Abstract
Lumenogenesis of small seamless tubes occurs through intracellular membrane growth and directed vesicle fusion events. Within the C. elegans excretory cell, which forms seamless intracellular tubes (canals) that mediate osmoregulation, lumens grow in length and diameter when vesicles fuse with the expanding lumenal surface. Here, we show that lumenal vesicle fusion depends on the small GTPase RAL-1, which localizes to vesicles and acts through the exocyst vesicle-tethering complex. Loss of either the exocyst or RAL-1 prevents excretory canal lumen extension. Within the excretory canal and other polarized cells, the exocyst co-localizes with the PAR polarity proteins PAR-3, PAR-6 and PKC-3. Using early embryonic cells to determine the functional relationships between the exocyst and PAR proteins, we show that RAL-1 recruits the exocyst to the membrane, while PAR proteins concentrate membrane-localized exocyst proteins to a polarized domain. These findings reveal that RAL-1 and the exocyst direct the polarized vesicle fusion events required for intracellular lumenogenesis of the excretory cell, suggesting mechanistic similarities in the formation of topologically distinct multicellular and intracellular lumens.
Keywords: exocyst, PAR proteins, lumenogenesis, tubulogenesis, vesicle trafficking, osmoregulation
INTRODUCTION
Epithelial tubes enable the rapid distribution of liquids, gases, and circulating cells throughout the body. In larger tubes, multiple cells connected by junctions surround a common extracellular lumen (Andrew and Ewald, 2010; Iruela-Arispe and Davis, 2009). By contrast, small intracellular tubes, such as capillaries, can form within the cytoplasm of a single cell or cells connected in series. Intracellular tube formation requires the polarized targeting and fusion of cytoplasmic vesicles. For example, vertebrate vascular endothelial cells form a lumen when cytoplasmic vesicles accumulate in a central region of the cell and fuse with one another, or fuse with an invading apical membrane domain (Herwig et al., 2011; Davis and Camarillo, 1996). In terminal cells within the Drosophila trachea, as well as within the C. elegans excretory cell, lumenal membrane with apical character grows distally from the cell body and expands in length and diameter as a result of intracellular vesicle targeting and fusion (Kolotuev et al., 2013; Gervais and Casanova, 2010; Schottenfeld-Roames and Ghabrial, 2012; Khan et al., 2013). The molecular mechanisms responsible for the polarized membrane fusion events needed to create intracellular tubes are not well understood.
Polarization in many cells is mediated by the proteins PAR-3 (a multi-PDZ domain scaffolding protein), PAR-6 (a PDZ and CRIB domain scaffolding protein) and aPKC (an atypical protein kinase C) (Johnston and Ahringer, 2010; Nance and Zallen, 2011), which are collectively called PAR proteins. During polarization, upstream polarity cues induce PAR proteins to segregate asymmetrically within the cell, resulting in spatially restricted interactions between PAR proteins and their effectors. The role of PAR proteins in lumenogenesis has been investigated in canine epithelial (MDCK) cells grown in culture to form three-dimensional cysts (Bryant et al., 2010). MDCK cell cysts are similar to multicellular tubes, in that their formation requires the creation and expansion of extracellular space, rather than the intracellular membrane growth that is needed to form seamless tubes. MDCK cyst formation follows the transient recruitment of Par3 and vesicles to the site of future lumen formation at the cell surface, and knockdown of Par3 results in cysts containing multiple disorganized lumen-like structures. These findings suggest that Par3 and associated PAR proteins help to direct targeted vesicle fusion at the site of lumen formation. It is not known whether this mechanism is used to create multicellular tubes in vivo.
The eight-protein exocyst complex is also required for lumen formation in MDCK cell cysts (Bryant et al., 2010). In a wide variety of organisms and cell types, the exocyst mediates vesicle tethering and subsequent fusion at specific sites on the cell membrane (Lipschutz et al., 2000; He and Guo, 2009; Liu and Guo, 2012). Six of the eight exocyst components are thought to associate with cytoplasmic exocytic vesicles (hereafter termed ‘core exocyst’ components), while Sec3 and Exo70 anchor the complex at the plasma membrane (Boyd, 2004). Small GTPases, including Ral, direct exocyst assembly and thereby promote vesicle tethering (Brymora et al., 2001; Sugihara et al., 2002; Moskalenko et al., 2002, 2003). Knockdown of exocyst function in MDCK cell cysts causes multiple disorganized lumens to form, similar to the phenotype of cysts lacking Par3. Moreover, Par3 and exocyst components show a mutually dependent localization during lumen formation (Bryant et al., 2010), and PAR proteins immunoprecipitate with exocyst components in multiple cell types (Lalli, 2009; Zuo et al., 2009, 2011; Rosse et al., 2009; Das et al., 2014). Recently, it was shown that the exocyst is also required for intracellular lumenogenesis within Drosophila terminal tracheal cells (Jones et al., 2014). These observations suggest that PAR proteins and the exocyst may cooperate to target vesicles to the cell surface during multicellular and intracellular lumenogenesis, although it remains unclear how the two protein complexes work together.
Here, using the C. elegans excretory cell as a model, we identify the exocyst as a downstream PAR effector responsible for driving vesicle fusion events that promote intracellular lumenogenesis. Required for maintaining osmotic balance (Nelson and Riddle, 1984), the excretory cell undergoes a rapid lumenal expansion during embryogenesis and the first larval stage (L1) to form an H-shaped seamless tube (canal) extending the length of the body (Figure 1A) (Nelson et al., 1983). Lumen formation and expansion occur through the fusion of specialized vesicles, called canalicular vesicles, which surround the lumenal surface (Kolotuev et al., 2013). A cytoskeletal scaffold coats the cytoplasmic face of the lumen, preserving its shape and aiding in canalicular vesicle tethering or fusion (Göbel et al., 2004; Khan et al., 2013). We show that the exocyst concentrates at the lumenal scaffold and its activity is required for the fusion of canalicular vesicles that promotes lumenogenesis. Using early embryonic cells, we demonstrate upstream roles for RAL-1/Ral in recruiting the exocyst to the membrane and for PAR proteins in promoting exocyst membrane asymmetry. Our findings reveal an in vivo pathway that directs vesicle fusion events required for seamless intracellular tube formation, and suggest that topologically distinct intracellular and multicellular tubes can form using similar molecular mechanisms.
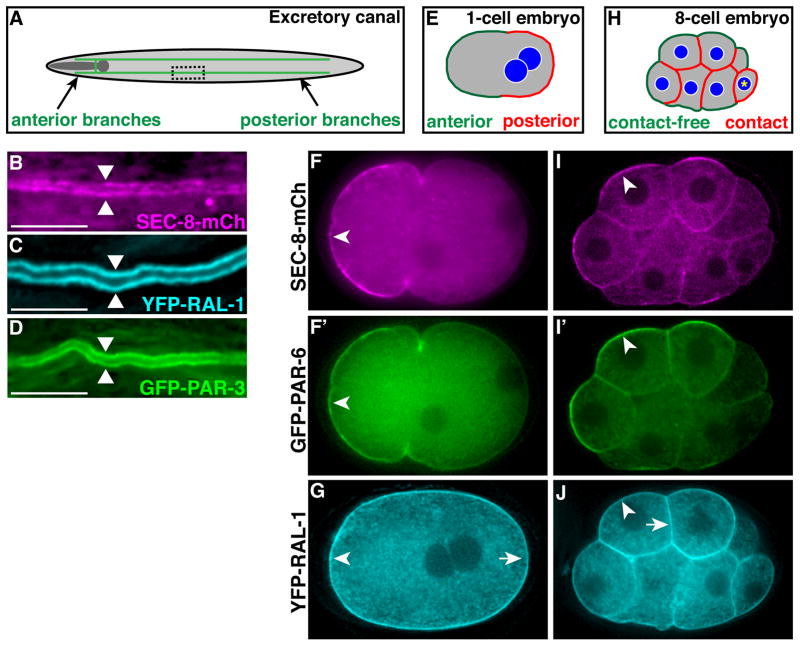
Figure 1. RAL-1, exocyst and PAR protein expression in polarized cells.
(A) Schematic of the excretory canal cell (green). The canal cell body and lateral branch are positioned adjacent the posterior pharynx (shaded dark gray). A representative region of posterior canal, depicted at higher magnification in B–D, is indicated by dashed rectangle. (B–D) Lateral view of excretory canal segment in L4 larvae expressing the indicated fusion proteins; arrowheads point towards canal lumen. (E) Schematic of a polarized 1-cell embryo displaying distinct anterior and posterior membrane domains. (F and F′) 1-cell embryo co-expressing SEC-8-mCherry (F) and PAR-6-GFP (F′), which are enriched at the anterior membrane (arrowheads). (G) 1-cell embryo expressing YFP-RAL-1, which localizes uniformly to both anterior (arrowhead) and posterior (arrow) membranes. (H) Schematic of a polarized 8-cell embryo with distinct contacted and contact-free cell surfaces; the germline precursor cell (asterisk) is unpolarized. (I and I′) 8-cell embryo co-expressing SEC-8-mCh (I) and PAR-6-GFP (I′), which are enriched at contact-free surfaces (arrowheads). (J) 8-cell embryo expressing YFP-RAL-1, which localizes uniformly to contact-free (arrowhead) and contacted (arrow) surfaces. In this figure and all subsequent figures, embryos and larvae are oriented anterior to the left, and whole embryos are ~50 μm in length. Scale bars in B–D are 10 μm.
RESULTS
The exocyst colocalizes with PAR proteins in the excretory cell and other polarized cell types
The expression and localization of the exocyst had not been described previously in C. elegans. There are C. elegans homologs of all eight exocyst components [sec-3, sec-5, sec-6, sec-8, sec-10, sec-15, exoc-7 (Exo70) and exoc-84 (Exo84)] as well as a single Ral homologue, ral-1 (see Materials and Methods). To determine whether the exocyst and RAL-1 are expressed during excretory canal morphogenesis, we created tagged fusion proteins for RAL-1 and the core exocyst components SEC-5, SEC-8, SEC-10 and SEC-15. Beginning at the onset of excretory canal formation and continuing through adult stages, tagged exocyst components and YFP-RAL-1 were present along the length of the excretory canal (Figure 1A–C; Figure S1A–C). Each tagged exocyst component and YFP-RAL-1 were also expressed maternally and therefore were present in cells of the early embryo. In particular, we noted a striking asymmetric localization of SEC-5-YFP, SEC-8-mCherry, mCherry-SEC-10 and SEC-15-YFP in polarized cells, including to the anterior membrane of one-cell embryos, the contact-free membrane of early embryonic cells, and the apical membrane of epithelial cells (Figure 1E, F, H, I; Figure S1E–G; data not shown). PAR-3, PAR-6 and PKC-3/aPKC have similar asymmetries in these polarized cells (Nance and Zallen, 2011), which we confirmed by analyzing embryos co-expressing both SEC-8-mCherry and PAR-6-GFP (Figure 1F, F′, I, I′; Figure S1G, G′). By contrast, YFP-RAL-1 displayed a more uniform plasma membrane enrichment (Figure 1G, J).
To determine whether PAR proteins are also present in the excretory canal, we examined PAR-3 and PAR-6 reporters. Similar to SEC-8-mCherry, fluorescently tagged PAR-3 and PAR-6 were present along the length of the canal lumen (Figure 1D; Figure S1D, D′), and we confirmed the excretory cell expression of endogenous PAR-6 and PKC-3 by immunostaining (Figure S1H–I‴). In summary, the exocyst and RAL-1 are found along the excretory canals together with PAR proteins, core exocyst components are asymmetric and co-localize with PAR proteins in polarized cells, and RAL-1 shows an overlapping but broader membrane localization.
ral-1 and the exocyst are essential for larval development and osmoregulation
To investigate the function of the exocyst, we examined mutations in ral-1, sec-5, and sec-8 predicted to be strong loss-of-function or null alleles (see Materials and Methods). ral-1(tm5205) lacks the start codon and first two exons of ral-1, which deletes a conserved region of the GTP-binding domain (van Dam and Robinson, 2006). sec-8(ok2187) deletes a central region of the sec-8 locus, resulting in a frame shift and premature stop. ral-1(tm5205) and sec-8(ok2187) mutants displayed similar phenotypes, with most animals becoming sterile adults (Table S1). sec-5(pk2358) contains a nonsense mutation approximately halfway through the sec-5 coding sequence (Frische et al., 2007). Most sec-5 mutants were fertile, but ruptured at the vulva shortly after producing a few eggs, and all progeny died as embryos or L1 larvae. Phenotypes associated with each mutant allele were rescued by the respective tagged wild-type transgene (Table S1).
To determine how ral-1, sec-5, and sec-8 mutant alleles affect the exocyst complex, we examined the localization of tagged exocyst components in each mutant, with the hypothesis that strong loss-of-function mutants would affect complex assembly. We focused on early embryonic cells because the exocyst complex shows a pronounced asymmetric localization in these cells, and because we previously developed tools to acutely remove gene function at this stage. To remove maternal contribution, we tagged yfp-ral-1, sec-5-yfp, and sec-8-mCherry transgenes with sequences encoding the PIE-1 protein ZF1 domain, which promotes rapid degradation of the tagged protein within early embryonic somatic cells (Reese et al., 2000; Nance et al., 2003). zf1-yfp-ral-1, sec-5-zf1-yfp, and sec-8-zf1-mCherry rescued the phenotypes of ral-1, sec-5, and sec-8 mutants, respectively (Table S2). In each case, the ZF1-tagged protein degraded rapidly in early embryos, leaving cells lacking all functional sources of the gene product (‘MZ’ mutants, see Materials and Methods). In sec-5(MZ) mutant embryos, exocyst marker mCherry-SEC-10 were lost from the membrane (Figure S2A, A′). ral-1(MZ) mutants showed a similar loss in SEC-8-mCherry and mCherry-SEC-10 (Figure S2A, A′ and data not shown), while exocyst markers were still present and asymmetric at the membrane in sec-8(MZ) early embryos (SEC-5-YFP, data not shown). Blocking degradation of ZF1-YFP-RAL-1 and SEC-5-ZF1-YFP by RNAi depletion of zif-1, which is required to destroy proteins tagged with the ZF1 domain (DeRenzo et al., 2003), rescued exocyst localization defects, indicating that these transgenes are functional at this stage (Figure S2B, B′, D, D′). Therefore, while the alleles ral-1(tm5205) and sec-5(pk2358) prevent exocyst membrane assembly, sec-8(ok2187) affects exocyst function distinctly.
In contrast to zygotic mutants, ral-1(MZ), sec-5(MZ) and sec-8(MZ) mutants arrested primarily at the L1 larval stage (Table S3), with a small percentage of mutants arresting during embryogenesis. sec-5(MZ) mutants obtained as embryos and larvae laid by sec-5 homozygous mothers displayed similar phenotypes and died at the same stage as those generated by ZF1-tagging (n=26). Therefore, despite the polarized localization pattern of the exocyst in early embryos, loss of exocyst function at this stage does not cause major developmental defects in most embryos. ral-1(MZ), sec-5(MZ) and sec-8(MZ) mutants that hatched were extremely sensitive to changes in osmolarity, accumulating fluid-filled cavities and dying quickly when placed in water (Figure 2A, B). This phenotype is characteristic of mutants with defects in excretory cell function (Nelson and Riddle, 1984; Buechner et al., 1999), suggesting a role for RAL-1 and the exocyst in formation or function of the excretory cell.
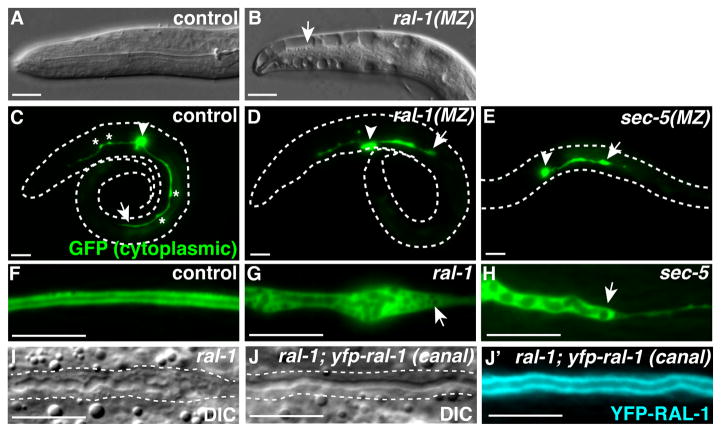
Figure 2. ral-1 and the exocyst are required for osmoregulation and excretory canal lumen extension.
(A–B) Anterior end of control (A) and ral-1(MZ) (B) L1 larvae placed in water; arrow indicates fluid-filled cavity. (C–E) Newly hatched L1 larvae (body outlined) expressing cytoplasmic GFP in the excretory canal cell. Arrowhead points to canal cell body, arrow indicates posterior extent of canal outgrowth, and asterisks in C highlight periodic cytoplasmic ‘pearls.’ (F–H) Segment of posterior excretory canal in L4 larvae of indicated genotypes expressing cytoplasmic GFP; mutant canals in G and H terminate prematurely (arrow). (I–J′) ral-1 mutant siblings lacking (I) or expressing (J and J′) canal-specific Ppgp-12::yfp-ral-1 transgene. Dashed lines in I and J outline the canal, which is discontinuous in ral-1 mutant (I) and smooth in rescued (J) canals; transgene expression in the rescued canal is shown in J′. Scale bars are 10 μm.
The exocyst is required for excretory cell canal lumenogenesis
We analyzed excretory cell morphology in ral-1(MZ) and sec-5(MZ) mutants by expressing cytoplasmic GFP from the excretory cell-specific pgp-12 promoter (Zhao et al., 2005). In newly hatched wild-type L1 larvae, short lumenized anterior canals extended toward the nose, and longer lumenized posterior canals extended approximately halfway to the posterior (Figure 2C) (Buechner et al., 1999; Kolotuev et al., 2013). A thin non-lumenized cytoplasmic projection extended just beyond the lumen as the canal elongated, as described previously (Kolotuev et al., 2013), and by the end of the L1 stage, the lumen and cytoplasm both extended the length of the body. Previously described (Kolotuev et al., 2013) periodic regions of cytoplasmic swelling were present along the canal length (Figure 2C, asterisks). In contrast, canals in ral-1(MZ) (n = 45) and sec-5(MZ) (n = 43) L1 larvae were disorganized and failed to properly elongate, containing much shorter and more irregular posterior canal branches (Figure 2D, E, Figure S3). In a small fraction of ral-1(MZ) (3/45) and sec-5(MZ) (11/43) mutant L1 larvae, we failed to detect even a rudimentary lumen. Mutant L1 worms left on agar plates for several days often developed posterior extensions that could reach the end of the body, but that failed to lumenize and contained vacuoles (Figure S3 and data not shown). These findings indicate that ral-1 and sec-5 are required for proper lumenogenesis within the canal, and are not essential for outgrowth of the non-lumenized canal basal extension. The presence of a rudimentary, disorganized and shortened lumen in mutants suggests either that canal lumen initiation occurs through an independent mechanism, or that ral-1(MZ) and sec-5(MZ) mutants retain sufficient function to allow for canal lumen initiation but not extension.
To assess exocyst function in less disorganized canals, we examined ral-1 and sec-5 zygotic mutants. In L4 larvae, the lumenized posterior canals of ral-1 and sec-5 mutants terminated prematurely, before reaching the tail (Figure 2F–H, Figure S4B). Lumens in both mutants typically ended with a bulge containing numerous vesicular structures followed by a thin cytoplasmic extension (Figure 2G, H), which in some animals extended well beyond the truncated lumen (Figure S4A). The lumenized portion of excretory canals in ral-1 and sec-5 mutants often displayed abnormalities including irregular shape, variable width and septa (Figure 2G–I). We tested whether ral-1 function in the canal was sufficient to rescue lumenogenesis phenotypes by expressing yfp-ral-1 from the pgp-12 promoter. ral-1; Ppgp-12::yfp-ral-1 animals formed normal, lumenized, and continuous canals [95%, n = 37 in ral-1; Ppgp-12::yfp-ral-1 versus 5%, n = 21 in ral-1 mutants alone, Chi-squared (p<0.0001)] (Figure 2I–J′). We conclude that ral-1 and the exocyst are required in the excretory cell for intracellular lumenization, but are dispensable for initial canal outgrowth.
Subcellular localization of RAL-1, the exocyst and PAR proteins within the excretory cell
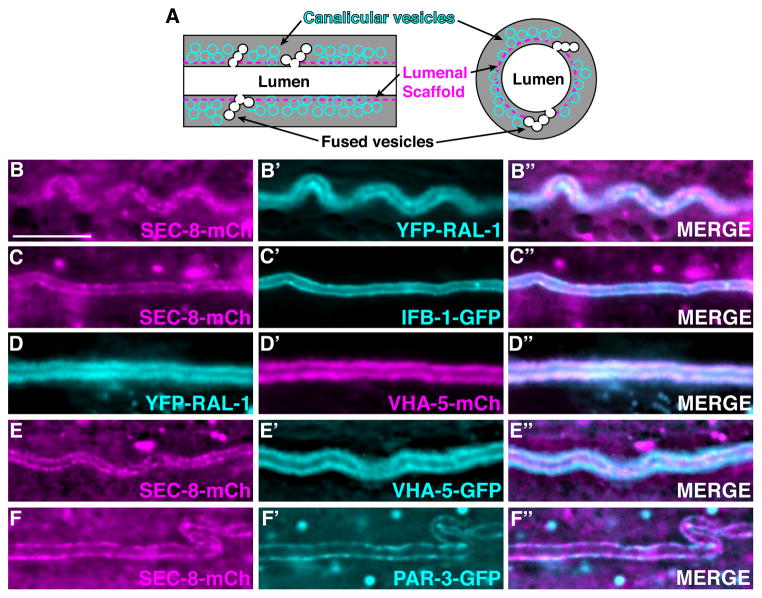
In order to better understand RAL-1 and exocyst function during canal lumen formation, we examined their subcellular localization within the excretory cell. We first compared the distributions of YFP-RAL-1 and SEC-8-mCherry with each other, then with known markers of canal structures. Examined by immunoelectron microscopy, the intermediate filament protein IFB-1 localizes to the lumenal scaffold, while V-type ATPase subunit VHA-5 is found on canalicular vesicles (Kolotuev et al., 2013). YFP-RAL-1 had a broader distribution along the canal than SEC-8-mCherry (Figure 3B–B″) but did not fill the width of the cell (Figure S5). SEC-8-mCherry showed a similar distribution to IFB-1-GFP but not VHA-5-mCherry (Figure 3C–C″, E–E″), while YFP-RAL-1 localized similarly to VHA-5-mCherry (Figure 3D–D″). To determine where PAR proteins are found within the excretory cell, we compared the distributions of PAR-3-GFP and SEC-8-mCherry. Similar to SEC-8-mCherry, PAR-3-GFP was lumenally restricted and punctate (Figure 3F–F″). We conclude that the exocyst and PAR proteins are likely present at the lumenal surface, while RAL-1 is also likely found on canalicular vesicles.
Figure 3. Subcellular localization of RAL-1, the exocyst, and PAR proteins within the excretory canal.
(A) Excretory canal schematic shown in lateral (left) and transverse (right) sections. (B–F″) Live images of excretory canals in L4 larvae co-expressing the indicated transgenes. Scale bar is 10 μm, and scale is equivalent in all images.
ral-1 and sec-5 are required for osmotic shock recovery
Given the localization of the exocyst to the lumenal scaffold and YFP-RAL-1 to canalicular vesicles, we hypothesized that exocyst activity was required for the formation or fusion of lumenal canalicular vesicles. Recovery from hyperosmotic shock triggers the fusion of additional canalicular vesicles with the lumen, increasing its surface area and promoting water and ion flux (Khan et al., 2013; Kolotuev et al., 2013). The additional vesicles are thought to arise from cytoplasmic ER-rich swellings named “pearls”, which form transiently along the canal in response to an increased salt load but resolve after worms return to isotonic conditions (Kolotuev et al., 2013; Hahn-Windgassen and Van Gilst, 2009). Pearls are thought to coincide with active areas of canal membrane growth, and also occur during normal canal outgrowth in L1 larvae (see Figure 2C). If the exocyst were required for the formation or lumenal fusion of canalicular vesicles, we reasoned that pearls induced by osmotic shock would persist in mutant larvae. Following osmotic shock, pearls induced in wild-type L4 larvae disappeared in 94% of animals within an hour after return to isotonic conditions (Figure 4A–C, G). By contrast, pearls disappeared in only 20% of ral-1 and 36% of sec-5 mutants within the same period (Figure 4D–G), but had fully resolved by the following day (data not shown). Given the role of membrane expansion in pearl recovery from hyperosmotic shock, these findings are consistent with a role for RAL-1 and the exocyst in the production and/or fusion of canalicular vesicles at the lumenal surface.
Figure 4. ral-1 and sec-5 are required for prompt recovery from hyperosmotic shock.
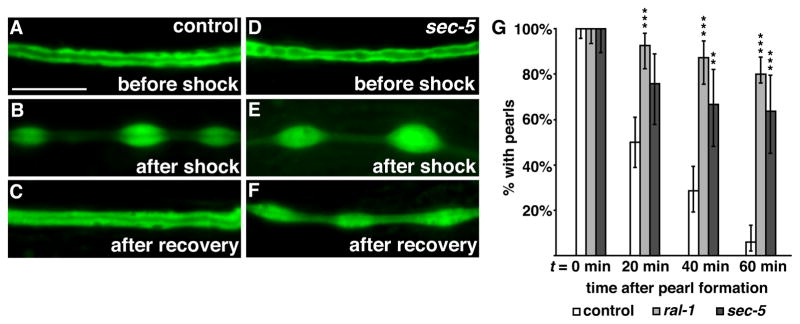
(A–C) Control larvae expressing canal-specific GFP before shock (A), 20 minutes after shock (B) and after one-hour recovery (C). (D–F) sec-5 mutant expressing canal-specific GFP; pearls remain after the one-hour recovery. (G) Percentage of control (n = 84), ral-1 (n = 55) and sec-5 (n = 33) mutants with cytoplasmic pearls following recovery from hyperosmotic shock; time zero corresponds to the first appearance of pearls following shock. Error bars represent the 95% confidence interval. **p < 0.001, *** p < 0.0001, Chi-squared test of mutants versus control. Scale bars are 10 μm, and scale is equivalent in all panels.
ral-1 promotes apical fusion of canalicular vesicles
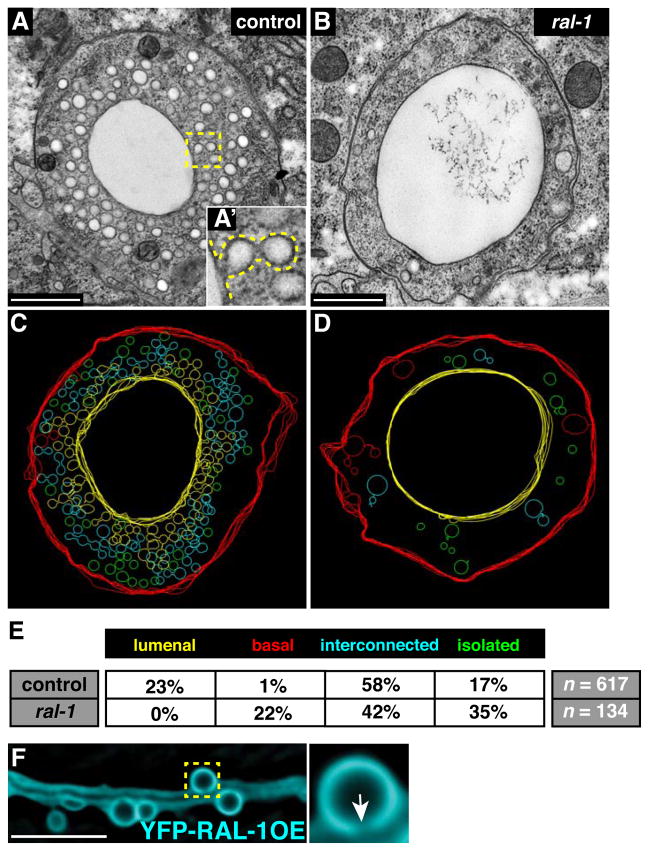
To visualize canalicular vesicles directly, we examined 200 nm thick sections of wild-type and ral-1 excretory canals using electron tomography. We chose to examine ral-1 zygotic mutants so that the architecture of the excretory canal was not severely abnormal, as in ral-1(MZ) mutants. Wild-type canals contained a single apical lumen and surrounding canalicular vesicles that occupied the majority of the canal cytoplasm (Figure 5A). Canalicular vesicles were present as isolated vesicles, interconnected vesicles, and vesicles connected to the lumenal or basal surface by a pore or thin membrane (Figure 5A′, C, E), similar to previous observations (Kolotuev et al., 2013). Canals in ral-1 mutants showed several prominent differences with wild type (Figure 5B, D, E, and Video S1, S2). First, we observed that ral-1 mutant canals had a variable lumen diameter (see Figure 2G, I) with significantly fewer vesicles along the canal than did wild type. A larger lumen diameter in ral-1 canals could reflect dysfunction of the canal itself, as other excretory cell mutants can develop canal cysts (Buechner et al., 1999). Second, no canalicular vesicles were connected to the lumen, in contrast to the 23% of wild-type vesicles that were connected lumenally. Finally, a substantial number of vesicles in ral-1 mutants were connected to the basal surface (22%) while basally connected vesicles were rare in wild type (1%). We conclude that ral-1 is required for canalicular vesicles to fuse with the lumenal surface, and to prevent vesicles from associating with the basal surface. The decrease in canalicular vesicle number in ral-1 mutants may indicate the presence of positive feedback between vesicle fusion and biogenesis, providing the cell with a mechanism to govern the number of canalicular vesicles needed for osmoregulation. Alternatively, ral-1 may have an additional role in canalicular vesicle formation.
Figure 5. ral-1 is needed for vesicle fusion with the canal lumen.
(A and A′) Representative transverse transmission electron microscopy (TEM) thin sections of the excretory canal in wild type (A) and ral-1 mutants (B); sections were taken from the posterior canal between the pharynx and distal gonad. A′ displays a higher magnification view of the boxed region in A; interconnected vesicles fused to the lumen are outlined by a dotted line. Scale bars in A and B are 500 nm. (C and D) Tracings from 200 nm thick section TEM tomograms (see Videos S1 and S2 for raw data Z-stacks) from regions immediately distal to the sections displayed in panels A and B. Each vesicle was outlined at its maximum diameter. Vesicles are classified as connected to the lumen (yellow), basal surface (red), interconnected (cyan), or isolated (green). (E) Summary of vesicle tracings from control (4 sections) and mutant (4 sections) tomograms; n refers to total number of vesicles from all sections combined. (F) L4 larval worm over-expressing YFP-RAL-1 specifically in the excretory canal. The magnified boxed region shows a cyst connected to the canal lumen (arrow). Scale bar is 10 μm.
To determine if increasing exocyst activity causes an increase in lumenogenesis, we over-expressed YFP-RAL-1 specifically in the excretory cell. Ppgp-12::yfp-ral-1 over-expression caused a dramatic expansion of the canal lumen, which accumulated numerous cysts connected to the lumen (Figure 5F). Cyst formation was reduced following partial depletion of SEC-8, indicating that RAL-1 regulates lumen expansion through the exocyst [11% with cysts in sec-8 RNAi (n=127) versus 35% in control (n=166), Chi-squared (p < 0.0001)]. Thus, while inactivation of the exocyst through ral-1 depletion prevents canalicular vesicle fusion and lumen extension, over-activation of the exocyst through ral-1 overexpression causes the lumen to expand.
PAR proteins function upstream to induce exocyst asymmetry in early embryonic cells
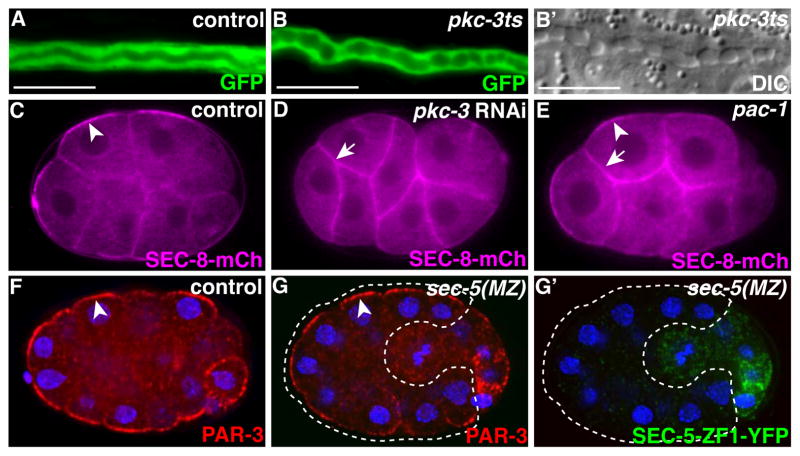
We hypothesized that PAR proteins polarize the exocyst and therefore promote vesicle fusion specifically at the lumenal surface. Addressing the role of PAR proteins in the excretory canal, which undergoes morphogenesis during late embryogenesis and larval stages, is complicated by the essential earlier requirements for these proteins during zygote polarization and epithelial cell formation (Totong et al., 2007; Achilleos et al., 2010). To circumvent these earlier requirements, we used a temperature-sensitive, partial loss-of-function allele of pkc-3 to acutely reduce PKC-3 function during excretory canal morphogenesis in larval worms (Fievet et al., 2012). Excretory canals in pkc-3ts mutants contained septations (51%, n=35; Figure 6A, B′), and in some cases terminated abruptly (11%, n=35) when compared to control larvae grown at the same temperature (n=22). Similar phenotypes were present in worms trans-heterozygous for pkc-3ts and a null allele of pkc-3 (10/12 displayed septations), indicating that they are specific to reduction in pkc-3 activity. Notably, the canal phenotypes of pkc-3ts mutants are similar to but less penetrant than those of sec-5 and ral-1 mutants (see Figure 2G–I), consistent with a reduction but not elimination of pkc-3 activity.
Figure 6. PAR proteins in canal morphogenesis and exocyst polarity.
(A–B′) Wild-type (A) and pkc-3ts mutant (B–B′) excretory canals from L4 larvae expressing GFP in the excretory canal and raised at 25°C after late embryogenesis; DIC image in B′ shows discontinuities of canal in B (compare to wild type in Figure S1D and to similar ral-1 mutant in Figure 2I). Scale bars are 10 μm. (C–D) 8-cell embryos from hermaphrodites expressing SEC-8-mCherry and fed with control bacteria containing empty vector (C) (n=66/66 polarized), or bacteria expressing pkc-3 dsRNA (n=19/20 not polarized). (E) pac-1 mutant 8-cell embryo expressing SEC-8-mCherry (n=16/16 not polarized). Arrowheads, SEC-8-mCherry at contact-free surfaces; arrows, SEC-8-mCherry at contacted surfaces. (F–G′) 26- to 28-cell stage control (n=24/24 polarized) (F) and mutant (n=13/13 polarized) (G, G′) embryos immunostained for indicated proteins. Dotted region in G and G′ outlines mutant cells depleted of maternally provided SEC-5-ZF1-YFP protein. Arrowheads, PAR-3 enriched at contact-free surfaces.
To determine the hierarchy between PAR proteins and the exocyst, we examined their relationship in early embryonic cells, where PAR proteins, RAL-1 and the exocyst complex displayed asymmetries similar to those seen in the excretory canal, and where PAR protein function could be removed completely. To test whether PAR proteins are essential for exocyst asymmetry, we knocked down PAR-3, PAR-6 or PKC-3 using RNAi and examined the localization of SEC-8-mCherry. Following PAR knockdown, SEC-8-mCherry remained associated with the plasma membrane but its asymmetry was lost (Figure 6C, D and Figure S6A, B). SEC-8-mCherry asymmetry was also lost in embryos depleted acutely of PAR-3 in early embryonic cells (Nance et al., 2003), suggesting a direct requirement for PAR proteins in exocyst asymmetry at this stage (Figure S5C). In pac-1 mutant early embryos, in which PAR-3, PAR-6, and PKC-3 are present but remain symmetric at the cell membrane, SEC-8-mCherry also remained symmetric (Figure 6E) (Anderson et al., 2008). We conclude that PAR proteins must be present and asymmetric in order to induce exocyst asymmetry.
To determine if the exocyst functions downstream of PAR proteins or in parallel, we analyzed PAR protein localization in ral-1(MZ) and sec-5(MZ) early embryos. Similar to control embryos, PAR-3, PAR-6 and PKC-3 remained asymmetrically localized (Figure 6F–G′ and Figure S6D–H′). To determine the contribution of active RAL-1 to exocyst membrane localization, we over-expressed constitutively active RAL-1 (RAL-1CA) (Hinoi et al., 1996; Frische et al., 2007) in early embryos expressing SEC-8-mCherry. Cells expressing GFP-RAL-1CA uniformly recruited SEC-8-mCherry to all cell surfaces (Figure 7A–B′) but had no effect on PAR protein asymmetry (Figure 7C–D′). Therefore, RAL-1 is necessary (see Figure S2) and active RAL-1 is sufficient to recruit the exocyst to the membrane, while PAR proteins are required to concentrate membrane-localized exocyst to a discrete cortical domain. We conclude that the exocyst functions downstream of RAL-1 and the PAR proteins.
Figure 7. Effect of RAL-1CA on the exocyst and PAR proteins.
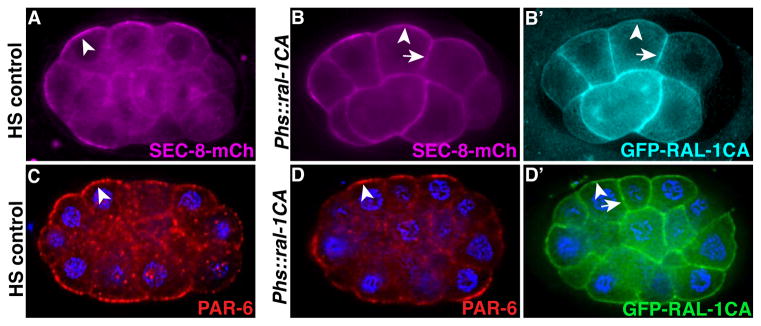
(A–B′) Heat-shock (HS) control embryo (A) or sibling embryos expressing heat-shock driven GFP-RAL-1CA (B and B′); all embryos express SEC-8-mCherry, which is enriched at contact-free surfaces (arrowheads) in control embryos (n = 50/50 polarized) and recruited additionally to contacted surfaces (arrows) in embryos expressing RAL-1CA (n = 24/26 ectopically recruited). (C–D′) HS control embryo immunostained for PAR-6 (n=11/11 polarized) (C), and sibling embryo expressing heat-shock driven GFP-RAL-1CA (n=12/12 polarized) (D and D′) co-immunostained for PAR-6 and GFP-RAL-1CA. Arrowheads show PAR-6 enrichment at contact-free surfaces, arrow indicates contacted surfaces.
DISCUSSION
Our findings reveal a molecular pathway, centered on the vesicle-tethering exocyst complex, which promotes lumenogenesis within a seamless intracellular tube. Previously, it had been shown that seamless lumen formation and expansion within the excretory cell result from intracellular vesicle fusion at the lumenal membrane (Kolotuev et al., 2013; Khan et al., 2013). We have shown that the exocyst and its activator RAL-1 are essential for excretory cell lumen outgrowth, and that compromising exocyst activity (through RAL-1 depletion) prevents canalicular vesicle fusions with the lumen. Based on previous studies and our observations, we propose that RAL-1 and the exocyst promote lumenogenesis by directing lumenal vesicle fusion events. Consistent with such a role, we observed that loss of exocyst function resulted in delayed pearl recovery from hyperosmotic stress, which normally causes increased vesicle fusion events with the canal lumen (Kolotuev et al., 2013). In addition, increasing exocyst activity (through overexpression of RAL-1) resulted in a dramatic expansion of the excretory cell lumen. Controlling the activity of RAL-1 may therefore provide a mechanism to regulate the timing and rate of membrane addition during lumenogenesis.
Using polarized early embryonic cells, we have defined the relative contributions of RAL-1 and PAR proteins to exocyst localization and function. Several observations indicate that active RAL-1 recruits the exocyst to the membrane. First, removing RAL-1 caused exocyst components to become cytoplasmic. Second, expressing constitutively active RAL-1 recruited core exocyst components to ectopic membrane domains. Finally, the larval arrest phenotype of ral-1(MZ) mutants was remarkably similar to that displayed by sec-5(MZ) and sec-8(MZ) mutants, suggesting that exocyst regulation is the primary function for RAL-1 in C. elegans. We define a distinct function for PAR proteins in regulating exocyst asymmetry: removing PAR function by depleting PAR-3, PAR-6, or PKC-3, or by preventing polarized PAR localization via pac-1 mutation, caused a loss of exocyst asymmetry without preventing membrane association. Thus, while RAL-1 promotes exocyst membrane recruitment, PAR proteins spatially restrict the exocyst to a membrane subdomain. Although we defined these relationships in experimentally accessible early embryonic cells, the analogous localization pattern of RAL-1, PAR proteins, and the exocyst within the excretory cell suggests that a similar relationship holds true in this cell as well. Testing this hypothesis is complicated by the earlier essential function of PAR proteins and by maternal PAR protein contribution, and will require the development of new genetic tools to acutely remove PAR function at later developmental stages. Notably, a recent synthetic lethal RNAi screen identified genetic interactions between pkc-3 and the exocyst, consistent with PAR proteins and the exocyst functioning together in multiple contexts during C. elegans development (Jiu et al., 2014).
The functional relationship between PAR proteins and the exocyst has been difficult to address in other systems. For example, knockdown experiments in MDCK cysts revealed that PAR proteins and the exocyst are mutually required for one another’s asymmetric localization to the site of lumen formation (Bryant et al., 2010); it is not clear if this reflects true interdependency in PAR and exocyst localization, or whether removing PAR or exocyst function prevents the formation of a membrane domain or structure where the two protein complexes normally localize. Evidence from neuronal cell culture studies suggests that RalA may facilitate a direct interaction between Par6 and exocyst component Exo84 (Das et al., 2014). In Drosophila terminal tracheal cells, activity of the PAR protein aPKC is required for membrane enrichment of the exocyst component SEC-8 (Jones et al., 2014), although it is not known in this system whether the exocyst is similarly required for PAR protein localization. Using C. elegans early embryos, where PAR and exocyst function can be rapidly and acutely removed, we have shown that the exocyst clearly functions downstream of PAR proteins. PAR protein localization was unaffected by ral-1 or sec-5 mutation, and these mutants did not show the dramatic polarity phenotypes evident in par mutants.
How PAR proteins control exocyst localization remains unknown. One possibility is that PAR proteins physically bind the complex, enriching membrane-bound exocyst proteins within a distinct cortical domain. In support of this model, physical interactions have been observed between PAR proteins and several different exocyst complex members (see Introduction). Alternatively, given that constitutively active RAL-1 ectopically recruits the exocyst to sites lacking PAR proteins in early embryos, it is possible that PAR proteins asymmetrically enrich a Ral-activating protein, such as a guanine-nucleotide exchange factor (GEF), that locally activates RAL-1. For instance, PAR-3 recruits the Rac GEF TIAM1 to dendritic spines in neurons, and to tight junctions in epithelial cells, to spatially restrict Rac activation (Mertens et al., 2005; Chen and Macara, 2005; Zhang and Macara, 2006). In this way, PAR proteins would spatially restrict both exocyst membrane recruitment and asymmetry.
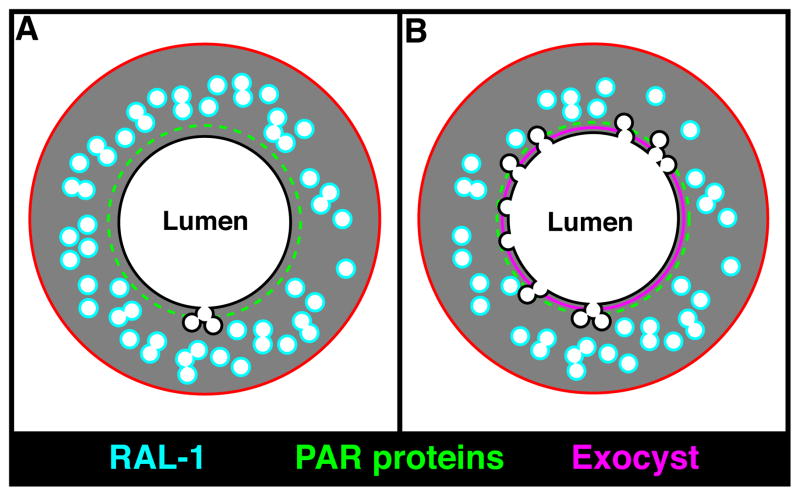
Based on these findings, we propose that lumen extension in the excretory cell occurs when the exocyst targets canalicular vesicles to dock at the growing lumenal surface, where RAL-1 and PAR proteins overlap (Figure 8). How do PAR proteins associate specifically along the lumenal membrane? One possibility is that an asymmetric PAR membrane domain, established in the excretory cell body, invades the cytoplasm to produce the canal lumen. For instance, lumenal membrane invasion occurs within the terminal cells of the Drosophila trachea (Gervais and Casanova, 2010), and the Ciona notochord (Denker et al., 2013). Following this initial polarization event, exocyst-mediated vesicle fusion would then function to extend and expand the growing lumenal membrane. This hypothesis is supported by the observation that the nascent excretory canal lumen appears to expand from its junction with the adjacent duct cell (Mancuso et al., 2012).
Figure 8. Model for excretory canal lumen formation.
(A) Schematic of the excretory canal shown in transverse section, with the PAR proteins (green) and RAL-1 (cyan) present at distinct but overlapping domains. (B) At regions containing both PAR proteins and active RAL-1, the exocyst (magenta) is recruited and triggers the docking and fusion of canalicular vesicles with the lumenal surface, leading to canal expansion and increased exchange of water and salt.
The mechanisms we have identified are likely to work in concert with the ezrin-radixin-moesin protein ERM-1, which localizes to the excretory canal lumenal scaffold and is essential for linear canal growth and structural maintenance (Göbel et al., 2004). Loss of ERM-1 results in short, cystic canals that fail to elongate (Khan et al., 2013). ERM-1 interacts with aquaporin AQP-8, which is present on canalicular vesicles, and is thought to help recruit AQP-8 to the lumenal surface. In turn, AQP-8 is thought to promote lumen expansion through water flux (Khan et al., 2013). Our findings show that RAL-1 and the exocyst are essential for canalicular fusion events, and are consistent with this model for ERM-1 function. However, aqp-8(MZ) null mutants are viable and form canals, in contrast to ral-1(MZ) and sec-5(MZ) mutants, indicating that ERM-1 recruitment of AQP-8 contributes to but is not essential for lumenogenesis.
Given the broad requirement for targeted vesicle fusion events in the lumenogenesis of seamless tubes, our findings suggest a mechanism for lumen formation that could operate in diverse cell types, including vertebrate vascular endothelial cells. Consistent with a conserved in vivo role for PAR proteins and the exocyst in seamless tube formation, par-6, aPKC, and exocyst components are required for intracellular lumenogenesis in Drosophila terminal tracheal cells (Jones and Metzstein, 2011; Jones et al., 2014). Together with findings in Drosophila tracheal cells, MDCK cysts (Bryant et al., 2010), and zebrafish intestinal cells (where aPKCλ is required for lumenogenesis) (Horne-Badovinac et al., 2001), our results suggest that intracellular and multicellular tubes, despite their fundamental differences in topology and organization, may form using common molecular mechanisms.
MATERIALS AND METHODS
Homolog identification
C. elegans Ral and exocyst homologs were determined by basic local alignment search tool (BLAST) search of the murine and Drosophila exocyst proteins against the C. elegans proteins (Wormbase release WS238). A single homolog was identified in the C. elegans genome for each gene. Reciprocal BLAST of C. elegans exocyst proteins against murine and Drosophila databases likewise identified highest scores for the respective homolog.
Strains
Strains used in this study are listed in Table S4. sec-8(ok2187) is an uncharacterized deletion allele isolated by the C. elegans Gene Knockout Consortium obtained from the Caenorhabditis Genetics Center (CGC, University of Minnesota), and was outcrossed four times. The ok2187 deletion was verified using primers flanking the deletion (5′-cggctcaattcgactctgcc-3′ and 5′-cctttccaagtgctactacttctgg-3′) and internal primers to confirm loss of the deleted sequence (5′-gacgcgatatatcatcatctgcagtc-3′ and 5′-gagaacttgcttctcgtattgaaggag-3′). ral-1(tm5205) is an uncharacterized deletion allele isolated by the National Bioresource Project (kindly provided by Shohei Mitani) and was outcrossed five times. The tm5205 deletion was verified with flanking primers (5′-ggcagaaatccaggaaaaagtgagg-3′ and 5′-gctttctgacgtaaaatacccaaaactcg-3′) and internal primers (5′-gtcgatttcccccgggttttc-3′ and 5′-cgacgtttttagagtctttgcatgcaaatttg-3′). The mutant sec-5(pk2358) was previously described (Frische et al., 2007) and was a kind gift from Fried Zwartkrius; we outcrossed the mutant three additional times. pkc-3(ne4250) was previously described (Fievet et al., 2012) and was a kind gift from Craig Mello; we outcrossed the mutant six times. All additional strains and mutants have been previously described, among which some were provided by the CGC.
Transgene construction
Psec-8::sec-8-mCherry, Psec-8::sec-8-zf1-mCherry, Psec-5::sec-5-zf1-yfp, Pral-1::yfp-ral-1, Pral-1::zf1-yfp-ral-1, Ppgp-12::mCherry were created by Gibson end-joining (Gibson et al., 2009). Fragments of genomic DNA or tags (see Table S5 for additional oligonucleotide information) were recombined into vector pJN566, which is a derivative of MosSCI vector pCFJ150 (Frøkjær-Jensen et al., 2008) modified to include a PmeI restriction site adjacent the unc-119 coding region (using primers 5′-PmeI-ggcctagttctagacattctc-3′ and 5′-PmeI- cactggccgtcgttttacac-3′). Prior to Gibson end-joining, pJN566 was linearized by digestion with PmeI. PCR fragments to be assembled contained 40 bp homology arms with adjacent fragments, or with vector sequences flanking the PmeI site.
Ppgp-12::yfp-ral-1, Ppgp-12::vha-5-gfp and Ppgp-12::vha-5-mCherry were generated by Gibson end-joining using vector pPD95.75 (Fire lab vector kit).
Psec-10::mCherry-sec-10 and Psec-15::sec-15-yfp were created by fosmid recombineering using galK selection and SW105 cells, as described (Tursun et al., 2009).
For Psec-10::mCherry-sec-10, the following homology arms were used to insert mCherry amplified from plasmid pBALU4 (Tursun et al., 2009):
5′-gtatattttatattcaaaagtggtttttcatgctttaggaacaaataattacag-3′,
5′-cggttcctgttctagatcctgtacatatgtcacatattgtccaccactcat-3′.
unc-119 was inserted into the LoxP site of Psec-10::mCherry-sec-10 fosmid using plasmid pLoxPunc119 and SW106 cells, as described (Zhang et al., 2008).
For Psec-15::sec-15-yfp, the following homology arms were used to insert yfp amplified from plasmid pBALU2 (Tursun et al., 2009):
5′-cgcaagaagcttctcgacacaattgtccgtcagttgaaactcctggaaatt-3′,
5′-taatcataccacagtaaaaggcaacataagtaacattaatttaaaaaatca-3′.
Psec-15::sec-15-yfp fosmid was then subcloned by gap repair into linearized pCFJ151 vector (Frøkjær-Jensen et al., 2008) using the following homology arms:
5′-gttcaaaaaaatagagggataaatgtttagttgtgttctgaactggtacac-3′,
5′-tactgagttgaataagtattgattcttctctaactgtccgtcattcataac-3′.
Phsp-16.2::gfp-ral-1CA was generated by Gateway cloning (Invitrogen). Endogenous ral-1 cDNA was amplified then cloned into the Gateway entry vector pDONR221, and then modified by PCR to generate a plasmid carrying ral-1G26V (ral-1CA) (Frische et al., 2007). This plasmid was then used in a Gateway LR reaction with a destination vector carrying the hsp-16.2 promoter driving GFP (Chan and Nance, 2013) and ral-1CA was inserted downstream of the GFP sequence.
par-3 RNAi is directed against an exon of par-3 and was cloned into the RNAi feeding vector pPD129.36 (Timmons et al., 2001).
Ppar-6::par-6-mCherry (Chan and Nance, 2013) and Ppar-3::par-3-gfp (Achilleos et al., 2010) plasmids and strains were described previously.
Worm transformation
Psec-8::sec-8-mCherry, Psec-8::sec-8-zf1-mCherry, Psec-5::sec-5-yfp, Psec-5::sec-5-zf1-yfp, Pral-1::yfp-ral-1, Pral-1::zf1-yfp-ral-1, Psec-10::mCherry-sec-10 were transformed into worms by microparticle bombardment as described (Praitis et al., 2001), with minor modifications. unc-119(ed3) mutant worms were bombarded with the above constructs, which all contain an unc-119 rescue cassette in cis. Plasmids were mixed 1:1 with pMA122 (Frøkjær-Jensen et al., 2012), which carries a heat-shock inducible peel-1 toxin. Transformed, non-Unc worms were propagated several generations, and then heat-shocked at 34°C for 2 hours to induce expression of the toxin, selecting against worms inheriting multicopy transgenes (those containing pMA122). At least two independent lines were analyzed for each construct.
Psec-8::sec-8-mCherry and Psec-15::sec-15-yfp were integrated into chromosome II in single copy by MosSCI (Frøkjær-Jensen et al., 2008). WM186 worms (Shirayama et al., 2012) (which contain the ttTi5605 Mos1 insertion on chromosome II, are unc-119(ed3), and are resistant to ivermectin) were injected with a DNA mixture including pTG96 (Psur-5::sur-5-gfp-NLS) (Yochem et al., 1998) (33 ng/μL), pJL44 (Phsp16.48::MosTase) (Frøkjær-Jensen et al., 2008) (33 ng/μL), pCCM416(Pmyo-2::avr-15) (Shirayama et al., 2012) (33 ng/μL) and the construct of interest (1 ng/μL); the vector backbone additionally includes unc-119(+) and homology arms flanking the ttTi5605 Mos1 site. Transmitting lines (non-Unc, SUR-5-GFP expressing) were heat-shocked for 2 hours at 34°C to induce germline transposase expression. After two subsequent generations, single-copy integrants were identified by ability to grow on NGM plates containing ivermectin (25 ng/mL) and by lack of SUR-5-GFP expression.
Ppgp-12::yfp-ral-1, Phsp-16.2::gfp-ral-1CA, Ppgp-12::vha-5-gfp, Ppgp-12::vha-5-mCherry, and Ppgp-12::mCherry were injected directly into the worm germline together with plasmids carrying either dominant rol-6(su1006) or sur-5-gfp to generate extrachromosomal arrays (Mello et al., 1991).
Heat shock over-expression of fluorescent transgenes
Worms were heat shocked as described previously (Chan and Nance, 2013). Embryos carrying an extrachromosomal array containing heat shock promoter hsp-16.2 driven transgenes were placed in a 34 degree C incubator for one hour, and then recovered at room temperature for 30 minutes. Then, embryos were either mounted on agarose pads and imaged live, or fixed then immunostained.
Immunostaining
Embryos were placed onto poly-L-lysine coated slides, freeze-cracked and fixed in methanol and paraformaldehyde as previously described (Anderson et al., 2008). The following primary antibodies and dilutions were used: rabbit anti-GFP, 1:2000 (Abcam), chicken anti-GFP, 1:1000 (Aves), rabbit anti-PAR-6, 1:20,000 (Schonegg and Hyman, 2006), mouse anti-PAR-3, 1:10 (Nance et al., 2003), rat anti-PKC-3, 1:400 (Tabuse et al., 1998), mouse anti-ERM-1, 1:50 (Developmental Hybridoma Studies Bank, University of Iowa). Fluorescently conjugated, species-specific, cross-adsorbed secondary antibodies were used to detect primary antibodies. Stained specimens were mounted in DABCO (Sigma) and imaged as described below.
Generation of maternal-zygotic mutants
Worms homozygous for ral-1, sec-5, or sec-8 mutations, and heterozygous for the respective ZF1-tagged rescuing transgene, were allowed to self-fertilize. 25% of the F1 progeny do not inherit the ZF1-tagged rescuing transgene (which is also expressed zygotically) and are therefore maternal-zygotic (MZ) mutants (protein inherited maternally from the transgene degrades in early embryos because of the ZF1 tag). A subset of embryos and larvae displaying the MZ phenotype were scored to confirm absence of transgene expression. sec-5(MZ) mutants were also obtained by allowing sec-5 homozygous mutants to self-fertilize and examining the resulting progeny.
RNAi
RNAi was performed using the feeding method (Timmons et al., 2001). For par RNAi, experiments were performed on HT115 bacteria carrying the empty vector pPD129.36, or derivatives carrying cDNA of par-3 (four repetitions) (this study, see above), par-6 (six repetitions) (Anderson et al., 2008) or pkc-3 (Chan and Nance, 2013). L4 worms were placed on plates seeded for 12 hours with bacterial cultures that had been grown for 9 hours at 37 C. Worms were left on plates for 24–48 hours and phenotypes were scored in the next generation in embryos. Analysis of PAR-6-GFP expression and localization was performed in parallel as a positive control for sufficient RNAi knockdown. zif-1 RNAi was performed as described (DeRenzo et al., 2003).
sec-8 RNAi clone was obtained from the Vidal cDNA RNAi library (clone ID Y106G6H.7). For RNAi dilution experiments, HT115 bacteria carrying the sec-8 RNAi plasmid were diluted with cultures grown simultaneously and carrying empty vector (pPD129.36). OD600 was measured after 9 hours in liquid culture (37°C) and serial dilutions were performed using equivalent amounts of bacteria from empty vector and sec-8 RNAi expressing bacteria. Strong RNAi (100% sec-8 RNAi) resulted in larval arrest in F1 worms, similar to sec-8(MZ) animals. Diluted RNAi (25%) produced no larval lethality, but sufficiently suppressed the yfp-ral-1 over-expression phenotype.
Microscopy & image processing
Differential interference contrast (DIC) using Nomarski optics and fluorescence images were collected using a Zeiss AxioImager, 63× 1.4 NA or 40× 1.3 NA objective, an Axiocam MRM camera and AxioVision software. In AxioVision software, all images were deconvolved with an identical algorithm. Images were cropped in ImageJ (NIH) and control and mutant images processed similarly using Photoshop (Adobe), with no γ adjustments. For all live imaging experiments, embryos and larvae were mounted onto pads made from 4% agarose in M9 or water at room temperature, except where noted. Immunostained embryos were mounted in DABCO (Sigma) mounting medium.
Hyperosmotic shock
The hyperosmotic shock protocol was performed as described with minor modifications (Kolotuev et al., 2013). L4 larval worms were transferred to NGM plates containing excess salt (500 mM NaCl) for 30 minutes. Worms were left on plates for 30 minutes and then transferred to standard NGM plates (50 mM NaCl). Within 20 minutes of return to NGM plates, pearl regions formed along the excretory canals in a subset of animals. Animals were scored for the persistence of pearls every 20 minutes on a Zeiss M2Bio fluorescence microscope. A small percentage of both ral-1 and sec-5 mutants on standard NGM plates had pearls along their canals prior to osmotic shock; only those lacking pearls were selected for the assay.
Electron microscopy
We collected L4 stage control and ral-1 mutant worms, where maternal protein in ral-1 mutants would be largely depleted. Sections were analyzed only in the anterior half of posterior canals, a region which by light microscopy appears largely normal in ral-1 mutants (see Figure 2). 10–15 worms were placed into 100 μm deep planchette hats with yeast paste. Hats were coated with hexadecane, sealed in the planchette holder and high pressure freezing was performed using a Wohlwend Compact HPF-01 High Pressure Freezer. Frozen hats were immediately transferred into liquid nitrogen, then to tubes containing 2% osmium tetroxide and 1% uranyl acetate in acetone at liquid nitrogen temperature. Samples in tubes were moved from liquid nitrogen into a Leica EM AFS2 freeze substitution unit and left at −90° C for 96 hours. Unit temperature was raised 5°C per hour to −60° C, incubated for 12 hours, raised to −30°C for an additional 12 hours, and finally raised to 0° C for 4 hrs. Three one hour exchanges of pure acetone at 0°C were used to remove osmium. Infiltration at room temperature began with a 1:1 mixture of acetone and Embed 812 (Electron Microscopy Sciences, Hatfield, PA) for 1 hour, followed by 1:2 overnight. Samples were allowed to sit in pure epon for 4 hours before embedding. Worms were flat embedded with aclar embedding film and polymerized at 60°C. Serial semi-thin sections were cut (UC6 microtome; Leica Microsystems) at 0.5 μm and stained with 1% Toluidine Blue to evaluate the quality of preservation and find the area of interest. Thin sections (60nm for morphology or 200nm for tomography) were cut and stained with uranyl acetate and lead citrate by standard methods. Stained grids were examined under a Philips CM-12 electron microscope and photographed with a Gatan (4k × 2.7k) digital camera.
Collection and Analysis of EM tomograms
For tomogram collection, samples were tilted between −70 degrees and +70 degrees at one-degree intervals and electron micrographs were recorded at 15,000 to 25,000-fold magnification with a CM200 microscope (FEI Corporation, Hillsboro, OR, USA) equipped with a 2k × 2k CCD camera (TVIPS, Gauting, Germany). Dual-axis tilt series were collected with a high-tilt tomography holder (Fischione, Export, PA, USA) and the serial EM program for automated data collection (Mastronarde, 2005). A second tilt series of the same area was collected after manually rotating the specimen support by ninety degrees. Dual-axis tomographic data were reconstructed by IMOD (Kremer et al., 1996). For modeling, features of interest within the tomogram volumes were segmented manually using the software AMIRA (Mercury Computer Systems, San Diego, CA, USA).
Statistics
For hyperosmotic shock experiments, total worms assayed over several experimental repetitions are represented in Figure 4G, and bars represent the 95% confidence interval of each percentage. The experiment was repeated six times for controls (all with <10% pearls at 60 min time point), five times for ral-1 (all with >50% pearls at 60 min), and twice for sec-5 (each with >50% pearls at 60 min). Statistical comparison was performed using a two-tailed Chi-squared test. For yfp-ral-1 over-expression and suppression, experiments were repeated three times, and each individual repetition contained a sample size of n>20. Samples did not vary significantly within each individual group between trials (as determined by Chi-squared test). Total n worms scored over the three trials were then grouped together and overall worms scored were compared between groups using a Chi-squared test.
Supplementary Material
Highlights.
The exocyst and RAL-1 are required for single-cell lumen extension
The exocyst and RAL-1 mediate polarized vesicle fusion in the canal cell
RAL-1 recruits the exocyst to the cell membrane
PAR proteins polarize the membrane distribution of the exocyst
Acknowledgments
We thank Jane Hubbard, Niels Ringstad and Diana Klompstra for critical reading of the manuscript, and Yuliya Zilberman for helpful discussions. We thank the NYULMC OCS Microscopy Core, especially Kristen Dancel and Chris Petzold, for their assistance with TEM. We thank the CGC, Eve Stringham, Fried Zwartkruis, Shohei Mitani, Christopher Trzepacz and Craig Mello for strains. S.A and J.N. designed the experiments and interpreted results. S.A. generated all strains and performed all experiments, except electron microscopy, which was performed by E.C. S.A. and J.N. wrote the manuscript. This work was funded by NIH grants R01GM098492 and R01GM078341 to J.N. and NIH NRSA fellowship F30DK093197 to S.A.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achilleos A, Wehman AM, Nance J. PAR-3 mediates the initial clustering and apical localization of junction and polarity proteins during C. elegans intestinal epithelial cell polarization. Development. 2010;137:1833–1842. doi: 10.1242/dev.047647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DC, Gill JS, Cinalli RM, Nance J. Polarization of the C. elegans embryo by RhoGAP-mediated exclusion of PAR-6 from cell contacts. Science. 2008;320:1771–1774. doi: 10.1126/science.1156063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew DJ, Ewald AJ. Morphogenesis of epithelial tubes: Insights into tube formation, elongation, and elaboration. Dev Biol. 2010;341:34–55. doi: 10.1016/j.ydbio.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd C. Vesicles carry most exocyst subunits to exocytic sites marked by the remaining two subunits, Sec3p and Exo70p. J Cell Biol. 2004;167:889–901. doi: 10.1083/jcb.200408124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DM, Datta A, Rodríguez-Fraticelli AE, Peränen J, Martín-Belmonte F, Mostov KE. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol. 2010;12:1035–1045. doi: 10.1038/ncb2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brymora A, Valova VA, Larsen MR, Roufogalis BD, Robinson PJ. The brain exocyst complex interacts with RalA in a GTP-dependent manner: identification of a novel mammalian Sec3 gene and a second Sec15 gene. J Biol Chem. 2001;276(32):29792–29797. doi: 10.1074/jbc.C100320200. [DOI] [PubMed] [Google Scholar]
- Buechner M, Hall DH, Bhatt H, Hedgecock EM. Cystic canal mutants in Caenorhabditis elegans are defective in the apical membrane domain of the renal (excretory) cell. Dev Biol. 1999;214:227–241. doi: 10.1006/dbio.1999.9398. [DOI] [PubMed] [Google Scholar]
- Chan E, Nance J. Mechanisms of CDC-42 activation during contact-induced cell polarization. J Cell Sci. 2013;126:1692–1702. doi: 10.1242/jcs.124594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Macara IG. Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat Cell Biol. 2005;7:262–269. doi: 10.1038/ncb1226. [DOI] [PubMed] [Google Scholar]
- Das A, Gajendra S, Falenta K, Oudin MJ, Peschard P, Feng S, et al. RalA promotes a direct exocyst-Par6 interaction to regulate polarity in neuronal development. J Cell Sci. 2014;127:686–699. doi: 10.1242/jcs.145037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GE, Camarillo CW. An a2b1 integrin-dependent pinocytic mechanism involving intracellular vacuole formation and coalescence regulates capillary lumen and tube formation in three-dimensional collagen matrix. Exp Cell Res. 1996;224:39–51. doi: 10.1006/excr.1996.0109. [DOI] [PubMed] [Google Scholar]
- DeRenzo C, Reese KJ, Seydoux G. Exclusion of germ plasm proteins from somatic lineages by cullin-dependent degradation. Nature. 2003;424:685–689. doi: 10.1038/nature01887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denker E, Bocina I, Jiang D. Tubulogenesis in a simple cell cord requires the formation of bi-apical cells through two discrete Par domains. Development. 2013;140:2985–2996. doi: 10.1242/dev.092387. [DOI] [PubMed] [Google Scholar]
- Fievet BT, Rodriguez J, Naganathan S, Lee C, Zeiser E, Ishidate T, Shirayama M, Grill SW, Ahringer J. Systematic genetic interaction screens uncover cell polarity regulators and functional redundancy. Nat Cell Biol. 2012;15:103–112. doi: 10.1038/ncb2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frische EW, Pellis-van Berkel W, van Haaften G, Cuppen E, Plasterk RHA, Tijsterman M, Bos JL, Zwartkruis FJT. RAP-1 and the RAL-1/exocyst pathway coordinate hypodermal cell organization in Caenorhabditis elegans. EMBO J. 2007;26:5083–5092. doi: 10.1038/sj.emboj.7601922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøkjær-Jensen C, Wayne Davis M, Jorgensen EM. Single-copy insertion of transgenes in Caenorhabditis elegans. Nat Genet. 2008;40:1375–1383. doi: 10.1038/ng.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøkjær-Jensen C, Davis MW, Ailion M, Jorgensen EM. Improved Mos1-mediated transgenesis in C. elegans. Nat Meth. 2012;9:117–118. doi: 10.1038/nmeth.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais L, Casanova J. In vivo coupling of cell elongation and lumen formation in a single cell. Curr Biol. 2010;20:359–366. doi: 10.1016/j.cub.2009.12.043. [DOI] [PubMed] [Google Scholar]
- Gibson DG, Young L, Chuang R-Y, Venter JC, Hutchison CA, Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Meth. 2009;6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- Göbel V, Barrett PL, Hall DH, Fleming JT. Lumen morphogenesis in C. elegans requires the membrane-cytoskeleton linker erm-1. Dev Cell. 2004;6:865–873. doi: 10.1016/j.devcel.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Hahn-Windgassen A, Van Gilst MR. The Caenorhabditis elegans HNF4alpha Homolog, NHR-31, mediates excretory tube growth and function through coordinate regulation of the vacuolar ATPase. PLoS Genet. 2009;5:e1000553. doi: 10.1371/journal.pgen.1000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Guo W. The exocyst complex in polarized exocytosis. Curr Opin Cell Biol. 2009;21:537–542. doi: 10.1016/j.ceb.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwig L, Blum Y, Krudewig A, Ellertsdottir E, Lenard A, Belting H-G, Affolter M. Distinct cellular mechanisms of blood vessel fusion in the zebrafish embryo. Curr Biol. 2011;21:1942–1948. doi: 10.1016/j.cub.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Hinoi T, Kishida S, Koyama S, Ikeda M, Matsuura Y, Kikuchi A. Post-translational modifications of Ras and Ral are important for the action of Ral GDP dissociation stimulator. J Biol Chem. 1996;271:19710–19716. doi: 10.1074/jbc.271.33.19710. [DOI] [PubMed] [Google Scholar]
- Horne-Badovinac S, Lin D, Waldron S, Schwarz M, Mbamalu G, Pawson T, Jan Y, Stainier DY, Abdelilah-Seyfried S. Positional cloning of heart and soul reveals multiple roles for PKC lambda in zebrafish organogenesis. Curr Biol. 2001;11:1492–1502. doi: 10.1016/s0960-9822(01)00458-4. [DOI] [PubMed] [Google Scholar]
- Iruela-Arispe ML, Davis GE. Cellular and molecular mechanisms of vascular lumen formation. Dev Cell. 2009;16:222–231. doi: 10.1016/j.devcel.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiu Y, Hasygar K, Tang L, Liu Y, Holmberg CI, Bürglin TR, Hietakangas V, Jäntti J. par-1, atypical pkc, and PP2A/B55 sur-6 are implicated in the regulation of exocyst-mediated membrane trafficking in Caenorhabditis elegans. G3. 2014;4:173–183. doi: 10.1534/g3.113.006718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston DS, Ahringer J. Cell polarity in eggs and epithelia: parallels and diversity. Cell. 2010;141:757–774. doi: 10.1016/j.cell.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Jones TA, Metzstein MM. A novel function for the PAR complex in subcellular morphogenesis of tracheal terminal cells in Drosophila melanogaster. Genetics. 2011;189:153–164. doi: 10.1534/genetics.111.130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Nikolova LS, Schjelderup A, Metzstein MM. Exocyst-mediated membrane trafficking is required for branch outgrowth in Drosophila tracheal terminal cells. Dev Biol. 2014 doi: 10.1016/j.ydbio.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan LA, Zhang H, Abraham N, Sun L, Fleming JT, Buechner M, Hall DH, Göbel V. Intracellular lumen extension requires ERM-1-dependent apical membrane expansion and AQP-8-mediated flux. Nat Cell Biol. 2013;15:143–156. doi: 10.1038/ncb2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolotuev I, Hyenne V, Schwab Y, Rodriguez D, Labouesse M. A pathway for unicellular tube extension depending on the lymphatic vessel determinant Prox1 and on osmoregulation. Nat Cell Biol. 2013;15:157–168. doi: 10.1038/ncb2662. [DOI] [PubMed] [Google Scholar]
- Kremer JR, Mastronarde DN, McIntosh JR. Computer visualization of three-dimensional image data using IMOD. J Struct Biol. 1996;116:71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- Lalli G. RalA and the exocyst complex influence neuronal polarity through PAR-3 and aPKC. J Cell Sci. 2009;122:1499–1506. doi: 10.1242/jcs.044339. [DOI] [PubMed] [Google Scholar]
- Lipschutz JH, Guo W, O’Brien LE, Nguyen YH, Novick P, Mostov KE. Exocyst is involved in cystogenesis and tubulogenesis and acts by modulating synthesis and delivery of basolateral plasma membrane and secretory proteins. Mol Biol Cell. 2000;11:4259–4275. doi: 10.1091/mbc.11.12.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Guo W. The exocyst complex in exocytosis and cell migration. Protoplasma. 2012;249:587–597. doi: 10.1007/s00709-011-0330-1. [DOI] [PubMed] [Google Scholar]
- Mancuso VP, Parry JM, Storer L, Poggioli C, Nguyen KCQ, Hall DH, Sundaram MV. Extracellular leucine-rich repeat proteins are required to organize the apical extracellular matrix and maintain epithelial junction integrity in C. elegans. Development. 2012;139:979–990. doi: 10.1242/dev.075135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronarde DN. Automated electron microscope tomography using robust prediction of specimen movements. J Struct Biol. 2005;152:36–51. doi: 10.1016/j.jsb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens AEE, Rygiel TP, Olivo C, van der Kammen R, Collard JG. The Rac activator Tiam1 controls tight junction biogenesis in keratinocytes through binding to and activation of the Par polarity complex. J Cell Biol. 2005;170:1029–1037. doi: 10.1083/jcb.200502129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskalenko S, Tong C, Rosse C, Mirey G, Formstecher E, Daviet L, Camonis J, White MA. Ral GTPases regulate exocyst assembly through dual subunit interactions. J Biol Chem. 2003;278:51743–51748. doi: 10.1074/jbc.M308702200. [DOI] [PubMed] [Google Scholar]
- Moskalenko S, Henry DO, Rosse C, Mirey G, Camonis JH, White MA. The exocyst is a Ral effector complex. Nature. 2002;4:66–72. doi: 10.1038/ncb728. [DOI] [PubMed] [Google Scholar]
- Nance J, Zallen JA. Elaborating polarity: PAR proteins and the cytoskeleton. Development. 2011;138:799–809. doi: 10.1242/dev.053538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance J, Munro EM, Priess JR. C. elegans PAR-3 and PAR-6 are required for apicobasal asymmetries associated with cell adhesion and gastrulation. Development. 2003;130:5339–5350. doi: 10.1242/dev.00735. [DOI] [PubMed] [Google Scholar]
- Nelson FK, Riddle DL. Functional study of the Caenorhabditis elegans secretory-excretory system using laser microsurgery. J Exp Zool. 1984;231:45–56. doi: 10.1002/jez.1402310107. [DOI] [PubMed] [Google Scholar]
- Nelson FK, Albert PS, Riddle DL. Fine structure of the Caenorhabditis elegans secretory-excretory system. J Ultrastruct Res. 1983;82:156–171. doi: 10.1016/s0022-5320(83)90050-3. [DOI] [PubMed] [Google Scholar]
- Praitis V, Casey E, Collar D, Austin J. Creation of low-copy integrated transgenic lines in Caenorhabditis elegans. Genetics. 2001;157:1217–1226. doi: 10.1093/genetics/157.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese KJ, Dunn MA, Waddle JA, Seydoux G. Asymmetric segregation of PIE-1 in C. elegans is mediated by two complementary mechanisms that act through separate PIE-1 protein domains. Mol Cell. 2000;6:445–455. doi: 10.1016/s1097-2765(00)00043-5. [DOI] [PubMed] [Google Scholar]
- Rosse C, Formstecher E, Boeckeler K, Zhao Y, Kremerskothen J, White MD, Camonis JH, Parker PJ. An aPKC-Exocyst Complex Controls Paxillin Phosphorylation and Migration through Localised JNK1 Activation. PLoS Biol. 2009;7:e1000235. doi: 10.1371/journal.pbio.1000235.g007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonegg S, Hyman AA. CDC-42 and RHO-1 coordinate acto-myosin contractility and PAR protein localization during polarity establishment in C. elegans embryos. Development. 2006;133:3507–3516. doi: 10.1242/dev.02527. [DOI] [PubMed] [Google Scholar]
- Schottenfeld-Roames J, Ghabrial AS. Whacked and Rab35 polarize dynein-motor-complex-dependent seamless tube growth. Nat Cell Biol. 2012;14:386–393. doi: 10.1038/ncb2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama M, Seth M, Lee H-C, Gu W, Ishidate T, Conte D, Mello CC. piRNAs Initiate an Epigenetic Memory of Nonself RNA in the C. elegans Germline. Cell. 2012;150:65–77. doi: 10.1016/j.cell.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara K, Asano S, Tanaka K, Iwamatsu A, Okawa K, Ohta Y. The exocyst complex binds the small GTPase RalA to mediate filopodia formation. Nature Publishing Group. 2002;4(1):73–78. doi: 10.1038/ncb720. [DOI] [PubMed] [Google Scholar]
- Tabuse Y, Izumi Y, Piano F, Kemphues KJ, Miwa J, Ohno S. Atypical protein kinase C cooperates with PAR-3 to establish embryonic polarity in Caenorhabditis elegans. Development. 1998;125:3607–3614. doi: 10.1242/dev.125.18.3607. [DOI] [PubMed] [Google Scholar]
- Timmons L, Court DL, Fire A. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene. 2001;263:103–112. doi: 10.1016/s0378-1119(00)00579-5. [DOI] [PubMed] [Google Scholar]
- Totong R, Achilleos A, Nance J. PAR-6 is required for junction formation but not apicobasal polarization in C. elegans embryonic epithelial cells. Development. 2007;134:1259–1268. doi: 10.1242/dev.02833. [DOI] [PubMed] [Google Scholar]
- Tursun B, Cochella L, Carrera I, Hobert O. A toolkit and robust pipeline for the generation of fosmid-based reporter genes in C. elegans. PLoS ONE. 2009;4:e4625. doi: 10.1371/journal.pone.0004625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam EM, Robinson PJ. Ral: mediator of membrane trafficking. Int J Biochem Cell Biol. 2006;38:1841–1847. doi: 10.1016/j.biocel.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Yochem J, Gu T, Han M. A new marker for mosaic analysis in Caenorhabditiselegans indicates a fusion between hyp6 and hyp7, two major components of the hypodermis. Genetics. 1998;149:1323–1334. doi: 10.1093/genetics/149.3.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Macara IG. The polarity protein PAR-3 and TIAM1 cooperate in dendritic spine morphogenesis. Nat Cell Biol. 2006;8:227–237. doi: 10.1038/ncb1368. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Nash L, Fisher AL. A simplified, robust, and streamlined procedure for the production of C. elegans transgenes via recombineering. BMC Dev Biol. 2008;8:119. doi: 10.1186/1471-213X-8-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Fang L, Chen N, Johnsen RC, Stein L, Baillie DL. Distinct regulatory elements mediate similar expression patterns in the excretory cell of Caenorhabditis elegans. J Biol Chem. 2005;280:38787–38794. doi: 10.1074/jbc.M505701200. [DOI] [PubMed] [Google Scholar]
- Zuo X, Fogelgren B, Lipschutz JH. The small GTPase Cdc42 is necessary for primary ciliogenesis in renal tubular epithelial cells. J Biol Chem. 2011;286:22469–22477. doi: 10.1074/jbc.M111.238469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X, Guo W, Lipschutz JH. The exocyst protein Sec10 is necessary for primary ciliogenesis and cystogenesis in vitro. Mol Biol Cell. 2009;20:2522–2529. doi: 10.1091/mbc.E08-07-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.