Abstract
Purpose
Treatment response in cancer has been monitored by measuring anatomic tumor volume (ATV) at various times without considering the inherent functional tumor heterogeneity known to critically influence ultimate treatment outcome: primary tumor control and survival. This study applied dynamic contrast-enhanced (DCE) functional MRI to characterize tumors’ heterogeneous subregions with low DCE values, at risk for treatment failure, and to quantify the functional risk volume (FRV) for personalized early prediction of treatment outcome.
Methods and Materials
DCE-MRI was performed in 102 stage IB2–IVA cervical cancer patients to assess tumor perfusion heterogeneity before and during radiation/chemotherapy. FRV represents the total volume of tumor voxels with critically low DCE signal intensity (<2.1 compared with precontrast image, determined by previous receiver operator characteristic analysis). FRVs were correlated with treatment outcome (follow-up: 0.2–9.4, mean 6.8 years) and compared with ATVs (Mann-Whitney, Kaplan-Meier, and multivariate analyses).
Results
Before and during therapy at 2–2.5 and 4–5 weeks of RT, FRVs >20, >13, and >5 cm3, respectively, significantly predicted unfavorable 6-year primary tumor control (p = 0.003, 7.3 × 10−8, 2.0 × 10−8) and disease-specific survival (p = 1.9 × 10−4, 2.1 × 10−6, 2.5 × 10−7, respectively). The FRVs were superior to the ATVs as early predictors of outcome, and the differentiating power of FRVs increased during treatment.
Discussion
Our preliminary results suggest that functional tumor heterogeneity can be characterized by DCE-MRI to quantify FRV for predicting ultimate long-term treatment outcome. FRV is a novel functional imaging heterogeneity parameter, superior to ATV, and can be clinically translated for personalized early outcome prediction before or as early as 2–5 weeks into treatment.
Keywords: Tumor heterogeneity, Magnetic resonance imaging (MRI), Dynamic contrast enhanced (DCE) MRI, Cervical cancer, Anatomic tumor volume, Functional tumor imaging
Introduction
A daily dilemma in managing cancer patients is the heterogeneous therapy responsiveness among different patients with the same tumor stage (1, 2) and even among different subregions within the same tumor (3). Contrary to routinely available “culture and sensitivity tests” to assess the effectiveness of a specific antibiotic agent for a specific infection in individual patients, “therapeutic sensitivity” testing to assess the effectiveness of a specific cancer therapy before or early during treatment is generally lacking.
Such tumor heterogeneity and the inability to reliably predict the long-term treatment outcome for individual patients presents a major challenge in the treatment of advanced cervical cancer (1, 2). Despite best standard-of-care concurrent radiation/chemotherapy, cure rates have not improved over the past decade, and treatment fails in approximately one third of patients (4, 5). Treatment regimens for Stages IIB–IVA are fairly uniform despite profound variability in tumor control and survival (6, 7). Although there is suggestion that more intense therapies may improve outcome (8), it remains a challenge to triage the use of intensified therapies for standard-of-care and clinical trial regimens (1, 2).
On the basis of the current established International Federation of Gynecology and Obstetrics (FIGO) staging and the anatomic tumor volume (ATV)-based response criteria (9, 10), treatment failure is frequently not detected until many months after the completion of primary therapy. At such delayed time, salvage treatment options have limited impact on the ultimate long-term treatment outcome: primary tumor control and survival (11). Therefore, early prediction of failure from an ongoing treatment is the key to enable a therapeutic window to target intensified therapy to those patients with a higher risk of failure.
Heterogeneity of tumor microenvironment has been reported with respect to regional vascular density and hypoxia, proliferation, energy metabolism, and gene expression of tumors (12–15). Although the concept of tumor heterogeneity has not been incorporated into the current clinical oncology practice nor the evidence-based cancer treatment paradigm, there is ample evidence that heterogeneous functional/biological properties of tumors profoundly influence treatment outcome (3, 12, 16, 17). With the notion of variable therapy responsiveness at different subregions within the same tumor (3, 16, 17), treatment failure is more critically influenced by at-risk subregions of the tumor, which possess unfavorable functional/biological properties, including poor perfusion and hypoxia (18–20). However, tumor heterogeneity is challenging to characterize and quantify in the clinical setting. Tissue biopsies are invasive and provide only limited sampling points of the entire heterogeneous tumor volume (21, 22). A novel noninvasive means to characterize functional heterogeneity throughout the entire tumor mass and quantify all at-risk subregions with unfavorable functional/biological properties, i.e., the tumor’s functional risk volume (FRV), would provide additional information to enhance the accuracy for early outcome prediction of a specific treatment in individual patients.
Dynamic contrast-enhanced (DCE) MRI has been valuable in assessing the microvascular structure and functional environment of tumors (23–26). DCE-MRI–based tumor perfusion status reflects the effective delivery of chemotherapy agents (12, 17, 27, 28) and oxygen (29) to tumor cells, both closely related to therapy responsiveness, tumor control and survival (17–19, 28). In cervical cancer, the well-established relationship between tumor vascularity, hypoxia, and radiation therapy response (18, 19) provides a unique tumor-biological basis to apply DCE perfusion imaging in clinical patients and explore DCE functional parameters to enhance the capability for early outcome prediction (30, 31). Poor perfusion status, reflected by low mean DCE value, has been associated with poor treatment outcome in cervical cancer (24, 31–36).
With the improved spatial and temporal resolution, clinical DCE-MRI would provide a unique opportunity to evaluate the heterogeneous functional/biological microenvironment throughout the entire tumor volume. High-precision three-dimensional (3D) anatomic and functional MRI are now available in the clinical setting (33). However, despite the promising reported correlations of tumor perfusion and treatment outcome (33–35, 37), clinical DCE imaging has not been routinely applied to characterize functional heterogeneity in clinical cervical cancer patients.
We have developed a voxelwise approach of DCE imaging to characterize each tumor voxel’s perfusion status and quantify variations in tumor perfusion throughout the heterogeneous tumor mass (36, 38). Such methodology can be readily translated into the clinical arena to assess DCE-MRI-based tumor heterogeneity and define at-risk tumor voxels with unfavorable DCE values. FRV can be derived from the summation of at-risk tumor voxels with low perfusion and hypoxia (29), which adversely affect radiation response and survival (17–20, 28, 39).
The purpose of this study was to (1) functionally characterize tumor heterogeneity and quantify the FRV, defined as the volume of critically low DCE voxels, within the heterogeneous tumor; (2) clinically validate the predictive power of FRV at different time points of treatment for ultimate treatment outcome by correlation with long-term tumor control and disease-specific survival; and (3) compare the independent power of FRV with that of ATV at different treatment time points for early prediction of outcome.
Methods and Materials
Patient population
DCE-MRI was performed in 102 Stage IB2–IVA cervical cancer patients accrued between 1994 and 2003. Patient characteristics are detailed in Table 1. Pretreatment evaluations included routine workup following the (FIGO) staging guidelines. Tumor therapy consisted of standard combined external beam radiation therapy with 6- to 24-MeV photon beams, concurrent weekly cisplatin-based chemotherapy, and standard brachytherapy. The external beam radiation dose to the pelvis ranged from 39.6 to 55.0 Gy (mean, 47.8 Gy) and was given at 1.8 to 2.0 Gy per fraction. All but four patients had brachytherapy, with Point A doses ranging from 14 to 61 Gy (mean, 39.5 Gy). Those without brachytherapy had external beam radiation therapy to a dose of 66.6 Gy using field reductions.
Table 1.
Patient characteristics
| Criteria | Patient number | Percentage |
|---|---|---|
| Age | ||
| Mean (years) | 52 | – |
| Range | 25–89 | |
| FIGO stage | ||
| I–II | 60 | 59 |
| III–IV | 42 | 41 |
| Tumor size | ||
| <6 cm | 33 | 32 |
| ≥6 cm | 69 | 68 |
| Lymph nodes | ||
| Uninvolved | 71 | 70 |
| Involved | 31 | 30 |
| Local control | ||
| Local control | 81 | – |
| Central failure* | 6 | |
| Pelvic failure† | 15 | |
| Death of disease | ||
| Alive | 62 | – |
| Dead of disease | 40 | |
Abbreviation: FIGO = International Federation of Gynecology and Obstetrics.
Failure in primary tumor/uterus without lateral pelvic or nodal failure.
Recurrence in pelvic nodes and or parametria with or without central failure.
MRI protocol
Three serial MRI studies were performed on an institutional review board–approved protocol at three defined time points: before radiation therapy, early during the treatment course at 2–2.5 weeks into treatment (corresponding to a dose of 20–25 Gy), and midway into treatment at 4–5 weeks (45–50 Gy). All MRI examinations were obtained from 1.5-T scanners. Precontrast imaging protocols included sagittal precontrast T2-weighted imaging (repetition time/effective echo time = 4000/104 msec, echo train length = 10, number of excitations = 2) for tumor delineation as described in detail earlier (32). For the initial phase of the study, DCE-MRI used T1-weighted fast spin-echo sequences (repetition time/effective echo time =150/18 msec, echo train length =4, number of excitations = 1) at 3-second intervals and a bolus injection of Gadolinium-based contrast agent (0.1 mmol/kg). More recently DCE-MRI acquisition consisted of a T1-weighted 3D gradient echo multislice sequence (echo time =5 ms, TR =12 ms, Flip = 25°, FOV = 25 × 40 cm2, Matrix = 138 × 256, Partition = 12, number of excitations =1, Slab =8.0 cm, Z oversample =40%) (31, 38). The tumor voxel size ranged from 20 to 65 mm3. The patients’ therapy was not influenced or modified by any MRI findings.
Image analysis
The tumor region was delineated on the T2-weighted image by three reviewers (NAM, WTY, and JZW). The anatomical 3D tumor volume (ATV) at the three time points (before treatment [ATV1], during early therapy at 2–2.5 weeks [ATV2], and midtherapy at 4–5 weeks [ATV3]) was derived by summation of all tumor voxels within the tumor region based on the tumor delineation from the T2-weighted images as described previously (Fig. 1a and 1e) (36). Using the first-pass DCE method (30), a time-signal intensity (SI) DCE curve was generated for each tumor voxel (Fig. 1c and 1g). Tumor voxel SI histograms (Fig. 1d and 1h), first developed in our laboratory, were derived for each individual tumor at all three time points to depict the distribution of the entire tumor voxel population (y axis) and their DCE values (plateau SI, x axis) as described in detail earlier (36). Tumor heterogeneity can be readily appreciated on the voxel SI histogram as a wide range distribution of SI values of the entire tumor voxel population.
Fig. 1.
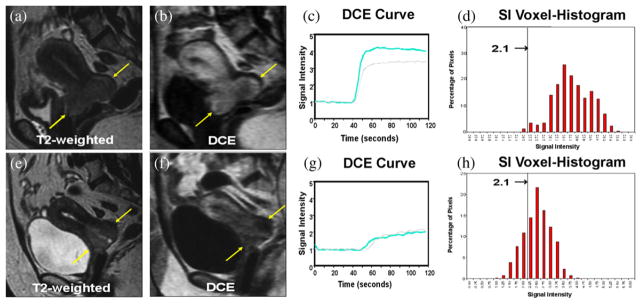
Heterogeneity of dynamic contrast-enhanced (DCE) and low perfusion. (a–d) Patient with a large Stage IIB cervical cancer (a, arrows) clinically expected to have poor outcome. At 2 weeks of therapy, this tumor shows intense enhancement (b, arrows), high perfusion on the DCE curve (c; gray: pretreatment, blue: early treatment). (d) Signal intensity (SI) voxel-histogram shows that most tumor voxels have high tumor perfusion (SI ≥2.1). This patient has been disease-free for more than 10 years. (e–h) Another Stage IIB patient with a smaller tumor (e, arrows) clinically expected to have excellent outcome. At 2 weeks, this tumor shows poor enhancement (f, arrows) and low perfusion on the DCE curve (g). (h) SI voxel-histogram shows that a much larger proportion of tumor voxels are classified as at-risk voxels (SI <2.1), compared with panel d. This patient had tumor recurrence 3 months after therapy and died 6 months later. Figure adapted with permission from Mayr NA et al. J Magn Reson Imaging 2000;12:1027–1033.
Functional risk volume
Tumor voxels with relatively lower DCE values were identified on the voxel- histogram (Fig. 1d and 1h). The functional at-risk tumor voxels were classified as those voxels with an SI < 2.1 (Fig. 1d and 1h), based on prior receiver operating characteristic (ROC) analysis that discriminated favorable from unfavorable treatment outcome (31). FRV was then derived by summation of the total number of the at-risk voxels within the tumor volume at each imaging time point (FRV1, FRV2, and FRV3) obtained at the pre-therapy, early-therapy, and mid-therapy time points, respectively.
Clinical follow-up and data collection
Patients were evaluated posttherapy in 3- to 12-month intervals until death or last contact. Median follow-up of surviving patients, calculated from therapy completion, was 6.8 years (range, 0.2–9.4 years). Primary (local) tumor control was defined as absence of histologically proven recurrence or progression of the primary tumor in the cervix, uterus, or pelvis. For disease-specific survival, death from cervical cancer or complications of cancer was scored as event, and death of intercurrent disease was censored.
Statistical analysis
FRVs at each treatment imaging time point (FRV1, FRV2, and FRV3) were correlated with primary tumor control and disease-specific survival endpoints (Mann-Whitney test). The optimal volume cutpoint values of FRVs at each imaging time point to differentiate the disease-specific survival vs. death were determined by ROC analysis. These optimal cutpoint values for FRVs were then applied for FRV1, FRV2, and FRV3, respectively, to correlate with primary tumor control and disease-specific survival (Kaplan-Meier analysis, log-rank test). The corresponding ATVs and FRVs at the same treatment time point were compared as independent predictors for the early prediction of long-term treatment outcome using multivariate analysis.
Results
FRVs and ATVs obtained at the different treatment time points are summarized in Table 2. Overall, the mean FRVs were smaller than the ATVs at the corresponding imaging time points. Both ATVs and FRVs showed a wide range within clinical tumor stages and steadily decreased during the treatment course (Table 3).
Table 2.
Mean, range, and SD of ATVs and FRVs at the three imaging time points
| ATV1 | ATV2 | ATV3 | FRV1 | FRV2 | FRV3 | |
|---|---|---|---|---|---|---|
| Mean | 74.421 | 42.097 | 17.187 | 42.513 | 19.633 | 8.941 |
| Range | 4.3–341.7 | 1.9–307.3 | 0–191.8 | 1.03–237.8 | 0.57–123.1 | 0–62.2 |
| SD | 62.062 | 44.798 | 24.683 | 45.314 | 23.359 | 12.149 |
Abbreviations: ATV = anatomic tumor volume; FRV = functional risk volume.
Table 3.
Correlation of FRV with primary tumor control
| FRV1 | FRV2 | FRV3 | |
|---|---|---|---|
| Below FRV cutpoint vs. Above FRV cutpoint | 97.3% vs. 73.0% | 98.3% vs. 55.8% | 100.0% vs. 54.8% |
| p (Kaplan-Maier) | 0.003 | 7.3 × 10−8 | 2.0 × 10−8 |
| p (Mann-Whitney) | 0.002 | 1.0 × 10−7 | 1.5 × 10−8 |
Abbreviation: FRV = functional risk volume.
The FRV cutpoints, determined by receiver operator characteristic analysis to differentiate between favorable and poor outcomes, are 20 cm3 (FRV1), 13 cm3 (FRV2), and 5 cm3 (FRV3), derived from DCE-MR obtained before, at 2–2.5 weeks, or 4–5 weeks into treatment, respectively. Intratreatment FRV2 and FRV3, which are obtained after exposure to cytotoxic treatment, show larger deferences than FRV1 in the predicted tumor treatment outcome.
ROC analysis identified the optimal cut-point values of the FRV at each treatment time point, which significantly discriminated disease-specific survival vs. death of cancer (Table 4). The optimal cutpoint values for FRV1, FRV2, and FRV3 were 20 cm3 (area under the curve [AUC] = 0.705), 13 cm3 (AUC = 0.799), and 5 cm3 (AUC = 0.796), respectively.
Table 4.
Correlation of FRV with disease-specific survival
| FRV1 | FRV2 | FRV3 | |
|---|---|---|---|
| Below FRV cutpoint vs. Above FRV cutpoint | 94.6% vs. 53.1% | 87.9% vs. 40.9% | 88.0% vs. 39.5% |
| p (Kaplan-Maier) | 1.9 × 10−4 | 2.1 × 10−6 | 2.5 × 10−7 |
| p (Mann-Whitney) | 1.8 × 10−4 | 7.3 × 10−7 | 4.6 × 10−6 |
Abbreviation: FRV = functional risk volume.
FRV cutpoints and timing as in Table 2.
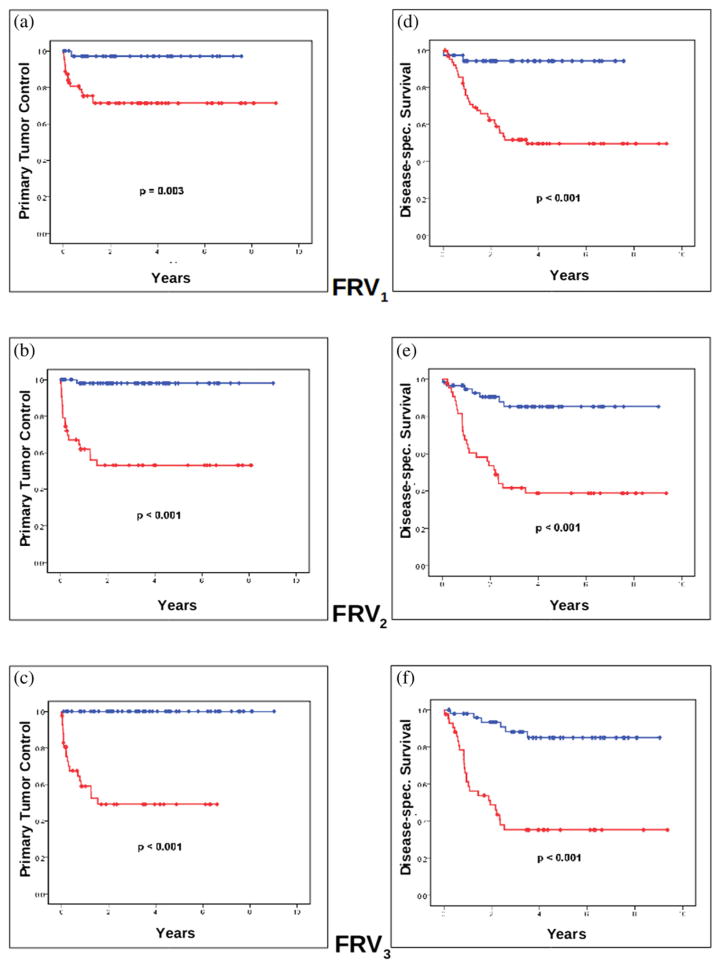
FRV1, FRV2, and FRV3 were significant early predictors of ultimate long-term treatment outcome, clinically validated by the outcome endpoints of primary tumor control and disease-specific survival rate (Tables 3 and 4, Fig. 2) with a mean posttherapy follow-up of 6.8 years. The predictive power of FRVs for treatment outcome also increased during the treatment course (Tables 3, and 4, Fig. 2). Pretreatment FRV1 had the least predictive power when compared with the intratreatment FRV2 and FRV3 based on the magnitude of differences in outcome. The differences discriminated by FRV in the predicted 6-year actuarial tumor control rate vs. primary tumor recurrence increased from 24.3% for FRV1 to 42.5% for FRV2 and 45.2% for FRV3 (Table 3, Fig. 2a–2c). The differences in disease-specific survival rate vs. cancer death increased from 41.5% to 47% and 48.5%, respectively (Table 4, Fig. 2d–2f). Multivariate analysis showed that FRV2 and FRV3 were superior to the ATVs as independent predictors of tumor control and disease-specific survival (Table 5).
Fig. 2.
Kaplan-Meier analysis of primary tumor control and disease-specific survival. Kaplan-Meier estimates differentiate primary tumor control rate vs. tumor recurrence for (a) FRV1, (b) FRV2, and (c) FRV3. Kaplan-Meier estimates for disease-specific survival rate vs. death from disease for (d) FRV1, (e) FRV2, and (f) FRV3. The blue graphs indicate groups with FRV above cutpoint, the red graphs groups below the FRV cutpoint. More detailed statistical analysis is summarized in Tables 3 and 4. FRV = functional risk volume; spec. = specific.
Table 5.
Comparison of ATVs and FRVs as independent predictors for primary tumor control and disease-specific survival
| ATV1 | ATV2 | ATV3 | FRV1 | FRV2 | FRV3 | |
|---|---|---|---|---|---|---|
| Local control | ||||||
| p | 0.567 | 0.175 | 0.077 | 0.065 | 0.020 | 0.004 |
| HR | 1.6 | 3.2 | 3.6 | 7.9 | 5.4 | 7.4 |
| CI | 0.32–8.02 | 0.59–17.38 | 0.87–14.69 | 0.88–68.96 | 1.30–22.61 | 1.92–28.74 |
| Disease-specific survival | ||||||
| p | 0.195 | 0.173 | 0.316 | 0.053 | 0.020 | <0.001 |
| HR | 1.9 | 2.0 | 0.6 | 4.5 | 3.3 | 7.9 |
| CI | 0.72–5.02 | 0.73–5.79 | 0.17–1.52 | 0.98–20.81 | 1.21–8.91 | 2.97–20.87 |
Abbreviations: ATV = anatomic tumor volume; CI = 95% confidence interval; FRV = functional risk volume; HR = hazard ratio.
Multivariate analysis incorporating ATV, FRV, stage and lymph node status shows that FRV2 and FRV3 are superior to ATVs as independent predictors of local control and disease-specific survival.
Discussion
Improvement of outcome by early prediction of treatment failure
Overall approximately one third of advanced cervical cancer patients fail standard first-line treatment (6, 40) despite extensive use of concurrent radiation and chemotherapy and high-end clinical imaging. Although heterogeneous treatment response throughout Stage IB2–IVA cervical cancer is well known, the treatment regimen remains largely uniform (“one size fits all”) for patients across these tumor stages (6, 7). The lack of personalized treatment strategy for individual patients, known to have heterogeneous treatment outcome (1, 2), likely plays an important role in the lack of improvement in treatment outcomes over the past decade (4). To provide personalized treatment strategy, early prediction of treatment outcome for each individual patient is key. Thus it is paramount to identify this significant number of high-risk patients early during their treatment course to provide a window of opportunity for adaptive therapy. In addition, low-risk patients, predicted early to have favorable treatment outcome, may benefit from less-intense therapy regimens, thereby reducing treatment-related morbidity, mortality, and health care cost.
Integration of morphologic and functional imaging methods to characterize tumor heterogeneity and target at-risk tumor subvolume (FRV) for early outcome prediction
Prediction of treatment failure is particularly challenging to make before or early during the treatment course, when targeted adjustments in therapy are more available and generally more effective (6, 11). By applying the known underlying pathophysiologic principles of low perfusion, hypoxia, and therapy resistance (17–20, 28, 39), our results support our hypothesis that the low-DCE subvolumes (FRV) within the heterogeneous tumor volume contribute to unfavorable treatment outcome. The at-risk tumor voxels with poor perfusion, critically low enough to result in therapy failure, were classified as having a DCE SI value <2.1, which had been validated previously (31). In our current research, both high-resolution 3D anatomic and functional imaging methodologies were integrated to target more effectively the essential underlying biology, and to more precisely derive FRV throughout the entire tumor with precision at the tumor voxel level. Such integrated imaging approach overcomes the challenge of irregular tumor morphology from the World Health Organization and Response Evaluation Criteria in Solid Tumors methodologies (9, 10), as well as the limitations of mean DCE values, averaged over the entire tumor volume, which do not characterize tumor heterogeneity (31). Furthermore, our methodology eliminates the need for tedious imaging manipulation to identify and match each tumor voxel sequentially at different treatment time points. Rather, our approach was to analyze the overall critical mass of at-risk voxels within the entire tumor at different treatment time points for outcome prediction. Although our methodology does not achieve precision at the microscopic/cellular level, it provides a noninvasive practical approach of functional heterogeneity assessment throughout the entire tumor region with a precision equivalent to single imaging voxel size currently available in the clinical setting.
Clinical validation and database
Advanced cervical cancer is an ideal model for ultra-early imaging and clinical validation of early outcome prediction, because it is not treated surgically, allowing sequential imaging before and during treatment. Because most treatment failures occur within 2 years, our patient cohort provided solid clinical endpoints for statistical analysis and clinical validation of the novel FRV concept and its predictive power. Although these results may be limited by the relatively small patient numbers and the relatively large number of imaging parameters, our study has the largest patient population with DCE MRI in cervical cancer with the longest follow-up reported to date.
FRV cutpoint values
ROC analysis defined the optimal cutpoint values of the FRV at different treatment time points (FRV1 = 20 cm3, FRV2 = 13 cm3, and FRV3 = 5 cm3) to differentiate patients with favorable vs. unfavorable treatment outcome (Tables 3 and 4, Fig. 2). Such integrated morphologic and functional imaging approach provides a robust methodology that can readily translate into the clinical setting to guide therapy strategy. Our results further suggest that treatment failure depends not only on how low the voxels’ DCE value is (quality of low perfusion) but also on the critical mass of low-DCE subvolume within the tumor (quantity of low perfusion; Tables 3 and 4, Fig. 2). FRV contains both these metrics and translates this critical functional imaging information into clinically significant therapy resistance to radiation and chemotherapy and unfavorable treatment outcome. Low-DCE volumes (FRV) as small as 13 cm3 in early therapy (20–25 Gy) and 5 cm3 in midtherapy (40–50 Gy) within the heterogeneous tumor were shown to impart primary tumor recurrence and cancer death (Tables 3 and 4, Fig. 2).
FRVs during treatment
In addition to FRV, the efficacy for early prediction of outcome also depends on the ability to incorporate essential information of the “therapeutic sensitivity” of a tumor to an ongoing therapy regimen. Such essential therapy-specific sensitivity information can only be reflected by the imaging parameters that are obtained during the treatment course, rather than those before treatment. FRVs decreased during the ongoing course of fractionated radiation therapy (Table 2), likely because of cell kill, both within the FRVs, and, to a greater degree, in the non-FRV tumor regions. Cell kill within the initial poorly perfused/oxygenated FRVs likely improves during ongoing radiotherapy through improvement of perfusion and reoxygenation. Patients with smaller subsequent FRVs had better outcome.
Our results show that the predictive power improved as early as 2 weeks into treatment (FRV2) compared with the pretherapy imaging (FRV1; Tables 3 and 4). Although the FRV3 at the fourth–fifth treatment week (40–50 Gy) suggests a slight trend of better outcome prediction (Tables 3 and 4, Fig. 2, Kaplan-Meier analysis), FRV2 enables treatment outcome prediction at a much earlier time point in the second treatment week, when only approximately one fourth of the total radiation dose and 2 of the 5–7 chemotherapy cycles have been given. At that time, there is greater latitude to implement very early adjustments of the cytotoxic therapy regimen. However, the comparative efficacy between FRV2 and FRV3 remains to be determined in future studies. In addition, the comparative or complementary value of other functional imaging modalities, e.g., diffusion MRI or PET, remains to be determined.
Comparison of FRV and ATV
Our results support the notion that the FRVs during therapy are superior to the ATVs obtained at the corresponding time points for early prediction of treatment outcome (Table 5). The addition of functional/biological information significantly improves the ability to predict tumor control and survival compared with morphologic tumor volume alone. Among our patient group, FRV and ATV were highly variable (Table 2), indicating that heterogeneity was pervasive across the traditional morphological tumor volume, clinical FIGO stage categories and imaging time points. This observation indirectly supports the concept that current established clinical criteria, including FIGO stage, World Health Organization, and Response Evaluation Criteria In Solid Tumors (9, 10), have inherent limitations for early outcome prediction and may play a role for our inability to implement timely personalized treatment strategy and the lack of improvement in treatment outcome.
Conclusion
Our results suggest that tumor-heterogeneity-based DCE parameters provide essential information of tumor biological/functional properties that critically influence long-term treatment outcome. The ability to characterize DCE heterogeneity by a voxelwise approach, classify the at-risk tumor voxels, and quantify FRV significantly improves early prediction of treatment outcome over the traditional morphology-based ATV. Therefore, FRVs are potential novel heterogeneity parameters that can augment the current established clinical staging and ATV response criteria. If reconfirmed with future studies, FRVs at various treatment time points can serve as noninvasive early outcome predictors for clinical translation and may guide therapy adaptation as a personalized theranostic paradigm to further improve treatment outcome. This new FRV concept and our methodology may also be applicable to derive tumor heterogeneity parameters in other tumors to enhance cancer therapy and to improve clinical trials by focusing novel cancer therapy regimens at more targeted patient cohorts with higher risk of therapy failure.
Summary.
The functional risk volume (FRV) is the absolute volume within the heterogeneous tumor that is characterized by low dynamic contrast enhancement, indicative of poor perfusion and hypoxia. This outcome study in cervical cancer patients shows that FRV correlates with local tumor control, disease specific and overall survival. FRV appears to be a better predictor of therapy outcome than the anatomical tumor volume.
Acknowledgments
Nina A. Mayr and William T. C. Yuh are supported by National Institute of Health Grant No. RO1 CA71906.
This work is dedicated to Jian Z. Wang, Ph.D., who critically contributed to this research. Dr. Wang tragically passed away in 2010.
Footnotes
Presented at American Society for Therapeutic Radiology and Oncology Annual Meeting, San Diego, CA, October 31, 2010.
Conflict of interest: none.
References
- 1.Eifel PJ. Problems with the clinical staging of carcinoma of the cervix. Semin Radiat Oncol. 1994;4:1–8. doi: 10.1053/SRAO00400001. [DOI] [PubMed] [Google Scholar]
- 2.Eifel PJ, Morris M, Wharton JT, et al. The influence of tumor size and morphology on the outcome of patients with FIGO stage IB squamous cell carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 1994;29:9–16. doi: 10.1016/0360-3016(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 3.Lyng H, Vorren AO, Sundfor K, et al. Intra- and intertumor heterogeneity in blood perfusion of human cervical cancer before treatment and after radiotherapy. Int J Cancer. 2001;96:182–190. doi: 10.1002/ijc.1019. [DOI] [PubMed] [Google Scholar]
- 4.International Agency for Research on Cancer, World Health Organization. Globocan 2008. Cervical cancer incidence and mortality worldwide in 2008. Summary. Available at: http://globocan.iarc.fr/factsheets/cancers/cervix.asp#MORTALITY.
- 5.American Cancer Society. Global cancer facts and figures 2007. 2007 Available at: http://www.cancer.org/downloads/STT/Global_Cancer_Facts_and_Figures_2007_rev.pdf.
- 6.Eifel PJ, Winter K, Morris M, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: An update of radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol. 2004;22:872–880. doi: 10.1200/JCO.2004.07.197. [DOI] [PubMed] [Google Scholar]
- 7.Torres MA, Jhingran A, Thames HD, Jr, et al. Comparison of treatment tolerance and outcomes in patients with cervical cancer treated with concurrent chemoradiotherapy in a prospective randomized trial or with standard treatment. Int J Radiat Oncol Biol Phys. 2008;70(1):118–125. doi: 10.1016/j.ijrobp.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 8.Chemoradiotherapy for Cervical Cancer Meta-analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: Individual patient data meta-analysis. J Clin Oncol. 2008;26:5802–5812. doi: 10.1200/JCO.2008.16.4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO handbook for reporting results of cancer treatment, Publication Number 48. Geneva: World Health Organization; 1979. Available at: http://whqlibdoc.who.int.publications/9241700483.pdf. [Google Scholar]
- 10.James K, Eisenhauer E, Christian M, et al. Measuring response in solid tumors: Unidimensional versus bidimensional measurement. J Natl Cancer Inst. 1999;91:523–528. doi: 10.1093/jnci/91.6.523. [DOI] [PubMed] [Google Scholar]
- 11.Kastritis E, Bamias A, Efstathiou E, et al. The outcome of advanced or recurrent non-squamous carcinoma of the uterine cervix after platinum-based combination chemotherapy. Gynecol Oncol. 2005;99:376–382. doi: 10.1016/j.ygyno.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 12.Brizel DM, Rosner GL, Prosnitz LR, et al. Patterns and variability of tumor oxygenation in human soft tissue sarcomas, cervical carcinomas and lymph node metastases. Int J Radiat Oncol Biol Phys. 1995;32:1121–1125. doi: 10.1016/0360-3016(95)00106-9. [DOI] [PubMed] [Google Scholar]
- 13.Koukourakis MI, Giatromanolaki A, Sivridis E, et al. Cancer vascularization: Implications in radiotherapy? Int J Rad Oncol Biol Phys. 2000;48:545–553. doi: 10.1016/s0360-3016(00)00677-5. [DOI] [PubMed] [Google Scholar]
- 14.Pugachev A, Ruan S, Carlin S. Dependence of FDG uptake on tumor microenvironment. Int J Radiat Oncol Biol Phys. 2005;62:545–553. doi: 10.1016/j.ijrobp.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Bachtiary B, Boutros PC, Pintilie M. Gene expression profiling in cervical cancer: An exploration of intratumor heterogeneity. Clin Cancer Res. 2006;12:5632–5640. doi: 10.1158/1078-0432.CCR-06-0357. [DOI] [PubMed] [Google Scholar]
- 16.Britten RA, Evans AJ, Allalunis-Turner MJ, et al. Intratumoral heterogeneity as a confounding factor in clonogenic assays for tumour radioresponsiveness. Radiother Oncol. 1996;39:145–153. doi: 10.1016/0167-8140(96)01719-7. [DOI] [PubMed] [Google Scholar]
- 17.Galmarini FC, Galmarini CM, Sarchi MI, et al. Heterogeneous distribution of tumor blood supply affects the response to chemotherapy in patients with head and neck cancer. Microcirculation. 2000;7:405–410. [PubMed] [Google Scholar]
- 18.Höckel M, Schlenger K, Mitze M, et al. Hypoxia and radiation response in human tumors. Semin Radiat Oncol. 1996;6:3–9. doi: 10.1053/SRAO0060003. [DOI] [PubMed] [Google Scholar]
- 19.Dunst J, Kuhnt T, Strauss HG, et al. Anemia in cervical cancers: impact on survival, patterns of relapse, and association with hypoxia and angiogenesis. Int J Radiat Oncol Biol Phys. 2003;56:778–787. doi: 10.1016/s0360-3016(03)00123-8. [DOI] [PubMed] [Google Scholar]
- 20.Vaupel P. Oxygenation of solid tumors. In: Teicher BA, editor. Drug resistance in oncology. New York: Marcel Dekker; 1993. pp. 53–85. [Google Scholar]
- 21.Latargau E, Randranarivelo HLM. Oxygen tension measurements in human tumors: The Institute Gustave-Roussy experience. Radiat Oncol Invest. 1994;1:285–291. [Google Scholar]
- 22.Urtasun RC, Chapman JD, JAR, et al. Binding of H-3-misonidazole to solid human tumors as a measure of tumor hypoxia. Int J Radiat Oncol Biol Phys. 1986;12:1263–1267. doi: 10.1016/0360-3016(86)90273-7. [DOI] [PubMed] [Google Scholar]
- 23.Brash RC, Li KC, Husband JE, et al. In vivo monitoring of tumor angiogenesis with MR imaging. Acad Radiol. 2000;7:812–823. doi: 10.1016/s1076-6332(00)80630-3. [DOI] [PubMed] [Google Scholar]
- 24.Mayr NA, Hawighorst H, Yuh WTC, et al. MR microcirculation in cervical cancer: Correlations with histomorphological tumor markers and clinical outcome. J Magn Reson Imaging. 1999;10:267–276. doi: 10.1002/(sici)1522-2586(199909)10:3<267::aid-jmri7>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 25.Brix G, Rempp K, Guckel F. Quantitative assessment of tissue microcirculation by dynamic contrast-enhanced MR imaging. Adv MRI Contrast. 1994;2:68–77. [Google Scholar]
- 26.Neeman M, Dafni H. Structural, functional, and molecular MR imaging of the microvasculature. Ann Rev Biomed Eng. 2003;5:29–56. doi: 10.1146/annurev.bioeng.5.040202.121606. [DOI] [PubMed] [Google Scholar]
- 27.Link KH, Leder G, Pillasch J. In vitro concentration response studies and in vitro Phase II tests as the experimental basis for regional chemotherapeutic protocols. Semin Surg Oncol. 1998;14:189–201. doi: 10.1002/(sici)1098-2388(199804/05)14:3<189::aid-ssu2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 28.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 29.Ellingsen C, Natvig I, Gaustad JV, et al. Human cervical carcinoma xenograft models for studies of the physiological microenvironment of tumors. J Cancer Res Clin Oncol. 2009;135:1177–1184. doi: 10.1007/s00432-009-0558-8. [DOI] [PubMed] [Google Scholar]
- 30.Yuh WTC. An exciting and challenging role for the advanced contrast MR imaging. J Magn Reson Imaging. 1999;10:221–222. doi: 10.1002/(sici)1522-2586(199909)10:3<221::aid-jmri1>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 31.Yuh WTC, Mayr NA, Jarjoura D, et al. Predicting control of primary tumor and survival by DCE MRI during early therapy in cervical cancer. Invest Radiol. 2009;44:343–350. doi: 10.1097/RLI.0b013e3181a64ce9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayr NA, Yuh WTC, Magnotta VA, et al. Tumor perfusion studies using fast magnetic resonance imaging technique in advanced cervical cancer—A new noninvasive predictive assay. Int J Radiat Oncol Biol Phys. 1996;36:623–633. doi: 10.1016/s0360-3016(97)85090-0. [DOI] [PubMed] [Google Scholar]
- 33.Gong QY, Brunt JN, Romaniuk CS, et al. Contrast enhanced dynamic MRI of cervical carcinoma during radiotherapy: Early prediction of tumour regression rate. Br J Radiol. 1999;72:1177–1184. doi: 10.1259/bjr.72.864.10703475. [DOI] [PubMed] [Google Scholar]
- 34.Loncaster JA, Carrington BM, Sykes JR, et al. Prediction of radiotherapy outcome using dynamic contrast enhanced MRI of carcinoma of the cervix. Int J Rad Oncol Biol Phys. 2002;54:759–767. doi: 10.1016/s0360-3016(02)02972-3. [DOI] [PubMed] [Google Scholar]
- 35.Yamashita Y, Baba T, Baba Y, et al. Dynamic contrast-enhanced MR imaging of uterine cervical cancer: Pharmacokinetic analysis with histopathologic correlation and its importance in predicting the outcome of radiation therapy. Radiology. 2000;216:803–809. doi: 10.1148/radiology.216.3.r00se07803. [DOI] [PubMed] [Google Scholar]
- 36.Mayr NA, Yuh WTC, Arnholt JC, et al. Pixel analysis of MR perfusion imaging in predicting radiation therapy outcome in cervical cancer. J Magn Reson Imaging. 2000;12:1027–1033. doi: 10.1002/1522-2586(200012)12:6<1027::aid-jmri31>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 37.Zahra MA, Tan LT, Priest AN, et al. Semiquantitative and quantitative dynamic contrast-enhanced magnetic resonance imaging measurements predict radiation response in cervix cancer. Int J Radiat Oncol Biol Phys. 2009;74:766–773. doi: 10.1016/j.ijrobp.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 38.Mayr NA, Yuh WTC, Jajoura D, et al. Ultra-early predictive assay for treatment failure using functional magnetic resonance imaging and clinical prognostic parameters in cervical cancer. Cancer. 2010;116:903–912. doi: 10.1002/cncr.24822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weidner N. Tumoral vascularity as a prognostic factor in cancer patients: The evidence continues to grow. J Pathol. 1998;184:119–122. doi: 10.1002/(SICI)1096-9896(199802)184:2<119::AID-PATH17>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 40.Green JA, Kirwan JM, Tierney JF, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: A systematic review and meta-analysis. Lancet. 2001;358:781–786. doi: 10.1016/S0140-6736(01)05965-7. [DOI] [PubMed] [Google Scholar]