Abstract
One in ten Americans suffer from chronic kidney disease, and close to 90,000 people die each year from causes related to kidney failure. Patients with end-stage renal disease are faced with two options: hemodialysis or transplantation. Unfortunately, the reach of transplantation is limited because of the shortage of donor organs and the need for immunosuppression. Bioengineered kidney grafts theoretically present a novel solution to both problems. Herein we discuss the history of bioengineering organs, the current status of bioengineered kidneys, considerations for the future of the field, and challenges to clinical translation. We hope that by integrating principles of tissue engineering, and stem cell and developmental biology, bioengineered kidney grafts will advance the field of regenerative medicine while meeting a critical clinical need.
Keywords: kidney, bioengineering, organ engineering, perfusion decellularization, transplantation
HISTORY OF BIOENGINEERING
The field of regenerative medicine started long before its name was coined by hospital administrator Leland Kaiser in 1992 in an article about future medical technologies that could “change the course of chronic disease…and regenerate tired and failing organ systems”1. In the 1920s, Nobel prize winner Alexis Carrel, a pioneer of cardiovascular and transplant surgery, collaborated with aviator and engineer Charles Lindbergh to create a pump oxygenator for the long-term perfusion of single organs; by 1935, they showed the first successful long-term ex vivo perfusion of organs by keeping cat thyroids alive outside the body for several weeks2,3. This perfusion pump would form the basis for bioreactors that are now widely used to preserve and grow organs ex vivo. However, it took a confluence of factors in the 1990s for regenerative medicine to fully mature into the burgeoning field that it is today. These factors include the discovery of embryonic stem (ES) cells, the more recent development of nuclear transfer technology, new insights into the expansion and differentiation of stem cells, and advances in tissue engineering, in which the principles of biology and engineering are applied to develop functional substitutes for damaged tissue4–6.
In a shift from two-dimensional to three-dimensional cellular adjuncts, in the 1990s, the first living tissue grafts were created based on synthetic polymers onto which cells were seeded7. These initial constructs contained hepatocytes, chondrocytes, and enterocytes8–10. Bioengineered trachea, bladders and vessels were the first constructs to be implanted into humans. In 2004, a 30 year old female with end-stage bronchomalacia underwent implantation of a totally bioengineered human trachea constructed from a deceased donor scaffold seeded with autologous cells derived from mesenchymal stem cells and epithelial cells11. In 2006, human bladder matrices were seeded with autologous bladder cells grown from culture and implanted into 10 patients who did well with a mean follow-up of nearly 4 years12. In 2007, blood vessels engineered from autologous skin and superficial vein cells were also successfully implanted into 10 patients with end-stage renal disease (ESRD) on hemodialysis13.
However, to go beyond tubular structures lined with single-cell layers to a more architecturally complex organ requires intra-organ organization of scaffolds, cells, and soluble factors along with intact vasculature for perfusion14,15. A significant advance came in 2008 with the discovery of perfusion-decellularization techniques that led to the development of a whole-heart scaffold with intact three-dimensional geometry and vasculature16. Using this approach, the native extracellular matrix of whole organs has been isolated from lung17–19, kidney20–22, liver23, and pancreas6,24. While this most recent vertical step in the field of organ engineering brings us closer to a bioengineered whole organ, this method relies upon donor organs to derive native extracellular matrices. Alternative techniques of fabricating three-dimensional biological structures, such as self-assembly and bioprinting, would avoid this issue15,25.
NEED FOR BIOARTIFICIAL KIDNEY
Chronic kidney disease (CKD) is a major healthcare challenge worldwide. In the United States, about 9.4% of adults suffer from chronic kidney disease, and the prevalence of ESRD is increasing26,27. Medical care for people with ESRD required $49.3 billion dollars in 2011: $32,922 Medicare costs per person per year for transplant patients and $87,945 Medicare costs per person per year for patients on hemodialysis27.
About 400,000 patients with end-stage renal disease depend on some form of dialysis28. Hemodialysis has revolutionized the care of these patients as a temporizing measure to remove toxic waste products and restore body fluid volume and electrolyte balance. Despite improvements in technology and patient care, one year on hemodialysis is associated with a 6% increase in the relative risk of death29. Adjusted rates of all-cause mortality are 6.5–7.9 times greater for patients on dialysis than for individuals in the general population; this is in contrast to kidney transplant recipients who have an adjusted all-cause mortality rate of 1.0–1.5 times the general population30. Long-term complications of hemodialysis include hypotension, malnutrition, access site infection, gastrointestinal bleeding, and depression. In addition, traditional dialytic modalities focus on solute clearance and volume management without providing the immunoregulatory, metabolic, and endocrine functions of native kidneys.
To expand the functionality of hemodialysis, cellular components have been added to renal replacement therapy. Development of the first extracorporeal bioartificial kidney (BAK) support systems started in the 1980s, when synthetic scaffolds were combined with cellular components31,32. In these experiments, human proximal renal tubular cells were cultured on hollow fiber scaffolds and then placed in series with a hemofiltration circuit. Phase I/II clinical trials demonstrated that bioartificial kidney systems were safely able to filter urine, improve metabolic parameters, reduce pro-inflammatory cytokine levels, and improve cardiovascular stability33,34. However, a significant impact on survival has been harder to discern as the multicenter, randomized, controlled, open-labeled Phase II clinical trial performed in 2004–2005 was likely underpowered35,36. Of the 58 critically ill patients with acute renal failure who were enrolled in the study, 21/40 completed BAK therapy and 4/18 completed conventional dialysis therapy35. Survival was significantly improved in patients who underwent BAK therapy at 180 days but not 28 days35. Currently, BAKs are limited by the survival of tubular cells and cost-effective manufacturing of the device37,38.
Organ transplantation represents a unique method of treatment to cure people with end-stage organ failure. Since the first successful kidney transplant in 1954, the field of transplantation has made substantial progress. However, transplant surgery still faces one fundamental problem—the number of people requiring organ transplants is simply higher than the number of organs available. In the United States, 18 people die on a transplant waiting list every day due to the critical organ shortage39. There are currently about 120,000 people on the waiting list for an organ; in 2013, there were only about 14,000 organ donors and only about 28,000 transplants performed40. In addition, despite an approximately 70% 5-year graft survival after kidney transplantation, patient survival is limited by cardiovascular disease, infection, malignancy, and chronic rejection41,42.
Fully implantable bioengineered kidneys have the potential to address these shortcomings by replacing a diseased organ with a newly functioning one. Bioengineered kidneys represent a new, theoretically inexhaustible supply of organs that could mitigate the ever-growing demand for transplantable organs and reduce waiting list mortality. Furthermore, if generated from patient-derived cells, bioengineered organs could also be transplanted without need for life-long immunosuppression, erasing the heavy burden associated with its side effects (infection, malignancy, drug toxicity) and cost (about $15,000 to $20,000 per year)43. With an increased donor pool, patients could be transplanted much earlier, when they have less comorbidities and a lower perioperative risk for adverse outcomes. In addition, regenerative medicine technology could be used to improve the quality of discarded donor grafts (currently about 40% of eligible donor grafts are not used for transplantation in the United States)44.
Thus, an ambitious goal for an alternative, efficacious renal replacement therapy is to generate a functional, self-sustaining, safe bioengineered kidney for transplantation (Tables 1 and 2).
Table 1. Minimum design criteria to generate a transplantable bioengineered kidney graft that can produce a filtrate.
This table outlines basic components needed to generate a single kidney that is capable of filtering.
| Structure |
Scaffold
|
Cells
| |
Organ assembly
| |
|
| |
| Function |
Filtration
|
|
| |
| Safety |
Implantation
|
Minimize antigenicity
| |
|
Sterility Clinical monitoring
| |
Table 2. Optimal design criteria to generate a fully functional transplantable bioengineered kidney graft.
This table outlines the ideal components of a bioengineered kidney that would be fully functional and clinically efficacious for use in humans.
| Structure | Scaffold
|
Cells
| |
Organ assembly
| |
|
| |
| Function | Filtration
|
Hormonal
| |
Other
| |
|
| |
| Safety | Implantation
|
Minimize antigenicity
| |
| Sterility Clinical monitoring
| |
CREATING A BIOENGINEERED KIDNEY
Following the principles of tissue engineering, the process of developing bioartifical organ grafts can be broken down into three stages: (1) creating an organ scaffold; (2) seeding the scaffold with cells; and (3) maturing the structure in a bioreactor.
Scaffolds
Initial efforts to engineer kidney tissue combined biological and synthetic components in extra-corporeal renal support systems. BAKs combine a hemofilter used in conventional dialysis with a bioreactor unit containing human primary renal proximal tubule cells derived from discarded donor kidneys to supplement current renal replacement technology with other physiological functions of the kidney. The first clinical trial using BAKs was reported in 200434 and a subsequent Phase II trial showed improved long-term but not short-term survival in ICU patients with acute renal failure treated with BAKs compared to continuous renal replacement alone35; however, this trial was underpowered and further studies are needed to confirm this result36. Current BAK research is focused on finding the best cell type to use and creating devices that contain growth factors and novel membrane materials to foster the optimal environment for cell viability37. While not implantable by design, these extracorporeal synthetic scaffolds provide very valuable information on tubular epithelial cell biology and function within an engineered construct.
A broad variety of biologic and/or synthetic materials could be used to create scaffolds for the regeneration of a whole organ. Conventional tissue fabrication techniques relied upon pre-formed acellular scaffolds that incorporated simple cell constructs of a single phenotype by photolithography or layer-by-layer deposition of ionic biopolymers45. Another technique distributes cells in patterns determined by laminar fluid flow in microfluidic channels46. Currently, fabrication resolution for photolithography is on the submicron scale47. Conventional three-dimensional printers have a spatial resolution of about 0.01 to 0.1 millimeters, but more recent technologies can reduce this to 65 nanometers48.
Bioprinting is promising method for creating scaffolds that do not rely upon a donor organ to supply the native extra-cellular matrix. It allows for precise spatial placement of different cells in the matrix in a manner that is low-cost and high throughput49. Bioprinting involves the use of a printing device that deposits cells and biomaterials into precise three-dimensional arrangements to generate structures that follow along a pre-determined blueprint50,51. The structure can then be matured in vitro or in vivo. For example, amniotic fluid-derived stem cells were bioprinted onto wound sites and accelerated wound healing in a mouse model52. Most recently, complex three-dimensional tissue constructs composed of stem cells, muscle cells, and endothelial cells were printed and retained their function in vivo49. The unique advantage of bioprinting, aside from its controlled composition and architectural construction, is the fact that both cells and matrix can be layered simultaneously. If further developed, this would allow a multitude of different cell phenotypes to be assembled in their physiologic three-dimensional relationship. Challenges in applying bioprinting approaches to organ engineering include the limited mechanical stability of the constructs, limited spatial resolution of current bioprinting devices, and the inability to create three-dimensional fiber structures through layering techniques.
Decellularization is an alternative process that generates three-dimensional structures without building an organ from the ground up. Cellular material is removed by mechanical, chemical, or enzymatic methods while the extracellular matrix remains intact53. Perfusion-decellularization, first reported in 2008, takes advantage of the intrinsic vascular structure of any organ (as well as the biliary tree, ureter, and airways) to efficiently and effectively deliver decellularization agents at a constant, low physiological pressure16,54.
Target organs can be harvested up to 4–6 hours postmortem, up to the point at which proteolysis begins. Treating target organs with detergents or acids through the innate vasculature results in a decellularized extracellular matrix composed of proteins and polysaccharides. Following decellularization, ethylene oxide or peracetic acid can be used to sterilize the matrix without destroying the matrix itself. The result is a natural, biocompatible backbone that is an ideal platform for organ bioengineering. These scaffolds are free of significant cellular content, retain major extracellular matrix proteins (such as collagens, laminins, fibronectins, and glycosaminoglycans), maintain tensile strength, and preserve geometric and spatial organization55.
Both regulatory signals and physical cues can determine cell phenotype and tissue function56. For example, decellularized rhesus monkey kidneys were more easily repopulated in younger donor kidneys compared with older donor kidneys57. Specifically engineering microenvironments can guide stem cell differentiation and function58. In some experiments, the renal scaffold itself supports embryonic stem cells to proliferate and differentiate into glomerular, vascular, and tubular pathways59. Further scaffold modifications can serve to encourage cell differentiation along a preferred pathway. For example, polyethylene glycol hydrogels can be modified with adhesion peptides to influence cellular interactions, such as the osteogenesis of bone marrow stromal cells60. Other peptides can be placed in peptide gels to control differentiation, such as the development of stem cells into neurons rather than astrocytes61. Soluble factors such as growth factors and cytokines can also be delivered in a controlled spatial and temporal fashion depending on how they are incorporated into the scaffold62. Conjugating a rodent bladder acellular matrix with basic fibroblast growth factor accelerated the cellularization and vascularization of the bladder after implantation63. Finally, fluid flow through the glomerulus and filtration across tubules generate forces that are essential for proper kidney cell function. Bioreactors that replicate the perfusion-based fluid flow aid in the long-term culture of bioengineered kidneys, especially in promoting the development of appropriate cell phenotypes.
Cells
The basic functional unit of the kidney is the nephron. The kidney contains about 1.2 million nephrons. Each nephron is a tube lined by a single cell layer that can be divided geographically into the renal corpuscle, proximal tubule, loop of Henle, distal tubule, and collecting duct system. The epithelial cells in each segment are highly specialized, varying in mitochondrial content and membrane properties depending on their function (e.g. proximal tubule cells have many mitochondria and a specialized brush border membrane, whereas cells in the thin loop of Henle do not). This allows for differential filtration, reabsorption, and secretion along the nephron to modulate urine volume and content. The renal corpuscle contains glomerular capillaries containing the afferent and efferent arterioles. The endothelial cells are lined by a basement membrane that in turn is surrounded by epithelial podocytes; together this forms the filtration barrier. Mesangial cells make up the remainder of the renal corpuscle and provide support to the glomerulus by secreting extracellular matrix, producing growth factors/cytokines, and exhibiting phagocytic activity.
Seeding a kidney scaffold requires epithelial cells, endothelial cells, and mesangial cells to make up the complex function of the kidney. Primary cultures of human tubular cells have been successfully integrated into bioartificial devices while continuing to provide metabolic, endocrine, and immunological properties64 but are difficult to expand in vitro to the numbers that would be required to repopulate a kidney of clinically relevant scale.
In our opinion, pluripotent cells such as ES cells and induced pluripotent stem (iPS) cells expand the repertoire for generating the necessary cell types and numbers for bioartificial organs, and offer the unique advantage of an autologous source. When using pluripotent cells, physiological development must be recapitulated to differentiate the necessary progenitor cells and ultimately the full spectrum of cellular phenotypes of any given organ.
Embryologically, the kidney is derived from two mesodermal structures: the ureteric bud and the metanephric mesenchyme. The ureteric bud gives rise to the calyceal system of the kidney while the rest of the functional components of the kidney including the glomeruli is derived from the metanephric mesenchyme. Following the course of physiologic development, generating metanephric mesenchyme is therefore the first goal in renal regeneration and bioengineering.
ES cells are pluripotent cells with unlimited self-renewal properties that have the capability to generate all cell types of the human body. Single-cell suspensions of murine embryonic cell lines can differentiate into renal epithelial cells and have been used to construct renal organoids in vitro65–67. Human ES cells also demonstrate the ability to form kidney-like structures, express genes associated with kidney development, and can be sorted into mesodermal populations enriched for intermediate mesoderm and putative renal progenitors68–71.
Pluripotent stem cells can be induced from somatic cells by the introduction of Oct3/4, Sox2, c-Myc, and Klf4, under embryonic stem cell culture conditions72. These iPS cells can be generated from renal cells, including adult mesangial cells73 or renal epithelial cells shed into the urine74, or even keratinocytes from patients with ESRD75. Delivery of iPS cells into rat kidneys with ischemia-reperfusion injury reduced inflammatory and apoptotic markers and improved the survival of rats with damaged kidneys76. The next step is to differentiate pluripotent stem cells into renal lineages. Renal lineage cells have been differentiated from murine ES and iPS cells77. Recently, iPS cells have been programmed to differentiate into intermediate mesoderm, the embryonic germ layer that gives rise to the kidneys, by treatment with a Wnt pathway activator and retinoids found by high-throughput chemical screening78.
Mesenchymal stem cells (MSC), found in adult bone marrow, also have multipotent properties and can differentiate into mesenchymal tissues such as osteoblasts, adipocytes, chondrocytes, tendon, muscle, and marrow stroma. MSCs have been used to improve kidney function in models of chronic renal failure by migrating to the site of damaged kidney tissue and exerting immunomodulatory and paracrine effects to restore kidney function79. Understanding and harnessing the renoprotective properties of MSCs could lead to another source of cells for a bioengineered kidney80,81.
Organ assembly
Tracheas, bladders, and blood vessels have been implanted successfully into more than 160 patients without the need for immunosuppressive medication82,83. However, these structures rely on diffusion to satisfy cellular metabolic demand. Without reconnection to a vascular supply, cells can only obtain nutrients and oxygen via diffusion across a distance of 1 to 3 mm84, thereby exposing the bioengineered organ to the risk of ischemia and/or graft failure. Indeed, the most proximal 1 cm of the first implanted graft trachea collapsed ventrally 8 months after implantation, likely because of insufficient blood supply11.
Bioengineering a viable kidney graft is more challenging because of the complex architecture and functionality of the kidney. The adult human kidney weighs between 115 to 170 grams, has a volume of about 200 cm3, and receives 25% of the total cardiac output (1.25L/min). The kidney functions as a filtration unit, endocrine organ (blood cell production, bone metabolism), immune regulator and modulator of cardiovascular physiology. Simplified kidney organoids, largely composed of a single cell type in a three dimensional matrix, have been used to study kidney disease, drug nephrotoxicity, and kidney development85,86, but are far from accomplishing the varied functions that the kidney must provide.
Whole kidney scaffolds have been derived through decellularization of cadaveric rat, pig, and rhesus monkey kidneys20,21. Renal extracellular matrices produced from porcine kidneys were implanted into pigs in vivo as proof-of-concept and demonstrated the ability to withstand physiologic blood pressure without extravasation; however, despite preservation of renal architecture at 2 weeks, the naked vasculature was thrombosed by 24 hours after implantation87. As further proof-of-principle, our group reported the regeneration of functional rat kidneys that were seeded with epithelial and endothelial cells and produced rudimentary urine when transplanted orthotopically in rats22. Future advances depend on further understanding the developmental biology of the kidney, including the role of growth factors, the regenerative/reparative properties of the kidney, the role of the extracellular matrix, and identification of potential renal progenitor cells88,89.
CHALLENGES AND OPPORTUNITIES IN TRANSLATING TO CLINICAL MEDICINE
Scaffolds
Perfusion-decellularization techniques have been successful in various organs from large animal models (swine, non-human primate) and humans, but these protocols need to be standardized and include steps to ensure clinical-grade quality complete with sterilization and preservation for future use. However, the current clinical use of decellularized bone, dermal, and heart valve allografts demonstrates the feasibility of scaling production of decellularized matrices to meet clinical demand.
Cells
The ideal clinically feasible cell source to generate progenitor cell populations on a large scale has yet to be identified. Cells from fetal tissues can properly differentiate and function; however, this source may have limited expansion capabilities and may meet with ethical concerns4. These same barriers are faced by human ES cells, use of which is surrounded by controversy. However, human ES cells also carry a theoretical oncogenic risk; for example survivin (BIRC5), an anti-apoptotic oncofetal gene, is highly expressed in human ES cells and may lead to teratoma formation90. The ectopic expression of transcription factors such as OCT4, SOX2, KLF4, c-MYC, NANOG, LIN28 can generate iPS cells for organ replacement and circumvent ethical concerns; however, these cells have tumorigenic traits since reprogramming is often accompanied by genetic and epigenetic alterations91. These autologous iPS cells provide a unique advantage in that they may potentially provide an inexhaustible source of patient- and tissue-specific stem cells
In addition, repopulating a scaffold requires an adequate number of viable cells (delivered during initial seeding or in situ expansion). In vitro expansion with the signals necessary to drive appropriate differentiation of multiple cell types into a primordial kidney has been accomplished such that transplanted embryonic metanephrons can grow and secrete concentrated filtrate, but this approach is limited in terms of scalability92. To circumvent the difficulties of large-scale cell culture, one approach could be to rely on repopulation by host cells in vivo, a process which has been successful in implanted dermal matrices and trachea4.
Xeno-bioengineered organs
An intriguing prospect is to combine the strengths of both xenotransplantation and bioengineering to generate “semi-xenografts” where the scaffold would be animal-derived and the repopulated cells would be human-derived3. This would make it easier to control the quality of the scaffold, as pathogen-free herds and post-harvest processing would eliminate most known pathogens4. For example, bioartificial human tissue with an innate vascularized network was created using a porcine small bowel platform83,93. Porcine small bowel was decellularized with preservation of vasculature, which was then reseeded with human endothelial cells and then implanted into the arm of a patient. After 1 week, the construct was viable and the vasculature was patent93. Though the majority of proteins in the extracellular matrix are highly conserved across species94, the immunological barriers facing xenotransplantation still pertain, such as the antigenicity of the Gal epitope on swine95.
Immune response
Surgical meshes—acellular dermal allografts—were first developed in the 1990s in order to treat full-thickness burn injuries96. These allografts were able to modulate tissue repair without antigenicity generating a specific immune response4. The host immune response to bioengineered organ constructs requires further study. What is known is that both the innate and acquired immune system are involved97, that the early response involves activation of PMNs and Th-2 pathways as a remodeling response rather than a rejecting one98, and that eventually a state of chronic inflammation is reached, where the foreign body is accepted and usually surrounded by a fibrous capsule6. The scaffold itself may be minimally immunogenic. Complete decellularization mitigates the immunogenicity of the scaffolds by removing cellular material containing antigenic epitopes97. Decellularized native extracellular matrix products currently in clinical use, such as dermal matrices, bone allografts, and heart valves, are fully biocompatible and are repopulated by host cells after implantation. However, implantation itself is accompanied by inflammatory, wound healing, and remodeling responses typical of any surgery.
Quality control and cost
Practically, bioengineered organs must meet standards of quality despite their patient-specific customization. Tools such as imaging, in vitro assays, and bioinformatics can aid in assessing the quality of the construct and the state of the tissue and cells within it. To date, the technology has not been successfully scaled up to a clinically relevant size, but first milestones towards clinically relevant graft dimensions have been met. In addition, post-operative care of the bioengineered organ to ensure long-term viability, and graft longevity will have to be determined. Lastly, reducing cost of personalized organ engineering will depend on developing cost effective reprogramming, differentiation, and regeneration strategies99.
CONCLUSIONS
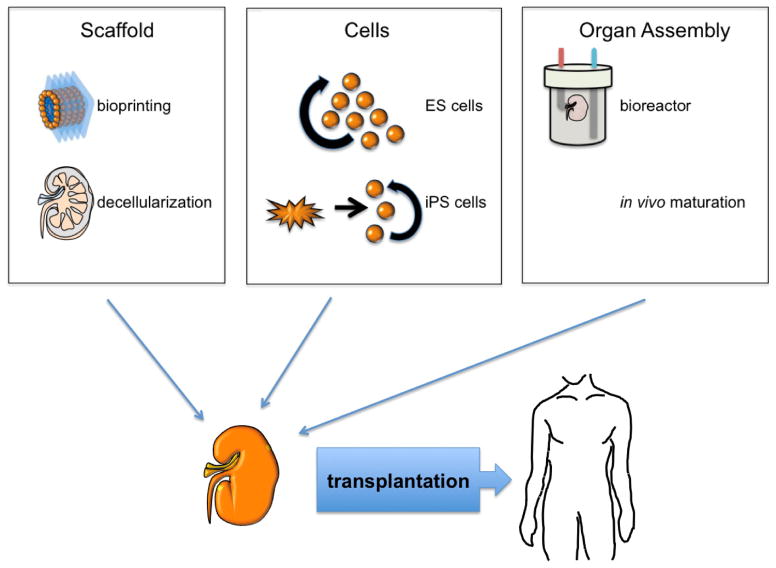
The demand for innovative and personalized renal replacement therapy is substantial. The generation of autologous bioengineered kidneys for transplantation is a promising concept for patients suffering from ESRD. This requires, in general, three main building blocks: an organ scaffold, cells for repopulation, and bioreactors for maturation (Figure 1). An overview of the current status of bioengineering organs demonstrates the exponential progress that has been made, with several regenerated constructs reaching clinical application. Recellularized kidney scaffolds are successfully transplanted into large animal models, and in small animal models, regenerated kidneys produce rudimentary urine. Major challenges such as derivation of all necessary cellular phenotypes from patient-derived cells, refined seeding strategies, and culture techniques to fully mature function remain. As a multidisciplinary community, we will be able to develop innovate solutions to overcome all of these hurdles, and bring bioengineered kidneys to clinical translation.
Figure 1. Current strategies for engineering a bioartificial kidney for transplantation.
Current strategies to create a bioarticial kidney can be divided into three stages: (1) generating a scaffold by bioprinting or decellularization; (2) populating the scaffold with cells from embryonic stem cells or induced pluripotent stem cells; (3) maturing the organ in vitro or in vivo. After the kidney has been matured, it can be transplanted into humans.
Acknowledgments
Financial support: Dr. Madariaga was supported by a fellowship from the International Heart and Lung Transplantation Society and Fellowship Award F32HL117540 from the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH). The kidney regeneration project was supported by the NIH Director’s New Innovator Award DP2 OD008749-01 (HC Ott).
Footnotes
Financial disclosure and conflict of interest statement: HC Ott is founder and stockholder of IVIVA Medical Inc. This relationship did not affect the content or conclusions contained in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaiser LR. The future of multihospital systems. Topics in health care financing. 1992 Summer;18(4):32–45. [PubMed] [Google Scholar]
- 2.Dutkowski P, de Rougemont O, Clavien PA. Alexis Carrel: genius, innovator and ideologist. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008 Oct;8(10):1998–2003. doi: 10.1111/j.1600-6143.2008.02364.x. [DOI] [PubMed] [Google Scholar]
- 3.Orlando G, Wood KJ, Stratta RJ, Yoo JJ, Atala A, Soker S. Regenerative medicine and organ transplantation: past, present, and future. Transplantation. 2011 Jun 27;91(12):1310–1317. doi: 10.1097/TP.0b013e318219ebb5. [DOI] [PubMed] [Google Scholar]
- 4.Soto Gutierrez A, Wertheim JA, Ott HC, Gilbert TW. Perspectives on whole-organ assembly: moving toward transplantation on demand. The Journal of clinical investigation. 2012 Nov 1;122(11):3817–3823. doi: 10.1172/JCI61974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langer R, Vacanti JP. Tissue engineering. Science. 1993 May 14;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 6.Orlando G, Baptista P, Birchall M, et al. Regenerative medicine as applied to solid organ transplantation: current status and future challenges. Transplant international: official journal of the European Society for Organ Transplantation. 2011 Mar;24(3):223–232. doi: 10.1111/j.1432-2277.2010.01182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cima LG, Vacanti JP, Vacanti C, Ingber D, Mooney D, Langer R. Tissue engineering by cell transplantation using degradable polymer substrates. Journal of biomechanical engineering. 1991 May;113(2):143–151. doi: 10.1115/1.2891228. [DOI] [PubMed] [Google Scholar]
- 8.Organ GM, Mooney DJ, Hansen LK, Schloo B, Vacanti JP. Transplantation of enterocytes utilizing polymer-cell constructs to produce a neointestine. Transplantation proceedings. 1992 Dec;24(6):3009–3011. [PubMed] [Google Scholar]
- 9.Vacanti CA, Langer R, Schloo B, Vacanti JP. Synthetic polymers seeded with chondrocytes provide a template for new cartilage formation. Plastic and reconstructive surgery. 1991 Nov;88(5):753–759. doi: 10.1097/00006534-199111000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Fontaine M, Hansen LK, Thompson S, et al. Transplantation of genetically altered hepatocytes using cell-polymer constructs. Transplantation proceedings. 1993 Feb;25(1 Pt 2):1002–1004. [PubMed] [Google Scholar]
- 11.Macchiarini P, Walles T, Biancosino C, Mertsching H. First human transplantation of a bioengineered airway tissue. The Journal of thoracic and cardiovascular surgery. 2004 Oct;128(4):638–641. doi: 10.1016/j.jtcvs.2004.02.042. [DOI] [PubMed] [Google Scholar]
- 12.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006 Apr 15;367(9518):1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 13.L’Heureux N, McAllister TN, de la Fuente LM. Tissue-engineered blood vessel for adult arterial revascularization. The New England journal of medicine. 2007 Oct 4;357(14):1451–1453. doi: 10.1056/NEJMc071536. [DOI] [PubMed] [Google Scholar]
- 14.Mikos AG, Herring SW, Ochareon P, et al. Engineering complex tissues. Tissue engineering. 2006 Dec;12(12):3307–3339. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jakab K, Norotte C, Marga F, Murphy K, Vunjak-Novakovic G, Forgacs G. Tissue engineering by self-assembly and bio printing of living cells. Biofabrication. 2010 Jun;2(2):022001. doi: 10.1088/1758-5082/2/2/022001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ott HC, Matthiesen TS, Goh SK, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nature medicine. 2008 Feb;14(2):213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 17.Ott HC, Clippinger B, Conrad C, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nature medicine. 2010 Aug;16(8):927–933. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 18.Petersen TH, Calle EA, Zhao L, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010 Jul 30;329(5991):538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Neill JD, Anfang R, Anandappa A, et al. Decellularization of human and porcine lung tissues for pulmonary tissue engineering. The Annals of thoracic surgery. 2013 Sep;96(3):1046–1055. doi: 10.1016/j.athoracsur.2013.04.022. discussion 1055–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baptista PM, Orlando G, Mirmalek Sani SH, Siddiqui M, Atala A, Soker S. Whole organ decellularization - a tool for bioscaffold fabrication and organ bioengineering. Conference proceedings: … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference; 2009; 2009. pp. 6526–6529. [DOI] [PubMed] [Google Scholar]
- 21.Nakayama KH, Batchelder CA, Lee CI, Tarantal AF. Decellularized rhesus monkey kidney as a three-dimensional scaffold for renal tissue engineering. Tissue engineering. Part A. 2010 Jul;16(7):2207–2216. doi: 10.1089/ten.tea.2009.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song JJ, Guyette JP, Gilpin SE, Gonzalez G, Vacanti JP, Ott HC. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nature medicine. 2013 May;19(5):646–651. doi: 10.1038/nm.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uygun BE, Soto-Gutierrez A, Yagi H, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nature medicine. 2010 Jul;16(7):814–820. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Carlo E, Baiguera S, Conconi MT, et al. Pancreatic acellular matrix supports islet survival and function in a synthetic tubular device: in vitro and in vivo studies. International journal of molecular medicine. 2010 Feb;25(2):195–202. [PubMed] [Google Scholar]
- 25.Campbell PG, Weiss LE. Tissue engineering with the aid of inkjet printers. Expert opinion on biological therapy. 2007 Aug;7(8):1123–1127. doi: 10.1517/14712598.7.8.1123. [DOI] [PubMed] [Google Scholar]
- 26.National Kidney F. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2002 Feb;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 27.Collins AJ, Foley RN, Chavers B, et al. US Renal Data System 2013 Annual Data Report. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2014 Jan;63(1 Suppl):A7. doi: 10.1053/j.ajkd.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 28. [Accessed 3/11/2014, 2014]; http://kidney.niddk.nih.gov/kudiseases/pubs/kustats/
- 29.Chertow GM, Johansen KL, Lew N, Lazarus JM, Lowrie EG. Vintage, nutritional status, and survival in hemodialysis patients. Kidney international. 2000 Mar;57(3):1176–1181. doi: 10.1046/j.1523-1755.2000.00945.x. [DOI] [PubMed] [Google Scholar]
- 30.System USRD. USRDS 2009 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2009. [Google Scholar]
- 31.Aebischer P, Ip TK, Panol G, Galletti PM. The bioartificial kidney: progress towards an ultrafiltration device with renal epithelial cells processing. Life support systems: the journal of the European Society for Artificial Organs. 1987 Apr-Jun;5(2):159–168. [PubMed] [Google Scholar]
- 32.Ip TK, Aebischer P. Renal epithelial-cell-controlled solute transport across permeable membranes as the foundation for a bioartificial kidney. Artificial organs. 1989 Feb;13(1):58–65. doi: 10.1111/j.1525-1594.1989.tb02833.x. [DOI] [PubMed] [Google Scholar]
- 33.Humes HD, Weitzel WF, Bartlett RH, Swaniker FC, Paganini EP. Renal cell therapy is associated with dynamic and individualized responses in patients with acute renal failure. Blood purification. 2003;21(1):64–71. doi: 10.1159/000067864. [DOI] [PubMed] [Google Scholar]
- 34.Humes HD, Weitzel WF, Bartlett RH, et al. Initial clinical results of the bioartificial kidney containing human cells in ICU patients with acute renal failure. Kidney international. 2004 Oct;66(4):1578–1588. doi: 10.1111/j.1523-1755.2004.00923.x. [DOI] [PubMed] [Google Scholar]
- 35.Tumlin J, Wali R, Williams W, et al. Efficacy and safety of renal tubule cell therapy for acute renal failure. Journal of the American Society of Nephrology: JASN. 2008 May;19(5):1034–1040. doi: 10.1681/ASN.2007080895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chertow GM, Waikar SS. Toward the promise of renal replacement therapy. Journal of the American Society of Nephrology: JASN. 2008 May;19(5):839–840. doi: 10.1681/ASN.2008030291. [DOI] [PubMed] [Google Scholar]
- 37.Humes HD, Buffington D, Westover AJ, Roy S, Fissell WH. The bioartificial kidney: current status and future promise. Pediatric nephrology. 2014 Mar;29(3):343–351. doi: 10.1007/s00467-013-2467-y. [DOI] [PubMed] [Google Scholar]
- 38.Sanechika N, Sawada K, Usui Y, et al. Development of bioartificial renal tubule devices with lifespan-extended human renal proximal tubular epithelial cells. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2011 Sep;26(9):2761–2769. doi: 10.1093/ndt/gfr066. [DOI] [PubMed] [Google Scholar]
- 39.U.S. Department of Health & Human Services. [Accessed 3/11/2014];2014 http://organdonor.gov/about/data.html.
- 40.2004 Annual Report of the US Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1994–2003. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; Rockville, MD: United Network for Organ Sharing; Richmond, VA: University Renal Research and Education Association; Ann Arbor, MI: 2004. [Google Scholar]
- 41.Cecka JM. Kidney transplantation in the United States. Clinical transplants. 2008:1–18. [PubMed] [Google Scholar]
- 42.Kahwaji J, Bunnapradist S, Hsu JW, Idroos ML, Dudek R. Cause of death with graft function among renal transplant recipients in an integrated healthcare system. Transplantation. 2011 Jan 27;91(2):225–230. doi: 10.1097/TP.0b013e3181ff8754. [DOI] [PubMed] [Google Scholar]
- 43.Gill JS, Tonelli M. Penny wise, pound foolish? Coverage limits on immunosuppression after kidney transplantation. The New England journal of medicine. 2012 Feb 16;366(7):586–589. doi: 10.1056/NEJMp1114394. [DOI] [PubMed] [Google Scholar]
- 44.Klein AS, Messersmith EE, Ratner LE, Kochik R, Baliga PK, Ojo AO. Organ donation and utilization in the United States, 1999–2008. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010 Apr;10(4 Pt 2):973–986. doi: 10.1111/j.1600-6143.2009.03008.x. [DOI] [PubMed] [Google Scholar]
- 45.Levenberg S, Langer R. Advances in tissue engineering. Current topics in developmental biology. 2004;61:113–134. doi: 10.1016/S0070-2153(04)61005-2. [DOI] [PubMed] [Google Scholar]
- 46.Chiu DT, Jeon NL, Huang S, et al. Patterned deposition of cells and proteins onto surfaces by using three-dimensional microfluidic systems. Proceedings of the National Academy of Sciences of the United States of America. 2000 Mar 14;97(6):2408–2413. doi: 10.1073/pnas.040562297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kasko AM, Wong DY. Two-photon lithography in the future of cell-based therapeutics and regenerative medicine: a review of techniques for hydrogel patterning and controlled release. Future medicinal chemistry. 2010 Nov;2(11):1669–1680. doi: 10.4155/fmc.10.253. [DOI] [PubMed] [Google Scholar]
- 48.Fischer J, Wegener M. Ultrafast polymerization inhibition by stimulated emission depletion for three-dimensional nanolithography. Advanced materials. 2012 Mar 8;24(10):OP65–69. doi: 10.1002/adma.201103758. [DOI] [PubMed] [Google Scholar]
- 49.Xu T, Zhao W, Zhu JM, Albanna MZ, Yoo JJ, Atala A. Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials. 2013 Jan;34(1):130–139. doi: 10.1016/j.biomaterials.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 50.Mironov V, Boland T, Trusk T, Forgacs G, Markwald RR. Organ printing: computer-aided jet-based 3D tissue engineering. Trends in biotechnology. 2003 Apr;21(4):157–161. doi: 10.1016/S0167-7799(03)00033-7. [DOI] [PubMed] [Google Scholar]
- 51.Derby B. Printing and prototyping of tissues and scaffolds. Science. 2012 Nov 16;338(6109):921–926. doi: 10.1126/science.1226340. [DOI] [PubMed] [Google Scholar]
- 52.Skardal A, Mack D, Kapetanovic E, et al. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem cells translational medicine. 2012 Nov;1(11):792–802. doi: 10.5966/sctm.2012-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gilbert TW. Strategies for tissue and organ decellularization. Journal of cellular biochemistry. 2012 Jul;113(7):2217–2222. doi: 10.1002/jcb.24130. [DOI] [PubMed] [Google Scholar]
- 54.Tapias LF, Ott HC. Decellularized scaffolds as a platform for bioengineered organs. Current opinion in organ transplantation. 2014 Apr;19(2):145–152. doi: 10.1097/MOT.0000000000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song JJ, Ott HC. Organ engineering based on decellularized matrix scaffolds. Trends in molecular medicine. 2011 Aug;17(8):424–432. doi: 10.1016/j.molmed.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 56.Salvatori M, Peloso A, Katari R, Orlando G. Regeneration and bioengineering of the kidney: current status and future challenges. Current urology reports. 2014 Jan;15(1):379. doi: 10.1007/s11934-013-0379-9. [DOI] [PubMed] [Google Scholar]
- 57.Nakayama KH, Batchelder CA, Lee CI, Tarantal AF. Renal tissue engineering with decellularized rhesus monkey kidneys: age-related differences. Tissue engineering. Part A. 2011 Dec;17(23–24):2891–2901. doi: 10.1089/ten.tea.2010.0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burdick JA, Vunjak-Novakovic G. Engineered microenvironments for controlled stem cell differentiation. Tissue engineering. Part A. 2009 Feb;15(2):205–219. doi: 10.1089/ten.tea.2008.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ross EA, Williams MJ, Hamazaki T, et al. Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. Journal of the American Society of Nephrology: JASN. 2009 Nov;20(11):2338–2347. doi: 10.1681/ASN.2008111196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang F, Williams CG, Wang DA, Lee H, Manson PN, Elisseeff J. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials. 2005 Oct;26(30):5991–5998. doi: 10.1016/j.biomaterials.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 61.Silva GA, Czeisler C, Niece KL, et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004 Feb 27;303(5662):1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 62.Sokolsky-Papkov M, Agashi K, Olaye A, Shakesheff K, Domb AJ. Polymer carriers for drug delivery in tissue engineering. Advanced drug delivery reviews. 2007 May 30;59(4–5):187–206. doi: 10.1016/j.addr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 63.Chen W, Shi C, Hou X, Zhang WW, Li L. Bladder acellular matrix conjugated with basic fibroblast growth factor for bladder regeneration. Tissue engineering. Part A. 2014 Feb 2; doi: 10.1089/ten.tea.2013.0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith PL, Buffington DA, Humes HD. Kidney epithelial cells. Methods in enzymology. 2006;419:194–207. doi: 10.1016/S0076-6879(06)19009-6. [DOI] [PubMed] [Google Scholar]
- 65.Vigneau C, Polgar K, Striker G, et al. Mouse embryonic stem cell-derived embryoid bodies generate progenitors that integrate long term into renal proximal tubules in vivo. Journal of the American Society of Nephrology: JASN. 2007 Jun;18(6):1709–1720. doi: 10.1681/ASN.2006101078. [DOI] [PubMed] [Google Scholar]
- 66.Kim D, Dressler GR. Nephrogenic factors promote differentiation of mouse embryonic stem cells into renal epithelia. Journal of the American Society of Nephrology: JASN. 2005 Dec;16(12):3527–3534. doi: 10.1681/ASN.2005050544. [DOI] [PubMed] [Google Scholar]
- 67.Xinaris C, Benedetti V, Rizzo P, et al. In vivo maturation of functional renal organoids formed from embryonic cell suspensions. Journal of the American Society of Nephrology: JASN. 2012 Nov;23(11):1857–1868. doi: 10.1681/ASN.2012050505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998 Nov 6;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 69.Gertow K, Wolbank S, Rozell B, et al. Organized development from human embryonic stem cells after injection into immunodeficient mice. Stem cells and development. 2004 Aug;13(4):421–435. doi: 10.1089/scd.2004.13.421. [DOI] [PubMed] [Google Scholar]
- 70.Batchelder CA, Lee CC, Matsell DG, Yoder MC, Tarantal AF. Renal ontogeny in the rhesus monkey (Macaca mulatta) and directed differentiation of human embryonic stem cells towards kidney precursors. Differentiation; research in biological diversity. 2009 Jul;78(1):45–56. doi: 10.1016/j.diff.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin SA, Kolle G, Grimmond SM, et al. Subfractionation of differentiating human embryonic stem cell populations allows the isolation of a mesodermal population enriched for intermediate mesoderm and putative renal progenitors. Stem cells and development. 2010 Oct;19(10):1637–1648. doi: 10.1089/scd.2010.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006 Aug 25;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 73.Song B, Niclis JC, Alikhan MA, et al. Generation of induced pluripotent stem cells from human kidney mesangial cells. Journal of the American Society of Nephrology: JASN. 2011 Jul;22(7):1213–1220. doi: 10.1681/ASN.2010101022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou T, Benda C, Duzinger S, et al. Generation of induced pluripotent stem cells from urine. Journal of the American Society of Nephrology: JASN. 2011 Jul;22(7):1221–1228. doi: 10.1681/ASN.2011010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thatava T, Armstrong AS, De Lamo JG, et al. Successful disease-specific induced pluripotent stem cell generation from patients with kidney transplantation. Stem cell research & therapy. 2011;2(6):48. doi: 10.1186/scrt89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee PY, Chien Y, Chiou GY, Lin CH, Chiou CH, Tarng DC. Induced pluripotent stem cells without c-Myc attenuate acute kidney injury via downregulating the signaling of oxidative stress and inflammation in ischemia-reperfusion rats. Cell transplantation. 2012;21(12):2569–2585. doi: 10.3727/096368912X636902. [DOI] [PubMed] [Google Scholar]
- 77.Morizane R, Monkawa T, Itoh H. Differentiation of murine embryonic stem and induced pluripotent stem cells to renal lineage in vitro. Biochemical and biophysical research communications. 2009 Dec 25;390(4):1334–1339. doi: 10.1016/j.bbrc.2009.10.148. [DOI] [PubMed] [Google Scholar]
- 78.Araoka T, Mae S, Kurose Y, et al. Efficient and rapid induction of human iPSCs/ESCs into nephrogenic intermediate mesoderm using small molecule-based differentiation methods. PloS one. 2014;9(1):e84881. doi: 10.1371/journal.pone.0084881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wise AF, Ricardo SD. Mesenchymal stem cells in kidney inflammation and repair. Nephrology. 2012 Jan;17(1):1–10. doi: 10.1111/j.1440-1797.2011.01501.x. [DOI] [PubMed] [Google Scholar]
- 80.de Almeida DC, Donizetti-Oliveira C, Barbosa-Costa P, Origassa CS, Camara NO. In Search of Mechanisms Associated with Mesenchymal Stem Cell-Based Therapies for Acute Kidney Injury. The Clinical biochemist. Reviews / Australian Association of Clinical Biochemists. 2013 Nov;34(3):131–144. [PMC free article] [PubMed] [Google Scholar]
- 81.Choi SJ, Kim JK, Hwang SD. Mesenchymal stem cell therapy for chronic renal failure. Expert opinion on biological therapy. 2010 Aug;10(8):1217–1226. doi: 10.1517/14712598.2010.500284. [DOI] [PubMed] [Google Scholar]
- 82.Orlando G, Soker S, Stratta RJ. Organ bioengineering and regeneration as the new Holy Grail for organ transplantation. Annals of surgery. 2013 Aug;258(2):221–232. doi: 10.1097/SLA.0b013e31829c79cf. [DOI] [PubMed] [Google Scholar]
- 83.Orlando G, Di Cocco P, D’Angelo M, Clemente K, Famulari A, Pisani F. Regenerative medicine applied to solid organ transplantation: where do we stand? Transplantation proceedings. 2010 May;42(4):1011–1013. doi: 10.1016/j.transproceed.2010.03.066. [DOI] [PubMed] [Google Scholar]
- 84.Folkman J, Hochberg M. Self-regulation of growth in three dimensions. The Journal of experimental medicine. 1973 Oct 1;138(4):745–753. doi: 10.1084/jem.138.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Desrochers TM, Palma E, Kaplan DL. Tissue-engineered kidney disease models. Advanced drug delivery reviews. 2013 Dec 17; doi: 10.1016/j.addr.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rosines E, Johkura K, Zhang X, et al. Constructing kidney-like tissues from cells based on programs for organ development: toward a method of in vitro tissue engineering of the kidney. Tissue engineering. Part A. 2010 Aug;16(8):2441–2455. doi: 10.1089/ten.tea.2009.0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Orlando G, Farney AC, Iskandar SS, et al. Production and implantation of renal extracellular matrix scaffolds from porcine kidneys as a platform for renal bioengineering investigations. Annals of surgery. 2012 Aug;256(2):363–370. doi: 10.1097/SLA.0b013e31825a02ab. [DOI] [PubMed] [Google Scholar]
- 88.Steer DL, Nigam SK. Developmental approaches to kidney tissue engineering. American journal of physiology. Renal physiology. 2004 Jan;286(1):F1–7. doi: 10.1152/ajprenal.00167.2003. [DOI] [PubMed] [Google Scholar]
- 89.Benigni A, Morigi M, Remuzzi G. Kidney regeneration. Lancet. 2010 Apr 10;375(9722):1310–1317. doi: 10.1016/S0140-6736(10)60237-1. [DOI] [PubMed] [Google Scholar]
- 90.Blum B, Bar-Nur O, Golan-Lev T, Benvenisty N. The anti-apoptotic gene survivin contributes to teratoma formation by human embryonic stem cells. Nature biotechnology. 2009 Mar;27(3):281–287. doi: 10.1038/nbt.1527. [DOI] [PubMed] [Google Scholar]
- 91.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007 Nov 30;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 92.Perin L, Da Sacco S, De Filippo RE. Regenerative medicine of the kidney. Advanced drug delivery reviews. 2011 Apr 30;63(4–5):379–387. doi: 10.1016/j.addr.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 93.Mertsching H, Schanz J, Steger V, et al. Generation and transplantation of an autologous vascularized bioartificial human tissue. Transplantation. 2009 Jul 27;88(2):203–210. doi: 10.1097/TP.0b013e3181ac15e1. [DOI] [PubMed] [Google Scholar]
- 94.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006 Jul;27(19):3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 95.Daly KA, Stewart-Akers AM, Hara H, et al. Effect of the alphaGal epitope on the response to small intestinal submucosa extracellular matrix in a nonhuman primate model. Tissue engineering. Part A. 2009 Dec;15(12):3877–3888. doi: 10.1089/ten.TEA.2009.0089. [DOI] [PubMed] [Google Scholar]
- 96.Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns: journal of the International Society for Burn Injuries. 1995 Jun;21(4):243–248. doi: 10.1016/0305-4179(95)93866-i. [DOI] [PubMed] [Google Scholar]
- 97.Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Seminars in immunology. 2008 Apr;20(2):109–116. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Allman AJ, McPherson TB, Badylak SF, et al. Xenogeneic extracellular matrix grafts elicit a TH2-restricted immune response. Transplantation. 2001 Jun 15;71(11):1631–1640. doi: 10.1097/00007890-200106150-00024. [DOI] [PubMed] [Google Scholar]
- 99.Platt JL, Cascalho M. New and old technologies for organ replacement. Current opinion in organ transplantation. 2013 Apr;18(2):179–185. doi: 10.1097/MOT.0b013e32835f0887. [DOI] [PMC free article] [PubMed] [Google Scholar]