Abstract
End stage kidney disease is a well-known complication of methylmalonic acidemia (MMA), and can be treated by dialysis, kidney transplant, or combined kidney-liver transplant. While liver and/or kidney transplantation in MMA may reduce the risk of metabolic crisis and end-organ disease, it does not fully prevent disease-related complications. We performed detailed metabolite and kinetic analyses in a 28-year-old patient with mut0MMA who underwent hemodialysis for 6 months prior to receiving a combined liver/kidney transplant. A single hemodialysis session led to a 54% reduction in plasma methylmalonic acid and yielded a plasma clearance of 103 ml/min and VD0.48 L/kg, which approximates the total body free water space. This was followed by rapid reaccumulation of methylmalonic acid over 24 hours to the predialysis concentration in the plasma. Following combined liver/kidney transplantation, the plasma methylmalonic acid was reduced to 3% of pre-dialysis levels (6965± 1638 (SD) µmol/Land 234± 100(SD) µmol/L)but remained >850 × higher than the upper limit of normal (0.27 ± 0.08 (SD) µmol/L). Despite substantial post-operative metabolic improvement, the patient developed significant neurologic complications including acute worsening of vision in the setting of pre-existing bilateral optic neuropathy, generalized seizures, and a transient, focal leukoencephalopathy. Plasma methylmalonic acid was stable throughout the post-operative course. The biochemical parameters exhibited by this patient further define the whole body metabolism of methylmalonic acid in the setting of dialysis and subsequent combined liver/kidney transplant.
Introduction
MMA (OMIM #251000) is an autosomal recessive disorder of organic acid metabolism characterized by impaired conversion of L-methylmalonyl-CoA into succinyl-CoA due to reduced activity of the enzyme methylmalonyl-CoA mutase (MUT) (Nyhan and Ozand 1998; Willard and Rosenberg 1980).Mutations that completely abolish MUT activity result in the mut0 class of MMA. Affected patients experience frequent and severe episodes of metabolic instability characterized by mental status changes, ketoacidosis and, at times, hyperammonemia (Manoli and Venditti 2010; Baumgartner and Viardot 1995). Basal ganglia infarction (mainly involving the globi palladi), pancreatitis, bone marrow suppression, and death can occur. Long-term morbidities include kidney failure, cardiomyopathy, poor growth, optic neuropathy, and developmental delay (Manoli and Venditti 2010; Baumgartner and Viardot 1995; Hörster et al 2007; Traber et al 2011; Prada at al 2011; Williams et al 2009).
Acute treatment of metabolic crises in MMA is supportive and includes rehydration, correction of electrolyte abnormalities, treatment of intercurrent infection, and the provision of intravenous glucose (Wendel and Baulny 2006). The longer-term management is likewise supportive and mainly consists of avoidance of known triggers of metabolic crises including infection and dehydration, dietary protein restriction, and cobalamin if a patient is determined to be vitamin B12 responsive (Wendel and Baulny 2006).
Liver and/or kidney transplantation has been utilized to improve metabolic control in this disease, with the goal of mitigating or eliminating metabolic crises (Lubrano et al 2007; Chakrapani et al 2002; Nagarajan et al 2005; Ho et al 2000; Nyhan et al 2002; Hsui et al 2003; Ogier et al 2005; van’t Hoff et al 1998; van’t Hofff et al 1999; Clothier et al 2011). Although transplantation can substantially reduce the plasma methylmalonic acid concentration, circulating metabolites remain more than 100 fold increased compared to controls after successful transplantation. Complications such as metabolic stroke and progression of chronic kidney disease can still occur (Chakrapani et al 2002; Nyhan et al 2002; Kaplan et al 2006; Chen et al 2010; Burdelski and Ullrich 1999; Leonard et al 2001; Kamei et al 2011). The precise indications for organ transplantation have not been defined, and controversy exists regarding whether an LKT or isolated KT is preferred in the setting of renal failure in a patient with mut0 MMA. A few reports suggest that isolated kidney transplantation may yield better metabolic control than solitary liver or combined organ transplantation (Lubrano et al 2007; Lubranoet al 2013) in patients with mut0 MMA. However, this claim is partially based on the misclassification of aB12 responsive cblA patient who was initially reported as mut0 (Lubrano et al 2007; Lubrano et al 2001; Lubrano et al 2013a; Lubrano et al 2013b). Another report has documented mixed outcomes in mut MMA after isolated KT, with some patients doing well, but others perishing in the face of multisystem failure or exhibiting metabolic instability after transplant (Brassier et al 2013).
Because guidelines surrounding all aspects of transplantation in MMA are lacking, the detailed description of the clinical course experienced by any given patient yields important information and unique insights needed to guide future clinical decisions. We report a 28-year-old female patient with mut0 MMA on hemodialysis (HD) for 6 months for end-stage renal disease caused by MMA-associated chronic tubulointerstitial nephritis who subsequently underwent combined LKT. To better characterize the effects of LKT on the metabolic phenotype of MMA, clearance (blood volume in unit volume per unit time cleared of metabolite by dialysis) of methylmalonic acid by intermittent HD was calculated, and plasma and cerebrospinal fluid (CSF) metabolites after transplantation were measured. Despite metabolic improvement following LKT, the patient developed numerous neurologic complications including worsening vision, seizures, and a focal leukoencephalopathy. Here, we present biochemical and imaging data surrounding this patient’s transplantation and describe acute neurological events following LKT in MMA, and review the literature.
Clinical Course
A 28-year-old female, who was the product of a first cousin union, presented at age 3 months with failure to thrive, developmental delay, and hypotonia. At 9 months of age, she was diagnosed with MMA following an episode of vomiting, dehydration, and metabolic acidosis. [14C] propionate incorporation and complementation studies performed on skin fibroblasts were consistent with mut0 MMA. She was found to be homozygous for c.2053dupCTC p.685insL in MUT (Adjalla et al 1998; Worgan et al 2006) and cobalamin unresponsive. Chronic kidney disease (CKD) was diagnosed at 4 years of age following a DTPA scan which showed a glomerular filtration rate of 26 ml/min/1.73 m2, and a kidney biopsy demonstrated tubulointerstitial nephritis with fibrosis consistent with MMA-associated kidney disease. She experienced a bilateral globus pallidus infarction at age 7 years that resulted in mild choreoathetosis. At age 11 years, she was diagnosed with secondary hyperparathyroidism and at 21 years she was diagnosed with hypothyroidism. Intellectual development has always been within the normal range. At 27 years of age, the patient developed an acute bilateral optic neuropathy, resulting in best-corrected visual acuity of 20/80 with the right eye and 20/400 with the left eye.
Because of deterioration of her visual function, worsening generalized debility, and increasing requirement for bicarbonate supplementation, HD was initiated to reduce MMA metabolites. At this time, the patient had a creatinine of 1.9 mg/dL and an estimated glomerular filtration rate (eGFR) of 23 ml/min/1.73m2 by the CKD-EPI Creatinine-Cystatin C (2012) equation (cystatin C 3.33 mg/L) (Inker etal 2012). As creatinine may be an inaccurate measure of kidney function in patients with MMA due to the low muscle mass, the combined use of cystatin C and creatinine may permit better estimation of GFR. Definitive studies, however, have yet to be performed in this patient population (Walter et al 1989; Kruzka et al 2013).
After extensive discussions with the patient and her family, a combined LKT was performed with the aim of preventing future complications of MMA and improving the quality of life without the need for HD. After 6 months of chronic HD, at age 28, she received a deceased donor LKT. Induction immunosuppression involved intraoperative basiliximab and methylprednisolone with mycophenolate mofetil initiated the evening of surgery. Intraoperative HD with a blood flow rate (QB) between 200 and 300 ml/min and dialysate flow rate of 600 ml/min was utilized to minimize accumulation of MMA and maintain normal blood pH. She experienced 11 minutes of intraoperative hypotension with a systolic blood pressure as low as 40 mmHg due to inferior vena cava (IVC) compression by the donor liver. Intravenous vasopressors and fluids were administered and repositioning of the liver ultimately relieved the hypotension. Immediately post-operatively, continuous veno-venous hemodialysis (CVVHD) (NxStage System One, Lawrence, MA) at QB 250 ml/min using bicarbonate dialysate at 1500 ml/hr (28 ml/kg/hr) was employed for 36 hours to minimize methylmalonic acid accumulation and to assist with fluid management. Plasma methylmalonic acid was 400 µmol/L while on CVVHD.
On post-operative day 1, abdominal ultrasonography revealed diminished flow in the main and right portal veins with no definite flow in the left portal vein and right hepatic vein. She returned to the operating room to relieve IVC compression by repositioning the donor liver and the surgical incision was left open to heal by secondary intention. On post-operative day 3, she developed acute, complete loss of vision in both eyes. Ophthalmologic examination revealed pale optic discs with non-reactive pupils bilaterally. On this day, plasma methylmalonic acid level was 390 µmol/L, which was not a significant change from the day prior (345 µmol/L) or the following day (358 µmol/L). On post-operative day 4, brain magnetic resonance imaging (MRI) and angiography (MRA) showed symmetric, small T2-hyperintese lesions in the posterior aspect of the globi pallidi which were felt to be a sequelae of her prior infarct (not shown), T2-hyperintense signal of the orbital segment of both optic nerves, and mild reduction in size of the right side of the optic chiasm (Figure 1) consistent with acute optic nerve damage superimposed on a chronic bilateral optic neuropathy. No other acute brain abnormalities were detected. At this time, her immunosuppression was methylprednisolone and mycophenolate mofetil. In the days leading to these events, urine output was between 1000 – 1500 ml/day with an even to net positive fluid balance. Electrolytes were within normal limits except for mild hypokalemia of 3.3 mEq/L (normal value 3.5–5.0 mEq/L), and arterial pH ranged from 7.34 to 7.44.
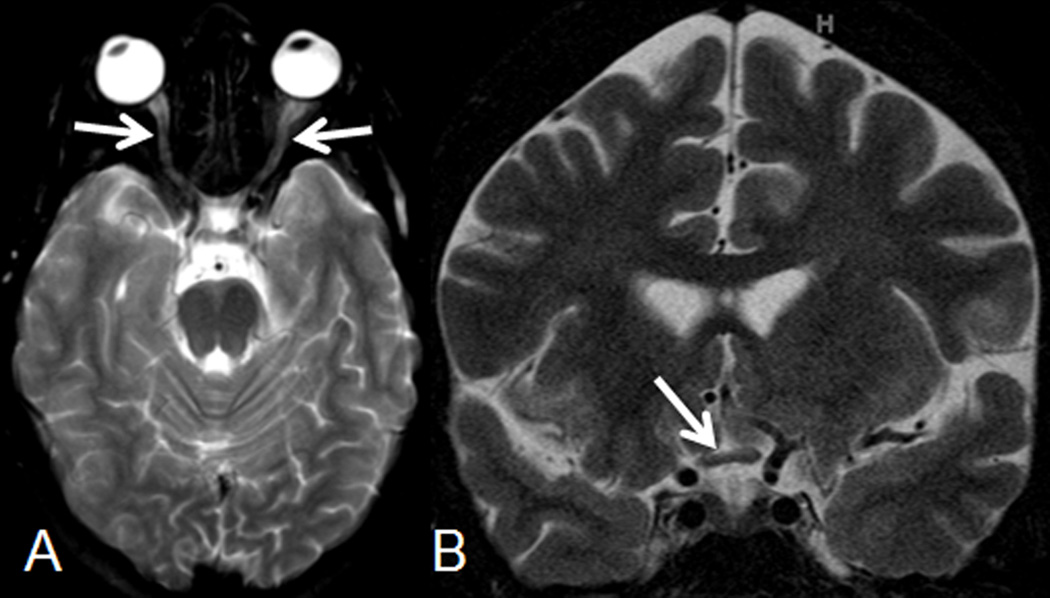
Figure 1.
(A) Axial and (B) coronal T2-weighted images on post-operative day 4 show mild diffuse hyperintense signal of the optic nerves (arrows on A) and an asymmetric decrease in size of the right lateral component of the optic chiasm (arrow on B).
Due to acute visual deterioration, a therapeutic trial of the antioxidant Epi-743 (Enns et al 2012; Sasun et al 2012) was started under a compassionate use protocol. Epi-743 was selected as it is an investigational drug with antioxidant properties that has shown promise in restoration of vision in individuals with Leber Hereditary Optic Neuropathy, as well as promise in the treatment of other mitochondrial disorders including Leigh Syndrome. Vision subsequently improved to 20/800 with the right eye and 20/400 with the left eye, associated with bilateral central scotomas. However, this medication was subsequently discontinued after 104 days to simplify her clinical management in the setting of the post-operative complications. At discharge, her vision was 20/800 bilaterally.
On post-operative day 6, tacrolimus was initiated. The patient’s clinical course began to show improvement; however, on post-operative day 28, she developed generalized seizures. Follow-up brain MRI revealed patchy T2-hyperintense signal in the pons and left midbrain with matching increased apparent diffusion coefficient (ADC) values (suggesting vasogenic edema), unchanged symmetric, small T2-hyperintese lesions in the posterior aspect of the globipallidi, and small T2-hyperintense lesions in the left thalamus and left corona radiate without matching diffusion abnormalities (Figure 2). Based on normal diffusion-weighted imaging (DWI), the T2-hyperintense lesions in the left thalamus and corona radiata were not consistent with ischemic lesions. Tacrolimus levels were at goal (trough 6.7ng/mL) and thought to be an unlikely etiology of the seizures. Nonetheless, this drug was discontinued as a precaution, and immunosuppression was maintained with mycophenolate mofetil, methylprednisolone, and monthly basiliximab. In addition, she was placed onoxcarbazepine, levetiracetam, and lacosamide, with temporary control of the seizures, and she was transferred to an inpatient rehabilitation facility.
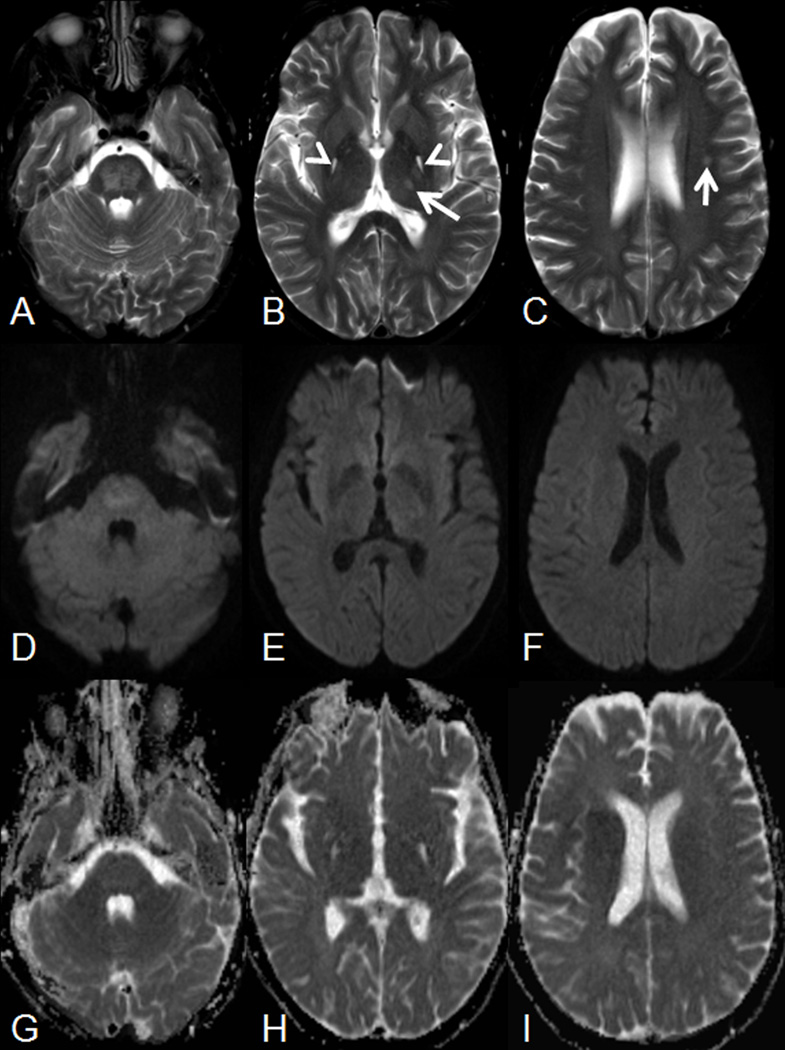
Figure 2.
(A–C) Axial T2-weighted images and matching (D–F) diffusion-weighted imaging (DWI) as well as (G–I) apparent diffusion coefficient (ADC) maps on post-operative day 28 demonstrate a patchy T2-hyperintense signal within the pons (A) with matching mild hyperintense signal on DWI (most likely due to T2 shine through effect) and increased ADC values, representing vasogenic edema. Additionally, symmetric T2-hyperintense lesions without matching diffusion abnormalities are noted in the posterior aspects of the globipallidi (arrowheads on B). Finally, small T2-hyperintense foci are noted in the left thalamus (arrow on B) and left corona radiate (arrow on C) without matching diffusion abnormalities.
Unfortunately, on post-operative day 48, seizures recurred and necessitated endotracheal intubation and mechanical ventilation. Repeat brain MRI showed improvement in the previously visualized changes in the thalamus and pons on T2 weighted images. On T2-weighted and fluid attenuation inversion recovery (FLAIR) images, there was minimally increased abnormal signal in the right cingulate gyrus with reduced diffusion on ADC maps. An etiology for the recurrence of seizures was not found. Monthly basiliximab was discontinued, and sirolimus was added to the regimen of mycophenolate mofetil and corticosteroids. On post-operative day 55, the patient developed persistent fever that resolved with drainage of an intraabdominal abscess. Her seizures were controlled with lacosamide, levetiracetam, and clonazepam. Throughout this course, both liver and kidney allograft functions were excellent.
After a 5-month hospital course, the patient was discharged to in-home rehabilitative care. Compared with her pre-transplant state, notable new comorbidities included decreased visual acuity of 20/800 bilaterally, an inability to ambulate independently due to muscle atrophy, and the presence of a facial tremor and seizure disorder. Eighteen months post-operatively, her tremor persists but is improved, her seizures are well controlled on lacosamide and clonazepam, her visual function is stable, and she is able to walk independently. The allografts are functioning well, with serum creatinine 0.6 mg/dL, near normal liver transaminases, normal bilirubin, albumin and total protein, and plasma MMA 266 µmol/L on an unrestricted diet.
Materials and Methods
The patient consented to all studies and reporting of results in accordance with approved Johns Hopkins Hospital IRB protocols: NA_00026986 “Investigation of metabolic disturbance in Methylmalonic Acidemia, Propionic Acidemia, Isovaleric Acidemia and Glutaric Aciduria Type 1” and NA_00036487 “Clinical Studies of Inborn Errors of Metabolism.”She was concurrently enrolled in National Institutes of Health study04-HG-0127 “Clinical and Basic Investigations of Methylmalonic Acidemia and Related Disorders”(clinicaltrials.gov identifier: NCT00078078).
Measurement of plasma, CSF, and HD effluent methylmalonic acid and acylcarnitines
Methylmalonic acid concentrations were determined by isotope dilution capillary-gas chromatography/mass spectrometry (GC/MS), using [methyl-2H3] methylmalonic acid as an internal standard (Stabler et al 1985; Stabler et al 1991). Acylcarnitine levels were determined by liquid chromatography/mass spectrometry via previously reported methods (Rinaldo et al 2008). Methylmalonic acid levels were measured in the plasma prior to and after HD, peri-transplant including on CVVHD, and periodically thereafter including during changes in clinical status. Plasma acylcarnitine profile was measured at the initiation of HD and periodically thereafter. CSF methylmalonic acid and acylcarnitine profile were measured on post-operative days 28, 48, and 55, which corresponded to the onset of generalized seizures (28 days), seizure escalation (48 days), and fever (55 days).
Measurement of amino acids
Plasma and CSF amino acid concentrations were determined by ion exchange chromatography. Samples were deproteinized by mixing with 1/10 the sample volume of 35% sulfosalicylic acid. The mixture was centrifuged for 3 min, and amino acids were assayed from the supernatant by ion-exchange liquid chromatography with ninhydrin detection on a Biochrom 30 Amino Acid Analyzer. Amino acids were assayed at the above noted time points.
Kinetics of methylmalonic acid during HD
Hemodialysis was initiated over three consecutive days via a tunneled dialysis catheter with bicarbonate-based dialysate at flow rate 600 ml/min. The 1st session was for 2 hours at QB 200 ml/min utilizing an F16 filter (Fresenius Medical Care North America, Waltham, MA), 2ndsession for 2.5 hours at QB 225 ml/min utilizing an F18 filter, and 3rd session for 3 hours at QB 400 ml/min utilizing an F18 filter. Pre-dialysis weight with the 3rd session was 48.2 kg, height 60.5 inches, and no ultrafiltration was performed. With the 3rd session, plasma methylmalonic acid was measured immediately before HD and at 2, 4, 22, and 52 hours after HD. As the increase in plasma methylmalonic acid post-dialysis appeared linear over the 52-hour time course, a methylmalonic acid concentration (0 hours) immediately post-HD was estimated by linear regression (Supplementary Methods). During this 3 hour HD session, effluent was collected from the 1st hour (36 L) and from the 3rd hour (36 L). Effluent concentrations of methylmalonic acid were measured from a 0.4 mL aliquot of each well-mixed36 L container. The concentration in the 36 L of effluent from the 2nd hour of dialysis was estimated at the midpoint of the exponential decline between the 1st and 3rd hour concentrations to permit calculation of the total quantity of methylmalonic acid removed by HD. Methylmalonic acid clearance by HD and the volume of distribution (VD) were calculated using dialysate-side kinetic equations(Supplementary Methods). Clearance of methylmalonic acid by residual renal function was unknown but likely low given an average urine output of 500 ml/day.
Results
Plasma methylmalonic acid measured multiple times over the 32 months before any medical intervention (dialysis or transplant) was 6965 ± 1638(SD) µmol/L (n=5).Plasma methylmalonic acid concentrations before and after the 3rd HD session are shown in Table 1. After a 3 hour hemodialysis session in this 48.2 kg patient utilizing an F18 filter at QB 400 ml/min and dialysate flow rate 600 ml/min, the methylmalonic acid reduction ratio was 54.2% for a plasma clearance of 103 ml/min. The VD was estimated at 0.48 L/kg, similar to total body water of 0.54L/kg as predicted by the Watson formula (Watson and Watson 1980; Guyton 1976). Similar patterns of reaccumulation were seen in the acylcarnitines C3 and C5DC (Table 1). Immediately prior to LKT, plasma methylmalonic acid was 4811 µmol/L, the patient having received HD the day before at QB 300 mL/min. While on CVVHD immediately post-transplant, plasma methylmalonic acid ranged 348 – 455 µmol/L. Average plasma methylmalonic acid from discontinuation of CVVHD to discharge from the hospital was 234± 100(SD) µmol/L (n=28). At 15 months post-transplant on an unrestricted diet, plasma methylmalonic acidwas266µmol/L.
Table 1.
Plasma metabolite measurements during first week of dialysis.
| day 1 | day 2 | day 3 | day 4 | day 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| pre-dialysis | 2h post dialysis |
pre-dialysis | 2h post dialysis |
pre-dialysis | 2h post dialysis |
4h post dialysis |
22 hours post dialysis |
52 hours post dialysis |
|
| plasma MMA (umol/L) | 6127 | 3914 | 5818 | 2986 | 4156 | 2012 | 2320 | 4580 | 6930 |
| plasma C3 (umol/L) | 57.62 | * | 51 | 39.51 | 51.41 | * | 37.73 | * | 61.87 |
| plasma C4DC (umol/L) | 8.66 | * | 7.25 | 3.03 | 4.76 | * | 3.38 | * | 9.23 |
indicates sample not available.
Simultaneous methylmalonic acid levels were measured in the plasma and CSF on post-operative days 28 (seizure onset) and 48 (seizure escalation), and from the CSF alone on day 55 (fever). Plasma methylmalonic acid was available from days 53 and 57 (Table 2) and did not vary significantly during these episodes from measurements made during clinical stability, although CSF methylmalonic acid was notably higher during episodes of seizure (days 28 and 48) as compared to fever (day 55). The CSF/plasma ratio remained approximately 2.5 – 5 times higher than in healthy controls at these time points. This is consistent with what has been described previously in a patient with mut0 MMA (Kaplan et al 2006). All CSF samples had normal or negative protein, glucose, cell counts, and cultures (Supplementary Table 1).
Table 2.
Plasma and CSF metabolites measured at post operative days (POD) 28, 48, 53, 55 and 57.
| Methylmalonate (umol/L) |
C3 (umol/L) | C4DC (umol/L) |
Glycine (umol/L) |
Alanine (umol/L) |
Glutamine (umol/L) |
Serine (umol/L) |
|
|---|---|---|---|---|---|---|---|
| normal range in CSF | 0.14–0.73 | * | * | 10.0–32.0 | 24.0–42.0 | 320–837 | 28–61 |
| normal range in plasma | 0.11–0.43 | <0.92 | <0.18 | 87.0–323.0 | 136.0–440.0 | 337.0–673.0 | 67–171 |
| Average CSF/plasma | 1.61 | * | * | 0.039 | 0.098 | 0.828 | 0.227 |
| CSF (POD 28) | 1439 | 5.97 | 0.49 | 5 | 49 | 399 | 16 |
| Plasma (POD 28) | 338 | 26.52 | 1.08 | 203 | 416 | 567 | 60 |
| CSF/plasma | 4.26 | 0.23 | 0.45 | 0.02 | 0.12 | 0.70 | 0.27 |
| CSF (POD 48) | 1274 | 2.22 | 0.42 | 5 | 51 | 376 | 16 |
| Plasma (POD 48) | 153 | 13.95 | 0.46 | 191 | 400 | 477 | 71 |
| CSF/plasma | 8.33 | 0.16 | 0.91 | 0.03 | 0.13 | 0.79 | 0.23 |
| CSF (POD 55) | 565 | 0.5 | 0.1 | 6 | 36 | 396 | 27 |
| Plasma (POD 53) | 135 | ** | ** | 165 | 334 | 541 | 63 |
| Plasma (POD 57) | 104 | 12.52 | 0.81 | 224 | 301 | 540 | 72 |
| CSF/plasma (D55+D57/2) | 5.43 | 0.04 | 0.12 | 0.03 | 0.12 | 0.73 | 0.40 |
Acylcarnitine profiles were measured simultaneously in the plasma and CSF on post-operative days 28 and 48, from the plasma on day 57, and from the CSF on day 55 (Table 2). Although normal CSF acylcarnitine levels have not been reported, the CSF levels remained lower than plasma in contrast to methylmalonic acid levels. Plasma C3 and C4DC remained appreciably elevated above the normal range.
Amino acid profiles in the plasma and CSF were measured at the same time points as methylmalonic acid (full profiles in Supplementary Table 2). Particular attention was given to glycine, glutamine, and alanine due to their role in nitrogen balance. The amino acid CSF/plasma ratios were consistent with previous descriptions in normal individuals (Table 2) (Kruse and Reiber 1985). The elevated alanine is possibly reflective of an increased CSF lactate, however this was not measured. Interestingly, the patient also had consistently reduced serine levels in her CSF and plasma, with a normal CSF to plasma ratio. Because her CSF serine was in the range of that observed in patients with serine deficiency (van der Crabben et al 2013), we elected to supplement her diet with serine in order to keep her plasma ratios in the high-normal range. The etiology of the low serine remains unknown. A retrospective analysis of her plasma serine levels prior to dialysis and surgery reveals levels that were generally in the normal range (86 µM +/− 32 (SD), n=11).
Discussion
We have described the kinetics and distribution of clinically relevant biochemical parameters in MMA through detailed metabolite analysis of a patient with mut0 MMA who developed end-stage kidney disease requiring HD followed by combined LKT. Our measurements indicate the VD of methylmalonic acid is similar to total body water, in agreement with a previous measurement of this value made by following kinetics of tritiated methylmalonic acid (Stokke et al 1967). HD induces massive flux in plasma methylmalonic acid concentration, and as demonstrated, by 52 hours post-HD, plasma methylmalonic acid, C3, and C4DC levels return to pre-HD baseline. Several brief reports have described the use of HD and/or peritoneal dialysis for periods ranging from 5 months to 5 years in the management of end-stage kidney disease due to MMA. Although the clearance of methylmalonic acid is markedly greater with HD than peritoneal dialysis, the number of reported patients and limited duration of therapy precludes definitive conclusions regarding the ability of any dialysis modality to mitigate or prevent acute complications of MMA (Nyhan et al 2002; van’t Hoff et al 1988; Moreno-Vega and Govantes 1985; Paik et al 2004; Etuwewe et al 2009).
It is unclear that peri-operative HD reduces transplant complications in MMA. We nonetheless used this therapy in an attempt to control methylmalonic acid load, to minimize the risk for metabolic acidosis, and for fluid management (Kameri et al 2011). Despite a stable post-operative reduction in plasma methylmalonic acid concentration, on post-operative day 3 the patient developed acute deterioration in vision due to further optic nerve damage superimposed on pre-existing chronic bilateral optic neuropathy, followed by generalized seizures on post-operative day 27. Neither neurologic event was seemingly related to a change in plasma methylmalonic acid, allograft function, or medications. Pre-transplant CSF metabolite levels were not performed in this patient, but have been previously reported to be approximately 5–10 times higher than plasma levels in other MMA patients who were not in renal failure (Kaplan et al 2006). Post-transplant CSF methylmalonic acid in this patient was 4.5 – 8 times higher than plasma, in agreement with previous work suggesting autonomous production of methylmalonic acid in the brain with inefficient transport across the blood brain barrier (Kaplan et al 2006, Oberholzer et al 1967). Interestingly, although a CSF/plasma gradient existed for methylmalonic acid, the CSF/plasma ratios of amino acids glycine, alanine, and glutamine were consistent with normal values.
Several etiologies were considered as the cause of the post-operative acute-on-chronic optic neuropathy, including the hypotensive episode during transplant surgery as well as metabolic stress associated with the transplant procedure itself. In attempting to treat potentially reversible pathology, we considered the possibility that there was a strong mitochondrial component to the visual loss. Acute opthic atrophy with properties similar to other mitochondrial causes of sudden vision loss are being increasingly reported in MMA (Traber, 2011), and this individual already had such an episode prior to surgery. Epi-743 was introduced into this patient’s care based on the evidence that the loss of vision could have a strong component of mitochondrial dysfunction, and the prior success of this investigational medication in restoring vision to individuals with mitochondrial etiology of loss of vision (Safun 2012). While this patient did have an initial, positive improvement of vision temporally associated with the introduction of Epi-743, it is difficult to interpret causation and this investigational medication was discontinued in consideration of her other pressing post-operative complications and an attempt to consolidate and simplify care.
These observations were all made during ongoing and often acute management of our patient’s clinical course, and as such, have several limitations. An immediately post-HD plasma methylmalonic acid concentration and the concentration of methylmalonic acid in the 2nd tertile of effluent were unavailable; however, any error introduced by estimating these values should be small and of minimal mathematical consequence. In addition, measures of residual renal function and urinary methylmalonic acid excretion were not available, potentially underestimating total body methylmalonic acid clearance. Given that there were many variables, it is not possible to know with certainty the etiology of the acute vision deterioration on post-operative day 3. Possible explanations include but are not limited to rapid changes in metabolite levels and the brief episode of intraoperative hypotension. Symptom presentation on the 3rd postoperative day seems more consistent with a metabolic complication of MMA than ischemic injury. Finally, pre-transplant CSF methylmalonic acid levels were not measured, and therefore it is not known with certainty the degree to which transplantation affected the CSF methylmalonic acid.
A better understanding of the distribution and kinetics of clinically relevant biochemical parameters is critical in developing effective treatment modalities for MMA and related disorders. This report provides evidence that intermittent HD is not an effective modality for stably reducing plasma or CSF methylmalonic acid and acylcarnitine levels. It also provides further evidence that plasma methylmalonic acid after LKT does not reflect CSF concentration, nor does lowering plasma methylmalonic acid necessarily prevent acute MMA related neurological complications. The role of CSF methylmalonic acid and acylcarnitines in the pathogenesis of MMA-related neurological complications has not been studied in detail and requires further investigation.
Supplementary Material
Take Home Message.
This report provides a better understanding of the distribution and kinetics of clinically relevant biochemical parameters in methylmalonic aciduria during initiation of dialysis and after combined liver/kidney transplant.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Footnotes
Hilary J. Vernon, C. John Sperati, Joshua D. King, Andrea Poretti, Neil R. Miller, Jennifer L. Sloan, Andrew M. Cameron, Donna Myers, Charles P. Venditti, David Valle have no conflicts of interest.
Compliance with Ethics Guidelines as described under “General Rules”.
Hilary J. Vernon conceptualized and designed of this study, collected and analyzed the biological samples, interpreted the data, and was the author primarily responsible for drafting the article. She is the Guarantor.
C. John Sperati interpreted the dialysis data, and was the author primarily responsible for drafting the supplementary sections and methodology of this article.
Joshua D. King interpreted the dialysis data, and revised the medical history and dialysis information critically for important intellectual content.
Andrea Poretti interpreted the neuroradiology data, and revised the medical history and radiologic information critically for important intellectual content.
Neil R. Miller interpreted the ophthalmology data, and revised the medical history and ophthalmologic information critically for important intellectual content.
Jennifer L. Sloan interpreted the metabolic data, and revised the medical history and metabolic information critically for important intellectual content.
Andrew M. Cameron interpreted the surgical data, and revised the medical history and surgical information critically for important intellectual content.
Donna Myers interpreted the dialysis data, and revised the medical history and dialysis information critically for important intellectual content.
Charles P. Venditti interpreted the metabolic data, and revised the medical history and metabolic information critically for important intellectual content.
David Valle conceptualized and designed this study with Dr. Vernon, and revised the medical history and metabolic data critically for important intellectual content.
Bibliography
- Adjalla CE, Hosack AR, Gilfix B, et al. Seven novel mutations in mut methylmalonic aciduria. Hum Mut. 1998;11:270–274. doi: 10.1002/(SICI)1098-1004(1998)11:4<270::AID-HUMU3>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Baumgartner ER, Viardot C. Long-term follow-up of 77 patients with isolated methylmalonic acidemia. JIMD. 1995;18:138–142. doi: 10.1007/BF00711749. [DOI] [PubMed] [Google Scholar]
- Brassier A, Boyer O, Valayannopoulos V, et al. Renal transplantation in 4 patients with methylmalonic aciduria: A cell therapy for metabolic disease. Mol Genet Metab. 2013;110:106–110. doi: 10.1016/j.ymgme.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Burdelski M, Ullrich K. Liver transplantation in metabolic disorders: summary of the general discussion. Eur J Pediatr. 1999;158s2:S95–S96. doi: 10.1007/pl00014331. [DOI] [PubMed] [Google Scholar]
- Chakrapani A, Sivakumar P, McKiernan PJ, Leonard JV. Metabolic stroke in methylmalonic acidemia five years after liver transplantation. J Pediatr. 2002;140:261–263. doi: 10.1067/mpd.2002.121698. [DOI] [PubMed] [Google Scholar]
- Chen PW, Hwu WL, Ho MC, et al. Stabilization of blood methylmalonic acid level in methylmalonic acidemia after liver transplantation. Pediatr Transplant. 2010;14:337–341. doi: 10.1111/j.1399-3046.2009.01227.x. [DOI] [PubMed] [Google Scholar]
- Clothier JC, Chakrapani A, Preece MA, et al. Renal transplantation in a boy with methylmalonic acidaemia. JIMD. 2011;34:695–700. doi: 10.1007/s10545-011-9303-y. [DOI] [PubMed] [Google Scholar]
- Enns GM, Kinsman SL, Perlman SL, et al. Initial experience in the treatment of inherited mitochondrial disease with EPI-743. Mol Genet Metab. 2012;105:91–102. doi: 10.1016/j.ymgme.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Etuwewe B, Jones CA, Mathur S, Wright KP, Morris AA. Peritoneal dialysis for chronic renal failure in a patient with methylmalonic acidaemia. Pediatr Nephrol. 2009;24:1085–1087. doi: 10.1007/s00467-008-1068-7. [DOI] [PubMed] [Google Scholar]
- Guyton AC. Textbook of Medical Physiology. fourth ed. Philadelphia: W.B. Saunders; 1976. [Google Scholar]
- Ho D, Harrison V, Street N. Anaesthesia for liver transplantation in a patient with methylmalonic acidaemia. Paediatr Anaesth. 2000;10:215–218. doi: 10.1046/j.1460-9592.2000.00451.x. [DOI] [PubMed] [Google Scholar]
- Hörster F, Baumgartner MR, Viardot C, et al. Long-term outcome in methylmalonic acidurias is influenced by the underlying defect (mut0, mut-, cblA, cblB) Pediatr Res. 2007;62:225–230. doi: 10.1203/PDR.0b013e3180a0325f. [DOI] [PubMed] [Google Scholar]
- Hsui JY, Chien YH, Chu SY. Living-related liver transplantation for methylmalonic acidemia: report of one case. Acta Paediatr Taiwan. 2003;44:171–173. [PubMed] [Google Scholar]
- Inker LA, Schmid CH, Tighiouart H, et al. CKD-EPI Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. New Eng J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei K, Ito S, Shigeta T, et al. Preoperative dialysis for liver transplantation in methylmalonic acidemia. Ther Apher Dial. 2011;15:488–492. doi: 10.1111/j.1744-9987.2011.00974.x. [DOI] [PubMed] [Google Scholar]
- Kaplan P, Ficicioglu C, Mazur AT, Palmieri MJ, Berry GT. Liver transplantation is not curative for methylmalonic acidopathy caused by methylmalonyl-CoA mutase deficiency. Mol Gen Metab. 2006;88:322–326. doi: 10.1016/j.ymgme.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Kruse T, Reiber H, Neuhoff V. Amino Acid Transport across the Human Blood-CSF Barrier An Evaluation Graph for Amino Acid Concentrations in Cerebrospinal Fluid. J Neurol Sci. 1985;70:129–138. doi: 10.1016/0022-510x(85)90082-6. [DOI] [PubMed] [Google Scholar]
- Kruzka PS, Manoli I, Sloan JL, Kopp JB, Venditti CP. Renal growth in isolated methylmalonic acidemia. Genet Med. 2013;15:990–6. doi: 10.1038/gim.2013.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard JV, Walter JH, McKiernan PJ. Workshop Report, The management of organic acidemias: the role of transplantation. JIMD. 2001;24:309–311. doi: 10.1023/a:1010395724012. [DOI] [PubMed] [Google Scholar]
- Lubrano R, Scoppi P, Barsotti P. Kidney transplantation in a girl with methylmalonic acidemia and end stage renal failure. Pediatr Nephrol. 2001;16:848–851. doi: 10.1007/s004670100688. [DOI] [PubMed] [Google Scholar]
- Lubrano R, Elli M, Rossi M, et al. Renal transplant in methylmalonic acidemia: could it be the best option? Report on a case at 10 years and review of the literature. Pediatr Nephrol. 2007;22:1209–1214. doi: 10.1007/s00467-007-0460-z. [DOI] [PubMed] [Google Scholar]
- Lubrano R, Bellelli E, Gentile I, et al. Pregnancy in a methylmalonic acidemia patient with kidney transplantation: a case report. Amer J Transplant. 2013a;13:1918–1922. doi: 10.1111/ajt.12282. [DOI] [PubMed] [Google Scholar]
- Lubrano R, Perez B, Elli M. Methylmalonic acidemia and kidney transplantation. Pediatr Nephrol. 2013b;10:2067–2068. doi: 10.1007/s00467-013-2536-2. [DOI] [PubMed] [Google Scholar]
- Manoli I, Venditti CP. Methylmalonic Acidemia. In: Gene Reviews™ [Internet] Pagon RA, Bird TD, Dolan CR, Stephens K, Adam MP, editors. Seattle: University of Washington; 2010. [Google Scholar]
- Moreno-Vega A, Govantes JM. Methylmalonic acidemia treated by continuous peritoneal dialysis. NEJM. 1985;312:1641–1642. [PubMed] [Google Scholar]
- Nagarajan S, Enns GM, Millan MT, Winter S, Sarwal MM. Management of methylmalonic acidaemia by combined liver–kidney transplantation. JIMD. 2005;28:517–524. doi: 10.1007/s10545-005-0517-8. [DOI] [PubMed] [Google Scholar]
- Nyhan WL, Ozand PT. Atlas of Metabolic Diseases. London: Chapman and Hall; 1998. pp. 13–23. [Google Scholar]
- Nyhan WL, Gargus JJ, Boyle K, Selby R, Koch R. Progressive neurologic disability in methylmalonic acidemia despite transplantation of the liver. Eur J Pediatr. 2002;161:377–379. doi: 10.1007/s00431-002-0970-4. [DOI] [PubMed] [Google Scholar]
- Oberholzer VG, Levin B, Burgess EA, Young WF. Methylmalonic aciduria An inborn error of metabolism leading to chronic metabolic acidosis. Arch Dis Child. 1967;42:492–504. doi: 10.1136/adc.42.225.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogier de Baulny H, Benoist JF, Rigal O, Touati G, Rabier D, Saudubray JM. Methylmalonic and propionic acidemias: management and outcome. JIMD. 2005;28:415–423. doi: 10.1007/s10545-005-7056-1. [DOI] [PubMed] [Google Scholar]
- Paik KH, Lee JE, Jin DK. Successful dialysis in a boy with methylmalonic acidemia. Pediatr Nephrol. 2004;19:1180–1181. doi: 10.1007/s00467-003-1409-5. [DOI] [PubMed] [Google Scholar]
- Prada CE, Al Jasmi F, Kirk EP, et al. Cardiac disease in methylmalonic acidemia. J Pediatr. 2011;159:862–864. doi: 10.1016/j.jpeds.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Rinaldo P, Cowan TM, Matern D. Acylcarnitine profile analysis. Gen in Med. 2008;10:151–156. doi: 10.1097/GIM.0b013e3181614289. [DOI] [PubMed] [Google Scholar]
- Sadun AA, Chicani CF, Ross-Cisneros FN, et al. Effect of EPI-743 on the clinical course of the mitochondrial disease Leber hereditary optic neuropathy. Arch Neurol. 2012;69:331–338. doi: 10.1001/archneurol.2011.2972. [DOI] [PubMed] [Google Scholar]
- Stabler SP, Podell ER, Allen RH. Quantitation of methylmalonic acid and other dicarboxylic acids in normal serum and urine using capillary gas chromatography–mass spectrometry. Anal Biochem. 1985;150:58–66. doi: 10.1016/0003-2697(85)90440-3. [DOI] [PubMed] [Google Scholar]
- Stabler SP, Allen RH, Barrett RE, Savage DG, Lindenbaum J. Cerebrospinal fluid methylmalonic acid levels in normal subjects and patients with cobalamin deficiency. Neuro. 1991;41:1627–1632. doi: 10.1212/wnl.41.10.1627. [DOI] [PubMed] [Google Scholar]
- Stokke O, Eldjarn L, Norum KR, Steen-Johnsen J, Halvorsen S. Methylmalonic acidemia A new inborn error of metabolism which may cause fatal acidosis in the neonatal period. Scand J Clin Lab Invest. 1967;4:313–328. [Google Scholar]
- Traber G, Baumgartner MR, Schwarz U, Pangalu A, Donath MY, Landau K. Subacute bilateral visual loss in methylmalonic acidemia. J Neuroophthalmol. 2011;31:344–346. doi: 10.1097/WNO.0b013e31822db480. [DOI] [PubMed] [Google Scholar]
- van der Crabben SN, Verhoeven-Duif NM, Brilstra EH, et al. An update on serine deficiency disorders. J Inherit Metab Dis. 2013;36:9592–9594. doi: 10.1007/s10545-013-9592-4. [DOI] [PubMed] [Google Scholar]
- van’t Hoff WG, Dixon M, Taylor J, et al. Combined liver–kidney transplantation in methylmalonic acidemia. J Pediatr. 1998;132:1043–1044. doi: 10.1016/s0022-3476(98)70407-x. [DOI] [PubMed] [Google Scholar]
- van’t Hoff WG, McKiernan PJ, Surtees RA, Leonard JV. Liver transplantation for methylmalonic acidaemia. Eur J Pediatr. 1999;158s2:S70–S74. doi: 10.1007/pl00014326. [DOI] [PubMed] [Google Scholar]
- Walter JH, Michalski A, Wilson WM, Leonard JV, Barratt TM, Dillon MJ. Chronic renal failure in methylmalonic acidaemia. Eur J Pediatr. 1989;148:344–348. doi: 10.1007/BF00444131. [DOI] [PubMed] [Google Scholar]
- Watson PE, Watson ID, Batt RD. Total body water volumes for adult males and females estimated from simple anthropometric measurements. Am J Clin Nutr. 1980;33:27–39. doi: 10.1093/ajcn/33.1.27. [DOI] [PubMed] [Google Scholar]
- Wendel U, Ogier de Baulny H. Branched-Chain Organic Acidurias/Acidemias. In: Fernandes, Saudubray, van den Berghe, Walter, editors. Inborn Metabolic Diseases. 4 ed. Heidelberg Germany: Springer; 2006. pp. 246–262. [Google Scholar]
- Willard HF, Rosenberg LE. Inherited methylmalonyl CoA mutase apoenzyme deficiency in human fibroblasts. Evidence for allelic heterogeneity, genetic compounds, and codominant expression. J Clin Invest. 1980;65:690–698. doi: 10.1172/JCI109715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ZR, Hurley PE, Altiparmak UE, et al. Late onset optic neuropathy in methylmalonic and propionic acidemia. Am J Ophthalmol. 2009;147:929–933. doi: 10.1016/j.ajo.2008.12.024. [DOI] [PubMed] [Google Scholar]
- Worgan LC, Niles K, Tirone JC, et al. Spectrum of mutations in mut methylmalonic acidemia and identification of a common Hispanic mutation and haplotype. Hum Mutat. 2006;27:31–43. doi: 10.1002/humu.20258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.