Abstract
Blastomyces dermatitidis, a dimorphic fungus and the causative agent of blastomycosis, is widely considered an extracellular pathogen, with little evidence for a facultative intracellular lifestyle. We infected mice with spores - the infectious particle - via the pulmonary route and studied intracellular residence, transition to pathogenic yeast and replication inside lung cells. Nearly 80% of spores were inside cells at 24 hours after infection with 104 spores. The majority of spores were located inside of alveolar macrophages, with smaller numbers in neutrophils and dendritic cells. Real time imaging showed rapid uptake of spores into alveolar macrophages, conversion to yeast, and intracellular multiplication during in vitro co-culture. The finding of multiple yeast in a macrophage was chiefly due to intracellular replication rather than multiple phagocytic events or fusion of macrophages. Depletion of alveolar macrophages curtailed infection in mice infected with spores, and lead to a 26-fold reduction in lung CFU by 6 days post-infection vs. non-depleted mice. Phase transition of the spores to yeast was delayed in these depleted mice over a time frame that correlated with reduced lung CFU. Spores cultured in vitro converted to yeast faster in the presence of macrophages than in medium alone. Thus, while advanced B. dermatitidis infection may exhibit extracellular residence in tissue, early lung infection with infectious spores reveals its unappreciated facultative intracellular lifestyle.
Keywords: Fungi, macrophages, dendritic cells, neutrophils, phagocytosis, lung
INTRODUCTION
Blastomyces dermatitidis is the causative agent of Blastomycosis, a potentially deadly fungal infection. The fungus is considered a primary pathogen that can infect immune-competent individuals, yet B. dermatitidis can also reactivate in previously infected patients that become immune-compromised (1, 2). B. dermatitidis is one of six dimorphic fungi that are collectively responsible for the majority of systemic fungal infections in the United Sates (3). Infections with the dimorphic fungi represent a growing public health problem, particularly in immune compromised patients (4), and limited measures are available to prevent their acquisition.
Blastomycosis is commonly reported in endemic regions of the United States, Canada, Africa and the Middle East (5-8). B. dermatitidis, like the other dimorphic fungi, grows in the soil or a similar environmental substrate as a mold, which bears spores (conidia). Primary pulmonary infection is initiated when spores are inhaled into the lungs of a susceptible host (9). There, spores enter alveoli and undergo a morphological transition into budding yeast. The phase transition to yeast is essential for pathogenesis of disease (10). Yeast cells are more resistant than spores to killing mediated by host immune cells such as neutrophils, macrophages and monocytes (11). Moreover, deletion of a global regulator of phase transition, DRK1 (Dimorphism Regulating Kinase), locks the fungus in the mold form and abrogates virulence (12).
B. dermatitidis is generally thought of as an “extracellular” pathogen. Histological sections of infected lung tissue and extrapulmonary sites following fungal dissemination support this premise by showing that most of the yeast are found in the extracellular space. Due to the long incubation period and the frequent delay in diagnosing blastomycosis, these data are often collected from human patients well after infection is initiated (13-15). This circumstance leaves a gap in the knowledge about the early stage of infection.
Some evidence points to the intracellular residence of B. dermatitidis. Sections of infected tissue have reported yeast inside of phagocytes (13, 15). In vitro, phagocytes quickly and efficiently internalize the small (2-5 μM) spores. The larger yeast (10-30 μM) are also phagocytosed, but to a lesser extent and at a slower rate (11). Whereas spores are more vulnerable to killing by phagocytes, yeast can replicate in vitro in their presence, and electron microscopy has revealed multiple yeast inside human monocytes during co-culture (16).
While prior work supports the idea that B. dermatitidis may grow inside of host phagocytes in vitro (16), this and other work did not exclude the possibilities that phagocytes may repeatedly internalize yeast from the extracelluar environment, nor that phagocytes may fuse upon exposure to yeast. Each of these events could also result in the presence of multiple yeast in phagocytes and give the erroneous conclusion of intracellular replication. Additionally, the in vitro studies above were done with yeast and not spores, the infectious particles that initiate infection. If spores are indeed rapidly internalized and highly sensitive to killing by leukocytes, this raises the question of where and how inhaled spores convert into yeast and replicate during early infection to establish disease. To our knowledge, studies of pulmonary blastomycosis have not been conducted with spores to initiate lung infection and interrogate their intracellular residence, transition, and replication during the early pathogenesis of disease.
Herein, we investigated the early pathogenesis of pulmonary blastomycosis in a model involving infection with spores. We investigated the host-pathogen interaction with emphasis on elucidating intracellular residence and replication of the fungus. We tackled several questions: 1) are spores taken into phagocytes in the lung, and if so, what are the cells and time course; 2) do spores convert to yeast inside lung phagocytes; 3) do yeast replicate inside these cells, and to what extent are multiple intracellular yeast due to replication, multiple phagocytic events or cell fusion; and 4) if B. dermatitidis replicates inside host lung phagocytes, do the phagocytes constrain or permit progression of early infection. We report that spores are rapidly taken up into alveolar macrophages where they convert to yeast and replicate intracellularly. Moreover, intracellular residence and replication in macrophages is required for initiation of disease.
METHODS
Mice
C57BL/6 wild type (WT) mice were obtained from The National Cancer Institute. CD45.1 C57BL/6 mice were obtained from Taconic. CD11c-Diphtheria Toxin Receptor (DTR) mice were obtained from The Jackson Laboratory and bred in house. Mice were housed and cared for according to guidelines from the University of Wisconsin Animal Care and Use Committee, who approved this work. Their guidelines are in compliance with Health and Human Services Guide for the Care and Use of Laboratory Animals.
Reagents and Cell Culture
Bone marrow was collected to generate bone marrow derived macrophages (BMM) and chimeric mice. Marrow was collected from femurs and tibias by rinsing and disruption through a 26-gauge needle and filtering via a 40 μM filter. BMM were differentiated in culture at 37°C using a 1:10 dilution of L929 supernatant and adherent cells were collected after one week. Alveolar macrophages were collected from exsanguinated mice by repeated lavage of the lung alveoli through a cannula with 0.6 mM EDTA in phosphate buffered saline (PBS) at 37°C. The lavage fluid was then placed on ice. Red blood cells (RBCs) were lysed using ammonium chloride/potassium bicarbonate (ACK) buffer. The murine alveolar like macrophage-cell line AMJ2-C11 was obtained from American Type Culture Collection (ATCC). Cells were counted on a hemocytometer using Trypan Blue (Sigma) to assay viability. Cells were cultured at 37°C and 5% CO2 and maintained in Roswell Park Memorial Institute (RPMI) 1640 medium (HyClone) with 10% heat-inactivated (56°C for 30 min) fetal bovine serum (Atlanta Biologicals), 100 units penicillin, and 100 μg streptomycin (Hyclone).
Fungi
Blastomyces dermatitidis yeast from strains 26199 and 14081 (ATCC) were taken in log-phase growth and suspended in PBS. Yeast were aspirated through a 26-G needle and passed through a 40 μm filter to reduce aggregated yeast. In some experiments, yeast were heat-killed at 65°C for one hour in PBS. Yeast CFU were counted from brain-heart infusion plates after five to seven days of growth at 37°C. Yeast from strain 14081 were plated onto potato dextrose agar and incubated for two weeks at 22°C. The resulting hyphal mat was lightly rubbed with a spreader and 5 mL PBS to collect spores. The resulting suspension was filtered through multiple layers of sterile miracloth (Millapore) and a 40 μM filter to reduce hyphal contamination (less than 2% in all experiments). Spore CFU were counted from Kelly's agar plates incubated at 30°C for 8-10 days. All work with spores was performed under biosafety level 3 conditions.
Fluorescent yeast were used in some experiments. A yeast strain expressing green fluorescent protein (GFP) under the control of a constitutive, histone H2B promoter has been described (17). We used mCherry fluorescent protein under the control a yeast phase-specific BAD-1 promoter (18) to create a “reporter” strain of 14081 in which spores fluoresced dimly and phase-transition lead to intense expression and bright red yeast via fluorescence microscopy or FACS. Thus, a dim mCherry signal was sufficient to allow tracking of spores in vivo by FACS, and the increased signal of >10 fold was used to confirm phase transition to yeast (Results).
Infection
Mice were anesthetized with isoflurane and suspended by their front incisors from a wire on a 45-degree plane. Mice were intubated with a BioLite Intubation System. Spores were delivered intratracheally through a cannula in a 20 μL suspension in PBS.
Flow cytometry
Lungs were diced by pressing them through a 40 μM filter with the plunger of a 5 mL syringe. Homogenates were digested using 1 mg/mL Collagenase D (Roche) and 10 ng/mL DNAse I (Sigma) for 20 min. at 37°C. RBCs were lysed with ACK buffer and the remaining cells were washed with 2mM EDTA and 0.5% BSA in PBS (fluorescence activated cell sorting [FACS] buffer). Staining was done in 100 μL of FACS buffer for 20 min at 4°C in the dark. Stained cells were washed with FACS buffer, fixed with 2% paraformaldehyde solution for 20 min, washed again and suspended in FACS buffer for analysis. All centrifugation was done at 1500 rpm for 5 min and 4°C in an Eppendorf 5825 centrifuge. Events were gated on FCS and SSC to exclude debris and LIVE/DEAD Fixable Yellow® (Molecular Probes) negative cells to exclude dead cells. Alveolar macrophages were defined phenotypically as CD11c+, Mac3+, CD11b−, CD103−, Ly6g−, and autofluorescent. Neutrophils were defined as CD11b+, Ly6g+ (clone 1A8), and CD11c−. Dendritic cells (DCs) were defined as CD11c+, MHCII+ with the following subsets: neutrophil-derived DCs were CD11c+, CD11b+, and Ly6g+; inflammatory DCs were CD11c+, CD11b+, and Ly6g−; and resident DCs were CD103+ and CD11c+ (19-21). Antibodies were conjugated to the following fluorophores FITC, PE, PerCP, PE-Cy7, APC, A700, or APC-Cy7, and were from BD Biosciences, eBiosciences, and BioLegend.
Spores and yeast were identified in FACS by low and high expression, respectively, of mCherry fluorescence as noted above and illustrated in Results. Extracellular fungi were stained with 10 μg/mL Uvitex 2B (PolySciences, Inc.). The percent of fungi that were intracellular was defined as the number of mCherry-positive/Uvitex 2B-negative events divided by the total number of fungi that stained with either dye. Data was collected on a LSRII cytometer (BD Biosciences) and analyzed by FlowJo software (Tree Star).
Microscopy
To characterize yeast inside of AMJ2C-11 macrophages, cells were cultured for 24 hours with 26199 yeast in a 24-well plate with cRPMI. The medium was removed and cells stained with 10μg/mL Uvitex 2B to identify extracellular yeast and 12 μM ethidium bromide to identify dead cells in PBS. Washed cells were fixed with 2% paraformaldehyde and permeabilized with 0.05% Saponin (Sigma) for 20 min. Cells were stained with anti-BAD-1-FITC to identify yeast. Anti-BAD-1 mAb (DD5-CB4) (22) was made from ascites, ammonium sulfate precipitated, purified on an A/G agarose column (Pierce Chemical) and then labeled with FITC (Molecular Probes) and purified by dialysis. Differential fluorescence microscopy was performed with an Olympus BX60 microscope. Images were captured with an Exi Aqua Camera (QImaging) and QCapture Pro 6.0 image software. Images were processed using Adobe Photoshop. Live Imaging was done on an Observer Z1 microscope with a humidified incubation chamber kept at 37°C and 5% CO2 and data was collected using AxioVision software (Zeiss). Movies were processed using iMovie.
Intracellular replication
In vitro intracellular replication was quantified by culturing 7×105 BMM or alveolar macrophages with 7×104 yeast at a multiplicity of infection of 0.1 on glass coverslips in a 24-well plate. We used yeast that express either red (mCherry) or green (GFP) fluorescence for these assays. We added the yeast at a 1:20 ratio of red to green yeast to reduce the likelihood of multiple yeast within a single macrophage arising from multiple phagocytic events. After allowing the yeast to be phagocytosed for four hours, free yeast were washed away with PBS. At 4-, 24-, 48-, and 72-hour time points, samples were collected by washing the coverslips with PBS and staining with either LIVE-DEAD Fixable Violet stain® (Molecular Probes) as per manufacturer instructions, or with 10 μg/mL Uvitex 2B for 20 min. to exclude extracellular yeast from analysis. Cells were then fixed using a 2% paraformaldehyde solution for 20 min., washed with PBS and stationed onto a glass slide cell-face down in Mowiol 4-88 anti-fade medium® (CalBioChem). Wells that were not collected at a time point were washed to remove extracellular yeast and replenished with fresh media. Fixed slides were maintained in the dark at 4°C until imaging.
Macrophage Fusion
BMM were stained with 15 μM 5-chloromethylfluorescein diacetate (CMFDA) (Invitrogen) or PKH26 (Sigma), as per manufacturer instructions, and equal parts of the stained cells were aliquoted into a 24-well plate containing glass coverslips for a total of 7×105 cells per well, as described above for quantification of intracellular replication. Fusion was stimulated with 7×104 yeast or, as a positive control, with the addition of 10 ng/mL IL-4 (PeproTech) and 1 μg/mL GMCSF (R&D Systems) (23).
Bone Marrow Chimeras
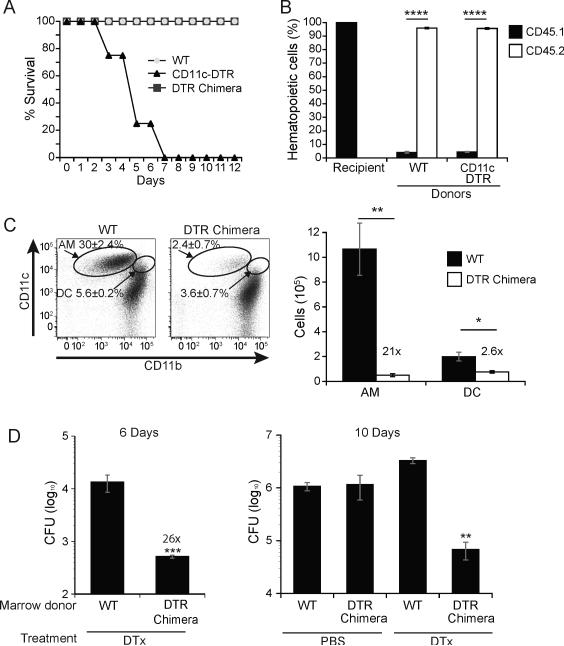
Administration of diphtheria toxin (DTx) (Sigma) to CD11c-DTR mice is lethal after more than two treatments of 100 ng (Figure 4). Therefore, we generated bone marrow chimeric mice as previously described (24). Briefly, chimeric mice were generated by lethally irradiating CD45.1 mice with two doses of 550 rads four hours apart on a X-rad 320 (Precision X-ray). Mice were injected with 1×107 bone marrow cells i.v. from either CD45.2 WT or CD11c-DTR donor mice. Chimeric mice were maintained on 0.5 mg/mL Baytril 100 (Bayer) and 2 mg/mL Neomycin sulfate (Sigma) in their drinking water for two weeks. Alveolar macrophages were allowed to reconstitute for 12 weeks. Mice were injected with 100 ng DTx i.p. every other day starting two days before infection with 3.2×104 spores. Lungs were collected every two days for 12 days and analyzed by FACS and for CFU.
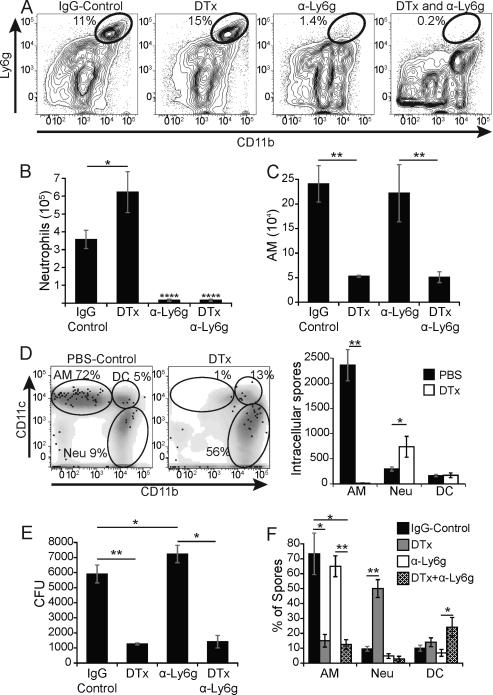
Figure 4. Lung CD11chigh cells permit progressive pulmonary infection.
(A) Uninfected mice were injected with 100 ng diphtheria toxin (DTx) i.p. every other day for up to 12 days. Results are representative of 5 independent experiments. (B) Lung homogenates of bone marrow chimeric recipients (CD45.1) that received congenic (CD45.2) WT control or CD11c-DTR bone marrow were assayed by flow cytometry for reconstitution of hematopoietic cells. Results are the mean±SEM of 2 experiments with 5-18 mice/group. (C) The efficiency of CD11c+ cell depletion in DTx treated mice after reconstitution with WT or CD11c-DTR bone marrow. The percent of alveolar macrophages and DCs and the absolute cell numbers following depletion are shown in flow plots (left) and a bar graph (right). Results are representative of 3 experiments with 5 mice per group. (D) Lung CFU of DTx- or PBS-control treated chimeric WT and CD11c-DTR mice 6 days and 10 days post infection with mCherry 14081 spores. Results are representative of 2 experiments with 3 chimeric mice per group. *P<0.05, **P<0.01, ****P <0.0001.
Neutrophil depletion
Neutrophils were depleted by i.v. injection every other day with 250 μg anti-Ly6g (clone 1A8) (BioXCell). Rat IgG was used as a control. Mice were treated with DTx and anti-Ly6g one day before infection and lungs were collected two days post infection.
Statistical Analysis
Experimental conditions were compared to controls using an unpaired student's t-test, Mann-Whitney U test, or ANOVA with Tukey's multiple comparison test where appropriate. p<0.05 was considered statistically significant. Analysis was performed with Prism software (Graph Pad). Data are presented as means and error bars represent standard error of the mean (SEM).
RESULTS
The interaction between spores and leukocytes early during infection
Pulmonary infection with B. dermatitidis begins with inhalation of infectious particles. Thus, we introduced spores into the lungs of mice to mimic the natural infection. Alveolar macrophages (CD11chigh, CD11b−, Mac3+) are the predominant leukocyte present in the lungs of naïve mice, with ≈5×105 cells, and they also accounted for the majority of the leukocytes in the lungs early during infection (Fig. 1A and Fig. S1). Infection with spores induced inflammation and the influx of other leukocytes (Fig. 1A) compared to naïve mice, which have ≈1×104 neutrophils (CD11b+, CD11c−, Ly6g+) and 5×104 DCs (CD11c+, MHCII+) in their lungs (data not shown). Higher inocula induced a more prominent influx of these cells. Infection with spores also led to the influx of a rarely described DC subset, neutrophil-derived DCs (CD11c+, CD11b+, Ly6g+) (Fig. 1A, inset and Fig. S1), which are barely detected in naïve mice (25).
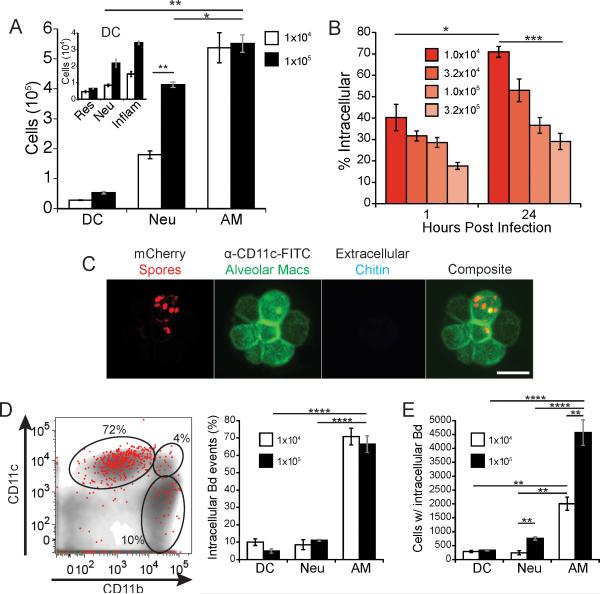
Figure 1. The majority of spores reside in alveolar macrophages early during infection.
Mice were infected with mCherry 14081 spores by intubation. (A) At 24 hours post infection, lung homogenates were analyzed for the total number and lineage of leukocytes. The gating markers and strategy are described in the Methods and illustrated in supplemental Fig. S1. Alveolar macrophages (AM); neutrophils (Neu), dendritic cells (DC). DC subsets (inlay) are resident (Res), neutrophil-derived (Neu), and inflammatory (inflam). (B) The proportion of total spores (mCherry+ events) in the lungs that are intracellular (Uvitex 2B−) at 1 and 24 hours post infection was measured at a range of inocula. Results are representative of three experiments each with five mice/group. (C) Intracellular residence of spores within alveolar macrophages from lung homogenates of mice infected with mCherry spores for 12 hours. Alveolar macrophages were identified with anti-CD11c-FITC. Uvitex 2B stain of chitin was used to determine if the spores were extracellular (only extracellular spores bind the dye). Bar in lower right panel = 25 microns. (D) Distribution of intracellular spores (mCherry+, Uvitex 2B−) 24 hours after mice were infected with 1×105 spores. A flow plot of a representative mouse illustrating intracellular spores (red dots) overlaid on total leukocytes (gray) with representative gates for alveolar macrophages (top left), DCs (top right), and neutrophils (bottom right). Distribution of spores within leukocytes (left panel) is based on the gating strategy shown in the Fig. S1; group mean±SEM analyzed with FACS (right panel). (E) The total number of lung leukocytes that contain spores 24 hours post infection. Results shown in panels D and E are representative of 3 experiments with 5 mice per group. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
We utilized B. dermatitidis that expresses mCherry protein fluorescence to let us track (dimly fluorescent) spores in the lungs of infected mice. We analyzed the intracellular residence of spores by identifying extracellular spores with the membrane-excludable, chitin-specific stain Uvitex 2B. We found a dose-dependent effect on intracellular residence, with lower spore inocula showing more intracellular infection than higher inocula (Fig. 1B). The lower limit of reliable detection by FACS was with an inoculum of 104 spores. At this inoculum, 70-80% of spores were located inside leukocytes by 24 hours post-infection. Although spores could not be reliably tracked at a lower inoculum, intracellular residence increased as the inoculum was reduced and thus could be higher at inocula of <104 spores.
We sought to identify the cells in which spores reside early during infection. Spores were readily identified inside alveolar macrophages upon microscopic analysis of homogenized lung tissue (Fig. 1C). To quantify the distribution of spores, we further analyzed whole lung homogenates by flow cytometry (Fig. 1D). Independent of the inoculum, the majority (>70%) of intracellular spores were detected in alveolar macrophages after 24 hours of infection, with only minor proportions in DCs and neutrophils (Fig. 1D). The selective association of spores with alveolar macrophages remained strong even at a higher inoculum of 105 spores, which leads to greater numbers of neutrophils in the lung. At 105 spores, there were about 4,700 alveolar macrophages with intracellular spores versus only 700 neutrophils and 250 DCs (Fig. 1E). Thus, most B. dermatitidis spores enter leukocytes early during infection and these spores reside predominantly within alveolar macrophages, not neutrophils or DCs.
The fate of intracellular spores and yeast
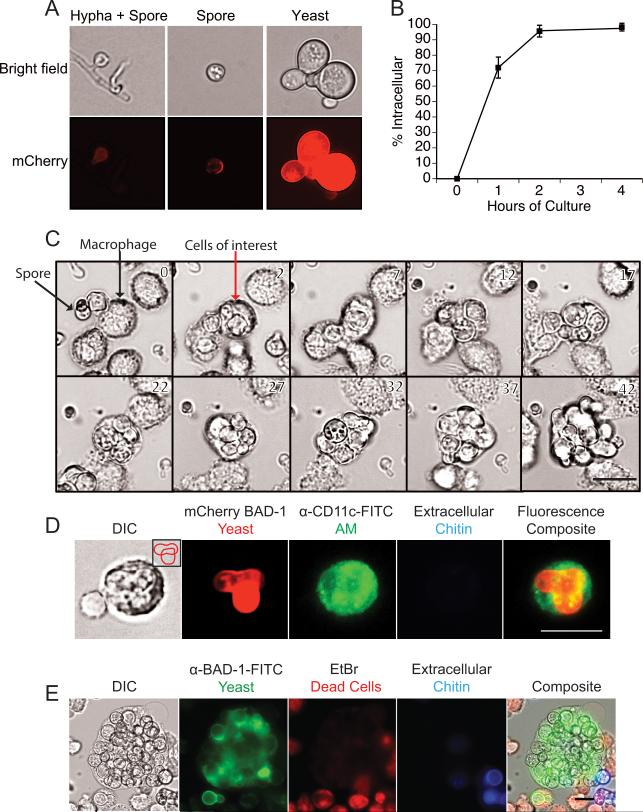
The high percentage of spores found inside alveolar macrophages suggests a likely role for macrophages early during infection – either in restricting growth of the fungus or providing a locale for intracellular replication and establishment of infection. We therefore investigated the ability of the fungus to survive, convert from spores to yeast, and replicate in alveolar macrophages. To monitor the phase transition of spores to yeast, we exploited the B. dermatitidis reporter strain. In this strain, the yeast phase-specific gene promoter (BAD-1) up-regulates mCherry fluorescence during the phase transition from mold or spore to yeast (18). Hyphae have no fluorescence, spores show dim expression, and yeast highly express mCherry (Fig. 2A). Spores of this strain were rapidly internalized by alveolar macrophages during in vitro co-culture, with 95% of spores inside macrophages by 2 hours (Fig. 2B). During live cell imaging of these spores cultured with primary alveolar macrophages, we observed intracellular transition of the spores to yeast and ensuing replication (Fig. 2C; and Video 1.Mov in supplement). We also detected budding yeast within individual alveolar macrophages found in lavage fluid from spore-infected mice (Fig. 2D), consistent with intracellular replication in vivo. Moreover, when yeast were cultured with AMJ2-C11 macrophages in vitro, some macrophages contained 30 or more yeast after 2 days of culture (Fig. 2E). Thus, spores can survive, germinate and even replicate as yeast inside of alveolar macrophages shortly after infection.
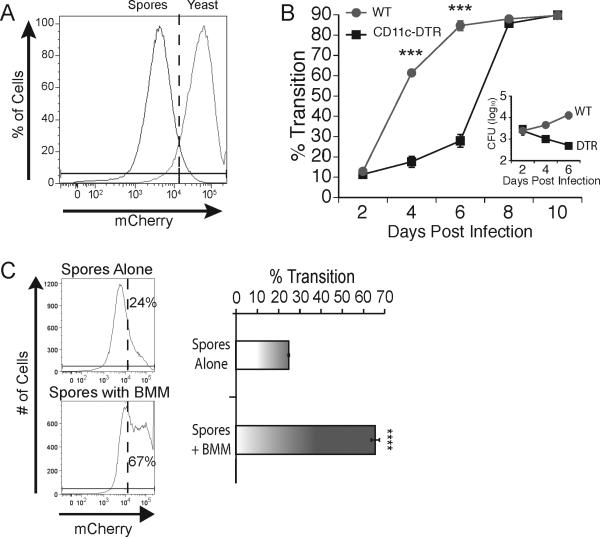
Figure 2. B. dermatitidis spores survive and replicate as yeast in alveolar macrophages.
(A) Bright field and fluorescence microscopy of spores and yeast of reporter strain 14081 that upregulates mCherry under the control of a yeast phase-specific BAD-1 promoter. mCherry fluorescence is quantified by FACS in Fig. 6A. (B) Uptake of mCherry spores by macrophages in vitro. Spores were cultured with primary alveolar macrophages at an MOI of 0.2. Uptake was quantified over 4 hours and analyzed by FACS. Extracellular spores stained Uvitex 2B+. Results are the mean of triplicate wells and representative of 3 experiments. (C) Live imaging of mCherry 14081 spores cultured with primary alveolar macrophages at a MOI of 0.2. Images shown are every 5 hours. The full movie is available online. (D) Mice were infected with mCherry 14081 spores and 3 days later BAL fluid was collected and stained with anti-CD11c-FITC to identify alveolar macrophages and Uvitex 2B to distinguish intracellular and extracellular yeast. Inlay in the left panel shows a budding yeast cell overlying another yeast. (E) Alveolar macrophage cell line AMJ2-C11 was cultured for 48 hours with 14081 yeast on coverslips. Cultures were treated with Uvitex 2B to stain the extracellular yeast, and with ethidium bromide to ascertain macrophage membrane integrity and cell viability. Cultures were fixed and then permeabilized to identify yeast with anti-BAD-1-FITC. Bar in lower right panels = 25 microns
Intracellular replication of yeast inside macrophages
Since B. dermatitidis is not considered a facultative intracellular pathogen that replicates inside macrophages, we further investigated the extent to which replication occurs intracellularly. The finding of multiple yeast in a single macrophage could be the result of multiple phagocytic events, fusion of macrophages, or replication of yeast inside of a macrophage. To maximize the likelihood of quantifying only intracellular replication events, we co-cultured macrophages in vitro with an inoculum containing red (red fluorescent protein) and green (green fluorescent protein) yeast in a ratio of 1 to 20, respectively. By interrogating macrophages that harbor only the less common red yeast, we greatly increased the probability that multiple, intracellular red yeast arose from replication in macrophages and not from multiple phagocytic events or macrophage fusion (Fig. 3A). For example, the probability that four red yeast in a macrophage arose from multiple phagocytic events is low - approximately 1/1.6×105. Briefly, we cultured BMM with yeast in vitro for four hours to enable phagocytosis, and then removed the non-adherent yeast. We incubated cultures for an additional 24 hours to allow yeast to replicate. Then, we counted the number of red yeast inside macrophages. We excluded extracellular yeast that stained positive for Uvitex 2B and macrophages that contained green yeast.
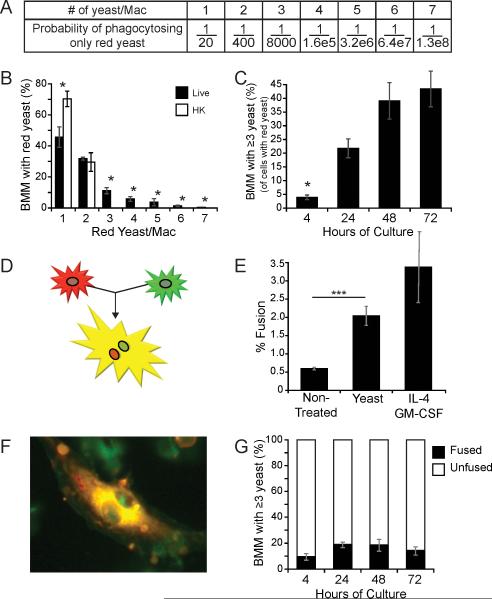
Figure 3. Intracellular replication by B. dermatitidis yeast.
(A) Probability of only red yeast being phagocytosed when they comprise 1/20th of the yeast inoculum. (B) BMM were cultured for 24 hours with two yeast strains, one expressing red fluorescent protein and the other, green fluorescent protein (ratio of 1:20, respectively); both strains in an assay were either live or heat-killed. The number of yeast per macrophage was enumerated for the BMM that contained only red yeast. 500 yeast-containing macrophages were enumerated per condition; results are the mean±SEM of five experiments. (C) BMM containing ≥3 red yeast were enumerated. 500 yeast-containing macrophages were counted per time point; results are the mean±SEM of three experiments (D) The fusion of a red macrophage with a green one results in a yellow, multinucleated giant cell. (E) Fusion between PKH26 (red) and CMFDA (green) stained BMM (1:1) was enumerated for cells cultured 24 hours with or without yeast (strain 26199) or with IL-4 and GM-CSF (control). Results are the mean±SEM of 4 experiments in which >400 macrophages were counted per condition. (F) A fused, yellow, multinucleated giant cell from a culture with yeast that does not contain intracellular yeast. (G) Red and green BMM were cultured with yeast over time. The proportion of fused BMM with ≥3 intracellular yeast is depicted. Results are the mean±SEM of 3 experiments in which >400 macrophages were counted per condition. *P<0.05, ***P <0.001.
Macrophages cultured with live yeast had more red yeast per macrophage than did macrophages cultured with heat-killed yeast (Fig. 3B). Since only co-cultures with live yeast resulted in 3 or more red yeast per macrophage (cultures with heat-killed yeast had only 1-2 red yeast per macrophage), we concluded that 3 or more yeast per macrophage was likely due to intracellular replication, not multiple phagocytic events. Some macrophages had 7 or more intracellular red yeast. The percentage of BMM that contained 3 or more red yeast grew steadily over time during 72-hours of incubation (Fig. 3C). Similar trends were observed in co-cultures of live yeast with primary alveolar macrophages (data not shown). The increasing proportion of macrophages with 3 or more yeast over time suggests that yeast frequently replicate inside macrophages.
The ratiometric analysis above reduced the chance that repeated phagocytic events could account for the finding of multiple intracellular red yeast, but fusion of macrophages could not be entirely excluded. We therefore assayed whether yeast induce fusion of macrophages and the extent to which this event explained our finding of multiple yeasts inside a macrophage. In this assay, BMM were stained with red or green fluorescent dye and mixed together in equal proportion; fusion of the two yielded a yellow, multinucleated giant cell (Fig. 3D). Yeast did induce macrophage fusion, but it occurred in only ≈2% of macrophages (Fig. 3E). This amount of fusion was similar to that found for BMM exposed to IL-4 and GM-CSF, which are known to induce fusion (26). Nevertheless, not all fused macrophages harbored intracellular yeast (Fig. 3F). In fact, fusion accounted for only a minority (≤20%) of BMM with 3 or more yeast (Fig. 3G). Thus, while co-culture with B. dermatitidis yeast induces macrophage fusion, the phenomenon does not contribute substantially to the finding of multiple yeast inside macrophages.
The role of macrophages during progressive pulmonary infection
Since we observed that most of the spore inoculum entered alveolar macrophages early during infection, and that these particles convert to yeast that replicate intracellularly, we sought to distinguish whether alveolar macrophages are required to constrain the early infection, or alternatively, enable replication and progression of infection. We sought to use CD11c-DTR mice to address the role of macrophages in the pathogenesis of early infection. We found that uninfected CD11c-DTR mice are too sensitive to DTx to survive multiple doses (Fig. 4A). Thus, we created bone-marrow chimeric mice in which WT CD45.1 mice were lethally irradiated and reconstituted with bone marrow from CD45.2 WT or CD45.2 CD11c-DTR mice (Fig. 4B) (24). Chimeric CD11c-DTR mice tolerated multiple doses of DTx, similar to WT mice (Fig. 4A). While CD11c is expressed on alveolar macrophages as well as DCs, toxin treatment reduced the macrophages by 21-fold and the DCs by only 2.6-fold (Fig. 4C). Depletion of CD11c-positive cells in chimeric mice that were challenged with B. dermatitidis spores resulted in a surprising 26 fold reduction in CFU by day 6 post-infection, compared to WT chimeric mice treated with DTx. This trend was seen across multiple time points (Fig. 4D). Toxin treatment of WT mice and DTR expression in the absence of toxin treatment did not independently reduce lung CFU (Fig 4D). Thus, CD11c-positive cells in the lung were essential for propagation and pathogenesis of infection, and their elimination unexpectedly stemmed the extent of pulmonary disease after infection with spores.
The role of neutrophils during spore infection
While we show above that yeast can replicate inside alveolar macrophages, it is also possible that residence inside these phagocytes protects the fungus from other more potent leukocyte effectors. Neutrophils are more effective than macrophages at killing B. dermatitidis spores and yeast (11, 27). Neutrophils also comprise the next largest population of leukocytes (after alveolar macrophages) in the lungs of spore-infected mice (Fig. 1A). We also observed that the proportion and number of lung neutrophils increased significantly after infection in toxin-treated vs. untreated control mice (Fig. 5A+B). This neutrophilia upon toxin treatment is consistent with previous work with these mice (28). Toxin treated CD11c-DTR mice showed a >6-fold increase in the percentage of leukocyte-associated spores that were associated with neutrophils (9% vs. 56%) (Fig. 5D, left panel); numbers showed corresponding trends (Fig. 5D, right panel).
Figure 5. Neutrophils do not account for reduced lung CFU in CD11c depleted mice.
CD11c-DTR mice were treated with rat IgG, 100 ng DTx i.p., anti-Ly6g antibody i.v. or both DTx and antibody. Mice were infected with mCherry 14081 spores and lung homogenates were analyzed 2 days later. (A) Flow plots showing the proportion of lung neutrophils in representative mice. (B) Neutrophil numbers were quantified by FACS and hemocytometer count. (C) The number of alveolar macrophages (AM) in mice given DTx alone or together with neutrophil depleting antibody. (D) Flow plot of the distribution of intracellular spores (mCherry+, Uvitex 2B−) denoted as black dots with respect to leukocytes (gray) and alveolar macrophages (AM; top left gate), DCs (DC; top right), and neutrophils (Neu; bottom right) in representative PBS- vs. toxin-treated CD11c-DTR mice (left panel). The right panel shows the number of intracellular spores associated with each cell type. (E) Lung CFU in mice corresponding to panels A-C. (F) The distribution of spores among leukocytes in depleted or control mice evaluated by FACS. All results are representative of 3 independent experiments with 5 mice/group. *P<0.05, **P<0.01, ****P<0.0001.
We hypothesized that; if macrophages offer a relatively “protected” locale for spores against neutrophils then an increased exposure to neutrophils in toxin-treated mice might explain the reduced lung CFU after depletion of CD11c-positive cells. To test this hypothesis, we depleted neutrophils with a Ly6g-specific antibody in toxin-treated CD11c-DTR mice before infection with spores (Fig. 5A+B). Neutrophil depletion did not affect the number of alveolar macrophages (compared to rat IgG treated controls) (Fig. 5C). Whereas the lung CFU after infection rose slightly in neutrophil-depleted wild-type mice vs. untreated mice, lung CFU did not increase significantly after neutrophils were depleted from toxin-treated CD11c-DTR mice (Fig. 5E). Spores did show a small increase in association with DCs in toxin-treated CD11c-DTR mice that were depleted of neutrophils (Fig. 5F). Thus, exposure of B. dermatitidis to neutrophils did not appear to explain the sharply reduced lung CFU in mice depleted of CD11c-positive cells, i.e. chiefly alveolar macrophages. This implies that B. dermatitidis spores may benefit directly from the growth environment inside macrophages.
The effect of macrophages on phase transition of spores to yeast
Since spores are more vulnerable than yeast to killing by leukocytes (11), the rapidity of phase transition would likely offer a selective advantage to the fungus in survival early during infection. By flow cytometric analysis, transition reporter yeast display a 10-fold higher mCherry fluorescence than spores (Fig. 6A). Using this reporter strain during infection, we found that the phase transition of spores to yeast is significantly delayed in the first week of infection in chimeric mice that are depleted of CD11c-positive cells (Fig. 6B); this delay in transition correlated with the time frame in which lung CFU is sharply curtailed in these mice.
Figure 6. Macrophages accelerate phase transition of spores to yeast.
(A) Flow cytometric analysis of mCherry expression on spores and yeast. (B) Chimeric WT and CD11c-DTR mice treated with DTx in figure 4 were infected with mCherry 14081 spores. The percentage of B. dermatitidis that underwent transition from spore to yeast in lung homogenates was defined as the proportion of mCherry events that were brighter than the threshold defined by FACS in panel A. The inset shows the CFU in these mice on the same time scale. Results are the mean±SEM of 3 mice/group, and are representative of two experiments. (C) mCherry 14081 spores were cultured in vitro in medium alone or with BMM at a MOI of 0.1 for 4 days and analyzed by flow cytometry for intensity of mCherry expression. Expression of mCherry beyond the dotted line was defined as transition to the yeast phase defined by FACS in panel A. Results in the left panel are that of representative wells. The right panel shows the percent of events that were mCherry high (yeast) quantified and averaged among triplicate wells. Results are the mean±SEM and representative of 3 experiments. ***P <0.001, ****P<0.0001.
We also used the reporter strain for in vitro studies with macrophages. The percentage of spores that demonstrated phase transition to yeast in vitro over four days nearly tripled during co-culture with BMM (Fig. 6C) and alveolar macrophages (data not shown), compared to culture in medium alone. During this time frame, little to no replication of the fungus occurred in vitro as determined by CFU analysis (data not shown). These data indicate that infectious spores undergo significantly faster transition to pathogenic yeast in vivo in the lungs of mice in which CD11c-positive cells – i.e. mainly alveolar macrophages - are present. Spores also evince more rapid transition to yeast when they are co-cultured in vitro with macrophages.
DISCUSSION
B. dermatitidis is generally considered an extracellular pathogen. Histological sections of infected patient tissues typically show a high proportion of the yeast in the extracellular space. However, most data from patients represents the late stage of infection once large numbers of yeast occupy tissue and neutrophils dominate. The large size of budding yeast can be hard for neutrophils to ingest, perpetuating the notion of extracellular residence. The role of intracellular residence in early pathogenesis and initiation of infection with spores has not been investigated.
Here, using a murine model of infection with spores, we observed that a high proportion of the spores that reach the lungs are located inside of alveolar macrophages within 24 hours of infection. Alveolar macrophages contained most of the spores after pulmonary infection despite a rapid influx of neutrophils, inflammatory DCs, and even neutrophil-derived DCs. This finding contrasts with reports of late-stage infection dominated by yeast and pyogranulomas (13). We observed that the lowest inoculum yielded the greatest association of spores with alveolar macrophages, approaching 80%. While our flow cytometric analysis was not sensitive enough to reliably detect leukocyte interactions with <104 spores, others have reported that as few as 70 spores are sufficient to cause disease and death in mice (29). While the inoculum of spores that causes disease in humans is unknown, our findings demonstrate that at low inocula most of the infectious spores enter alveolar macrophages, a niche in which they undergo morphogenesis to initiate infection and suppress innate host defense such as NO and TNF-α production (30, 31).
We used live cell imaging to investigate the uptake of spores by naïve alveolar macrophages, phase transition to yeast and intracellular replication in real time. Although spores are vulnerable to killing by macrophages, and infection has been thought to arise from extracellular spores (32), we found that over 95% of spores were taken up by two hours of incubation, and that spores converted to yeast, which replicated inside alveolar macrophages. Although we are currently unable to monitor spore transition in vivo in real time, our imaging in vitro established intracellular transition and replication inside primary alveolar macrophages. Based on our findings that 3 or more yeast within a single macrophage is most likely due to replication, and not multiple phagocytic events nor macrophage fusion, our finding of 3 or more yeast and budding within single alveolar macrophages from the broncho-alveolar lavage fluid of infected mice supports the premise that intracellular replication occurs in vivo.
Previous investigators have sought to evaluate intracellular replication of B. dermatitidis yeast within macrophages in vitro (16, 33). Their findings of an increase in CFU may have been confounded by the potential growth of extracellular or partially internalized yeast. Our live imaging data demonstrates unequivocal intracellular replication by yeast, and is further supported by our ratiometric studies. Using quantitative analysis involving red and green yeast at a defined ratio of 1:20, we established that the increasing number of live yeast per macrophage was due to intracellular replication over time. We chose the ratiometric method of investigation over an alternate method of measuring the budding index, where the proportion of budded yeast is quantified to identify growth under different conditions (34-36). We found that regardless of the intracellular or extracellular location of yeast a high proportion of them (>70%) are budding (data not shown). This prevented us from observing a change in the proportion that bud under different conditions and using the budding index to evaluate intracellular replication.
We considered the possibility that our quantitation of intracellular replication could be confounded by cell fusion and formation of giant cells. Multinucleated giant cells are observed during histological analysis of B. dermatitidis infected human lungs. These cells are commonly seen in proximity to yeast and occasionally with intracellular yeast (14). We did find evidence that yeast induce macrophage fusion and multinucleated giant cells. However, this was a rare occurrence and did not alter our conclusions because the vast majority of macrophages with three or more yeast were not fused. Furthermore, ratiometric analysis reduced the likelihood that fusion confounded our analysis: a macrophage containing a red yeast was 20 times more likely to fuse with a macrophage containing a green yeast than one with another red yeast and macrophages that contained the more prevalent green yeast were excluded from analysis.
In view of finding most spores within alveolar macrophages in vivo, and strong in vitro evidence of intracellular phase transition and replication, we tested whether macrophages constrain or facilitate early pathogenesis of infection. CD11c-DTR mice were useful to address this question, although the intolerance of these mice to multiple toxin treatments required the generation of chimeric mice. Depletion of CD11chigh cells sharply curtailed lung CFU in the toxin- treated chimeric mice. We interpret this finding as evidence that the entry of spores into alveolar macrophages permits initiation of infection. We sought to restore lung macrophages by adoptive transfer back into depleted mice, but were unsuccessful in reconstituting the numbers; thus we lack formal proof for the role of alveolar macrophages per se. Cells other than alveolar macrophages express CD11c, for example DCs, and they are also depleted in CD11c-DTR mice. However, DCs are unlikely to account for the reduction in CFU we observed. There are many more alveolar macrophages in the lungs and spores chiefly associated with them and not DCs. Moreover, DTx was 10-fold more efficient in depleting alveolar macrophages than DCs. Finally, some monocyte-derived DCs are insensitive to depletion by this toxin (37). Still, we cannot exclude that DCs might play a role in the outcome we observed in toxin treated mice.
Neutrophils can kill B. dermatitidis, particularly the spore form (32). One explanation for the reduction in lung CFU after depletion of CD11chigh cells is that intracellular residence inside macrophages protects spores from attack by other leukocytes such as neutrophils. Neutrophils are the next most prevalent leukocyte in the lungs after alveolar macrophages. Furthermore, toxin treatment of CD11c-DTR mice induces an influx of neutrophils (28). However, depleting neutrophils at the same time as CD11chigh cells did not restore CFU to the levels of untreated mice. It is possible that other leukocytes compensated and killed spores in the absence of neutrophils and macrophages. For example, spores associated more with DCs in these double depleted mice. Thus, enhanced uptake by DCs could contribute to reduced CFU in these mice. Alternatively, the intracellular environment in alveolar macrophages may independently foster better phase transition or growth of B. dermatitidis.
Spores converted to yeast more rapidly in vivo in wild-type mice than in mice depleted of CD11chigh cells. These in vivo data are supported by our in vitro findings in which spore transition was hastened by co-culture with macrophages versus culture in medium alone. These findings support the idea that delayed phase transition to yeast may have contributed to the reduction in CFU we observed in CD11c depleted mice. Faster transition to the yeast form offers a selective growth advantage to B. dermatitidis since this form is much more resistant to elements of host defense (11).
Our work offers strong evidence that B. dermatitidis is capable of intracellular survival, phase transition, and replication within alveolar macrophages, and that these events direct the pathogenesis of early infection. Thus, B. dermatitidis displays a facultative intracellular lifestyle. In recent years, Cryptococcus neoformans has been reclassified as a facultative intracellular pathogen of macrophages (36). This reevaluation of C. neoformans has lead to new insight about survival strategies of the fungus in the mammalian host and inside macrophages (36, 38-40). The entry of B. dermatitidis spores into alveolar macrophages likewise gives the fungus a clear-cut advantage during the early pathogenesis of blastomycosis. Our findings set the stage for further work into the mechanisms for intracellular survival and replication and the elevated rate of spore transition of B. dermatitidis within naive macrophages.
In summary, we provide new insight about the early stages of B. dermatitidis infection after inhalation of spores. Although intracellular residence and replication appears to be an integral part of early infection, the extent to which such events play a role in late infection remain unknown. Intracellular residence could enable B. dermatitidis to spread from the lungs to extra-pulmonary tissue. This “Trojan Horse” method of dissemination has been proposed for other intracellular fungi such as C. neoformans (41-45), where in a murine model of cerebral infection yeast have been detected in monocytes circulating in blood and in leptomeningeal capillaries of brain sections. New insight into how B. dermatitidis grows in host cells may likewise improve our understanding of how the fungus establishes itself in the lung and disseminates to visceral sites.
Supplementary Material
ACKNOWLEDGMENTS
We thank Lori Neal for her advice, Robert Gordon for assistance with graphic illustrations, and Tom Sullivan and Hugo Paes for engineering of the mCherry reporter strain of B. dermatitidis.
Abbreviations used in this paper
- AM
Alveolar macrophage
- BAD-1
Blastomyces adhesin 1
- BMM
Bone marrow macrophages
- DC
Dendritic cell
- DTR
Diphtheria toxin receptor
- DTx
Diphtheria toxin
- Neu
Neutrophil
Footnotes
This study was supported with funding from NIH grant AI-035681 and from an American fellowship from the American Association of University Women (AAUW).
REFERENCES
- 1.Lemos LB, Baliga M, Guo M. Blastomycosis: The great pretender can also be an opportunist. Initial clinical diagnosis and underlying diseases in 123 patients. Annals of diagnostic pathology. 2002;6:194–203. doi: 10.1053/adpa.2002.34575. [DOI] [PubMed] [Google Scholar]
- 2.Laskey W, Sarosi GA. Endogenous activation in blastomycosis. Annals of internal medicine. 1978;88:50–52. doi: 10.7326/0003-4819-88-1-50. [DOI] [PubMed] [Google Scholar]
- 3.Chu JH, Feudtner C, Heydon K, Walsh TJ, Zaoutis TE. Hospitalizations for endemic mycoses: a population-based national study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2006;42:822–825. doi: 10.1086/500405. [DOI] [PubMed] [Google Scholar]
- 4.McNeil MM, Nash SL, Hajjeh RA, Phelan MA, Conn LA, Plikaytis BD, Warnock DW. Trends in mortality due to invasive mycotic diseases in the United States, 1980-1997. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2001;33:641–647. doi: 10.1086/322606. [DOI] [PubMed] [Google Scholar]
- 5.Baily GG, Robertson VJ, Neill P, Garrido P, Levy LF. Blastomycosis in Africa: clinical features, diagnosis, and treatment. Reviews of infectious diseases. 1991;13:1005–1008. doi: 10.1093/clinids/13.5.1005. [DOI] [PubMed] [Google Scholar]
- 6.Kuttin ES, Beemer AM, Levij J, Ajello L, Kaplan W. Occurrence of Blastomyces dermatitidis in Israel. First autochthonous Middle Eastern case. The American journal of tropical medicine and hygiene. 1978;27:1203–1205. doi: 10.4269/ajtmh.1978.27.1203. [DOI] [PubMed] [Google Scholar]
- 7.Kane J, Righter J, Krajden S, Lester RS. Blastomycosis: a new endemic focus in Canada. Canadian Medical Association journal. 1983;129:728–731. [PMC free article] [PubMed] [Google Scholar]
- 8.McDonough ES. Blastomycosis-epidemiology and biology of its etiologic agent Ajellomyces dermatitidis. Mycopathologia et mycologia applicata. 1970;41:195–201. doi: 10.1007/BF02051495. [DOI] [PubMed] [Google Scholar]
- 9.Schwarz J, Baum GL. Blastomycosis. American journal of clinical pathology. 1951;21:999–1029. doi: 10.1093/ajcp/21.11.999. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Lin X. Morphogenesis in fungal pathogenicity: shape, size, and surface. PLoS pathogens. 2012;8:e1003027. doi: 10.1371/journal.ppat.1003027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drutz D, Frey CL. Intracellular and extracellular defenses of human phagocytes against Blastomyces dermatitidis conidia and yeast. J Lab Clin Med. 1985;105:737–750. [PubMed] [Google Scholar]
- 12.Nemecek JC, Wuthrich M, Klein BS. Global control of dimorphism and virulence in fungi. Science. 2006;312:583–588. [Google Scholar]
- 13.Williams JE, Moser SA, Turner SH, Standard PG. Development of pulmonary infection in mice inoculated with Blastomyces dermatitidis conidia. Am J Respir Crit Care Med. 1994;149:500–509. doi: 10.1164/ajrccm.149.2.8306053. [DOI] [PubMed] [Google Scholar]
- 14.Schwarz J, Salfelder K. Blastomycosis. A review of 152 cases. Curr Top Pathol. 1977;65:165–200. [PubMed] [Google Scholar]
- 15.Vanek J, Schwarz J, Hakim S. North American blastomycosis: a study of ten cases. American journal of clinical pathology. 1970;54:384–400. doi: 10.1093/ajcp/54.3.384. [DOI] [PubMed] [Google Scholar]
- 16.Bradsher R, Ulmer W, Marmer D, Townsend J, Jacobs R. Intracellular Growth and Phagocytosis of Blastomyces dermatitidis by Monocyte-Derived Macrophages from Previously Infected and Normal Subjects. J Infect Dis. 1985;151:57–64. doi: 10.1093/infdis/151.1.57. [DOI] [PubMed] [Google Scholar]
- 17.Krajaejun T, Gauthier GM, Rappleye CA, Sullivan TD, Klein BS. Development and application of a green fluorescent protein sentinel system for identification of RNA interference in Blastomyces dermatitidis illuminates the role of septin in morphogenesis and sporulation. Eukaryot Cell. 2007;6:1299–1309. doi: 10.1128/EC.00401-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rooney PJ, Sullivan TD, Klein BS. Selective expression of the virulence factor BAD1 upon morphogenesis to the pathogenic yeast form of Blastomyces dermatitidis: evidence for transcriptional regulation by a conserved mechanism. Mol Microbiol. 2001;39:875–889. doi: 10.1046/j.1365-2958.2001.02300.x. [DOI] [PubMed] [Google Scholar]
- 19.Zaynagetdinov R, Sherrill TP, Kendall PL, Segal BH, Weller KP, Tighe RM, Blackwell TS. Identification of myeloid cell subsets in murine lungs using flow cytometry. American journal of respiratory cell and molecular biology. 2013;49:180–189. doi: 10.1165/rcmb.2012-0366MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misharin AV, Morales-Nebreda L, Mutlu GM, Budinger GR, Perlman H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. American journal of respiratory cell and molecular biology. 2013;49:503–510. doi: 10.1165/rcmb.2013-0086MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geng S, Matsushima H, Okamoto T, Yao Y, Lu R, Page K, Blumenthal RM, Ward NL, Miyazaki T, Takashima A. Emergence, origin, and function of neutrophil-dendritic cell hybrids in experimentally induced inflammatory lesions in mice. Blood. 2013;121:1690–1700. doi: 10.1182/blood-2012-07-445197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wuthrich M, Klein BS. Investigation of anti-WI-1 adhesin antibody-mediated protection in experimental pulmonary blastomycosis. J Infect Dis. 2000;181:1720–1728. doi: 10.1086/315473. [DOI] [PubMed] [Google Scholar]
- 23.Helming L, Gordon S. Molecular mediators of macrophage fusion. Trends Cell Biol. 2009;19:514–522. doi: 10.1016/j.tcb.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Zaft T, Sapoznikov A, Krauthgamer R, Littman DR, Jung S. CD11chigh dendritic cell ablation impairs lymphopenia-driven proliferation of naive and memory CD8+ T cells. J Immunol. 2005;175:6428–6435. doi: 10.4049/jimmunol.175.10.6428. [DOI] [PubMed] [Google Scholar]
- 25.Oehler L, Majdic O, Pickl WF, Stockl J, Riedl E, Drach J, Rappersberger K, Geissler K, Knapp W. Neutrophil granulocyte-committed cells can be driven to acquire dendritic cell characteristics. The Journal of experimental medicine. 1998;187:1019–1028. doi: 10.1084/jem.187.7.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helming L, Gordon S. Macrophage fusion induced by IL-4 alternative activation is a multistage process involving multiple target molecules. Eur J Immunol. 2007;37:33–42. doi: 10.1002/eji.200636788. [DOI] [PubMed] [Google Scholar]
- 27.Brummer E, Stevens DA. Opposite effects of human monocytes, macrophages, and polymorphonuclear neutrophils on replication of Blastomyces dermatitidis in vitro. Infect Immun. 1982;36:297–303. doi: 10.1128/iai.36.1.297-303.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tittel AP, Heuser C, Ohliger C, Llanto C, Yona S, Hammerling GJ, Engel DR, Garbi N, Kurts C. Functionally relevant neutrophilia in CD11c diphtheria toxin receptor transgenic mice. Nat Methods. 2012;9:385–390. doi: 10.1038/nmeth.1905. [DOI] [PubMed] [Google Scholar]
- 29.Sugar AM, Picard M. Experimental blastomycosis pneumonia in mice by infection with conidia. J Med Vet Mycol. 1988;26:321–326. doi: 10.1080/02681218880000461. [DOI] [PubMed] [Google Scholar]
- 30.Rocco NM, Carmen JC, Klein BS. Blastomyces dermatitidis yeast cells inhibit nitric oxide production by alveolar macrophage inducible nitric oxide synthase. Infect Immun. 2011;79:2385–2395. doi: 10.1128/IAI.01249-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finkel-Jimenez B, Wüthrich M, Brandhorst T, Klein BS. The WI-1 adhesin blocks phagocyte TNF-alpha production, imparting pathogenicity on Blastomyces dermatitidis. J Immunol. 2001;166:2665–2673. doi: 10.4049/jimmunol.166.4.2665. [DOI] [PubMed] [Google Scholar]
- 32.Sugar AM, Picard M. Macrophage- and oxidant-mediated inhibition of the ability of live Blastomyces dermatitidis conidia to transform to pathogenic yeast phase: implications for the pathogenesis of dimorphic fungal infections. J Infect Dis. 1991;163:371–375. doi: 10.1093/infdis/163.2.371. [DOI] [PubMed] [Google Scholar]
- 33.Bradsher RW. Live Blastomyces dermatitidis yeast-induced responses of immune and nonimmune human mononuclear cells. Mycopathologia. 1984;87:159–166. doi: 10.1007/BF00436902. [DOI] [PubMed] [Google Scholar]
- 34.Zettel MF, Garza LR, Cass AM, Myhre RA, Haizlip LA, Osadebe SN, Sudimack DW, Pathak R, Stone TL, Polymenis M. The budding index of Saccharomyces cerevisiae deletion strains identifies genes important for cell cycle progression. FEMS microbiology letters. 2003;223:253–258. doi: 10.1016/S0378-1097(03)00384-7. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz DA. Characterization of the biological activity of Cryptococcus infections in surgical pathology. The Budding Index and Carminophilic Index. Annals of clinical and laboratory science. 1988;18:388–397. [PubMed] [Google Scholar]
- 36.Feldmesser M, Kress, Yvonne, Novikoff, Phyllis, Casadevall, Arturo Cryptococcus neoformans Is a Facultative Intracellular Pathogen in Murine Pulmonary Infection. Infect Immun. 2000;68:4225–4237. doi: 10.1128/iai.68.7.4225-4237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bar-On L, Steffen J. Defining In Vivo Dendritic Cell Functions Using CD11c-DTR Transgenic Mice. Methods Mol Biol. 2010;595:429–442. doi: 10.1007/978-1-60761-421-0_28. [DOI] [PubMed] [Google Scholar]
- 38.Johnston SA, May RC. Cryptococcus interactions with macrophages: evasion and manipulation of the phagosome by a fungal pathogen. Cellular microbiology. 2013;15:403–411. doi: 10.1111/cmi.12067. [DOI] [PubMed] [Google Scholar]
- 39.Marodi L, Schreiber S, Anderson DC, MacDermott RP, Korchak HM, Johnston RB., Jr. Enhancement of macrophage candidacidal activity by interferon- gamma. Increased phagocytosis, killing, and calcium signal mediated by a decreased number of mannose receptors. J Clin Invest. 1993;91:2596–2601. doi: 10.1172/JCI116498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shao X, Mednick A, Alvarez M, van Rooijen N, Casadevall A, Goldman DL. An innate immune system cell is a major determinant of species-related susceptibility differences to fungal pneumonia. J Immunol. 2005;175:3244–3251. doi: 10.4049/jimmunol.175.5.3244. [DOI] [PubMed] [Google Scholar]
- 41.Fonseca FL, Guimaraes AJ, Kmetzsch L, Dutra FF, Silva FD, Taborda CP, Araujo GD, Frases S, Staats CC, Bozza MT, Schrank A, Vainstein MH, Nimrichter L, Casadevall A, Rodrigues ML. Binding of the wheat germ lectin to Cryptococcus neoformans chitooligomers affects multiple mechanisms required for fungal pathogenesis. Fungal genetics and biology : FG & B. 2013;60:64–73. doi: 10.1016/j.fgb.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drevets DA, Leenen PJ. Leukocyte-facilitated entry of intracellular pathogens into the central nervous system. Microbes and infection / Institut Pasteur. 2000;2:1609–1618. doi: 10.1016/s1286-4579(00)01317-4. [DOI] [PubMed] [Google Scholar]
- 43.Chretien F, Lortholary O, Kansau I, Neuville S, Gray F, Dromer F. Pathogenesis of cerebral Cryptococcus neoformans infection after fungemia. J Infect Dis. 2002;186:522–530. doi: 10.1086/341564. [DOI] [PubMed] [Google Scholar]
- 44.Charlier C, Nielsen K, Daou S, Brigitte M, Chretien F, Dromer F. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect Immun. 2009;77:120–127. doi: 10.1128/IAI.01065-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang YC, Stins MF, McCaffery MJ, Miller GF, Pare DR, Dam T, Paul- Satyaseela M, Kim KS, Kwon-Chung KJ. Cryptococcal yeast cells invade the central nervous system via transcellular penetration of the blood-brain barrier. Infect Immun. 2004;72:4985–4995. doi: 10.1128/IAI.72.9.4985-4995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.