Abstract
Oxygen supply and diffusion into tissues are necessary for survival. The oxygen partial pressure (pO2), which is a key component of the physiological state of an organ, results from the balance between oxygen delivery and its consumption. In mammals, oxygen is transported by red blood cells circulating in a well-organized vasculature. Oxygen delivery is dependent on the metabolic requirements and functional status of each organ. Consequently, in a physiological condition, organ and tissue are characterized by their own unique ‘tissue normoxia’ or ‘physioxia’ status. Tissue oxygenation is severely disturbed during pathological conditions such as cancer, diabetes, coronary heart disease, stroke, etc., which are associated with decrease in pO2, i.e. ‘hypoxia’. In this review, we present an array of methods currently used for assessing tissue oxygenation. We show that hypoxia is marked during tumour development and has strong consequences for oxygenation and its influence upon chemotherapy efficiency. Then we compare this to physiological pO2 values of human organs. Finally we evaluate consequences of physioxia on cell activity and its molecular modulations. More importantly we emphasize the discrepancy between in vivo and in vitro tissue and cells oxygen status which can have detrimental effects on experimental outcome. It appears that the values corresponding to the physioxia are ranging between 11% and 1% O2 whereas current in vitro experimentations are usually performed in 19.95% O2, an artificial context as far as oxygen balance is concerned. It is important to realize that most of the experiments performed in so-called normoxia might be dangerously misleading.
Keywords: oxygen, normoxia, physioxia, hypoxia, oxygen partial pressure
Introduction
Oxygen is vital for living cells; it plays a fundamental role in their metabolism. However, the simple molecular diffusion of gases in tissues, as well as nutrients is not sufficient for the metabolic needs of large, complex and active multicellular organisms. Consequently, it is necessary to provide tissues and cells with optimal oxygen concentrations.
Several strategies have been developed to maximize oxygen capture and oxygen transport into organs. In mammals, oxygen is absorbed by the lungs. Because of its poor solubility, oxygen is bound to haemoglobin which is packaged within red blood cells. A well-organized vasculature enables the delivery of oxygenated red blood cells to the tissues. The macrovasculature allows a rapid blood circulation and the microvasculature which irrigates each organ provides locally the optimal oxygen supply. A highly controlled oxygenation of cells is vital, particularly in animals with very high metabolic requirements.
Recent knowledge, about the microenvironment significance both for normal and pathological issues, implies the tight control of in vitro experimental conditions to which the cells are exposed.
Oxygen is a basic component of the microenvironment required for cell activity; remarkably, in vitro cultures are mostly performed in a 95% air (79% N2/21% O2) atmosphere, supplemented by 5% of carbon dioxide, thus providing 19.95% O2. As described in this review, these conditions are largely overestimated in terms of O2 content of the atmosphere and do not represent the endogenous oxygen pressure of the organ the cells come from. Knowing the drastic consequences of an oxidative stress on the cell physiology, it appears fundamental to determine what the exact levels of oxygen pressure in various tissues in normal physiology are. This will allow comparing pathological situations to normal ones and point to the importance of the oxygen levels in experimental settings that represents an essential parameter.
The present review is to exemplify how the oxygen partial pressure (pO2) in tissues influences the molecular and subsequent cellular behaviours. As a result, the pO2 used in in vitro experiments should be tightly controlled, in order to get as close as possible to the physiological conditions. We first provide an overview of the methods available until now for assessing tissue oxygenation: imaging techniques usually used in the tumour context and pO2 quantification techniques. The second part compiles the state of the art of the pO2 in tumour, then, by comparison, the physiological status in various human organs. In the last part, to illustrate the primordial role of oxygen in physiology, we present and discuss several examples of significantly modulated cellular activities that depend on the oxygen level. Several units are used to define pO2. In the international system, the pressure unit is the Pascal (Pa). Other units include: the bar (1 bar = 100 kPa), the atmosphere (1 atm = 101.325 kPa), the Torr (1 torr = 133.322 Pa) and two units mostly used in medicine: the millimetre of mercury (1 mmHg = 133.322 Pa) and the percentage of oxygen (1%= 1.013 kPa).
To distinguish between the various levels of oxygenation, the following terms are currently used: ‘normoxia’ corresponding to atmospheric oxygen pressure, the commonly used oxygen pressure for cell cultures (i.e. around 150 mmHg or 19.95% oxygen, 20.3 kPa); ‘tissue normoxia’ that we also call ‘physioxia’ for the pO2 measured in different organs in a physiological condition; ‘hypoxia’ representing a pO2 lower than physioxia and indicative for a lack of oxygenation in the tissue.
Imaging of hypoxic areas
The chemical and physical properties of oxygen enable a wide variety of methods adequate to mapping oxygen content in vivo. Techniques to visualize oxygenation are highly described in cancer studies, because hypoxia in tissues is a characteristic pathophysiological property of advanced solid tumours [1]. In this context, imaging tools that specifically target hypoxic environments are imperative for cancer diagnosis and treatment. The choice of the method, in the following studies, is based on applicability to the experimental model and the nature of information sought.
Hypoxia markers
In the development of imaging probes for tumour hypoxia, nitroimidazoles have received particular attention as hypoxia markers because of their unique behaviour in hypoxic environments. 2-nitroimidazole (azomycin) was originally found to be an antibiotic against anaerobic bacteria and protozoa in 1953 [2]. Nitroaromatics are selectively reduced by nitroreductase enzymes under hypoxic conditions to form hydroxylamine intermediates that can bind irreversibly to cellular nucleophilic groups in proteins or DNA. This property permits their antibacterial action. The irreversible retention of nitroimidazoles in the cells is also a prerequisite to differentiate between normoxic and hypoxic tissues [3] (Fig. 1A).
Fig 1.
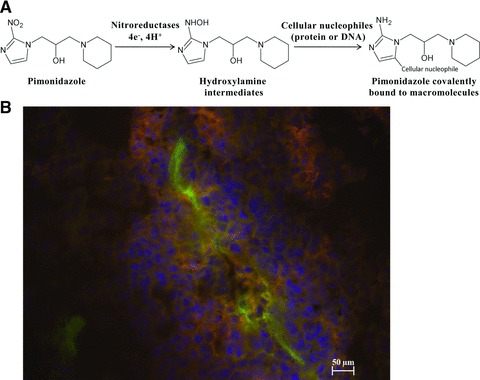
Nitroimidazole derivatives used as hypoxia markers. (A) Schematic of the proposed mechanism underlying the binding of pimonidazole to cellular macromolecules, as an example of nitroimidazole tracer. (B) Hypoxic sites detection in B16-melanoma induced tumour by pimonidazole binding, visualized in red. More cells are proliferating [4′,6′-diamidino-2-phenylindole (DAPI)-labelled cells in blue] close to the blood vessel. Endothelial cells lining the blood vessel wall are stained in green after incubation with a fluorescent antibody specific for PECAM-1 (CD31).
The detection of these exogenous hypoxia markers are based on immunohistochemical methods. 2-nitroimidazole derivatives, such as pimonidazole or 2-(2-nitro-1-H-imidazol-1-yl)-N-(2,2,3,3,3-pentafluoropropyl) acetamide (EF5), are injected into the patient before surgical resection of tumour tissue. Immunohistochemical methods yield microscopic information on hypoxia in relation to tissue histology. However, such data provide only relative information on pO2 values (Fig. 1B).
The pimonidazole is considered to be the ‘standard’ exogenous hypoxic marker. This injectable molecule is commercially available. The binding of exogenous hypoxia markers such as pimonidazole to cellular macromolecules has been shown to increase dramatically below an oxygen concentration of 10 mmHg [4]. In fact, oxygen-independent staining with anti-pimonidazole antibodies was reported, indicating that other hypoxia-independent events may occur in exogenous hypoxia markers.
Positron emission tomography (PET)
PET is an in vivo tomographic imaging technique. It is based on the detection of anti-parallel 511 keV photons emitted during the annihilation of positrons with electrons. PET radiotracers are physiologically and pharmacologically relevant compounds labelled with short-lived positron-emitting radionuclides. After internalization by injection or inhalation, the tracer reaches the target and the quantity is then detected with a PET scanner, with a ring-shaped array of photoelectric crystals [5] (Fig. 2).
Fig 2.
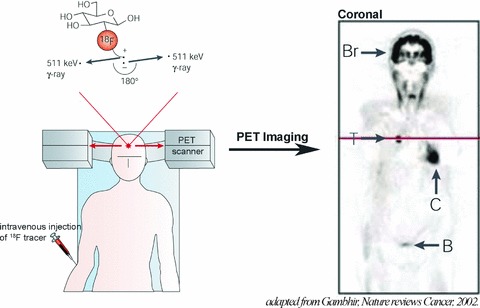
Principles of PET. 18F radiotracer is intravenously injected. The tracer decays by emitting a positron, which annihilates with a nearby electron to produce two γ-rays. The PET scanner can detect the coincident γ-rays, and images can be reconstructed showing the location(s) and concentration of the tracer of interest. Sectional PET image is shown: normal uptake in brain (Br) and myocardium (C), and renal excretion into the urinary bladder (B) are visible. Also seen is a tumour (T) in the lungs that takes up more 18F radiotracer than the surrounding tissues. Adapted with permission from Macmillan Publishers Ltd: Nature Reviews Cancer, Gambhir, copyright 2002[5].
The main strategy for tracer imaging of tissue hypoxia to date has involved the use of compounds that are preferentially absorbed and trapped within cells in the reduced state. As the first such agent to be developed, 18F- fluoromisonidazole is the gold standard and has been used to identify hypoxic tumours in human patients [6]. Because it has many disadvantages (slow clearance and low cellular uptake), newer radiotracers for hypoxia imaging have been intensively investigated. For example, Copper(II)-diacetyl-bis(N4-methylthiosemicarbazone) (Cu-ATSM) is a hypoxia-targeted PET agent, which displays a higher uptake (<1 hr) in hypoxic tissues than the nitroimidazoles, allowing better quality images. After successful pre-clinical studies, Cu-ATSM has also been evaluated in human beings [7]. Even so, the anatomical resolution of PET (approximately 4–8 mm3 in clinical and 1–2 mm3 in small animal imaging systems) is noticeably poorer than that achieved by magnetic resonance imaging (MRI). Moreover, PET can be used to calculate oxygen consumption after 15O-radioisotope administration [8] or to show hypoxic area, but this technique does not allow direct pO2 evaluation.
Near-infrared spectroscopy (NIRS)
NIRS is a non-invasive, optical technique applying the principles of light transmission and absorption to investigate dynamic changes in tissue oxygenation by measuring haemoglobin saturation [9]. NIRS uses visible light (700–1000 nm) in the near-infrared region which crosses readily through biological tissues. The NIRS signal is obtained from the absorption of light by haemoglobin in the vasculature (small arterioles, capillaries and venules). For clinical studies, this method can be used under normal conditions without a major restriction of motion. Its main advantage is the ability to perform real-time measurements and their repeatability [10]. One drawback of the NIRS method is that it does not measure tissue pO2 but provides information on vascular oxygenation (oxygen saturation), which results from the balance between oxygen delivery and oxygen consumption [11].
Magnetic resonance spectroscopy (MRS)
MRS is an application of MRI hereby spectra of metabolic changes in living tissue are obtained. In 1988, Busse et al. [12] showed that MRI could be used for imaging tumour pO2 in vivo, after intravenous injection of perfluorocarbon (PFC) probes. This technique is referred as 19F-MRI. Mason et al. [13] used hexafluorobenzene because it gives a single, narrow 19F-MRI signal and is highly sensitive to oxygen concentration compared to the rest of PFCs. In fact, the 19F spin lattice relaxation rate (R1) of PFCs varies linearly with the dissolved oxygen concentration; thus, the 19F-based oximetry reports absolute values of oxygen concentration [14]. An example of monitoring hypoxia of rat tumour is presented in Figure 3.
Fig 3.
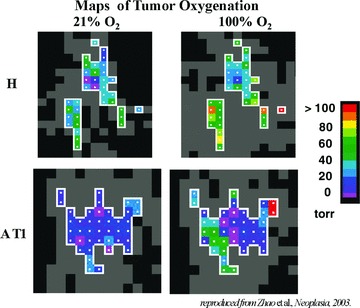
pO2 maps of rat tumours obtained using 19F nuclear magnetic resonance using hexafluorobenzene as the reporter molecule. The H tumour (a well-differentiated and slow-growing tumour) was significantly better oxygenated than the AT1 tumour (an anaplastic and faster-growing subline). In response to oxygen inhalation, pO2 increased significantly in both the H and the AT1 tumours. Adapted with permission from Neoplasma press, Zhao et al., 2003[14].
Two other MRI techniques such as blood oxygen level-dependent MRI (BOLD-MRI) [15] and dynamic contrast-enhanced MRI (DCE-MRI) [16] have been applied to measure oxygenation into inaccessible tissue. BOLD-MRI, also referred to flow and oxygenation dependent MRI, reflects the changes in the amount of oxygen bound to haemoglobin in blood. The blood content of deoxyhaemoglobin (a paramagnetic species) compared to oxyhaemoglobin (non-paramagnetic) induces differences in susceptibility (i.e. changes in the local magnetic field) around the blood vessels. This affects the relaxation properties of the surrounding protons. The major disadvantage of the BOLD oximetry is that it does not provide the absolute oxygen concentration in tissue but only the changes in blood oxygenation [17].
In the last several years, DCE-MRI has emerged as a promising method for measuring perfusion including blood flow and blood volume in tissue. This technique makes the use of paramagnetic contrast agent, mostly consisting of a low-molecular-weight gadolinium (Gd)-based tracer. However, by itself, DCE-MRI cannot provide a comprehensive picture of oxygen status. Much of the success of DCE-MRI, to date, can be attributed to its ability to provide high-resolution images of tumour that depict perfusion and permeability of the smallest vessels, especially the capillary network.
Electron paramagnetic resonance spectroscopy (EPR)
EPR (or electron spin resonance) presents the unique capability to detect unpaired electrons. Thus, it can provide information about the composition and chemical features of a milieu or a tissue including oxygenation. The measurement of oxygen concentration by EPR (EPR oximetry) involves the use of an external probe (most commonly lithium phthalocyanine) consisting of either implantable paramagnetic particles, or soluble probe molecules (nitroxides) that physically interact with, but do not consume oxygen [18]. The changes in the EPR signal caused by the interaction of two paramagnetic species, molecular oxygen and the probe, allow determining pO2. The EPR characteristics which appear to be potentially advantageous include the capability of making repeated measurements at the same sample, site specificity and accuracy of the obtained information. However, although paramagnetic ions that, such as nitroxides, are likely to be used in EPR studies and unlikely to have significant toxicity, they have not yet been fully tested in human patients [19]. Also, with existing instrumentation, the sensitivity of the non-invasive method is restricted to 10 mm from the surface of the body.
Oxygen partial pressure measurement
The previous techniques achieve a precise imaging of hypoxic area, most often by labelling the oxygen content in a tissue. However, these tools provide information on the relative oxygenation, but they fail quantifying pO2 and allowing comparable data between techniques. On the contrary, the following techniques directly measure pO2. As previously, the choice of the method is based on its applicability to the experimental model and the nature of information sought.
Polarographic sensor
The polarographic pO2 electrode has been considered as the ‘gold standard’ for measuring oxygen tension [20]. Oxygen electrodes for the measurement in tissue and microcirculation are conventionally based on the classical Clark’s electrode [21]. This type of electrodes consists of a noble metal (e.g. silver, gold and platinum) which reduces oxygen due to a negative polarizing voltage. The difference in voltage between the reference electrode (anode) and the measuring electrode (cathode) is proportional to the amount of oxygen molecules being reduced on the cathode. In the Clark’s electrode, both the anode and cathode are placed behind an oxygen-permeable and electrically insulating membrane.
Measurements with the polarographic electrode probe are made by rapidly stepping a 300 μm needle electrode through the tissue, thus providing an assessment of oxygenation in the local microenvironment. This technology has been used for several decades and was, up to recent dates, the only technique that could directly measure tissue pO2.
However, despite the relative ease to use this device, several important disadvantages need to be reported. First, the needle electrode cannot measure oxygenation in a given micro-region over time because it consumes oxygen during the measurement process. To obtain reliable measurements, O2 consumption by the electrode should be minimal compared to O2 tension within the tissue, otherwise the electrodes alter their own O2 environment. Second, the use of a needle is invasive and potentially damages the tissue. Miniaturized electrochemical sensors have two major advantages compared to larger electrodes: shorter time of responses and better spatial resolution. Third, as needle tip oxygen electrodes measure pO2 at a single point, the heterogeneous oxygen distribution in the tissues is not represented. Lübbers and coworkers [22] overcame this problem by using a device which punctures the tissue in a stepwise manner; they were thus able to show the expected heterogeneity and distribution of quantitatively measured pO2. Last, the median pO2 values vary widely among the different laboratories although they are using the same model, thus requiring several comparative studies [23].
Optical sensor
Recently, an optic fibre-based system has been developed based on pO2-dependent changes in lifetime of the pulse of a fluorescent dye placed at the tip of a probe. The most commonly used fluorescent dye is ruthenium chloride. The oxygen in tissue or fluids quenches the light emitted by ruthenium; the light quenching is proportional to oxygen tension in the vicinity of the dye. Oxygen is not consumed during the reaction, so a continuous readout of tissue pO2 can be obtained [24]. An example of pO2 measurement is presented in Figure 4.
Fig 4.
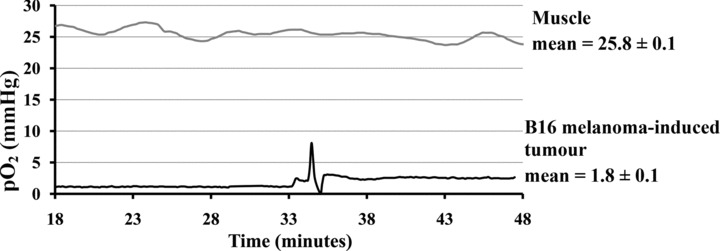
B16-melanoma-induced tumour and muscle pO2 measured using polarographic needle, in mouse. B16 tumour cells are injected subcutaneously in mouse and pO2 tumour is measured after 14 days, in comparison to the healthy muscle tissue. Experiment representative of 5 (mean ± S.E.M.).
The emitted fluorescent light is transmitted through an optical fibre to a microprocessor which quantifies the changes compared to calibration data [25]. This process does not consume oxygen, but relies solely on the amount of oxygen present. The optic-fibre probe can stay in a position for a long period of time, which allows measuring both temporal pO2 changes as well as the effect of tissue oxygen modifying agents.
Mass spectrometry
The mass spectrometry principle consists of ionizing chemical compounds to generate charged molecules and the measurement of their mass-to-charge ratios. Moreover, this technique permits the quantification of the amount of a compound in a sample. Consequently, molecular oxygen can be identified and measured quantitatively by mass spectrometry. For example, Seylaz et al. [26] were able to measure gas partial pressures, including pO2, of rabbit brain. They used a single cannula implanted stereotaxically in a given structure, then sampled a part of the organ and analysed gases in it. The measurements of pO2 were continuous and quantitative. The invasiveness and the time of response make mass spectrometry less attractive than the other methods [27].
What does tumour hypoxia mean?
As described above, numerous techniques have been developed over the past decades to evaluate the oxygenation status of tumours, but none has entered widespread clinical use. The critical pO2 in tumours is proposed to be 8–10 mmHg [28]. Below this level, detrimental changes associated with reduced oxygen consumption have been observed (ATP depletion, intracellular acidosis, apoptosis…). For example, this is confirmed in human renal carcinoma, in which mean pO2 value was measured at 9.6 mmHg [29] using a polarographic oxygen sensor probe. In the same way, median pO2 was 6 mmHg within human liver tumours [1]. Even if tumours could present higher level of oxygenation than 10 mmHg, pO2 value is always lower in the tumour than in the respective normal tissue, which is the true definition of hypoxia. Oxygenation in primary brain tumours has been measured at 13 mmHg in 104 cases, the fraction of strong hypoxia (≤2.5 mmHg) representing 26%[1]. In comparison, normal oxygenation of brain tissue is assured when pO2 reaches 35 mmHg, as reported in the next section.
In fact, these discrepancies in the oxygenation pattern are partially explained by strong inter-tumour variability. Measurements of tumour oxygenation within 28 tumours with 12 different histologies [30] exhibited an overall pO2 ranging from 0 to 54 mmHg (median = 24 mmHg). Moreover, an inadequate perfusion in the tumour, due to severe abnormalities of the tumour microcirculation, causes a heterogeneous distribution of hypoxic and/or anoxic tissue area.
In conclusion, hypoxia is an independent negative prognostic indicator in solid tumours, but intratumoral pO2 cannot be predicted by size, grade or histology of the tumour, but is, in part, dependent on the initial oxygenation status of the host tissue.
Small molecules and hypoxia
Chemotherapeutic drugs
Tumour cells are highly unresponsive to most anticancer drugs [31]. Tumour blood vessels are chaotic, leaky and disorganized. This results in a poor perfusion efficacy, reduced oxygen delivery to tumour cells and induction of chronic hypoxia. This lack of O2 in tumours tends to select cells with stronger malignant phenotype. Moreover increased DNA mutations in tumour cells activate genes that influence the uptake, metabolism and export of drugs, in favour of tumour survival. For example, in tumour cells P-glycoprotein expression and multidrug resistance receptors are enhanced [32]. Furthermore, modifications in sensitivity to p53-mediated apoptosis and in DNA mismatch repair make cells resistant to platinum-based chemotherapeutic agents (as carboplatin or cisplatin) [33, 34]. Transient hypoxia can also disturb protein folding in the endoplasmic reticulum, which confers to tumour cells resistance to topoisomerase II-targeted drugs [35] as etoposide, doxorubicin, etc.
The abnormal structure and function of tumour vasculature make it inefficient for red blood cells mediated oxygen supply as well as blood-borne drug delivery. The distribution of many drugs within tumours is also heterogeneous; therefore only a portion of the target tumour cells is exposed to a potentially lethal concentration of the cytotoxic agent.
Moreover cell proliferation decreases as a function of its distance from blood vessels, an effect that is at least partially due to hypoxia [36]. This relatively low rate of cell proliferation in hypoxia limits the effectiveness of chemotherapeutic drugs active mainly against highly proliferative cells. Thus, many anticancer agents such as methotrexate, 5-fluorouracil, doxorubicin, carboplatin, melphalan, bleomycin, etoposide, etc. have reduced in vitro cytotoxicity in experimental hypoxic conditions.
Radiation sensitizers: the nitroimidazoles
Nevertheless, by its specificity and its major role in drug resistance, tumour hypoxia represents a unique and attractive target to develop strategies for cancer therapy. For this reason studies were conducted on drugs that are selectively toxic against hypoxic cells. For example, nitroimidazoles could mimic the effects of oxygen and thereby sensitize hypoxic cells to radiation. In clinical trials, radiotherapy added to nitroimidazoles (metronidazole, misonidazole and etanidazole) did not result in significant improvements over radiotherapy alone, mainly because the overall toxicity of these derivatives prevented them from being given at high enough doses [37].
Hypoxia prodrugs: tirapazimine and anthraquinone
Another strategy uses hypoxia-activated prodrugs. Tirapazimine is the first compound to be developed specifically as a hypoxic cytotoxin and whose clinical development has been the most important [38] (Table 1). This benzotriazine di-N-oxide is selectively activated by multiple reductases to form free radicals in hypoxic cells thereby resulting in radical damage directly to the topoisomerase II enzyme [39] or to DNA. Despite the very promising results obtained in various preclinical studies, survival benefit is not clearly demonstrated in clinical trials [40, 41].
Table 1.
Examples of small molecules targeting or mimicking hypoxia: their chemical structure and mechanism.
| Small molecules | |||
|---|---|---|---|
| Structure | Mechanism | References | |
| pO2 modulator | |||
| myo-inositol trispyrophosphate (ITPP) | 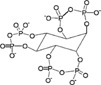 |
Allosteric effector of haemoglobin | [43–45] |
| Hypoxia-activated prodrugs | |||
| Tirapazimine | 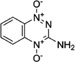 |
Forms free radicals when activated | [38–41] |
| anthraquinone (AQ4N) | 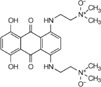 |
Cytotoxic when reduced to AQ4 | [42] |
| Hypoxia mimetics | |||
| Dimethyloxallyl glycine (DMOG) | 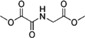 |
Inhibition of prolyl-4-hydroxylase by competition with the substrate | [48, 49] |
| Desferrioxamine | 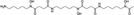 |
Inhibition of prolyl hydroxylase by Fe2+ chelation of the catalytic core | [50, 51] |
| Metal ions (for example, Co2+ and Cu2+) | Inhibition of prolyl hydroxylase by substitution for Fe2+ of the catalytic core | [51, 52] | |
The only other hypoxia-activated prodrug now in clinical trials is the anthraquinone AQ4N (Table 1). AQ4N is a prodrug of a potent DNA intercalator/topoisomerase poison, AQ4. AQ4N has substantial activity against hypoxic cells in various transplanted tumours [42] and has recently completed a Phase I clinical trial.
pO2 modulator: myo-inositol trispyrophosphate (ITPP)
At the opposite of these drugs whose activity is modified by hypoxia, ITPP is, as we know, the only compound able to directly modulate pO2. ITPP acts as an allosteric effector, by enhancing the capacity of haemoglobin to release bound oxygen [43]. This leads to higher oxygen tension in the hypoxic environment, and thus inhibits hypoxia-induced angiogenesis. ITPP is a promising molecule for cancer [44] as well as heart failure [45] therapies, by restoring physiological level of oxygenation in hypoxic tissues (Table 1). Such a molecule could be beneficially used to combine and potentialize drugs that preferentially induce apoptosis of endothelial cells in the tumour as 5,6-dimethylxanthenone-4-acetic acid (DMXAA) [46] and that was recently shown to act through the redox pathway [47].
Hypoxia mimetics: dimethyloxallyl glycine, desferrioxamine and metal ions
For tumour hypoxia modelling in vitro, the use of a hypoxia chamber, in which 95% N2/5% CO2 gas mixture was introduced up to obtain the desired pO2, was the technique of choice. Small molecules mimicking the hypoxic signal were also attractive tools. Dimethyloxallyl glycine [48, 49], desferrioxamine [50, 51] and metal ions [51, 52] were commonly used as hypoxia mimetics (Table 1). In general, these molecules block the catalytic activity of prolyl-hydroxylases, an oxygen sensor able to inactivate hypoxia inducible factor (HIF)-1α activity in normoxic conditions.
This shows how meaningful the knowledge of the oxygen status in normal compared to pathological tissues can be for the design of diagnosis settings, on the one hand, and for therapeutic strategies, on the other hand. This led us to define the physioxia concept.
What does physioxia mean?
The fundamental purpose of the vasculature is to deliver oxygen and nutrients to the cells and to remove dioxide (and other metabolic products) from them. Therefore, oxygenated blood is distributed in each tissue according to its function and its needs. These differ from one tissue to the other. A balance is consequently established between the supply/consumption and retrieval/loss. This is the reason why studying the biology of distinct cell types in the same so-called normoxia is not scientifically correct.
From air to blood
Oxygen is carried through the body from the breathed air to the various organs. This transport occurs in two different media: a gaseous medium (air and airways), then a liquid medium (blood). First, the oxygen transport is primarily achieved by breathing movements, helping the oxygen to go into lungs during the inspiration. Then, oxygen diffuses passively across the alveolar-capillary membrane in the blood, according to concentration gradients. pO2 during the transport from air to the blood that are considered as physiological in human beings are indicated in Figure 5.
Fig 5.
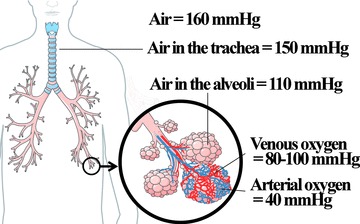
Delivery of oxygen from the atmosphere to the blood, in human, and normal pO2 in mmHg. Figure was produced using Servier Medical Art. (http://www.servier.com).
In brain
The inability of the brain to store metabolic products, despite its high oxygen and glucose requirements, makes it very susceptible to ischemic alterations/damages. It is the reason why brain is actively studied in terms of oxygen monitoring.
The pO2 in the brain extracellular fluid results from the balance between oxygen delivery and consumption, and is influenced by changes in capillary perfusion. It reflects the availability of oxygen for oxidative energy-dependent (ATP dependent) activity and, therefore, brain activity. To measure pO2 in the brain, the two most commonly used systems to date are electrodes (Licox and Neurotrend). Using probes, Assad et al. [53] first reported average pO2 values to be 33–36 mmHg in the brain of three patients. Meixensberger et al. [54] measured the brain tissue pO2 after craniotomy and opening of the dura mater in 26 patients and determined a pressure of 48 mmHg as normal values. In the same way, Hoffman et al. [55] used a sensor inserted 4 cm deep into the cortex of patients after craniotomy and opening of the dura. Out of six patients with a non-compromised brain circulation, were found a tissue pO2 of 37 ± 12 mmHg.
Dings et al. [56] in 1998 undertook complete monitoring of brain tissue pO2 as a function of the depth underneath the dura level. The heterogeneity of brain pO2 levels was determined by measurements performed on 27 patients, by removing the brain-inserted probe in a stepwise manner. The mean pO2 was shown to decrease with the brain depth (23.8 ± 8.1 mmHg at 22 to 27 mm below the dura, 25.7 ± 8.3 mmHg at 17 to 22 mm, 33.0 ± 13.3 mmHg at 12 to 17 mm and 33.3 ± 13.3 mmHg at 7 to 12 mm).
Scientific community agrees to say that if the brain tissue pO2 is above 35 mmHg (4.6%), normal oxygenation of the brain tissue should be assured.
In lungs
In clinical analysis, evaluation of tidal breathing (air volume inhaled and exhaled during restful/normal breathing) and alveolar O2 pressure are the most studied oxygen status-related parameters. While breathing in, pO2 ranges around 160 mmHg. It moves down along a gradient to about 100–120 mmHg in the alveolus. These data concern air oxygen. In fact, only few papers discuss the pO2 inside the tissue. Using a polarographic electrode in 20 patients the median normal lung pO2 was found to range from 23 to 656 mmHg (median, 42.8) [57]. Variability was explained by three patients with extremely high lung pO2 resulting from incomplete lung breathing out during measurements using Eppendorf type microelectrode.
In skin
Much of the experimental work on skin has been limited to estimation of mean tissue pO2 from measurements of transcutaneous pO2 or has used needle electrodes that were large relative to the dermal papilla. Therefore we focused on the spatial distribution of pO2 related to the arrangement of the cutaneous microcirculation.
Wang et al. [58] published the study of spatial variations of pO2 measurements in human skin using microelectrodes. The outer layers of human skin consist of dermal papillae and epidermis. The sub-papillary plexus gives rise to a single papillary loop in each dermal papilla. Epidermis is the outer layer of the skin, composed of terminally differentiated stratified squamous epithelium and not vascularized.
In the superficial region of the skin (5–10 μm), pO2 was approximately 8.0 ± 3.2 mmHg (n= 6). The value increased to 24.0 ± 6.4 mmHg (n= 8) in dermal papillae (45–65 μm), and at the depth just above the sub-papillary plexus (100–120 μm), pO2 was approximately 35.2 ± 8.0 mmHg (n= 9). These data demonstrate that pO2 increases with depth from the surface of the skin to the dermis.
Although there is now a strong evidence that O2 may enter the skin from the atmosphere, this paper shows that, when this is prevented, oxygen supply to the epidermis and superficial dermis can be maintained by the papillary microcirculation, suggesting a regulation of the papillary microcirculation to achieve the O2 requirements of the tissue at a very local level [58].
In intestinal tissue
From 1985, Thermann et al. [59] have determined the local pO2 of the small bowel wall, using a multiwire Pt surface electrode. Local pO2 values of the serosal site of the small bowel of 12 patients were measured at 61.2 (53.0–71.0) mmHg.
Interestingly, few studies evaluated the effects of volatile anaesthetics on oxygenation. Müller et al. [60] investigated the effect of bupivacaine, a local anaesthetic drug, on pO2 by polarography, using a multiwire surface probe. At the serosal side of the colon wall the tissue pO2 increased from 34 to 51 mmHg after epidural bupivacaine administration. This molecule blocks spinal nerve, which certainly produces sympathetic denervation of the bowel with an increase of regional nutritive blood flow. Later, the same group evaluated the effects of desflurane, a highly fluorinated methyl ethyl ether, and isoflurane, a halogenated ether, both used for maintenance of general anaesthesia. Tissue oxygen pressure was measured on the serosal side of the large intestine. No difference in oxygenation was observed for these molecules and, again, pO2 mean values were high: desfluorane group 60.7 ± 21.7 mmHg versus isoflurane group: 57.7 ± 20.2 mmHg [61].
In liver
Data on tissue pO2 in human liver tissue were limited until the clinical study of Leary et al. [62] in 2002. They used a multiparameter sensor to enable continuous monitoring of liver tissue oxygen tension in the early post-operative period in 12 patients after liver transplantation. Tissue oxygen tension values decreased in the first 24 hrs and subsequently increased to a mean of 55.5 ± 21.3 mmHg at 48 hrs after surgery. This was associated with a decrease in the degree of acidosis.
In 2004, liver tissue oxygenation was measured in healthy and anaesthetized patients. Measurements were done by a sensor inserted under the liver capsule before liver resection. In these conditions, the median liver pO2 was 30.7 mmHg [63]. Another study was published describing the changes that occur in liver tissue pO2 during hepatic ischemia consequently to hepatic vascular occlusion. Thirteen patients took part in this study, distributed in two groups depending of the clamp period. Before clamping, in group 1, the median pre-clamp of pO2 was 42.04 mmHg and of 34.53 mmHg in group 2. There was no significant change in pO2 whatever clamping or reperfusion durations in both groups [64].
In kidney
In kidneys, oxygen was necessary for the organ function. Within the medulla, tubules and vasa recta are disposed in a hairpin pattern to maximize the concentration of urine by countercurrent exchange. Oxygen diffuses from arterial to venous vasa recta, which leaves the outer medulla deficient in oxygen. In this region, the medullary thick ascending limb is responsible for the generation of an osmotic gradient by active reabsorption of sodium, a process that requires a large amount of oxygen.
Medullary physioxia under normal conditions has been documented in several mammalian species. The medullary pO2 is in the range of 10 to 20 mmHg, contrasting with the pO2 in the cortex, which is about 50 mmHg [65]. In human beings, Müller et al. [66] have reported a study in patients undergoing living donor kidney transplantation. The tissue oxygen pressure was measured in the superficial cortex of the kidney before nephrectomy by using a multiwire tissue surface pO2 electrode. The pO2 mean value in the cortex was 72 ± 20 mmHg, which is slightly higher than the previously found values. Data about human medulla are not yet available.
In muscle
Muscle oxygenation was highly documented and studied for 30 years. Bylund-Fellenius et al. [67] have precisely determined the pO2 into the medial head of the gastrocnemius muscle, using an oxygen probe. Normal values for healthy patient groups were 28.9 ± 3.4 and 29.6 ± 1.8 mmHg. Using the same technique, several laboratories have published similar data. Beerthuizen et al. [68], have positioned a polarographic needle electrode in the vastus lateralis of the quadriceps femoris muscle. The electrode was withdrawn stepwise and, after each 200 μm step, the pO2 value was measured. In this way 100 pO2 values from 100 different places in the same skeletal muscle were obtained. The skeletal muscle pO2 assessments in 31 healthy human beings showed a mean of 4.2 ± 1.8 kPa (31.9 ± 13.7 mmHg). Boekstegers et al. [69] have determined a mean muscular pO2 of 25 mmHg within biceps muscle of 29 patients. More recently, Ikossi et al. [70] have evaluated deltoid muscle tissue oxygenation by insertion of a Licox polarographic probe and determined a mean pO2 of 34 ± 11 mmHg.
Two other techniques were described by the literature allowing the evaluation of pO2 in skeletal muscle: mass spectrometry and MRS. The first technique was used since 1988 by Kiaer and Kristensen to measure pO2 in the resting anterior tibial muscle of healthy adults. The pO2 was 21 ± 3.6 mmHg [71]. The second method, MRS, was not used to directly measure pO2 but for measurement of deoxymyoglobin as a marker of skeletal muscle oxygenation. 1H-MRS of myoglobin is non-invasive and is clearly limited to muscle tissue, the only site in which this endogenous intracellular marker of oxygenation is found. The experimental protocol was focused on resting muscle oxygenation in the muscles of the lower leg of 10 volunteers. This investigation has shown a 9 ± 1% Mb desaturation and a calculated intracellular pO2 of 34 ± 6 mmHg [72].
In bone marrow
Several articles have reported evaluation of the pO2 in bone marrow compartment and the impact of oxygen in stem cell biology. Accessibility of the marrow explained the quantity of available data about this tissue. Indeed, this tissue can be easily obtained by aspiration into a syringe and analysed using a blood gas analyser. In Textbook of the medical physiology, Guyton estimated the tissue oxygen tension in vivo to be approximately 40 mmHg [73]. In fact, pO2 seems to be a little higher. Ischikawa and Ito have determined a pressure of 51.8 ± 14.5 mmHg [74]. More recently, Harrison et al. [75] performed gas analysis in a series of five healthy volunteer bone marrow donors. The mean pO2 of the marrow aspirates was 54.9 ± 0.98 mmHg. Although oxygenation is a critical reference point for determining the effects of hypoxemia upon the bone marrow microenvironment in clinical situations, two studied pathologies (leukaemia [76] and chronic bronchitis with respiratory insufficiency [77]) showed no significant modification of bone marrow pO2.
In umbilical cord blood
For few decades, utility of cord blood was established: it contains undifferentiated stem cells, which can be used for bone marrow transplant. Since 1989 and the first cord blood transplant in a French boy with Fanconi’s anaemia [78], aspiration from the umbilical cord at the time of childbirth was accentuated. It is the raison why analyses of cord blood gas were commonly done and physiological values of pO2 were determined. pO2 in umbilical vein blood should be normally between 20 and 30 mmHg, whereas blood pO2 within umbilical artery is around 10–15 mmHg.
To summarize physioxia
In this review, we clearly point to the characteristic fact that each tissue presents a distinct/specific mixture of gases balance, depending on its vascularization and its supply. Mean values of pO2 in several human tissues are presented in Table 2.
Table 2.
Normal values of pO2 in various human tissues, expressed in mmHg and in percentage of oxygen in the microenvironment
| pO2 | |||
|---|---|---|---|
| mmHg | % | References | |
| Air | 160 | 21.1 | |
| Inspired air (in the tracheus) | 150 | 19.7 | |
| Air in the alveoli | 110 | 14.5 | |
| Arterial blood | 100 | 13.2 | |
| Venous blood | 40 | 5.3 | |
| Cell | 9.9–19 | 1.3–2.5 | [92] |
| Mitochondria | <9.9 | <1.3 | [92] |
| Brain | 33.8 ± 2.6 | 4.4 ± 0.3 | [53–56, 91] |
| Lung | 42.8 | 5.6 | [57] |
| Skin (sub-papillary plexus) | 35.2 ± 8 | 4.6 ± 1.1 | [58] |
| Skin (dermal papillae) | 24 ± 6.4 | 3.2 ± 0.8 | [58] |
| Skin (superficial region) | 8 ± 3.2 | 1.1 ± 0.4 | [58] |
| Intestinal tissue | 57.6 ± 2.3 | 7.6 ± 0.3 | [59–61] |
| Liver | 40.6 ± 5.4 | 5.4 ± 0.7 | [62–64] |
| Kidney | 72 ± 20 | 9.5 ± 2.6 | [66] |
| Muscle | 29.2 ± 1.8 | 3.8 ± 0.2 | [67–72] |
| Bone marrow | 48.9 ± 4.5 | 6.4 ± 0.6 | [73–75] |
Cellular and molecular consequences of physioxia versus normoxia and hypoxia
In the latter part of this review, we describe several modulation processes shown to be involved in the response to hypoxia. We propose and discuss that they may also be the result of controlled physioxia. In fact, physiological phenotypes could be hidden during studies conducted in so-called normoxia and further investigations have been done in the newly defined oxygen conditions, to validate our hypothesis.
Hypoxia inducible factors actions
As mentioned above, physioxia presents pO2 values close to those used in numerous in vitro studies for mimicking hypoxia. The HIF is now recognized to play a central role in a variety of systemic and local adaptive responses, including the control of red blood cell production, regulation of angiogenesis, modulation of vascular tone, enhancement of glycolysis and cellular glucose uptake [79]. In mammalian cell lines, two isoforms of HIF-α, HIF-1α and HIF-2α are induced by hypoxia within minutes and show very similar regulation characteristics.
HIF-1α stabilization in strong hypoxic conditions is primarily an acute response to hypoxia and the HIF-1α protein levels are reduced or disappear for prolonged hypoxia. HIF-2α levels, on the other hand, continue to increase with time in hypoxia and become more important during the later phases of hypoxia [80]. Moreover, prolonged moderate hypoxia (5% O2 or 38 mmHg) seems to be sufficient to stabilize HIF-2α, but not to induce HIF-1α as showed in Figure 6.
Fig 6.
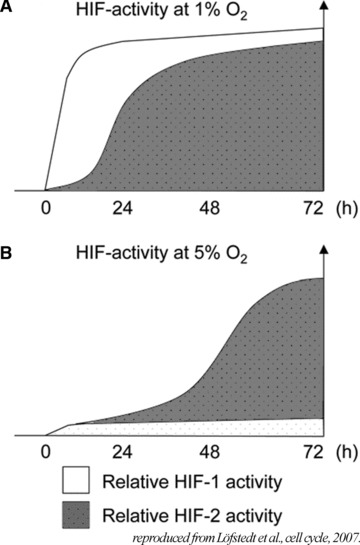
Relative HIF-1 and HIF-2 contributions to the hypoxic response over time at 1% (A) and 5% (B) oxygen as demonstrated in human neuroblastoma cells. Model based on a summary of data obtained from protein level variations (Western blot), chromatin immunoprocipitations (ChIP) and activation of target genes ± siRNA treatment. Reproduced with permission from Lofstedt et al., Cell Cycle, 2007[80].
Because the majority of tissue pO2 ranges between 3% and 10% (23–70 mmHg), HIF-2α may be responsible for the transcription of genes involved in physioxia. Consequently, cells express a protein pattern in physiological conditions distinct than in ‘classical’/21% oxygen culture conditions. Anyway, it is clear that HIFs are under a precise control that is dependent on any change in the oxygen level. A variation, as low as the difference between physioxia and hypoxia, becomes highly significant. Recently, Ben-Shoshan et al. [81] evaluated precisely the effect of HIF-1α and HIF-2α expression on the endothelial cell physiology. Both factors enhance cellular mechanisms, but their contributions are distinct. Together, these data highlight the crucial role of HIF-2α in cell physiology in tissue conditions.
Role of microRNAs in hypoxia-dependent regulations
MicroRNAs have emerged as crucial players regulating the magnitude of gene expression in a variety of organisms [82]. MicroRNAs are short (20–24 nucleotides) non-coding RNAs that negatively regulate gene expression with complementary RNA sequence. Extensive research has revealed the existence of more than 1000 different human microRNAs, which represent approximately 3% of the eukaryotic transcriptome. Numerous reports have demonstrated the importance of microRNA-mediated regulation in key processes, such as proliferation, apoptosis, differentiation and development, cellular identity and pathogen–host interactions. Consequently, their deregulation is observed in different human pathologies including cancer, heart disease and neurodegeneration [83].
Study from Kulshreshtha’s group identified a set of hypoxia-regulated microRNAs (HRMs), providing an additional link between a hypoxic-specific stress factor and gene expression control. The HRM group includes: miR-21, 23a, 23b, 24, 26a, 26b, 27a, 30b, 93, 103, 103, 106a, 107, 125b, 181a, 181b, 181c, 192, 195, 210 and 213, which were consistently induced in response to hypoxia in breast and colon cancer cells [84]. In addition, few microRNAs were identified as down-regulated in hypoxic cells, including miR-15b, 16, 19a, 20a, 20b, 29b, 30b, 30e-5p, 101, 141, 122a, 186, 197 and 320. These gene expression regulators were also dependent of the oxygen percentage. Few microRNAs were shown to be expressed from 38 mmHg (5%) of oxygen pressure [85]. Candidate targets for HRM were searched by in silico analyses and revealed genes involved in proliferation, apoptosis, DNA repair, chromatin remodelling, metabolism and migration. Each HRM is predicted to down-regulate an amount of 10 genes, sometimes as many as 200, which could confound the effort to identify biologically relevant targets [86]. There is growing evidence that microRNAs are strong regulators of the transcriptome, especially in hypoxia and physioxia. However further investigations are necessary to determine the exact role of microRNAs in cellular physiology. Importantly microRNAs are studied extensively and show promises as selective targets for future therapeutic strategies.
Cell adhesion molecules: their regulation by oxygen partial pressure
Cell adhesion molecules (CAMs) are cell surface glycoproteins that mediate physical interactions between adjacent cells and between cells and the surrounding extracellular matrix. CAMs belong to different protein families, including integrins, cadherins, selectins, addresins and immunoglobulin-like cell molecules [87] and their expression is restricted to specific cell types. Besides playing a key homeostatic role in maintaining the architecture of quiescent tissues, CAMs have also to adapt to the microenvironmental changes that occur during some physiological and pathological processes [88]. We have previously shown that endothelial CAMs are modulated by nitric oxide [89] whose production is dependent on oxygen pressure. To visualize the direct impact of oxygen tension on the cell surface phenotype of endothelial cells, we studied intercellular adhesion molecules (ICAM) and vascular cell adhesion molecule (VCAM) which are involved in inflammatory processes: ICAM-1/CD54, ICAM-2/CD102 and VCAM-1/CD106. As shown in Figure 7, ICAM-1/CD54 expression is stable whatever was the oxygen level. But skin physioxia (26.6 mmHg or 3.5% O2) decreases VCAM-1/CD106 whereas only hypoxia (less than 7.6 mmHg or 1% O2) is able to inhibit ICAM-2/CD102.
Fig 7.
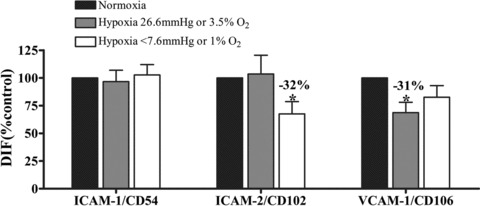
pO2 dependence of the expression of three CAMs in skin microvascular endothelial cells: ICAM-1/CD54, ICAM-2/CD102 and VCAM-1/CD106. Cells were maintained in normoxia, skin physioxia (26.6 mmHg or 3.5% O2) or hypoxia (less than 7.6 mmHg or 1% O2) during 24 hrs. Skin microvascular endothelial cells coming mostly from the dermis, physioxia was considered to be around 3–3.5% O2[58]. Then they were incubated with specific antibodies and labelled with a fluorescent secondary antibody. Fluorescence was quantified by flow cytometry. Changes in adhesion molecules expression are shown as percentage of fluorescence intensity related to fluorescence intensity of non-treated cells (DIF, mean from three experiments ± S.E.M.; *P < 0.05).
From this example, we can conclude that CAMs expression is sensitive to oxygen and, that CAMs do not present the same sensitivity. Thus, results coming from experiments carried out in the classical 21% O2 culture condition do not represent the physiological state of cells.
Soluble molecules: example of angiogenin
Another example that illustrates the crucial role of physiological oxygen pressure is the secretion of soluble factors. They are fundamental molecules for cell communication, allowing information delivery from one cell to another by a paracrine signalling. Soluble factors are constituents of the microenvironment and a variation in their concentrations is prerequisite of a rapid cellular and tissue response.
As an example, angiogenin is up-regulated in various types of human cancers and takes part in tumour progression by stimulating both angiogenesis and cancer cell proliferation [90].The release of angiogenin from various cells present in the skin is very sensitive to oxygen pressure (Fig. 8). Strong hypoxia (less than 7.6 mmHg or 1% O2) induces an increase in angiogenin secretion for the cell lines derived from fibroblasts, human skin microvascular endothelial cells, normal melanocytes and keratinocytes. But skin physioxia (22.8 mmHg of O2 or 3% O2) is already able to lead to the same increase of angiogenin by keratinocytes and normal melanocyte cell line. On the opposite, this pO2 does not modify the angiogenin expression by human skin microvascular endothelial cell line or fibroblasts. Here again, results from normoxia does not reflect the real behaviour of cells in physioxia. Furthermore, this example points out the difference of sensitivity to oxygen pressure of distinct cell lines.
Fig 8.
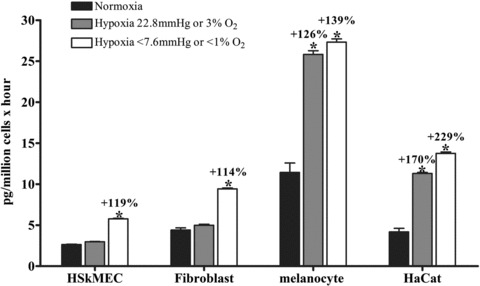
Oxygen dependent modulation of angiogenin secretion by four distinct cell lines constituting the skin. Human skin endothelial microvascular cell line (HSkMEC), fibroblasts MSU 1.1, normal melanocyte cell line and HaCat keratinocytes have been maintained in normoxia, skin physioxia (22.8 mmHg or 3% O2) or hypoxia (less than 7.6 mmHg or 1% O2) during 16 hrs. Supernatants have been collected and angiogenin content assessed by the cytometric bead assay (CBA) technique. Results are expressed as picograms of angiogenin secreted by 10 × 6 cells in one hour (mean ± S.E.M.); *P < 0.05.
Conclusion
In this review, we have pointed to the importance of taking into account the physioxia. First, for in vitro studies, it is important to know the organ pO2 we want to mimic, to be closer to the physiology. Each organ has its own oxygenation status, depending of its function. Second, we have reported that cell activity could be modulated by oxygen from the gene level to the proteome expression, with a fine regulation. A literature survey points out that only few experiments were done in physioxia. Thus, we should consider differently the biological experimental approaches in order to take into account the real value of pO2 in tissues and be relevant of in vivo situation. Third, in in vivo experiments, pO2‘normal’ values can be used to evaluate the metabolic state of an organ. For instance in brain, pO2 value is more sensitive and relevant than others parameters to diagnose and follow injuries [91].
Nevertheless, in addition to physioxia, we must take into account the blood stream distribution which is dynamically adjusted to the needs of different organs. Whereas large organs with high oxygen consumption primarily receive a greater proportion of the cardiac output than smaller or metabolically less demanding tissues, the distribution for tissues may vary greatly depending on the time-related functional requirements. Although some tissues (such as the brain) have very uniform energy requirements, the perfusion and energy expense of other tissues (such as liver and bowel) varies largely depending on their functional state. Therefore, precise knowledge of the functional changes in oxygen delivery is mandatory to, first, correctly interpret data from monitoring tissue oxygenation and, second, culture cell in oxygen conditions corresponding to ‘natural’ tissue/organ in vivo environment. The impact of such knowledge is particularly illustrated by the tumour tissue biology and its consequences on therapy efficacy.
In conclusion, physioxia is different and involves distinct cellular aspects compared to so-called normoxia and consequently, distinct reactions to hypoxia. We would like to highlight the importance of opening a new way of research, using reliable oxygen conditions that will allow interpretation and translation of in vitro scientific findings into real life settings.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Vaupel P, Höckel M, Mayer A. Detection and characterization of tumor hypoxia using pO2 histography. Antioxid Redox Signal. 2007;9:1221–5. doi: 10.1089/ars.2007.1628. [DOI] [PubMed] [Google Scholar]
- 2.Maeda K, Osato T, Umezawa H. A new antibiotic, azomycin. J Antibiot. 1953;6:182. [PubMed] [Google Scholar]
- 3.Nunn A, Linder K, Strauss HW. Nitroimidazoles and imaging hypoxia. Eur J Nucl Med. 1995;22:265–80. doi: 10.1007/BF01081524. [DOI] [PubMed] [Google Scholar]
- 4.Kizaka-Kondoh S, Konse-Nagasawa H. Significance of nitroimidazole compounds and hypoxia-inducible factor-1 for imaging tumor hypoxia. Cancer Science. 2009;100:1366–73. doi: 10.1111/j.1349-7006.2009.01195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002;2:683–93. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 6.Jensen RL. Brain tumor hypoxia: tumorigenesis, angiogenesis, imaging, pseudoprogression, and as a therapeutic target. J Neurooncol. 2009;92:317–35. doi: 10.1007/s11060-009-9827-2. [DOI] [PubMed] [Google Scholar]
- 7.Holland JP, Lewis JS, Dehdashti F. Assessing tumor hypoxia by positron emission tomography with Cu-ATSM. Q J Nucl Med Mol Imaging. 2009;53:193–200. [PMC free article] [PubMed] [Google Scholar]
- 8.Ter-Pogossian MM, Herscovitch P. Radioactive oxygen-15 in the study of cerebral blood flow, blood volume, and oxygen metabolism. Semin Nucl Med. 1985;15:377–94. doi: 10.1016/s0001-2998(85)80015-5. [DOI] [PubMed] [Google Scholar]
- 9.Boushel R, Piantadosi CA. Near-infrared spectroscopy for monitoring muscle oxygenation. Acta Physiol Scand. 2000;168:615–22. doi: 10.1046/j.1365-201x.2000.00713.x. [DOI] [PubMed] [Google Scholar]
- 10.Intes X, Chance B. Non-PET functional imaging techniques: optical. Radiol Clin North Am. 2005;43:221–34. doi: 10.1016/j.rcl.2004.07.002. , xii. [DOI] [PubMed] [Google Scholar]
- 11.Boushel R, Langberg H, Olesen J, et al. Monitoring tissue oxygen availability with near infrared spectroscopy (NIRS) in health and disease. Scand J Med Sci Sports. 2001;11:213–22. doi: 10.1034/j.1600-0838.2001.110404.x. [DOI] [PubMed] [Google Scholar]
- 12.Busse LJ, Pratt RG, Thomas SR. Deconvolution of chemical shift spectra in two- or three-dimensional [19F] MR imaging. J Comput Assist Tomogr. 1988;12:824–35. doi: 10.1097/00004728-198809010-00020. [DOI] [PubMed] [Google Scholar]
- 13.Mason RP, Antich PP, Babcock EE, et al. Non-invasive determination of tumor oxygen tension and local variation with growth. Int J Radiat Oncol Biol Phys. 1994;29:95–103. doi: 10.1016/0360-3016(94)90231-3. [DOI] [PubMed] [Google Scholar]
- 14.Zhao D, Ran S, Constantinescu A, et al. Tumor oxygen dynamics: correlation of in vivo MRI with histological findings. Neoplasia. 2003;5:308–18. doi: 10.1016/S1476-5586(03)80024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howe FA, Robinson SP, McIntyre DJ, et al. Issues in flow and oxygenation dependent contrast (FLOOD) imaging of tumours. NMR Biomed. 2001;14:497–506. doi: 10.1002/nbm.716. [DOI] [PubMed] [Google Scholar]
- 16.Taylor JS, Tofts PS, Port R, et al. MR imaging of tumor microcirculation: promise for the new millennium. J Magn Reson Imaging. 1999;10:903–7. doi: 10.1002/(sici)1522-2586(199912)10:6<903::aid-jmri1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 17.Baudelet C, Gallez B. How does blood oxygen level-dependent (BOLD) contrast correlate with oxygen partial pressure (pO2) inside tumors? Magn Reson Med. 2002;48:980–6. doi: 10.1002/mrm.10318. [DOI] [PubMed] [Google Scholar]
- 18.Swartz HM. Using EPR to measure a critical but often unmeasured component of oxidative damage: oxygen. Antioxid Redox Signal. 2004;6:677–86. doi: 10.1089/152308604773934440. [DOI] [PubMed] [Google Scholar]
- 19.Swartz HM, Walczak T. Developing in vivoEPR oximetry for clinical use. Adv Exp Med Biol. 1998;454:243–52. doi: 10.1007/978-1-4615-4863-8_29. [DOI] [PubMed] [Google Scholar]
- 20.Stone HB, Brown JM, Phillips TL, et al. Oxygen in human tumors: correlations between methods of measurement and response to therapy. Summary of a workshop held November 19–20, 1992, at the National Cancer Institute, Bethesda, Maryland. Radiat Res. 1993;136:422–34. [PubMed] [Google Scholar]
- 21.Clark LC. Monitor and Control of Blood and Tissue Oxygen Tensions. ASAIO J. 1958;2:41–8. [Google Scholar]
- 22.Lübbers DW, Baumgärtl H, Zimelka W. Heterogeneity and stability of local PO2 distribution within the brain tissue. Adv Exp Med Biol. 1994;345:567–74. doi: 10.1007/978-1-4615-2468-7_75. [DOI] [PubMed] [Google Scholar]
- 23.Nozue M, Lee I, Yuan F, et al. Interlaboratory variation in oxygen tension measurement by Eppendorf “histograph” and comparison with hypoxic marker. J Surg Oncol. 1997;66:30–8. doi: 10.1002/(sici)1096-9098(199709)66:1<30::aid-jso7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 24.Vanderkooi JM, Maniara G, Green TJ, et al. An optical method for measurement of dioxygen concentration based upon quenching of phosphorescence. J Biol Chem. 1987;262:5476–82. [PubMed] [Google Scholar]
- 25.Griffiths JR, Robinson SP. The OxyLite: a fibre-optic oxygen sensor. Br J Radiol. 1999;72:627–30. doi: 10.1259/bjr.72.859.10624317. [DOI] [PubMed] [Google Scholar]
- 26.Seylaz J, Pinard E, Meric P, et al. Local cerebral PO2, PCO2, and blood flow measurements by mass spectrometry. Am J Physiol. 1983;245:H513–8. doi: 10.1152/ajpheart.1983.245.3.H513. [DOI] [PubMed] [Google Scholar]
- 27.Ndubuizu O, LaManna JC. Brain tissue oxygen concentration measurements. Antioxid Redox Signal. 2007;9:1207–19. doi: 10.1089/ars.2007.1634. [DOI] [PubMed] [Google Scholar]
- 28.Höckel M, Vaupel P. Biological consequences of tumor hypoxia. Semin Oncol. 2001;28:36–41. [PubMed] [Google Scholar]
- 29.Lawrentschuk N, Poon AMT, Foo SS, et al. Assessing regional hypoxia in human renal tumours using 18F-fluoromisonidazole positron emission tomography. BJU Int. 2005;96:540–6. doi: 10.1111/j.1464-410X.2005.05681.x. [DOI] [PubMed] [Google Scholar]
- 30.Aquino-Parsons C, Luo C, Vikse CM, et al. Comparison between the comet assay and the oxygen microelectrode for measurement of tumor hypoxia. Radiother Oncol. 1999;51:179–85. doi: 10.1016/s0167-8140(99)00035-3. [DOI] [PubMed] [Google Scholar]
- 31.Trédan O, Galmarini CM, Patel K, et al. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99:1441–54. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- 32.Ledoux S, Yang R, Friedlander G, et al. Glucose depletion enhances P-glycoprotein expression in hepatoma cells. Cancer Res. 2003;63:7284–90. [PubMed] [Google Scholar]
- 33.Graeber TG, Osmanian C, Jacks T, et al. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 34.Kondo A, Safaei R, Mishima M, et al. Hypoxia-induced enrichment and mutagenesis of cells that have lost DNA mismatch repair. Cancer Res. 2001;61:7603–7. [PubMed] [Google Scholar]
- 35.Gray MD, Mann M, Nitiss JL, et al. Activation of the unfolded protein response is necessary and sufficient for reducing topoisomerase IIα protein levels and decreasing sensitivity to topoisomerase-targeted drugs. Molecular Pharmacology. 2005;68:1699–707. doi: 10.1124/mol.105.014753. [DOI] [PubMed] [Google Scholar]
- 36.Tannock IF. The relation between cell proliferation and the vascular system in a transplanted mouse mammary tumour. Br J Cancer. 1968;22:258–73. doi: 10.1038/bjc.1968.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown JM. Clinical trials of radiosensitizers: what should we expect? Int J Radiat Oncol Biol Phys. 1984;10:425–9. doi: 10.1016/0360-3016(84)90063-4. [DOI] [PubMed] [Google Scholar]
- 38.Zeman EM, Brown JM, Lemmon MJ, et al. SR-4233: a new bioreductive agent with high selective toxicity for hypoxic mammalian cells. Int J Radiat Oncol Biol Phys. 1986;12:1239–42. doi: 10.1016/0360-3016(86)90267-1. [DOI] [PubMed] [Google Scholar]
- 39.Peters KB, Brown JM. Tirapazamine: a hypoxia-activated topoisomerase II poison. Cancer Res. 2002;62:5248–53. [PubMed] [Google Scholar]
- 40.von Pawel J, von Roemeling R, Gatzemeier U, et al. Tirapazamine plus cisplatin versus cisplatin in advanced non-small-cell lung cancer: a report of the international CATAPULT I study group. Cisplatin and tirapazamine in subjects with advanced previously untreated non-small-cell lung tumors. J Clin Oncol. 2000;18:1351–9. doi: 10.1200/JCO.2000.18.6.1351. [DOI] [PubMed] [Google Scholar]
- 41.Williamson SK, Crowley JJ, Lara PN, et al. Phase III trial of paclitaxel plus carboplatin with or without tirapazamine in advanced non-small-cell lung cancer: Southwest Oncology Group Trial S0003. J Clin Oncol. 2005;23:9097–104. doi: 10.1200/JCO.2005.01.3771. [DOI] [PubMed] [Google Scholar]
- 42.Patterson LH. Bioreductively activated antitumor N-oxides: the case of AQ4N, a unique approach to hypoxia-activated cancer chemotherapy. Drug Metab Rev. 2002;34:581–92. doi: 10.1081/dmr-120005659. [DOI] [PubMed] [Google Scholar]
- 43.Fylaktakidou KC, Lehn J, Greferath R, et al. Inositol tripyrophosphate: a new membrane permeant allosteric effector of haemoglobin. Bioorg Med Chem Lett. 2005;15:1605–8. doi: 10.1016/j.bmcl.2005.01.064. [DOI] [PubMed] [Google Scholar]
- 44.Kieda C, Greferath R, Crola da Silva C, et al. Suppression of hypoxia-induced HIF-1alpha and of angiogenesis in endothelial cells by myo-inositol trispyrophosphate-treated erythrocytes. Proc Natl Acad Sci USA. 2006;103:15576–81. doi: 10.1073/pnas.0607109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biolo A, Greferath R, Siwik DA, et al. Enhanced exercise capacity in mice with severe heart failure treated with an allosteric effector of hemoglobin, myo-inositol trispyrophosphate. Proc Natl Acad Sci USA. 2009;106:1926–9. doi: 10.1073/pnas.0812381106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ching L, Cao Z, Kieda C, et al. Induction of endothelial cell apoptosis by the antivascular agent 5,6-Dimethylxanthenone-4-acetic acid. Br J Cancer. 2002;86:1937–42. doi: 10.1038/sj.bjc.6600368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brauer R, Wang LS, Woon S, et al. Preferential labelling of oxidisable proteins with a photoactivatable analogue of the anti-tumor agent DMXAA and evidence for redox signalling in its mode of action. Neoplasia. 2010;12:755–65. doi: 10.1593/neo.10636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baader E, Tschank G, Baringhaus KH, et al. Inhibition of prolyl 4-hydroxylase by oxalyl amino acid derivatives in vitro, in isolated microsomes and in embryonic chicken tissues. Biochem J. 1994;300:525–30. doi: 10.1042/bj3000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ivan M, Haberberger T, Gervasi DC, et al. Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc Natl Acad Sci USA. 2002;99:13459–64. doi: 10.1073/pnas.192342099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang GL, Semenza GL. Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: implications for models of hypoxia signal transduction. Blood. 1993;82:3610–5. [PubMed] [Google Scholar]
- 51.Gleadle JM, Ebert BL, Firth JD, et al. Regulation of angiogenic growth factor expression by hypoxia, transition metals, and chelating agents. Am J Physiol Cell Physiol. 1995;268:C1362–8. doi: 10.1152/ajpcell.1995.268.6.C1362. [DOI] [PubMed] [Google Scholar]
- 52.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–54. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 53.Assad F, Scultheiss R, Leniger-Follert E, et al. Measurement of local oxygen partial pressure (PO2) of the brain cortex in cases of tumors. Adv Neurosurg. 1984;12:263–6. [Google Scholar]
- 54.Meixensberger J, Dings J, Kuhnigk H, et al. Studies of tissue PO2 in normal and pathological human brain cortex. Acta Neurochir Suppl. 1993;59:58–63. doi: 10.1007/978-3-7091-9302-0_10. [DOI] [PubMed] [Google Scholar]
- 55.Hoffman WE, Charbel FT, Edelman G. Brain tissue oxygen, carbon dioxide, and pH in neurosurgical patients at risk for ischemia. Anesth Analg. 1996;82:582–6. doi: 10.1097/00000539-199603000-00027. [DOI] [PubMed] [Google Scholar]
- 56.Dings J, Meixensberger J, Jäger A, et al. Clinical experience with 118 brain tissue oxygen partial pressure catheter probes. Neurosurgery. 1998;43:1082–95. doi: 10.1097/00006123-199811000-00045. [DOI] [PubMed] [Google Scholar]
- 57.Le Q, Chen E, Salim A, et al. An evaluation of tumor oxygenation and gene expression in patients with early stage non-small cell lung cancers. Clin Cancer Res. 2006;12:1507–14. doi: 10.1158/1078-0432.CCR-05-2049. [DOI] [PubMed] [Google Scholar]
- 58.Wang W, Winlove CP, Michel CC. Oxygen partial pressure in outer layers of skin of human finger nail folds. J Physiol. 2003;549:855–63. doi: 10.1113/jphysiol.2002.037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thermann M, Jostarndt L, Eberhard F, et al. [Oxygen supply of the human small intestine in mechanical ileus] Langenbecks Arch Chir. 1985;363:179–84. doi: 10.1007/BF01261291. [DOI] [PubMed] [Google Scholar]
- 60.Müller M, Schück R, Erkens U, et al. Effects of lumbar peridural anesthesia on tissue pO2 of the large intestine in man. Anasthesiol Intensivmed Notfallmed Schmerzther. 1995;30:108–10. doi: 10.1055/s-2007-996457. [DOI] [PubMed] [Google Scholar]
- 61.Müller M, Schindler E, Roth S, et al. Effects of desflurane and isoflurane on intestinal tissue oxygen pressure during colorectal surgery. Anaesthesia. 2002;57:110–5. doi: 10.1046/j.0003-2409.2001.02363.x. [DOI] [PubMed] [Google Scholar]
- 62.Leary TS, Klinck JR, Hayman G, et al. Measurement of liver tissue oxygenation after orthotopic liver transplantation using a multiparameter sensor. A pilot study. Anaesthesia. 2002;57:1128–33. doi: 10.1046/j.1365-2044.2002.02782_5.x. [DOI] [PubMed] [Google Scholar]
- 63.Brooks AJ, Eastwood J, Beckingham IJ, et al. Liver tissue partial pressure of oxygen and carbon dioxide during partial hepatectomy. Br J Anaesth. 2004;92:735–7. doi: 10.1093/bja/aeh112. [DOI] [PubMed] [Google Scholar]
- 64.Brooks AJ, Hammond JS, Girling K, et al. The effect of hepatic vascular inflow occlusion on liver tissue pH, carbon dioxide, and oxygen partial pressures: defining the optimal clamp/release regime for intermittent portal clamping. J Surg Res. 2007;141:247–51. doi: 10.1016/j.jss.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 65.Brezis M, Rosen S. Hypoxia of the renal medulla – its implications for disease. N Engl J Med. 1995;332:647–55. doi: 10.1056/NEJM199503093321006. [DOI] [PubMed] [Google Scholar]
- 66.Müller M, Padberg W, Schindler E, et al. Renocortical tissue oxygen pressure measurements in patients undergoing living donor kidney transplantation. Anesth Analg. 1998;87:474–6. doi: 10.1097/00000539-199808000-00045. [DOI] [PubMed] [Google Scholar]
- 67.Bylund-Fellenius AC, Walker PM, Elander A, et al. Energy metabolism in relation to oxygen partial pressure in human skeletal muscle during exercise. Biochem J. 1981;200:247–55. doi: 10.1042/bj2000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beerthuizen GI, Goris RJ, Kreuzer FJ. Skeletal muscle Po2 during imminent shock. Arch Emerg Med. 1989;6:172–82. doi: 10.1136/emj.6.3.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boekstegers P, Riessen R, Seyde W. Oxygen partial pressure distribution within skeletal muscle: indicator of whole body oxygen delivery in patients? Adv Exp Med Biol. 1990;277:507–14. doi: 10.1007/978-1-4684-8181-5_57. [DOI] [PubMed] [Google Scholar]
- 70.Ikossi DG, Knudson MM, Morabito DJ, et al. Continuous muscle tissue oxygenation in critically injured patients: a prospective observational study. J Trauma. 2006;61:780–90. doi: 10.1097/01.ta.0000239500.71419.58. [DOI] [PubMed] [Google Scholar]
- 71.Kiaer T, Kristensen KD. Intracompartmental pressure, PO2, PCO2 and blood flow in the human skeletal muscle. Arch Orthop Trauma Surg. 1988;107:114–6. doi: 10.1007/BF00454498. [DOI] [PubMed] [Google Scholar]
- 72.Richardson RS, Duteil S, Wary C, et al. Human skeletal muscle intracellular oxygenation: the impact of ambient oxygen availability. J Physiol. 2006;571:415–24. doi: 10.1113/jphysiol.2005.102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guyton A. Textbook of medical physiology. 6th ed. Philadelphia: Saunders; 1981. [Google Scholar]
- 74.Ishikawa Y, Ito T. Kinetics of hemopoietic stem cells in a hypoxic culture. Eur J Haematol. 1988;40:126–9. doi: 10.1111/j.1600-0609.1988.tb00808.x. [DOI] [PubMed] [Google Scholar]
- 75.Harrison JS, Rameshwar P, Chang V, et al. Oxygen saturation in the bone marrow of healthy volunteers. Blood. 2002;99:394. doi: 10.1182/blood.v99.1.394. [DOI] [PubMed] [Google Scholar]
- 76.Fiegl M, Samudio I, Clise-Dwyer K, et al. CXCR4 expression and biologic activity in acute myeloid leukemia are dependent on oxygen partial pressure. Blood. 2009;113:1504–12. doi: 10.1182/blood-2008-06-161539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Skouby AR. Haematologic adaptation in patients with chronic bronchitis and pulmonary insufficiency. Acta Med Scand. 1976;199:185–90. doi: 10.1111/j.0954-6820.1976.tb06714.x. [DOI] [PubMed] [Google Scholar]
- 78.Gluckman E, Broxmeyer HA, Auerbach AD, et al. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174–8. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- 79.Wiesener MS, Jürgensen JS, Rosenberger C, et al. Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. FASEB J. 2003;17:271–3. doi: 10.1096/fj.02-0445fje. [DOI] [PubMed] [Google Scholar]
- 80.Löfstedt T, Fredlund E, Holmquist-Mengelbier L, et al. Hypoxia inducible factor-2alpha in cancer. Cell Cycle. 2007;6:919–26. doi: 10.4161/cc.6.8.4133. [DOI] [PubMed] [Google Scholar]
- 81.Ben-Shoshan J, Maysel-Auslender S, Luboshits G, et al. Hypoxia-inducible factor-1alpha and -2alpha additively promote endothelial vasculogenic properties. J Vasc Res. 2009;46:299–310. doi: 10.1159/000181546. [DOI] [PubMed] [Google Scholar]
- 82.Ambros V. MicroRNA pathways in flies and worms: growth, death, fat, stress, and timing. Cell. 2003;113:673–6. doi: 10.1016/s0092-8674(03)00428-8. [DOI] [PubMed] [Google Scholar]
- 83.Suárez Y, Sessa WC. MicroRNAs as novel regulators of angiogenesis. Circ Res. 2009;104:442–54. doi: 10.1161/CIRCRESAHA.108.191270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kulshreshtha R, Ferracin M, Wojcik SE, et al. A microRNA signature of hypoxia. Mol Cell Biol. 2007;27:1859–67. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hebert C, Norris K, Scheper MA, et al. High mobility group A2 is a target for miRNA-98 in head and neck squamous cell carcinoma. Mol Cancer. 2007;6:5–15. doi: 10.1186/1476-4598-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kulshreshtha R, Davuluri RV, Calin GA, et al. A microRNA component of the hypoxic response. Cell Death Differ. 2008;15:667–71. doi: 10.1038/sj.cdd.4402310. [DOI] [PubMed] [Google Scholar]
- 87.Mousa SA. Cell adhesion molecules: potential therapeutic & diagnostic implications. Mol Biotechnol. 2008;38:33–40. doi: 10.1007/s12033-007-0072-7. [DOI] [PubMed] [Google Scholar]
- 88.Juliano RL. Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu Rev Pharmacol Toxicol. 2002;42:283–323. doi: 10.1146/annurev.pharmtox.42.090401.151133. [DOI] [PubMed] [Google Scholar]
- 89.Carreau A, Kieda C, Grillon C. Nitric oxide modulates the expression of endothelial cell adhesion molecules involved in angiogenesis and leukocyte recruitment. Exp Cell Res. 2011;317:29–41. doi: 10.1016/j.yexcr.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 90.Gao X, Xu Z. Mechanisms of action of angiogenin. Acta Biochim Biophys Sin. 2008;40:619–24. doi: 10.1111/j.1745-7270.2008.00442.x. [DOI] [PubMed] [Google Scholar]
- 91.Korsic M, Jugović D, Kremzar B. Intracranial pressure and biochemical indicators of brain damage: follow-up study. Croat Med J. 2006;47:246–52. [PMC free article] [PubMed] [Google Scholar]
- 92.Gleadle J, Ratcliffe PJ. Encyclopedia of life sciences. Chichester: John Wiley & Sons, Ltd; 2001. Hypoxia. [Google Scholar]


