Abstract
Subarachnoid hemorrhage (SAH) is a devastating condition with high morbidity and mortality rates due to the lack of effective therapy. Early brain injury (EBI) and cerebral vasospasm (CVS) are the two most important pathophysiological mechanisms for brain injury and poor outcomes for patients with SAH. CVS has traditionally been considered the sole cause of delayed ischemic neurological deficits after SAH. However, the failure of antivasospastic therapy in patients with SAH supported changing the research target from CVS to other mechanisms. Currently, more attention has been focused on global brain injury within 3 days after ictus, designated as EBI. The dysfunction of subcellular organelles, such as endoplasmic reticulum stress, mitochondrial failure, and autophagy–lysosomal system activation, has developed during EBI and delayed brain injury after SAH. To our knowledge, there is a lack of review articles addressing the direction of organelle dysfunction after SAH. In this review, we discuss the roles of organelle dysfunction in the pathogenesis of SAH and present the opportunity to develop novel therapeutic strategies of SAH via modulating the functions of organelles.
Keywords: Organelles, Subarachnoid Hemorrhage, Early Brain Injury, Cerebral Vasospasm, Therapy
Introduction
Subarachnoid hemorrhage (SAH), which only accounts for 5 % of stroke, is often a devastating condition because of significant morbidity and mortality [58, 73]. Among SAH, 85 % are caused by intracranial ruptured aneurysms, termed spontaneous aneurysmal SAH [63]. Although much progress has been made with the surgical clip and endovascular coil for intracranial ruptured aneurysms over the last decade, long-term outcomes for patients with SAH are still unsatisfactory [9, 49]. Further elucidation of the pathogenesis is helpful for developing novel therapeutic interventions for SAH.
To date, early brain injury (EBI) and cerebral vasospasm (CVS) are the two most important determinants for poor outcome in patients with SAH [43, 55]. CVS, which occurs between 3 and 14 days after SAH [11], is traditionally considered the sole cause of delayed ischemic neurological deficits (DINDs) [23, 51]. Endothelin (ET)-1, a potent vaso-constrictor, plays a key role in CVS after SAH [53]. However, randomized, double-blind, placebo-controlled trials demonstrated that the ET-1 receptor antagonist, clazosentan, which can significantly ameliorate angiographic vasospasm, failed to improve functional outcomes in patients with SAH [35, 36]. Furthermore, CVS was a common imaging finding in approximately 70 % of patients with SAH, but only one-third of those patients went on to suffer from DINDs [1]. Those findings suggest that SAH-induced DINDs may be a result of multiple factors. The importance of EBI (which occurs within the first 72 h after SAH) has recently been emphasized because of its potentially critical role in the pathophysiology of SAH [5]. Inflammation, oxidative stress, excitotoxicity, and impaired ionic homeostasis (but not mechanical force) have all been proposed as having a role in EBI and other types of stroke [56, 60]. To date, studies of the alteration of organelles after SAH have included endoplasmic reticulum (ER) stress, mitochondrial dysfunction, and activation of the autophagy–lysosomal system. Neurobehavioral deficits were dependent on the disturbance of organelles in several kinds of cerebral cells. A new review focusing on disturbance or alteration of organelle function is helpful in understanding the pathophysiology of SAH [16]. Targeting organelles may provide a novel therapeutic potential for SAH treatment.
Appropriate animal models are imperative in understanding the pathogenesis of and treatment strategies for SAH [61]. This review summarizes preclinical evidence of the functional alteration of subcellular organelles in the pathogenesis of SAH. This is followed by a discussion of future research directions in developing new therapeutic strategies of SAH via modulating the organelles.
The Functional Alteration of Organelles Within the Progression of SAH
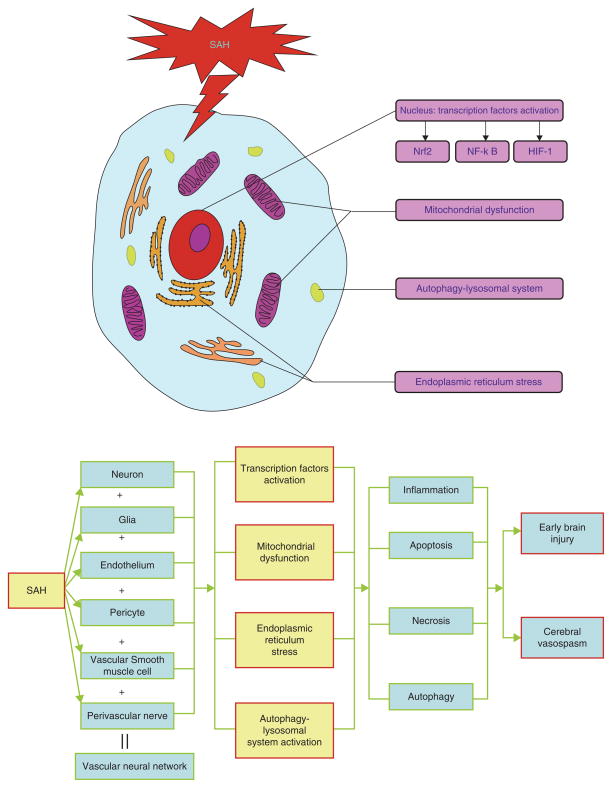
The main subcellular organelles in central nervous system (CNS) cells are the nucleus, ER, mitochondria, lysosomes, ribosomes, and Golgi body. Experimental studies have demonstrated that some subcellular organelles, including the ER, mitochondria, and autophagy–lysosomal system, have altered functions after SAH and are implicated in the pathophysiology of SAH. In the following sections, we describe the underlying roles of these organelles in SAH (Fig. 1).
Fig. 1.
The functional disturbance of organelles in the pathogenesis of SAH. The components of the vascular neural network of the brain, including neurons, glia, endothelium, pericytes, vascular smooth muscle cells, and perivascular nerves, all suffer from SAH-induced injuries. The dysfunctions/functional alterations of the organelles that take place are in the transcription factors (e.g., Nrf2, NF-κB, and HIF-1), mitochondrial dysfunction, endoplasmic reticulum stress, and the autophagy–lysosomal system. These pathophysiologic cascades play a critical role in inflammation, apoptosis, necrosis, and autophagy in brain parenchyma after SAH
Nucleus: Transcription Factor Activation
The Nuclear Factor-Erythroid 2-Related Factor 2 (Nrf2)-Antioxidant Response Element (ARE) Signaling
The nuclear factor-erythroid 2-related factor 2 (Nrf2)–antioxidant response element (ARE) pathway was the key regulator in maintaining cellular homeostasis via antioxidant defense, making it a therapeutic candidate for SAH [3]. Nrf2 is a cap ‘n’ collar (CNC) transcription factor, which possesses a basic region leucine zipper structure [76]. In the latent state, Nrf2 is sequestered by Kelch-like erythroid cell-derived protein with CNC homology-associated protein 1 (Keap1)-dependent ubiquitination–proteasomal degradation in the cytoplasm. Modifying critical cysteine thiols of Keap1 and Nrf2 by some oxidants promotes Nrf2 dissociation from the Keap1/Nrf2 complex and translocation into nuclei. Subsequently, Nrf2 binds to ARE in the promoter of cytoprotective genes leading to upregulated expression of relevant proteins, such as heme oxygenase-1, NAD(P)H:quinone oxidoreductase 1, and glutathione-S-transferase [34, 64].
Evidence from experimental SAH research indicates a protective role of the Nrf2/ARE pathway in EBI and CVS after SAH. Nrf2/ARE signaling was activated during the EBI period after SAH [66, 74]. Post-SAH treatment with melatonin and recombinant human erythropoietin reduced brain edema and improved neurobehavioral outcome via activating the Nrf2/ARE pathway and modulating oxidative stress after SAH, making these drugs promising for treatment. In addition, an elevated level of Nrf2 was detected in endothelial and smooth muscle cells in the basilar arterial walls [65]. The activation of Nrf2 increased in the arterial wall, parallel to the development of basilar artery vasospasm, in a double-injection SAH rabbit model. Because of the elevated expression of Nrf2, the Nrf2/ARE pathway was hypothesized to prevent CVS after SAH [78]. In an in vitro SAH model, sulforaphane, an agonist of the Nrf2–ARE pathway, can inhibit oxyhemoglobin (OxyHb)-induced inflammatory cytokine, such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α, release in vascular smooth muscle cells [77]. Oxyhemoglobin-induced inflammation was aggravated in astrocytes from Nrf2-knockout mice via nuclear factor (NF)-κB signaling [45]. Thus, the Nrf2/ARE pathway may exert anti-inflammatory and antioxidative effects that contribute to alleviation of EBI and CVS after SAH. However, additional in vivo experiments using Nrf2 agonists or antagonists are required to further investigate the role of Nrf2 on SAH-induced brain injury.
NF-κB Signaling
NF-κB signaling is involved in various CNS disorders because it regulates immune and inflammatory responses including infection, brain trauma, neurodegenerative diseases, and stroke [48, 54]. In mammalian cells, the NF-κB family of transcription factors consists of five members, Rel A (p65), c-Rel, Rel B, p50, and p52. Under inactive conditions, NF-κB is sequestered in the cytoplasm by binding with IκB family members. The IκB kinase (IKK) enzyme complex can phosphorylate IκB proteins to release active NF-κB, leading to translocation of NF-κB into the nucleus. Subsequently, NF-κB dimmers (p50–p65) are free to bind to the promoters of genes of inflammatory mediators and increase the release of those mediators [42]. An in vitro study demonstrated that the phosphorylation of IκB diminishes its association with NF-κB, leading to NF-κB translocation into the nucleus, where NF-κB binds to the promoter of nitric oxide synthase (NOS)-2 in endothelial cells [8].
NF-κB signaling in SAH has been explored and some trials are ongoing trials. Toll-like receptor (TLR)-4 is an important upstream receptor of NF-κB. At the acute stage of SAH, TLR4/NF-κB signaling is significantly activated, suggesting that this pathway may regulate the inflammatory response in experimental SAH [33]. Post-SAH administration of progesterone attenuated EBI via suppressing the activation of TLR4/NF-κB signaling in the cortex after SAH [68]. p65, a nuclear NF-κB subunit, was overexpressed in the basilar artery, which indicated that the NF-κB-mediated inflammatory response may also facilitate the development of CVS after SAH. Intracisternal administration of pyrrolidine dithiocarbamate, an inhibitor of NF-kB, reduced the levels of TNF-α, IL-1β, intercellular adhesion molecule (ICAM)-1, and vascular cell adhesion molecule (VCAM)-1 and alleviated CVS in a rat model of SAH [79]. In addition, Nle4, DPhe7-α-MSH (NDP-MSH) and trehalose exerted protective effects on CVS via inhibiting NF-κB signaling in the basilar artery [13, 15]. Similarly, 6-mercaptopurine increased the level of IκB, downregulating NF-κB activity. Thus, 6-mercaptopurine was capable of hindering the production of inflammatory cytokines (e.g., IL-1β, IL-6, and TNF-α) after SAH. The anti-inflammatory effect of 6-mercaptopurine contributed to its antivasospastic property in SAH animals [7]. Furthermore, because of the association of p65 with the estrogen receptor, 17β-estradiol blocked the binding of p65 to the gene target inducible NOS (iNOS). Therefore, this hormone drug reduced iNOS and showed a neuroprotective effect on CVS [57]. The activation of NF-κB was biphasic in a single injection rabbit SAH model. The peaks of NF-κB activity occurred around day 3 and day 10 after SAH. The first peak plays a prominent role in neuronal injury, but the exact role of the second peak requires additional investigation [72].
In conclusion, these data indicated an essential role of the NF-κB pathway in the pathogenesis of EBI and CVS after SAH.
Hypoxia-Inducible Factor (HIF)-1
HIF-1 is a critical regulator of cellular adaptation to hypoxic stress and is a heterodimeric DNA-binding complex composed of one α- and one β-subunit [30, 37]. Cytoplasmic HIF-1α is continuously degraded via ubiquitination in normoxic conditions. However, in hypoxia, the proteasomal degradation of HIF-1α is inhibited, leading to HIF-1α accumulation. Nondegraded HIF-1α recruits HIF-1β to form the functional HIF complex, which enters the nucleus. HIF-1 binds in the location of hypoxia response elements to induce the transcription activation of these genes (e.g., erythropoietin, vascular endothelial growth factor (VEGF), and heme oxygenase (HO)-1) [2].
During the EBI, the expression of HIF-1α, VEGF, and BNIP3 were increased in the hippocampus and cortex [44]. Hyperbaric oxygen reduced the expression of HIF-1α and its target genes (including VEGF and BNIP3), which resulted in fewer apoptotic cells [12]. A recent study demonstrated that HIF-1α may exert a deleterious effect in EBI by upregulating its downstream proteins BNIP3 and VEGF, resulting in cell apoptosis, blood brain barrier (BBB) disruption, and brain edema [69]. However, 3-(5′-hydroxymethyl-2′-furyl)-1-benzylindazole (YC-1), a HIF-1α inhibitor, increased cell apoptosis in the hippocampus and increased cognitive function damage in SAH rats, suggesting that HIF-1α might exert beneficial effects in SAH [10].
In the brainstem, an upregulated HIF-1α protein level, but not mRNA level, was detected in the acute (10 min) and chronic (7 days) phases of SAH. The difference in levels between protein and mRNA is not yet clear. Deferoxamine promoted the expression and activity of HIF-1α, which ameliorated basilar artery vasospasm [20]. Additionally, isoflurane significantly attenuated vasospasm by increasing endothelial HIF-1 and iNOS in SAH mice [38]. Conversely, HIF-1α was suggested as an important contributor in the development of CVS in other studies. 2-Methoxyestradiol might reduce CVS and improve neurological deficient via inhibiting HIF-1α/VEGF and HIF-1α/BNIP3 apoptotic pathways after SAH [70].
To date, both beneficial and detrimental effects of HIF-1α have been found in SAH. Therefore, the exact mechanism of HIF-1α in the pathogenesis of SAH is not yet fully elucidated.
Mitochondrial Dysfunction
Mitochondria, the double-membrane organelle, are the primary energy-generating systems in most eukaryotic cells. Mitochondria play a vital role in cellular bioenergetics, function, and survival [29]. Mitochondrial dysfunction leads to a serial of detrimental consequences, including collapse of the mitochondrial inner transmembrane potential, disruption of mitochondrial biogenesis, overproduction of reactive oxygen species, outflow of matrix calcium, and release of apoptogenic proteins [6, 25]. Mitochondrial disturbance, as a starting mechanism, results in apoptosis and necrosis.
Mitochondrial dysfunction in neuronal cells of the cortex has been described in EBI. SB203580, a p38-specific inhibitor, might prevent mitochondrial depolarization, increase ATP content and decrease cytochrome c release. SB203580 administration attenuated mitochondrial impairment-induced neuronal apoptosis [21]. However, the molecular mechanism of SB203580 in the amelioration of mitochondrial dysfunction is not yet fully elucidated. Tea polyphenols inhibited mitochondrial membrane potential polarization, leading to increased ATP content, and blocked cytochrome c release in the cerebral cortex [41]. Taken together, mitochondrial dysfunction likely plays an important role in the pathogenesis of SAH, especially in apoptosis.
Autophagy–Lysosomal System
The lysosome, an acidic organelle, is the terminal proteolytic compartment in cells. It can degrade macromolecules from endocytosis, phagocytosis, and autophagy [32, 52]. Autophagy, a lysosomal degradation pathway, is involved in protein degradation and clearing, defective organelle turnover, and cellular remodeling [39]. Some sequential processes of autophagy are phagophores, autophagosomes, the fusing of autophagosomes with lysosomes, degradation, and recycling/reuse of degradative products [40, 62]. Microtubule-associated protein light chain-3 (LC-3) is an autophagosome biomarker. Beclin-1 is a Bcl-2-interacting protein required for autophagy [26].
Increasing attention has been paid to the diverse role of autophagy in CNS disorders. Appropriate autophagic activity can facilitate the clearance of the dysfunctional/aging macromolecules and organelles, thus it can promote neuronal survival, whereas excessive autophagy induces cell death and is detrimental [59].
The activation of autophagy in neurons was detected in the EBI period after SAH [28]. The autophagy activity in cortical neurons peaked at 24 h and recovered at 48 h after SAH. Rapamycin, an autophagy activator, ameliorated cortical neuronal apoptosis, brain edema, and BBB breakdown by increasing autophagy-related signaling (LC-3 and beclin-1) 24 h after SAH. Conversely, 3-methyladenine, an autophagy inhibitor, decreased the level of autophagy-related proteins and worsened neurological deficits [67]. Furthermore, simvastatin suppressed apoptosis and attenuated EBI via enhancing autophagy [75]. The activation of autophagy prevented activation of SAH-induced neuronal caspase-dependent and -independent pathways to inhibit apoptosis [22]. However, the mutual link between autophagy and apoptosis after SAH is still unclear and needs to be further investigated.
The role of autophagy in the pathogenesis of cerebral CVS after SAH also has been investigated. Cystatin C increased LC-3 in the artery wall 48 h after SAH, which attenuated SAH-induced CVS [31].
Taken together, these data indicate that autophagy may be a potential effective target for preventing EBI and CVS after SAH, but that more investigation focusing on the precise mechanism is required.
ER Stress
The ER is a cellular organelle with a network of tubular membranes and is responsible for calcium storage and signaling as well as for protein folding and processing [46]. Once the ER is impaired by some pathophysiological insult, unfolded proteins accumulate in the lumen of the ER [24]. To cope with lethal conditions, the ER has a variety of stress responses, including unfolded protein response, ER overload response, and ER-associated degradation. Those responses can block the new synthesis of unfolded proteins, but can also promote the degradation of unfolded or misfolded proteins, which is important for restoring normal ER function. At the same time, ER stress can also cause a disturbance in ER function and eventually lead to apoptosis of the affected cells [47].
ER dysfunction is involved in the pathogenesis of CNS disorders, including SAH [46, 50]. The p53-upregulated modulator of apoptosis (PUMA) promotes apoptosis of endothelial cell and results in BBB disruption after SAH. PUMA siRNA suppressed the expression of ER-related proteins in microvascular endothelial cells of the hippocampus [71]. Further studies are likely to yield the exact mechanisms behind PUMA, ER stress, and apoptosis. C/EBP homologous protein (CHOP) overexpression was recently found to possibly play an important role in the ER stress-induced apoptotic cascades after SAH. CHOP silencing by small interfering RNA is capable of inhibiting apoptosis, reducing BBB disruption, and improving neurological function after SAH [17].
ER stress plays a critical role in the development of CVS as well. CHOP was elevated in the basilar artery after SAH. CHOP knockout by its siRNA could reduce bim and cleaved caspase-3 while increasing bcl-2 in vascular tissues; therefore, suppressing endothelial apoptosis and ameliorating CVS after SAH [18]. Overall, ER stress may be an important response in EBI and CVS after SAH.
Future Directions and Conclusion
The incomplete knowledge of the mechanisms of SAH and loose translational research hinder the development of targeted therapies for this devastating disease [27]. Currently, the significance of functional disturbance of organelles in the pathophysiology of SAH is emerging. Further identification of the precise roles of each organelle in SAH pathogenesis will help to elucidate the exact molecular mechanisms and create hope in discovering effective treatments for this devastating form of stroke. Electron microscopy or other imaging technologies will be useful in observing the phenotypic transformation of organelles after SAH. Organelle-specific manipulations may be effective for SAH therapy. Furthermore, because organelles are a collection of interrelated components, multitarget therapeutic strategies that focus on multiple organelles is likely to be more efficient for SAH treatment. Moreover, considering the significance of EBI on the outcome of SAH, more efforts on EBI are required to develop a novel treatment paradigm [14, 51]. Finally, sex differences in the organelles need to be emphasized in experimental studies [4, 19].
In conclusion, the functional disturbance of organelles contributes to the pathogenesis of EBI and CVS after SAH by transcription factor entry into the nucleus, ER stress, mitochondrial dysfunction, and autophagy–lysosomal system activation. The crosstalk among these organelles and their exact roles in SAH remain unclear. Further exploration to address these issues will broaden our knowledge of the pathogenesis of SAH and facilitate the development of novel therapeutic strategies for SAH.
Acknowledgments
This study was supported by a National Institutes of Health grant (NS053407) to JH Zhang and by a National Natural Science Foundation of China grant (No.81171096) to JM Zhang.
Footnotes
Conflict of Interest Statement We declare that we have no conflict of interest.
Contributor Information
Sheng Chen, Department of Neurosurgery, Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang, China. Department of Physiology and Pharmacology, Loma Linda University, 11041 Campus St, Loma Linda, CA 92354, USA.
Haijian Wu, Department of Neurosurgery, Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang, China.
Jiping Tang, Department of Physiology and Pharmacology, Loma Linda University, 11041 Campus St, Loma Linda, CA 92354, USA.
Jianmin Zhang, Department of Neurosurgery, Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang, China.
John H. Zhang, Email: johnzhang3910@yahoo.com, Department of Physiology and Pharmacology, Loma Linda University, 11041 Campus St, Loma Linda, CA 92354, USA.
References
- 1.Alaraj A, Charbel FT, Amin-Hanjani S. Peri-operative measures for treatment and prevention of cerebral vasospasm following subarachnoid hemorrhage. Neurol Res. 2009;31:651–659. doi: 10.1179/174313209X382395. [DOI] [PubMed] [Google Scholar]
- 2.Bain JM, Moore L, Ren Z, Simonishvili S, Levison SW. Vascular endothelial growth factors A and C are induced in the SVZ following neonatal hypoxia-ischemia and exert different effects on neonatal glial progenitors. Transl Stroke Res. 2013;4:158–170. doi: 10.1007/s12975-012-0213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baird L, Dinkova-Kostova AT. The cytoprotective role of the Keap1-Nrf2 pathway. Arch Toxicol. 2011;85:241–272. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- 4.Bramlett HM. Importance of sex in the pathophysiology and treatment of acute CNS repair. Transl Stroke Res. 2013;4:379–380. doi: 10.1007/s12975-013-0264-3. [DOI] [PubMed] [Google Scholar]
- 5.Caner B, Hou J, Altay O, Fuj M, 2nd, Zhang JH. Transition of research focus from vasospasm to early brain injury after sub-arachnoid hemorrhage. J Neurochem. 2012;123(Suppl 2):12–21. doi: 10.1111/j.1471-4159.2012.07939.x. [DOI] [PubMed] [Google Scholar]
- 6.Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Chang CZ, Wu SC, Lin CL, Hwang SL, Kwan AL. Purine anti-metabolite attenuates nuclear factor kappaB and related pro-inflammatory cytokines in experimental vasospasm. Acta Neurochir (Wien) 2012;154:1877–1885. doi: 10.1007/s00701-012-1452-8. [DOI] [PubMed] [Google Scholar]
- 8.Chen LC, Hsu C, Chiueh CC, Lee WS. Ferrous citrate up- regulates the NOS2 through nuclear translocation of NFkappaB induced by free radicals generation in mouse cerebral endothelial cells. PLoS One. 2012;7:e46239. doi: 10.1371/journal.pone.0046239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coppadoro A, Citerio G. Subarachnoid hemorrhage: an update for the intensivist. Minerva Anestesiol. 2011;77:74–84. [PubMed] [Google Scholar]
- 10.Dong Y, Li Y, Feng D, Wang J, Wen H, Liu D, Zhao D, Liu H, Gao G, Yin Z, Qin H. Protective effect of HIF-1alpha against hippocampal apoptosis and cognitive dysfunction in an experimental rat model of subarachnoid hemorrhage. Brain Res. 2013;1517:114–121. doi: 10.1016/j.brainres.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 11.Dorsch NW, King MT. A review of cerebral vasospasm in aneurysmal subarachnoid haemorrhage part I: incidence and effects. J Clin Neurosci. 1994;1:19–26. doi: 10.1016/0967-5868(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 12.Dzietko M, Derugin N, Wendland MF, Vexler ZS, Ferriero DM. Delayed VEGF treatment enhances angiogenesis and recovery after neonatal focal rodent stroke. Transl Stroke Res. 2013;4:189–200. doi: 10.1007/s12975-012-0221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Echigo R, Shimohata N, Karatsu K, Yano F, Kayasuga-Kariya Y, Fujisawa A, Ohto T, Kita Y, Nakamura M, Suzuki S, Mochizuki M, Shimizu T, Chung UI, Sasaki N. Trehalose treatment suppresses inflammation, oxidative stress, and vasospasm induced by experimental subarachnoid hemorrhage. J Transl Med. 2012;10:80. doi: 10.1186/1479-5876-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujii M, Yan J, Rolland WB, Soejima Y, Caner B, Zhang JH. Early brain injury, an evolving frontier in subarachnoid hemorrhage research. Transl Stroke Res. 2013;4:432–446. doi: 10.1007/s12975-013-0257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gatti S, Lonati C, Acerbi F, Sordi A, Leonardi P, Carlin A, Gaini SM, Catania A. Protective action of NDP-MSH in experimental subarachnoid hemorrhage. Exp Neurol. 2012;234:230–238. doi: 10.1016/j.expneurol.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 16.Gump W, Laskowitz DT. Management of post-subarachnoid hemorrhage vasospasm. Curr Atheroscler Rep. 2008;10:354–360. doi: 10.1007/s11883-008-0054-7. [DOI] [PubMed] [Google Scholar]
- 17.He Z, Ostrowski RP, Sun X, Ma Q, Huang B, Zhan Y, Zhang JH. CHOP silencing reduces acute brain injury in the rat model of subarachnoid hemorrhage. Stroke. 2012;43:484–490. doi: 10.1161/STROKEAHA.111.626432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He Z, Ostrowski RP, Sun X, Ma Q, Tang J, Zhang JH. Targeting C/EBP homologous protein with siRNA attenuates cerebral vasospasm after experimental subarachnoid hemorrhage. Exp Neurol. 2012;238:218–224. doi: 10.1016/j.expneurol.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herson PS, Palmateer J, Hurn PD. Biological sex and mechanisms of ischemic brain injury. Transl Stroke Res. 2013;4:413–419. doi: 10.1007/s12975-012-0238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hishikawa T, Ono S, Ogawa T, Tokunaga K, Sugiu K, Date I. Effects of deferoxamine-activated hypoxia-inducible factor-1 on the brainstem after subarachnoid hemorrhage in rats. Neurosurgery. 2008;62:232–240. doi: 10.1227/01.NEU.0000311082.88766.33. discussion 240–231. [DOI] [PubMed] [Google Scholar]
- 21.Huang L, Wan J, Chen Y, Wang Z, Hui L, Li Y, Xu D, Zhou W. Inhibitory effects of p38 inhibitor against mitochondrial dysfunction in the early brain injury after subarachnoid hemorrhage in mice. Brain Res. 2013;1517:133–140. doi: 10.1016/j.brainres.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Jing CH, Wang L, Liu PP, Wu C, Ruan D, Chen G. Autophagy activation is associated with neuroprotection against apoptosis via a mitochondrial pathway in a rat model of subarachnoid hemorrhage. Neuroscience. 2012;213:144–153. doi: 10.1016/j.neuroscience.2012.03.055. [DOI] [PubMed] [Google Scholar]
- 23.Kassell NF, Sasaki T, Colohan AR, Nazar G. Cerebral vaso-spasm following aneurysmal subarachnoid hemorrhage. Stroke. 1985;16:562–572. doi: 10.1161/01.str.16.4.562. [DOI] [PubMed] [Google Scholar]
- 24.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 25.Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 26.Kumari S, Anderson L, Farmer S, Mehta SL, Li PA. Hyperglycemia alters mitochondrial fission and fusion proteins in mice subjected to cerebral ischemia and reperfusion. Transl Stroke Res. 2012;3:296–304. doi: 10.1007/s12975-012-0158-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lapchak PA, Zhang JH, Noble-Haeusslein LJ. RIGOR guidelines: escalating STAIR and STEPS for effective translational research. Transl Stroke Res. 2013;4:279–285. doi: 10.1007/s12975-012-0209-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JY, He Y, Sagher O, Keep R, Hua Y, Xi G. Activated autophagy pathway in experimental subarachnoid hemorrhage. Brain Res. 2009;1287:126–135. doi: 10.1016/j.brainres.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 29.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 30.Lisy K, Peet DJ. Turn me on: regulating HIF transcriptional activity. Cell Death Differ. 2008;15:642–649. doi: 10.1038/sj.cdd.4402315. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Cai H, Wang Z, Li J, Wang K, Yu Z, Chen G. Induction of autophagy by cystatin C: a potential mechanism for prevention of cerebral vasospasm after experimental subarachnoid hemorrhage. Eur J Med Res. 2013;18:21. doi: 10.1186/2047-783X-18-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat Rev Mol Cell Biol. 2007;8:622–632. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- 33.Ma CX, Yin WN, Cai BW, Wu J, Wang JY, He M, Sun H, Ding JL, You C. Toll-like receptor 4/nuclear factor-kappa B signaling detected in brain after early subarachnoid hemorrhage. Chin Med J (Engl) 2009;122:1575–1581. [PubMed] [Google Scholar]
- 34.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macdonald RL, Higashida RT, Keller E, Mayer SA, Molyneux A, Raabe A, Vajkoczy P, Wanke I, Bach D, Frey A, Marr A, Roux S, Kassell N. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: a randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2) Lancet Neurol. 2011;10:618–625. doi: 10.1016/S1474-4422(11)70108-9. [DOI] [PubMed] [Google Scholar]
- 36.Macdonald RL, Kassell NF, Mayer S, Ruefenacht D, Schmiedek P, Weidauer S, Frey A, Roux S, Pasqualin A. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double- blind, placebo-controlled phase 2 dose-finding trial. Stroke. 2008;39:3015–3021. doi: 10.1161/STROKEAHA.108.519942. [DOI] [PubMed] [Google Scholar]
- 37.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milner E, Harries MD, Vellimana AK, Gidday JM, Han BH, Zipfel GJ. Post-conditioning with isoflurane reduces SAH- induced vasospasm and microthrombosis via hypoxia-inducible factor 1 and nitric oxide synthase. Neurosurgery. 2013;60(Suppl 1):181. [Google Scholar]
- 39.Mizushima N. The pleiotropic role of autophagy: from protein metabolism to bactericide. Cell Death Differ. 2005;12(Suppl 2):1535–1541. doi: 10.1038/sj.cdd.4401728. [DOI] [PubMed] [Google Scholar]
- 40.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 41.Mo H, Chen Y, Huang L, Zhang H, Li J, Zhou W. Neuroprotective effect of tea polyphenols on oxyhemoglobin induced subarachnoid hemorrhage in mice. Oxid Med Cell Longev. 2013;2013:743938. doi: 10.1155/2013/743938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Napetschnig J, Wu H. Molecular basis of NF-kappaB signaling. Annu Rev Biophys. 2013;42:443–468. doi: 10.1146/annurev-biophys-083012-130338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naraoka M, Munakata A, Matsuda N, Shimamura N, Ohkuma H. Suppression of the Rho/Rho-kinase pathway and prevention of cerebral vasospasm by combination treatment with statin and fasudil after subarachnoid hemorrhage in rabbit. Transl Stroke Res. 2013;4:368–374. doi: 10.1007/s12975-012-0247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ostrowski RP, Colohan AR, Zhang JH. Mechanisms of hyperbaric oxygen-induced neuroprotection in a rat model of sub-arachnoid hemorrhage. J Cereb Blood Flow Metab. 2005;25:554–571. doi: 10.1038/sj.jcbfm.9600048. [DOI] [PubMed] [Google Scholar]
- 45.Pan H, Wang H, Zhu L, Mao L, Qiao L, Su X. Depletion of Nrf2 enhances inflammation induced by oxyhemoglobin in cultured mice astrocytes. Neurochem Res. 2011;36:2434–2441. doi: 10.1007/s11064-011-0571-6. [DOI] [PubMed] [Google Scholar]
- 46.Paschen W. Endoplasmic reticulum: a primary target in various acute disorders and degenerative diseases of the brain. Cell Calcium. 2003;34:365–383. doi: 10.1016/s0143-4160(03)00139-8. [DOI] [PubMed] [Google Scholar]
- 47.Paschen W, Mengesdorf T. Cellular abnormalities linked to endoplasmic reticulum dysfunction in cerebrovascular disease–therapeutic potential. Pharmacol Ther. 2005;108:362–375. doi: 10.1016/j.pharmthera.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 48.Pasparakis M. Regulation of tissue homeostasis by NF-kappaB signalling: implications for inflammatory diseases. Nat Rev Immunol. 2009;9:778–788. doi: 10.1038/nri2655. [DOI] [PubMed] [Google Scholar]
- 49.Rinkel GJ, Algra A. Long-term outcomes of patients with aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2011;10:349–356. doi: 10.1016/S1474-4422(11)70017-5. [DOI] [PubMed] [Google Scholar]
- 50.Roussel BD, Kruppa AJ, Miranda E, Crowther DC, Lomas DA, Marciniak SJ. Endoplasmic reticulum dysfunction in neurological disease. Lancet Neurol. 2013;12:105–118. doi: 10.1016/S1474-4422(12)70238-7. [DOI] [PubMed] [Google Scholar]
- 51.Rowland MJ, Hadjipavlou G, Kelly M, Westbrook J, Pattinson KT. Delayed cerebral ischaemia after subarachnoid haemorrhage: looking beyond vasospasm. Br J Anaesth. 2012;109:315–329. doi: 10.1093/bja/aes264. [DOI] [PubMed] [Google Scholar]
- 52.Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol. 2009;10:623–635. doi: 10.1038/nrm2745. [DOI] [PubMed] [Google Scholar]
- 53.Saggu R. Characterisation of Endothelin-1-Induced intrastriatal lesions within the juvenile and adult rat brain using MRI and 31P MRS. Transl Stroke Res. 2013;4:351–367. doi: 10.1007/s12975-013-0258-1. [DOI] [PubMed] [Google Scholar]
- 54.Sarkar FH, Li Y, Wang Z, Kong D. NF-kappaB signaling pathway and its therapeutic implications in human diseases. Int Rev Immunol. 2008;27:293–319. doi: 10.1080/08830180802276179. [DOI] [PubMed] [Google Scholar]
- 55.Sehba FA, Hou J, Pluta RM, Zhang JH. The importance of early brain injury after subarachnoid hemorrhage. Prog Neurobiol. 2012;97:14–37. doi: 10.1016/j.pneurobio.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sehba FA, Pluta RM, Zhang JH. Metamorphosis of sub-arachnoid hemorrhage research: from delayed vasospasm to early brain injury. Mol Neurobiol. 2011;43:27–40. doi: 10.1007/s12035-010-8155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shih HC, Lin CL, Lee TY, Lee WS, Hsu C. 17beta-Estradiol inhibits subarachnoid hemorrhage-induced inducible nitric oxide synthase gene expression by interfering with the nuclear factor kappa B transactivation. Stroke. 2006;37:3025–3031. doi: 10.1161/01.STR.0000249008.18669.5a. [DOI] [PubMed] [Google Scholar]
- 58.Simard JM, Tosun C, Ivanova S, Kurland DB, Hong C, Radecki L, Gisriel C, Mehta R, Schreibman D, Gerzanich V. Heparin reduces neuroinflammation and transsynaptic neuronal apoptosis in a model of subarachnoid hemorrhage. Transl Stroke Res. 2012;3:155–165. doi: 10.1007/s12975-012-0166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith CM, Chen Y, Sullivan ML, Kochanek PM, Clark RS. Autophagy in acute brain injury: feast, famine, or folly? Neurobiol Dis. 2011;43:52–59. doi: 10.1016/j.nbd.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Switzer JA, Sikora A, Ergul A, Waller JL, Hess DC, Fagan SC. Minocycline prevents IL-6 increase after acute ischemic stroke. Transl Stroke Res. 2012;3:363–368. doi: 10.1007/s12975-012-0150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tajiri N, Dailey T, Metcalf C, Mosley YI, Lau T, Staples M, van Loveren H, Kim SU, Yamashima T, Yasuhara T, Date I, Kaneko Y, Borlongan CV. In vivo animal stroke models: a rationale for rodent and non-human primate models. Transl Stroke Res. 2013;4:308–321. doi: 10.1007/s12975-012-0241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Uchiyama Y, Shibata M, Koike M, Yoshimura K, Sasaki M. Autophagy-physiology and pathophysiology. Histochem Cell Biol. 2008;129:407–420. doi: 10.1007/s00418-008-0406-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369:306–318. doi: 10.1016/S0140-6736(07)60153-6. [DOI] [PubMed] [Google Scholar]
- 64.Vomhof-Dekrey EE, Picklo MJ., Sr The Nrf2-antioxidant response element pathway: a target for regulating energy metabolism. J Nutr Biochem. 2012;23:1201–1206. doi: 10.1016/j.jnutbio.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 65.Wang Z, Chen G, Zhu WW, Zhou D. Activation of nuclear factor-erythroid 2-related factor 2 (Nrf2) in the basilar artery after subarachnoid hemorrhage in rats. Ann Clin Lab Sci. 2010;40:233–239. [PubMed] [Google Scholar]
- 66.Wang Z, Ma C, Meng CJ, Zhu GQ, Sun XB, Huo L, Zhang J, Liu HX, He WC, Shen XM, Shu Z, Chen G. Melatonin activates the Nrf2-ARE pathway when it protects against early brain injury in a subarachnoid hemorrhage model. J Pineal Res. 2012;53:129–137. doi: 10.1111/j.1600-079X.2012.00978.x. [DOI] [PubMed] [Google Scholar]
- 67.Wang Z, Shi XY, Yin J, Zuo G, Zhang J, Chen G. Role of autophagy in early brain injury after experimental subarachnoid hemorrhage. J Mol Neurosci. 2012;46:192–202. doi: 10.1007/s12031-011-9575-6. [DOI] [PubMed] [Google Scholar]
- 68.Wang Z, Zuo G, Shi XY, Zhang J, Fang Q, Chen G. Progesterone administration modulates cortical TLR4/NF-kappaB signaling pathway after subarachnoid hemorrhage in male rats. Mediators Inflamm. 2011;2011:848309. doi: 10.1155/2011/848309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu C, Hu Q, Chen J, Yan F, Li J, Wang L, Mo H, Gu C, Zhang P, Chen G. Inhibiting HIF-1alpha by 2ME2 ameliorates early brain injury after experimental subarachnoid hemorrhage in rats. Biochem Biophys Res Commun. 2013;437:469–474. doi: 10.1016/j.bbrc.2013.06.107. [DOI] [PubMed] [Google Scholar]
- 70.Yan J, Chen C, Lei J, Yang L, Wang K, Liu J, Zhou C. 2-Methoxyestradiol reduces cerebral vasospasm after 48 hours of experimental subarachnoid hemorrhage in rats. Exp Neurol. 2006;202:348–356. doi: 10.1016/j.expneurol.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 71.Yan J, Li L, Khatibi NH, Yang L, Wang K, Zhang W, Martin RD, Han J, Zhang J, Zhou C. Blood-brain barrier disruption following subarchnoid hemorrhage may be facilitated through PUMA induction of endothelial cell apoptosis from the endoplasmic reticulum. Exp Neurol. 2011;230:240–247. doi: 10.1016/j.expneurol.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 72.You WC, Li W, Zhuang Z, Tang Y, Lu HC, Ji XJ, Shen W, Shi JX, Zhou ML. Biphasic activation of nuclear factor-kappa B in experimental models of subarachnoid hemorrhage in vivo and in vitro. Mediators Inflamm. 2012;2012:786242. doi: 10.1155/2012/786242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zacharia BE, Hickman ZL, Grobelny BT, DeRosa P, Kotchetkov I, Ducruet AF, Connolly ES., Jr Epidemiology of aneurysmal subarachnoid hemorrhage. Neurosurg Clin N Am. 2010;21:221–233. doi: 10.1016/j.nec.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 74.Zhang J, Zhu Y, Zhou D, Wang Z, Chen G. Recombinant human erythropoietin (rhEPO) alleviates early brain injury following subarachnoid hemorrhage in rats: possible involvement of Nrf2-ARE pathway. Cytokine. 2010;52:252–257. doi: 10.1016/j.cyto.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 75.Zhao H, Ji Z, Tang D, Yan C, Zhao W, Gao C. Role of autophagy in early brain injury after subarachnoid hemorrhage in rats. Mol Biol Rep. 2013;40:819–827. doi: 10.1007/s11033-012-2120-z. [DOI] [PubMed] [Google Scholar]
- 76.Zhao X, Aronowski J. Nrf2 to pre-condition the brain against injury caused by products of hemolysis after ICH. Transl Stroke Res. 2013;4:71–75. doi: 10.1007/s12975-012-0245-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao XD, Zhou YT, Lu XJ. Sulforaphane enhances the activity of the Nrf2-ARE pathway and attenuates inflammation in OxyHb-induced rat vascular smooth muscle cells. Inflamm Res. 2013;62:857–863. doi: 10.1007/s00011-013-0641-0. [DOI] [PubMed] [Google Scholar]
- 78.Zhao XD, Zhou YT, Zhang X, Wang XL, Qi W, Zhuang Z, Su XF, Shi JX. Expression of NF-E2-related factor 2 (Nrf2) in the basilar artery after experimental subarachnoid hemorrhage in rabbits: a preliminary study. Brain Res. 2010;1358:221–227. doi: 10.1016/j.brainres.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 79.Zhou ML, Shi JX, Hang CH, Cheng HL, Qi XP, Mao L, Chen KF, Yin HX. Potential contribution of nuclear factor-kappaB to cerebral vasospasm after experimental sub-arachnoid hemorrhage in rabbits. J Cereb Blood Flow Metab. 2007;27:1583–1592. doi: 10.1038/sj.jcbfm.9600456. [DOI] [PubMed] [Google Scholar]