Abstract
Regenerative medicine seeks to repair or replace damaged tissues or organs, with the goal to fully restore structure and function without the formation of scar tissue. Cell based therapies are promising new therapeutic approaches in regenerative medicine. By using mesenchymal stem cells, good results have been reported for bone engineering in a number of clinical studies, most of them investigator initiated trials with limited scope with respect to controls and outcome. With the implementation of a new regulatory framework for advanced therapeutic medicinal products, the stage is set to improve both the characterization of the cells and combination products, and pave the way for improved controlled and well-designed clinical trials. The incorporation of more personalized medicine approaches, including the use of biomarkers to identify the proper patients and the responders to treatment, will be contributing to progress in the field. Both translational and clinical research will move the boundaries in the field of regenerative medicine, and a coordinated effort will provide the clinical breakthroughs, particularly in the many applications of bone engineering.
Keywords: regenerative medicine, stem cell therapy, bone regeneration, clinical studies, ethics
Introduction
Bone grafting is widely used in hospitals to repair injured, aged or diseased skeletal tissue. In Europe, about one million patients undergo a surgical bone reconstruction annually and this number is increasing, also due to our ageing population. Bone autograft is the safest and most effective grafting procedure, because it uses patient’s own autologous and thus ‘safe’ bone, and provides a natural substrate for new cells to grow into the graft, to be replaced by remodelling new bone. However, it adds another surgical ‘donor’ site (typically the iliac crest), with often additional morbidity including pain and infections and is particularly limited in volume (about 20 cm3). Allograft bone coming from tissue banks may transfer disease or lead to immunological rejection [1].
Because both autograft and allograft have drawbacks, scientists have long been searching for materials that could be used to replace the transplanted bone [2–4]. Although most synthetic bone substitutes available possess some of the beneficial properties of autograft, none yet has all the benefits of autologous bone. For instance, calcium phosphate (CaP) bioceramics do not possess sufficient osteoinductive properties to allow reconstruction of large bone defects [5]. In view of these limitations and due to the increasing number of bone grafting procedures, surgeons are looking for more advanced therapies [6–9]. The use of recombinant bone morphogenetic proteins (BMPs) has added another promising dimension to bone healing. Underpinned by solid scientific research, BMPs induce and promote critical steps of mostly endochondral bone formation. BMP devices have now been approved for clinical use in spinal fusion, non-unions and severely compromised long bone fractures. However, BMP technology has its limitations in particular when the microenvironment is compromised with poor or no vascularization [10, 11]. Therefore, current research focuses on tissue engineering incorporating also (stem) cell therapy into the strategy. Although significant basic research has been generated about this issue, the practical transfer of this research into the clinical field deserves major attention from the European and world health authorities and the general public.
In this review, we explore the present status of (stem) cell based therapies with specific focus on human bone regeneration, and subsequent progress towards bone tissue engineering. Present clinical targets under research in adults and children will be discussed. Finally, ethical issues and European regulations about the transfer of cell therapy research into patients with bone defects will be reviewed, with closing remarks about the present and future status of this remarkable resource.
Characterization of cells for bone regeneration in human beings
The novel field of regenerative medicine has recently expanded with the use of cells as effective therapeutic tools also for bone repair. These innovative medicinal products including mesenchymal stem cells (MSCs), require an adequate regulatory framework, with the dual aim to promote development of new therapies to significant health problems and to protect the safety of the individuals to whom these products are administered. Besides safety, attention must be given to several scientific and technical aspects to set up standards for the final quality, and efficacy of these novel products. In particular, the possibility to produce a high number of MSCs from a single donor [12] and their potential lower immunogenicity [13] make them particularly attractive as ‘off-the shelf’ products, to be used in an autologous or in an allogeneic setting. In this context, there is a need to guarantee the traceability of the product, from the donation of the starting material to the use of the final product perhaps in several different recipients.
For these reasons, the regulatory agencies in different countries have chosen to adapt two consolidated sets of already existing rules to cellular products: the rules governing the pharmaceutical production and the regulation on the donation, manipulation and distribution of cells, tissues and organs. Thus, a new regulatory category of ‘advanced therapy medicinal products’ (ATMP) was proposed, finally going into effect by the end of 2007. In Europe, MSCs or combination products are thus considered ATMP, as defined by the European Regulation (European Commission [EC]) No. 1394/2007. ATMP include: (i) gene therapy medicinal products as defined in part IV of Annex I to Directive 2001/83/EC; (ii) somatic cell therapy medicinal products as defined in Part IV to Directive 2001/83/EC and (iii) tissue-engineered products, intended as products that contain or consist of engineered cells or tissues, and presented as having properties for, or administered to human beings in order to regenerate, repair or replace human tissues. MSCs may fall under EC No. 1394/2007 as somatic cell therapy products or tissue-engineered products depending on the source, manufacturing process and proposed indication for use. Due to the wide use of osteofixation biomaterials used for fracture and bone tissue repair, often in long-term contact with living bone tissue and cells, one clear-cut and specific issue related to the use of MSCs for bone repair is the possibility to use them in combination with biomaterials, thus identifying them as a typical tissue-engineered product. On the other hand, the EU Regulation is also in compliance with the 2004/23/EC directive on donation, procurement and testing of human cells and tissues and with directive 2002/98/EC on human blood and blood components.
Besides the need to guarantee the safety of the donor, the traceability from the donor to the recipient and the quality of the production process at any step, the main objective of these regulations is to obtain products with standards of pre-defined quality (quality control), depending on the identity of the product (or in other words, demands for a certain quality standard in an intended use). Identity is generally defined as the collective aspects of those characteristics that make one specific cellular product recognizable. These characteristics can be defined by the production process and the immunophenotype of the final product. On this basis, Dominici et al. [14] proposed the minimal criteria to define a cell as an MSC. These parameters included the property to adhere to the plastic (that is still the most common aspect exploited for MSC production from different sources) and the classic membrane marker (CD) phenotype (absence of e.g. haematopoietic or immunogenic markers, such as CD45, CD34, CD14 or CD11b, CD79α or CD19 and HLA-DR – negative criteria – and presence of the typical MSC markers CD90, CD105, CD73 – positive criteria). In addition, stem cells need to be characterized by multipotency and stemness, i.e. they need to be able to differentiate along several different stem cell specific lineages, and at the same time, when based on asymmetric cellular divisions, they are able to retain their stemness. These aspects, even thought important to define the identity of MSCs, are at our present level of knowledge not sufficient to explain differences in behaviour, both in vitro and in vivo, of MSCs obtained from different sources or individuals and require further investigation. For practical reasons, the current identity definition of MSCs is mostly based on plastic adherence, on the immunophenotypic (CD markers) pattern and the differentiation potential in vitro. This definition is also used for the scope of this review.
The production of MSCs for clinical applications requires adhering to good manufacturing practices (GMP) to ensure the product safety and efficacy. This is a complex and often expensive process that starts from the qualification of the starting material, the definition of the culture process and of the quality controls to be applied as ‘in process’ controls and on the final product. Regarding the potential source of MSCs, it has been demonstrated that their precursors are typically associated with the blood vessels, and found in most of the human tissues [15], thus making it theoretically possible to obtain MSCs from an unlimited number of organs and tissues. In spite of this, only few tissues are currently considered as a source material for the clinical grade production of MSCs, due to their ease of collection, their wide availability and the safety of the donor. These widely used sources include bone marrow (BM), fat (adipose) tissue and cord blood. To be compliant to the GMP and the rules governing the safety of donation, the collection of the starting material should follow a donor-validation step and procedures addressed to guarantee the compliance to several pre-defined quality standards. This aspect is important because its consequences can dramatically interfere with the outcome of MSC production. As an example, in case of cord blood, it has been demonstrated that several parameters of the starting material, such as the time from collection to processing, the volume and the cell content, can affect the probability to obtain MSCs [16]. For BM, the age of the donor has been found to be inversely correlated to the yield of MSCs that can be obtained [17], whereas controversial evidence has been reported for adipose tissue derived MSCs with regard to the age of the donor [18, 19] and to the site and the procedure used for harvesting [20]. Because the standard culture media for obtaining MSCs have been historically represented by DMEM or α-Minimum Essential Medium (MEM) medium supplemented with foetal bovine serum, besides the many general aspects of MSC production, an important issue is represented by the possibility to use alternatives to animal products in the culture medium, such as human platelet lysate or plasma. In particular, platelet lysate contains a number of growth factors related to osteoblastic differentiation such as BMP2–4-6, transforming growth factor-β1, insulin-like growth factors, fibroblast growth factor-β, platelet-derived epidermal growth factor, platelet factor-4, interleukin-1 and osteonectin [21]. This could explain why the use of such animal-free culture systems could result in some limited induction of differentiation with up-regulation of several late osteoblastic genes such as alkaline phosphatase, bone sialoprotein and osteopontin when compared to MSCs grown and expanded in the presence of foetal calf serum based media [22].
The manufacturing of MSCs combined with biomaterials is generally achieved by expanding MSCs on plastic, seeding them on the selected scaffolds and culturing the seeded cells inside the scaffold, with the aim of obtaining a homogeneous cell distribution of viable cells that are metabolically active, and this aspect represents an important challenge for the GMP-compliant manufacturing process. It is therefore critical not only to understand and control parameters that influence the behaviour of the seeded cells, but also to set up validated methods for the quality control of these combination products. Several parameters that are usually part of the quality controls of cellular products should be adapted to the combination of cells with biomaterial and this issue poses important technical challenges. As an example, some currently used viability assays such as chromogenic methods [e.g. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT)] or other viable-dye based methods (e.g. neutral red staining), could lead to false positive results, due to the presence of a ‘background noise’ of the dye adsorbed by the biomaterial [23, 24]. For that reason, the validation of these assays should include the calculation of this background noise from a blank control that must be subtracted from the results obtained with the whole hybrid sample. Definition of lineage potency assays should be chosen with attention. In fact, the evaluation of the osteogenic differentiation inside the scaffold that can be made indirectly, by measuring osteo-specific secreted proteins, such as osteocalcin, or bone-specific m-RNA by PCR, is of great importance to understand and exploit the biomaterial-specific effect on osteogenesis [25, 26].
In conclusion, the characterization of cell populations including MSCs alone or in combination with biomaterials for bone repair is a new challenging field of regenerative medicine in the wider context of biotechnology and innovation, thus opening new perspectives to cellular therapy. A multidisciplinary approach is therefore needed to make cell-based bone regeneration available to patients.
State of the art in bone tissue engineering
Tissue engineering combines MSCs, synthetic scaffolds and molecular signals (growth or differentiating factors) in order to form hybrid constructs. In a classical approach, bone tissue engineering consists of harvesting BM from a patient, culturing those cells in vitro to a sufficient number (amplification) and then seeding onto a suitable scaffold prior to implantation into the same patient for differentiation and/or tissue regeneration [7, 8]. The sketch in Figure 1 illustrates the initial concept of bone tissue engineering.
Fig 1.
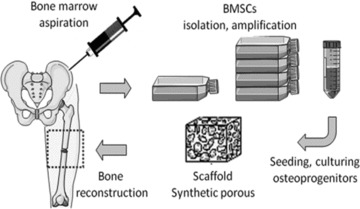
Principle of bone tissue engineering.
MSCs, originally also called BM stromal cells, were for the first time isolated from BM by Friedenstein et al. in 1976 and have been considered since then progenitor cells for skeletal tissues [27]. MSCs are clonogenic and multipotent cells that are capable of differentiating into several mesodermal cell lineages including osteoblasts, chondrocytes, adipocytes, tenocytes and myoblasts. From a small volume of BM, MSCs can be isolated and expanded into large numbers due to their high proliferative capacity. Although moving towards senescence, they maintain their functionality after culture expansion and cryopreservation [28]. When subcutaneously implanted in immunocompromised mice, MSCs cultured on a three-dimensional bioceramic scaffold can form bone tissue and haematopoiesis-supportive stroma (niche) [29]. BM is considered a readily available and abundant source of MSCs for bone tissue engineering applications.
Recent studies have shown that liposuction aspirates contain pluripotent adipose tissue-derived stem cells that can differentiate into various mesodermal cell types, including osteoblasts, myoblasts, chondroblasts and preadipocytes [30, 31]. Apart the easiness of harvesting these cells for clinical applications, there are limited data regarding the osteogenic potential of adipose tissue-derived stem cells in vivo.
Human MSCs from BM or adipose tissue have a great potential for bone regeneration. Other tissue sources such as periost have also been reported as containing progenitors for bone engineering [32, 33]. Significant growth opportunities exist for MSCs on synthetic or natural biomaterials as bone tissue-engineered substitutes.
Reconstruction of segmental large bone defect still represents a challenge in orthopaedic and oral oncology situations. CaP ceramics alone have failed to provide enough capacity for induction of new bone formation and/or as bone substitutes for bridging large or critical size bone defects. Hybrid materials made of autologous MSCs and synthetic bone substitutes have been scarcely used in clinical situations. The reasons of a limited clinical success may be related to several bottlenecks in the multidisciplinary field of bone tissue engineering:
Biomaterials used as bone void fillers are inspired by the bone extracellular matrix (hydroxyapatite [HA], collagen I) but they need to be actively or passively colonized by cells and vascularized in order to promote real bone tissue formation and healing. The regenerative capabilities of current biomaterials are still limited to small bone defects.
The autologous approach for isolation and osteogenic differentiation of MSCs is highly demanding in terms of logistics, production and safety of culture conditions leading to a costly therapeutic procedure.
The selection of a restricted and well-defined population of cells from different donors with age and genetic diversity remains a challenge for regenerative medicine at this early stage of research.
The association of biomaterials and osteoprogenitor cells raises technical challenges (i.e. cell sources, types, doses, timing) and regulatory issues (devices with medicinal drugs) for the implementation in clinical trials.
Bone formation requires different cell populations that cooperate to set up a complex 3D tissue under the guidance of biomechanical cues and vascularization plays a major role in such complete tissue healing. Bone integration and remodelling, critical for functional recovery, requires additional cell types, including osteoclasts.
Biomaterials for scaffolding MSCs
Depending on the clinical targets, different biomaterials such as CaP ceramics, functionalized hydrogels or advanced composites of CaP and bioresorbable polymers could be used to maintain cells and to allow regeneration of bone tissue.
Scientists have focused for many years in the development of materials mimicking the mature bone tissue. They have prepared porous materials that resemble both to their composition and 3D structure the trabecular bone. Macroporous CaP ceramics, particularly HA, β-tricalcium phosphate (β-TCP) or biphasic mixtures (BCP), have been widely used for scaffolding cells. Others have used collagen or polymeric biodegradable sponges. For minimal invasive surgery, injectable formulations of CaP particles suspended in hydrogels would be useful. Examples of such biomaterials are shown in Figure 2.
Fig 2.
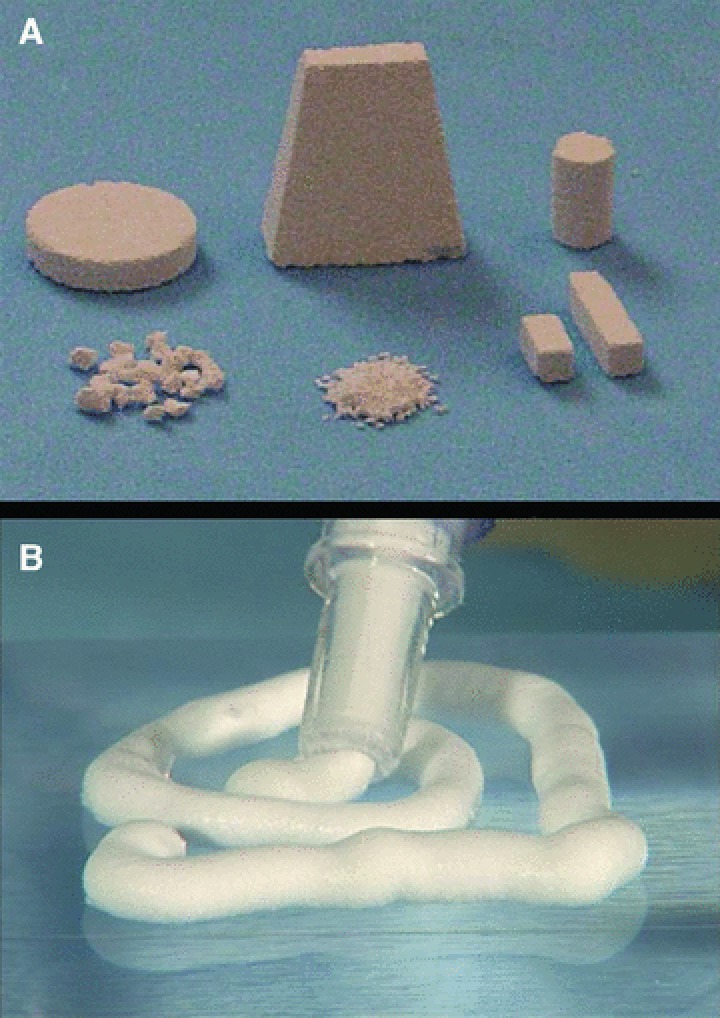
Examples of biomaterials for scaffolding of human MSCs. (A) Porous BCP ceramics and (B) injectable paste made of CaP particles suspended in hydrogel for minimal invasive surgery.
The adhesion, proliferation and osteogenic differentiation of MSCs on scaffolds are expected to be critical steps in the development of bone tissue engineering. The scaffold should support MSC adhesion with a relatively high efficiency. As shown in Figure 3, MSCs attached, proliferated and produced an abundant extracellular matrix on CaP ceramics. However, the cell/biomaterial volume ratio is highly important for osteoinduction in hybrid constructs. High porosity and permeability of scaffolds are extremely important for a uniform cell invasion, in vivo and in loco tissue ingrowth, vascularization and osteogenesis. Intergranular spaces between particulate scaffolds can provide an interconnected porosity for cell seeding and a high degree of freedom for tissue ingrowth. Intergranular spaces may be further increased by suspending CaP particles in hydrogels. Another advantage of hydrogels is that they allow cell injection to complex shaped bone defects or minimal invasive surgery without damaging cell viability.
Fig 3.
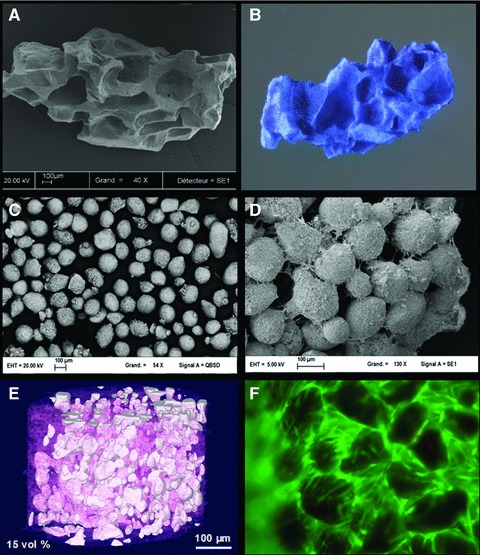
Examples of biomaterials for MSC scaffolds. (A) Porous BCP granules, (B) with human MSCs (methylene blue staining), (C) BCP particles of 100–200 μm, (D) 3D constructs made of particles, cells and extracellular matrix, (E) BCP particles suspended in polysaccharide/collagen hydrogel and (F) MSCs cultured in 3D hydrogels (live/dead staining).
Hydrogels are promising materials for tissue engineering as they contain a relatively low amount of dry mass (1–20%) causing little inflammation and foreign body reaction during degradation. They are appealing materials for tissue engineering because they can homogeneously suspend cells while allowing rapid diffusion of nutrients and metabolites [34]. Ideally, the hydrogel should form a temporary mechanical support for the cells and degradation should keep pace with new tissue formation and the reciprocal extracellular matrix production. Hydrogels based on extracellular matrix components (collagen, fibrin, polypeptides) provide an adhesive surface for the cells, but their composition, mechanical properties and degradation rates are difficult to control. Polysaccharides such as agarose, alginate, hyaluronic acid, chitosan and hydroxylpropylmethyl cellulose, are interesting candidates for cell encapsulation but their chemical modifications are difficult due to their insolubility in most of organic solvents.
Future trends in bone tissue engineering
For several years, the research has attempted to mimic both the composition and structure of trabecular bone. Considering the volume, scaffolds occupy a large part whereas only a layer of cells is present on the surface of biomaterial. This poor scaffold-to-cell volume ratio may be one of the reasons that only a limited amount of bone tissue is formed in vivo by such hybrid constructs. Indeed, mimicking ‘the end product’ and not the natural process leading to its formation or bone tissue healing may not be the most appropriate approach because nature proceeds in the reverse way, starting with cells which increasingly form matrix.
During osteogenesis, a relatively high cellular content prevails with numerous osteoprogenitors producing extracellular matrix whereas a limited number of cells can be found in readymade mature bone tissue. Furthermore, bone tissue is able to heal and is constantly remodelled by cellular activity originating from the periost and BM reservoir. In this process, multiple cell types cooperate to fabricate this complex 3D tissue whereas the extracellular matrix is degraded as part of the renewal (remodelling) process by multinucleated osteoclast cells. The balance between the osteoblast-mediated formation and osteoclast-mediated resorption is under biochemical and biomechanical control. Bone tissue is also a highly vascularized tissue with blood capillaries supplying oxygen and nutrients to the cells. It is therefore a complex 3D tissue containing different types and high numbers of cells at the early stage of its formation, but only a few cells in an abundant extracellular matrix at its mature state.
The research in tissue engineering has recently moved to the fabrication of artificial extracellular matrices resembling those encountered at the early stage of tissue development. In this respect, a developmental engineering approach has been proposed and described in detail [35, 36]. Recent experimental data have supported this new conceptual framework [37].
To support this new developmental engineering approach, new biologically relevant biomaterials, better responding to the requirements of the developmental processes and their modular design, need to be developed. The group of Stupp has designed biomimetic peptides that self-assemble into supramolecular structures under physiological conditions [38]. Hydrogels formed by crosslinking hydrophilic polymer chains have also been developed as three-dimensional cell and tissue culture environments. These hydrogels cross-link into 3D network in response to changes in temperature, pH, ionic environment or via chemical moieties, enzymatic reaction or UV light. The ability to control the synthesis of hydrogels is attractive for building complex 3D structures that mimic those of tissues. Ladet et al. have recently reported the design and fabrication of multilayered polysaccharide-based hydrogels with highly controlled physical properties [39]. Our group has developed injectable bone fillers using silanized-cellulose hydrogels combined with solid CaP particles that gel in situ at physiological pH [40]. These pH sensitive cross-linking hydrogels may be used as artificial extracellular matrix for culturing various cell types [34].
Bioceramics with special microstructure and intrinsic osteoinductivity may be able to trigger stem cells to form bone. Although basic research progress in these and other interesting future targets has been done, available clinical trials are still scarce but on the other hand, deeper understanding of the involved processes will facilitate the potential transfer of such technologies to clinical use.
Clinical targets for cell therapy in orthopaedics
The ability of bone to regenerate and to undergo repair may be compromised by the size and location of the bone defects, and by associated vascular or soft tissue injuries. When the in situ repair process is jeopardized and impaired, surgery may be required. Conventional reference treatment to augment bone healing is based on cancellous bone autograft application, or larger, even vascularized, segmental bone graft (frequently constructed out of the fibula) when the defect exceeds some centimetres. It may be necessary to associate such implantation on bone fixation by internal or external devices. Cell therapy is an alternative to bone graft, in which cells with osteogenic potential are transferred to the defect, alone or with a scaffold. A major advantage of such cell therapies is the preservation of the original bone stock and avoidance of pain in the autograft donor site, but other advantages include in theory unrestricted availability, higher cellular concentration, shorter surgical time and decreased associated morbidity. Various MSCs have osteogenic potential and, as mentioned, are present in BM and other tissues. They can be obtained by BM aspiration of the iliac crest. When there is a bone defect, a space-filling and mechanically supporting biocompatible regeneration scaffold will be necessary, the characteristics of which are discussed in the previous section.
The clinical indications for the cell or a hybrid cell and scaffold graft have to be precise and well defined. In this part, we discuss potential clinical indications for stem cell therapy, and the choice of type of cell therapy approach that may be appropriate for which clinical indication (Fig. 4).
Fig 4.
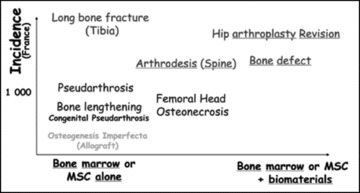
Relationship between bone diseases, cell therapy and biomaterials.
Current clinical problems and therapeutic approaches
Local bone defects, whatever the cause of the defects, are at this point the main indications for cell/combination therapy in adults. Osteoporosis, a systemic metabolic bone disease, may be a target in the future due to the risk for associated fractures and related local problems. The main orthopaedic problems in adults are non-union or delayed consolidation following fracture (as an example, Fig. 5), osteotomy, arthrodesis or limb lengthening; bone defects subsequent to trauma, infection, prosthesis loosening or tumour resection and finally, bone necrosis (particularly, avascular necrosis [AVN] of the femoral head, as shown in Fig. 6).
Fig 5.

Computerized (CT) reconstruction of a non-union in a tibial diaphysis fracture.
Fig 6.
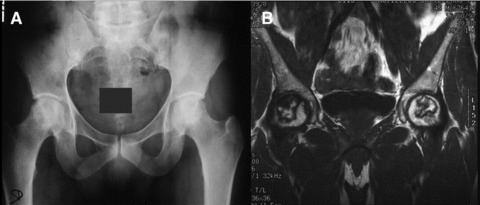
(A) Early AVN of the hip, radiological images; (B) Magnetic resonance imaging of hips in the frontal plane, the same patient as in (A) showing characteristic images of AVN of both femoral heads, Ficat and Arlet stage II.
Non-union or delayed consolidation is a potential complication of a long bone fracture, even if there is no bone loss. Apart from stability, that may require intra- or extramedullary fixation, autologous bone graft is the treatment of preference. For non-union after arthrodesis (mainly spine fusion) or lengthening, a new bone graft may be necessary. Alternative non-surgical treatments with pulsed electromagnetic fields or low-intensity ultrasound are currently in the clinical armamentarium.
Bone defects pose an important clinical problem. Surgeons attempt to fill bone gaps with autograft promoting the formation of new bone and allowing integration with the host bone at the margins (graft-host bone interface). Current treatment depends on the length or the volume of the defect and there are no clear treatment algorithms in effect. Autogenous bone grafting, harvested from iliac crest, is still considered the gold standard, especially in small defects (2–3 cm) where the success rate of bone grafting is high. For large bone defects, the success is limited also due to the amount of graft material that can be collected. For larger defects (3–10 cm), the Masquelet technique evolves as being quite useful; the defect is first filled with acrylic cement, which promotes the formation of a pseudo-membrane and, 6 weeks later, the cement is replaced with autologous bone graft in which situation the pseudo-membrane appears to increase the implantation yield, possibly also due to the local expression of cytokines and growth factors [41]. The Masquelet technique indirectly demonstrates the importance of the control of the microenvironment or implantation site for the successful outcome of bone grafting. For defects larger than 6–8 cm, particularly in long bones, vascularized bone grafts may be necessary. An alternative to those bone grafting techniques is the Ilizarov distraction technique, known as bone transport, where the bone defect is filled utilizing the displacement of a contiguous diaphyseal segment with the help of an external fixation device. This procedure takes several months, usage of an external fixator is necessary and often a bone graft is required at the end of procedure due to non-union at the junction with the mobilized diaphyseal segment. Large allogenic bone grafts may be used to fill large defects; however, they are only very partially recolonized by host cells and may be resorbed, as well as potential risk of pathogen transmission. Bone cement may be used as permanent filler but this non-biological solution does not regenerate bone.
In bone necrosis, a significant bone defect may not be the problem, because the bone scaffold is still present. Surgical conservative treatments to enhance bone repair have been proposed, from the least invasive core decompression to interventions with possible morbidity such as bone grafting, osteotomy or free vascularized bone graft.
Potential clinical applications of cell based therapies for bone repair
The use of stem cells, alone or associated with biomaterials, may be an alternative to the limited amount of bone autografts available and the morbidity induced at the harvest site. Autologous BM is rich in osteoprogenitors and growth factors, as well as cell populations such as stromal cells present in the mononuclear cellular fraction of the BM. BM-derived MSCs can be easily obtained by bone aspiration at the iliac crest, prepared and used in different ways (Fig. 7):
Fig 7.
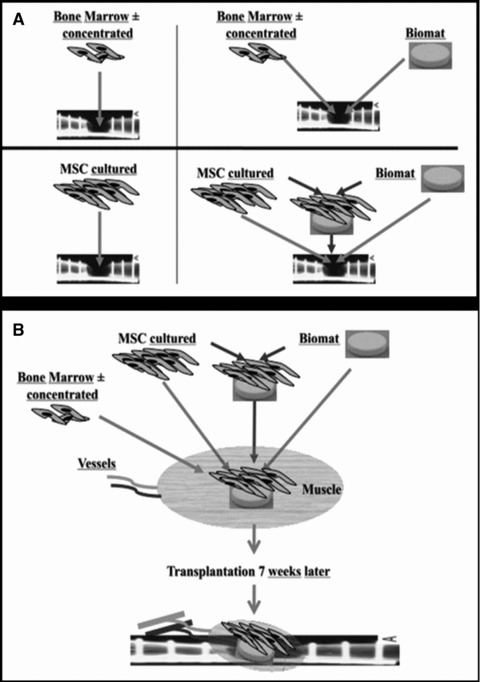
Different possibilities for cellular therapies for bone regeneration (A and B).
Mononuclear cells with MSCs may be used directly. The aspirated BM, without other manipulation, can be percutaneously injected at the affected site. To increase the number of mononuclear cells and thus of MSCs in the volume to be injected, it is possible to separate by centrifugation the mononuclear cells and concentrate them 8- to 10-fold. After aspiration with or without concentration, mononuclear cells can be mixed in the operating room with an osteoconductive scaffold and implanted at the defect site.
Mononuclear cells may be cultured in vitro to allow selection and expansion of an adherent fraction corresponding to MSCs. This increases the number of MSCs to millions of cells. MSCs can be percutaneously injected alone or extemporaneously mixed with scaffolds during surgery, so that this composite material is used in the same way as bone grafts. Prior to implantation, cultured MSCs may be expanded in vitro on scaffolds during several days/weeks, allowing for scaffold colonization and for cell differentiation, before grafting of this processed composite material at the affected site.
An alternative to the two previous ways is to implant the composite material (cell + scaffold) in a heterotopic site, e.g. in a richly vascularized muscle, to promote angiogenesis and blood vessel growth into the construct during some weeks and then to transfer it to the affected site with vascular anatomoses for the transferred muscle flap containing the implant.
Data reported in clinical studies
Clinical trials, clinical series and case reports have been published on the above listed possible uses of MSCs and are summarized in Tables 1, 2 and 3.
Table 1.
Clinical studies on non-union or delayed union treated by cell therapy
| References | Cases | Treatment | Outcome and conclusions |
|---|---|---|---|
| [43] | 20 cases, tibia non-union | Percutaneous injection of BM | Consolidation in 8/10 immobilized with cast and 10/10 with nail |
| [44] | 20 cases, long bone non-union | 2 percutaneous injection of BM with an interval of 3 weeks | Consolidation of 17/20 (85%) in 5 months |
| [45] | 60 tibia non-union | Injection (mean 20 cm3) of concentrated BM (5 to 6 times more nucleated cell in 50 ml). Injected | Bone union in 53/60 (88%) in 4 months. Positive correlation between bone union and concentration of MSCs |
| [46] | 2 groups of 25 patients, prospective study in lumbar spinal fusion | One group of Iliac crest autograft and one of type I collagen HA matrix soaked with BM aspirate | Equivalent fusion rate for post-erolateral fusion. Biomaterial group with no complications on donor site |
| [47] | 15 infected tibia non-union | Infection-free environment, allogenic cancellous bone graft ‘vitalized’ with autologous BM to perform fibula and tibia fusion | Infection control in 14/15 (93.3%). Consolidation in 11/15 (73.3%) |
| [48] | 33 patients HTO, prospective in 3 groups | A: lyophilized bone chips with platelet gel were implanted (11); B: lyophilized bone chips with platelet gel and BM stromal cells (12); C: lyophilized bone chips without gel (10),controls | Increased osteoblasts, bone apposition and osseointegration from 6 weeks in groups A and B. Adding platelet gel or platelet gel combined with BM stromal cells to lyophilized bone chips increases osteogenic potential |
| [49] | 41 patients, posterior instrumented spinal fusion | Concentrated BM combined with porous β-TCP | Good spinal fusion 95.1% after 34.5 months |
| [50] | Lengthening of 56 bones in 20 patients | Injection of ex vivo expanded autologous MSCs mixed with platelet-rich plasma (PRP) in 24 bones Controls in 32 bones | Significantly shorter consolidation period with MSC-PRP. Average index of healing 27.1 ± 6.89 days/cm. Control healing: 36.2 ± 10.4 days/cm |
| [52] | RCT 64 long bone closed simple fractures delayed union in 2 groups | One group of standard treatment. One group of injection, ex vivo cultured MSCs with medium for osteoblast differentiation | The group with injection showed at 2 months significant better callus formation |
| [53] | 1 case of tibia pseudoarthrosis resistant to six previous surgical procedures | Autologous BM stromal cells expanded to 5 × 10(6) cells after three weeks. Combined in surgery with calcium sulphate (CaSO4) in pellet form | Clinically and radiologically healed 2 months after implantation |
Table 2.
Clinical studies on bone defect treatment with cells and a scaffold
| References | Cases | Treatment | Outcome and conclusions |
|---|---|---|---|
| [54] | 90 patients with simple bone cysts BM (39, 2 year FU) Methylprednisolone acetate (38, 2 year FU) | BM or methylprednisolone acetate injection, at random. | 9/39 (23%) with BM healed 16/38 (42%) with methylprednisolone healed. Superior healing rate with esteroid injection. The cells of BM in a cavity without matrix may not induce healing. |
| [55] | 28 patients with simple bone cyst | Aspiration and percutaneous autogenous BM injection (single injection in 16, 2 or 3 injections in 12) | Healing in 23/28 cysts (82%), mean FU 34.7 ± 6.87 months. Autogenous BM injection, safe and effective treatment method for simple bone cysts, but sometimes repeated injections are necessary |
| [56] | 48 patients (39 with 19 months av. FU) with different bone defects | DBM used alone and with BM (DBM-BM) | 30/39 patients, osseous union (77%). Fracture non-union, most recalcitrant group (union achieved in only 61%). Comparable to iliac crest autograft |
| [57] | 23 calcaneal unicameral cysts in 20 patients (av. FU of 49.4 months) | Lyophilized irradiated chip allogeneic bone and autogenous BM (13 cysts in 11 patients). Percutaneous injection of irradiated allogeneic demineralized bone powder and autogenous BM (10 cysts in 9 patients). | Comparable results, advantage of safety for the percutaneous treatment. |
| [58] | 78 patients (79 hips) with acetabular defects at revision THR. 87% (69 hips), type III AAOS defects | Standard frozen non-irradiated bone bank allograft (group A). Freeze-dried irradiated bone allograft, vitalized with autologous marrow (group B). | Results on incorporation of the allograft were not different, with the advantage of microbiological safety for the irradiated allograft |
| [59] | 10 patients with volumetric bone defects (curettage of 7 benign tumours, 2 pseudoarthrosis, 1 aseptic loosening) | Concentrated BM in association with a collagen matrix | Bone healing in 7 of 10 patients |
| [60] | Bone defects 4–7 cm in 3 patients (tibia, humerus and ulna) | Osteoprogenitor cells from BM and expanded ex vivo. Placed during the operation on macroporous HA scaffold | Success confirmed at 6.5 years FU [61], but scaffold remained without resorption |
| [62] | One case of avulsed distal phalange of the thumb | Ex vivo expanded cells shed from periosteum, injected in a porous coral block inserted with no contact with bone | Good functional result Biopsy: lamellar bone and ossified endochondral tissue |
| [63] | 3 cases of defect after curettage for benign tumours | Composite of ex vivo expanded MSCs on scaffold during several days before operation | Satisfactory osseointegration at 29 months FU |
| [64] | 6 cases of mandible defect | Composite of ex vivo expanded MSCs from BM for 7 days on a bone substitute in osteogenic culture medium | Biopsies at 4 months: bone formation in 3 patients (in 2, unrelated to the tissue-engineered construct) |
| [65] | One patient with subtotal mandilectomy of 7 cm for tumour 8 years before | Titanium mesh cage filled with bone mineral blocks infiltrated with 7 mg rh BMP7 +20 ml autologous BM. Transplant implanted into latissimus dorsi 7 weeks. Transplanted as a free bone-muscle flap to repair the mandibular defect. | Heterotopic bone induction to form a mandibular replacement inside the latissimus dorsi muscle in a human being (patient = bioreactor). |
FU: follow-up; AAOS: American Academy of Orthopaedic Surgeons.
Table 3.
Clinical studies on AVN treated by cell therapy
| Reference | Cases | Treatment | Outcome and conclusions |
|---|---|---|---|
| [68] | 189 hips (116 patients) with AVN of femoral head | Concentrated BM after forage decompression | Higher number of progenitor cells transplanted in their hips had better outcome |
| [70] | 18 hips (13 patients) stage I/II femoral head AVN | Core decompression alone or core decompression with concentrated BM (randomized) | At 24 months, significantly better clinical and radiological with BM |
| [69] | 534 hips (342 patients) with AVN (stages I and II) | Core decompression and autologous BM grafting obtained from the iliac crest of patients | Severe collapse and total hip replacement in 94/534 at FU 8 to 18 years after treatment |
| [71] | 30 hips (22 patients) and 9 hips (8 patients) in 2 groups | Concentrated BM seeded into porous HA cylinder after core decompression (30 hips). HA cylinder without cells (9 hips). | At a mean 29 months FU, severe collapse in 3/22 patients with BM and in 6/8 patients without BM. |
FU: follow-up; AAOS: American Academy of Orthopaedic Surgeons.
Regarding therapies for bone healing problems with no significant bone defect (Table 1, reporting on non-union or delayed union in long bone fractures or osteotomies, lengthening or arthrodesis), Connolly et al. [42] demonstrated back in 1989 a positive correlation between the osteogenic capacity and BM cell concentration, and published in 1991 on 20 cases of tibial non-union treated by percutaneous injection of BM, with consolidation in 8/10 if immobilized with cast, and 10/10 with intramedullary nailing [43]. Garg et al. [44] reported on 20 cases of long bone non-unions treated with two percutaneous injections of BM with an interval of 3 weeks : 17/20 (85%) healed in 5 months. To improve the effectiveness of injections, Hernigou [45] used injections of concentrated BM which allowed an enrichment of about 5-fold of nucleated cells in the same 50 ml volume. With this technique, bone union was obtained in 53 of 60 tibia non-unions (88%) and a significantly positive correlation was concluded between bone union and the MSC concentration in the injected graft [45]. Before concentration, the aspirate contained an average of 612 ± 134 progenitors/cm3 but after concentration, the average was 2579 ± 1121/cm3. For the patients with bone union, more than 1500 progenitors/cm3 were injected, with an average total of 54,962 ± 17,431 progenitors. A mean of 20 cm3 was injected in this study.
Non-concentrated BM combined with a scaffold during surgery is probably a more frequently used technique in clinical practice than has been published. Neen et al. [46], in a prospective study, compared two groups of 25 patients for lumbar spinal fusion with either iliac crest autograft or type I collagen HA matrix soaked with BM aspirate. The fusion rate was equivalent for post-erolateral fusion, but the group with biomaterial had no donor site morbidity. Ateschrang et al. [47] reported on 15 infected tibia non-unions, after establishing an infection-free environment, using allogenic cancellous bone graft ‘vitalized’ through the injection of autologous BM. Infection control was obtained in 14/15 (93.3%) and consolidation in 11/15 (73.3%).
Concentrated BM may be combined with a scaffold during surgery. Dallari et al. [48], in a prospective study on high tibial osteotomy, compared three methods to fill the defect : (i) lyophilized bone chips with platelet gel; (ii) lyophilized bone chips with platelet gel and concentrated BM stromal cells and (iii) lyophilized bone chips alone as a control. Consolidation was best in group (ii) and in (i) better than in (iii). Gan et al. [49] used concentrated BM combined with porous β-TCP for posterior instrumented spinal fusion in 41 patients. After 34.5 months, 95.1% cases had good spinal fusion. This was as effective as autologous bone graft without the morbidity associated with the harvesting of graft from the iliac crest.
MSCs can be culture expanded ex vivo to allow injection of a greater number of cells than after concentration. A particular application is delayed union/consolidation on which different studies are available. Kitoh et al. [50] demonstrated, in a retrospective study of 56 bones in 20 patients on delayed complication after limb lengthening, that the group with injection of ex vivo expanded autologous MSCs (differentiated in osteoblasts) mixed with platelet-rich plasma had a significantly shorter consolidation period than the control (average index of healing 27.1 ± 6.89 days/cm versus control 36.2 ± 10.4 days/cm). The average number of transplanted cells was 3.2 ± 1.37 × 107. In 2009, Kitoh et al. [51] analysed the difference between the femur and the tibia, and the healing index was lower for the femur. Their results suggested that regionally varying bone-forming processes by cell transplantation might be related to local blood supply and soft tissue covering. Kim et al. [52] performed a prospective study on 64 cases of long bone with closed and simple fractures with delayed union randomized in two groups: standard treatment and injection of ex vivo cultured MSCs with a medium facilitating osteoblast differentiation. The group with injection showed at 2 months a significantly better callus formation. Bajada et al. [53] reported one case of tibia pseudoarthrosis treated with the same procedure but using expanded cells mixed with CaSO4 pellets.
The filling of a (larger) bone defect is a more challenging situation, as cell suspensions alone cannot be used and it is necessary to deliver them locally and have some containment on a scaffold. The available clinical studies are summarized in Table 2. Wright et al. [54] randomized 90 patients with simple bone cyst in two groups of treatment, comparing methylprednisolone acetate steroid injection and injection of non-concentrated BM alone. The steroid provided better healing rate (42%) than the BM (23%), suggesting that BM cells in a cavity without matrix cannot contribute significantly to healing. Zamzan et al. [55] obtained healing in 82% of 28 cysts treated by aspiration and one to three percutaneous injections of autogenous BM. Treatment of a bone defect by a composite implant of non-concentrated, non-expanded BM with scaffold or demineralized bone matrix (DBM) has also been reported. Tiedeman et al. [56] used BM and DBM to treat osseous defects in 39 patients with comparable results to iliac crest bone graft (61% to 77% success rate). Park et al. [57] compared the treatment of 23 unicameral bone cysts of the calcaneus associating autologous BM with an open chip allogenic bone graft deposit or with a closed percutaneous injection of demineralized bone powder. Results were similar with the advantage of the low morbidity associated with a percutaneous treatment. Ochs et al. [58] compared two matched cohorts of patients with deficient acetabular bone stock (type III according to the American Academy of Orthopaedic Surgeons classification) at revision hip replacement, treated by impaction bone grafting and a reinforcement ring. A control group with standard frozen non-irradiated bone bank allograft was compared to a group with freeze-dried irradiated bone allograft vitalized with BM aspirated from iliac crest or tibial metaphysis. The results on allograft incorporation were not different, but with the advantage of microbiological safety for the irradiated allograft. Jäger et al. [59] used concentrated BM in association with a collagen matrix in 10 patients (including filling after curettage of 7 benign tumours, 2 pseudoarthrosis and one aseptic loosening), and obtained bone healing in 7/10. Quarto et al. [60] first reported the use of osteoprogenitor cells isolated from BM, expanded ex vivo and placed on a macroporous HA scaffold during surgery to fill bone defects (4 to 7 cm) in 3 patients (tibia, humerus and ulna). The success of these three cases was confirmed with a follow-up of 6.5 years by Marcacci et al. [61], but the scaffold, essentially unresorbable, remained unchanged. The same year, Vacanti et al. [62] reported the replacement of an avulsed distal phalanx of the thumb by cells harvested from the periostum and expanded ex vivo, injected in a porous coral (porous HA) block inserted in a pocket beneath a flap at the extremity of the thumb, with no contact with bone tissue. The functional result was good and a biopsy revealed lamellar bone and ossified endochondral tissue.
The next step in therapeutic approach would be to expand MSCs on a scaffold ex vivo before surgery during several days/weeks, and then implant this composite in the defect. Morishita et al. [63] reported three cases of defects treated with this technique after curettage of benign tumours. The BM MSCs after 2 weeks proliferation were culture expanded on HA blocks or granules during 2 more weeks with osteoblastic differentiation medium, before implantation. At a minimum follow up of 29 months, the osseointegration was satisfactory without radiolucent zones. In maxillofacial surgery, Meijer et al. [64] reported six cases of a mandible defects filled using this principle and biopsied 4 months later. Biopsies showed bone formation in only three out of six patients, and in only one out of six patients bone formation was induced by the tissue-engineered construct. He concluded that it is important to differentiate between bone formation induced by the cells from the border of the osseous defect and by implanted cells.
Another approach does not use ex vivo expanded cells but the patient acts as his own bioreactor. Warnke et al. [65, 66] reported the case of a patient with subtotal mandibulectomy of 7 cm, due to a tumour treated 8 years before, by reconstruction with a bone muscle flap prefabricated in vivo. A titanium mesh, computer-designed to fit within the defect region, was loaded with HA blocks coated with rhBMP-7 and aspirated BM. This composite was implanted in his latissimus dorsi muscle to allow for heterotopic bone growth and vessels ingrowth from the muscle vessels. After 7 weeks, the composite was transplanted into the mandible defect and vascular pedicles were anastomosed onto the external carotid vessels. Bone density and mineralization improved with time and bone formation was detected in all parts of the replaced mandible. Unfortunately the initial good results were not durable with fracture and infection. The patient died from cardiac arrest 15 months after implantation.
Regarding femoral head avascular osteonecrosis, many techniques of core decompression with bone graft have been described for its treatment. Hernigou et al. [67] found a decrease in the number of MSCs in the upper end of the femur in patients with corticosteroid-induced osteonecrosis. Because osteonecrosis may thus be a ‘stromal disease’, the possibility of injecting/implanting MSCs or BM in the femoral head may be a potential treatment for this condition. Hernigou et al. [68], after decompression, used grafting with concentrated BM and reported results in 189 hips of 116 patients. Patients with higher numbers of progenitor cells transplanted in their hips had better outcome. In 2009, Hernigou et al. [69] reported satisfactory results on 534 hips with avascular osteonecrosis at early stages (stages I and II) treated by this technique, with only 94 total hip replacements at a follow up of 8–18 years. Gangji et al. [70] studied 18 hips (13 patients) with stage I or II osteonecrosis of the femoral head. Hips were randomized in two groups, core decompression alone or core decompression with implantation of concentrated BM. After 24 months, there were significantly better clinical and radiological results with the BM graft. Yamasaki et al. [71] studied 30 hips (22 patients) using concentrated BM seeded into a porous HA cylinder to fill the drilled hole after core decompression in osteonecrosis of femoral head. These were compared to a control group of nine hips (eight patients) with the HA cylinder without cells. At the mean follow-up of 31 months, a reduction in the osteonecrotic lesion was observed in the group with cells, where only three progressed to collapse. In the control, a majority of patients had a severe collapse of the femoral head (see Table 3).
The reported trials and studies have established the feasibility and reasonable safety of cell therapy approaches, and some measures of efficacy in obtaining bone healing. In all these cases, no autologous bone grafts were harvested. However, only small numbers of patients have so far been included in such studies. Larger trials should be implemented to better define/characterize the implant (e.g. the optimal cell numbers, parameters for the cell and scaffold combinations) and the long-term efficacy.
Selected paediatric bone disorders and cellular therapies
Two major groups of paediatric bone disorders have been focused in paediatric patients treated using cellular therapies. These are inborn errors of bone metabolism and degenerative bone disorders in childhood.
Inborn errors of bone metabolism and cellular therapy
Plasticity, mineralization and bone remodelling are particularly important during childhood. The critical role of the balance between bone formation and bone resorption becomes evident in a number of inborn errors of bone metabolism [72]. Osteopetrosis was one of the first paediatric bone disorders in which molecular analysis identified the heterogeneous genetic background of a single clinical disorder [73]. This helped significantly to identify patients who will benefit from BM transplantation (BMT) [74, 75]. Although the hallmark of osteopetrosis is excessive bone mass, osteogenesis imperfecta is characterized by greatly reduced bone formation and severe fragility of these bones [76]. Interestingly, treatment by BMT in this disease was attempted much later [77]. However, in this disease, which is mainly caused by a collagen type I synthesis defect, not only haematopoietic stem cells became of interest, but also stromal cell populations [78, 79]. In fact, the first systemic application of isolated human MSCs was performed in osteogenesis imperfecta. Although the growth rate significantly improved during the first months and years, it slowed down subsequently [80]. This correlated with in vitro findings suggesting that systemic osteopoiesis is more difficult to achieve by current BMT strategies than haematopoiesis [81]. These limitations become evident, when children undergo BMT for inborn errors of metabolism, which affect several tissues, e.g. mucopolysaccharidosis [82, 83]. If the toxicity of BMT can be decreased in non-malignant diseases, this technique becomes the most promising alternative for novel cell therapy approaches [84]. Novel cell types and targeted conditioning regimens of reduced intensity will allow for systemic cellular therapies of inborn errors of bone metabolism [85, 86].
Degenerative bone disorders in childhood
Osteonecrosis in paediatric patients is frequently caused by steroid-based therapies of haematological malignancies [87]. Furthermore, alterations of blood rheology, e.g. in sickle cell anaemia, render children susceptible to AVN of the bone [88]. Predominantly, femoral bones are affected with distal epiphyseal lesions being more frequent than proximal lesions. The incidence of AVN in children under the age of 10 years is below 0.2% after treatment in the acute lymphoblastic leukaemia – Berlin–Frankfurt–Münster (ALL-BFM) protocol 95 (trial ALL-BFM 95). However, 16% of the teenagers older than 15 years treated using this protocol are affected by AVN. Thus, AVN is a frequent complication of steroid-based therapies for haematological malignancies. Similarly, juvenile idiopathic arthritis or other autoimmune diseases in children may lead to AVN, when glucocorticosteroids are added to the treatment regimen [89]. Current treatment strategies aim primarily at elimination of pain, restoration of function and prevention of disease progression. Conservative measures are limited to immobilization and physiological rehabilitation, which is often complicated in children due to compliance problems [90]. Surgical measures include core decompression and ultimately prosthetic replacement. The outcome after core decompression is very variable and often dependent on risk factors, localization and surgical accessibility [91–93]. In order to improve the efficacy of core decompression, healthy BM or BM mononuclear cells from an aspirate at a distant site have been instilled in the interventions [68, 70, 94]. The most potent osteogenic cells in the BM known to date are MSCs [95–97]. Recent analyses of this population in the marginal zone of steroid-induced AVN showed a significant reduction in their numbers, viability and plasticity [67]. However, these cells are of critical importance for regenerating bony tissue via secretion of factors modulating the hypoxic and inflammatory environment as well as stimulating angiogenesis, which seems equally important for bone regeneration as the transdifferentiation of MSCs [98]. It is a matter of debate, whether MSCs may differentiate into tissues of interest, although this proof of principle has been verified in various animal model experiments [99–101]. In some studies, the beneficial effect of MSCs was rather attributed to the secretion of cytokines and growth factors at the site of injury [102, 103]. Obviously, for the use of MSCs in regenerative medicine a sustained engraftment is desirable. In fact, in the osteonecrosis model experiments advantage is taken of both mechanisms, secretion of tissue-repair modulating factors as well as cell engraftment with osteogenic differentiation.
Osteonecrotic lesions are bradytrophic areas with low concentrations of oxygen and nutrients. Interestingly, MSCs produce vascular endothelial growth factors and insulin-like growth factor binding proteins which are anti-apoptotic and involved in neovascularization and osteogenesis [104]. It is noteworthy that MSCs grow in culture media with low glucose content and thus are adapted to limited nutrient supply at the time when they are injected into the necrotic lesion.
Taken together, cellular therapy approaches are quite promising for several inherited as well as acquired bone diseases during childhood, because a durable cure of the musculoskeletal system for a lifetime is of crucial importance for development and quality of life.
Ethical aspects of EU clinical trials
Continuous development of the new fields in medicine led to a boost of studies involving both human samples and animal experiments used for clinical and preclinical trials. The European Union seeks to unify all the ethical and legal aspects regarding such concerns under a common framework, so that the design of the studies, procedures for their approval and other related issues would be similar throughout the Union. Current research activities in the field of human health raise various ethical concerns. Worldwide there is a continuing interest in ethical aspects, including research intervention and clinical trials conducted in human beings, the use of human adult/embryonic stem cell (ESC) and/or foetal cells and the experiments on non-human primates and other animals.
The main ethical issues related to the scientific research is the need to carry out all types of research activities using such cells, because there is few other feasible alternative and the need to evaluate the benefit/burden balance, including the scientific aspects and the social and cultural gains. Induced pluripotent stem cells and somatic cell nuclear transfer (therapeutic and reproductive cloning) are examples of innovations, which can perhaps, to an extent, solve some of the important immunological and ethical issues, but will also create new ones [105].
The development of scientific research based on experiments on human beings (the human model) raised a social problem – patients’ desire to have access to the latest discoveries in the medical field and researchers’ desire to discover new therapies, investigative methods and drugs. The risks of research are acceptable/accepted, as it is believed that the outcome contribute to the public good. The purpose of research is therefore the improvement of healthcare [106]. Whereas scientists claim academic freedom, self-direction and self-regulation, the public responses ranged between wonder and awe and fear and anger. As science is publicly funded and performed for the benefit of the society, it is expected to act responsibly in exchange of fulfilling its demands for resources and autonomy, and that the researchers will abide to the highest ethical standards [107].
The ethical framework presumes two essential elements: provision of information regarding the ethics of research on human beings and the fundamental principles (rules and ideas) that will influence the conduct of research and the establishment of procedures which have been designed to facilitate the application of the ethical principles. Research should respect human life and dignity, and the integrity of scientists is the basis of the privilege of research freedom granted by society to such undertakings [107].
A special ethical concern is raised by ESC research. Many authors argued that ESC research is opposed to human dignity, as it requires the destruction of human embryos, considered to be human beings at a very early stage of their development. Instead, it promotes research with adult stem cells, as this does not involve the destruction of human embryos, and that adult stem cells appear equally promising when compared to ESC in the context of specific clinical applications such as bone regeneration [108–111]. This position considers that moral status of an embryo is absolute at all stages of its development [109], and therefore embryos should be considered persons, involving the respect of their rights which are the same as those of all human beings. Another position deems the moral status to gradually increase, therefore ESC research needing careful consideration [112, 113]. Finally, some authors acknowledge no moral status of embryos, so the research that uses ESC would be moral for them, and even they claim not conducting ESC research would be immoral [114, 115]. Moreover, different countries hold different opinions on the subject, not always translated in their laws. In a global survey performed in 2006 involving 50 countries, 23 allow research on human embryos under strict conditions, out of which 16 have laws in force, 7 conduct ESC research by guidelines. Some countries, such as Austria, Ireland, Cyprus, Costa Rica and Italy explicitly prohibit ESC research. US law allows the procurement of human ESC lines and research on supernumerary embryos by guidelines. Japan, as well as Belgium, Singapore, South Korea, Sweden and UK have adopted national laws allowing embryo cloning for therapeutic or research purposes. The remaining countries have no explicit policy on the topic [111].
Nationally and internationally, there has been a proliferation of laws, regulations and guidelines, generated by different regulatory bodies, which has resulted in disharmony in national, regional and international recommendations for research. A multitude of different organizations provide multinational guidelines, standards, regulations and opinions including the World Health Organization, World Medical Association (WMA), EC, European Medicines Agency (EMEA), European Science Foundation with a recent Science Policy Briefing [116], International Conference on Harmonization (ICH), Council for International Organizations of Medical Sciences, United Nations Educational, Scientific and Cultural Organization, European Group on Ethics in Science and New Technologies (EGE), International Society for SC Research, Human Genome Organization, cleric organizations, etc.
Among these documents, only the most significant ones are mentioned in this review. The Code of Nuremberg [117], published in 1947 as a result of the medical experimentation during World War II, states the voluntary participation based on informed consent [118]. The Declaration of Helsinki [119] was developed by the WMA in 1964, in order to provide a set of principles to physicians and other participants in medical research involving human beings. The Declaration focused on the researcher’s responsibility to protect the individuals of research [118]. The Belmont Report, published in 1979, provides an ethical foundation on the protection of human beings and it became a symbol of a fight against racism and abuse of vulnerable individuals of medical research. Written by the National Commission for the Protection of Human Subjects of Biomedical and Behavioural Research, drafted first in the Belmont Conference Centre, the Report discusses six key ethical principles and their application in research: informed consent, beneficence, justice, fidelity, non-malfeasance and veracity [118]. In 1990, the ICH of Technical Requirements for Registration of Pharmaceuticals for Human Use was founded by the FDA (Food and Drug Administration), the EC (European Commission) and the MHLW (Japanese Ministry of Health, Labor and Social Affairs) in alliance with the pharmaceutical industry. As a result, the EMEA was established by the European Commission [110]. ICH deals with quality, safety, efficacy and multidisciplinary and other relevant aspects of performing clinical trials are approached. The council of Europe Convention on Human Rights and Biomedicine [120] signed on 4 April, 1997 in Oviedo that ‘an intervention in the health field may only be carried out after the person concerned has given free and informed consent to it’. The scientific research in the field of biology and medicine shall be carried out freely and by ensuring the protection of the individual. The Directive 2004/23/EC defines the standards of quality and safety for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells [121].
The fundamental ethical principles applicable to human stem cell research, stipulated in the Opinion #15 of EGE in Sciences and New Technologies regarding ‘Ethical aspects of human stem cell research and use’[122] involve the principles of respect for human dignity, individual autonomy, justice and beneficence (with regard to the improvement and protection of health), freedom of research and proportionality. It also recommends taking into account, based on a precautionary approach, the potential long-term consequences of stem cell research and use for individuals and the society. The basic ethical issues connected with clinical trials are: (1) the procedure for obtaining informed consent of the individuals of the clinical trial; (2) approval of the studies; (3) data protection, confidentiality and anonymization; (4) risk-benefit assessment; (5) protection of the health of persons involved in clinical trials and (6) transparency regarding research results.
Ethics related to information and consent
Persons who take part in the research are entitled to be informed about and to consent or not to clinical research [123]. EGE Opinion #15 [122] states the ethical aspects of clinical trials regarding the protection of the recipients of transplantation. It argues ‘the potential long-term consequences of stem cell research and use for individuals and the society’ must be taken into account. Information should be provided as clear and precise as possible by the doctor supervising the procurement: arrangements, in particular on the ‘free nature of the donation’, and its anonymity; possible tissue storage time and conditions; registration of data in databases, in conformity with requirements of private life protection and medical confidentiality; foreseeable use of the tissues (diagnostic, allograft or autograft, pharmaceutical products, research, cell lines production for various uses, etc.); the donor may at any time withdraw her/his consent, without any negative consequences for the person. There are two key issues that must be included in the informed consent forms: who benefits and what happens to data, samples and animals at the end. Only persons able to freely understand and question the protocols may/must provide consent. Vulnerable individual categories are considered to be pregnant women, human foetuses, neonates, children, prisoners, persons who are physically handicapped, mentally disabled, economically or educationally disadvantaged, racial minorities, the very sick and the institutionalized. These populations are generally excluded, but there are possibilities of including them in order to avoid loss of opportunity.
Informed consent is a requirement for ethical conduct of clinical trials, but it is not sufficient [118]. In order to obtain an informed consent, the investigators must determine the culture and literacy of the individuals. The notion of individuality is lacking in some cultures. The individuals must be adults who show literacy and responsibility. Written documents are not always provided. The World Health Organization provides templates for different informed consent forms. All of these consist of two main sections: an information sheet, and a certificate of consent (or certificate of assent for children).
Approval of the studies
All clinical trial must be approved by the Research Ethics Committee. In this respect the requirement of scientific journals to mention the ethical approval and the name of the approving committee has improved the situation although in one recent study 31% of manuscripts published in high impact journals lacked the mention of the ethical approval [124]. More demanding studies, such as multicenter, multinational or ethically complicated studies, may require approval by national competent authority. Similarly, for the commercially orientated parties and sponsors, the requirement of FDA- and EMEA-like agencies for ethical approval before registration of new drugs and ATMPs provides a good incentive.
Ethics related to privacy/data protection
The Charter of Fundamental Rights refers to protection of personal data and non-discrimination, which also included genetic status. EGE Opinion #11, point 2.4, states: ‘In order to reconcile the traceability requirement and the need to protect the donor’s rights (medical confidentiality and privacy), tissue banks must take the necessary steps to protect confidentiality of the data by developing appropriate coding systems’[125].
EGE Opinion #13 considers that personal health data form part of the personality of the individual, and must not be treated as mere objects of commercial transaction [126]. The human right to respect for private life requires that confidentiality of personal health data is guaranteed at all times and that informed consent of the individual is required for the collection and release of such data. The principles of self-determination, legitimate purpose, security and the right to participate in the medical decision-making process are addressed. At the time, EGE considered a directive on medical data protection was needed within the existing Data Protection Directive to address the issues related to use of medical data through information and communications technologies means [126]. This led to the establishment of Directive 2002/58/EC of the European Parliament and of the Council of 12 July 2002 concerning the processing of personal data and the protection of privacy in the electronic communications sector (directive on privacy and electronic communications). Personal data, such as health information, criminal justice, financial, genetic and location information must be protected.
The investigator’s challenge is to process (obtain, hold, disclose) data while protecting identity. This is achieved by fair and lawful processing, and only for limited purposes. The processing must be adequate, relevant and not excessive. The data must be recorded accurately and securely, not kept longer than necessary and processed in accordance with the individual’s rights. It is not to be transferred to countries without adequate data protection.
Ethics related to the risk-benefit assessment
This is critical in stem cell research, as in any research, but is more difficult as the uncertainties are considerable given the gaps in our knowledge. Attempts to minimize the risks and increase the benefits have been made, and strategies for safety have been elaborated. These have utmost importance in the transplantation of genetically modified cells and when stem cells are derived from somatic cells (the risks that transplanted stem cells cause abnormalities or induce tumours or cancer). The potential benefits for the patients should be taken into account, but not exaggerated. The risk-benefit assessment for each individual is an independent ethical requirement for a trial to be acceptable [127]. Clinical research represents a context with distinctive risk and benefit characteristics, which requires application of patient standard rather than professional standard regarding the uncertainty of the risks and the limited benefits to the patient [123].
Ethics related to protection of the health of persons involved in clinical trials
This must be made in order to minimize the possibility that irreversible and potentially harmful changes are introduced in clinical applications of stem cell research. Techniques enhancing the possibilities of reversibility should be used whenever possible. Usually some groups, such as prisoners, mentally impaired persons, severely injured patients and very young children are excluded from clinical trials. ‘The general social goal should be to ensure that all decisions about biomedical research are consistent with standards for research integrity and public health protection’[128].
Ethics related to transparency regarding research results
EGE recommended in the context of funding stem cell research within the EU Framework Programs for Research that ‘the EU should insist that the results of such research be widely disseminated and not hidden for reasons of commercial interest’. There is also a need to avoid unnecessary replication of research. When researchers conceal the presence of selected trials, these studies cannot influence the thinking of patients, clinicians, other researchers and experts who write practice guidelines. Pharmaceutical companies will have to reveal some information, as descriptions of studies on drugs [128]. If every trial’s existence is part of the public record the stakeholders in clinical research can explore the full range of clinical evidence. The clinical trial registries were introduced to address this issue. Research sponsors have the opportunity to show the public they carefully consider the ethical issues related to research. They may submit information on clinical trials they initiate and report the outcome in the registries [128, 129].
Future directions and remarks
There is a high interest for biomedical research on innovative (stem) cell therapeutic approaches. New cell based technologies and stem cell research arouse fears and worries in many people including the issues of embryo research and cell nuclear replacement. The development of a policy for research and therapy in this field within the EU should be broadly permissive, but with rigorous ethical and legal oversight, enabling the policy to evolve in a rational way and with public support.
Among the potential cell therapy approaches that can promote bone healing in a clinical scenario, the easiest and current technique is the use of aspirated BM. Hernigou et al. [45], using BM concentrates, demonstrated positive correlation between successful bone union and the number of the injected progenitor cells. The concentration of BM aspirates is a rather simple procedure, but to assess its efficacy, it is necessary to know the quantity of injected mononuclear cells and of the MSCs. Even after concentration, we need more information on the purity i.e. not only nucleated cells are present, but also other cell types, platelets and an array of secreted factors are also included and may have a role in the effectiveness of the graft. It is a simple procedure, during surgery, to co-implant native BM aspirates with a scaffold, which are particularly needed in bone defect surgery. However, it is not presently possible to ascertain the optimum number of cells and the volume to be used, and how many and which cells are really sufficient and required for an improved clinical outcome.
The culture expansion of cells ex vivo is at first a more complex technique, but easier for subsequent characterization and quantification, and the number of cells obtained for transplantation is more than a 1000 times greater than in concentrates. Correlation with clinical outcome therefore should be more achievable. The exact quantification also allow for an improved seeding on a wider scaffold surface. The currently available data favour the opinion that to make this all more predictable in clinical outcome, expanded, well-characterized MSC populations and robustly manufactured combination products are the best long-term solution. However, this requires some serious additional research and preclinical work, and the implementation of these processes along GMP guidelines.
In addition, quite some steps have to be taken with regard to the clinical studies and their outcome. For bone healing studies more in particular, the evolution of the graft is often controlled only by imaging, because biopsies may be hazardous on a healed bone union. There is little histological information on the osseointegration. Probably, the main part of the graft undergoes necrosis, similarly to the autologous cancellous bone graft. The development of new imaging modalities will certainly be instrumental to better understand the processes. To improve the vascularization of the forming bone, the currently available options include the use of vascularized bone grafts (fibula) and the two steps procedure as proposed by Warnke et al. [66], technically demanding and not possible in all localizations. In view of all this, adaptive clinical trial design and evaluation with combined outcome are aspects that will need to be integrated with these new cell based therapeutics. Identifying the proper indication, patients at risk and responders to treatment are part of the clinical challenges.
In future, using more advanced developmental engineering approaches will lead to the manufacturing of robust tissue intermediates enhancing many critical aspects of bone healing including vascularization, tissue integration and remodelling. Finally, because MSCs appear to have some immune modulatory/suppressive effects, an allogenic approach may become feasible. The use of progenitor cell population must always take safety into account including caution when this therapeutic approach is used in bone defects after tumour surgery.
Acknowledgments
The authors are grateful to the European Science Foundation for the Research Network Program (RNP) funding the Regenerative Medicine Network (REMEDIC), which took the initiative to this review. The European Union support by means of the 7th Frame Program is appreciated with the funding of the REBORNE project (‘Regenerating Bone defects using New biomedical Engineering approaches’, EC-GA n°241879, HEALTH-2009–1.4.2); the Danish Council for Strategic Research of the ‘Individualized musculoskeletal regeneration and reconstruction’ and EU MATERA projects/TEKES for stem cell research and new generation titania.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Burchardt H. Biology of bone transplantation. Orthop Clin North Am. 1987;18:187–96. [PubMed] [Google Scholar]
- 2.De Groot K. Bioceramics of calcium phosphate. ca Raton FL: CRC Press; 1983. Ceramics of calcium phosphates: preparation and properties. [Google Scholar]
- 3.Daculsi G. Biphasic calcium phosphate concept applied to artificial bone, implant coating and injectable bone substitute. Biomaterials. 1998;19:1473–8. doi: 10.1016/s0142-9612(98)00061-1. [DOI] [PubMed] [Google Scholar]
- 4.Laurencin C, Khan Y, El-Amin SF. Bone graft substitutes. Expert Rev Med Devices. 2006;3:49–57. doi: 10.1586/17434440.3.1.49. [DOI] [PubMed] [Google Scholar]
- 5.Fellah BH, Gauthier O, Weiss P, et al. Osteogenicity of biphasic calcium phosphate ceramics and bone autograft in a goat model. Biomaterials. 2008;29:1177–88. doi: 10.1016/j.biomaterials.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 6.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 7.Petite H, Viateau V, Bensaid W, et al. Tissue-engineered bone regeneration. Nat Biotechnol. 2000;18:959–63. doi: 10.1038/79449. [DOI] [PubMed] [Google Scholar]
- 8.Derubeis AR, Cancedda R. Bone marrow stromal cells (BMSCs) in bone engineering: limitations and recent advances. Ann Biomed Eng. 2004;32:160–5. doi: 10.1023/b:abme.0000007800.89194.95. [DOI] [PubMed] [Google Scholar]
- 9.Khan Y, Yaszemski MJ, Mikos AG, et al. Tissue engineering of bone: material and matrix considerations. J Bone Joint Surg Am. 2008;90:36–42. doi: 10.2106/JBJS.G.01260. [DOI] [PubMed] [Google Scholar]
- 10.Giannoudis P, Einhorn T. Bone morphogenetic proteins – applications in orthopaedic and trauma surgery. London: Elsevier; 2010. [Google Scholar]
- 11.Garrison KR, Shemilt I, Donell S, et al. Bone morphogenetic protein (BMP) for fracture healing in adults. Cochrane Database Syst Rev. 2010;6:CD006950. doi: 10.1002/14651858.CD006950.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schallmoser K, Rohde E, Reinisch A, et al. Rapid large-scale expansion of functional mesenchymal stem cells from unmanipulated bone marrow without animal serum. Tissue Eng Part C Methods. 2008;14:185–96. doi: 10.1089/ten.tec.2008.0060. [DOI] [PubMed] [Google Scholar]
- 13.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–43. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 14.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 15.Crisan M, Chen CW, Corselli M, et al. Perivascular multipotent progenitor cells in human organs. Ann N Y Acad Sci. 2009;1176:118–23. doi: 10.1111/j.1749-6632.2009.04967.x. [DOI] [PubMed] [Google Scholar]
- 16.Bieback K, Kern S, Kluter H, et al. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004;22:625–34. doi: 10.1634/stemcells.22-4-625. [DOI] [PubMed] [Google Scholar]
- 17.Xin Y, Wang YM, Zhang H, et al. Aging adversely impacts biological properties of human bone marrow-derived mesenchymal stem cells: implications for tissue engineering heart valve construction. Artif Organs. 2010;34:215–22. doi: 10.1111/j.1525-1594.2009.00824.x. [DOI] [PubMed] [Google Scholar]
- 18.Shi YY, Nacamuli RP, Salim A, et al. The osteogenic potential of adipose-derived mesenchymal cells is maintained with aging. Plast Reconstr Surg. 2005;116:1686–96. doi: 10.1097/01.prs.0000185606.03222.a9. [DOI] [PubMed] [Google Scholar]
- 19.van Harmelen V, Skurk T, Rohrig K, et al. Effect of BMI and age on adipose tissue cellularity and differentiation capacity in women. Int J Obes Relat Metab Disord. 2003;27:889–95. doi: 10.1038/sj.ijo.0802314. [DOI] [PubMed] [Google Scholar]
- 20.Jurgens WJ, Oedayrajsingh-Varma MJ, Helder MN, et al. Effect of tissue-harvesting site on yield of stem cells derived from adipose tissue: implications for cell-based therapies. Cell Tissue Res. 2008;332:415–26. doi: 10.1007/s00441-007-0555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marx RE, Carlson ER, Eichstaedt RM, et al. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–46. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 22.Chevallier N, Anagnostou F, Zilber S, et al. Osteoblastic differentiation of human mesenchymal stem cells with platelet lysate. Biomaterials. 2010;31:270–8. doi: 10.1016/j.biomaterials.2009.09.043. [DOI] [PubMed] [Google Scholar]
- 23.Anselme K, Broux O, Noel B, et al. In vitro control of human bone marrow stromal cells for bone tissue engineering. Tissue Eng. 2002;8:941–53. doi: 10.1089/107632702320934047. [DOI] [PubMed] [Google Scholar]
- 24.Materna T, Rolf HJ, Napp J, et al. In vitro characterization of three-dimensional scaffolds seeded with human bone marrow stromal cells for tissue engineered growth of bone: mission impossible? A methodological approach. Clin Oral Implants Res. 2008;19:379–86. doi: 10.1111/j.1600-0501.2007.01483.x. [DOI] [PubMed] [Google Scholar]
- 25.Giannoni P, Muraglia A, Giordano C, et al. Osteogenic differentiation of human mesenchymal stromal cells on surface-modified titanium alloys for orthopedic and dental implants. Int J Artif Organs. 2009;32:811–20. doi: 10.1177/039139880903201107. [DOI] [PubMed] [Google Scholar]
- 26.Mastrogiacomo M, Scaglione S, Martinetti R, et al. Role of scaffold internal structure on in vivo bone formation in macroporous calcium phosphate bioceramics. Biomaterials. 2006;27:3230–7. doi: 10.1016/j.biomaterials.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 27.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–50. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 28.Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64:278–94. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 29.Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–36. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 30.de Girolamo L, Sartori MF, Albisetti W, et al. Osteogenic differentiation of human adipose-derived stem cells: comparison of two different inductive media. J Tissue Eng Regen Med. 2007;1:154–7. doi: 10.1002/term.12. [DOI] [PubMed] [Google Scholar]
- 31.Lee JH, Rhie JW, Oh DY, et al. Osteogenic differentiation of human adipose tissue-derived stromal cells (hASCs) in a porous three-dimensional scaffold. Biochem Biophys Res Commun. 2008;370:456–60. doi: 10.1016/j.bbrc.2008.03.123. [DOI] [PubMed] [Google Scholar]
- 32.De Bari C, Dell’Accio F, Vanlauwe J, et al. Mesenchymal multipotency of adult human periosteal cells demonstrated by single-cell lineage analysis. Arthritis Rheum. 2006;54:1209–21. doi: 10.1002/art.21753. [DOI] [PubMed] [Google Scholar]
- 33.Eyckmans J, Luyten FP. Species specificity of ectopic bone formation using periosteum-derived mesenchymal progenitor cells. Tissue Eng. 2006;12:2203–13. doi: 10.1089/ten.2006.12.2203. [DOI] [PubMed] [Google Scholar]
- 34.Vinatier C, Guicheux J, Daculsi G, et al. Cartilage and bone tissue engineering using hydrogels. Biomed Mater Eng. 2006;16:S107–13. [PubMed] [Google Scholar]
- 35.Lenas P, Moos M, Luyten FP. Developmental engineering: a new paradigm for the design and manufacturing of cell-based products. Part I: from three-dimensional cell growth to biomimetics of in vivo development. Tissue Eng B Rev. 2009;15:381–94. doi: 10.1089/ten.TEB.2008.0575. [DOI] [PubMed] [Google Scholar]
- 36.Lenas P, Moos M, Luyten FP. Developmental engineering: a new paradigm for the design and manufacturing of cell-based products. Part II: from genes to networks: tissue engineering from the viewpoint of systems biology and network science. Tissue Eng B Rev. 2009;15:395–422. doi: 10.1089/ten.TEB.2009.0461. [DOI] [PubMed] [Google Scholar]
- 37.Scotti C, Tonnarelli B, Papadimitropoulos A, et al. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci USA. 2010;107:7251–6. doi: 10.1073/pnas.1000302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang S, Greenfield MA, Mata A, et al. A self-assembly pathway to aligned monodomain gels. Nat Mater. 2010;9:594–601. doi: 10.1038/nmat2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ladet S, David L, Domard A. Multi-membrane hydrogels. Nature. 2008;452:76–9. doi: 10.1038/nature06619. [DOI] [PubMed] [Google Scholar]
- 40.Fellah BH, Weiss P, Gauthier O, et al. Bone repair using a new injectable self-crosslinkable bone substitute. J Orthop Res. 2006;24:628–35. doi: 10.1002/jor.20125. [DOI] [PubMed] [Google Scholar]
- 41.Masquelet AC, Begue T. The concept of induced membrane for reconstruction of long bone defects. Orthop Clin North Am. 2010;41:27–37. doi: 10.1016/j.ocl.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 42.Connolly JF, Guse R, Tiedeman J, et al. Autologous marrow injection for delayed unions of the tibia: a preliminary report. J Orthop Trauma. 1989;3:276–82. doi: 10.1097/00005131-198912000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Connolly JF, Guse R, Tiedeman J, et al. Autologous marrow injection as a substitute for operative grafting of tibial nonunions. Clin Orthop Relat Res. 1991:259–70. [PubMed] [Google Scholar]
- 44.Garg NK, Gaur S, Sharma S. Percutaneous autogenous bone marrow grafting in 20 cases of ununited fracture. Acta Orthop Scand. 1993;64:671–2. doi: 10.3109/17453679308994595. [DOI] [PubMed] [Google Scholar]
- 45.Hernigou P, Poignard A, Beaujean F, et al. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87:1430–7. doi: 10.2106/JBJS.D.02215. [DOI] [PubMed] [Google Scholar]
- 46.Neen D, Noyes D, Shaw M, et al. Healos and bone marrow aspirate used for lumbar spine fusion: a case controlled study comparing healos with autograft. Spine. 2006;31:E636–40. doi: 10.1097/01.brs.0000232028.97590.12. [DOI] [PubMed] [Google Scholar]
- 47.Ateschrang A, Ochs BG, Ludemann M, et al. Fibula and tibia fusion with cancellous allograft vitalised with autologous bone marrow: first results for infected tibial non-union. Arch Orthop Trauma Surg. 2009;129:97–104. doi: 10.1007/s00402-008-0699-2. [DOI] [PubMed] [Google Scholar]
- 48.Dallari D, Savarino L, Stagni C, et al. Enhanced tibial osteotomy healing with use of bone grafts supplemented with platelet gel or platelet gel and bone marrow stromal cells. J Bone Joint Surg Am. 2007;89:2413–20. doi: 10.2106/JBJS.F.01026. [DOI] [PubMed] [Google Scholar]
- 49.Gan Y, Dai K, Zhang P, et al. The clinical use of enriched bone marrow stem cells combined with porous beta-tricalcium phosphate in posterior spinal fusion. Biomaterials. 2008;29:3973–82. doi: 10.1016/j.biomaterials.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 50.Kitoh H, Kitakoji T, Tsuchiya H, et al. Distraction osteogenesis of the lower extremity in patients with achondroplasia/hypochondroplasia treated with transplantation of culture-expanded bone marrow cells and platelet-rich plasma. J Pediatr Orthop. 2007;27:629–34. doi: 10.1097/BPO.0b013e318093f523. [DOI] [PubMed] [Google Scholar]
- 51.Kitoh H, Kawasumi M, Kaneko H, et al. Differential effects of culture-expanded bone marrow cells on the regeneration of bone between the femoral and the tibial lengthenings. J Pediatr Orthop. 2009;29:643–9. doi: 10.1097/BPO.0b013e3181b2afb2. [DOI] [PubMed] [Google Scholar]
- 52.Kim SJ, Shin YW, Yang KH, et al. A multi-center, randomized, clinical study to compare the effect and safety of autologous cultured osteoblast(Ossron) injection to treat fractures. BMC Musculoskelet Disord. 2009;10:20–9. doi: 10.1186/1471-2474-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bajada S, Harrison PE, Ashton BA, et al. Successful treatment of refractory tibial nonunion using calcium sulphate and bone marrow stromal cell implantation. J Bone Joint Surg Br. 2007;89:1382–6. doi: 10.1302/0301-620X.89B10.19103. [DOI] [PubMed] [Google Scholar]
- 54.Wright JG, Yandow S, Donaldson S, et al. A randomized clinical trial comparing intralesional bone marrow and steroid injections for simple bone cysts. J Bone Joint Surg Am. 2008;90:722–30. doi: 10.2106/JBJS.G.00620. [DOI] [PubMed] [Google Scholar]
- 55.Zamzam MM, Abak AA, Bakarman KA, et al. Efficacy of aspiration and autogenous bone marrow injection in the treatment of simple bone cysts. Int Orthop. 2009;33:1353–8. doi: 10.1007/s00264-008-0619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tiedeman JJ, Garvin KL, Kile TA, et al. The role of a composite, demineralized bone matrix and bone marrow in the treatment of osseous defects. Orthopedics. 1995;18:1153–8. doi: 10.3928/0147-7447-19951201-05. [DOI] [PubMed] [Google Scholar]
- 57.Park IH, Micic ID, Jeon IH. A study of 23 unicameral bone cysts of the calcaneus: open chip allogeneic bone graft versus percutaneous injection of bone powder with autogenous bone marrow. Foot Ankle Int. 2008;29:164–70. doi: 10.3113/FAI.2008.0164. [DOI] [PubMed] [Google Scholar]
- 58.Ochs BG, Schmid U, Rieth J, et al. Acetabular bone reconstruction in revision arthroplasty: a comparison of freeze-dried, irradiated and chemically-treated allograft vitalised with autologous marrow versus frozen non-irradiated allograft. J Bone Joint Surg Br. 2008;90:1164–71. doi: 10.1302/0301-620X.90B9.20425. [DOI] [PubMed] [Google Scholar]
- 59.Jäger M, Jelinek EM, Wess KM, et al. Bone marrow concentrate: a novel strategy for bone defect treatment. Curr Stem Cell Res Ther. 2009;4:34–43. doi: 10.2174/157488809787169039. [DOI] [PubMed] [Google Scholar]
- 60.Quarto R, Mastrogiacomo M, Cancedda R, et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344:385–6. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 61.Marcacci M, Kon E, Moukhachev V, et al. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng. 2007;13:947–55. doi: 10.1089/ten.2006.0271. [DOI] [PubMed] [Google Scholar]
- 62.Vacanti CA, Bonassar LJ, Vacanti MP, et al. Replacement of an avulsed phalanx with tissue-engineered bone. N Engl J Med. 2001;344:1511–4. doi: 10.1056/NEJM200105173442004. [DOI] [PubMed] [Google Scholar]
- 63.Morishita T, Honoki K, Ohgushi H, et al. Tissue engineering approach to the treatment of bone tumors: three cases of cultured bone grafts derived from patients’ mesenchymal stem cells. Artif Organs. 2006;30:115–8. doi: 10.1111/j.1525-1594.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- 64.Meijer GJ, de Bruijn JD, Koole R, et al. Cell based bone tissue engineering in jaw defects. Biomaterials. 2008;29:3053–61. doi: 10.1016/j.biomaterials.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 65.Warnke PH, Springer IN, Wiltfang J, et al. Growth and transplantation of a custom vascularised bone graft in a man. Lancet. 2004;364:766–70. doi: 10.1016/S0140-6736(04)16935-3. [DOI] [PubMed] [Google Scholar]
- 66.Warnke PH, Wiltfang J, Springer I, et al. Man as living bioreactor: fate of an exogenously prepared customized tissue-engineered mandible. Biomaterials. 2006;27:3163–7. doi: 10.1016/j.biomaterials.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 67.Hernigou P, Beaujean F, Lambotte JC. Decrease in the mesenchymal stem-cell pool in the proximal femur in corticosteroid-induced osteonecrosis. JBone Joint SurgBr. 1999;81:349–55. doi: 10.1302/0301-620x.81b2.8818. [DOI] [PubMed] [Google Scholar]
- 68.Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. ClinOrthopRelat Res. 2002:14–23. doi: 10.1097/00003086-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 69.Hernigou P, Poignard A, Zilber S, et al. Cell therapy of hip osteonecrosis with autologous bone marrow grafting. Indian J Orthop. 2009;43:40–5. doi: 10.4103/0019-5413.45322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gangji V, Hauzeur JP, Matos C, et al. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. A pilot study. J Bone Joint Surg Am. 2004;86-A:1153–60. doi: 10.2106/00004623-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 71.Yamasaki T, Yasunaga Y, Ishikawa M, et al. Bone-marrow-derived mononuclear cells with a porous hydroxyapatite scaffold for the treatment of osteonecrosis of the femoral head: a preliminary study. J Bone Joint Surg Br. 2010;92:337–41. doi: 10.1302/0301-620X.92B3.22483. [DOI] [PubMed] [Google Scholar]
- 72.Cohen MM., Jr The new bone biology: pathologic, molecular, and clinical correlates. Am J Med Genet A. 2006;140:2646–706. doi: 10.1002/ajmg.a.31368. [DOI] [PubMed] [Google Scholar]
- 73.Stark Z, Savarirayan R. Osteopetrosis. Orphanet J Rare Dis. 2009;4:5–17. doi: 10.1186/1750-1172-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Steward CG. Hematopoietic stem cell transplantation for osteopetrosis. Pediatr Clin North Am. 2010;57:171–80. doi: 10.1016/j.pcl.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 75.Driessen GJ, Gerritsen EJ, Fischer A, et al. Long-term outcome of haematopoietic stem cell transplantation in autosomal recessive osteopetrosis: an EBMT report. Bone Marrow Transplant. 2003;32:657–63. doi: 10.1038/sj.bmt.1704194. [DOI] [PubMed] [Google Scholar]
- 76.Shapiro JR, Sponsellor PD. Osteogenesis imperfecta: questions and answers. Curr Opin Pediatr. 2009;21:709–16. doi: 10.1097/MOP.0b013e328332c68f. [DOI] [PubMed] [Google Scholar]
- 77.Horwitz EM, Prockop DJ, Gordon PL, et al. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood. 2001;97:1227–31. doi: 10.1182/blood.v97.5.1227. [DOI] [PubMed] [Google Scholar]
- 78.Le Blanc K, Gotherstrom C, Ringden O, et al. Fetal mesenchymal stem-cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation. 2005;79:1607–14. doi: 10.1097/01.tp.0000159029.48678.93. [DOI] [PubMed] [Google Scholar]
- 79.Horwitz EM, Prockop DJ, Fitzpatrick LA, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–13. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 80.Horwitz EM, Gordon PL, Koo WK, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci USA. 2002;99:8932–7. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dominici M, Marino R, Rasini V, et al. Donor cell-derived osteopoiesis originates from a self-renewing stem cell with a limited regenerative contribution after transplantation. Blood. 2008;111:4386–91. doi: 10.1182/blood-2007-10-115725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aldenhoven M, Sakkers RJ, Boelens J, et al. Musculoskeletal manifestations of lysosomal storage disorders. AnnRheumDis. 2009;68:1659–65. doi: 10.1136/ard.2008.095315. [DOI] [PubMed] [Google Scholar]
- 83.Sauer M, Meissner B, Fuchs D, et al. Allogeneic blood SCT for children with Hurler’s syndrome: results from the German multicenter approach MPS-HCT 2005. Bone Marrow Transplant. 2009;43:375–81. doi: 10.1038/bmt.2008.328. [DOI] [PubMed] [Google Scholar]
- 84.Willasch A, Hoelle W, Kreyenberg H, et al. Outcome of allogeneic stem cell transplantation in children with non-malignant diseases. Haematologica. 2006;91:788–94. [PubMed] [Google Scholar]
- 85.Kwan MD, Slater BJ, Wan DC, et al. Cell-based therapies for skeletal regenerative medicine. Hum Mol Genet. 2008;17:R93–8. doi: 10.1093/hmg/ddn071. [DOI] [PubMed] [Google Scholar]
- 86.Jethva R, Otsuru S, Dominici M, et al. Cell therapy for disorders of bone. Cytotherapy. 2009;11:3–17. doi: 10.1080/14653240902753477. [DOI] [PubMed] [Google Scholar]
- 87.Burger B, Beier R, Zimmermann M, et al. Osteonecrosis: a treatment related toxicity in childhood acute lymphoblastic leukemia (ALL) – experiences from trial ALL-BFM 95. Pediatr Blood Cancer. 2005;44:220–5. doi: 10.1002/pbc.20244. [DOI] [PubMed] [Google Scholar]
- 88.Adekile AD, Gupta R, Yacoub F, et al. Avascular necrosis of the hip in children with sickle cell disease and high Hb F: magnetic resonance imaging findings and influence of alpha-thalassemia trait. Acta Haematol. 2001;105:27–31. doi: 10.1159/000046529. [DOI] [PubMed] [Google Scholar]
- 89.Kobayakawa M, Rydholm U, Wingstrand H, et al. Femoral head necrosis in juvenile chronic arthritis. Acta Orthop Scand. 1989;60:164–9. doi: 10.3109/17453678909149245. [DOI] [PubMed] [Google Scholar]
- 90.Castro FP, Jr, Barrack RL. Core decompression and conservative treatment for avascular necrosis of the femoral head: a meta-analysis. Am J Orthop. 2000;29:187–94. [PubMed] [Google Scholar]
- 91.Beltran J, Knight CT, Zuelzer WA, et al. Core decompression for avascular necrosis of the femoral head: correlation between long-term results and preoperative MR staging. Radiology. 1990;175:533–6. doi: 10.1148/radiology.175.2.2326478. [DOI] [PubMed] [Google Scholar]
- 92.Low K, Mont MA, Hungerford DS. Steroid-associated osteonecrosis of the knee: a comprehensive review. Instr Course Lect. 2001;50:489–93. [PubMed] [Google Scholar]
- 93.Simank HG, Brocai DR, Strauch K, et al. Core decompression in osteonecrosis of the femoral head: risk-factor-dependent outcome evaluation using survivorship analysis. Int Orthop. 1999;23:154–9. doi: 10.1007/s002640050335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yan ZQ, Chen YS, Li WJ, et al. Treatment of osteonecrosis of the femoral head by percutaneous decompression and autologous bone marrow mononuclear cell infusion. Chin J Traumatol. 2006;9:3–7. [PubMed] [Google Scholar]
- 95.Datta N, Holtorf HL, Sikavitsas VI, et al. Effect of bone extracellular matrix synthesized in vitro on the osteoblastic differentiation of marrow stromal cells. Biomaterials. 2005;26:971–7. doi: 10.1016/j.biomaterials.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 96.Kotobuki N, Hirose M, Machida H, et al. Viability and osteogenic potential of cryopreserved human bone marrow-derived mesenchymal cells. Tissue Eng. 2005;11:663–73. doi: 10.1089/ten.2005.11.663. [DOI] [PubMed] [Google Scholar]
- 97.Kraus KH, Kirker-Head C. Mesenchymal stem cells and bone regeneration. Vet Surg. 2006;35:232–42. doi: 10.1111/j.1532-950X.2006.00142.x. [DOI] [PubMed] [Google Scholar]
- 98.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair–current views. Stem Cells. 2007;25:2896–902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 99.Liechty KW, MacKenzie TC, Shaaban AF, et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6:1282–6. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 100.Meyerrose TE, De Ugarte DA, Hofling AA, et al. In vivo distribution of human adipose-derived mesenchymal stem cells in novel xenotransplantation models. Stem Cells. 2007;25:220–7. doi: 10.1634/stemcells.2006-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang Z, Goh J, Das DS, et al. Efficacy of bone marrow-derived stem cells in strengthening osteoporotic bone in a rabbit model. Tissue Eng. 2006;12:1753–61. doi: 10.1089/ten.2006.12.1753. [DOI] [PubMed] [Google Scholar]
- 102.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. JCell Biochem. 2006;98:1076–84. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 103.Noiseux N, Gnecchi M, Lopez-Ilasaca M, et al. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther. 2006;14:840–50. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 104.Müller I, Vaegler M, Holzwarth C, et al. Secretion of angiogenic proteins by human multipotent mesenchymal stromal cells and their clinical potential in the treatment of avascular osteonecrosis. Leukemia. 2008;22:2054–61. doi: 10.1038/leu.2008.217. [DOI] [PubMed] [Google Scholar]
- 105.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 106.Cave E, Nichols C. Clinical audit and reform of the UK research ethics review system. Theor Med Bioeth. 2007;28:181–203. doi: 10.1007/s11017-007-9034-0. [DOI] [PubMed] [Google Scholar]
- 107.Jones NL. A code of ethics for the life sciences. Sci Eng Ethics. 2007;13:25–43. doi: 10.1007/s11948-006-0007-x. [DOI] [PubMed] [Google Scholar]
- 108.Oduncu FS. Stem cell research in Germany: ethics of healing versus human dignity. Med Health Care Philos. 2003;6:5–16. doi: 10.1023/a:1022585217710. [DOI] [PubMed] [Google Scholar]
- 109.Doerflinger R. Destructive stem-cell research on human embryos. Origins. 1999;28:71–73. [PubMed] [Google Scholar]
- 110.Lo B, Parham L. Ethical issues in stem cell research. Endocr Rev. 2009;30:204–13. doi: 10.1210/er.2008-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Isasi RM, Knoppers BM. Beyond the permissibility of embryonic and stem cell research: substantive requirements and procedural safeguards. Hum Reprod. 2006;21:2474–81. doi: 10.1093/humrep/del235. [DOI] [PubMed] [Google Scholar]
- 112.Mertes H, Pennings G, Van Steirteghem A. An ethical analysis of alternative methods to obtain pluripotent stem cells without destroying embryos. Hum Reprod. 2006;21:2749–55. doi: 10.1093/humrep/del233. [DOI] [PubMed] [Google Scholar]
- 113.Holm S. The ethical case against stem cell research. Camb Q Healthc Ethics. 2003;12:372–83. doi: 10.1017/s0963180103124061. [DOI] [PubMed] [Google Scholar]
- 114.Cregan K. Ethical and social issues of embryonic stem cell technology. Intern Med J. 2005;35:126–7. doi: 10.1111/j.1445-5994.2004.00766.x. [DOI] [PubMed] [Google Scholar]
- 115.McCullough LB, Chervenak FA. Ethics in obstetrics and gynecology. Oxford, UK: Oxford University Press; 1994. [Google Scholar]
- 116.Human Stem Cell Research and Regenerative Medicine. A European Perspective on Scientific, Ethical and Legal Issues. . Available at. http://www.esf.org/publications/science-policy-briefings. . (Accessed 2 Feb 2011)
- 117.Code of Nuremberg . Available at. http://ohsr.od.nih.gov/guidelines/nuremberg.html. . (Accessed 2 Feb 2011)
- 118.Slavicek G, Forsdahl G. Ethics and regulatory aspects in medical research. Int J Stomat Occ Med. 2009;2:45–9. [Google Scholar]
- 119.Declaration of Helsinki . Available at. http://www.wma.net/en/20activities/10ethics/10helsinki/index.html. . (Accessed 2 Feb 2011)
- 120.Convention of the Council of Europe for the Protection of Human Rights and Dignity of the Human Being regarding the Application of Biology and Medicine. Convention on Human Rights and Biomedicine signed in Oviedo on 4th April 1997, and the additional protocols. . Available at. http://conventions.coe.int/Treaty/EN/Treaties/Html/164.htm. . (Accessed 2 Feb 2011)
- 121.Directive 2004/23/EC . Available at. http://www.eortc.be/Services/Doc/clinical-EU-directive-04-04-01.pdf. . (Accessed 2 Feb 2011)
- 122.Opinion of EGE No. 15/2000. Ethical aspects of human stem cell research and use. . Available at. http://ec.europa.eu/european_group_ethics/docs/avis15_en.pdf. . (Accessed 2 Feb 2011)
- 123.Berry RM. Informed consent law, ethics, and practice: from infancy to reflective adolescence. HEC Forum. 2005;17:64–81. doi: 10.1007/s10730-005-4951-7. [DOI] [PubMed] [Google Scholar]
- 124.Schroter S, Plowman R, Hutchings A, et al. Reporting ethics committee approval and patient consent by study design in five general medical journals. J Med Ethics. 2006;32:718–23. doi: 10.1136/jme.2005.015115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Opinion of EGE No. 11/1998. Ethical aspects of human tissue banking. . Available at. http://ec.europa.eu/european_group_ethics/docs/avis11_en.pdf. . (Accessed 2 Feb 2011)
- 126.Opinion of EGE No. 13/1999. Ethical issues on healthcare in the information society. . Available at. http://ec.europa.eu/european_group_ethics/docs/avis13_en.pdf. . (Accessed 2 Feb 2011)
- 127.Hansson SO. Uncertainty and the ethics of clinical trials. Theor Med Bioeth. 2006;27:149–67. doi: 10.1007/s11017-006-0001-y. [DOI] [PubMed] [Google Scholar]
- 128.Dresser R. Private-sector research ethics: marketing or good conflicts management? The 2005 John J. Conley Lecture on Medical Ethics. Theor Med Bioeth. 2006;27:115–39. doi: 10.1007/s11017-005-5289-5. [DOI] [PubMed] [Google Scholar]
- 129.De Angelis C, Drazen JM, Frizelle FA, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. Circulation. 2005;111:1337–8. doi: 10.1161/01.CIR.0000158823.53941.BE. [DOI] [PubMed] [Google Scholar]


