Abstract
Airway inflammation and mucus hyperproduction play the central role in the development of asthma, although the mechanisms remain unclear. The aquaporin (AQP)-5 may be involved in the process due to its contribution to the volume of liquid secreted from the airways. The present study firstly found the overexpression of AQP5 in the airway epithelium and submucosal glands of asthmatics. Furthermore, we aimed at evaluating the role of AQP5 in airway inflammation and mucous hyperproductions during chronic allergic responses to house dust mite (HDM). Bronchoalveolar lavage levels of interleukin (IL)-2, IL-4, IL-10, interferon-γ and Mucin 5AC (MUC5AC), and number of peribronchial and perivascular cells were measured in AQP5 wild-type and AQP5 knockout (KO) mice. We found that HDM induced airway inflammation, lung Th2 cell accumulation and mucin hypersecretion in C57BL/6 mice rather than AQP5 KO mice. Expression of MUC5AC and MUC5B proteins and genes in the lung tissue was significantly lower in AQP5 KO mice. Thus, our results implicate involvement of AQP5 in the development of airway inflammation and mucous hyperproduction during chronic asthma.
Keywords: aquaporins, mucin, house dust mite, chronic allergy, inflammation
Introduction
Allergic asthma is a complex disorder, which involves a significant contribution of environmental stimuli for the manifestation of symptoms and degree of clinical severity. Chronic asthma is pathologically characterized by airway inflammation, increased mucus production, airway remodelling and airway hyper-responsiveness to bronchoconstrictive agents [1].
The aquaporins (AQPs) are a family of small transmembrane proteins that facilitate osmotically driven water transport, and in some cases the transportation of small solutes such as glycerol [2]. AQP5 is expressed in the apical membrane of type I alveolar epithelial cells, acinar epithelial cells in submucosal glands and large airway epithelia [3, 4]. The volume of liquid secreted from the nasopharynx and the upper airways was lower by 2-fold in AQP5 knockout (KO) mice, resulting in hypertonic fluid with elevated protein concentration [5]. In addition, the previous studies found AQP5 might be involved in the regulation of MUC5AC production [6], airway hyper-responsiveness to cholinergic stimulation and altered lung resistance and dynamic compliance [7], lung infection [8], airway inflammation [9] and acute lung injury [10].
House dust mite (HDM) represents one of the most common aeroallergens in allergy and asthma, influencing approximately 10% of the population. Chronic exposure to HDM extract in mice could lead to persistent airway inflammation, hyper-responsiveness and remodelling, as a accepted model of chronic asthma [11]. In the present study, we tried to validate if AQP5 as the disease-related target could be overexpressed in the asthmatics and furthermore investigate the potential role of AQP5 in airway inflammation and mucin hypersecretion induced by chronic exposure to HDM. We compared the effects of chronic HDM exposure on the development of airway eosinophilia, cytokine production and mucin secretion in AQP5 KO wild-type (WT) mice. Our results firstly showed the overexpression of AQP5 in the airway epithelium and submucosal glands of asthmatics and found that the expression of MUC5AC and MUC5B genes and proteins, number of inflammatory cells and production of Th2 cytokines were markedly lower in AQP5 KO mice. Thus, our data indicate that AQP5 may act as a novel regulator of the allergic response and a therapeutic target for treating mucin hypersecretion and airway inflammation in chronic asthma.
Materials and methods
Human bronchial tissues
Human bronchi were obtained from patients with lung cancer or with lung cancer and chronic asthma who underwent lung surgery in Zhongshan hospital (Shanghai, China) from 2007 to 2010. The patients (n= 6) were diagnosed as chronic asthma according to the Global Initiative for Asthma launched in 1993 in collaboration with the National Heart, Lung, and Blood Institute, National Institutes of Health, USA, and the World Health Organization. All of the lower airway samples from the lung cancer patients who had the treatment of steroid were obtained from the lobe where the cancer was located. However, all of the cancer lesions were located peripherally and the large airway samples did not include any cancer lesions. The present study was proved by the local ethics committee of Fudan University Zhongshan Hospital, and all patients agreed to participate by signing a written informed consent.
Animals
AQP5 KO mice (gift from Dr. A. S. Verkmann, California University) were generated as previously described [12] and back-crossed to a C57BL/6 background for 6 to 10 generations. The experiments were performed on litter-matched (age 7–8 weeks, body weight 20–25 g, female) WT (AQP5 WT) and AQP5-null (AQP5 KO) mice. Mice were genotyped from DNA isolated by tail clips with PCR primers to the AQP5 gene [12]. The mice were kept in a 12 : 12 hr night–day rhythm, fed with standard mice chow and provided water ad libitum. This study was approved by the Animal Care Committee of Fudan University Zhongshan Hospital.
Chronic asthma model
Mice were challenged by the intranasal administration of 25 μg of purified HDM extract (Dermatophagoides pteronyssinus; ALK, Hørsholm, Demark) in a total volume of 10 μl of saline for once a day, 5 days a week, for up to five consecutive weeks. Phosphate-buffered solution (PBS) at the same volume without HDM was used in the control group. There were 8–10 mice in each terminate group.
Bronchoalveolar lavage (BAL)
The trachea was cannulated and the bronchoalveolar space was lavaged with 0.5 ml PBS and withdrawn with 0.3 ml twice. BAL samples were centrifuged at 500 ×g for 5 min. at 4°C and stored at −70°C until analysis. Levels of interleukin (IL)-2, IL-4, IL-10 and interferon (IFN)-γ were determined using specific ELISA as suggested by the manufacturer manual (ELISA kits, eBioscience, San Diego, CA, USA). The concentrations of cytokine were determined by the comparison of ELISA readings with the standard curve using recombinant cytokine of known concentrations. The amount of MUC5AC in the supernatant of BAL was measured using ELISA (USCN Life Science & Technology Company, Missouri City, TX, USA).
Histological evaluation
Twenty-four hours after the last HDM challenge, lungs were harvested, fixed in 10% neutral-buffered formalin and embedded in paraffin. Sections (4 μm) of specimens were put onto 3-amino propyltriethoxy saline-coated slides. The morphology and leucocyte infiltration in the tissue were assessed using haematoxylin and eosin staining. Inflammatory changes were graded by a scale of 0–5 for perivascular, bronchiolar and submucosal gland eosinophilia [13]. Quantitative analysis of pathology was performed by the scoring system, e.g. 0: normal; 1: low grade of cell influx, diffuse oedema and cell loss; 2: low to middle grade of cell influx, tissue damage, alveolar and bronchiolar oedema, and cell loss; 3: clear changed grade of cell influx, tissue damage, regional and focal oedema, and cell loss; 4: obvious changed and pronounced grade of cell influx, tissue damage, oedema and cell loss; 5: most obvious changed grade of cell influx, tissue pathology, pneumonic-type oedema, epithelial metaplasia and mucus cell hyperplasia. The results for each lung from the three observers were averaged to obtain a mean value for each parameter. Tissues were sliced and assessed with the periodic acid–Schiff staining to demonstrate the presence of mucin within goblet cells
Immunohistochemistry
Tissue sections laid on silane-coated slides were stained by immunohistochemistry with mucin 5AC Rabbit polyclonal IgG (H-160, SC-20118, Lot: B0403; Santa Cruz Biotechnology, Santa Cruz, CA, USA), mucin 5B mouse monoclonal IgG (5B#19–2E, SC-21768 Lot: L2203; Santa Cruz Biotechnology) and the antibodies (rabbit anti-AQP5 affinity purified polyclonal antibody; Chemicon, Temecula, CA, USA). The samples were dewaxed in xylene and dehydrated in a graded ethanol series. Endogenous peroxidase activity was inhibited by incubating the slides with 3% H2O2 for 30 min., followed by washing in PBS and microwave heating (750 W) for 10 min. in 10 mmol/l citric acid at pH 6.0 for AQP5 and pH 9.0 for MUC5AC and MUC5B. The sections were inactivated with normal goat serum at 37°C for 15 min. After washing thrice with PBS, the sections were incubated with the primary antibodies, placed in a humid chamber at 37°C for 1 hr, washed in PBS and incubated at 37°C for 30 min. with biotinylated goat antimouse or rabbit (1:100; Maixin-Bio, Shanghai, China) according to manufacturer’s instructions. The tissue was incubated with Streptavidin Peroxidase (Maixin-Bio) reagents at 37°C for 30 min., stained with freshly prepared DAB (Maixin-Bio) and counterstained with haematoxylin (Maixin-Bio).
Morphometric quantification of the stained sections was performed with a customized digital image analysis system (IMAGE-Pro plus 4.5). Analysis of MUC5AC and MUC5B in the airway and capturing images of airway wall was performed. A line was drawn along the basal border of the airway epithelium from which a 20 μm band of tissue was projected in a basal direction. The software then calculated the percentage of each band that was positively stained for the individual stains on the basis of previously determined colour plane settings. The final score for each stained section was expressed as a weighted mean, with each result weighted in proportion to the total area examined. Goblet cells were identified as periodic acid-Schiff+ cells and counted manually and expressed as cells per millimetre length of airway wall.
Quantitative real-time PCR
The right lung was frozen in liquid nitrogen immediately after harvest and homogenized in TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) using an ULTRA-TURRAX power homogenizer. Total RNA was isolated, as recommended by the manufacturer. The amount of RNA in aqueous solution was determined by absorbance at 260 nm. Equal amounts (1 μg) of total cellular RNA were reverse-transcribed using Moloney murine leukemia virus (MMLV) first-strand synthesis kit (BBI, Madison, WI, USA). Before cDNA synthesis, each RNA sample was treated with DNase I (Invitrogen Life Technologies) to eliminate any potential contamination with genomic DNA. PCRs were carried out in 25 μl containing 2 μl cDNA, 10 μM of each forward and reverse primer. Mouse glyceraldehyde 3-phosphate dehydrogenase (GADPH) was used as the internal control to quantify initial cellular transcripts. Primer sequences included: mouse GAPDH – sense: 5′TGTGTCCGTCGTGGATCTGA3′, antisense: 5′CCTGCTTCACCACCTTCTTGAT3′, MUC5AC – sense: 5′AATGCAGCATCATCAACAGCG3′ antisense: 5′AGGCACAGGCGTCATTCACA3′, MUC5B – sense: 5′CTGTACGGGAACCCTAAGGAAA3′, antisense: 5′ACCGCAAGACAGTCGCATTTA3′. Real-time PCR conditions for amplification of target genes were as follows: The PCR mixture was denatured at 95°C for 10 sec., annealed and extended at 60°C for 1 min., and the above processes was repeated till it totalled 45 cycles. Amplification data measured by fluorescence were collected in real time and analysed by Rotor-Gene 6.0.14 Software.
Statistical analysis
Data were expressed with means ± S.D. Differences between groups were analysed using software SPSS for windows (version 12.0) by unpaired two-tailed parametric Student’s t-test, after ANOVA test. P-values less than 0.05 were considered statistically significant.
Results
Expression of AQP5 protein in human bronchi
The lower airways without cancer were selected for pathological evaluation. The player of airway epithelial cells in patients with chronic asthma was obviously thicker than those without chronic asthma. Hypertrophy of epithelial cells in the mucosal players and submucosal glands was noticed in asthmatics. Immunohistochemical analysis demonstrated that AQP5 was localized in normal airway epithelium (Fig. 1A) and submucosal glands (Fig. 1C) of the bronchi. The expression of AQP5 proteins was obviously higher in the epithelial cells of airway mucosa (Fig. 1B) and submucosal glands (Fig. 1D) in asthmatics.
Fig 1.
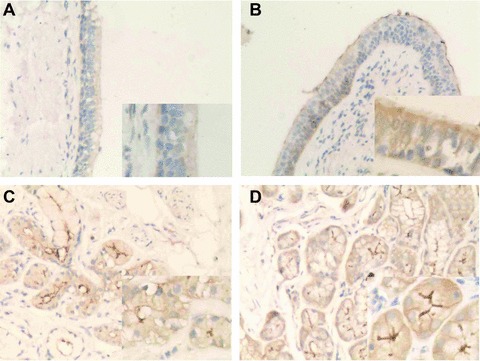
Immunohistochemical localization of AQP5 protein in human bronchi. AQP5 protein (brown staining) was localized in normal airway epithelium (A) and submucosal glands (C) of the bronchi, whereas the expression of AQP5 increased in the airway epithelium (B) and submucosal glands (D) of asthmatics. (Magnification ×200.)
Allergic inflammation in HDM-treated mice
Administration of HDM for 5 weeks to C57BL/6 WT mice led to a significant increase in the amount of pulmonary oedema and epithelial hypertrophy in the terminal airway and leucocyte infiltration (Fig. 2B), although not in AQP5 KO animals (Fig. 2D). The total scores of histopathology in HDM-treated AQP5 WT mice were significantly higher than those in PBS-treated animals (Table 1), whereas AQP5 KO mice had significantly less inflammation at the airway as compared with AQP5 WT mice after HDM exposure. To determine whether AQP5 KO mice had reduced airway eosinophilia, we analysed the cell number in the peribronchial and perivascular tissues after HDM exposure. The percentage of airway eosinophils was significantly increased in WT mice with HDM challenge for 5 weeks as compared with those with PBS. HDM-treated AQP5 KO mice had significantly fewer eosinophils as compared with WT animals (Table 1). There was no significant difference of neutrophil numbers in the lung tissue between WT and AQP5 KO mice (data not shown).
Fig 2.
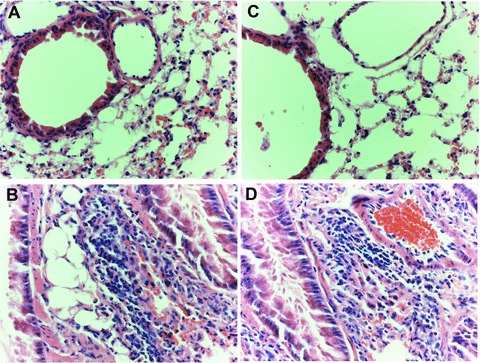
Histological findings of lung tissues with peripheral airways (haematoxylin and eosin, ×400 origin) from WT mice with PBS (A) or HDM (B) and AQP5 KO mice with PBS (C) or HDM (D) after the intranasal challenges, once a day, 5 days a week for 5 weeks.
Table 1.
Severity of inflammatory cellular infiltration in lung tissue after immunization
| Groups | Perivascular eosinophilia | Peribronchiolar eosinophilia | Oedema | Epithelial damage |
|---|---|---|---|---|
| Control | 0(0–0) | 0(0–0) | 0(0–0) | 0(0–1) |
| WT challenged with HDM | 4(3–4)* | 4(3–4)* | 4(3–4)* | 4(3–5)* |
| AQP5 KO mice challenged with HDM | 3(2–3)*† | 4(3–4)* | 2(1–2)*† | 3(2–3)*† |
Inflammatory changes were graded by histopathological assessment using a semi-quantitative scale of 0–5. Results are presented as median, with range in parenthesis (n= 8/group).
P < 0.01 versus animals challenged with PBS, †P < 0.05 versus WT mice challenged with HDM.
Role of AQP5 in HDM-induced cytokine production
BAL levels of IL-4 (Fig. 3A) and IL-10 (Fig. 3B) in animals challenged with HDM were significantly increased as compared with those with PBS (P < 0.05 and 0.01, respectively). AQP5 KO mice had significantly lower levels of IL-4 and IL-10 than WT mice after chronic exposure to HDM (P < 0.05). BAL levels of IL-2 (Fig. 3C) and IFN-γ (Fig. 3D) in AQP5 KO mice were significantly higher than those in WT mice (P < 0.05), whereas levels in both AQP5 KO and WT mice challenged with HDM were significantly lower than those with PBS (P < 0.01). There was no significant difference between WT and AQP5 KO after the challenge with PBS.
Fig 3.
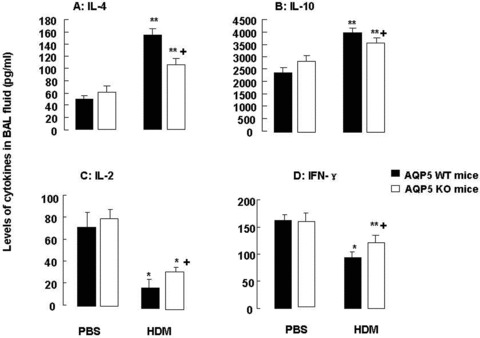
Levels of IL-4 (A), IL-10 (B), IL-2 (C) and IFN-γ (D) in BAL fluid harvested from WT and AQP5 KO mice (n= 8/group) after intranasal challenges with HDM, once a day, 5 days a week for 5 weeks; ‘*’ and ‘**’ stand for the P-values less than 0.05 and 0.01, respectively, as compared with WT and AQP5 KO mice challenged with PBS. ‘+’ stands for the P-values less than 0.05, as compared with WT mice challenged with HDM.
Airway epithelial mucin changes after chronic HDM exposure
Chronic exposure to HDM induced obvious metaplasia (Fig. 4A-2), hyperplasia and hypertrophy of goblet cells with staining positively with periodic acid–Schiff for mucin in WT mice, as compared with animals challenged with PBS (Fig. 4A-1) and AQP5 KO mice with HDM (Fig. 4A-4). There was no significant difference of goblet cell metaplasia between mice with PBS and AQP5 KO mice with PBS. However, the number of goblet cells in the small airway of HDM-challenged WT animals measured by morphometric system was significantly higher than that in PBS- and HDM-challenged AQP5 KO animals (P < 0.01 and 0.05, respectively, Fig. 2B). There was no significant difference of goblet cell alterations between PBS-challenged WT and AQP5 KO animals.
Fig 4.
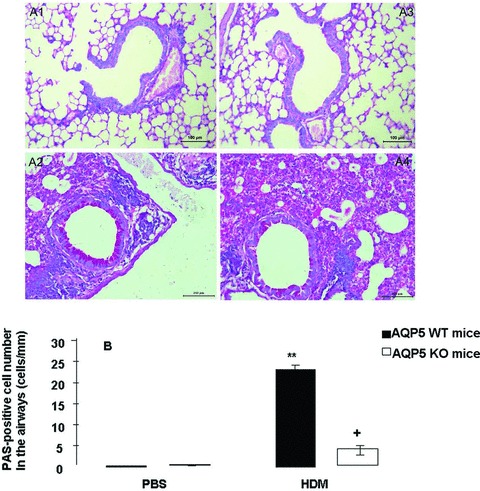
Histological findings (A) of PAS-stained sections of airways of WT mice challenged with PBS (A-1) or HDM (A-2) or AQP5 KO mice with PBS (A-3) or HDM (A-4) after the intranasal challenges, once a day, 5 days a week for 5 weeks. Morphometric quantification of PAS+ cells (B) in the airway wall of WT or AQP5 KO mice after chronic exposure to PBS or HDM. ‘**’ stands for the P-values less than 0.01, as compared with PBS-challenged WT mice, and ‘+’ stands for the P-values less than 0.05, as compared with WT mice with HDM, respectively.
Airway MUC5AC and MUC5B changes after chronic allergen exposure
Figure 5 demonstrates that more cells with positive staining of MUC5AC (Fig. 5A) and MUC5B (Fig. 5B) were observed in the small airway of WT mice after chronic exposure to HDM, as compared with AQP5 KO animals. The number of MUC5AC (Fig. 6A) – and MUC5B (Fig. 6B) – positive epithelial cells in the airway was significantly higher in WT mice as compared with in AQP5 KO mice after chronic HDM challenge (P < 0.01 and 0.05, respectively) and in animals challenged with BPS (P < 0.01, respectively). BAL levels of MUC5AC proteins were significantly increased in HDM-challenged WT (P < 0.01) and AQP5 KO animals (P < 0.05), respectively, as shown in Figure 7A. AQP5 KO mice exhibited a significant decrease about 51.7% in BAL levels of MUC5AC after chronic HDM challenge as compared with WT mice (P < 0.05, Fig. 7A). To confirm the expression of MUC5AC and MUC5B in the lung tissue, mRNA levels of MUC5AC and MUC5B were assessed by quantitative real-time PCR. There was a significant increase in gene expression of MUC5AC (Fig. 7B) and MUC5B (Fig. 7C) in the lung tissue of WT (P < 0.010 and AQP5 KO animals (P < 0.05) challenged with HDM, as compared with those with PBS, respectively. The expression of both MUC5AC and MUC5B in AQP5 KO mouse lung tissue was significantly lower than that in WT mice after HDM challenge.
Fig 5.
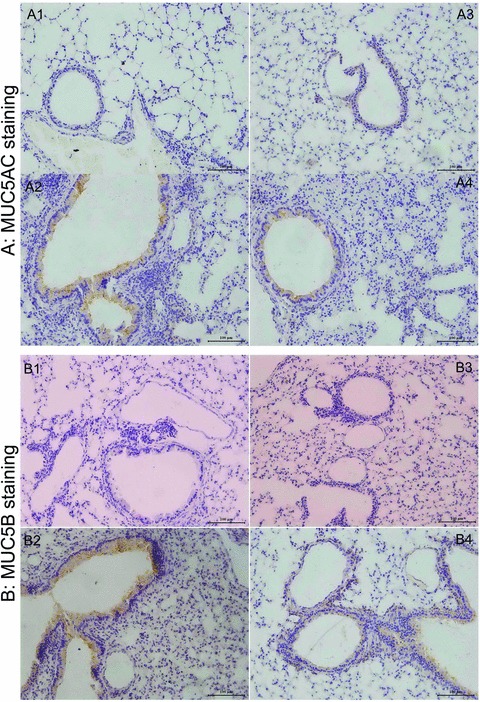
The photomicrographs of MUC5AC-stained (A) and MUC5B-stained sections (B) of airways from WT mice with PBS (A-1, B-1) or HDM (A-2, B-2) and AQP5 KO mice with PBS (A-3, B-3) or HDM (A-4, B-4) after intranasal challenges, once a day, 5 days a week for 5 weeks. Mucin 5AC (H-160, SC-20118, Lot: B0403; Santa Cruz Biotechnology). Rabbit polyclonal IgG, mucin 5B (5B#19–2E, SC-21768 Lot: L2203, Santa Cruz Biotechnology) mouse monoclonal IgG. Mucin 5AC Ab-1 (45M1, MS-145-PO Lot: 145; NeoMarkers, Fremont, CA, USA) have been used as controls for the specific monoclonal antibodies.
Fig 6.
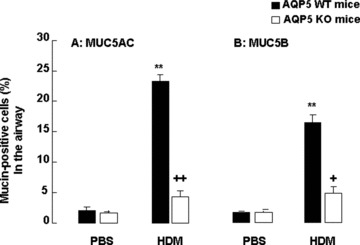
Morphometric measurements of percentage of MUC5AC+ (A) and MUC5B+ cells (B) in the airway of WT and AQP5 KO mice (n= 10/group) after intranasal challenges with PBS or HDM, once a day, 5 days a week for 5 weeks. ‘**’ stands for the P-values less than 0.01, as compared with PBS-challenged WT mice, and ‘+’ and ‘++’ stand for the P-values less than 0.05 and 0.01, respectively, as compared with WT mice with HDM, respectively.
Fig 7.
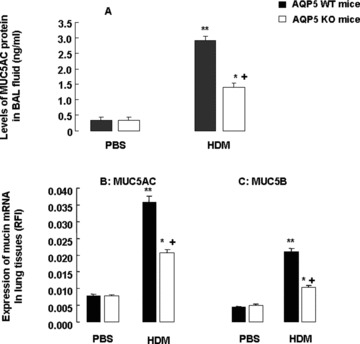
Levels of MUC5AC proteins in BAL fluid (A) and mRNA expression of MUC5AC (B) and MUC5B (C) in the lung tissue measured by relative fluorescence intensity in WT and AQP5 KO mice (n= 8/group) after intranasal challenges with PBS or HDM, once a day, 5 days a week for 5 weeks. The relative fluorescence intensity was calculated by comparing the measured wavelength-integrated intensity of an unknown sample to that of a standard with known fluorescence intensity. ‘*’ and ‘**’ stand for the P-values less than 0.05 and 0.01, respectively, as compared with PBS-challenged WT mice, and ‘+’ stands for the P-values less than 0.05, as compared with WT mice with HDM, respectively.
Discussion
The AQPs are a family of small (30 kD monomer) integral membrane proteins that function as selective water transporters, and in some cases they also transport glycerol (aquaglyceroporins). Of 13 related AQPs in mammals, at least four are expressed in the lung and airways, e.g. AQP1 in microvascular endothelia, AQP3 and AQP4 in airway epithelia and AQP5 in the apical membrane of type I alveolar epithelial cells, acinar epithelial cells in submucosal glands or large airway epithelia [2]. There was no clinical study on the changes of AQP5 expression in the asthmatics, and only one study demonstrated that the gene and protein expressions of AQP5 were altered in patients with chronic obstructive pulmonary disease [14]. The present study firstly examined the expression of AQP5 proteins by immunohistochemistry and found the overexpression of AQP5 protein in the asthmatic airway epithelium and submucosal glands of the bronchi as compared to controls. Due to the lack of reliable, specific and nontoxic inhibitors against AQPs, the significance of AQPs has been examined through the use of gene KO mice or primary cell culture extracted from AQP KO mice. In the present study, we investigate potential involvements of AQP5 in chronic HDM-induced mucus hyperproduction by using AQP KO mice.
Inflammatory cytokines have been found to be involved in overproduction and overexpression of mucus proteins and genes in the airway epithelium [15, 16]. For example, Th2 cytokines like IL-4 were suggested to regulate mucin gene expression [17] and were down-regulated in the AQP5 KO mouse, suggesting that AQP5 may be involved in robust Th2-driven responses to allergens. The fact that AQP5 deletion resulted in increased levels of IFN-γ and IL-2 suggests that there may be a switch from Th2 towards Th1 responses where AQP5 plays a critical role. Th2 cytokines were found to be important in eosinophil recruitment, airway hyper-responsiveness and mucus hypersecretion [18, 19]. It is possible that AQP5 may be involved in the development of HDM-induced chronic airway inflammation and mucus hyperproduction through the overproduction of those inflammatory mediators. We found the formation of metaplasia, hyperplasia and hypertrophy of airway goblet cells and peri-bronchial accumulation of inflammatory cells in WT mice following chronic HDM exposure, although obviously less in AQP5 KO mice. Although there is a potential that animals with the genetic modification may have physiological difference and changes, the present study did not find the significant alternations of any measured parameters between WT and AQP5 KO mice challenged with PBS.
Of mucin genes in adult human lung [20], MUC5AC and MUC5B appear to be the predominant genes expressed and the most abundant in mucus secretions [21]. Our data provide solid evidence to support the involvement of AQP5 in overproduction of MUC5AC and MUC5B during chronic lung inflammation. We investigated metaplasia, hyperplasia and hypertrophy of goblet cells by immunochemical staining, hyperproduction and hypersecretion of mucins by both morphometry and BAL levels measured and hyperexpression of MUC5AC and MUC5B proteins and genes in the lung tissue. AQP5 KO mice had significantly less alterations in those measurements after chronic challenge with HDM, as compared with WT mice, but still higher than in those challenged with PBS. It indicates that the AQP5 is, at least, partially, involved in development of mucus hyperproduction in chronic asthma. The previous results demonstrated that AQP5 KO mice had low inflammatory responses to ovalbumin, including leucocyte infiltration in lung tissues and cytokine expression [22]. Like acute allergic reaction, the role of AQP5 in chronic airway inflammation and mucosal hyperproduction may be involved in down-regulation of antigen presenting cells, because dendritic cells in AQP5 KO mice had less antigen-presenting function [22–24].
Mucins, cystic fibrosis transmembrane conductance regulator protein (CFTR) and AQP5 are involved in the formation and composition of the airway surface liquid, essential for proper mucociliary clearance. Expressions of AQP5 and CFTR were found in the airway [25], of which CFTR expression was enhanced whereas AQP5 expression was completely abolished after IL-13 treatment. However, amiloride administration did not inhibit water permeability of airway AQPs [26]. IL-13 did not affect AQP3 or AQP4 expression, whereas it completely inhibited the expression of both AQP5 mRNA and protein [25].
Our findings are supported by a number of previous studies, e.g. pilocarpine-induced fluid secretion [5] and AQP-deletion-impaired osmotic equilibration [27]. However, another study showed that mice deficient in AQP5 were hyper-responsive to cholinergic stimulation and had high lung resistance and low dynamic compliance [7]. The differences between present and previous study [7] include the genetic background, e.g. 129svj mice in the previous and CD1/C57 in the present, and the models, e.g. bronchostimulator-induced fluid secretion in the previous and HDM-induced chronic asthma in the present. Previous studies showed that a bronchodilating β-adrenergic agonist increased AQP5 abundance and redistribution of AQP5 to the apical membrane of mouse lung epithelial cells (MLE-12) through an adenosine 3′, 5′- monophosphate (cAMP)-protein kinase A (PKA) dependent pathway [28]. However, other studies demonstrated that siRNA-induced AQP5 down-regulation was associated with MUC5AC over-expression in SPC-1 cell line [29]. We believe the discrepancy between our findings and those of other investigators may be due to the differences between the in vitro and animal models. There is a great need to investigate and understand the role of AQP5 in acute and chronic airway diseases.
In conclusion, our data demonstrated that the chronic challenge with HDM resulted in the development of airway inflammation and mucus hyperproduction in WT mice, although significantly less in AQP5 KO mice. Levels of leucocyte recruitment, cytokine production and mucus hyperproduction in AQP5 KO mice after HDM challenge were still higher than those with PBS challenge. Thus, our data indicate that AQP5 may act as one of new and novel therapeutic targets to treat chronic airway diseases.
Acknowledgments
The authors acknowledge Wang Xun for technical assistance. This project was supported by Shanghai Leading Academic Discipline Project, Project Number: B115, Fudan University (Distinguished Professor Grant), Shanghai Science & Technology Committee (08PJ1402900) and the Research Fund for the Dectoral Program of Higher Education: 20060246073.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Bochner BS, Busse WW. Allergy and asthma. J Allergy Clin Immunol. 2005;115:953–9. doi: 10.1016/j.jaci.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 2.Verkman AS. Role of aquaporins in lung liquid physiology. Respir Physiol Neurobiol. 2007;159:324–30. doi: 10.1016/j.resp.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Funaki H, Yamamoto T, Koyama Y, et al. Localization and expression of AQP5 in cornea, serous salivary glands, and pulmonary epithelial cells. Am J Physiol. 1998;275:C1151–7. doi: 10.1152/ajpcell.1998.275.4.C1151. [DOI] [PubMed] [Google Scholar]
- 4.Ishida N, Hirai SI, Mita S. Immunolocalization of aquaporin homologs in mouse lacrimal glands. Biochem Biophys Res Commun. 1997;238:891–5. doi: 10.1006/bbrc.1997.7396. [DOI] [PubMed] [Google Scholar]
- 5.Song YL, Verkman AS. Aquaporin-5 dependent fluid secretion in airway submucosal glands. J Biol Chem. 2001;276:41288–92. doi: 10.1074/jbc.M107257200. [DOI] [PubMed] [Google Scholar]
- 6.Chen ZH, Wang XD, Gao L, et al. Regulation of MUC5AC mucin secretion by depletion of AQP5 in SPC-A1 cells. Biochem Biophys Res Commun. 2006;342:775–81. doi: 10.1016/j.bbrc.2006.01.103. [DOI] [PubMed] [Google Scholar]
- 7.Krane CM, Fortner CN, Hand AR, et al. Aquaporin 5-deficient mouse lungs are hyperresponsive to cholinergic stimulation. Proc Natl Acad Sci USA. 2001;98:14114–9. doi: 10.1073/pnas.231273398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Towne JE, Harrod KS, Krane CM, et al. Decreased expression of aquaporin (AQP)1 and AQP5 in mouse lung after acute viral infection. Am J Respir Cell Mol Biol. 2000;22:34–44. doi: 10.1165/ajrcmb.22.1.3818. [DOI] [PubMed] [Google Scholar]
- 9.Towne JE, Krane CM, Bachurski CJ, et al. Tumor necrosis factor-alpha inhibits aquaporin 5 expression in mouse lung epithelial cells. J Biol Chem. 2001;276:18657–64. doi: 10.1074/jbc.M100322200. [DOI] [PubMed] [Google Scholar]
- 10.Jiao GY, Li ER, Yu RJ. Decreased expression of AQP1 and AQP5 in acute injured lungs in rats. Chin Med J. 2002;115:963–7. [PubMed] [Google Scholar]
- 11.Cates EC, Fattouh R, Wattie J, et al. Intranasal exposure of mice to house dust mite elicits allergic airway inflammation via a GM-CSF-mediated mechanism. J Immunol. 2004;173:6384–92. doi: 10.4049/jimmunol.173.10.6384. [DOI] [PubMed] [Google Scholar]
- 12.Moore M, Ma TH, Yang BX, et al. Tear secretion by lacrimal glands in transgenic mice lacking water channels AQP1, AQP3, AQP4 and AQP5. Exp Eye Res. 2000;70:557–62. doi: 10.1006/exer.1999.0814. [DOI] [PubMed] [Google Scholar]
- 13.Underwood S, Foster M, Raeburn D, et al. Time-course of antigen-induced airway inflammation in the guinea-pig and its relationship to airway hyperresponsiveness. Eur Respir J. 1995;8:2104–13. doi: 10.1183/109031936.95.08122104. [DOI] [PubMed] [Google Scholar]
- 14.Wang K, Feng YL, Wen FQ, et al. Decreased expression of human aquaporin-5 correlated with mucus overproduction in airways of chronic obstructive pulmonary disease. Acta Pharmacol Sin. 2007;28:1166–74. doi: 10.1111/j.1745-7254.2007.00608.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee C, Kolesnik TB, Caminschi I, et al. Suppressor of cytokine signalling 1 (SOCS1) is a physiological regulator of the asthma response. J Allergy Clin Immunol. 2009;39:897–907. doi: 10.1111/j.1365-2222.2009.03217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krane CM, Deng BJ, Mutyam V, et al. Altered regulation of aquaporin gene expression in allergen and IL-13-induced mouse models of asthma. Cytokine. 2009;46:111–8. doi: 10.1016/j.cyto.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karras JG, Crosby JR, Guha M, et al. Anti-inflammatory activity of inhaled IL-4 receptor-alpha antisense oligonucleotide in mice. Am J Respir Cell Mol Biol. 2007;36:276–85. doi: 10.1165/rcmb.2005-0456OC. [DOI] [PubMed] [Google Scholar]
- 18.Walter DM, McIntire JJ, Berry G, et al. Critical role for IL-13 in the development of allergen-induced airway hyperreactivity. J Immunol. 2001;167:4668–75. doi: 10.4049/jimmunol.167.8.4668. [DOI] [PubMed] [Google Scholar]
- 19.Tomkinson A, Duez C, Cieslewicz G, et al. A murine IL-4 receptor antagonist that inhibits IL-4-and IL-13-induced responses prevents antigen-induced airway eosinophilia and airway hyperresponsiveness. J Immunol. 2001;166:5792–800. doi: 10.4049/jimmunol.166.9.5792. [DOI] [PubMed] [Google Scholar]
- 20.Rogers DF. Physiology of airway mucus secretion and pathophysiology of hypersecretion. Respir Care. 2007;52:1134–49. [PubMed] [Google Scholar]
- 21.Henke MO, John G, Germann M, et al. MUC5AC and MUC5B mucins increase in cystic fibrosis airway secretions during pulmonary exacerbation. Am J Respir Crit Care Med. 2007;175:816–21. doi: 10.1164/rccm.200607-1011OC. [DOI] [PubMed] [Google Scholar]
- 22.Wang GF, Dong CL, Tang GS, et al. Membrane water permeability related to antigen-presenting function of dendritic cells. J Allergy Clin Immunol. 2008;153:410–9. doi: 10.1111/j.1365-2249.2008.03702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moon C, Rousseau R, Soria JC, et al. Aquaporin expression in human lymphocytes and dendritic cells. Am J Hematol. 2004;75:128–33. doi: 10.1002/ajh.10476. [DOI] [PubMed] [Google Scholar]
- 24.Bousquet J, Bieber T, Fokkens W, et al. Important questions in allergy: novel research areas. Allergy. 2008;63:143–7. doi: 10.1111/j.1398-9995.2007.01615.x. [DOI] [PubMed] [Google Scholar]
- 25.Skowron-Zwarg M, Boland S, Caruso N, et al. Interleukin-13 interferes with CFTR and AQP5 expression and localization during human airway epithelial cell differentiation. Exp Cell Res. 2007;313:2695–702. doi: 10.1016/j.yexcr.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 26.Pedersen PS, Braunstein TH, Jorgensen A, et al. Stimulation of aquaporin-5 and transepithelial water permeability in human airway epithelium by hyperosmotic stress. Pflugers Arch. 2007;453:777–85. doi: 10.1007/s00424-006-0157-3. [DOI] [PubMed] [Google Scholar]
- 27.Ma TH, Song YL, Gillespie A, et al. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J Biol Chem. 1999;274:20071–4. doi: 10.1074/jbc.274.29.20071. [DOI] [PubMed] [Google Scholar]
- 28.Sidhaye V, Hoffert JD, King LS. cAMP has distinct acute and chronic effects on Aquaporin-5 in lung epithelial cells. J Biol Chem. 2005;280:3590–6. doi: 10.1074/jbc.M411038200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen ZH, Zhu R, Bai L, et al. Downregulation of aquaporin 5 induced by vector-based short hairpin RNA and its effect on MUC5AC gene expression in human airway submucosal gland cells. Respir Physiol Neurobiol. 2006;152:197–203. doi: 10.1016/j.resp.2005.08.005. [DOI] [PubMed] [Google Scholar]


