Abstract
Despite current guidelines and the range of available treatments, over a half of patients with asthma continue to suffer from poor symptomatic control and remain at risk of future worsening. Although a number of non-pharmacological measures are crucial for good clinical management of asthma, new therapeutic controller medications will have a role in the future management of the disease. Several long-acting anticholinergic bronchodilators are under investigation or are available for the treatment of respiratory diseases, including tiotropium bromide, aclidinium bromide, glycopyrronium bromide, glycopyrrolate and umeclidinium bromide, although none is yet licensed for the treatment of asthma. A recent Phase III investigation demonstrated that the once-daily long-acting anticholinergic bronchodilator tiotropium bromide improves lung function and reduces the risk of exacerbation in patients with symptomatic asthma, despite the use of inhaled corticosteroids (ICS) and long-acting β2-agonists (LABAs). This has prompted the question of what the rationale is for long-acting anticholinergic bronchodilators in asthma. Bronchial smooth muscle contraction is the primary cause of reversible airway narrowing in asthma, and the baseline level of contraction is predominantly set by the level of ‘cholinergic tone’. Patients with asthma have increased bronchial smooth muscle tone and mucus hypersecretion, possibly as a result of elevated cholinergic activity, which anticholinergic compounds are known to reduce. Further, anticholinergic compounds may also have anti-inflammatory properties. Thus, evidence suggests that long-acting anticholinergic bronchodilators might offer benefits for the maintenance of asthma control, such as in patients failing to gain control on ICS and a LABA, or those with frequent exacerbations.
Introduction
Asthma affects over 300 million individuals worldwide, a figure that is estimated to grow by 100 million by 2025.1 A chronic inflammatory disease of the airways, asthma has multifactorial pathophysiological causes and considerable heterogeneity in the classification of the disease by phenotype, aetiology, severity and interventional control.
Current guidelines recommend stepwise management to gain and maintain control, in which the clinical definition of full ‘control’ is daytime symptoms or use of reliever medication less than twice a week, no limitations of activity, no nocturnal symptoms and normal lung function.2 Furthermore, the American Thoracic Society and the European Respiratory Society state that any definition or measure of control must take into account the management of a patient’s future risk.3 Thus, in clinical management of asthma, consideration must be given to reducing the frequency of exacerbations, preserving lung function, preventing reduced lung growth in children and minimising the adverse effects of any treatment.4
For those receiving low-dose inhaled corticosteroids (ICS), current step-up treatment involves the addition of a long-acting β2-agonist (LABA) or leukotriene receptor antagonist as controller therapy. In patients unable to attain or maintain control with ICS and LABA—those in Global Initiative for Asthma treatment steps 3–5 (Figure 1)—upward titration of ICS dose, leukotriene modifiers, sustained-release theophylline, oral glucocorticosteroids and anti-immunoglobulin E (omalizumab) are all further or alternative treatment options.2
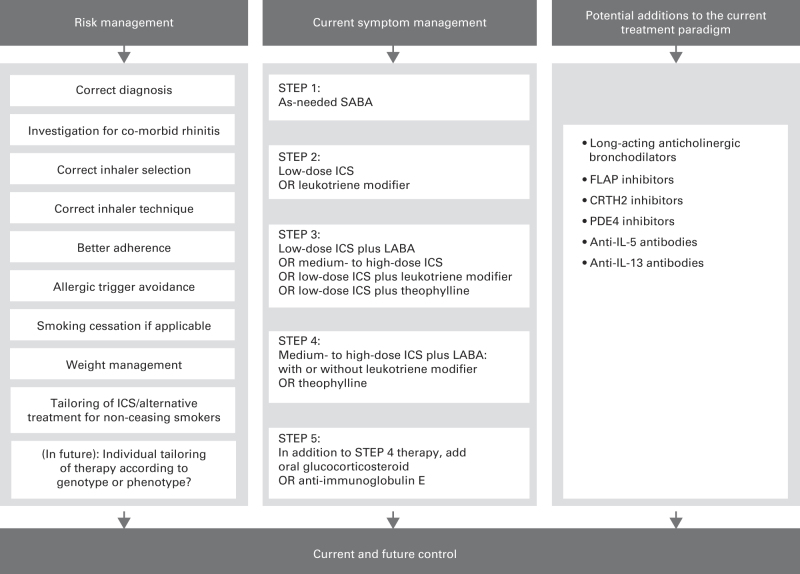
Figure 1.
Combined approaches for the management of control in asthma.2,5,10–16 FLAP, 5-lipoxygenase-activating protein; ICS, inhaled corticosteroids; IL, interleukin; LABA, long-acting β2-agonist; PDE4, phosphodiesterase-4; SABA, short-acting β2-agonist.
Despite these guidelines and the wide range of therapies available, poor control of current asthma symptoms, and of future asthma exacerbations, continues to affect >50% of patients,5–9 with exacerbations placing significant strain on their quality of life and on health-care systems.10 Risk factors associated with future exacerbations include previous exacerbations, poor control, inhaler technique and adherence, co-morbid allergic rhinitis, gastro-oesophageal reflux disease, psychological dysfunction, smoking and obesity.10 The same factors, in addition to incorrect diagnosis, poor choice of inhaler, variation in individual treatment responses or genetic components, have been attributed to the underlying poor control.11 There are a number of actions available in the primary care setting to reduce the impact of these factors (Figure 1).10,11
In the light of such concerns around risk and poor control, it is appropriate to consider the rationale for investigating additional controller medications. A number of new therapies are under investigation,12 including long-acting anticholinergic bronchodilators (the focus of this review), anti-prostaglandin D2 CRTH2 antagonists,13 phosphodiesterase-4 inhibitors,5 anti-leukotriene 5-lipoxygenase-activating protein antagonists14 and the monoclonal antibodies mepolizumab and lebrikizumab (which are raised against interleukin-515 and interleukin-13,16 respectively).
Short-acting anticholinergic agents, particularly ipratropium bromide (ipratropium) and oxitropium bromide (oxitropium), have been used in asthma for many years,17,18 although they have not become widespread because they are generally considered to be less effective than short-acting β2-agonists (SABAs) for acute bronchodilation.17 This, coupled with a perception that longer-term antagonism of cholinergic receptors induces little bronchodilation above that induced by LABAs,19,20 has meant that, in contrast to chronic obstructive pulmonary disease,21,22 long-acting anticholinergic bronchodilators have not been considered or thoroughly investigated as potential controller medication in asthma. Early studies demonstrated mild bronchodilation and protection, over 48 h, against methacholine-induced bronchoconstriction in male patients with asthma,23 and, in patients with severe persistent asthma, small improvements in lung function were observed with the LABA salmeterol plus the long-acting anticholinergic bronchodilator tiotropium bromide (tiotropium), with a halved dose of fluticasone propionate.24
Recently, Phase I–III clinical investigation with long-acting anticholinergic bronchodilators in asthma has begun: two Phase II trials of umeclidinium bromide (umeclidinium) have completed (NCT01641692; NCT01573624), and Phase II and III trials with tiotropium, as add-on therapy, have demonstrated improvements in lung function and a reduction in exacerbation risk in patients with poorly controlled asthma despite the use of ICS or ICS plus a LABA.25–28
In this review, we consider the pathophysiological and clinical rationales for use of long-acting anticholinergic agents in the broader management of asthma, and the clinical evidence reported to date. Please see Box 1 for a description of the literature search and appraisal methods.
Literature evaluation methods.
Clinical evidence around long-acting anticholinergic bronchodilators
We performed searches in November 2013 of PubMed, Google Scholar and Cochrane databases and ClinicalTrials.gov (www.clinicaltrials.gov).
PubMed searches
All terms restricted to title and abstract, with restriction of results to clinical trials:
(1) Asthma* AND (anticholinergic OR antimuscarinic OR cholinergic OR muscarinic OR parasympathetic)
(2) Asthma* AND (tiotropium OR umeclidinium OR aclidinium OR glycopyrronium OR darotropium OR QVA149 OR glycopyrrolate)
In November 2013 the searches yielded 209 results; search 2 yielded 25 results. PubMed search results were manually reviewed for articles or studies relevant to the topic of short-acting muscarinic agonists or long-acting muscarinic agonists for acute or maintenance therapy
Cochrane database searches
‘Asthma AND anticholinergic’, limited to title, abstract and keywords, yielding 39 hits, the titles and abstracts of which were manually reviewed
In November 2013 the searches yielded one review19 relating to the use of anticholinergics for asthma management, and eight reviews of anticholinergics in a variety of acute settings
www.clinicaltrials.gov searches
Asthma AND tiotropium OR umeclidinium OR aclidinium OR glycopyrronium OR darotropium OR glycopyrrolate OR QVA149
Pathophysiology and pharmacology
PubMed and Google scholar searches
The following terms in Boolean strings: asthma; respiratory; cholinergic; muscarinic; parasympathetic; autonomic; tone; pathophysiology; anticholinergic; antimuscarinic; β-agonist; phenotype; genotype; inflammation; bronchoconstriction; and bronchodilation
As this article is not a systematic review, certain articles within the pathophysiology and pharmacology sections were reviewed and cited based on their adjudged relevance to the topic
The Role of Cholinergic Activity in the Pathophysiology of Asthma
The symptoms of asthma, and of acute exacerbations, are attributed to airway narrowing that occurs as a consequence of chronic inflammation and associated hyper-responsiveness.2 Local influx of inflammatory cells and high levels of inflammatory mediators result in airway oedema, airway thickening, mucus hypersecretion and bronchial smooth muscle contraction (Table 1).2 Although multiple pathophysiological mechanisms are thought to contribute to the characteristic narrowing of airways and the hyper-responsiveness found in asthma (Table 1),2 bronchial smooth muscle contraction represents the primary cause of reversible airway obstruction in asthma.29,30 The degree of basal airway smooth muscle contraction (airway smooth muscle ‘tone’) is under autonomic nervous regulation (Figure 2), although the mechanisms are not fully understood. During normal ventilation, adrenergic sympathetic nerves and parasympathetic cholinergic and non-cholinergic nerves are all active,29,31,32 but cholinergic activity is thought to be the predominant driver of bronchoconstriction (Figure 2, Box 2).31 Acute treatment with the anticholinergic compounds atropine and ipratropium is known to reduce basal airway smooth muscle tone.33,34
Table 1. Mechanisms of airway narrowing and hyper-responsiveness in asthma2 .
| Process | Consequence |
|---|---|
| Increased volume and/or contractility of airway smooth muscle cells | Excessive contractility of airway smooth muscle |
| Secretion of multiple bronchoconstriction mediators such as histamine, prostaglandin D2 and neurotransmitters | Airway smooth muscle contraction |
| Uncoupling of airway smooth muscle contraction as a result of inflammatory changes in the airway wall | Excessive narrowing of the airways; loss of maximum plateau of contraction when a bronchodilator is administered |
| Oedema due to microvascular leakage in response to inflammatory mediators and structural changes to airway smooth muscle | Thickening of airway wall; amplification of airway narrowing due to contraction of airway smooth muscle for geometric reasons |
| Sensitisation of sensory nerves leading to afferent activity and autonomic reflex | Increased parasympathetic, cholinergic and airway smooth muscle tone, with consequent exaggerated bronchoconstriction in response to sensory stimuli |
Figure 2.
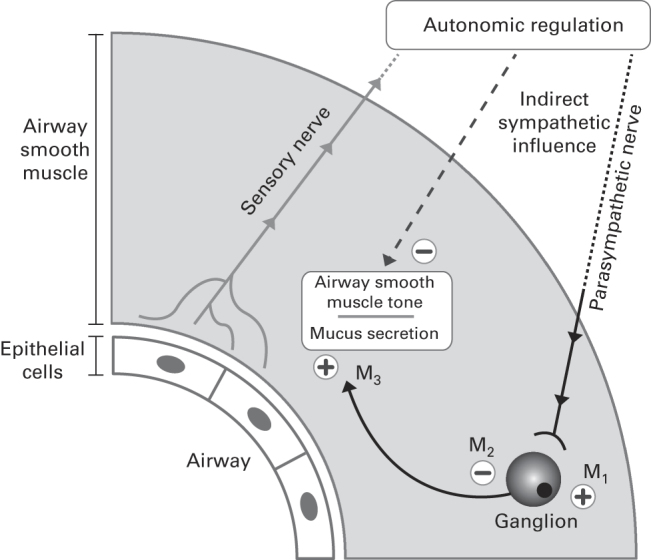
Autonomic regulation of airway smooth muscle tone.29,32,49,50 M1, M2, M3, muscarinic acetylcholine receptors 1, 2 and 3. + and − symbols represent signals increasing and decreasing airway smooth muscle tone, respectively. Note that non-adrenergic non-cholinergic autonomic pathways have been omitted for simplicity. Adapted from the study by Cazzola, et al.,32 with permission from the American Society for Pharmacology and Experimental Therapeutics.
Possible pathophysiological reasons why long-acting anticholinergic bronchodilators may be beneficial for the control of asthma.
Cholinergic activity is the predominant driver of bronchial smooth muscle contraction, the primary cause of reversible airway obstruction in asthma29–31
Patients with asthma have increased basal airway smooth muscle tone, possibly as a result of increased cholinergic tone30,35
Acute treatment with anticholinergic compounds reduces basal airway smooth muscle tone33,34
Local airway inflammatory mediators may have a role in inducing increased cholinergic tone29,31,36–39
Cholinergic activity may have a prominent role in airway smooth muscle remodelling17,46,47
Cholinergic receptors on lung submucosal cells regulate mucus secretion49,50,52
Increased cholinergic and smooth muscle tone may contribute to airway hyper-responsiveness39,44,45
Cholinergic antagonists may have non-neuronal anti-inflammatory actions51
Patients with asthma may have abnormal muscarinic receptor expression40
Patients with asthma may have increased release of acetylcholine from cholinergic nerve endings41
Patients with asthma may have reduced levels of neuromodulators that attenuate cholinergic neurotransmission42,43
Patients with asthma have increased basal airway smooth muscle tone,35 and there is evidence to suggest that this is a result of increased basal activity of pulmonary parasympathetic cholinergic nerves, hereinafter described as ‘cholinergic tone’. Molfino et al. 30 demonstrated that bronchoconstriction induced by breath-holding is significantly inhibited by ipratropium in asthmatic patients but not in healthy volunteers. It is thought that cholinergic tone, at least, is driven by afferent nervous activity arising in the airways,29,36,37 and it has been hypothesised that local airway inflammatory mediators may have a role in inducing afferent activity and an autonomic reflex response, thereby driving an increase in cholinergic tone (Figure 2).31,38,39 Other proposed mechanisms for increased cholinergic tone in asthmatic patients include abnormal muscarinic receptor expression,40 increased release of acetylcholine from cholinergic nerve endings41 and reduced levels of neuromodulators that attenuate cholinergic neurotransmission.42,43
The degree to which cholinergic tone contributes to airway narrowing in asthma, either at basal state or during exacerbations, is unclear. However, the fact that airway hyper-responsiveness can persist in asthmatic patients, possibly even in the absence of airway inflammation following long-term ICS use,44 suggests that other pathophysiological factors, such as increased cholinergic and smooth muscle tone, have a role in asthma.39,45 It has been proposed that acetylcholine has a prominent role in allergen-induced airway smooth muscle remodelling.46–48 In a guinea pig model of ongoing allergic asthma, treatment with tiotropium inhibited increases in airway smooth muscle mass and contractility induced by allergic challenge; it has thus been hypothesised that anticholinergic drugs could help prevent airway smooth muscle remodelling in human asthma.17
Cholinergic activity is also believed to regulate non-smooth muscle and non-neuronal cells within the lungs, including inflammatory cells and those controlling mucus secretion.49,50 In a guinea pig model, tiotropium was shown to reduce allergen-induced mucus gland hypertrophy and goblet cell number,50 suggesting that anticholinergic bronchodilators might also reduce airflow obstruction by reducing mucus hypersecretion. Expression of cholinergic receptors on inflammatory cells raises the additional question of whether there are any non-neuronal anti-inflammatory actions of cholinergic antagonists, although a review of studies on chronic obstructive pulmonary disease failed to identify robust evidence of this.51
Pharmacology of Anticholinergic Bronchodilators
Anticholinergic bronchodilators are antagonistic to parasympathetic activity and exert their effects on acetylcholine receptors on airway smooth muscle and pulmonary parasympathetic nerves (Figure 2). Acetylcholine receptors fall into two families—nicotinic and muscarinic—and it is the M1, M2 and M3 subtypes of the latter that are thought to be primarily involved in the regulation of bronchoconstriction.17 All subtypes of muscarinic receptors are widely expressed in the brain, the parasympathetic nervous system and the body’s smooth muscle tissues. M1 receptors are broadly distributed throughout the parasympathetic ganglia and regulate cholinergic transmission. M2 receptors are found in prejunctional membranes of neuromuscular junctions of airway smooth muscle and regulate negative feedback to reduce acetylcholine transmission. In a pulmonary context, M3 receptors are predominantly expressed in smooth muscle cells, where they regulate contraction, and also within lung submucosal glands, where they regulate mucus secretion52 (Figure 2). Thus, it is preferable for antimuscarinic bronchodilators to have a relatively high affinity for M1 and M3 receptors and low affinity for the M2 receptor.17
Currently, there are five anticholinergic drugs available for bronchodilation in respiratory disease. Ipratropium and oxitropium are short-acting non-selective antagonists of M1, M2 and M3 receptors.53 In contrast, tiotropium, aclidinium bromide (aclidinium) and glycopyrronium bromide (glycopyrronium) are long-acting compounds, with comparative selectivity for the M1/M3, M2/M3 and M3 receptors, respectively.17,53,54
Short-acting anticholinergics are generally considered less effective acute bronchodilators than SABAs, and their short duration of action makes them broadly unsuitable as controller medication. Thus, evidence of increased cholinergic tone in patients with asthma indicates that the longer-acting bronchodilator compounds may be more suitable as controller medications in asthma.
There is some rationale to suggest that the addition of long-acting anticholinergic bronchodilators to LABAs might provide advantages in the treatment of asthma (Box 2). It is reasonable to hypothesise that by simultaneously antagonising parasympathetic smooth muscle contraction and stimulating adrenergic smooth muscle relaxation, it is possible to achieve greater bronchodilation compared with either strategy in isolation. To date, there has been little thorough clinical investigation of this hypothesis in asthma, but a study in a guinea pig model found that bronchodilation induced by the LABA carmoterol was significantly augmented by the addition of tiotropium.55 In vitro studies have also found that the LABA indacaterol can synergistically increase the inhibitory effects of glycopyrronium on methacholine-induced airway smooth muscle contraction.56 As discussed below, improvements in lung function have been observed in asthmatic patients receiving tiotropium as add-on therapy to LABA plus ICS.26,28
It has been suggested that anticholinergic/LABA combination therapy might offer advantages in mitigating daily variation, based on evidence that sympathetic activity may be elevated during the daytime, relative to the parasympathetic system, which may predominate at night.57–60 For example, it was shown in a small study in patients with nocturnal asthma that ipratropium is more effective than salbutamol in the prevention of morning reductions in peak expiratory flow.59 It is also possible that a combined approach to bronchodilation might reduce the impact of inter-patient variability in the relative responses to anticholinergic or adrenergic interventions. Finally, tachyphylaxis to the effects of β-agonists is known to occur (although the clinical relevance of this remains unclear),61–63 and it has been proposed that crosstalk between muscarinic receptor signalling and adrenergic receptor signalling in smooth muscle cells might interfere with tachyphylactic mechanisms. This would provide a further rationale for the investigation of LABA/long-acting anticholinergic bronchodilator combination therapy in asthma, as add-on to ICS.64
Clinical Evidence of Anticholinergic Bronchodilators in Asthma
Historically, short-acting anticholinergic bronchodilators have not been considered appropriate for the control of asthma, except in some cases for the acute treatment of asthma attacks in patients with chronic stable asthma,17,18 and in those who experience adverse events from SABAs, such as tachycardia, arrhythmia and tremor.1,43 Although short-acting anticholinergics are considered less effective rapid bronchodilators than SABAs such as salbutamol,17,19 there are data to suggest that, for acute exacerbations, ipratropium in combination with a SABA as reliever medication improves lung function to a greater extent than a SABA alone.34,65,66 In a double-blind, randomised trial, Rodrigo and Rodrigo65 investigated the effects of high-dose ipratropium plus the SABA albuterol (registered generic name for salbutamol in the USA) in adults with acute asthma, in the emergency department. Patients receiving high-dose ipratropium plus albuterol had a greater improvement in peak expiratory flow and forced expiratory volume in 1 s compared with patients who received albuterol alone. The risk of hospital admission was 49% lower in the ipratropium/albuterol arm.65 Further, a meta-analysis has indicated that the addition of a short-acting anticholinergic to a SABA is associated with a significant reduction in the risk of hospitalisation in children.67 Thus, in adults or children, the main justification for the use of short-acting anticholinergic drugs in acute asthma is reduction of the elevated airway smooth muscle and cholinergic tone during an acute crisis, although administration of multiple doses has been associated with a reduction in hospitalisations and risk of hospitalisation.34,65–68
Although tiotropium has been indicated for the treatment of chronic obstructive pulmonary disease for over a decade, no long-acting anticholinergic bronchodilators are currently approved in asthma. A number of compounds exist, including aclidinium, glycopyrronium, glycopyrrolate and darotropium bromide, but, as mentioned, presently only tiotropium and umeclidinium have clinical trials in asthma listed on ClinicalTrials.gov. The latter has been under investigation in two dose-ranging Phase II trials in patients with asthma, as a monotherapy (NCT01641692) and in combination with fluticasone furoate (NCT01573624), although to our knowledge no results from these trials have yet been published.
Early studies with long-acting anticholinergics in asthma were small and underpowered, and failed to detect meaningful responses. However, studies of tiotropium and of glycopyrrolate indicated that long-acting anticholinergics can provide sustained bronchodilation and bronchoprotection.23,24,69,70
To date, more thorough clinical evaluation has been performed with tiotropium only, in six Phase II or III studies, involving over 3,500 patients (Table 2). In an investigator-initiated three-way crossover trial (14 weeks per treatment) in 210 patients with asthma inadequately controlled by low-dose ICS (twice-daily beclomethasone 80 μg), tiotropium delivered via the Spiriva HandiHaler device (Boehringer Ingelheim Pharmaceuticals, Ridgefield, CT, USA) was shown to be superior to a doubling of ICS dose and equal to the addition of salmeterol, as assessed by improvements in lung function (Table 2).27
Table 2. Comparison of lung function and clinical findings from clinical trialsa with long-acting anticholinergic bronchodilators in asthma.
| Authors | Severity b | Duration per treatment, weeks | N | Study drug(s) | Comparator(s) | Primary and key secondary end points | Difference from comparator c |
|---|---|---|---|---|---|---|---|
| Peters et al. 27 | Mild to moderate asthma inadequately controlled by low-dose ICS | 14 | 210 | Once-daily tiotropium 18 μg, via Spiriva HandiHaler | Doubling ICS dose | Morning PEF | 25.8 l/min (95% CI: 14.4–37.1; P<0.001) |
| Doubling ICS dose | Daily symptom score | −0.11 points (P<0.001) | |||||
| Salmeterol | Morning PEF | No significant difference | |||||
| Salmeterol | Daily symptom score | No significant difference | |||||
| Kerstjens et al. 26 | Severe asthma inadequately controlled by high-dose ICS + LABA | 8 | 107 | Once-daily tiotropium 5 μg, via Respimat SoftMist | Placebo | Tiotropium 5 μg, peak FEV1 | 139 ml (95% CI: 96–181; P<0.0001) |
| Asthma-related health status or symptoms | No significant difference | ||||||
| Once-daily tiotropium 10 μg, via Respimat SoftMist | Tiotropium 10 μg, peak FEV1 | 170 ml (95% CI: 128–213; P<0.001) | |||||
| Asthma-related health status or symptoms | No significant difference | ||||||
| Bateman et al. 25 | Mild to moderate asthma uncontrolled by ICS alone | 16 | 38 | Once-daily tiotropium 5 μg, via Respimat SoftMist | Placebo (following run-in with salmeterol)d | Morning pre-dose PEF | −20.70 l/min (95% CI: −33.24 to −8.16; P=0.001 for superiority) |
| Salmeterol (following run-in with salmeterol)d | Morning pre-dose PEF | −0.78 l/min (95% CI: −13.096 to 11.530; P=0.002 for non-inferiority) | |||||
| Kerstjens et al. 28 | Poorly controlled asthma despite use of ICS + LABA | 48 | 912 | Once-daily tiotropium 5 μg, via Respimat SoftMist | Placebo | Peak FEV1 at week 24 | 86±34 ml (P=0.01) (trial 1); 154±32 ml (P<0.001) (trial 2) |
| Trough FEV1 at week 24 | 88±31 ml (P=0.01) (trial 1); 111±30 ml (P=0.001) (trial 2) | ||||||
| Reduction in risk of severe exacerbation at week 48 | 21% (hazard ratio 0.79; P<0.03) (pooled population) | ||||||
| Difference in AQLQ | 0.04 units, NS (trial 1)e 0.18 units, P=0.02 (trial 2)e | ||||||
| Difference in ACQ-7 | −0.13, NS (trial 1)e −0.2, P=0.003 (trial 2)e |
Abbreviations: ACQ-7, seven-question Asthma Control Questionnaire; AQLQ, Asthma Quality of Life Questionnaire; CI, confidence interval; FEV1, forced expiratory volume in 1 s; ICS, inhaled corticosteroids; LABA, long-acting β2-agonist; NS, not significant; PEF, peak expiratory flow.
Only studies published in journal primary publication format have been included (Kerstjens et al. 76,77 and Beeh et al. 75 not shown).
All studies were in adults.
All lung function values are mean change from baseline, unless otherwise stated.
Active treatments were evaluated as maintenance therapies following a 4-week run-in period with salmeterol.
Minimal clinically important difference not achieved.
Subsequent published investigations of tiotropium have all involved administration via the Respimat SoftMist inhaler (Boehringer Ingelheim Pharma, Ingelheim am Rhein, Germany). In an 8-week crossover trial, once-daily tiotropium at a dose of 5 or 10 μg improved lung function, compared with placebo, in 107 patients with severe persistent poorly controlled asthma already receiving ICS and LABA (Table 2).26 In a 16-week trial in patients with arginine/arginine homozygosity at amino acid 16 of the β2-adrenergic receptor (B16-Arg/Arg) and moderate poorly controlled asthma (already receiving ICS), once-daily tiotropium at a dose of 5 μg was superior to placebo and non-inferior to twice-daily salmeterol at a dose of 50 μg for maintenance of improvements in lung function (Table 2).25 The rationale for performing the latter study was based on suggestions that the adverse-event profile of β2-agonists is worse, and the efficacy lower, in patients with the B16-Arg/Arg polymorphism,71,72 although prospective investigation has revealed that there are no such concerns.73,74 A subsequent Phase II dose-ranging study tested tiotropium at doses of 5 μg, 2.5 μg and 1.25 μg as add-on to ICS and found the 5 μg dose to provide the greatest bronchodilator effect.75
Data from the first Phase III trial on a long-acting anticholinergic bronchodilator in asthma were published in 2012.28 In two replicate trials including a total of 912 patients with poorly controlled asthma despite the use of LABA and high-dose ICS (⩾800 μg budesonide or equivalent), tiotropium 5 μg administered via the Respimat SoftMist inhaler as add-on therapy significantly reduced the risk of severe exacerbations compared with placebo (values provided in Table 2). Small but statistically significant improvements in lung function were also observed.28 Surprisingly, given the changes in lung function and exacerbation rate, improvements in symptomatic benefit (seven-question Asthma Control Questionnaire [ACQ-7] and Asthma Quality of Life Questionnaire) were small and inconsistent. Of adverse events reported in ⩾2% of patients, only allergic rhinitis occurred at a statistically significantly higher rate in the tiotropium group compared with the placebo group. Dry mouth, a typical side effect associated with anticholinergic drugs, was reported in ⩽2% of patients.28
More recently, a Phase III replicate trial of once-daily tiotropium at a dose of 5 or 2.5 μg, versus placebo, as add-on to medium-dose ICS (400–800 μg budesonide or equivalent) was conducted in 2,103 patients with poorly controlled asthma.76,77 An active comparator arm of salmeterol 50 μg versus placebo was also included. Again, statistically significant improvements in lung function were observed with tiotropium, which were comparable in magnitude with those seen with salmeterol. A statistically significant improvement over placebo in ACQ-7 responder rate was observed in all three active arms, although, as is common in analyses of ACQ-7 in asthma clinical trials,78 there was also a large placebo effect.76,77 We await the full primary publication from this trial.
Is there a role for long-acting Anticholinergic Bronchodilators in Asthma?
Is it possible to determine to which patients, and in which clinical situations, long-acting anticholinergic bronchodilators might offer clinical benefits? Phase III investigation has found that tiotropium add-on therapy offers advantages to adults with severe asthma who are failing to gain control on ICS and LABA combinations.28 This, and the fact that the benefit:risk ratio of ICS falls at high ICS doses,2,7,17,79,80 suggests that addition of long-acting anticholinergic bronchodilators to ICS plus a LABA is likely to be a useful option for patients with poorly controlled severe asthma, and an alternative to further increases in ICS dose.
Whether long-acting anticholinergics will be appropriate as alternatives to LABAs is a harder question to answer. Nevertheless, tiotropium add-on to medium-dose ICS has been shown to provide lung function and ACQ-7 improvements that were comparable with those of salmeterol,76,77 indicating that, in patients for whom LABAs may be unsuitable, long-acting anticholinergics could be a helpful alternative.
Although the ACQ-7 effects reported in clinical trials thus far are relatively small, it will be interesting to see to what extent in practice patients gain clinically relevant benefits in control or future risk. Further, one might expect that the demonstrated reduction of exacerbation risk with tiotropium as add-on to ICS plus LABA28 might translate into long-term improvements in overall control. We anticipate that lung function improvements of the magnitude observed in the trials we have described will translate into clinically relevant benefits to patients in a real-world setting. At the time of writing, few real-world studies have been performed, as long-acting anticholinergic bronchodilators are yet to be approved in asthma. However, in a retrospective study of the UK Optimum Patient Care Research Database, off-label use of tiotropium in patients predominantly in Global Initiative for Asthma step 3 or 4 was found to be associated with a reduction in the number of exacerbations and a reduced risk of severe exacerbation or lower respiratory tract infection.81
It is yet to be determined in Phase III investigation whether long-acting anticholinergic bronchodilators offer similar benefits to adults with mild asthma or to children or adolescents, although several Phase III trials are underway with tiotropium in these populations (NCT01316380; NCT01634139; NCT01634152; NCT01277523).
There are some physiological (Box 2) and clinical rationales that allow us to suggest groups of patients for whom long-acting anticholinergic bronchodilators might be appropriate. A few small studies with short-acting anticholinergic bronchodilators have indicated that responses to anticholinergics are more likely in older patients82,83 or in those with intrinsic (non-allergic) asthma.84 It has also been suggested that patients intolerant of β2-adrenergic agents or with nocturnal asthma might respond better to anticholinergic bronchodilators.17 Further, there is evidence that patients with non-eosinophilic sputum profiles85,86 or neutrophilic inflammation do not gain the same benefit from ICS as those with eosinophilic inflammation,87 and hence may be candidates for additional treatments such as long-acting anticholinergic bronchodilators, as may groups in which steroid resistance is known to occur, such as smokers or obese patients.88,89
It is currently unclear why long-acting anticholinergic bronchodilators might reduce the rate of exacerbations. However, one can hypothesise that a contributing factor to exacerbations might be an increase in afferent sensory nerve activity, resulting in an increase in parasympathetic tone and subsequent bronchoconstriction. If this were the case, treatment with long-acting anticholinergic therapies may attenuate such autonomic effects and provide additional bronchodilation.
Conclusions
It has long been apparent from clinical and preclinical investigations of the pathophysiology of asthma that cholinergic parasympathetic tone contributes to contraction of bronchial smooth muscle and narrowing of the airways. The extent to which increased parasympathetic tone is a consequence of reflex to the inflammatory state or is a pathophysiological mechanism in itself is unclear. Regardless, the raised parasympathetic tone does provide a rationale for the use of long-acting anticholinergic bronchodilators in asthma, and recent Phase III trial results have demonstrated clinical benefits and lung function improvements with tiotropium as add-on therapy to ICS alone or ICS plus LABA in adult patients with poorly controlled asthma. In light of the evidence, we believe that anticholinergic bronchodilators will be a useful add-on therapy for patients at high risk of future worsening or exacerbations, and in patients whose asthma remains uncontrolled on a broad range of treatments and/or for whom other alternative therapies are unsuitable.
Whether tiotropium or other long-acting anticholinergic bronchodilators will offer clinical advantages in younger patients, or in those with less severe asthma than studied thus far, is under investigation. As we gain clinical experience in asthma with long-acting anticholinergics, if approved, it will be interesting to see whether and to what extent certain subgroups and phenotypes benefit from their use as controller medications.
Acknowledgments
The authors acknowledge the medical writing assistance received from Sam Yarwood, PhD, of Complete HealthVizion, in the form of literature searches and preparation and revision of the draft manuscript.
DP: a member of advisory boards for Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Meda, Merck, Mundipharma, Napp, Novartis, Nycomed, Pfizer, Sandoz and Teva; grants and support for research in respiratory disease from the following organisations in the past 5 years: UK National Health Service, Aerocrine, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Merck, Mundipharma, Novartis, Nycomed, Orion, Pfizer and Teva; consultancy for Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Meda, Merck, Mundipharma, Napp, Novartis, Nycomed, Pfizer, Sandoz and Teva; speaker fees from Activaero, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Merck, Mundipharma, Novartis, Pfizer and Teva; payment for manuscript preparation from Merck, Mundipharma and Teva; payment for the development of educational materials from GlaxoSmithKline; stock/stock options in AKL International; payment for travel/accommodations/meeting expenses from Boehringer Ingelheim, Mundipharma, Napp and Novartis. LF: speaker bureau for Boehringer Ingelheim. AK: advisory boards or speaker bureau for AstraZeneca, Boehringer Ingelheim, Merck Frosst, Novartis, Pfizer, Purdue, Sanofi and Takeda. TvdM: research grants from Almirall, AstraZeneca, GlaxoSmithKline, MSD and Nycomed; consultancy fees for advisory boards from Almirall, AstraZeneca, MDS, Novartis and Nycomed; speaker fees from AstraZeneca, GlaxoSmithKline, MDS, Novartis and Nycomed. MRR: consultancy for Almirall, Boehringer Ingelheim, Chiesi and Novartis; speaker fees from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline and Novartis.
References
- Masoli M, Fabian D, Holt S, Beasley R, The Global Initiative for Asthma . Global burden of asthma. Available at http://www.ginasthma.org/local/uploads/files/GINABurdenReport_1.pdf (accessed 6 December 2013).
- Global Initiative for Asthma . Global strategy for asthma management and prevention. Updated 2012. Available at http://www.ginasthma.org/local/uploads/files/GINA_Report_2012Feb13.pdf (accessed 6 December 2013).
- Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute . Expert Panel Report 3: Guidelines for the diagnosis and management of asthma. Available at http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm (accessed 15 November 2013).
- Barnes PJ. New therapies for asthma: is there any progress? Trends Pharmacol Sci. 2010;31:335–343. doi: 10.1016/j.tips.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Canonica GW, Baena-Cagnani CE, Blaiss MS, Dahl R, Kaliner MA, Valovirta EJ, GAPP Survey Working Group Unmet needs in asthma: Global Asthma Physician and Patient (GAPP) Survey: global adult findings. Allergy. 2007;62:668–674. doi: 10.1111/j.1398-9995.2007.01352.x. [DOI] [PubMed] [Google Scholar]
- Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med. 2004;170:836–844. doi: 10.1164/rccm.200401-033OC. [DOI] [PubMed] [Google Scholar]
- Adams RJ, Fuhlbrigge A, Guilbert T, Lozano P, Martinez F. Inadequate use of asthma medication in the United States: results of the asthma in America national population survey. J Allergy Clin Immunol. 2002;110:58–64. doi: 10.1067/mai.2002.125489. [DOI] [PubMed] [Google Scholar]
- Partridge MR, van der Molen T, Myrseth SE, Busse WW. Attitudes and actions of asthma patients on regular maintenance therapy: the INSPIRE study. BMC Pulm Med. 2006;6:13. doi: 10.1186/1471-2466-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims EJ, Price D, Haughney J, Ryan D, Thomas M. Current control and future risk in asthma management. Allergy Asthma Immunol Res. 2011;3:217–225. doi: 10.4168/aair.2011.3.4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughney J, Price D, Kaplan A, Chrystyn H, Horne R, May N. Achieving asthma control in practice: understanding the reasons for poor control. Respir Med. 2008;102:1681–1693. doi: 10.1016/j.rmed.2008.08.003. [DOI] [PubMed] [Google Scholar]
- O'Byrne PM, Naji N, Gauvreau GM. Severe asthma: future treatments. Clin Exp Allergy. 2012;42:706–711. doi: 10.1111/j.1365-2222.2012.03965.x. [DOI] [PubMed] [Google Scholar]
- Barnes N, Pavord I, Chuchalin A, Bell J, Hunter M, Lewis T. A randomized, double-blind, placebo-controlled study of the CRTH2 antagonist OC000459 in moderate persistent asthma. Clin Exp Allergy. 2012;42:38–48. doi: 10.1111/j.1365-2222.2011.03813.x. [DOI] [PubMed] [Google Scholar]
- Iwona S, Tomasz G. Antileukotriene treatment in children with asthma - new patents. Recent Pat Inflamm Allergy Drug Discov. 2008;2:202–211. doi: 10.2174/187221308786241938. [DOI] [PubMed] [Google Scholar]
- Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- Corren J, Lemanske RF, Jr, Hanania NA, Korenblat PE, Parsey MV, Arron JR. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–1098. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- Restrepo RD. Use of inhaled anticholinergic agents in obstructive airway disease. Respir Care. 2007;52:833–851. [PubMed] [Google Scholar]
- Meier CR, Jick H. Drug use and pulmonary death rates in increasingly symptomatic asthma patients in the UK. Thorax. 1997;52:612–617. doi: 10.1136/thx.52.7.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westby MJ, Benson MK, Gibson PG. Anticholinergic agents for chronic asthma in adults. Cochrane Database Syst Rev. 2004. [DOI] [PMC free article] [PubMed]
- Novelli F, Malagrinò L, Dente FL, Paggiaro P. Efficacy of anticholinergic drugs in asthma. Expert Rev Respir Med. 2012;6:309–319. doi: 10.1586/ers.12.27. [DOI] [PubMed] [Google Scholar]
- Vogelmeier C, Hederer B, Glaab T, Schmidt H, Rutten-van Mölken MP, Beeh KM. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364:1093–1103. doi: 10.1056/NEJMoa1008378. [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- O'Connor BJ, Towse LJ, Barnes PJ. Prolonged effect of tiotropium bromide on methacholine-induced bronchoconstriction in asthma. Am J Respir Crit Care Med. 1996;154 (4 Pt 1):876–880. doi: 10.1164/ajrccm.154.4.8887578. [DOI] [PubMed] [Google Scholar]
- Fardon T, Haggart K, Lee DKC, Lipworth BJ. A proof of concept study to evaluate stepping down the dose of fluticasone in combination with salmeterol and tiotropium in severe persistent asthma. Respir Med. 2007;101:1218–1228. doi: 10.1016/j.rmed.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Bateman ED, Kornmann O, Schmidt P, Pivovarova A, Engel M, Fabbri LM. Tiotropium is noninferior to salmeterol in maintaining improved lung function in B16-Arg/Arg patients with asthma. J Allergy Clin Immunol. 2011;128:315–322. doi: 10.1016/j.jaci.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Kerstjens HAM, Disse B, Schröder-Babo W, Bantje TA, Gahlemann M, Sigmund R. Tiotropium improves lung function in patients with severe uncontrolled asthma: a randomized controlled trial. J Allergy Clin Immunol. 2011;128:308–314. doi: 10.1016/j.jaci.2011.04.039. [DOI] [PubMed] [Google Scholar]
- Peters SP, Kunselman SJ, Icitovic N, Moore WC, Pascual R, Ameredes BT. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715–1726. doi: 10.1056/NEJMoa1008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstjens HAM, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med. 2012;367:1198–1207. doi: 10.1056/NEJMoa1208606. [DOI] [PubMed] [Google Scholar]
- Canning BJ. Reflex regulation of airway smooth muscle tone. J Appl Physiol. 2006;101:971–985. doi: 10.1152/japplphysiol.00313.2006. [DOI] [PubMed] [Google Scholar]
- Molfino NA, Slutsky AS, Julià-Serdà G, Hoffstein V, Szalai JP, Chapman KR. Assessment of airway tone in asthma. Comparison between double lung transplant patients and healthy subjects. Am J Respir Crit Care Med. 1993;148:1238–1243. doi: 10.1164/ajrccm/148.5.1238. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Neural mechanisms in asthma. Br Med Bull. 1992;48:149–168. doi: 10.1093/oxfordjournals.bmb.a072531. [DOI] [PubMed] [Google Scholar]
- Cazzola M, Page CP, Calzetta L, Matera MG. Pharmacology and therapeutics of bronchodilators. Pharmacol Rev. 2012;64:450–504. doi: 10.1124/pr.111.004580. [DOI] [PubMed] [Google Scholar]
- Douglas NJ, Sudlow MF, Flenley DC. Effect of an inhaled atropinelike agent on normal airway function. J Appl Physiol. 1979;46:256–262. doi: 10.1152/jappl.1979.46.2.256. [DOI] [PubMed] [Google Scholar]
- Rodrigo G, Rodrigo C, Burschtin O. A meta-analysis of the effects of ipratropium bromide in adults with acute asthma. Am J Med. 1999;107:363–370. doi: 10.1016/s0002-9343(99)00243-0. [DOI] [PubMed] [Google Scholar]
- Hashimoto A, Maeda H, Yokoyama M. Augmentation of parasympathetic nerve function in patients with extrinsic bronchial asthma—evaluation by coefficiency of variance of R-R interval with modified long-term ECG monitoring system. Kobe J Med Sci. 1996;42:347–359. [PubMed] [Google Scholar]
- Kesler BS, Canning BJ. Regulation of baseline cholinergic tone in guinea-pig airway smooth muscle. J Physiol. 1999;518:843–855. doi: 10.1111/j.1469-7793.1999.0843p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jammes Y, Mei N. Assessment of the pulmonary origin of bronchoconstrictor vagal tone. J Physiol. 1979;291:305–316. doi: 10.1113/jphysiol.1979.sp012814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ. Neuroeffector mechanisms: the interface between inflammation and neuronal responses. J Allergy Clin Immunol. 1996;98 (5 Pt 2):S73–S81. [PubMed] [Google Scholar]
- Goyal M, Jaseja H, Verma N. Increased parasympathetic tone as the underlying cause of asthma: a hypothesis. Med Hypotheses. 2010;74:661–664. doi: 10.1016/j.mehy.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Ayala LE, Ahmed T. Is there loss of protective muscarinic receptor mechanism in asthma? Chest. 1989;96:1285–1291. doi: 10.1378/chest.96.6.1285. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Modulation of neurotransmission in airways. Physiol Rev. 1992;72:699–729. doi: 10.1152/physrev.1992.72.3.699. [DOI] [PubMed] [Google Scholar]
- Kanazawa H, Kawaguchi T, Shoji S, Fujii T, Kudoh S, Hirata K. Synergistic effect of nitric oxide and vasoactive intestinal peptide on bronchoprotection against histamine in anesthetized guinea pigs. Am J Respir Crit Care Med. 1997;155:747–750. doi: 10.1164/ajrccm.155.2.9032223. [DOI] [PubMed] [Google Scholar]
- Park HW, Yang MS, Park CS, Kim TB, Moon HB, Min KU. Additive role of tiotropium in severe asthmatics and Arg16Gly in ADRB2 as a potential marker to predict response. Allergy. 2009;64:778–783. doi: 10.1111/j.1398-9995.2008.01876.x. [DOI] [PubMed] [Google Scholar]
- Lundgren R, Söderberg M, Hörstedt P, Stenling R. Morphological studies of bronchial mucosal biopsies from asthmatics before and after ten years of treatment with inhaled steroids. Eur Respir J. 1988;1:883–889. [PubMed] [Google Scholar]
- An SS, Bai TR, Bates JHT, Black JL, Brown RH, Brusasco V. Airway smooth muscle dynamics: a common pathway of airway obstruction in asthma. Eur Respir J. 2007;29:834–860. doi: 10.1183/09031936.00112606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosens R. Inhibition of allergen-induced airway remodeling by tiotropium and budesonide: a comparative study. Abstract A269 presented at the 103rd Annual International Conference of the American Thoracic Society: San Francisco, CA, USA; 2007. [Google Scholar]
- Gosens R, Bos IST, Zaagsma J, Meurs H. Protective effects of tiotropium bromide in the progression of airway smooth muscle remodeling. Am J Respir Crit Care Med. 2005;171:1096–1102. doi: 10.1164/rccm.200409-1249OC. [DOI] [PubMed] [Google Scholar]
- Milara J, Serrano A, Peiró T, Gavaldà A, Miralpeix M, Morcillo EJ. Aclidinium inhibits human lung fibroblast to myofibroblast transition. Thorax. 2012;67:229–237. doi: 10.1136/thoraxjnl-2011-200376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker B, Peatfield AC, Richardson PS. Nervous control of mucin secretion into human bronchi. J Physiol. 1985;365:297–305. doi: 10.1113/jphysiol.1985.sp015773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos IST, Gosens R, Zuidhof AB, Schaafsma D, Halayko AJ, Meurs H. Inhibition of allergen-induced airway remodelling by tiotropium and budesonide: a comparison. Eur Respir J. 2007;30:653–661. doi: 10.1183/09031936.00004907. [DOI] [PubMed] [Google Scholar]
- Bateman ED, Rennard S, Barnes PJ, Dicpinigaitis PV, Gosens R, Gross NJ. Alternative mechanisms for tiotropium. Pulm Pharmacol Ther. 2009;22:533–542. doi: 10.1016/j.pupt.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Belmonte KE. Cholinergic pathways in the lungs and anticholinergic therapy for chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:297–304. doi: 10.1513/pats.200504-043SR. [DOI] [PubMed] [Google Scholar]
- Cazzola M, Matera MG. Emerging inhaled bronchodilators: an update. Eur Respir J. 2009;34:757–769. doi: 10.1183/09031936.00013109. [DOI] [PubMed] [Google Scholar]
- Sykes DA, Dowling MR, Leighton-Davies J, Kent TC, Fawcett L, Renard E. The influence of receptor kinetics on the onset and duration of action and the therapeutic index of NVA237 and tiotropium. J Pharmacol Exp Ther. 2012;343:520–528. doi: 10.1124/jpet.112.194456. [DOI] [PubMed] [Google Scholar]
- Rossoni G, Manfredi B, Razzetti R, Civelli M, Berti F. Positive interaction of the novel β2-agonist carmoterol and tiotropium bromide in the control of airway changes induced by different challenges in guinea-pigs. Pulm Pharmacol Ther. 2007;20:250–257. doi: 10.1016/j.pupt.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Kume H, Imbe S, Iwanaga T, Tohda Y. Synergistic effects between glycopyrronium bromide and indacaterol on a muscarinic agonist-induced contraction in airway smooth muscle. Abstract P4835 presented at the European Respiratory Society Annual Congress: Vienna, Austria; 2012. [Google Scholar]
- Gaultier C, Reinberg A, Girard F. Circadian rhythms in lung resistance and dynamic lung compliance of healthy children. Effects of two bronchodilators. Respir Physiol. 1977;31:169–182. doi: 10.1016/0034-5687(77)90100-1. [DOI] [PubMed] [Google Scholar]
- Furlan R, Guzzetti S, Crivellaro W, Dassi S, Tinelli M, Baselli G. Continuous 24-hour assessment of the neural regulation of systemic arterial pressure and RR variabilities in ambulant subjects. Circulation. 1990;81:537–547. doi: 10.1161/01.cir.81.2.537. [DOI] [PubMed] [Google Scholar]
- Cox ID, Hughes DTD, McDonnell KA. Ipratropium bromide in patients with nocturnal asthma. Postgrad Med J. 1984;60:526–528. doi: 10.1136/pgmj.60.706.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JF, Pearson SB. The parasympathetic nervous system and the diurnal variation of lung mechanics in asthma. Respir Med. 1991;85:285–289. doi: 10.1016/s0954-6111(06)80098-3. [DOI] [PubMed] [Google Scholar]
- Larj MJ, Bleecker ER. Effects of β2-agonists on airway tone and bronchial responsiveness. J Allergy Clin Immunol. 2002;110 (6 Suppl):S304–S312. doi: 10.1067/mai.2002.130045. [DOI] [PubMed] [Google Scholar]
- Haney S, Hancox RJ. Recovery from bronchoconstriction and bronchodilator tolerance. Clin Rev Allergy Immunol. 2006;31:181–196. doi: 10.1385/CRIAI:31:2:181. [DOI] [PubMed] [Google Scholar]
- Abramson MJ, Walters J, Walters EH. Adverse effects of beta-agonists: are they clinically relevant? Am J Respir Med. 2003;2:287–297. doi: 10.1007/BF03256657. [DOI] [PubMed] [Google Scholar]
- Casarosa P, Pieper MP, Gantner F. Cross-talk between the human muscarinic M3 and β2 receptors: evidence for heterologous desensitization. Am J Respir Crit Care Med. 2010;181 [Google Scholar]
- Rodrigo GJ, Rodrigo C. First-line therapy for adult patients with acute asthma receiving a multiple-dose protocol of ipratropium bromide plus albuterol in the emergency department. Am J Respir Crit Care Med. 2000;161:1862–1868. doi: 10.1164/ajrccm.161.6.9908115. [DOI] [PubMed] [Google Scholar]
- Rodrigo GJ, Rodrigo C. The role of anticholinergics in acute asthma treatment: an evidence-based evaluation. Chest. 2002;121:1977–1987. doi: 10.1378/chest.121.6.1977. [DOI] [PubMed] [Google Scholar]
- Griffiths B, Ducharme FM. Combined inhaled anticholinergics and short-acting beta2-agonists for initial treatment of acute asthma in children. Cochrane Database Syst Rev. 2013. [DOI] [PMC free article] [PubMed]
- Plotnick L, Ducharme F. Combined inhaled anticholinergics and beta2-agonists for initial treatment of acute asthma in children. Cochrane Database Syst Rev. 2000. [DOI] [PubMed]
- Hansel TT, Neighbour H, Erin EM, Tan AJ, Tennant RC, Maus JG. Glycopyrrolate causes prolonged bronchoprotection and bronchodilatation in patients with asthma. Chest. 2005;128:1974–1979. doi: 10.1378/chest.128.4.1974. [DOI] [PubMed] [Google Scholar]
- Terzano C, Petroianni A, Ricci A, D'Antoni L, Allegra L. Early protective effects of tiotropium bromide in patients with airways hyperresponsiveness. Eur Rev Med Pharmacol Sci. 2004;8:259–264. [PubMed] [Google Scholar]
- Israel E, Chinchilli VM, Ford JG, Boushey HA, Cherniack R, Craig TJ. Use of regularly scheduled albuterol treatment in asthma: genotype-stratified, randomised, placebo-controlled cross-over trial. Lancet. 2004;364:1505–1512. doi: 10.1016/S0140-6736(04)17273-5. [DOI] [PubMed] [Google Scholar]
- Kazani S, Israel E. Long-acting β-agonists and inhaled corticosteroids: is the whole greater than the sum of its parts? J Allergy Clin Immunol. 2010;125:357–358. doi: 10.1016/j.jaci.2009.12.021. [DOI] [PubMed] [Google Scholar]
- Bleecker ER, Postma DS, Lawrance RM, Meyers DA, Ambrose HJ, Goldman M. Effect of ADRB2 polymorphisms on response to longacting β2-agonist therapy: a pharmacogenetic analysis of two randomised studies. Lancet. 2007;370:2118–2125. doi: 10.1016/S0140-6736(07)61906-0. [DOI] [PubMed] [Google Scholar]
- Bleecker ER, Nelson HS, Kraft M, Corren J, Meyers DA, Yancey SW. β2-receptor polymorphisms in patients receiving salmeterol with or without fluticasone propionate. Am J Respir Crit Care Med. 2010;181:676–687. doi: 10.1164/200809-1511OC. [DOI] [PubMed] [Google Scholar]
- Beeh KM, Ablinger O, Moroni-Zentgraf P, Hollaenderova Z, Pivovarova A, Engel M. Tiotropium in asthma: a dose-finding study in adult patients with moderate persistent asthma. Poster A1283 presented at the American Thoracic Society International Conference: Philadelphia, PA, USA; 2013. [Google Scholar]
- Kerstjens HAM, Bleecker E, Meltzer E, Casale T, Pizzichini E, Schmidt O. Tiotropium as add-on therapy to inhaled corticosteroids for patients with symptomatic asthma: lung function and safety. Eur Respir J. 2013;42 (Suppl 57):980s. [Google Scholar]
- Kerstjens HAM, Bleecker E, Meltzer E, Casale T, Pizzichini E, Schmidt O. Tiotropium as add-on to inhaled corticosteroids significantly improves asthma control as reflected by the ACQ responder rate. Eur Respir J. 2013;42 (Suppl 57):876s. [Google Scholar]
- Bateman ED, Esser D, Chirila C, Fernandez M, Fowler A, Moroni-Zentgraf P. Systematic review and meta-analysis of the magnitude of the effect on the AQLQ and ACQ in asthma clinical trials. Poster P4113 presented at the European Respiratory Society Annual Congress: Barcelona, Spain; 2013. [Google Scholar]
- Powell H, Gibson PG. Inhaled corticosteroid doses in asthma: an evidence-based approach. Med J Aust. 2003;178:223–225. doi: 10.5694/j.1326-5377.2003.tb05167.x. [DOI] [PubMed] [Google Scholar]
- Szefler SJ, Martin RJ, King TS, Boushey HA, Cherniack RM, Chinchilli VM. Significant variability in response to inhaled corticosteroids for persistent asthma. J Allergy Clin Immunol. 2002;109:410–418. doi: 10.1067/mai.2002.122635. [DOI] [PubMed] [Google Scholar]
- Price DB, Kaplan A, Jones R, Freeman D, Burden A, Gould SE. Real-life prescribing and outcomes of long-acting anticholinergic therapy in adult asthma patients in UK clinical practice. Am J Respir Crit Care Med. 2013;187 [Google Scholar]
- Ullah MI, Newman GB, Saunders KB. Influence of age on response to ipratropium and salbutamol in asthma. Thorax. 1981;36:523–529. doi: 10.1136/thx.36.7.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly MJ. Ageing, late-onset asthma and the beta-adrenoceptor. Pharmacol Ther. 1993;60:389–404. doi: 10.1016/0163-7258(93)90029-d. [DOI] [PubMed] [Google Scholar]
- Partridge MR, Saunders KB. Site of action of ipratropium bromide and clinical and physiological determinants of response in patients with asthma. Thorax. 1981;36:530–533. doi: 10.1136/thx.36.7.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M, Morgan A, Shaw DE, Parker D, Green R, Brightling C. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax. 2007;62:1043–1049. doi: 10.1136/thx.2006.073429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavord ID, Brightling CE, Woltmann G, Wardlaw AJ. Non-eosinophilic corticosteroid unresponsive asthma. Lancet. 1999;353:2213–2214. doi: 10.1016/S0140-6736(99)01813-9. [DOI] [PubMed] [Google Scholar]
- Bradding P, Green RH. Subclinical phenotypes of asthma. Curr Opin Allergy Clin Immunol. 2010;10:54–59. doi: 10.1097/ACI.0b013e32833489a9. [DOI] [PubMed] [Google Scholar]
- Lazarus SC, Chinchilli VM, Rollings NJ, Boushey HA, Cherniack R, Craig TJ. Smoking affects response to inhaled corticosteroids or leukotriene receptor antagonists in asthma. Am J Respir Crit Care Med. 2007;175:783–790. doi: 10.1164/rccm.200511-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri R, Livingston E, McMahon AD, Lafferty J, Fraser I, Spears M. Effects of smoking cessation on lung function and airway inflammation in smokers with asthma. Am J Respir Crit Care Med. 2006;174:127–133. doi: 10.1164/rccm.200510-1589OC. [DOI] [PubMed] [Google Scholar]