Abstract
SUR2A is an ATP-binding protein that serves as a regulatory subunit of cardioprotective ATP-sensitive K+ (KATP) channels. Based on signalling pathway regulating SUR2A expression and SUR2A role in regulating numbers of fully assembled KATP channels, we have suggested that nicotinamide-rich diet could improve physical endurance by stimulating SUR2A expression. We have found that mice on nicotinamide-rich diet significantly improved physical endurance, which was associated with significant increase in expression of SUR2A. Transgenic mice with solely overexpressed SUR2A on control diet had increased physical endurance in a similar manner as the wild-type mice on nicotinamide-rich diet. The experiments focused on action membrane potential and intracellular Ca2+ concentration have demonstrated that increased SUR2A expression was associated with the activation of sarcolemmal KATP channels and steady Ca2+ levels in cardiomyocytes in response to β-adrenergic stimulation. In contrast, the same challenge in the wild-type was characterized by a lack of the channel activation and rise in intracellular Ca2+. Nicotinamide-rich diet was ineffective to increase physical endurance in mice lacking KATP channels. This study has shown that nicotinamide-rich diet improves physical endurance by increasing expression of SUR2A and that this is a sole mechanism of the nicotinamide-rich diet effect. The obtained results suggest that oral nicotinamide is a regulator of SUR2A expression and has a potential as a drug that can improve physical endurance in conditions where this effect would be desirable.
Keywords: nicotinamide, SUR2A, endurance, KATP channels
Introduction
Improving physical endurance is crucial for improving quality and duration of life in people suffering from different conditions including old age, heart failure, chronic obstructive pulmonary disease, cystic fibrosis, anticancer treatment and many others. At the moment there is no well-established strategy that can improve physical endurance, apart on physical exercise (reviewed in [1]). However, it is acknowledged that physical exercise is labour intensive with compliance being an important problem. In addition, some population of patients cannot perform the intensity of physical exercise required to achieve a desirable effect (reviewed in [2]). Therefore, a pill that would improve physical endurance and produce similar effect of those produced physical exercise would be welcomed in context of many conditions and diseases.
The ATP-sensitive K+ (KATP) channel was originally discovered in membrane patches excised from ventricular cardiomyocytes [3]. Structurally, sarcolemmal KATP channels are heteromultimers composed of, at least, two distinct subunits. The pore-forming inwardly rectifying K+ channel core, Kir6.2, is primarily responsible for K+ permeance, whereas the regulatory subunit, also known as the sulfonylurea receptor, or SUR2A, has been implicated in ligand-dependent channel gating [4]. More recently, it has been suggested that the sarcolemmal KATP channel protein complex may be composed of more proteins than just Kir6.2 and SUR2A, including Kir6.1 and enzymes regulating intracellular ATP levels and glycolysis [5–10]. Sarcolemmal KATP channels have been shown to play a crucial role in ischaemic preconditioning (a phenomenon when brief episodes of ischaemia/reperfusion protect the heart against myocardial infarction, [11]) and myocardial resistance to ischaemia (reviewed in [12]). More recently, it has been suggested that these channels might play a central role in response to acute-exercise stress where they seem to contribute to the adaptation of cardiac function to match physical demand. It has been shown that mice lacking these channels are characterized by decreased physical endurance, which was manifested by their inability to sustain increased physical activity [13].
If a lack of sarcolemmal KATP channels is associated with decreased physical endurance, does it mean that an increase in number of sarcolemmal KATP channels would be associated with increased physical endurance? One of the problems in testing such strategy was finding a way to increase the numbers of sarcolemmal KATP channels in vivo. This was particularly complex as KATP channels are heteromultimers composed of more than one protein. It was recently proposed that SUR2A, a KATP channel regulatory subunit, is a rate-limiting factor in forming fully assembled KATP channels (reviewed in [14]). We have recently found that nicotinamide-rich diet up-regulates SUR2A [15].
If nicotinamide-rich diet can up-regulate SUR2A then it could also improve physical endurance. Testing this hypothesis would be clinically important, as it could lead to a safe and efficient strategy of improving physical performance in many different conditions. Mice are experimental models successfully used in preclinical studies of nicotinamide and KATP channels and findings on this species have been successfully interpolated in human beings many times [16–18]. Therefore, we have undertaken this research to test whether nicotinamide-rich diet would improve physical endurance in mice. If our working hypothesis is confirmed, this study would open the door for a clinical trial that would test oral nicotinamide as a therapy against range of conditions associated with impaired physical performance and fitness, including old age, heart failure, cystic fibrosis and many others.
Methods
Nicotinamide-rich diet
C57/BL6J male mice (8–10 weeks old) or Kir6.2 knockout mice were fed ad libitum with RM-1 diet (control diet) or RM-1 + 0.1g/kg nicotinamide (nicotinamide-rich diet; Special Diets Services, Witham, UK). Each mouse was fed for a week before used for experimentation. All experiments conform to the Home Office Regulations in United Kingdom and were conducted according to the principles of the Declaration of Helsinki. The experiments on C57/BL6J mice have been done under authority of Project Licences 60/3152 and 60/3925.
SUR2A and Kir6.2 knockout mice
Generation, breeding and genotyping of these mice have previously been described in detail [13, 19]. All experiments conform to the Home Office Regulations in United Kingdom and were conducted according to the principles of the Declaration of Helsinki. The experiments with SUR2A mice have been done under authority of Project Licences 60/3152 and 60/3925.
Real time RT-PCR
Total RNA was extracted from cardiac ventricular and atrial tissue, whole brain and thoracic aorta of mice using TRIZOL reagent (Invitrogen, Paisley, UK) according to the manufacturer’s recommendations. Extracted RNA was further purified with RNeasy Mini Kit (Qiagen, Crawley, UK) according to the manufacturer’s instruction. The specific primers for mouse SUR2A, Kir6.2 and Kir6.1 were designed as follows: For SUR2A: sense, ACTATGGAGTCCGAGAACTA, antisense, AGGTTTGGACCAGTATCACA; for Kir6.2: sense; ACATGCAGGTGGTGCGCAAG, antisense, AGGGCATCCAGCAGACTGCG; for Kir6.1: sense, 5′-GTCACACGCTGGTCATCTTCAC-3, antisense, 5-GGCATTCCTCAGTCATCATTCTCC-3′. The reverse transcription (RT) reaction was carried out with ImProm-II Reverse Transcriptase (Promega, Southampton, UK). A final volume of 20 μl of RT reaction containing 4 μl of 5× buffer, 3 mM MgCl2, 20 U of RNasin® Ribonuclease inhibitor, 1 U of ImProm-II reverse transcriptase, 0.5 mM each of dATP, dCTP, dGTP and dTTP, 0.5 μg of oligo(dT), and 1μg of RNA was incubated at 42°C for 1 hr and then inactivated at 70°C for 15 min. The resulting cDNA was used as a template for real-time PCR. A SYBR Green I system was used for the RT-PCR and the 25 μl reaction mixture contained: 12.5 μl of iQ™ SYBR® Green Supermix (2×), 7.5 nM each primers, 9 μl of ddH2O and 2 μl of cDNA. In principle, the thermal cycling conditions were as follows: an initial denaturation at 95°C for 3 min., followed by 40 cycles of 10 sec. of denaturing at 95°C, 15 sec. of annealing at 56°C and 30 sec. of extension at 72°C. The real-time PCR was performed in the same wells of a 96-well plate in the iCycler iQ™ Multicolor Real-Time Detection System (Bio-Rad, Hercules, CA, USA). Data were collected following each cycle and displayed graphically (iCycler iQ™ Real-time Detection System Software, version 3.0A, Bio-Rad). Primers were tested for their ability to produce no signal in negative controls by dimer formation and then with regard to the efficiency of the PCR reaction. Efficiency is evaluated by the slope of the regression curve obtained with several dilutions of the cDNA template. Melting curve analysis tested the specificity of primers. Threshold cycle values, PCR efficiency (examined by serially diluting the template cDNA and performing PCR under these conditions) and PCR specificity (by constructing the melting curve) were determined by the same software. Each mouse cDNA sample was measured at three different quantities (and duplicated at each concentration, the corresponding no-RT mRNA sample was included as a negative control (blank).
Treadmill test
A six lane treadmill (Columbus Instruments, Columbus, OH, USA) was used to perform treadmill tests and determine energy expenditure [20–22]. The treadmill endurance test consisted of a step-wise increase in velocity over time at constant incline (C57/BL6J and SUR2A mice) or step-wise increase in velocity and incline over time (Kir6.2 knockout mice). Before the experiments, animals were acclimatized to the treadmill. Inability to continue with physical activity was determined by the animal being unable to continue test irrespective of encouragement by electric shock. Tolerated workload was defined as the sum of kinetic (Ek= m.v2/2) and potential energy (Ep = m.g.v.t.sinΦ), where m is animal mass, v is running velocity, g is acceleration due to gravity, t is time elapsed at a given protocol level and Φ is the angle of incline [13].
Isolation of single cardiomyocytes
Ventricular cardiomyocytes were dissociated from the mouse using an established enzymatic procedure [23]. In brief, hearts were retrogradely perfused (at 37°C) with medium 199, followed by Ca2+-ethylene glycol-bis(b-aminoethyl ether)-N,N,N',N'-tetraacetic acid (EGTA)-buffered low-Ca2+ medium (pCa = 7), and finally low-Ca2+ medium containing pronase E (8 mg per 100 ml), proteinase K (1.7 mg per 100 ml), bovine albumin (0.1 g per 100 ml, fraction V) and 200 μM CaCl2. Ventricles were cut into fragments in the low-Ca2+ medium enriched with 200 μM CaCl2. Cells were isolated by stirring the tissue (at 37°C) in a solution containing pronase E and proteinase K supplemented with collagenase (5 mg per 10 ml). The first aliquot was removed, filtered through a nylon sieve, centrifuged for 60 sec. (at 300–400 rpm), and washed. Remaining tissue fragments were re-exposed to collagenase, and isolation continued for two to three such cycles.
Patch-clamp electrophysiology
For perforated patch-clamp electrophysiology, a method of measurement whole cell K+ current without disturbing intracellular milieu, cells were superfused with Tyrode solution without glucose [in mM:136.5 NaCl, 5.4 KCl, 1.8 CaCl2, 0.53 MgCl2 and 5.5 N-(2-Hydroxyethyl)piperazine-N'-(ethanesulfonic acid) (HEPES)-NaOH, pH 7.4]. Pipettes were filled with (in mM) 140 KCl, 1 MgCl2, amphotericin B (240 mg/ml; Sigma-Aldrich, Dorset, UK) and 5 HEPES-KOH, pH 7.3. The membrane potential was normally held at –40 mV, and the currents evoked by a series of 400 msec. depolarizing and hyperpolarizing current steps (–100 to 80 mV in 20 mV steps) were recorded directly to hard disk using an Axopatch-200B amplifier, Digidata-1321 interface and pClamp8 software (Axon Instruments, Foster City, CA). The capacitance compensation was adjusted to null the additional whole-cell capacitative current. The slow capacitance component measured by this procedure was used as an approximation of the cell surface area and allowed normalization of current amplitude (i.e. current density). Currents were low-pass filtered at 2 kHz and sampled at 100 msec. intervals.
Measurement of intracellular Ca2+
Ventricular myocytes were loaded with 3.5 μM of the Ca2+-selective fluorescent probe, Fluo-3 acetoxymethyl ester (Fluo-3AM), for 30 min. at room temperature as previously described [24]. Myocytes loaded with Fluo-3 were plated on glass cover slips which were transferred to an experimental chamber mounted on the stage of a Zeiss LSM-510 laser-scanning confocal microscope (LSM-510, Zeiss, Göttingen, Germany) filled up with Tyrode’s solution (136.5 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.53 mM MgCl2, 5.5 mM glucose and 5.5 mM HEPES-NaOH, pH 7.4) and paced to beat by field stimulation (parameters of the stimulation: 5–20 mV depending on cellular threshold, 5 msec., 0.5 Hz). Intracellular Ca2+, in the absence and presence of isoprenaline, was continuously imaged with a Zeiss LSM-510 laser-scanning confocal microscope (LSM-510, Zeiss) using Ar/UV laser to ‘excite’ the dye at 488 nm. Emission light by photomultiplying tubes was detected at 520 nm. The parameters of image acquisition were similar for all examined cells (mid-cell section thickness was 1 μm and gain was set always at ∼700 arbitrary units). Images were acquired, viewed, and analysed using Zeiss Image Examiner Software. Only beating rod-shaped cells with clear striations were used for experimentation.
Measurement of action membrane potential
Sarcolemmal membrane potential was monitored in cells in the absence and presence of isoprenaline (500 nM)/glybenclamide (10 μM). Cells were loaded with di-8-ANEPPS according to the manufacturer’s instruction (Invitrogen) and the sarcolemma imaged using laser confocal microscopy in line-scan mode (LSM-510, Zeiss; [25]). Cells were stimulated as described in above section and scanned under control conditions, then exposed to drugs. Cells were scanned at 5 min. intervals in the absence and presence of isoprenaline or isoprenaline/glybenclamide. Fluorescence was detected/imaged at 488 nM excitation wavelength and emission was captured at >505 nM.
Statistical analysis
Data are presented as mean ± S.E.M, with n representing the number of analysed mice or cells in single cell experiments. Mean values were compared by the Student’s t-test, Mann-Whitney rank sum test or by Chi-square test where appropriate using SigmaStat program (Jandel Scientific, Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
Nicotinamide-rich diet increases SUR2A mRNA levels in heart ventricles
It has been previously shown that the level of SUR2A mRNA correlates well with the number of fully assembled sarcolemmal KATP channels and that this parameter could be used as a proxy measurement of the level of sarcolemmal KATP channels [19, 26–29]. We have analysed levels of SUR2A mRNA as well as mRNAs of two other KATP channel subunits, Kir6.2 and Kir6.1, in mice fed with control and nicotinamide-rich diet. Real time RT-PCR revealed that nicotinamide-rich diet significantly increased the levels of SUR2A mRNA in the heart as the threshold cycle for mice on control and nicotinamide-rich diet was 32.0 ± 0.4 and 22.0 ± 0.9, respectively (n = 6 for each; P < 0.01; Fig. 1). In contrast, no difference was found in mRNA levels of pore-forming KATP channel subunits, Kir6.2 and Kir6.1 (Kir6.2: the threshold cycle was 21.5 ± 0.5 for control and 22.0 ± 0.2 for nicotinamide-rich diet, n = 4, P = 0.35; Kir6.1: 21.2 ± 0.2 for control and 20.7 ± 0.1 for nicotinamide-rich diet, n = 4, P = 0.11; Fig. 1). Also, no significant difference was found in glyceraldehyde 3-phosphate dehydrogenase (GADPH) mRNA levels (Fig. 1; threshold cycle was 14.4 ± 0.2 for mice on control and 13.5 ± 0.8 on nicotinamide-rich diet, n = 6 for each, P = 0.24). As nicotinamide has a similar structure as a KATP channels opener nicorandil, we have tested whether nicotinamide would have any direct effect on KATP channels activity by using perforated patch clamp electrophysiology. Nicotinamide (1 mM) did not have any significant effect on whole cell K+ current in ventricular cardiomyocytes (the current density at 80 mV was 1.84 ± 0.20 pA/pF in the absence and 1.77 ± 0.15 pA/pF in the presence of nicotinamide; n = 4; P = 0.79, Fig. 2A). We have also elucidated possibility that nicotinamide-rich diet affects expression of other subtypes of sulfonylurea receptors (SUR1 and SUR2B) in ventricular tissue as well as expression of SUR1, SUR2A and SUR2B in some non-ventricular tissues. Real time RT-PCR did not reveal any statistically significant differences in level of expression of these subunits between mice on different diets in tested tissues (Fig. 2B).
Fig 1.
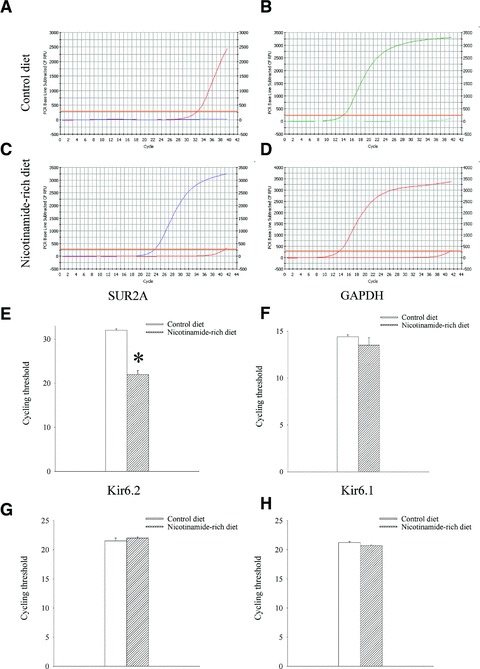
Expression of KATP channel subunits in hearts of mice on control and nicotinamide-rich diet. Representative progress curves for the real-time PCR amplification of SUR2A (A and C) and GAPDH (B and D) cDNA from mice on control or nicotinamide-rich diet (as labelled in the figure) and a corresponding bar graphs (E and F) as well as bar graphs depicting cycle threshold values for Kir6.2 and Kir6.1. Each bar represents mean ± S.E. of the mean (n = 4–6). *P < 0.05.
Fig 2.
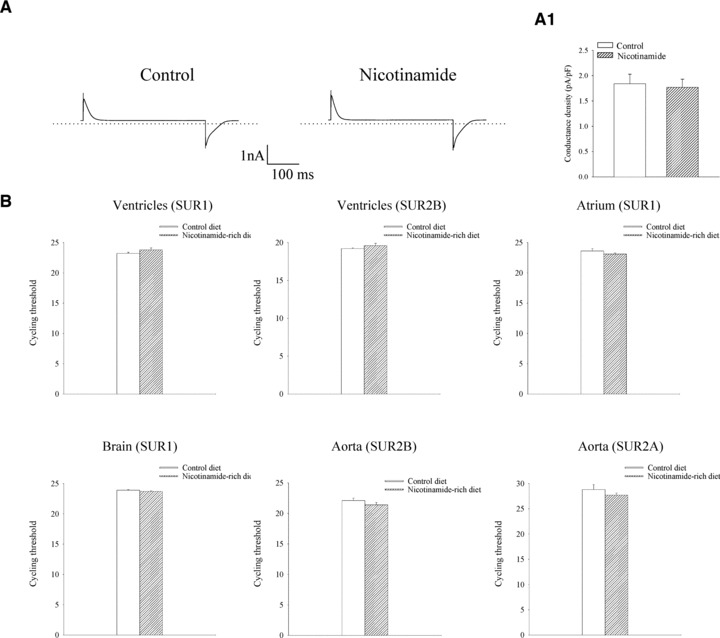
Acute electrophysiological effect of nicotinamide on isolated cardiomyocytes and expression of different SUR types in cardiac and some extra-cardiac tissues. (A) Typical whole cell currents at 80 mV in a cardiomyocyte in the absence (control) and presence (nicotinamide) of nicotinamide (1 mM) and a current density graph corresponding to conditions (A1). Each bar represents mean ± S.E. of the mean (n = 4). (B) Bar graphs showing real time RT-PCR cycle threshold values for depicted tissues and genes. Each bar represents mean ± S.E. of the mean (n = 3–6).
Nicotinamide-rich diet increases physical endurance
To test physical endurance of mice on control and nicotinamide-rich diet, we have used treadmill as described previously [13]. The tolerated workload, a parameter that incorporates time of effort with speed and incline of the treadmill, was significantly higher in mice on nicotinamide-rich diet compared with the mice on control diet (tolerated workload was 54.7 ± 5.1 J in mice on control and 104 ± 8.2 J in mice on nicotinamide-rich diet, n = 6 for each, P < 0.01; Fig. 3). Mice on control diet were able to stay on treadmill for 67.3 ± 6.2 min. (n = 6), while this time was significantly increased in mice on nicotinamide-rich diet (to 144.0 ± 14.9 min.; Fig. 3; P < 0.01).
Fig 3.
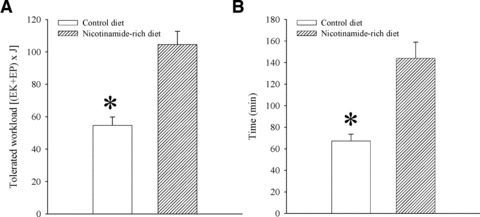
Physical endurance in mice on control and nicotinamide-rich diet. Bar graphs showing energy expenditure (A) and time spent (B) on treadmill of mice on control and nicotinamide-rich diet. Each bar represents mean ± S.E. of the mean (n = 6). *P < 0.05.
A sole increase in SUR2A mimics the effect of nicotinamide-rich diet on physical endurance
To determine whether a sole increase in SUR2A would have similar effect on physical endurance as a nicotinamide-rich diet, we have taken advantage of transgenic phenotype overexpressing SUR2A alone [19]. Under the exercise-stress test, mice in which the SUR2A was under control of cytomegalovirus promoter performed at a significantly increased level than the littemate controls (Fig. 4). The tolerated workload was ∼2-fold higher in transgenic mice compared with the wild-type animals (tolerated workload was 45.6 ± 2.2 J in wild-type mice and 91.6 ± 13.4 J in SUR2A mice, n = 5–7, P = 0.01; Fig. 4). Time spent on treadmill was significantly increased in SUR2A mice when compared to the wild-type (Fig. 4).
Fig 4.
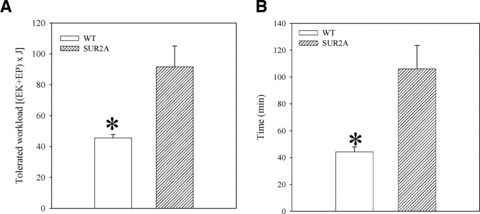
Physical endurance in wild-type (WT) and SUR2A mice on control diet. Bar graphs showing energy expenditure (A) and time spent (B) on treadmill of WT and SUR2A mice. Each bar represents mean ± S.E. of the mean (n = 6). *P < 0.05.
A shortening in action membrane potential in response to β-adrenergic stress in cardiomyocytes from SUR2A mice
It is generally accepted that the activation of β-adrenergic receptors and increased cardiac output and blood/oxygen supply underlies general adaptation to stress and ability of mammalians to sustain increased physical activity [30]. The activation of KATP channels induces shortening of the action membrane potential. Therefore, we have assessed the duration of action membrane potential in cells from both phenotypes under control conditions and when stimulated with a β-adrenoceptor agonist, isoprenaline. Under control conditions, action membrane potential duration remained stable over time in both phenotypes, i.e. at the beginning of field stimulation duration of action membrane potential was 228 ± 11 msec. in wild-type and 223 ± 44 msec. in SUR2A mice (Fig. 4; n = 6–10; P = 0.90) and these values were not significantly changed after 30 min. of stimulation (action membrane potential duration; wild-type: 234 ± 17 msec., n = 10, P = 0.74; SUR2A: 218 ± 22 msec., n = 6, P = 0.89; Fig. 4). When isoprenaline (500 nM), an agonist of β-adrenergic receptors used to induce stress, was added, no changes in action membrane potential duration was observed in wild-type (from 243 ± 17 msec. in the absence to 254 ± 24 msec. in the presence of isoprenaline, n = 7, P = 0.61; Fig. 5). In contrast to the wild-type, in SUR2A mice action membrane potential was significantly shortened after exposure to 500 nM isoprenaline (from 240 ± 19 msec. in the absence to 163 ± 13 msec. in the presence of isoprenaline, n = 6, P = 0.0007; Fig. 5). To test whether this shortening is induced by the activation of sarcolemmal KATP channels, we have used glybenclamide, a blocker of KATP channels. When applied alone, glybenclamide (10 μM) did not alter duration of action membrane potential in SUR2A cells (data not shown), but glybenclamide (10 μM) blocked the shortening of action membrane potential in SUR2A cell (from 238 ± 15 msec. in the absence to 228 ± 37 msec. in the presence of isoprenaline and glybenclamide, n = 6, P = 0.76; Fig. 5).
Fig 5.
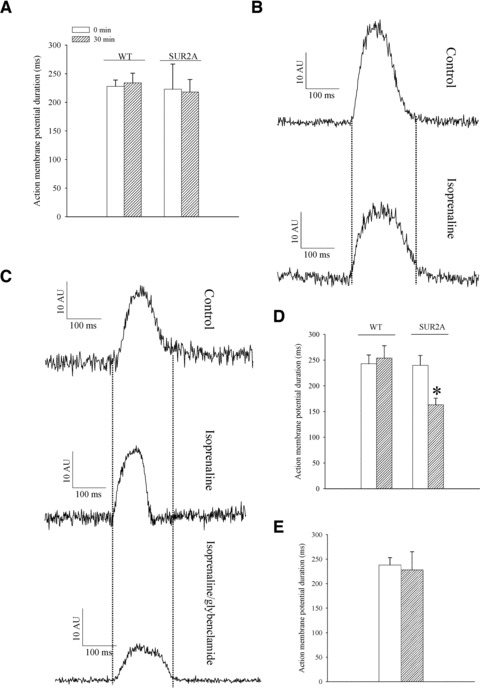
Action membrane potential duration in wild-type and SUR2A cardiomyocytes. (A) Duration of action membrane potential in cardiomyocytes from wild-type (WT) and SUR2A (SUR2A) at depicted time-points. Original action membrane potentials from wild-type (B) and SUR2A cardiomyocyte (C) in the absence (control) and presence of isoprenaline (500 nM) and isoprenaline (500 nM)/glybenclamide (10 μM). (D–E) Graphs corresponding to (B) and (C). Each bar represents mean ± S.E. of the mean (n = 6–10). *P < 0.05.
An improved Ca2+ homeostasis in SUR2A cardiomyocytes exposed to β-adrenergic stress
It was suggested that KATP channels regulate Ca2+ homeostasis during β-adrenergic stress and that this is crucial for regulation of physical endurance [13]. A shortening of cardiac action membrane potential is known to be associated with decreased Ca2+ influx and prevention of Ca2+ loading. Therefore, it was possible that Ca2+ homeostasis during stress could be improved in cardiac cells expressing more SUR2A. To test this hypothesis, we have measured intracellular Ca2+ levels in cardiac cells from the wild-type and SUR2A mice; a build up of Ca2+ over protracted time period would suggest a strained Ca2+ homeostasis. Thus, we have used isoprenaline (500 nM) and monitored Ca2+ levels in both cellular phenotypes. In cells from wild-type, there was a slow build up of intracellular Ca2+ during stimulation with isoprenaline (Fig. 6) and the average increase in Fluo-3 fluorescence under these conditions was 40.4 ± 2.3% (n = 12). In contrast, in SUR2A cells isoprenaline did not increase intracellular concentration at all (the average changes in intracellular Ca2+ concentration over 40 min. was –3.4 ± 2.3%, n = 8). The difference between wild-type and SUR2A mice was statistically significant (P < 0.01).
Fig 6.
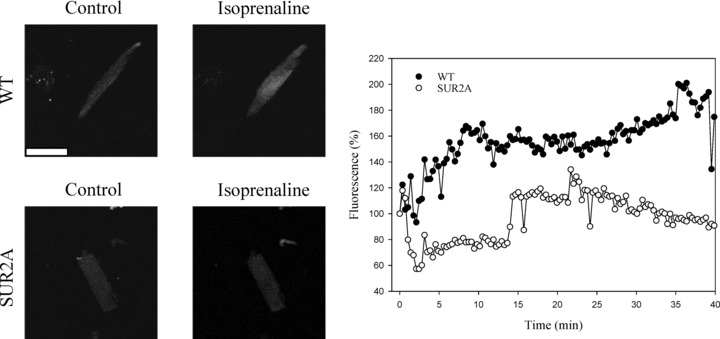
Intracellular Ca2+ in cardiac cells in response to long lasting stimulation with isporenaline. Left panels: Laser confocal images of Fluo 3-loaded cardiac cells from wild-type (WT) and SUR2A (SUR2A) mice in the absence and presence of isoprenaline (500 nM). White bar corresponds to 40 μm. Graph: Time course of Fluo-3 fluorescence in cardiac cells from wild-type (WT) and SUR2A (SUR2A) mice in the presence of isoprenaline (500 nM). Each point represents mean ± S.E.M. (n = 8–12).
Nicotinamide-rich diet has no effect on physical endurance in Kir6.2 knockout mice
In addition to up-regulating SUR2A expression, nicotinamide-rich diet could have other cardiac effects [31] and these might have effect on physical endurance. It was, therefore, possible that some mechanisms additional to up-regulation of SUR2A and sarcolemmal KATP channels could mediate the effect of nicotinamide-rich diet on physical endurance. To confirm or exclude this possibility, we have used Kir6.2 knockout mice. These mice have disrupted Kir6.2 gene and are lacking KATP channels in the heart and any intervention that alter the number/activity of KATP channels is ineffective in these mice. Consequently, if nicotinamide-rich diet improves physical endurance solely due to up-regulation of SUR2A and sarcolemmal KATP channels, this intervention would have no effect on physical endurance of Kir6.2 knockout mice. In contrast, if there is non-KATP channels mechanism involved, nicotinamide-rich diet would improve physical endurance in Kir6.2 knockout mice. Here, we have found that the tolerated workload was not different between mice on control and nicotinamide-rich diet; tolerated workload was 28.4 ± 1.4 J in mice on control and 28.0 ± 1.8 J in mice on nicotinamide-rich diet (n = 6–7, P = 0.95; Fig. 7). Time spent on treadmill was also not significantly different (Fig. 7).
Fig 7.
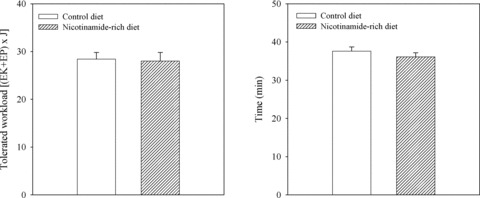
Physical endurance in Kir6.2 knockout (Kir6.2 KO) mice on control and nicotinamide-rich diet. Bar graphs showing energy expenditure (A) and time spent (B) on treadmill of Kir6.2 KO mice on control and nicotinamide-rich diet. Each bar represents mean ± S.E. of the mean (n = 6–7).
Discussion
In the present study we have shown that nicotinamide-rich diet increases physical endurance by up-regulating SUR2A in the heart. It seems that this is sole mechanism of nicotinamide-rich diet-induced improvement of physical endurance.
Nicotinamide is the amide of nicotinic acid and a member of water-soluble vitamin B group. In cells, nicotinamide is incorporated into nicotinamide adenine dinucleotide (NAD) and NAD phosphate, coenzymes in various enzymatic oxidation-reduction reactions (reviewed in [28]). It has been reported that exposure of cardiac cells to mild hypoxia and consequent increase in intracellular NAD stimulate SUR2A expression by activating PI3-kinase signalling pathway that stimulates SUR2 promoter via c-jun transcription factor [29]. Most interestingly, this signalling pathway has solely regulated SUR2A expression without affecting any other genes, as revealed by gene array technology [29]. Further experiments have supported the idea that increase in intracellular NAD seem to triggers signalling pathway that up-regulate SUR2A. In turn, an increase in SUR2A expression leads to increase in number of sarcolemmal KATP channels and cardiac phenotype with increased resistance to ischaemia reperfusion [29]. Nicotinamide-rich diet increases levels of NAD in many tissues, although this has not been specifically reported for the heart [32]. We have recently found that nicotinamide-rich diet up-regulates SUR2A and increases myocardial resistance to ischaemia reperfusion [15], which is in agreement with the effect of (1) nicotinamide-rich diet on intracellular NAD levels [32], (2) intracellular NAD on SUR2A expression [29] and (3) increased SUR2A on heart resistance to ischaemia reperfusion [19, 33]. The effect of increased SUR2A and sarcolemmal KATP channels on physical endurance has never been studied before. In the present study, nicotinamide-rich diet has increased the expression of SUR2A and numbers of sarcolemmal KATP channels, which is in agreement with the effect of nicotinamide-rich diet described in Ref. [15].
It has been demonstrated that a lack of sarcolemmal KATP channels is associated with decreased physical endurance due to heart inability to respond to increased metabolic demand and to increase cardiac output. Here we have shown that nicotinamide-rich diet has increased physical endurance in mice and that would (1) confirm a crucial role of sarcolemmal KATP channels in regulating physical endurance, (2) show that by manipulating KATP channel numbers physical endurance can be increased considerably over the physiological level and (3) demonstrate a potential of nicotinamide-rich diet as a strategy against conditions associated with impaired physical performance.
It is appreciated that nicotinamide-rich diet might have other cardiac and non-cardiac effects and that SUR2 is not necessarily only gene regulated by nicotinamide-rich diet [31]. On the other hand, we have previously shown that there are conditions, like mild hypoxia is, that could regulate the expression of SUR2A alone [29]. Indeed, in the present study, SUR2A was the only gene regulated by nicotinamide-rich diet out of all genes we tested. If up-regulation of SUR2A is the main mechanism of nicotinamide-rich diet increase in physical endurance, then the same phenomenon should be observed in transgenic mice with solely increased SUR2A (this phenotype is published in [19]). The treadmill endurance test has demonstrated that a sole increase in SUR2A levels in transgenic mice has mimicked the effect of nicotinamide-rich diet in the wild-type suggesting that up-regulation of SUR2A could be indeed the mechanism by which nicotinamide-rich diet improves physical performance. In study by Zingman et al. [13], it has been shown that a lack of sarcolemmal KATP channels lead to impaired Ca2+ homeostasis in cardiomyocytes during stimulation with 500 nM of isoprenaline, which is a β-adrenergic stress; a difficulty to maintain Ca2+ homeostasis in the absence of sarcolemmal KATP channels seems to be the main factor leading to decreased physical endurance in Kir6.2 knockout phenotype [13]. Here, we have shown that 500 nM of isoprenaline was associated with shortening of action membrane potential in a glybenclamide-sensitive manner in SUR2A, but not wild-type, cells. This indicates that there is opening of sarcolemmal KATP channels in cells with increased numbers of sarcolemmal KATP channels under the applied level of stress. It has been previously shown that increased number of sarcolemmal KATP channels is associated with earlier activation of KATP channels [19, 21], which is in accord with our present findings. The activation of KATP channels prevents intracellular Ca2+ loading, which is the main cause of impaired cellular function during adrenergic stress. Our results that Ca2+ homeostasis was improved in SUR2A cells when compared to the wild-type ones is in agreement with a role of sarcolemmal KATP channels as regulators of intracellular Ca2+ (reviewed in [12]). It also supports a theory that the regulation of Ca2+ homeostasis in the heart is the cellular mechanism underlying KATP channels-mediated regulation of physical endurance [13].
The final test for our hypothesis that SUR2A up-regulation is the main/sole mechanism of nicotinamide-rich diet was in determining whether nicotinamide-rich diet can improve physical endurance of Kir6.2 knockout mice. These mice have disrupted Kir6.2 gene and they lack all components of KATP channels in sarcolemma as this subunit is crucial for SUR2A trafficking [34]. Consequently, no intervention that manipulates with the sarcolemmal KATP channels number would be successful in Kir6.2 knockout mice. Conversely, if there is a KATP channel-independent mechanism involved, it would be revealed in Kir6.2 knockout mice. The fact that nicotinamide-rich diet did not affect physical endurance in Kir6.2 knockout mice suggest that increase in sarcolemmal KATP channels is the main mechanism involved in nicotinamide-rich diet increase in physical endurance.
It has been previously shown that a small change in intracellular NAD might regulate the expression of SUR2A without affecting expression of other genes [29]. In the present study, a dose of nicotinamide per os used in mice was low and it is much lower than the dose that was required to protect the heart against ischaemia reperfusion [15]. The human equivalent dose of nicotinamide to increase physical endurance would be lower than any other dose of nicotinamide that is currently used in clinical practice for any other indication. This means that oral nicotinamide has a clear potential as a drug that can regulate SUR2A expression and improve physical endurance in conditions where this effect would be desirable.
Conflict of interest
The authors confirm that there are no conflicts of interest.
Acknowledgments
This research was supported by grants from BBSRC, British Heart Foundation, MRC, TENOVUS-Scotland, Wellcome Trust and Anonymous Trust.
References
- 1.Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scan J Med Sci Sports. 2006;16:3–63. doi: 10.1111/j.1600-0838.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- 2.Rhodes RE, Fiala B. Building motivation and sustainability into the prescription and recommendations for physical activity and exercise therapy: the evidence. Physiother Theory Pract. 2009;25:424–41. doi: 10.1080/09593980902835344. [DOI] [PubMed] [Google Scholar]
- 3.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–8. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 4.Inagaki N, Gonoi T, Clement JP, et al. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–7. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- 5.Cui Y, Giblin JP, Clapp LH, et al. A mechanism for ATP-sensitive potassium channel diversity: functional coassembly of two pore-forming subunits. Proc Natl Acad Sci USA. 2001;98:729–34. doi: 10.1073/pnas.011370498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrasco AJ, Dzeja PP, Alekseev AE, et al. Adenylate kinase phosphotransfer communicates cellular energetic signals to ATP-sensitive potassium channels. Proc Acad Natl Sci A. 2001;98:7623–8. doi: 10.1073/pnas.121038198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crawford RM, Ranki HJ, Booting CH, et al. Creatine kinase is physically associated with the cardiac ATP-sensitive K+ channel in vivo. FASEB J. 2002;16:102–4. doi: 10.1096/fj.01-0466fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crawford RM, Budas GR, Jovanović S, et al. M-LDH serves as a sarcolemmal KATP channel subunit essential for cell protection against ischemia. EMBO J. 2002;21:3936–48. doi: 10.1093/emboj/cdf388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jovanović S, Jovanović A. High glucose regulates the activity of cardiac sarcolemmal KATP channels via 1,3-bisphosphoglycerate novel link between cardiac membrane excitability and glucose metabolism. Diabetes. 2005;54:383–93. doi: 10.2337/diabetes.54.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jovanović S, Du Q, Crawford RM, et al. Glyceraldehyde 3-phosphate dehydrogenase serves as an accessory protein of the cardiac sarcolemmal KATP channel. EMBO Rep. 2005;6:848–52. doi: 10.1038/sj.embor.7400489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 12.Kane GC, Liu XK, Yamada S, et al. Cardiac KATP channels in health and disease. J Mol Cell Cardiol. 2005;38:937–43. doi: 10.1016/j.yjmcc.2005.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zingman LV, Hodgson DM, Bast PH, et al. Kir6.2 is required for adaptation to stress. Proc Natl Acad Sci USA. 2002;99:13278–83. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jovanović A, Jovanović S. SUR2A targeting for cardioprotection. Curr Opin Pharmacol. 2009;9:189–93. doi: 10.1016/j.coph.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Sukhodub A, Du Q, Jovanović S, et al. Nicotinamide-rich diet protects the heart against ischaemia-reperfusion in mice: a crucial role for cardiac SUR2A. Pharmacol Res. 2010;61:564–70. doi: 10.1016/j.phrs.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaanders JH, Bussink J, van der Kogel AJ. ARCON: a novel biology-based approach in radiotherapy. Lancet Oncol. 2002;3:728–37. doi: 10.1016/s1470-2045(02)00929-4. [DOI] [PubMed] [Google Scholar]
- 17.Reddy S, Bibby NJ, Wu D, et al. A combined casein-free-nicotinamide diet prevents diabetes in the NOD mouse with minimum insulitis. Diabetes Res Clin Pract. 1995;29:83–92. doi: 10.1016/0168-8227(95)01109-9. [DOI] [PubMed] [Google Scholar]
- 18.Crinò A, Schiaffini R, Manfrini S, et al. A randomized trial of nicotinamide and vitamin E in children with recent onset type 1 diabetes (IMDIAB IX) Eur J Endocrinol. 2004;150:719–24. doi: 10.1530/eje.0.1500719. [DOI] [PubMed] [Google Scholar]
- 19.Du Q, Jovanović S, Clelland A, et al. Overexpression of SUR2A generates a cardiac phenotype resistant to ischaemia. FASEB J. 2006;20:1131–41. doi: 10.1096/fj.05-5483com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kane GC, Behfar A, Yamada S, et al. ATP-sensitive K+ channel knockout compromises the metabolic benefit of exercise training resulting in cardiac deficits. Diabetes. 2004;53:S165–75. doi: 10.2337/diabetes.53.suppl_3.s169. [DOI] [PubMed] [Google Scholar]
- 21.Reyes S, Kane GC, Zingman LV, et al. Targeted disruption of KATP channels aggravates cardiac toxicity in cocaine abuse. Clin Transl Sci. 2009;2:361–5. doi: 10.1111/j.1752-8062.2009.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alekseev AE, Reyes S, Yamada S, et al. Sarcolemmal ATP-sensitive K+ channels control energy expenditure determining body weight. Cell Metab. 2010;11:58–69. doi: 10.1016/j.cmet.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sukhodub A, Jovanović S, Du Q, et al. AMP-activated protein kinase mediates preconditioning in cardiomyocytes by regulating activity and trafficking of sarcolemmal ATP-sensitive K+ channels. J Cell Physiol. 2007;210:224–36. doi: 10.1002/jcp.20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jovanović S, Jovanović N, Jovanović A. High glucose protects single beating adult cardiomyocytes against hypoxia. Biochem Biophys Res Commun. 2006;341:57–66. doi: 10.1016/j.bbrc.2005.12.147. [DOI] [PubMed] [Google Scholar]
- 25.Budas GR, Sukhodub A, Alessi DR, et al. 3’Phosphoinositide-dependent kinase-1 is essential for ischemic preconditioning of the myocardium. FASEB J. 2006;20:2556–8. doi: 10.1096/fj.06-6252fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ranki HJ, Budas GR, Crawford RM, et al. Gender-specific difference in cardiac ATP-sensitive K+ channels. J Am Coll Cardiol. 2001;38:906–15. doi: 10.1016/s0735-1097(01)01428-0. [DOI] [PubMed] [Google Scholar]
- 27.Ranki HJ, Budas GR, Crawford RM, et al. 17β-estradiol regulates expression of KATP channels in heart-derived H9c2 cells. J Am Coll Cardiol. 2002;40:367–74. doi: 10.1016/s0735-1097(02)01947-2. [DOI] [PubMed] [Google Scholar]
- 28.Ranki HJ, Crawford RM, Budas GR, et al. Ageing is associated with decrease in number of sarcolemmal ATP-sensitive K+ channels in a gender-dependent manner. Mech Ageing Dev. 2002;123:695–705. doi: 10.1016/s0047-6374(01)00415-8. [DOI] [PubMed] [Google Scholar]
- 29.Crawford RM, Jovanović S, Budas GR, et al. Chronic mild hypoxia protects heart-derived H9c2 cells against acute hypoxia/reoxygenation by regulating expression of the SUR2A subunit of the ATP-sensitive K+ channels. J Biol Chem. 2003;278:31444–55. doi: 10.1074/jbc.M303051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ament W, Verkerke GJ. Exercise and fatigue. Sports Med. 2009;39:389–422. doi: 10.2165/00007256-200939050-00005. [DOI] [PubMed] [Google Scholar]
- 31.Bogan KL, Brenner C. Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu Rev Nutr. 2008;28:115–30. doi: 10.1146/annurev.nutr.28.061807.155443. [DOI] [PubMed] [Google Scholar]
- 32.Jackson TM, Rawling JM, Roebuck BD, et al. Large supplements of nicotinic acid and nicotinamide increase tissue NAD+ and poly(ADP-ribose) levels but do not affect diethylnitrosamine-induced altered hepatic foci in Fischer-344 rats. J Nutr. 1995;125:1455–61. doi: 10.1093/jn/125.6.1455. [DOI] [PubMed] [Google Scholar]
- 33.Du Q, Jovanović S, Sukhodub A, et al. Infection with AV-SUR2A protects H9C2 cells against metabolic stress: a mechanism of SUR2A-mediated cytoprotection independent from the KATP channels activity. Biochem Biophys Acta-Mol Cell Res. 2010;1803:405–15. doi: 10.1016/j.bbamcr.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwappach B, Zerengue N, Jan YN, et al. Molecular basis for K(ATP) assembly: transmembrane interactions mediate association of a K+ channel with an ABC transporter. Neuron. 2000;26:155–67. doi: 10.1016/s0896-6273(00)81146-0. [DOI] [PubMed] [Google Scholar]


