Abstract
We have investigated the blood levels of sub-classes of stem cells (SCs) [mesenchymal stem cells (MSCs), haematopoietic stem cells (HSCs), endothelial progenitor cells/circulating endothelial cells (EPCs/CECs) and tissue-committed stem cells (TCSCs)] in heart failure (HF) patients at different stage of pathology and correlated it with plasmatic levels of proangiogenic cytokines. Peripheral blood level of SCs were analysed in 97 HF patients (24 in NYHA class I, 41 in class II, 17 in class III and 15 in class IV) and in 23 healthy controls. Plasmatic levels of PDGF-BB, bFGF, HGF, vascular endothelial growth factor (VEGF), SDF-1α, TNF-α and NTproBNP were also measured. Compared with healthy individuals, MSC, and in particular the sub-classes CD45−CD34−CD90+, CD45−CD34−CD105+ and CD45−CD34−CXCR4+ were significantly enhanced in NYHA class IV patients (16.8-, 6.4- and 2.7-fold, respectively). Level of CD45−CD34−CD90+CXCR4+cells progressively increased from class II to class IV (fold increases compared with controls: 8.5, 12 and 21.5, respectively). A significant involvement of CXCR4+ subpopulation of HSC (CD45+CD34+CD90+CXCR4+, 1.4 versus 13.3 cells/μl in controls and NYHA class III patients, respectively) and TCSC (CD45−CD34+CXCR4+, 1.5 cells/ μl in controls versus 12.4 and 28.6 cells/μl in NYHA classes II and IV, respectively) were also observed. All tested cytokines were enhanced in HF patients. In particular, for PDGF-BB and SDF-1α we studied specific ligand/receptors pairs. Interestingly, the first one positively correlated with TCSCs expressing PDGFR (r = 0.52, P = 0.001), whereas the second one correlated with TCSCs (r = 0.34, P = 0.005) and with MSCs CD90+ expressing CXCR4 (r = 0.39, P = 0.001). HF is characterized by the increase in the circulating levels of different MSC, HSC, EPC and TCSC subsets. Both the entity and kinetic of this process varied in distinct cell subsets. Specifically, differently from HSCs and EPCs/CECs, MSCs and TCSCs significantly increased with the progression of the disease, suggesting a possible distinct role of these cells in the pathophysiology of HF.
Keywords: heart failure, mesenchymal stem cells, haematopoietic stem cells, endothelial progenitor cells, cytokines, myocardial repair
Introduction
Transplantation of mononuclear fraction of bone marrow stem cells (M-BMSCs) capable of multi-lineage differentiation has been considered to restore function of acutely injured myocardium [1, 2]. Some properly randomized clinical trials in post-myocardial infarction (AMI) patients showed improvement of cardiac function after infusion of M-BMSCs, whereas others failed to do so [3–9]. All these studies have been conducted in patients with well-preserved left ventricular function (ejection fraction ranging from 40% to 50%). It has been argued that these patients need little more than evidence-based pharmacological therapy, whereas patients with more severely reduced left ventricular function and/or chronic heart failure (HF) should benefit more from M-BMSCs transplantation.
Data on stem cells in HF are scanty. A progressive decline of circulating endothelial progenitors cells (EPCs) with increasing severity of HF has been reported [10].
However, in addition to EPCs, bone marrow haematopoietic (HSCs), mesenchymal (MSCs) and the so-called tissue-committed stem cells (TCSCs) may have the potential to be recruited into the blood stream and to differentiate in endothelial, cardiac and skeletal muscle cells, thus facilitating regeneration of new myocardium. Data on the overall recruitment of these cells in HF patients are not available, and yet they are crucial to understand the mechanism of cardiovascular repair.
The aim of this study was to analyse the spontaneous increase of the blood levels of all these classes of BMSCs in 23 healthy individuals and in 97 patients affected by different degrees of HF. Possible candidate cytokines associated with this process were evaluated by measuring both the CXCR4-SDF-1α and PDGF-BB-PDGFR axes. The role of TNF-α and of other specific pro-angiogenetic cytokines [bFGF, vascular endothelial growth factor (VEGF) and HGF] was also determined.
Materials and methods
Patients
Ninety-seven patients (81% males, mean age 68 ± 9 years) were consecutively enrolled. The control group consisted of 23 healthy individuals who were matched as to gender and age (87% males; aged 65 ± 2 years) with the patients. Ethics committee approval was obtained as was the informed consent by both patients and controls, according to the World Medical Association Declaration of Helsinki. Table 1 shows clinical details of the studied population. Diagnosis of HF was based on history of HF of at least six months duration, reduced exercise tolerance, objective left ventricular functional impairment and raised level of NTproBNP above the normal range of 486 pg/ml at entry. Diagnosis of idiopathic dilated cardiomyopathy was based on accepted criteria [11]. HF staging was performed by the New York Heart Association (NYHA) classification and on the basis of NTproBNP value.
Table 1.
Baseline clinical parameters of the study population
| Variables | Controls n = 23 | All (n = 97) | NYHA I (n = 24) | Patients NYHA II (n = 41) | NYHA III (n = 17) | NYHA IV (n = 15) | p1 | p2 |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 65.3 ± 2.2 | 68.3 ± 9.5 | 64.9 ± 8.4 | 69.2 ± 9.6 | 69.5 ± 10.1 | 69.8 ± 9.9 | NS | NS |
| Males (%) | 87.0 | 81.4 | 95.8 | 82.9 | 70.6 | 66.7 | NS | NS |
| WBC (103/ml) | 2.3 ± 0.4 | 2.3 ± 1.0 | 2.5 ± 1.1 | 2.3 ± 1.0 | 2.5 ± 1.2 | 2.0 ± 0.7 | NS | NS |
| LVFE (%) | – | 33.2 ± 8.3 | 38.7 ± 4.8 | 33.2 ± 8.3 | 32.5 ± 7.4 | 25.1 ± 7.5 | – | <0.0001 |
| VO2 peak (ml/kg/min) | – | 14.2 ± 5.4 | 17.5 ± 5.3 | 15.4 ± 3.2 | 9.8 ± 0.9 | 7.8 ± 1.0 | – | <0.0001 |
| NTproBNP (fmol/ml) | 3.5 ± 3.9 | 78.5 ± 93.1 | 27.9 ± 19.6 | 65.6 ± 55.8 | 58.8 ± 40.1 | 216.8 ± 148.9 | <0.0001 | <0.0001 |
| Risk factors (%) | ||||||||
| Diabetes | – | 39.0 | 40.9 | 25.0 | 40.0 | 50.0 | – | NS |
| Hypercholesterolaemia | – | 68.2 | 81.8 | 67.5 | 66.7 | 37.5 | – | NS |
| Smoking habits | – | 63.5 | 63.6 | 62.5 | 73.3 | 50.0 | – | NS |
| History of hypertension | – | 57.6 | 63.6 | 52.5 | 73.3 | 37.5 | – | NS |
| CAD familiarity | – | 43.5 | 45.5 | 42.5 | 46.7 | 37.5 | – | NS |
| Therapy (%) | ||||||||
| ACE inhibitors | – | 68.0 | 79.2 | 63.4 | 76.5 | 53.5 | – | NS |
| ARB | – | 64.9 | 54.2 | 73.2 | 76.5 | 46.7 | – | NS |
| β-Blockers | – | 92.8 | 91.7 | 97.6 | 82.4 | 93.3 | – | NS |
| Antialdosterone | – | 36.1 | 25.0 | 41.5 | 23.5 | 53.3 | – | NS |
| Diuretics | – | 88.7 | 75.0 | 92.7 | 88.2 | 100 | – | NS |
| Digitalis | – | 21.7 | 12.5 | 14.6 | 41.2 | 33.3 | – | NS |
| Nitrates | – | 19.6 | 16.7 | 14.6 | 41.2 | 13.3 | – | NS |
| Calcium antagonists | – | 10.3 | 33.3 | 2.4 | 5.9 | 0 | – | 0.0008 |
p1: patients versus controls; p2: intra NYHA classes analysis; NS: not significative; WBC: white blood cells; LVEF: left ventricular ejection fraction; VO2 peak: peak oxygen consumption; CAD: coronary artery diseases; ACE: angiotensin-converting enzyme; ARB: angiotensin II receptors blockers. Risk factors were evaluated in 85 of 97 patients.
Patients were receiving standard evidence-based guided pharmacological treatment. Statins have been discontinued in all patients at least 3 weeks before blood collection. The control group consisted of healthy individuals without any cardiovascular risk who were receiving no treatment.
Stem cells quantification
Blood samples were systematically collected from patients after statin washout from an antedecubital vein performed with a 21-gauge needle and immediately utilized for BMSC assay or duly centrifuged to obtain plasma to be frozen at −80° for subsequent cytokines and NTproBNP determination. Un-fractioned blood samples were incubated with a panel of directly conjugated monoclonal antibodies (Abs): either FITC- or PERCP-CD45, either FITC- or PE-CD34 and PE-CD90 (BD Biosciences, Franklin Lakes, NJ, USA), PE-CD105 (AbD Serotec, Oxford, UK), PE-CD133 (Miltenyi Biotec, Bergisch Gladbach, Germany), PE-CD144, PE-PDGFR-α and PE-PDGFR-β (Santa Cruz Biotechnology, Santa Cruz, CA, USA) FITC-CXCR4. Samples were lysed by FACS Lysing Solution (BD Biosciences) and are acquired by CyAn (Dako, Glostrup, Denmark) or FACScan (BD Biosciences), 400,000 cells/sample were collected.
PDGFR expression was evaluated in 48 patients and 13 controls. Each experiment included negative controls. For multi-colour staining, single-colour stained controls were included to create a compensation matrix (Summit Software, Dako). Analyses were performed utilizing SummitSoftware (Dako), CellQuest (BD Biosciences) and Flow-Jo (Tree Star, Ashland, OR, USA).
Analytical gates were used to enumerate total number and subsets of circulating SCs. Circulating cell concentrations are expressed as number of cells/μl of blood.
Cytokines analysis
Plasma levels of angiogenic cytokines (VEGF, HGF, bFGF, PDGF-BB) were analysed performed with a Searchlight human angiogenesis array 2-multiplex assay (Tema Ricerca, Bologna, Italy), according to manufacturer’s instructions. The colorimetric reaction was blocked and sent for reading to the TEMA Ricerca laboratories, where plates were read with a Search Light CCD Image and Analysis System. The sensitivity of the assay was 4.9, 3.1, 2 and 1 pg/ml for VEGF, HGF, bFGF and PDGF-BB, respectively. Intra- and inter-assay coefficients of variations (CVs) were 8% and 6% for VEGF, 2.5% and 5% for HGF, 6% and 5.5% for bFGF and 7.7% and 3.6% for PDGF-BB.
TNF-α was determined according to manufacturer’s instructions (EASIA kit Biosource TNF-α-Biosource Europe S.A.) by a solid phase enzyme amplified sensitivity immunoassay in which the use of a blend of monoclonal Abs allowed the measurement of the total circulating TNF-α. The sensitivity of the assay was 3 pg/ml. Intra- and inter-assay coefficients of variations (CVs) were 5.2% and 9.9%, respectively.
NT-proBNP was evaluated by Nt-proBNP direct ELISA, a sandwich enzyme immunoassay for the determination of N-terminal fragment of proBNP in human EDTA plasma or serum. This assay was developed by DRG Instruments GmbH, Marburg, Germany and distributed by Technogenetics S.r.l., Milan, Italy. The minimum detection limit was 3 fmol/mL; CV intra-assay (n = 16) and inter-assay (n = 10) from 5% to 8%, and from 7% to 10%, respectively.
To measure circulating levels of SDF-1α, an additional centrifugation step of the separated plasma at 10,000 × g for 10 min. at 4°C was performed for complete platelet removal.
SDF-1α quantification was performed by Quantikine Human CXCL12/SDF-1α based on a quantitative enzyme immunoassay technique. This assay was developed by R&D Systems Europe and distributed by Space Import-Export S.r.l., Milan, Italy. The assay sensitivity was 18 pg/ml considering it as mean of 22 evaluations of minimum detectable dose; the assay precision revealed by a CV intra-assay (n = 20) <3.9% and a CV inter-assay (n = 40) <13.4%.
Statistical analysis
The groups were compared with respect to demographic characteristics by ANOVA or Fisher’s exact tests (P = 0.05, two-tailed). The specific classes of SC were compared among HF groups and healthy individuals with a multivariate analysis of variance model, performed with SAS GLM Procedure. Contrast among healthy individual and HF patients and single HF severity group were also planned in the procedure. Descriptive statistics and graphical analyses were used to summarize data and results as appropriate to the type of data. All analyses were conducted performed with SAS (SAS Institute, Cary, NC, USA).
Results
Patients’ characterization
The characteristics of the studied population, including cardiovascular risk factors, cardiac functionality parameters and therapy, are shown in Table 1. Sixty-six patients (68%) had ischaemic aetiology, whereas 15 (15,5%) satisfied the criteria for idiopathic dilated cardiomiopathy. The remaining 16 patients had HF because of hypertension (n = 8), valvular disorders (n = 3), myocarditis (n = 2) and alcohol abuse (n = 3). All patients were receiving guidelines pharmacological therapy consisting of ACE inhibitors (68%), angiotensin II receptors blockers (64.9%), ˜β-blockers (92.8%), antialdosterone drugs (36,1%), diuretics (88.7%) and digitalis (21.7%). As expected, this multitherapy regime did vary according to the severity of HF (NYHA). Patients with ischaemic heart disease also received antianginal drugs such as nitrates and calcium antagonists.
There were no major differences between groups as for HF aetiology and the most common cardiovascular risk factors: age, diabetes, hypercholesterolemia, smoking habits, history of hypertension and coronary diseases familiarity. As expected % LVEF and VO2 peak significantly decreased and plasma levels of NTproBNP progressively increased with the deterioration on the NYHA class.
Stem cells analysis
Table 2 summarizes the Abs combination utilized to identify the different subpopulation of each class of BMSC. Figure 1 shows a typical example of analytical gates used to count total numbers and subsets of circulating stem cells. Putative MSCs were identified as CD45−CD34− cells, expressing either CD90 or CD105 [12]; HSCs as CD45+ and CD34+ cells co-expressing either CD90 or CD105 [13]; EPCs, a very heterogeneous group of cells, were characterized as CD45− with or without the surface markers CD133 and CD144 [14–16]. TCSCs were identified as CD34+CD45+ or CD45− cells co-expressing the CXCR4 receptor [17]. In view of the role of SDF-1α-CXCR4 interaction in homing, repopulation and recruitment of human stem cells [18], the expression of CXCR4 receptor was also evaluated in all groups except for the EPCs, because this was already evaluated in the CD45−CD34+ component of the TCSCs. Because of the role of PDGF-BB in MSC recruitment, we evaluated the axis PDGF-BB/PDGFR as well [19].
Table 2.
Stem cells classes and antibody combination used to identify different circulating subsets
| Stem cells class | Phenotype |
|---|---|
| A) CD45−CD34−CD90+ | |
| B) CD45−CD34−CD105+ | |
| C) CD45−CD34−CXCR4+ | |
| Mesenchymal stem cells | D) CD45−CD34−CD90+CXCR4+ |
| E) CD45−CD34−CD105+CXCR4+ | |
| F) CD45−CD34−PDGFR+ | |
| G) CD45−CD34−CXCR4+PDGFR+ | |
| A) CD45+CD34+CD90+ | |
| B) CD45+CD34+CD105+ | |
| Haematopoietic stem cells | C) CD45+CD34+CD90+CXCR4+ |
| D) CD45+CD34+CD105+CXCR4+ | |
| E) CD45+CD34+PDGFR+ | |
| F) CD45+CD34+CXCR4+PDGFR+ | |
| A) CD45−CD133+CD144+ | |
| Endothelial progenitors/endothelial cells | B) CD45−CD133+CD144− |
| C) CD45−CD133− CD144+ | |
| A) CD45+CD34+CXCR4+ | |
| Tissue committed stem cells | B) CD45−CD34+CXCR4+ |
| C) CD45−CD34+CXCR4+PDGFR+ |
Fig 1.
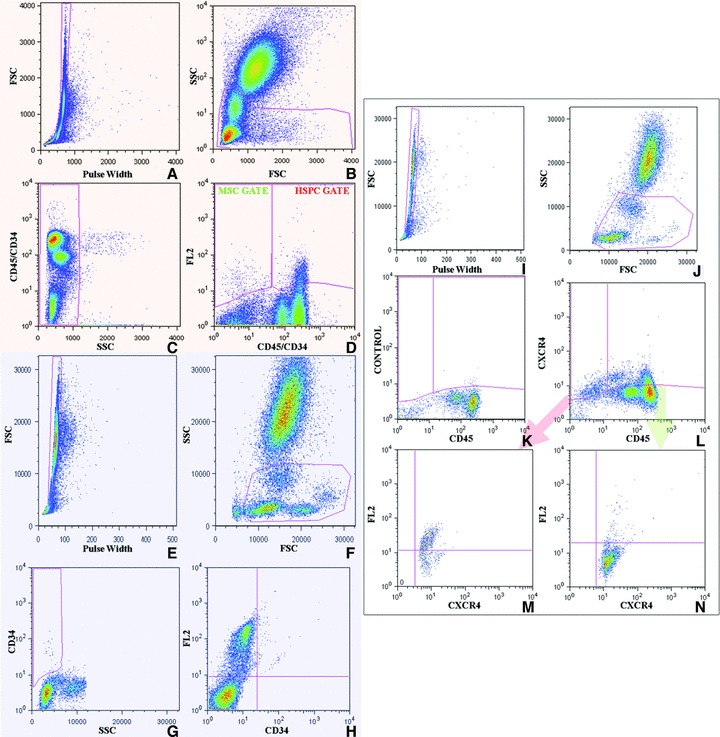
Gating strategy. Orange box: MSC and HSPC enumeration. Pulse width versus FSC (A), FSC versus SSC (B), and CD45/CD34 (C) dot plots were utilized to exclude cell doublets, platelets, erythrocytes, dead cells, debris and neutrophilis, restricting the analysis to the lympho-monocyte fraction. On the basis of the level of expression of CD45 and CD34, we recognized, within the gated blood cells, a CD45+CD34+ population and a CD45−CD34− population (D). HSC were recognized within the CD45+CD34+ fraction, FL2-CD133+, FL2-CD90+ or FL2-CD105+ cells (HSC gate, D). MSC were studied within the CD45/CD34low/neg cells, as either FL2-CD90+ or FL2-CD105+ (MSC gate, D). Blue box: CD34lowSSC and EPC/EC enumeration. To count CD34+SSClow cells and EPC/EC, cells were first gated on pulse width versus FSC (E) and FSC versus SSC (F) dot plots. CD34 versus SSC dot plots were exploited to establish the gate identifying the CD34+ fraction characterized by low SSC (G). CD34 versus FL2-CD144 dot plots were utilized to recognize circulating CD144+ EPC/EC and to distinguish, within this class, the subpopulation of cells co-expressing CD144 and CD34 (H). White box: CXCR4+ cell enumeration and characterization. Regarding CXCR4+SCs, we first gated cells on the basis of pulse width versus FSC (I) and FSC versus SSC (J) dot plots. Samples stained with CD45 and isotype-matched antibodies were then plotted on CD45 versus CXCR4 charts to establish the gates identifying both the CXCR4+CD45+ and the CXCR4+CD45− populations (K). These two latter populations (L) were then analysed separately plotting CXCR4 versus either FL2-CD105, FL2-CD90 (M and N).
The results obtained for each class of stem cells are reported in Figures 2 (MSC), 3 (HSC), 4 (EPC/CEC) and 5 (TCSC), respectively.
Fig 2.
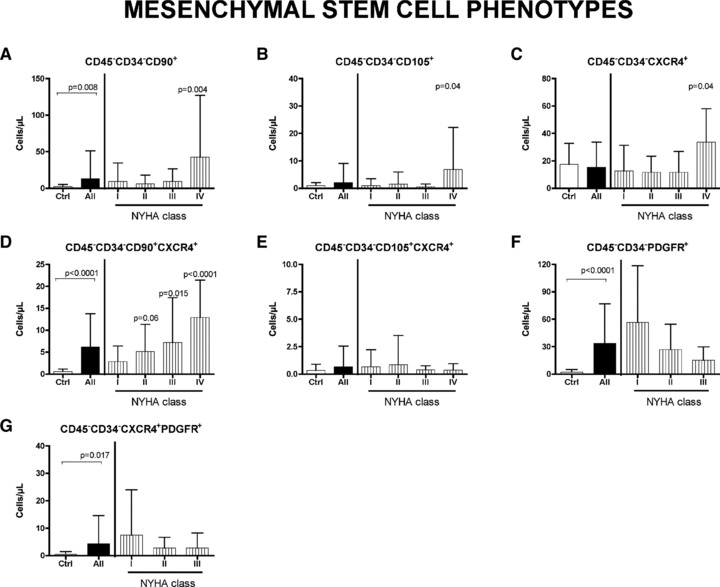
Circulating mesenchymal stem cells in HF patients. Peripheral blood level of CD45−CD34−CD90+ cells (A) are significantly increased in HF patients, and in particular in NYHA class IV patients compared with healthy donors (Ctrl). CD45−CD34−CD105+ cells (B) are significantly higher in NYHA class IV patients compared with controls. The CXCR4+ subpopulation of CD45−CD34− cells is also highly recruited (C) and, in particular, the subset CD45−CD34−CD90+CXCR4+ shows a significative increment in worsening of pathology (D). No differences between controls and patients are present regarding CD45−CD34−CD105+CXCR4+ subset (E). Also the level of PDGFR-positive MSCs and the CXCR4-positive component are higher in patients than in controls (F and G, respectively). Significative differences are reported as P value. Data are expressed as mean ± S.D.
Fig 5.
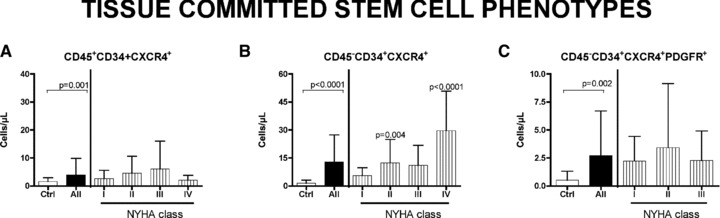
Circulating tissue committed stem cells in HF patients. Specific subset (CD45− and CD45+ and CD45−PDGFR+) of CD34+CXCR4+ SCs are analysed. Significative difference are found between all patients and controls in all populations. Differences in specific NYHA classes was found in CD45−CD34+CXCR4+ (B), but not in CD45−CD34+CXCR4+ (A) and in PDGFR-positive (C) cells. Significative difference between controls and patients are reported as P value. Data are expressed as mean ± S.D.
Figure 2 shows that all subpopulation of MSCs, with exception of the CD45−CD34− CD105+CXCR4+, are significantly increased in NYHA class IV patients versus controls. A specific subset of MSCs, the CD45−CD34−CD90+CXCR4+, seems to be most recruited (Fig. 2D), with a clear, significant step-wise progressive increase as HF deteriorates (P for trend <0.0001). The number of circulating MSCs expressing either PDGFR or PDGFR and CXCR4 resulted to be significantly increased in patients with respect to controls (Fig. 2F and G).
Conversely, HSCs (Fig. 3), although significantly augmented in patients with respect to controls, did not show a significant trend to increase with NYHA class. Emblematically, the fraction characterized as CD45+CD34+CD90+ expressing the CXCR4 receptor (Fig. 3C), peaks in class III patients and returns to control level in class IV. CD45+CD34+CD105+ cells expressing the CXCR4 and CD45+CD34+ cells expressing PDGFR were also increased in patients (Fig. 3D and E).
Fig 3.

Circulating haematopoietic stem cells in HF patients. Level of CD90+ and CD105+ HSC (A and B, respectively) are analysed in peripheral blood of controls and patient. No significative difference are observed between healthy donors and patients considered for New York Association class. In the CXCR4+ subtype, only the subset CD45+CD34+ CD90+CXCR4+ differs significatively between controls and patients in NYHA class III (C). No differences are reported in CD45+CD34+CD105+CXCR4+ cells (D). Regarding PDGFR-positive cells, statistically significative increase is observed in CD45+CD34+ class (E), but not in CXCR4+ component (F). Significative difference between controls and patients are reported as P value. Data are expressed as mean ± S.D.
Figure 4 shows the behaviour of the EPC fractions. Mean values in patients were significantly increased versus controls. The behaviour of TCSCs is reported in Figure 5. Interestingly, the CD45−CD34+CXCR4+ cells, which are committed to myocytes and endothelium, show a significant trend to increase with the severity of HF (P < 0.0001), similarly to the MSCs (Fig. 5B). Conversely, the CD45+CD34+CXCR4+ cells (Fig. 5A), which are committed to form lymphocytes and monocytes, although showing a significant increase in patient with respect to controls, did not present such a significative progressive raise.
Fig 4.
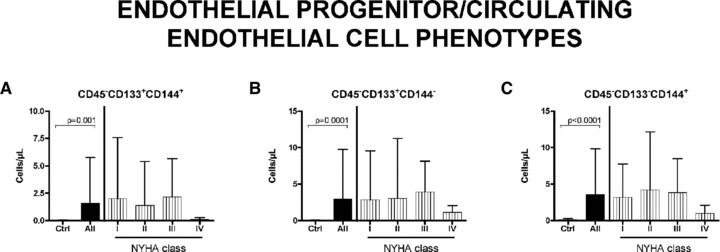
Circulating endothelial precursors/cells in HF patients. Differences in EPCs/ECs level between all patients and control is significative. Number of cells, compared with controls, is higher in I–III NYHA classes and decreases in IV NYHA class, even if differences are not significative in any tested point. (A) Endothelial precursors cells; (B) differentiating endothelial cells; (C) mature endothelial cells. Data are expressed as mean ± S.D.
Figure 6 shows the plasma behaviours of all the measured cytokines. PDGF-BB, TNF-α and SDF-1α (Fig. 6A, E and F) show a significant progressive increase with the deterioration of HF (P < 0.0001, P = 0.0004 and P < 0.0001, respectively). HGF and bFGF (Fig. 6B and C) are also significantly raised but do not show a progressive increase. VEGF is significantly elevated in NYHA I, but not in the more advanced ones (Fig. 6D).
Fig 6.

Cytokines plasmatic level in HF patients. Histograms represent plasma concentration of PDGF-BB (A), HGF (B), bFGF (C), VEGF (D), TNF-α (E) and SDF-1α (F) in healthy individuals and HF patients. Significative difference between controls and patients are reported as P value. Data are expressed as mean ± S.D.
Correlations
Table 3 shows the correlation between cytokine release and circulating levels of specific SC subsets. In particular, PDGF-BB positively correlates with peripheral blood level of MSC CD90+ (r = 0.34, P = 0.0001), CD105+ (r = 0.3, P = 0.0012) and CD90+CXCR4+ (r = 0.51, P < 0.0001); with TCSC CD45−CD34+CXCR4+ (r = 0.46, P < 0.0001) and with the subfraction of TCSC expressing PDGFR (r = 0.52, P = 0.001).
Table 3.
Correlation between cytokines and stem cell subsets
| Cytokines | Related cells | r | P |
|---|---|---|---|
| PDGF-BB | CD45−CD34−CD90+ | 0.34 | 0.0001 |
| CD45−CD34−CD105+ | 0.3 | 0.0012 | |
| CD45−CD34−CD90+CXCR4+ | 0.51 | <0.0001 | |
| CD45−CD34+CXCR4+ | 0.46 | <0.0001 | |
| CD45−CD34+CXCR4+PDGFR+ | 0.52 | 0.001 | |
| TNF-α | CD45−CD34−CD90+ | 0.52 | <0.0001 |
| CD45−CD34−CD105+ | 0.26 | 0.004 | |
| CD45−CD34−CD90+CXCR4+ | 0.22 | 0.018 | |
| CD45−CD34−CD105+CXCR4+ | 0.46 | <0.0001 | |
| CD45−CD34+CXCR4+PDGFR+ | 0.59 | 0.0002 | |
| bFGF | CD45−CD34−CD90+CXCR4+ | 0.33 | 0.0003 |
| CD45−CD34+CXCR4+ | 0.40 | <0.0001 | |
| CD45−CD34−PDGFR+ | 0.51 | 0.001 | |
| HGF | CD45−CD133+CD144+ | 0.34 | 0.0003 |
| CD45−CD133+CD144− | 0.29 | 0.003 | |
| CD45−CD34−PDGFR+ | 0.47 | 0.002 | |
| VEGF | CD45−CD133+CD144+ | 0.3 | 0.001 |
| CD45−CD133+CD144− | 0.28 | 0.004 | |
| CD45−CD34−PDGFR+ | 0.47 | 0.002 | |
| SDF-1α | CD45−CD34−CD90+CXCR4+ | 0.39 | 0.001 |
| CD45−CD34+CXCR4+ | 0.34 | 0.005 | |
| CD45+CD34+CD90+ | 0.36 | 0.003 | |
| CD45−CD133+CD144+ | 0.37 | 0.007 | |
| CD45−CD133−CD144+ | 0.52 | <0.0001 | |
| CD45−CD133+CD144− | 0.40 | 0.003 |
TNF-α correlates with MSC CD90+ (r = 0.52, P < 0.0001), CD105+ (r = 0.26, P = 0.004), CD90+CXCR4+ (r = 0.22, P = 0.018), CD105+CXCR4+ (r = 0.46, P < 0.0001) and with TCSC CD45−CD34+CXCR4+PDGFR+ (r = 0.59, P = 0.0002).
bFGF positively correlates with the increase of MSC CD90+CXCR4+ (r = 0.33, P = 0.0003), with CD45−CD34+CXCR4+ cells (r = 0.4, P < 0.0001) and with CD45−CD34−PDGFR+ cells (r = 0.51, P = 0.001).
The pro-angiogenic role of HGF and VEGF is confirmed by the correlation of their cytoplasmic level with the number of circulating endothelial progenitors cells CD133+CD144+ and CD133+CD144−.
SDF-1α correlates with circulating levels of MSC CD90+CXCR4+ (r = 0.39, P = 0.001), CD45−CD34+CXCR4+ (r = 0.34, P = 0.005), HSC CD90+ (r = 0.36, P = 0.003) and all the considered EPC subsets.
Finally, we evaluated the correlation between circulating BMSC and NTproBNP. As expected, a statistically significant, positive correlation exists between the levels of NTproBNP and the severity of HF (P < 0.0001, P = 0.003, P < 0.0001 for II–IV NYHA class, respectively).
NTproBNP was also associated with the recruitment of all subtype of MSCs (CD105+, r = 0.22, P = 0.015; CD90+ r = 0.27, P = 0.003; CXCR4+ r = 0.24, P = 0.009; CD90+CXCR4+, r = 0.34, P = 0.0001) and TCSC (CD45−CD34+CXCR4+, r = 0.3, P = 0.001). No correlation was observed between NTproBNP and HSCs and EPCs.
Discussion
This is a descriptive study on different classes and relative subpopulations of circulating SCs in HF. These data, never reported before in HF, are important because, according to their marker combination, circulating SCs are likely to generate a preferential progeny of cardiac cells. This, in turn, could contribute to the comprehension of the pathophysiological events associated with the progressive and irreversible evolution of HF. Our findings suggest that, in HF, the recruitment of SCs into the bloodstream is a complex phenomenon. MSCs seem to be preferentially increased in HF. This is surprising as they are less than 0.001% of total number of bone marrow mononuclear cells [20]. At present there is no accepted definition for MSCs, apart that they are non haematopoietic stem cells [21]. Therefore, MSCs are negative for markers of the haematopoietic lineage (CD34 and CD45), whereas positive for CD90 and CD105, which are important determinants for mesenchymal phenotype [22]. We found that both these subsets of cells are recruited in NYHA class IV patients. Only the CD90+CXCR4+ MSCs show an early (NYHA class II) and progressive increase which correlates with clinical (NYHA) and biological (NTproBNP) deterioration of HF, as well as with SDF-1α. This finding is relevant as the axis CXCR4-SDF-1α is considered to be crucial for homing of primitive cells to stem cells niches or to injured tissues [23]; moreover, evidence is accumulating on the importance of this axis in promoting myocardial repair after acute injury [24–26]. Another axis we have investigated is the PDGF-BB/PDGFR one. Its role in MSC recruitment is well established [27], whereas its involvement in cardiac pathology is emerging [28]. In failing patients, the significant increase in the plasmatic level of PDGF-BB is paralleled by the raise in both MSCs and TCSCs. In this latter class we have also demonstrated a correlation between the level of PDGF-BB and the TCSC subset expressing PDGFR. Therefore, both SDF-1α and PDGF-BB seem to play a relevant role in the recruitment of specific SC subsets expressing on their surface the respective receptors.
MSC plasmatic levels do not correlate with gender, aging and all the other confounding factors tested, confirming previous observations that the frequency of MSCs is not dictated by chronological age of the individual [22].
Whether the increased recruitment of MSCs constitutes a natural reparative response to chronic HF is difficult to say. MSCs are considered important for reconstructive and gene-targeting therapy [21]. Reports in animals have shown that BMSCs home to the heart and promote regeneration of the infracted myocardium in different species, possibly involving a paracrine effect [29]. However, intracoronary delivery of MSCs to dogs after infarction led mainly to the differentiation into fibroblast in the scarred region [30]. Therefore, the progressive increase with severity of the HF we found could reflect either a possible natural repairing response or a contribution to HF deterioration by inducing further fibrosis.
HSCs are multipotent and possess the properties of self-renewal and multilineage differentiation. The antigens CD34 and CD45 are commonly used to sort human HSCs co-expressing either CD90 or CD105. HSCs are significantly increased in HF patients with respect to controls, although a clear trend towards an increase with NYHA progression could not be demonstrated.
CD45+CD34+CD90+CXCR4+ cells display an emblematic behaviour because they peak in class III patients returning to control level in class IV.
A similar behaviour was found for both EPCs and circulating endothelial cells (CECs), defined, in this study, as CD45−CD133+VE-cadherin (CD144)+/− and CD45−CD133−CD144+, respectively [31]. EPCs were also investigated in the past as CD34+, CD133+ co-expressing VEGF receptor-2 and as e-CFUs (endothelial colony-forming units) [14]. We deliberately did not use this characterization as these data are already available in similar set of patients with HF [10]. Interestingly, although EPCs and CECs were significantly elevated in HF patients with respect to controls, in class IV patients EPCs their circulating levels are very low. To explain this contradictory behaviour it was suggested that increase of EPCs during early phases of HF is triggered by the HF-induced severe endothelial damage and apoptosis [10], whereas in the more advanced phase of the disease (NYHA IV) it is suppressed by TNF-α[32]. Indeed, we previously found an inverse correlation between TNF-α and CD133+[10]. Interestingly, it should be noted that in this study, TNF-α positively correlates with NYHA and with MSC CD90+, CD105+ and CD90+CXCR4+ and CD105+CXCR4+ level. The more severe the disease, the more TNF-α release, the more rise of MSCs. Thus, as for other aspect of its activity, it is possible that this cytokine exerts different action of BMSC movement: suppression for EPCs and a stimulus for MSC production and mobilization [33, 34]. A still unresolved issue is whether for clinical application is better to use undifferentiated or tissue committed stem cells (TCSCs). The latter could already express nuclear and/or cytoplasmatic-specific mRNA of the myocytes, endothelial and smooth muscle cell lineages. It has been postulated that these partially differentiated cells could exert a faster and greater regenerative response and acquire the adult phenotype more rapidly. They have been used experimentally immediately after MI and, recently, in a randomized multicentre clinical trial [6]. Although the REGENT trial failed to reach the primary end point, there was a benefit in a subset of patients with severely depressed LVEF. We found a different behaviour according to the subset of TCSCs studied. Only the CD45−CD34+CXCR4+ show a significant constant increase with the progression of the disease and a positive correlation with NTproBNP, bFGF, SDF-1α and PDGF-BB. It is tempting to speculate that the increase of cells committed for myocytes and endothelium is a natural reparative response to HF, controlled by the bone marrow via circulating cytokines. Obviously such response is insufficient as if circulating BMCs had the ability to spontaneously repair the damaged cells, HF will not progress. Interestingly, the CD45+CD34+CXCR4+, which are committed for lympho and monocytes, were not significantly increased in HF patients. It is however possible that the high level of TNF-α is responsible for the biphasic behaviour of this subpopulation of TCSCs.
There are several limitations that should be considered: the limited number of patient studied, particularly in class IV. The casual contribution of BMSCs recruitment to HF remains to be determined as well the significance of their increase.
In conclusion, both MSCs and TCSCs are the subsets of stem cells that constantly increase with severity of HF.
Acknowledgments
The authors wish to thank Simonetta Piva for performing statistical analysis and Blood Center of the University Hospital of Udine for supplying blood from healthy donors. This work was supported by a grant from ‘Programma di Ricerca Regione-Università 2007/2009’ (Regione Emilia Romagna) and grant LR26/2005 (Regione Friuli Venezia Giulia).
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 2.Li TS, Hayashi M, Ito H, et al. Regeneration of infarcted myocardium by intramyocardial implantation of ex-vivo trasforming growth factor-beta-programmed bone marrow stem cells. Circulation. 2005;111:2438–45. doi: 10.1161/01.CIR.0000167553.49133.81. [DOI] [PubMed] [Google Scholar]
- 3.Schaefer A, Meyer GP, Fuchs M, et al. Impact of intracoronary bone marrow cell transfer on diastolic function in patients after acute myocardial infarction: results from the BOOST trial. Eur Heart J. 2006;27:929–35. doi: 10.1093/eurheartj/ehi817. [DOI] [PubMed] [Google Scholar]
- 4.Meyer GP, Wollert KC, Lotz J, et al. Intracoronary bone marrow cell transfer after myocardial infarction; eighteen months’ follow-up data from the randomized, controlled BOOST (Bone marrow transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113:1287–94. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 5.Lunde K, Solheim S, Aakhus R, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;335:1199–209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 6.Tendera M, Wojakowsky W, Ruzytto W, et al. for the REGENT investigators. Intracoronary infusion of bone marrow-derived selected CD34+CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre myocardial regeneration by Intracoronary infusion of selected population of stem cells in acute myocardial infarction (REGENT) trial. Eur Heart J. 2009;30:1313–21. doi: 10.1093/eurheartj/ehp073. [DOI] [PubMed] [Google Scholar]
- 7.Beitnes JO, Hopp E, Lunde K, et al. Long-term results after intracoronary injection of autologous mononuclear bone marrow cells in acute myocardial infarction: the ASTAMI randomized, controlled study. Heart. 2009;95:1983–9. doi: 10.1136/hrt.2009.178913. [DOI] [PubMed] [Google Scholar]
- 8.Schächinger V, Assmus B, Erbs S, et al. for the REPAIR-AMI investigators. Intracoronary infusion of bone marrow-derived mononuclear cells abrogates adverse left ventricular remodeling post-acute myocardial infarction: insight from the reinfusion of enriched progenitor cells and infarct remodeling in acute myocardial infarction (REPAIR-AMI) trial. Eur J Heart Fail. 2009;11:973–9. doi: 10.1093/eurjhf/hfp113. [DOI] [PubMed] [Google Scholar]
- 9.Assmus B, Rolf A, Erbs S, et al. for the REPAIR-AMI Investigators. Clinical outcome 2 years after intracoronary administration of bone marrow-derived progenitor cells in acute myocardial infarction. Circ Heart Fail. 2010;3:89–96. doi: 10.1161/CIRCHEARTFAILURE.108.843243. [DOI] [PubMed] [Google Scholar]
- 10.Valgimigli M, Rigolin GM, Fucili A, et al. CD34+ and endothelial progenitor cells in patients with various degree of congestive heart failure. Circulation. 2004;110:1209–12. doi: 10.1161/01.CIR.0000136813.89036.21. [DOI] [PubMed] [Google Scholar]
- 11.Task Force for Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of European Society of Cardiology: Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur J Heart Fail. 2008;10:933–89. doi: 10.1016/j.ejheart.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 12.He Q, Wan C, Li G. Concise review: multipotent mesenchymal stromal cells in blood. Stem cells. 2007;25:69–77. doi: 10.1634/stemcells.2006-0335. [DOI] [PubMed] [Google Scholar]
- 13.Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am J Pathol. 2006;169:338–46. doi: 10.2353/ajpath.2006.060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peichev M, Naiyer A, Pereira D, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–8. [PubMed] [Google Scholar]
- 15.Handgretinger R, Gordon P, Leimig T, et al. Biology and plasticity of CD133+ hematopoietic stem cells. Ann N Y Acad Sci. 2003;996:141–51. doi: 10.1111/j.1749-6632.2003.tb03242.x. [DOI] [PubMed] [Google Scholar]
- 16.Dejana E, Bazzoni G, Lampugnani MG. Vascular endothelial (VE)-cadherin: only an intercellular glue. Exp Cell Res. 1999;252:13–9. doi: 10.1006/excr.1999.4601. [DOI] [PubMed] [Google Scholar]
- 17.Kucia M, Dawn B, Hunt G, et al. Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood after myocardial infarction. Circ Res. 2004;95:1191–9. doi: 10.1161/01.RES.0000150856.47324.5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petit I, Jin D, Rafii S. The SDF-1α-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol. 2007;28:299–307. doi: 10.1016/j.it.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamizo A, Marini F, Amano T, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–18. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 20.Ingram DA, Caplice NM, Yoder MC. Unresolved questions, changing definitions and novel paradigms for defining endothelial progenitor cells. Blood. 2005;106:1525–31. doi: 10.1182/blood-2005-04-1509. [DOI] [PubMed] [Google Scholar]
- 21.Pittenger M, Martin B. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 22.Leri A, Kajstura J, Anversa P. Cardiac stem cells and mechanism of myocardial generation. Physiol Rev. 2005;85:1373–1416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- 23.Pitchford SC, Furze RC, Jones CP, et al. Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell. 2009;4:62–72. doi: 10.1016/j.stem.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Zhao T, Zhang D, Millard RW, et al. Stem cell homing and angiomyogenesis in transplanted hearts are enhanced by combined intramyocardial SDF-1alpha delivery and endogenous cytokine signaling. Am J Physiol Heart Circ Physiol. 2009;296:H976–86. doi: 10.1152/ajpheart.01134.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haider H, Jiang S, Idris NM, et al. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1alpha/CXCR4 signaling to promote myocardial repair. Circ Res. 2008;103:1300–8. doi: 10.1161/CIRCRESAHA.108.186742. [DOI] [PubMed] [Google Scholar]
- 26.Abbott JD, Huang Y, Liu D, et al. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–5. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 27.Tokunaga A, Oya T, Ishii Y, et al. PDGF receptor beta is a potent regulator of mesenchymal stromal cell function. J Bone Miner Res. 2008;23:1519–28. doi: 10.1359/jbmr.080409. [DOI] [PubMed] [Google Scholar]
- 28.Das H, George JC, Joseph M, et al. Stem cell therapy with overexpressed VEGF and PDGF genes improves cardiac function in a rat infarct model. PLoS One. 2009;4:e7325. doi: 10.1371/journal.pone.0007325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 30.Vulliet PR, Greeley M, Halloran SM, et al. Intra-coronary arterial injection of the mesenchymal stromal cells and microinfarction in dogs. Lancet. 2004;363:783–4. doi: 10.1016/S0140-6736(04)15695-X. [DOI] [PubMed] [Google Scholar]
- 31.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–12. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 32.Dufour C, Corcione A, Svahn J, et al. TNF-alpha and IFN-gamma are overexpressed in the bone marrow of Fanconi anemia patients and TNF-alpha suppresses erytropoiesis in vitro. Blood. 2003;102:2053–9. doi: 10.1182/blood-2003-01-0114. [DOI] [PubMed] [Google Scholar]
- 33.Grisar J, Aletaha D, Steiner CW, et al. Endothelial progenitor cells in active rheumatoid arthritis: effects of tumour necrosis factor and glucocorticoid therapy. Ann Rheum Dis. 2007;66:1284–8. doi: 10.1136/ard.2006.066605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crisostomo PR, Wang Y, Markel TA, et al. Human mesenchymal stem cells stimulated by TNF-alpha, LPS, or hypoxia produce growth factors by an NF kappa B- but not JNK-dependent mechanism. Am J Physiol Cell Physiol. 2008;294:C675–82. doi: 10.1152/ajpcell.00437.2007. [DOI] [PubMed] [Google Scholar]


