Abstract
Endothelial cells (ECs) line the microvasculature and constitute a barrier between the vessel lumen and surrounding tissues. ECs inform circulating immune cells of the health and integrity of surrounding tissues, recruiting them in response to pathogens and tissue damage. ECs play an active role in the transmigration of immune cells across the vessel wall. We have discovered important differences in the properties of ECs on soft hydrogel substrates of varying stiffness, in comparison to glass. Primary ECs from several human sources were tested; all formed monolayers normally on soft substrates. EC monolayers formed more mature cell-cell junctions on soft substrates, relative to glass, based on increased recruitment of vinculin and F-actin. EC monolayers supported transendothelial migration (TEM) on soft substrates. Immune cells, including peripheral blood lymphocytes (PBLs) and natural killer cells, showed decreasing numbers of paracellular (between-cell) transmigration events with decreasing substrate stiffness, while the number of transcellular (through-cell) events increased for PBLs. Melanoma cancer cells showed increased transmigration with decreased stiffness. Our findings demonstrate that endothelial monolayers respond to the mechanical properties of their surroundings, which can regulate the integrity and function of the monolayer independently from inflammatory signals. Soft hydrogel substrates are a more appropriate and physiological model for tissue environments than hard substrates, with important implications for the experimental analysis of
Keywords: transendothelial migration, soft substrates, endothelial cells, lymphocytes, NK cells, cancer cells
Introduction
Microvascular blood vessels are important conduits for exchange of nutrients and waste products. In addition, they provide access for immune cells to tissues for maintenance, repair, and response to pathogens. Endothelial cells (ECs) line these vessels and inform circulating immune cells of the state of the surrounding tissue. ECs recruit and activate specific immune cells to migrate across the endothelium, into the surrounding tissue, under a variety of physiological and pathological conditions [Vestweber, 2007]. To maintain the integrity of the blood vessel during immune cell transmigration, ECs play an active role—creating, enlarging and closing conduits that allow cells to enter and exit the bloodstream without drastic changes in the permeability of the vessel [Muller, 2009; Sage and Carman, 2009; Wittchen, 2009].
The route for transendothelial migration (TEM) may be between ECs (paracellular) or directly through one EC (transcellular) [Engelhardt and Wolburg, 2004]. The paracellular route involves controlled relaxation of EC-cell junctions to create an opening through which cells can pass [Muller, 2009]. The transcellular route requires exquisite control of membrane remodeling [Muller, 2014]; the EC opens and then closes a conduit through which a single immune cell passes [Engelhardt and Wolburg, 2004].
TEM of immune cells occurs in response to pathogenic tissue damage. Immune cell TEM can be part of an adaptive immune response, such as rapid homing of T lymphocytes to sites of inflammation, or an innate immune response, such as recruitment of natural killer cells (NK cells), a class of lymphocytes designed to kill pathogen-infected or cancerous cells [Vivier et al., 2011]. Both paracellular and transcellular routes of TEM are taken by T lymphocytes and NK cells while crossing the endothelium to reach affected tissues [Muller, 2014].
In cancer, the TEM pathway is co-opted to allow metastasizing cells to transit the vessel wall, into and out of the circulation [Fazakas et al., 2011; Zervantonakis et al., 2012; Reymond et al., 2013]. Many of these transmigration events occur in the absence of inflammatory signals, suggesting that other factors, such as mechanical stress or properties of the surrounding tissue, may regulate transmigration [Stroka and Aranda-Espinoza, 2011]. We hypothesized that the mechanical properties of the underlying tissue could alter EC monolayers and influence the rates and routes of TEM of immune cells and cancer cells.
Here, to test this hypothesis, we investigated the effects of substrate stiffness on the integrity and function of endothelial monolayers, using tunable hydrogels. Polyacrylamide gels can serve as substrates to support mammalian cell growth in culture [Pelham and Wang, 1997], with the elasticity of the gel being proportional to the extent of crosslinking by bis-acrylamide, allowing one to tune the stiffness of the hydrogel [Discher et al., 2005]. We tested a range of bis-acrylamide concentrations corresponding to a range of Young’s modulus of elasticity from ~10 kPa (0.1% bis-acrylamide) to ~90 kPa (0.8% bis-acrylamide) [Tse and Engler, 2010]. Human tissues range in stiffness from 1 kPa (e.g., brain) to 180 kPa (e.g., kidney) [McKee et al., 2011]. Cartilage and bone have elastic moduli in the GPa range [Bar-On and Wagner, 2013], and even these substances are 10–20 times softer than glass. We tested how the stiffness of hydrogel substrates affected the properties of endothelial monolayers comparing primary human ECs derived from umbilical vein (HUVEC), dermal microvasculature (HDMVEC), and brain microvasculature (HBMEC).
Results
ECs From Different Tissues on Substrates of Varying Stiffness
We asked how ECs from three different human sources—umbilical vein, dermal microvasculature, and brain microvasculature—responded to inflammatory and mechanical cues. First, for monolayers on glass, treatment of HUVEC, HDMVEC, and HBMEC cells with TNFα led to similar levels of increased expression of ICAM-1 (Fig. 1). We observed no significant differences in actin cytoskeleton organization, based on F-actin staining, when comparing the three cell types with each other or when comparing plus and minus TNFα (Fig. 1A).
Fig. 1.

Induction of ICAM-1 by TNFα treatment of ECs. A: Anti-ICAM-1 staining shows upregulation in EC monolayers from three different primary sources in response to treatment with TNFα. Scale bar, 50 μm. B: Expression of ICAM-1 by immunoblot.
We asked how the three EC types would adapt to changes in their mechanical environment by plating them on hydrogels of varying stiffness (Fig. 2). ECs from each source formed visually similar monolayers on glass and polyacrylamide hydrogels with 0.2–0.8% bis-acrylamide, corresponding to 20–90 kPa. However, monolayers on 0.1% bis-acrylamide hydrogels (10 kPa) were often incomplete; cells did not cover the entire surface of the hydrogel despite being present at the same density. On lower stiffness substrates, with elasticity 0.5–2 kPa, ECs did not form monolayers; instead, they formed networks with numerous large gaps. On 0.2 kPa substrates, ECs grew as clumps and did not spread.
Fig. 2.
ECs do not form monolayers on the softest substrates. DIC images of HDMVEC ECs on polyacrylamide hydrogels of 1 and 0.2 kPa. Cells form clumps and networks but not monolayers. Scale bar, 100 μm.
Substrate Stiffness Influences EC Junction Formation
We asked whether loss of monolayer integrity at low substrate stiffnesses was accompanied by fewer or weaker cell-cell adhesions. Among EC junction components, vinculin is important to form immature focal adherens cell-cell junctions [Huveneers et al., 2012]. Interactions of vinculin with VE-cadherin and F-actin play important roles in mechano-sensing and mechanotransduction during formation of EC junctions [Gulino-Debrac, 2013; Huveneers and de Rooij, 2013]. As junctions mature and get larger, they become linear. Focal adherens junctions have a serrated morphology. They are composed of patches of VE-cadherin oriented perpendicular to the cell edge, and they have vinculin-rich adhesion complexes at their ends. Linear junctions have a continuous line of VE-cadherin parallel to the cell edge. They contain relatively little vinculin, except at focal adhesions that are not attached to the junction [Huveneers et al., 2012]. Using these criteria, we assessed the maturation state of junctions based the pattern and intensity of fluorescence staining for VE-cadherin, vinculin and F-actin in EC monolayers allowed to form on glass and on 0.1–0.8% bis-acrylamide hydrogels.
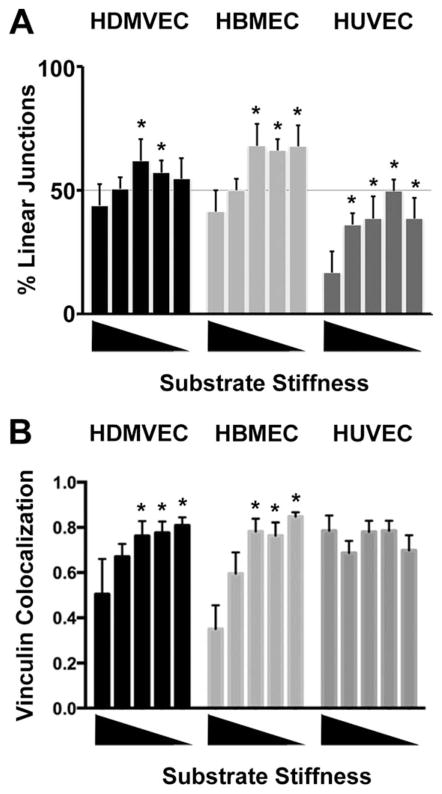
The morphology and molecular composition of junctions was different for ECs grown on glass compared with hydrogels. On glass, the morphology of cell junctions was predominantly serrated as opposed to linear for all three types of ECs—HDMVECs, HBMECs and HUVECS (Figs. 3–5, top rows). In addition, the intensity of vinculin staining was greater at serrated junctions than at linear junctions (Figs. 3–5, top rows). In contrast, when ECs were grown on hydrogel substrates, the morphology of the junctions was predominantly linear, for HDMVECs and HBMECs (Fig. 6A). HUVECs showed a significantly greater percentage of linear junctions on hydrogel substrates than on glass (Fig. 6A). The intensity of vinculin staining at cell junctions was greater for cells on hydrogels than on glass, for HDMVECs and HBMECs but not HUVECs (Fig. 6B). The presence of stress fibers in close proximity and parallel to the linear junctions prevented accurate Pearson’s coefficient analysis, even at high magnification.
Fig. 3.
Cell junction formation in HDMVEC monolayers on soft substrates. Characterization of junction structure and molecular composition by fluorescence imaging with anti-VE-cadherin, anti-vinculin and fluorescent phalloidin. Percentages indicate bis-acrylamide concentrations. Scale bar, 50 μm.
Fig. 5.
Cell junction formation in HUVEC monolayers on soft substrates. Characterization of junction structure and molecular composition by fluorescence imaging with anti-VE-cadherin, anti-vinculin and fluorescent phalloidin. Percentages indicate bis-acrylamide concentrations. Scale bar, 50 μm.
Fig. 6.
Junction morphology and vinculin content on soft substrates. A: The lengths of linear and serrated junctions were measured for each cell type on each substrate and the percentages of linear junctions are plotted. B: Pearson’s coefficients of correlation for vinculin and VE-cadherin at cell junctions for each cell type on each substrate. For both panels, the mean value is plotted, along with one SEM. Substrates were ordered by stiffness from highest (glass) to lowest (0.1% bis-acrylamide PA hydrogel). Values that are statistically significant (P <0.01) compared to the value for glass are indicated by asterisks.
We visualized the intensity of vinculin staining specifically at linear junctions, by comparing anti-vinculin with anti-VE-cadherin intensities, at higher magnification. For HDMVECs, the content of vinculin at linear junctions appeared highest for hydrogels with bis-acrylamide concentrations of 0.4% and lower (Fig. 7). For HBMECs, the vinculin content of linear junctions appeared highest for bis-acrylamide concentrations of 0.2% and lower (Fig. 8). In contrast, HUVECs showed high vinculin content at linear junctions on all substrate stiffnesses, including all bis-acrylamide concentration gels and even glass (Fig. 9).
Fig. 7.
Effect of substrate stiffness on mature junctions in HDMVECs. Fluorescence images reveal that the recruitment of vinculin and F-actin to VE-cadherin-positive mature junctions between adjacent cells (arrows) increases as substrate stiffness decreases. Percentages indicate bis-acrylamide concentrations. Scale bar, 10 μm.
Fig. 8.
Effect of substrate stiffness on mature junctions in HBMECs. Fluorescence images reveal that the recruitment of vinculin and F-actin to VE-cadherin-positive mature junctions between adjacent cells (arrows) increases as substrate stiffness decreases. Percentages indicate bis-acrylamide concentrations. Scale bar, 10 μm.
Fig. 9.
Effect of substrate stiffness on mature junctions in HUVECs. Fluorescence images reveal that the recruitment of vinculin and F-actin to VE-cadherin-positive mature junctions between adjacent cells (arrows) does not change as substrate stiffness decreases. Percentages indicate bis-acrylamide concentrations. Scale bar, 10 μm.
Unexpectedly, the results for F-actin differed from those for vinculin. The content of F-actin at linear junctions, based on fluorescent phalloidin staining, was similar for each of the three types of EC and each of the substrates of different stiffness (Figs. 7–9).
Transendothelial Migration
We asked whether differences in EC cell-cell junctions on substrates of varying stiffness would be accompanied by functional changes in the process of TEM. Immune cells, including peripheral blood lymphocytes (PBLs) and natural killer lymphocytes (NK cells), transmigrate through endothelial monolayers via both paracellular and transcellular routes [Muller, 2011]. We hypothesized that differences in cell-cell junctions would differentially affect the paracellular and transcellular routes, with stronger cell-cell junctions on lower stiffness substrates leading to a decrease in the efficiency of paracellular transmigration. We found that for PBLs, the median number of paracellular-route transmigration events decreased from five per high-power field on glass to two on 0.1% bis-acrylamide PA gels, while the median number of transcellular-route events increased from one to five (Fig. 10, left panel). These values corresponded to an increase in the percentage of transcellular-route events from 17% on glass to 73% on 0.1% bis-acrylamide PA gels (Fig. 10, right panel).
Fig. 10.

Effect of substrate stiffness on transendothelial migration of PBLs. PBLs were added to the top of HDMVEC monolayers and allowed to transmigrate. The number of transmigration events and the route taken is plotted on the left, with percentage on the right. Red circles represent the transcellular route, and blue circles represent the paracellular route. Each plotted point corresponds to one experiment, in which five high power fields were scored. Black bars indicate the mean of the values for the three experiments. Statistically significant differences (P <0.01) compared to glass are indicated by asterisks.
For NK cells, we found that the median number of paracellular-route TEM events decreased from 21 per high-power field (hpf) on glass to six/hpf on 0.1% bis-acrylamide PA gels, while the median number of transcellular-route events decreased from 35/hpf to 21/hpf (Fig. 11, left panel). The decrease in paracellular TEM events was greater than the decrease in transcellular events, such that the percentage of transcellular-route events increased from 60% on glass to 76% on 0.1% bis-acrylamide PA gels (Fig. 11, right panel).
Fig. 11.
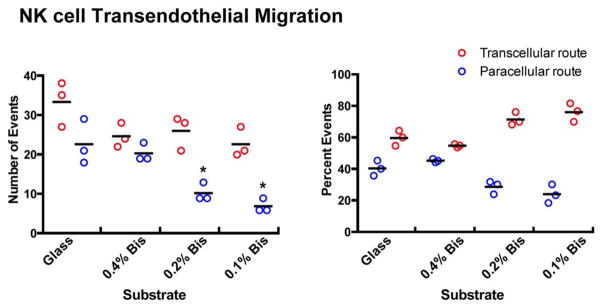
Effect of substrate stiffness on transendothelial migration of NK cells. NK cells were added to the top of HDMVEC monolayers and allowed to transmigrate. The number of transmigration events and the route taken is plotted on the left, with percentage on the right. Each plotted point corresponds to one experiment, in which five high power fields were scored. Black bars indicate the mean of the values for the three experiments. Statistically significant differences (P <0.01) compared to glass are indicated by asterisks. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
We asked how substrate stiffness affected TEM by cancer cells, in comparison with immune cells. Melanoma cells from the uvea of the eye metastasize to distant sites via the bloodstream [Clarijs et al., 2001]. We examined TEM of melanoma cells through HDMVEC monolayers on glass versus hydrogels of different stiffnesses, without treating the monolayer with TNFα as we did for immune cells. Here, time-lapse DIC movies were used to follow individual migrating cancer cells and determine the route of TEM. In contrast to immune cells, melanoma cells migrated only via the paracellular route; no transcellular-route events were observed. On glass, 2.3% ± 1.2% (mean ± SEM) of melanoma cells per field transmigrated. The values were higher on soft substrates: 27% ± 3% on 0.8% bis-acrylamide PA and 41% ± 9% on 0.1% bis-acrylamide PA (Fig. 12). Therefore, melanoma cells showed increased levels of TEM, via the paracellular route, when endothelial monolayers were cultured on soft substrates, compared to glass, in striking contrast to our findings with migrating immune cells.
Fig. 12.
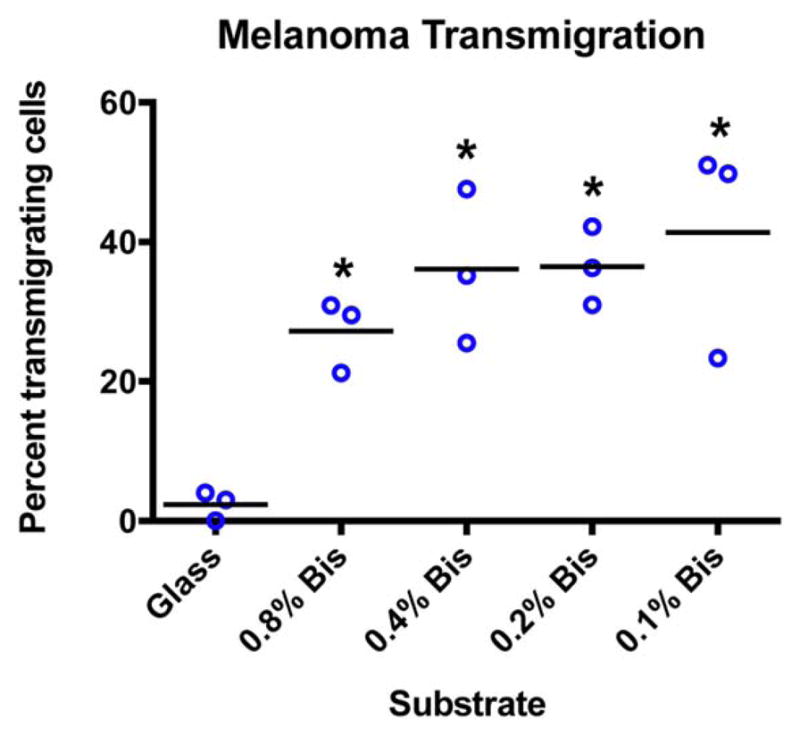
Effect of substrate stiffness on transendothelial migration of melanoma cancer cells. Melanoma cells (92.1) were added to the top of HDMVEC monolayers and allowed to transmigrate. No transcellular transmigration by melanoma cells was detected in any experiments. Graph shows percentage of cells that transmigrated. Each plotted point represents data collected from one low-power field. Black bars indicate the mean of the values for the three experiments. The values for all soft substrates differed significantly from the value for glass (P <0.01). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Discussion
We examined the role of the mechanical environment on the formation and function of EC monolayers, using tunable polyacrylamide hydrogels as soft substrates. We made three important discoveries. First, soft substrates alter the molecular composition and structure of the junctions between ECs. Second, soft substrates shift the route of TEM for immune cells from paracellular to transcellular, by decreasing the efficiency of the paracellular route. Third, soft substrates increase the efficiency of TEM for melanoma cells, which migrate exclusively by the paracellular route.
Formation and Composition of Cell-Cell Junctions
We found that decreasing substrate elasticity to physiological levels altered the molecular composition of EC cell-cell junctions and the ability of ECs to form monolayers. Nascent EC-cell junctions form primarily through the homophilic binding of VE-cadherin molecules on adjacent ECs. F-actin plays a critical role in junction formation, in coordination with VE-cadherin through vinculin [Huveneers et al., 2012]. As EC junctions mature, catenins are recruited to the cytoplasmic portion of VE-cadherin, and vinculin becomes less abundant [Huveneers et al., 2012]. For ECs on glass, the mechanical stress of fluid shear across the surface of the monolayer increases the recruitment of vinculin to VE-cadherin, and this in turn recruits F-actin and Arp2/3 complex to remodel the junctions [Gulino-Debrac, 2013; Abu Taha and Schnittler, 2014].
Here, we observed vinculin recruitment and the accumulation of F-actin as markers of junction maturity and the cellular response to the mechanical properties of the substrate. On glass, we found that HDMVEC and HBMEC monolayers formed both linear and serrated junctions, with less vinculin and F-actin staining on the linear than the serrated junctions, consistent with previous findings on glass [Huveneers et al., 2012]. Our results were similar for HUVEC monolayers, with less pronounced differences in vinculin staining among junctions.
For ECs cultured on soft substrates, we observed higher vinculin levels at linear junctions than on glass, similar to the levels seen with serrated junctions. We observed similar F-actin content at linear junctions in ECs on soft substrates and on glass, overlapping both VE-cadherin and vinculin staining. Vinculin staining showed loci of stronger staining along many linear junctions, and F-actin staining was stronger at these loci. On glass, ECs have been observed to recruit vinculin and cortical actin to mature junctions only in response to high tension [Gulino-Debrac, 2013]. However, our findings suggest that on soft substrates, vinculin is present, even in the absence of tension.
Effects on the Route of TEM: Immune vs. Cancer Cells
One important function of ECs is to facilitate the migration of immune cells to sites of inflammation. We used PBLs as a T-lymphocyte pool to examine adaptive immune cell transmigration and cultured NK cells to examine innate immune cell transmigration. Our results with PBLs and NK cells show that EC monolayers grown on glass do not faithfully model this function. We found that PBLs and NK cells were more likely to migrate via the transcellular route versus the paracellular route on soft substrates than on glass, suggesting that this may be the predominant route of extravasation in the setting of a tissue. The transcellular route may provide the EC with a greater degree of control over the process of transmigration and extravasation, while maintaining strong junctions and a high level of barrier function. Similar findings have been reported recently by the Carman lab [Martinelli et al., 2014], who suggest that stronger EC junctions make transcellular transmigration the path of least resistance. However, for NK cells, we did not observe increased transcellular transmigration with decreased substrate stiffness. This would suggest that ECs can regulate the paracellular and transcellular routes independently for specific immune cell populations. These findings are providing us with the basis for more extensive molecular analyses of mechanisms of immune cell TEM on soft substrates [Mooren et al., 2014; Mukherjee et al., 2014].
In the setting of inflammation, induction of ICAM-1 on ECs is an important parameter controlling recruitment and transmigration of immune cells. We found similar levels of ICAM-1 induction among HDMVECs, HBMECs and HUVECs, each isolated from different tissues. A previous study using only HUVECs showed no change in induction of ICAM-1 in response to changing substrate stiffness [Stroka et al., 2012], further demonstrating that ICAM-1 is induced similarly among ECs in all tissues regardless of stiffness.
Circulating cancer cells also migrate across endothelia and into tissues, but they use different factors and pathways than immune cells do [Fazakas et al., 2011; Zervantonakis et al., 2012]. We found that melanoma cells migrated quickly and efficiently through EC monolayers grown on physiologically soft substrates, and that this transmigration was much less efficient when ECs were grown on glass. Therefore, as with immune cell TEM, the use of hard substrates to study cancer cell extravasation may fail to faithfully model cell behavior and interactions.
One striking conclusion from our results is that the efficiency of paracellular-route TEM by melanoma cancer cells increases as substrate stiffness decreases, which is the opposite of what occurs with immune cells Although the junctions between ECs appear to be stronger on soft substrates, the melanoma cells move through them more efficiently, raising the possibility that junction molecules help mediate the process.
Concluding Remarks
Taken together, our data show substantial and important changes in EC monolayers grown on soft substrates versus glass, including the ability of ECs to support TEM by different cell types. EC monolayers on soft substrates offer a more accurate model of physiological conditions, superior to glass. Based on these results, we speculate that, in the organism, the mechanical environment of endothelial monolayers created by surrounding tissue may serve as an important regulator of monolayer integrity and function, one that is distinct from inflammatory mediators and processes.
Materials and Methods
Chemicals and reagents were obtained from Fisher Scientific (Pittsburgh, PA) or Sigma-Aldrich (Saint Louis, MO), unless stated otherwise.
Cell Culture
Cells were cultured at 37°C in 5% CO2. Human dermal microvascular ECs (HDMVECs) and human umbilical vein ECs (HUVECs) were obtained from Lonza (Allendale, NJ). Primary human brain microvascular ECs (HBMECs) were obtained from ScienCell (Carlsbad, CA). A human brain microvascular cell line, CMEC/D3 [Daniels et al., 2013], was tested, but the cells did not express VE-cadherin and did not form monolayers, so they were not included in this study. Primary ECs were cultured in EBM-2 base medium supplemented with the EGM-2 kit for HUVECs or the EGM-2 MV kit for HDMVECs and HBMECs. HBMECs were not used after four passages; HDMVECs and HUVECs were not used after nine passages. Human PBLs were isolated as described [Mooren et al., 2014]. Human NK-92 cells (ATCC, Manassas, VA) were cultured as described [Mukherjee et al., 2014]. Human 92.1 uveal melanoma cells, a generous gift of Dr. Martine Jager (Laboratory of Ophthalmology, Leiden University), were grown in RPMI 1640 medium (Life Technologies, Carlsbad, CA) supplemented with 10% FBS and antibiotics.
Soft Substrate Preparation
Polyacrylamide hydrogels were prepared as described [de Rooij et al., 2005]. Briefly, 20-mm glass-bottom culture dishes were coated with 3-aminopropyltriethoxysilane and activated by treatment with 0.5% glutaraldehyde for 30 min. Polyacrylamide mixtures were prepared in PBS with 7% acrylamide, 3% acrylamidopropyltrimethyl ammonium chloride, and varying concentrations of bis-acrylamide (0.1–0.8%). Ammonium persulfate and TEMED were added to final concentrations of 0.1% each, and 20 μL of the acrylamide mixture was placed in the center of the treated glass-bottom dish. An 18-mm coverslip was placed on top to create a smooth, even gel, sandwiched between the coverslip and the glass-bottom dish. The acrylamide was allowed to polymerize for 30 min at RT, the coverslip was removed, and the gel was washed three times with PBS. The substrates were coated with fibronectin (10 μg/mL in PBS) by overnight incubation at 4°C and washed with PBS.
For PA gels, Young’s modulus of elasticity is linearly proportional to the concentration of bis-acrylamide [Tse and Engler, 2010]. We prepared PA gels with bis-acrylamide concentrations of 0.1–0.8%, corresponding to a Young’s modulus of 10–90 kPa [Tse and Engler, 2010]. At lower bis-acrylamide concentrations, the PA gels we prepared were inhomogeneous; therefore, we obtained gels with Young’s modulus of <10 kPa from a commercial source (Matrigen Life Technologies, Brea, CA).
Immunostaining and Antibodies
Fixation was carried out by adding 2× fixative (PBS containing 4% paraformaldehyde and 0.4% glutaraldehyde) directly to live cells in tissue culture medium. After 15 min at 37°C, cells were permeabilized with 0.1% Triton X-100 in PBS for 5 min, washed with PBS, and blocked with 3% BSA in PBS. Primary and secondary antibodies were diluted in 3% BSA. Primary antibodies included rabbit anti-VE-cadherin (Cell Signaling, Danvers, MA), mouse anti-human vinculin (Sigma-Aldrich), and mouse anti-human ICAM-1 (R&D Systems, Minneapolis, MN). F-actin was visualized using Alexa-fluor conjugated phalloidin (Life Technologies). Secondary antibodies were Alexa-fluor conjugates (Life Technologies), and the mounting agent was ProLong Gold (Life Technologies). Cells were imaged with an inverted microscope (Olympus IX72) using 40× and 100× objectives.
Quantification of Monolayers
Images of monolayers were analyzed using ImageJ [Schneider et al., 2012]. To determine the percentage of linear junctions, the line tool was used on 40× images to measure the lengths of linear and non-linear junctions as identified by VE-cadherin staining. Pixels were converted to μm. For each experimental condition, the values were summed to give a total length of linear versus non-linear junctions, and the fraction of linear junctions relative to the whole was calculated. For vinculin localization, boxes were drawn around linear junctions (10 μm along each junction, with 2 μm on either side). Colocalization analysis was used to compare the fluorescent profiles of vinculin and VE-cadherin in 10 separate boxes for each cell type and substrate. Pearson correlation coefficients >0.5 were considered significant for presence of vinculin at junctions.
Transendothelial Migration Assays
ECs were plated on soft substrates and incubated overnight to allow formation of monolayers. Monolayers were monitored visually by phase-contrast imaging to ensure that they covered the substrate completely, without defects, before transmigration assays were performed.
HDMVEC monolayers were treated with TNFα (20 ng/mL) overnight. PBLs and NK cells were added and incubated for 15 min and 1 h, respectively. Preparations were fixed and stained for VE-cadherin and ICAM-1 to identify transmigration events and determine whether the route was paracellular versus transcellular. Events were counted individually across 5–10 different fields of view using a 40× objective. Experiments were performed in duplicate or triplicate, with each data point representing an average of two or three plates.
Melanoma cells were added to HDMVEC monolayers in EGM-2 MV culture medium and placed into an environmental chamber (Stage Top Incubator, Tokai Hit, Shizuokaken, Japan) with 5% CO2 at 37°C on an inverted microscope (Olympus IX72). DIC images were captured at 12-s intervals with a 10× objective. Time-lapse movies were reviewed to follow individual tumor cells as they migrated into the endothelial monolayer.
Fig. 4.
Cell junction formation in HBMVEC monolayers on soft substrates. Characterization of junction structure and molecular composition by fluorescence imaging with anti-VE-cadherin, anti-vinculin and fluorescent phalloidin. Percentages indicate bis-acrylamide concentrations. Scale bar, 50 μm.
Acknowledgments
This work was supported by NIH grant GM38542 to JAC.
References
- Abu Taha A, Schnittler HJ. Dynamics between actin and the VE-cadherin/catenin complex: novel aspects of the ARP2/3 complex in regulation of endothelial junctions. Cell Adh Migr. 2014;8(2):125–135. doi: 10.4161/cam.28243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-On B, Wagner HD. Structural motifs and elastic properties of hierarchical biological tissues—a review. J Struct Biol. 2013;183:149–164. doi: 10.1016/j.jsb.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Clarijs R, Schalkwijk L, Ruiter DJ, de Waal RM. Lack of lymphangiogenesis despite coexpression of VEGF-C and its receptor Flt-4 in uveal melanoma. Invest Ophthalmol Vis Sci. 2001;42:1422–1428. [PubMed] [Google Scholar]
- Daniels BP, Cruz-Orengo L, Pasieka TJ, Couraud PO, Romero IA, Weksler B, Cooper JA, Doering TL, Klein RS. Immortalized human cerebral microvascular endothelial cells maintain the properties of primary cells in an in vitro model of immune migration across the blood brain barrier. J Neurosci Methods. 2013;212:173–179. doi: 10.1016/j.jneumeth.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij J, Kerstens A, Danuser G, Schwartz MA, Waterman-Storer CM. Integrin-dependent actomyosin contraction regulates epithelial cell scattering. J Cell Biol. 2005;171:153–164. doi: 10.1083/jcb.200506152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Wolburg H. Mini-review: Transendothelial migration of leukocytes: through the front door or around the side of the house? Eur J Immunol. 2004;34:2955–2963. doi: 10.1002/eji.200425327. [DOI] [PubMed] [Google Scholar]
- Fazakas C, Wilhelm I, Nagyoszi P, Farkas AE, Hasko J, Molnar J, Bauer H, Bauer HC, Ayaydin F, Dung NT, Siklos L, Krizbai IA. Transmigration of melanoma cells through the blood-brain barrier: role of endothelial tight junctions and melanoma-released serine proteases. PLoS One. 2011;6:e20758. doi: 10.1371/journal.pone.0020758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulino-Debrac D. Mechanotransduction at the basis of endothelial barrier function. Tissue Barriers. 2013;1(2):e24180. doi: 10.4161/tisb.24180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huveneers S, de Rooij J. Mechanosensitive systems at the cadherin-F-actin interface. J Cell Sci. 2013;126:403–413. doi: 10.1242/jcs.109447. [DOI] [PubMed] [Google Scholar]
- Huveneers S, Oldenburg J, Spanjaard E, van der Krogt G, Grigoriev I, Akhmanova A, Rehmann H, de Rooij J. Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. J Cell Biol. 2012;196:641–652. doi: 10.1083/jcb.201108120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli R, Zeiger AS, Whitfield M, Sciuto TE, Dvorak A, Van Vliet KJ, Greenwood J, Carman CV. Probing the biomechanical contribution of the endothelium to lymphocyte migration: diapedesis by the path of least resistance. J Cell Sci. 2014;127:3720–3734. doi: 10.1242/jcs.148619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee CT, Last JA, Russell P, Murphy CJ. Indentation versus tensile measurements of Young’s modulus for soft biological tissues. Tissue Eng Part B Rev. 2011;17:155–164. doi: 10.1089/ten.teb.2010.0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooren OL, Li J, Nawas J, Cooper JA. Endothelial cells use dynamic actin to facilitate lymphocyte transendothelial migration and maintain the monolayer barrier. Mol Biol Cell. 2014;25:4115–4129. doi: 10.1091/mbc.E14-05-0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Kim J, Mooren OL, Cooper JA. Role of cortactin homolog HS1 in transendothelial migration of natural killer cells. PLoS One. 2014 doi: 10.1371/journal.pone.0118153. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WA. Mechanisms of transendothelial migration of leukocytes. Circ Res. 2009;105:223–230. doi: 10.1161/CIRCRESAHA.109.200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WA. Mechanisms of leukocyte transendothelial migration. Annu Rev Pathol. 2011;6:323–344. doi: 10.1146/annurev-pathol-011110-130224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WA. How endothelial cells regulate transmigration of leukocytes in the inflammatory response. Am J Pathol. 2014;184:886–896. doi: 10.1016/j.ajpath.2013.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham RJJ, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond N, d’Agua BB, Ridley AJ. Crossing the endothelial barrier during metastasis. Nat Rev Cancer. 2013;13:858–870. doi: 10.1038/nrc3628. [DOI] [PubMed] [Google Scholar]
- Sage PT, Carman CV. Settings and mechanisms for transcellular diapedesis. Front Biosci. 2009;14:5066–5083. doi: 10.2741/3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroka KM, Aranda-Espinoza H. Effects of morphology vs. cell-cell interactions on endothelial cell stiffness. Cell Mol Bioeng. 2011;4:9–27. doi: 10.1007/s12195-010-0142-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroka KM, Levitan I, Aranda-Espinoza H. OxLDL and substrate stiffness promote neutrophil transmigration by enhanced endothelial cell contractility and ICAM-1. J Biomech. 2012;45:1828–1834. doi: 10.1016/j.jbiomech.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse JR, Engler AJ. Preparation of hydrogel substrates with tunable mechanical properties. Curr Protoc Cell Biol. 2010;Chapter 10(Unit 10.16) doi: 10.1002/0471143030.cb1016s47. [DOI] [PubMed] [Google Scholar]
- Vestweber D. Adhesion and signaling molecules controlling the transmigration of leukocytes through endothelium. Immunol Rev. 2007;218:178–196. doi: 10.1111/j.1600-065X.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen ES. Endothelial signaling in paracellular and transcellular leukocyte transmigration. Front Biosci. 2009;14:2522–2545. doi: 10.2741/3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zervantonakis IK, Hughes-Alford SK, Charest JL, Condeelis JS, Gertler FB, Kamm RD. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc Natl Acad Sci USA. 2012;109:13515–13520. doi: 10.1073/pnas.1210182109. [DOI] [PMC free article] [PubMed] [Google Scholar]