Abstract
Introduction
Statins influence immune system activities through mechanisms independent of their lipid-lowering properties. T cells can be subdivided based on cytokine secretion patterns into two subsets: T-helper cells type 1 (Th1) and type 2 (Th2). Independent laboratory studies have shown statins to be potent inducers of a Th2 switch in immune cell response and be neuroprotective in several models of central nervous system (CNS) disease. This study was the first to evaluate the immune modulating effects of statins in subarachnoid hemorrhage (SAH).
Methods
Simvastatin was administered to rats intraperitoneally in two dosages (1 and 20 mg/kg) 30 min after the induction of SAH using endovascular perforation. Neurological scores were assessed 24 h later. Animals were then sacrificed, and samples of cortex and brain stem were tested for expression of the T-regulatory cell cytokine transforming growth factor (TGF) β1, as well as interleukin (IL) 1β, a proinflammatory cytokine associated with Th1 immune responses. The presence of TGF-β1 secreting T cells was evaluated with the use of brain slices.
Results
SAH significantly impaired neurological function in all SAH groups (treated and untreated) versus sham. Animals treated with high-dose simvastatin had less neurological impairment than both untreated and low-dose groups. Cortical and brain-stem levels of TGF-β1 were significantly elevated following SAH in the high-dose group. IL-1βwas significantly elevated following the induction of SAH but was inhibited by high-dose simvastatin. Double-labeled fluorescent immunohistochemical data demonstrated the presence of lymphocytes in the subarachnoid and perivascular spaces following SAH. Expression of TGF-β1 by lymphocytes was markedly increased following treatment with high-dose simvastatin.
Conclusion
The present study elucidated the potential role of a Th2 immune switch in statin provided neuroprotection following SAH.
Keywords: Endovascular perforation model, Subarachnoid hemorrhage, Simvastatin, Early brain injury, TGF-β1, Th2 lymphocytes, Th3 lymphocytes
Introduction
Aneurysmal subarachnoid hemorrhage (SAH), accounting for roughly 3% of all strokes, is a devastating neurological event that has been the focus of scientific investigation for decades [13,38]. Significant advances in the surgical treatment and intensive care for these patients have resulted in significantly improved mortality and functional outcome [20]. However, the disease still has mortality as high as 40%, and a majority of the survivors still have significant disability [34]. An inflammatory response occurs within the central nervous system (CNS) following SAH and likely plays a significant role in the early brain injury following SAH [4]. Whether the infiltration of lymphocytes into the CNS following injury has a neuroprotective and regenerative role versus being a response that is detrimental to recovery is still being intensively investigated [19]. Resolution appears to lie with understanding the roles of subpopulations of cells, particularly T-helper cells type 1 (Th1), type 2 (Th2), and type 3 (Th3) subsets. Th1-dominated immune responses are associated with significant CNS injury [7,8]. These responses are exacerbated or increased in frequency under certain conditions, such as systemic infection in conjunction with CNS injury [7]. The ability to inhibit Th1 immune responses has been found to reduce infarct size and improve outcomes following ischemic stroke [8]. In addition, the augmentation of Th2 and Th3 immune responses has been associated with neuroprotection and may augment neuroregeneration [15,19]. Statins have been described as being able to elicit a Th2 immune response, and they been found to promote neuronal recovery in models of spinal cord injury and traumatic brain injury [2,18]. The immunomodulating properties of statins may help explain their reported efficacy in treating SAH patients [25,28,32,42]. In this experiment, we hypothesized that statins would provide neuroprotection against the neurological injury following experimental SAH that is associated with a Th2 immune switch in the CNS characterized by the infiltration of leukocytes producing the immunosuppressive cytokine transforming growth factor (TGF) β1. We also predicted that this immune modulation would result in the suppression of proinflammatory cytokines, such as interleukin (IL) 1β.
Materials and Methods
Experimental Animals and Groups
One hundred fifteen adult male Sprague–Dawley rats (Harlan, Indianapolis, IN) weighing between 250 and 350 g were divided randomly into four weight-matched groups: shamoperated treated with vehicle (sham, n = 24), SAH treated with vehicle (SAH, n = 32), SAH treated with low-dosage simvastatin (Calbiochem, CA, USA) (1 mg/kg, S-1, n = 33) or high-dosage simvastatin (20 mg/kg, S-20, n = 26). Our group’s previous experiments have shown that 20 mg/kg simvastatin had no effect on cerebral physiology in sham animals [39]; therefore, a sham group treated with 1 mg/kg or 20 mg/kg simvastatin was not evaluated in this experiment.
Animals experiencing mild SAH (grades 0–7) were excluded from the study as per the SAH grading system criteria because mild SAH does induce detectable neurological deficits in experimental studies [40]. In brief, the SAH grading system is as follows: The basal cistern is divided into six segments, and each segment is allotted a grade from 0 to 3 depending on the amount of subarachnoid blood clot in the segment: grade 0, no subarachnoid blood; 1, minimal subarachnoid blood; 2, moderate blood clot with recognizable arteries; 3, blood clot obliterating all arteries within the segment. The animals received a total score ranging from 0 to 18 after adding the scores from all six segments. Mild SAH was categorized as all animals that received a total score of five or less. The SAH grading was done in a blinded fashion.
Induction of SAH
All procedures and experiments were approved by the Institutional Animal Care and Use Committee of Loma Linda University. The endovascular perforation model of SAH in rats was used for this study as previously described [9,30]. Briefly, general anesthesia was induced with ketamine (100 mg/kg ip) and xylazine hydrochloride (10 mg/kg ip) followed by atropine (0.1 mg/kg sc). After intubation, the animals were ventilated with an animal ventilator (Harvard Apparatus). A heating pad and a heating lamp were used to maintain the rectal temperature at 36.0 ± 0.5°C. SAH was induced by endovascular perforation of the internal carotid artery (ICA) bifurcation with a sharpened 4-0 nylon suture. After exposing the left common carotid artery (CCA), external carotid artery (ECA), and ICA through a midline skin incision, the ECA was ligated, cut, and shaped into a 3-mm stump. The suture was advanced rostrally into the ICA from the ECA stump until resistance was felt (~18 mm from the common carotid bifurcation) and then pushed 3 mm further to perforate the bifurcation of the anterior cerebral and middle cerebral arteries. Immediately after puncture, the suture was withdrawn into the ECA stump, and the ICA was reperfused. Operative procedures were exactly same for the sham group, except that the suture was removed once resistance was felt without puncture. The incision was then closed, and rats were housed individually following their recovery from anesthesia. All rats received 3 ml normal saline intraperitoneally to prevent dehydration, and animals had free access to food and water until euthanization.
Drug Administration
Thirty minutes after the procedure, treatment groups received either a high (20 mg/kg) or low (1 mg/kg) dose of simvastatin via an intraperitoneal injection. High-dose simvastatin (20 mg/ kg) was selected on guidance from previous literature [29], whereas the low dosage (1 mg/kg) is comparable to that used in current clinical settings. All simvastatin dosages were dissolved in ethanol and adjusted to a final concentration of 10%, with a total volume of 1.5 ml when they were administered. Sham and SAH groups received vehicle (1.5 ml of 10% ethanol in normal saline). This concentration of ethanol was not likely to affect any of the physiologic parameters assessed during this experiment [3,5,10].
Neurological Scoring
Neurological scores were evaluated in a blinded fashion 24 h after SAH with a modification of the scoring system reported by Garcia et al. [14]. An 18-point scoring system was used to evaluate the sensorimotor deficits.
Western Blotting Analysis
A standard Western blotting protocol using the following antibodies: anti-TGF-β1 (Cell Signaling, cat. 3711) and IL-1β (Santa Cruz Biotechnology, sc-74138) was performed on brain tissue collected 24 h after SAH [22]. Briefly, animals were euthanized under anesthesia (5% isoflurane anesthetic in 70% medical air with 30% oxygen), and brains were immediately removed and stored at −80°C until analysis (n = 6 for each group). Protein extraction from the brain obtained by gently homogenizing in RIPA lysis buffer (Santa Cruz Biotechnology, sc-24948) and further centrifuged at 14,000 g at 4°C for 30 min. The supernatant was used as whole-cell protein extract, and the protein concentration was determined by using a detergent-compatible assay (Bio-Rad, Dc protein assay). Equal amounts of protein (50 µg) were loaded on a sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel. Protein was electrophoresed and transferred to a nitrocellulose membrane; the membrane was then blocked and incubated with one of two primary antibodies, anti-TGFβ1 (Cell Signaling, cat. 3711) or IL-1β (Santa Cruz Biotechnology, sc-74138) overnight at 4°C. Nitrocellulose membranes were then incubated with secondary antibodies (Santa Cruz Biotechnology) for 1 h at room temperature. Immunoblots were then probed with an ECL Plus chemiluminescence reagent kit (Amersham Biosciences, Arlington Heights, IL) and exposed to films. The data were analyzed by the software ImageJ 1.41 (National Institutes of Health).
Fluorescence Immunohistochemical Staining
Double-fluorescence labeling for TGF-β1 and T cells in the brain slices mounted on histological glass slides was carried out as previously described [12]. Four 10-µm thick sections per brain, from the central portion of the subarachnoid blood clot, were used in each group (n = 4). Brain slices were immersed in the citrate buffer and boiled in a microwave oven for 10 min for antigen retrieval, then washed three times with phosphate-buffered saline (PBS) as described elsewhere [41]. This was followed by incubation with 5% donkey serum for 1 h at room temperature (RT). After removing excess blocking serum, primary antibodies were applied overnight at 4°C followed by secondary antibodies for 2 h at RT. Histological preparations were coverslipped with antifade reagent (Millipore) and observed under an Olympus BX51 epifluorescent microscope. The primary antibodies used were rabbit anti-TGF-β1 (Cell Signaling) and mouse anti-T-cell maker (Santa Cruz), each diluted 1:100. The respective secondary antibodies (Jackson Immunoresearch Labs) were diluted 1:200. All other histological regents were obtained from Fisher Scientific Company. Photomicrographs were captured with a computer-assisted camera and merged with aid of Magna Fire Software (Systat Co.) [11].
Statistical Analysis
The data are expressed as mean plus or minus SEM. Statistical differences between the various groups were assessed with a one-way analysis of variance (ANOVA) with Holm-Sidak post hoc analysis. Statistical differences between two groups were assessed with Student t test. Mortality was analyzed using a chi-square test. A value of p < 0.05 was considered statistically significant.
Results
Physiological Data
Physiological parameters were monitored before, during, and after surgery. No statistical differences were observed between the SAH group (n = 6) and the S-20 group (n = 6) with regard to mean arterial blood pressure, arterial blood gases, paO2 and paCO2, pH levels, and glucose levels before, immediately after puncture, and 30 min after SAH (data not published).
Mortality and Exclusion
Twelve rats were excluded from further evaluation following the grading of their SAH at 24 h. Eleven were due to mild grade SAH (0–7), and one was due to the presence of an acute subdural hematoma. This resulted in the following number of animals in each experimental group: Sham n = 24, SAH n = 29, S-1 n = 27, S-20 n = 25. Nine rats died before intended sacrifice time and were excluded from assessment of vasospasm and neurological deficits at 24 h. However, these rats were included in mortality statistics. The 24-h mortality rates in each group were as follows: sham, 0.0% (0/24); SAH, 17.2% (5/29); S-1, 11.1% (3/27); S-20, 4.0% (1/25). This resulted in 24 animals being in each experimental group at 24 h. The SAH groups (both treated and untreated) did not differ significantly in their mortality rates (p > 0.05, ANOVA).
High-Dose Simvastatin
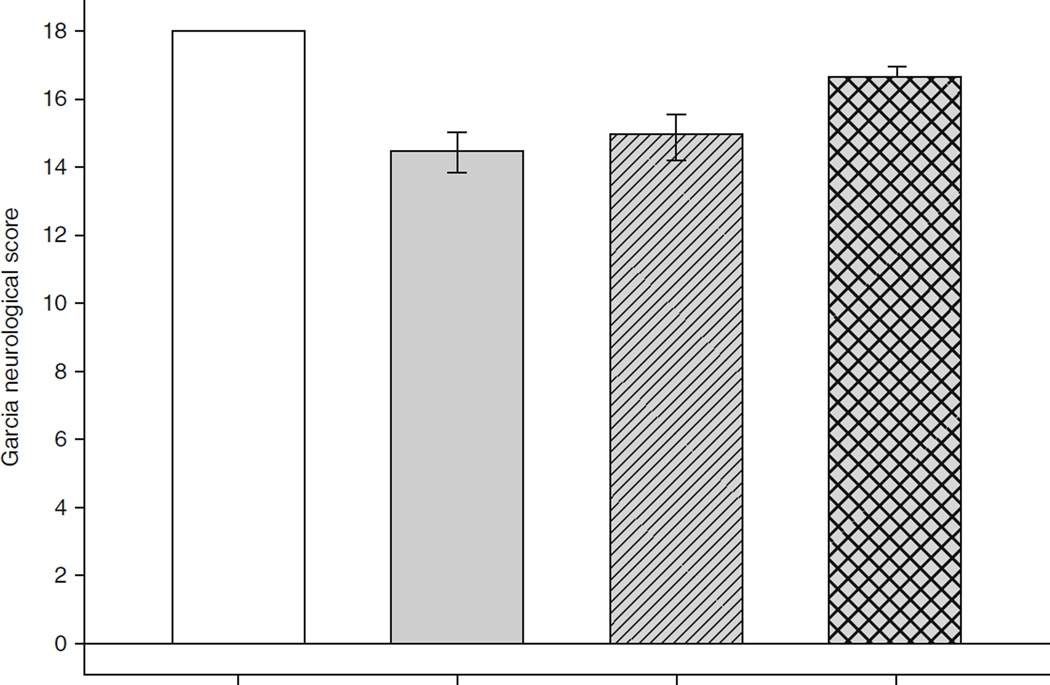
High-dose simvastatin provided neuroprotection following SAH. SAH created significant neurological injury in all groups (Fig. 1. Animals treated with 20 mg/kg of simvastatin had improved neurological scores over vehicle or groups treated with 1 mg/kg simvastatin 24 h after SAH (Fig. 1).
Fig. 1.
Effects of statin treatment on neurological score 24 h following subarachnoid hemorrhage (SAH). A modified Garcia Neurological Score was used, which ranges from 0 to 18. Eighteen represents normal neurological function. n = 24 per group; error bar, SE. #p < 0.05 versus sham; *p < 0.05 versus vehicle; @p < 0.05 versus S-1; analysis of variance (ANOVA). LPS lipopolysaccharide, MCA middle cerebral artery
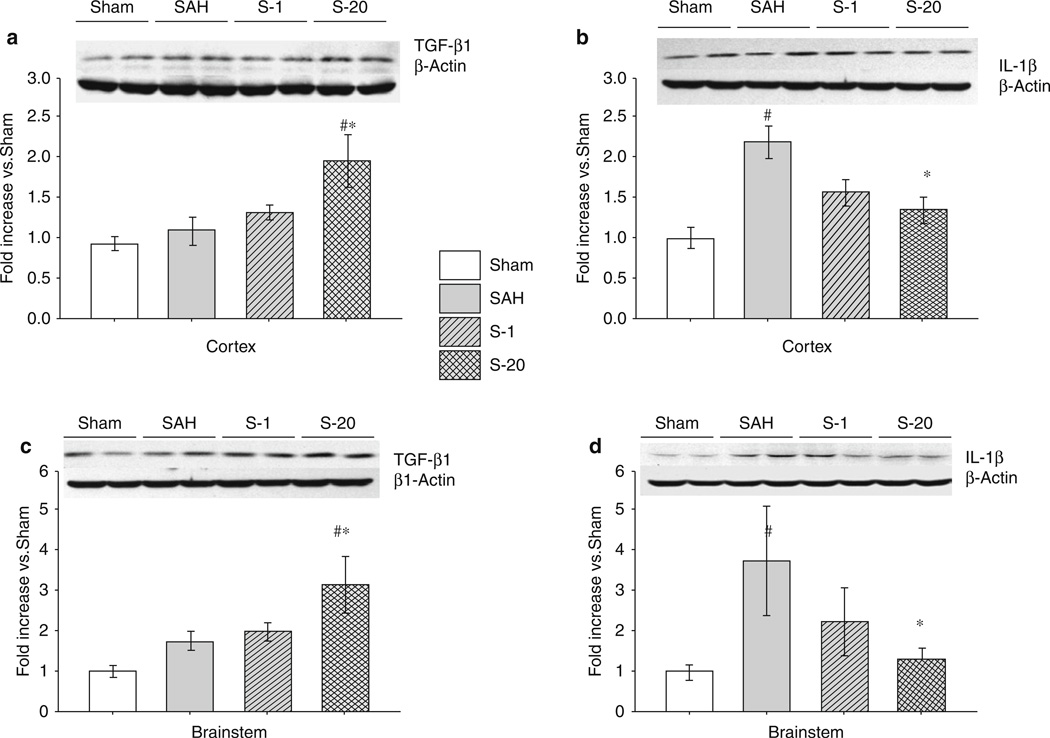
High-dose simvastatin administration was associated with increased expression of the anti-inflammatory cytokine TGF-β and inhibition of the proinflammatory cytokine IL-1β. Cortical and brain-stem samples demonstrated a differential expression of IL-1β and TGF-β1 following SAH that was affected by simvastatin treatment. TGF-β1 levels were not significantly increased by the induction of experimental SAH, and low-dose simvastatin (1 mg/kg) did not have a significant effect on TGF-β1 levels following SAH (Fig. 2a, c). High-dose simvastatin (20 mg/kg) resulted in a significant increase in the expression of TGF-β1 following SAH (Fig. 2a, c). Following SAH, cortical brain samples showed a significant increase in the expression of IL-1β versus sham (Fig. 2b). Treatment with simvastatin appeared to reduce IL-1β expression following SAH in a dose-dependent manner, with significant reduction seen at a dose of 20 mg/kg (Fig. 2b). Similar results were found in brain-stem samples from the same animals (Fig. 2d).
Fig. 2.
Effects of statin treatment on transforming growth factor (TGF)β1 and interleukin (IL) 1β on expression in the cortex and brain stem 24 h after subarachnoid hemorrhage (SAH). Western blots for cortex TGF-β1 (a) and IL-1β (b), as well as brain stem TGF-β1 (c) and IL-1β (d). Expression levels of each protein in Western blot are expressed as a ratio of β-actin levels for normalization. n = 6 rats per groups; error bar, SE. #p < 0.05 versus sham; *p < 0.05 versus vehicle; analysis of variance (ANOVA)
Fluorescence Immunohistochemical Evidence of Th2 Switch
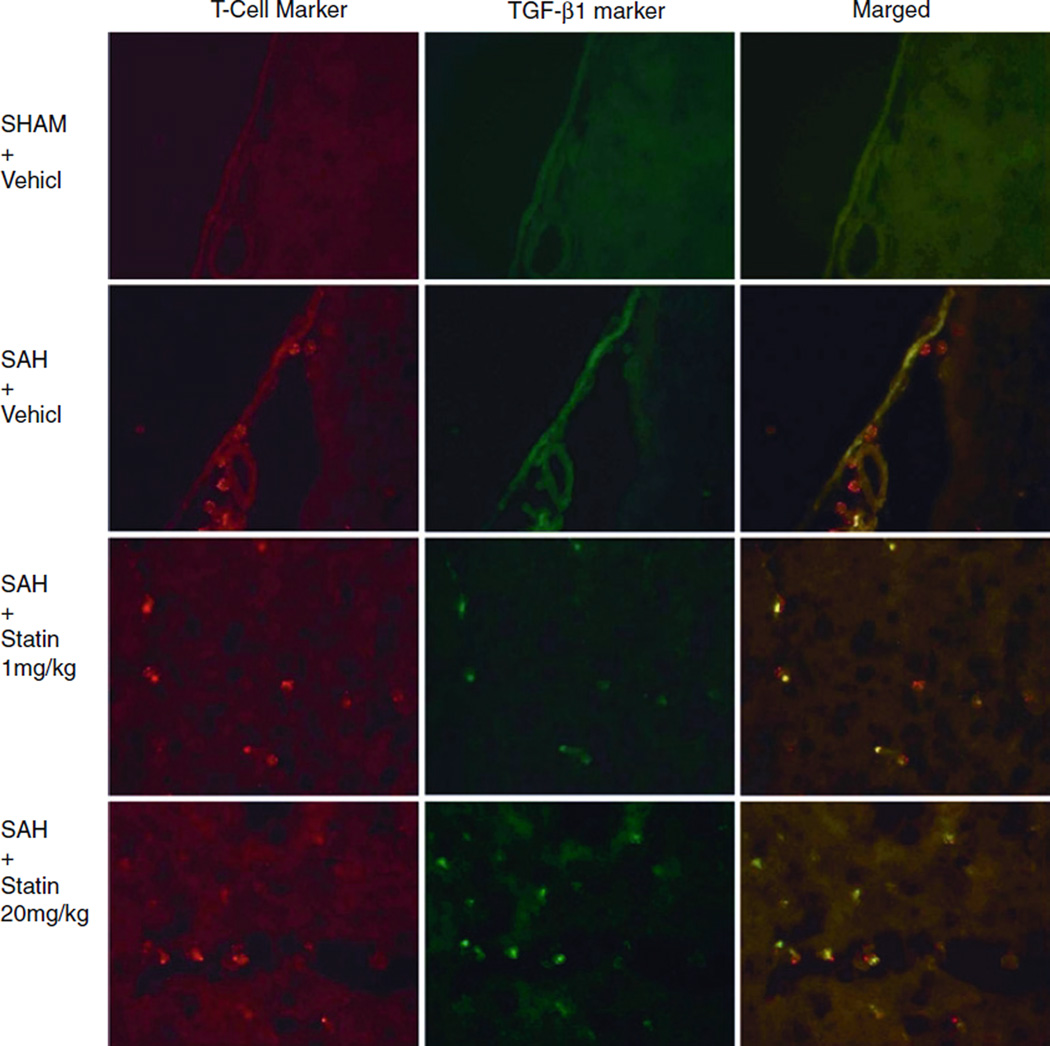
Representative photos of double-fluorescence labeling for TGF-β1 and T cells in the brain slices mounted on histological glass slides demonstrated that lymphocytes were not visible in the subarachnoid space of the basal cisterns following sham surgery (Fig. 3). Lymphocytes were present following SAH, and their number did not appear to be affected by the administration of simvastatin (Fig. 3). Cells expressing TGF-β1 were present in the subarachnoid space in the statin-treated groups (Fig. 3). Evaluation of the merged images demonstrated that these cells were lymphocytes (Fig. 3). Comparison of the SAH and S-20 groups clearly demonstrated that leukocytes were only expressing TGF-β1 in the simvastatin-treated animals (Fig. 3).
Fig. 3.
Representative photographs of double-fluorescence labeling for transforming growth factor (TGF) β1 and T cells in the brain slices mounted on histological glass slides. The mouse anti-T-cell maker indicated T cells are not visualized in the subarachnoid space following sham surgery but become present following subarachnoid hemorrhage (SAH). The TGF-β1 expression by leukocytes was detected following treatment with simvastatin with the use of rabbit anti-TGFβ-1 antibodies. Samples collected 24 h after SAH
Discussion
Subarachnoid hemorrhage, like nearly all CNS injuries, results in an inflammatory reaction. Several investigators have characterized the timeline and population of cells infiltrating the CNS following subarachnoid hemorrhage, indicating that SAH may elicit its own characteristic inflammatory reaction [23,27]. Kubot et al. looked at several populations of leukocytes, including macrophages and lymphocytes, and found that populations of these cells peaked between 24 and 48 h [23]. Lymphocyte subpopulations, such as Th1, Th2, and Th3, have been found to play critical roles in determining the extent of injury in ischemic stroke and other experimental CNS injuries [7,9,19]. Under circumstances such as concordant systemic infection or history of previous CNS antigen exposure, one can determine which type of lymphocyte population dominates a CNS inflammatory reaction [7,9,19]. Typically, Th1-dominant responses are associated with greater degrees of neuronal apoptosis and CNS injury, while Th2- or Th3-dominant responses are associated with neuroregeneration and attenuated detrimental inflammatory reactions [15,19]. Manipulation of the immune system following CNS injury to produce Th2 and Th3 lymphocyte-dominated immune responses, which are characterized by the secretion of immunomodulatory cytokines such as IL-4, IL-10, and TGF-β1, has been sought after by investigators with the goal of providing neuroprotection [15,19]. Wolf et al. showed that Th2 cells support neuronal survival better than Th1 cells in vitro [44]. Gimsa et al. demonstrated the Th2 cells mediated suppression of inflammatory signals in brain slices [16]. In addition, vaccination for the treatment of experimental CNS injury showed that Th2-inducing adjuvants promote axon regeneration better than the Th1-inducing adjuvant [21,35]. Becker et al. demonstrated that a Th1 immune response following stroke resulted in larger infarctions in ischemic stroke models, while smaller infarctions were observed when Th2 immune responses were elicited [7,8].
Statins, the drugs widely recognized as 3-hydroxy-3- methylglutaryl-coenzyme A–coenzyme A (HMG-CoA) reductase inhibitors and cholesterol-lowering agents, have been shown to be able to promote Th2- and Th3-dominated immune responses in various experimental conditions [1,2,18,45]. For example, Youssef et al. showed that atorvastatin induced a Th2 switch characterized by TGF-β upregulation, interleukin downregulation, and reversed paralysis in experimental autoimmune encephalomyelitis [45]. Other investigators have found statins to be neuroprotective in several CNS diseases, including Alzheimer’s disease, multiple sclerosis (MS), stroke, and traumatic brain injury [7,8,24,31,33,36]. Pannu et al. found that statin treatment reduced IL-1β expression in the spinal cord while preventing neuronal apoptosis [31]. We have previously demonstrated that simvastatin reverses vasospasm following experimental SAH in rats, and that this treatment is associated with less-severe neurological injury [39]. However, many authors have previously noted that vasospasm does not induce neurological deficits in rats due to their abundant collateral circulation and neuronal physiology [17]. Therefore, we sought to investigate the potential mechanisms of this statin-mediated neuroprotection. In this study, we have shown that simvastatin in a dosage of 20 mg/kg results in a significant reduction in the neurological injury induced by SAH. In addition, high-dose simvastatin increased the cortical and brain-stem expression of TGF-β1 following SAH. TGF-β1 is a cytokine that is expressed in the normal adult brain by parenchymal microglial cells, exerting a trophic anti-inflammatory effect [26]. It has been shown to promote the survival of neurons and inhibit microglial and astrocyte proliferation in the setting of CNS inflammatory reactions [27]. TGF-β1 is a potent inhibitor of cell-mediated immunity and a key marker for Th2 lymphocyte-dominated inflammatory responses [43]. Our results also showed that simvastatin reduces the expression of IL-1β. IL-1β is known to be markedly elevated following CNS trauma, infection, and stroke and is also a marker of Th1-mediated inflammatory reactions [6,37].
Our fluorescence immunohistochemical staining from cortex and brain-stem samples helped to provide a potential explanation for the marked elevation of TGF-β1. Lymphocytes were observed to accumulate in the subarachnoid space following SAH (Fig. 3). In untreated SAH animals, infiltrating lymphocytes did not demonstrate TGF-β1 expression. However, animals treated with high-dose simvastatin following SAH showed marked expression of TGF-β1 by lymphocytes in the subarachnoid space and brain parenchyma (Figs. 2 and 3). These observations help to support the hypothesis that simvastatin induces a Th2 immune switch in these animals, and that infiltrating Th2 lymphocytes are responsible for the measured increase in TGF-β1 expression in the brain following treatment.
Conclusion
The present study provided evidence for the neuroprotective role of statins following SAH and suggested that statins induce the presence of regulatory T lymphocytes in the subarachnoid space following SAH. The use of immune modulation through the use of statins or other inducers of Th2 switches has the potential to provide better outcomes for patients who suffer aneurysmal SAH.
Acknowledgment
This study was supported by grants (NS053407) from the National Institutes of Health to J.H.Z.
Footnotes
Conflicts of Interest Statement We declare that we have no conflict of interest.
Contributor Information
Robert E. Ayer, Department of Neurosurgery, Loma Linda University Medical Center, 11234 Anderson Street, Room 2562B, Loma Linda, CA, USA
Robert P. Ostrowski, Department of Physiology and Pharmacology, Loma Linda University Medical Center, Loma Linda, CA, USA
Takashi Sugawara, Department of Neurosurgery, National Hospital Organization Disaster Medical Center, Tokyo, Japan
Qingy Ma, Department of Physiology and Pharmacology, Loma Linda University Medical Center, Loma Linda, CA, USA.
Nazanin Jafarian, Department of Anesthesiology, Loma Linda University Medical Center, Loma Linda, CA, USA
Jiping Tang, Department of Physiology and Pharmacology, Loma Linda University Medical Center, Loma Linda, CA, USA
John H. Zhang, Department of Neurosurgery, Loma Linda University Medical Center, 11234 Anderson Street, Room 2562B, Loma Linda, CA, USA Department of Anesthesiology, Loma Linda University Medical Center, Loma Linda, CA, USA; Department of Physiology and Pharmacology, Loma Linda University Medical Center, Loma Linda, CA, USA johnzhang3910@yahoo.com.
References
- 1.Aktas O, Waiczies S, Smorodchenko A, Dorr J, Seeger B, Prozorovski T, Sallach S, Endres M, Brocke S, Nitsch R, Zipp F. Treatment of relapsing paralysis in experimental encephalomyelitis by targeting Th1 cells through atorvastatin. J Exp Med. 2003;197:725–733. doi: 10.1084/jem.20021425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arora M, Chen L, Paglia M, Gallagher I, Allen JE, Vyas YM, Ray A, Ray P. Simvastatin promotes Th2-type responses through the induction of the chitinase family member Ym1 in dendritic cells. Proc Natl Acad Sci USA. 2006;103:7777–7782. doi: 10.1073/pnas.0508492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayer RE, Sugawara T, Chen W, Tong W, Zhang JH. Melatonin decreases mortality following severe subarachnoid hemorrhage. J Pineal Res. 2008;44:197–204. doi: 10.1111/j.1600-079X.2007.00508.x. [DOI] [PubMed] [Google Scholar]
- 4.Ayer RE, Zhang JH. The clinical significance of acute brain injury in subarachnoid hemorrhage and opportunity for intervention. Acta Neurochir Suppl. 2008;105:179–184. doi: 10.1007/978-3-211-09469-3_35. [DOI] [PubMed] [Google Scholar]
- 5.Barry KJ, Scott RM. Effect of intravenous ethanol on cerebral vasospasm produced by subarachnoid blood. Stroke. 1979;10:535–537. doi: 10.1161/01.str.10.5.535. [DOI] [PubMed] [Google Scholar]
- 6.Basu A, Krady JK, Levison SW. Interleukin-1: a master regulator of neuroinflammation. J Neurosci Res. 2004;78:151–156. doi: 10.1002/jnr.20266. [DOI] [PubMed] [Google Scholar]
- 7.Becker KJ, Kindrick DL, Lester MP, Shea C, Ye ZC. Sensitization to brain antigens after stroke is augmented by lipopolysaccharide. J Cereb Blood Flow Metab. 2005;25:1634–1644. doi: 10.1038/sj.jcbfm.9600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker KJ, McCarron RM, Ruetzler C, Laban O, Sternberg E, Flanders KC, Hallenbeck JM. Immunologic tolerance to myelin basic protein decreases stroke size after transient focal cerebral ischemia. Proc Natl Acad Sci USA. 1997;94:10873–10878. doi: 10.1073/pnas.94.20.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bederson JB, Germano IM, Guarino L. Cortical blood flow and cerebral perfusion pressure in a new noncraniotomy model of subarachnoid hemorrhage in the rat. Stroke. 1995;26:1086–1091. doi: 10.1161/01.str.26.6.1086. [DOI] [PubMed] [Google Scholar]
- 10.Biros MH, Kukielka D, Sutton RL, Rockswold GL, Bergman TA. The effects of acute and chronic alcohol ingestion on outcome following multiple episodes of mild traumatic brain injury in rats. Acad Emerg Med. 1999;6:1088–1097. doi: 10.1111/j.1553-2712.1999.tb00109.x. [DOI] [PubMed] [Google Scholar]
- 11.Cheng O, Ostrowski RP, Wu B, Liu W, Chen C, Zhang JH. Cyclooxygenase-2 mediates hyperbaric oxygen preconditioning in the rat model of transient global cerebral ischemia. Stroke. 2011;42:484–490. doi: 10.1161/STROKEAHA.110.604421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fathali N, Ostrowski RP, Lekic T, Jadhav V, Tong W, Tang J, Zhang JH. Cyclooxygenase-2 inhibition provides lasting protection against neonatal hypoxic-ischemic brain injury. Crit Care Med. 2010;38:572–578. doi: 10.1097/CCM.0b013e3181cb1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2:43–45. doi: 10.1016/s1474-4422(03)00266-7. [DOI] [PubMed] [Google Scholar]
- 14.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- 15.Gee JM, Kalil A, Shea C, Becker KJ. Lymphocytes: potential mediators of postischemic injury and neuroprotection. Stroke. 2007;38(2 Suppl):783–788. doi: 10.1161/01.STR.0000248425.59176.7b. [DOI] [PubMed] [Google Scholar]
- 16.Gimsa U, Wolf SA, Haas D, Bechmann I, Nitsch R. Th2 cells support intrinsic anti-inflammatory properties of the brain. J Neuroimmunol. 2001;119:73–80. doi: 10.1016/s0165-5728(01)00343-5. [DOI] [PubMed] [Google Scholar]
- 17.Gules I, Satoh M, Clower BR, Nanda A, Zhang JH. Comparison of three rat models of cerebral vasospasm. Am J Physiol Heart Circ Physiol. 2002;283:H2551–H2559. doi: 10.1152/ajpheart.00616.2002. [DOI] [PubMed] [Google Scholar]
- 18.Hakamada-Taguchi R, Uehara Y, Kuribayashi K, Numabe A, Saito K, Negoro H, Fujita T, Toyo-oka T, Kato T. Inhibition of hydroxymethylglutaryl-coenzyme a reductase reduces Th1 development and promotes Th2 development. Circ Res. 2003;93:948–956. doi: 10.1161/01.RES.0000101298.76864.14. [DOI] [PubMed] [Google Scholar]
- 19.Hendrix S, Nitsch R. The role of T helper cells in neuroprotection and regeneration. J Neuroimmunol. 2007;184:100–112. doi: 10.1016/j.jneuroim.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 20.Hop JW, Rinkel GJ, Algra A, van Gijn J. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke. 1997;28:660–664. doi: 10.1161/01.str.28.3.660. [DOI] [PubMed] [Google Scholar]
- 21.Huang DW, McKerracher L, Braun PE, David SA. A therapeutic vaccine approach to stimulate axon regeneration in the adult mammalian spinal cord. Neuron. 1999;24:639–647. doi: 10.1016/s0896-6273(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 22.Jadhav V, Matchett G, Hsu FP, Zhang JH. Inhibition of Src tyrosine kinase and effect on outcomes in a new in vivo model of surgically induced brain injury. J Neurosurg. 2007;106:680–686. doi: 10.3171/jns.2007.106.4.680. [DOI] [PubMed] [Google Scholar]
- 23.Kubota T, Handa Y, Tsuchida A, Kaneko M, Kobayashi H, Kubota T. The kinetics of lymphocyte subsets and macrophages in subarachnoid space after subarachnoid hemorrhage in rats. Stroke. 1993;24:1993–2000. doi: 10.1161/01.str.24.12.1993. [DOI] [PubMed] [Google Scholar]
- 24.Lu D, Goussev A, Chen J, Pannu P, Li Y, Mahmood A, Chopp M. Atorvastatin reduces neurological deficit and increases synaptogenesis, angiogenesis, and neuronal survival in rats subjected to traumatic brain injury. J Neurotrauma. 2004;21:21–32. doi: 10.1089/089771504772695913. [DOI] [PubMed] [Google Scholar]
- 25.Lynch JR, Wang H, McGirt MJ, Floyd J, Friedman AH, Coon AL, Blessing R, Alexander MJ, Graffagnino C, Warner DS, Laskowitz DT. Simvastatin reduces vasospasm after aneurysmal subarachnoid hemorrhage: results of a pilot randomized clinical trial. Stroke. 2005;36:2024–2026. doi: 10.1161/01.STR.0000177879.11607.10. [DOI] [PubMed] [Google Scholar]
- 26.Makwana M, Jones LL, Cuthill D, Heuer H, Bohatschek M, Hristova M, Friedrichsen S, Ormsby I, Bueringer D, Koppius A, Bauer K, Doetschman T, Raivich G. Endogenous transforming growth factor beta 1 suppresses inflammation and promotes survival in adult CNS. J Neurosci. 2007;27:11201–11213. doi: 10.1523/JNEUROSCI.2255-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathiesen T, Lefvert AK. Cerebrospinal fluid and blood lymphocyte subpopulations following subarachnoid haemorrhage. Br J Neurosurg. 1996;10:89–92. doi: 10.1080/02688699650040584. [DOI] [PubMed] [Google Scholar]
- 28.McGirt MJ, Blessing R, Alexander MJ, Nimjee SM, Woodworth GF, Friedman AH, Graffagnino C, Laskowitz DT, Lynch JR. Risk of cerebral vasospasm after subarachnoid hemorrhage reduced by statin therapy: a multivariate analysis of an institutional experience. J Neurosurg. 2006;105:671–674. doi: 10.3171/jns.2006.105.5.671. [DOI] [PubMed] [Google Scholar]
- 29.McGirt MJ, Lynch JR, Parra A, Sheng H, Pearlstein RD, Laskowitz DT, Pelligrino DA, Warner DS. Simvastatin increases endothelial nitric oxide synthase and ameliorates cerebral vasospasm resulting from subarachnoid hemorrhage. Stroke. 2002;33:2950–2956. doi: 10.1161/01.str.0000038986.68044.39. [DOI] [PubMed] [Google Scholar]
- 30.Ostrowski RP, Colohan AR, Zhang JH. Mechanisms of hyperbaric oxygen-induced neuroprotection in a rat model of subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2005;25:554–571. doi: 10.1038/sj.jcbfm.9600048. [DOI] [PubMed] [Google Scholar]
- 31.Pannu R, Barbosa E, Singh AK, Singh I. Attenuation of acute inflammatory response by atorvastatin after spinal cord injury in rats. J Neurosci Res. 2005;79:340–350. doi: 10.1002/jnr.20345. [DOI] [PubMed] [Google Scholar]
- 32.Parra A, Kreiter KT, Williams S, Sciacca R, Mack WJ, Naidech AM, Commichau CS, Fitzsimmons BF, Janjua N, Mayer SA, Connolly ES., Jr Effect of prior statin use on functional outcome and delayed vasospasm after acute aneurysmal subarachnoid hemorrhage: a matched controlled cohort study. Neurosurgery. 2005;56:476–484. doi: 10.1227/01.neu.0000153925.96889.8a. [DOI] [PubMed] [Google Scholar]
- 33.Qu C, Lu D, Goussev A, Schallert T, Mahmood A, Chopp M. Effect of atorvastatin on spatial memory, neuronal survival, and vascular density in female rats after traumatic brain injury. J Neurosurg. 2005;103:695–701. doi: 10.3171/jns.2005.103.4.0695. [DOI] [PubMed] [Google Scholar]
- 34.Schievink WI. Intracranial aneurysms. N Engl J Med. 1997;336:28–40. doi: 10.1056/NEJM199701023360106. [DOI] [PubMed] [Google Scholar]
- 35.Sicotte M, Tsatas O, Jeong SY, Cai CQ, He Z, David S. Immunization with myelin or recombinant Nogo-66/MAG in alum promotes axon regeneration and sprouting after corticospinal tract lesions in the spinal cord. Mol Cell Neurosci. 2003;23:251–263. doi: 10.1016/s1044-7431(03)00053-8. [DOI] [PubMed] [Google Scholar]
- 36.Stepien K, Tomaszewski M, Czuczwar SJ. Neuroprotective properties of statins. Pharmacol Rep. 2005;57:561–569. [PubMed] [Google Scholar]
- 37.Stojanov S, Lapidus S, Chitkara P, Feder H, Salazar JC, Fleisher TA, Brown MR, Edwards KM, Ward MM, Colbert RA, Sun HW, Wood GM, Barham BK, Jones A, Aksentijevich I, Goldbach-Mansky R, Athreya B, Barron KS, Kastner DL. Periodic fever, aphthous stomatitis, pharyngitis, and adenitis (PFAPA) is a disorder of innate immunity and Th1 activation responsive to IL-1 blockade. Proc Natl Acad Sci USA. 2011;A108:7148–7153. doi: 10.1073/pnas.1103681108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sudlow CL, Warlow CP. Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. International Stroke Incidence Collaboration. Stroke. 1997;28:491–499. doi: 10.1161/01.str.28.3.491. [DOI] [PubMed] [Google Scholar]
- 39.Sugawara T, Ayer R, Jadhav V, Chen W, Tsubokawa T, Zhang JH. Simvastatin attenuation of cerebral vasospasm after subarachnoid hemorrhage in rats via increased phosphorylation of Akt and endothelial nitric oxide synthase. J Neurosci Res. 2008;86:3635–3643. doi: 10.1002/jnr.21807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugawara T, Ayer R, Jadhav V, Zhang JH. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods. 2008;167:327–334. doi: 10.1016/j.jneumeth.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Titova E, Kevil CG, Ostrowski RP, Rojas H, Liu S, Zhang JH, Tang J. Deficiency of CD18 gene reduces brain edema in experimental intracerebral hemorrhage in mice. Acta Neurochir Suppl. 2008;105:85–87. doi: 10.1007/978-3-211-09469-3_17. [DOI] [PubMed] [Google Scholar]
- 42.Tseng MY, Czosnyka M, Richards H, Pickard JD, Kirkpatrick PJ. Effects of acute treatment with pravastatin on cerebral vasospasm, autoregulation, and delayed ischemic deficits after aneurysmal subarachnoid hemorrhage: a phase II randomized placebo-controlled trial. Stroke. 2005;36:1627–1632. doi: 10.1161/01.STR.0000176743.67564.5d. [DOI] [PubMed] [Google Scholar]
- 43.Weiner HL. Oral tolerance: immune mechanisms and the generation of Th3-type TGF-beta-secreting regulatory cells. Microbes Infect. 2001;3:947–954. doi: 10.1016/s1286-4579(01)01456-3. [DOI] [PubMed] [Google Scholar]
- 44.Wolf SA, Fisher J, Bechmann I, Steiner B, Kwidzinski E, Nitsch R. Neuroprotection by T-cells depends on their subtype and activation state. J Neuroimmunol. 2002;133:72–80. doi: 10.1016/s0165-5728(02)00367-3. [DOI] [PubMed] [Google Scholar]
- 45.Youssef S, Stüve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM, Bravo M, Mitchell DJ, Sobel RA, Steinman L, Zamvil SS. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]