after the 7. 1-MPa Hydra 10 record human dive. Eur J Appl Physiol. 2000;81:325–328. doi: 10.1007/s004210050050. [DOI] [PubMed] [Google Scholar]
- 27.Kolias AG, Sen J, Belli A. Pathogenesis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage: Putative mechanisms and novel approaches. J Neurosci Res. 2009;87:1–11. doi: 10.1002/jnr.21823. [DOI] [PubMed] [Google Scholar]