Abstract
We advance the hypothesis that the telocyte might be the cell of origin of both PEComas (perivascular epithelioid cell tumours) and GISTs (gastro-intestinal and extra-gastrointestinal stromal tumours). The hypothesis is supported by data from the literature reporting that both PEComas and GISTs, as well as telocytes, share the expression of several markers. These data were supplemented by original immunohistochemical tests on selected series. Specifically: (1) Melanoma markers (Melan A, MiTF) typical of PEComas are expressed by a substantial fraction of GISTs. A fraction of GISTs was also found positive for CD63, a tetraspanin protein originally described in melanomas and marking exosomes. (2) c-KIT (CD117), proper of the vast majority of GISTs, can be expressed by PEComas (as well as by telocytes). (3) Markers described in telocytes (CD34, S-100, smooth muscle actin and vascular endothelial growth factor) have been reported as positive in cases of PEComas and GISTs. Telocytes show distinctive ultrastructural features with thin, extended, telopodes and are likely involved in inter-cellular signalling via paracrine secretion as well as by shed vesicles and exosomes. These cells have been described in many locations (cavitary and non-cavitary organs) and might display potentialities of a wide spectrum of differentiation (and function). In conclusion we propose that telocytes could be the common cells of origin for both PEComas and GISTs.
Keywords: telocytes, immunohistochemistry, GIST, PEComa, c-kit, CD34, VEGF
Introduction
The term ‘telocytes’[1] has been selected to designate cells characterized by thin, extended, moniliform projections best recognized in electron microscopy which have been described in the stroma of several organs as heart [2–5] and skeletal muscles [6], vessels [7, 8], placenta [9], duodenal lamina propria [10], pleura [11] and lungs [12], being mainly located in perivascular areas. Their light-microscopical detection is linked to the expression of several markers such as c-kit (CD117), CD34, smooth muscle actin (SMA), S-100 protein, vimentin and vascular endothelial growth factor (VEGF).
Although they seemed similar to interstitial cells of Cajal, they are not identical. However, they (or perhaps only a fraction) might be engaged in the process of stem cell proliferation, because the presence of exosomes suggests that they share features with mesenchymal stem cells [13] and direct physical nanocontacts were reported [12].
No pathological lesions have so far been referred to neoplastic or reactive alterations of telocytes.
We advance the hypothesis that these cells might indeed share a prominent interest in pathology, and that they might represent the common cell of origin of a variety of stromal tumours and non-neoplastic conditions, whose cell of origin is presently unclear, comprehensive of tumours supposedly derived from perivascular epithelioid cells (s.c. PEComas) and of gastrointestinal and extra-gastrointestinal (GISTs, and E-GISTs) [14]. Our hypothesis surmises that a phenotypic spectrum (likely linked to different specific functions) is acquired by telocytes in different organs and locations, and suggests a common histogenesis of different pathological lesions. This hypothesis finds support in a series of serendipitous observations in the literature (see below) which otherwise would remain unexplained. In addition, we looked, in a limited number of selected cases, for additional experimental data supporting our hypothesis and thus we checked, in a series of lesions and foetal organs for the alternative expression of markers presently regarded as proper, if not specific, of either PEComas or GISTs.
Material and methods
Tissue specimens were routinely fixed in buffered 10% formalin and embedded in paraffin.
We collected from the archives of our institutions: five cases of pulmonary lymphangioleiomyomatosis, five cases of angiomyolipomas of the kidney, 25 cases of GISTs and thoracic block (comprehensive of heart and lungs) from three cases of 17-week-old foetuses (from medically oriented abortions).
Four-micron-thick paraffin sections were stained using the FLEX-EnVision visualization system by Dako (Glostrup, Denmark) following the procedure recommended by the producer. The following reagents were used as primary antibodies:
HMB45 (clone HMB45, 1:50; Dako), Melan A (clone A 103, 1:50; Dako), microphtalmia transcription factor (MiTF) (clone 34CA5, 1:20; Novocastra, Newcastle-upon-Tyne, UK), CD63 (clone NKI/C3, 1:100; Novocastra), CD117 (polyclonal, 1:400; Dako), S-100 protein (polyclonal, 1:400; Dako), smooth muscle actin (SMA, clone 1 A4, 1:50; Dako), CD34 (clone Qbend, 1:50; Dako).
The immunohistochemical reaction was developed using DAB as chromogen and nuclei were counterstained in Haemalum.
Results
We first checked in GISTs for the presence of melanoma markers, which are regarded as diagnostic of PEComas. In a preliminary study on 10 cases of GIST, none was found positive for HMB45. We then tested additional melanoma markers on a selected tumour series (Table 1) and 58.33% were found positive for MiTF (14 out of 24 cases of GISTs of the spindle, epithelioid or mixed type) and 68.18% were positive for CD63 (15 out of 22 tested cases). The staining was either limited to a few dispersed cells or extended to the vast majority of tumour cells (Fig. 1A and B). The kidney PEComas tested for CD63 were also positive, similar to other melanocytic markers.
Table 1.
Cases of GISTs tested for immunohistochemical markers
| Nr crt | Nr case | Histological type | MITF | CD63 | CD117 | CD34 | Others |
|---|---|---|---|---|---|---|---|
| 1 | 122476/12524 | Spindle cell | + | ++ | +++ | + | DOG1+, Tau+ |
| 2 | 125572/170691 | Spindle cell | ++ | +++ | +++ | − | DOG1+Nest+D2-40+ |
| 3 | 122620/158407 | Spindle cell | ++ | +++ | + | +++ | Nest+, Tau+Act-S-100- |
| 4 | 172861/19229 | Spindle cell | + | + | nd | nd | DOG1+, Act+, S-100+, PDGFRA− |
| 5 | 121339/9042 | Epithelioid | ++ | − | ++ | + | |
| 6 | 125039/97356 | Spindle cell | + | +++ | ++ | ++ | Act+, S-100+, Tau+ |
| 7 | 120007/7556 | Spindle cell | − | + | − | ++ | DOG1+, Nest+, Act+, S-100+, Tau+ |
| 8 | 140581/194914 | Spindle cell | − | nd | ++ | ++ | Nest+, Act+, S-100+ |
| 9 | 119213/736 | Epithelioid | − | nd | +++ | +++ | Nest−, Act−, Tau+, S-100- |
| 10 | 159971/141910 | Spindle cell | − | − | +++ | +++ | Nest+++ D2-40 +/−, Cav + PDGFRA +/− Act−, S-100− |
| 11 | 176172/83466 | Spindle cell | ++ | − | − | +++ | S-100−, Act+ |
| 12 | 171246/169333 | Spindle cell | − | ++ | +++ | +++ | DOG1+, Nest+, S-100+, Act+, D2-40−, PDGFRA− |
| 13 | 162922/147189 | Spindle cell | − | +++ | +++ | nd | |
| 14 | 172056/172088 | Mixed | − | − | +++ | +++ | PDGFRA+, Nest+, S-100+, Act−, D2-40− |
| 15 | 172238/148390 | Mixed | ++ | +++ | +++ | +++ | Nest+, PDGFRA+, D2-40+ |
| 16 | 180373/83467 | Spindle cell | + | + | +++ | +++ | Act+, S-100- |
| 17 | 167839/67394 | Epithelioid | − | + | − | ++ | Act+, S-100+ |
| 18 | 167842/148389 | Mixed | + | + | − | + | Act+, S-100+ |
| 19 | 172240/159475 | Epithelioid | − | − | +++ | − | Nest+, PDGFRA+, D2-40− |
| 20 | 148649/176069 | Spindle cell | + | nd | +++ | +++ | Nest+, Act+, S-100+, Tau+ |
| 21 | 172242/172909 | Spindle cell | + | − | +++ | +++ | DOG1+, Nest−, D2-40-, PDGFRA− |
| 22 | 148504/113074 | Mixed | + | ++ | ++ | nd | DOG1+, Nest+, D2-40− |
| 23 | 121339/9042 | Epithelioid | + | − | + | ++ | Nest+, Tau+ |
| 24 | 149944/5032 | Epithelioid | − | ++ | +++ | − | DOG1+, Nest+, Act+, D2-40− |
| 25 | 152303/37203 | Spindle cell | nd | +++ | +++ | − | DOG1+, Nest+, Act+, S-100+, PKC?+, HMB45− |
−: negative; +: sparse positivity; ++: zonal positivity; +++: diffuse positivity; nd: not done
Fig 1.
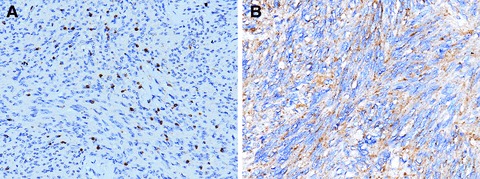
Cases of gastro-intestinal stromal tumours (GISTs) stained with anti- microphtalmia transcription factor (MiTF) (A) and with anti-CD63 (B). Numerous tumour cells are positive (staining for MiTF located in the nucleus). Nuclei counterstained with Haemalum (200 × ).
Then we evaluated CD117 expression in PEComa-related lesions. Cytoplasmic projections or sparse spindle cells, morphologically reminding of telocytes, were CD117-positive in focal intra-alveolar areas typical of lymphangioleiomyomatosis of the lung.
In the foetal heart we detected by CD63 staining some elongated telocyte-like cells in perivascular areas (Fig. 2B), presence of MiTF-positive elongated cells in the atrial wall and a very limited number of cells, mainly located in sub-pericardial areas, were found to be CD117-positive. On the contrary, in areas of foetal lung, no CD63-positive cells were observed, while numerous haphazardly arranged spindle-shaped cells, with long and thin projections, were detected in the interstitium by CD117 immunostaining (Fig. 2B).
Fig 2.
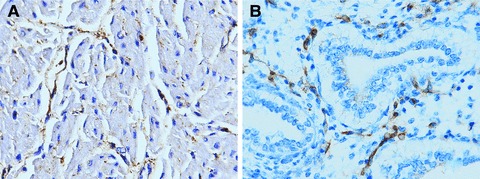
In the foetal heart, elongated cells sharing the structure and location of telocytes are marked by CD63 staining (A). In the foetal lung, numerous irregularly arranged spindle-shaped cells, with long and thin projections are detected in the interstitium by CD-117 staining (B). Nuclei counterstained with Haemalum (400 × ).
Discussion
PEComas is a general term indicating a family of tumours including renal and hepatic angiomyolipomas, clear cell sugar tumours of the lung and clear cell myomelanocytic tumours, as well as pulmonary lymphangioleiomyomatosis [15]. These lesions are supposedly originating from perivascular epithelioid cells, but their normal histological counterpart is presently unknown [15, 16]. The common and unifying feature of PEComas is the remarkable (and so far unexplained) modulation of immunohistochemical markers, with co-expression of SMA and myosin and melanocytic markers (HMB 45, Melan A). The histotype of the tumours varies, from spindle to epithelioid. Focal and occasionally remarkable expression of S-100 protein, SMA, desmin, CD117 (c-KIT) and CD 34 has been noted in these tumours [16, 17]. PEComas have a widespread distribution, with reports of cases occurring in sites such as gastro-intestinal tract, soft tissues and female genital organs, but also in the heart [18], laryngopharyx [19] and pancreas [17].
Occasional and so far unexplained observations, in both PEComas and GISTs, have been reported in the literature:
Expression of melanoma antigens, particularly of Melan A, has been noted in a substantial fraction of epithelioid GISTs [20]. We have here confirmed and further expanded these findings. In agreement with data of the literature [20] we did not detect positivity for HMB45, but a considerable number of GISTs were found positive with MiTF and especially with CD63, a marker characteristic, though not specific, of melanomas [21]. The significance of these findings, as far as type and clinical evolution of melanoma-marker positive GISTs, is presently unknown and warrants experimental study.
CD117 (c-KIT) expression is proper of the vast majority (over 95%) of GISTs, but it can occasionally be detected in PEComas [16, 17, 22]. In the present work we observed CD117-positive cytoplasmic projections or sparse spindle cells, morphologically reminiscent of telocytes, in focal areas of lymphangioleiomyomatosis of the lung.
SMA is expressed in GISTs [23], as well as in the vast majority of PEComas [16] (telocytes have positive expression of αSMA) [9].
S-100 expression has been reported in both PEComas [18] and spindle cell GISTs [20, 24] (telocytes are also positive for S-100) [5].
PEComas as well as GISTs are occasionally CD34-positive [17, 23] (telocytes have positive expression for CD34) [5, 9, 25, 26].
VEGF is expressed in GISTs, in angiomyolipomas and in lymphangioleiomyomatosis [27, 28] (telocytes have positive expression for VEGF [6, 9, 29, 30].
All these data, which would remain unexplained, are fitting with the hypothesis that the common cell of origin of both PEComas and GISTs has to be referred to telocytes, a cell type with ubiquitous distribution and distinctive structural features, likely involved in inter-cellular signalling via paracrine secretion. Telocytes would then give rise, in several organs, to a spectrum of lesions variably expressing at least some of the cells’ differentiation markers. A schematic representation of this concept is presented (Table 2).
Table 2.
Schematic representation of the expression in PEComas and GISTs of immunohistochemical markers proper of Telocytes
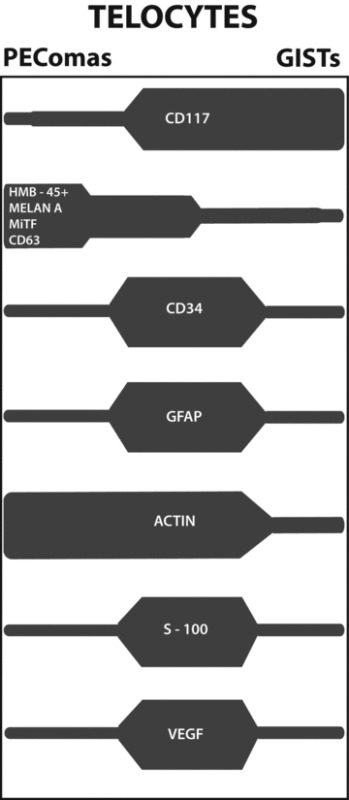 |
The present hypothesis suggests a common cell of origin of PEComas and GISTs, two tumour types with apparently unrelated pathogenesis, because the former are associated to genetic alterations of tuberous sclerosis complex (TSC), an autosomal dominant genetic disease due to losses of TSC1 (9q34) or TSC2 (16p13.3) genes which seem to have a role in the regulation of the Rheb/mTOR/p70S6K pathway, while the vast majority of the latter are due to mutations of KIT and PDGFRA genes. However, evidence that these tumour types share common metabolic pathways derives from evidence that they both are sensitive to agents targeting the mTOR pathway [31, 32].
Our hypothesis carries as well a prospect on the definition and significance of telocytes, which should then be regarded as an archetypal entity with potentialities switching from a perivascular stromal cell to a differentiated cell type with contractile and signalling properties. In the heart interstitium, a site where ultrastructural investigations detected a vast number of telocytes [25, 33, 34], we observed MiTF, CD117 and/or CD63-positive cells. The morphology of these cells closely matches that of telocytes already described in this location. No data are presently available on telocyte-related pathological lesions of the heart, but the present hypothesis might imply a reconsideration of the histogenesis of cardiac stromal tumours whose nature and cell of origin are presently questioned, such as cardiac myxomas [35, 36]. The significance of the expression in GISTs and PEComas of CD63, a tetraspanin protein known to be typical of exosomes [37], remains to be established. It remains to be defined if the micro-vescicles filling up the thin, extended, cytoplasmic projections characterizing telocytes are indeed CD63-positive. Our finding of a remarkable number of CD117-positive stromal cells, morphologically fitting as telocytes, in the interstitium of foetal lung is equally awaiting further investigations. So far, telocytes have been described in the sub-pleural areas of the lung [7], and recently in lung [12].
In conclusion, a series of data from the literature as well as our focused immuno-histochemical investigations concurrently fit with the hypothesis that a phenotypic spectrum (likely linked to different specific functions) can be acquired by telocytes in different organs and locations, and that these cells might constitute the cell of origin of pathological lesions occurring in different organs and specifically designated as PEComas and GISTs.
Acknowledgments
Study was conducted within the frame and with the support of the Project PERSOTHER (SMIS-CSNR: 549/12.024). Prof. Anna Sapino (University of Turin, Italy) critically reviewed the manuscript.
References
- 1.Popescu LM, Faussone-Pellegrini MS. TELOCYTES – a case of serendipity: the winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to TELOCYTES. J Cell Mol Med. 2010;14:729–40. doi: 10.1111/j.1582-4934.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Popescu LM, Gherghiceanu M, Manole CG, et al. Cardiac renewing: interstitial Cajal-like cells nurse cardiomyocyte progenitors in epicardial stem cell niches. J Cell Mol Med. 2009;13:866–86. doi: 10.1111/j.1582-4934.2009.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gherghiceanu M, Popescu L. Cardiomyocyte precursors and Telocytes in epicardial stem cell niche. Electron microscope images. J Cell Mol Med. 2010;14:871–7. doi: 10.1111/j.1582-4934.2010.01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gherghiceanu M, Manole CG, Popescu LM. Telocytes in endocardium: electron microscope evidence. J Cell Mol Med. 2010;14:2330–4. doi: 10.1111/j.1582-4934.2010.01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Popescu LM, Manole CG, Gherghiceanu M, et al. Telocytes in human epicardium. J Cell Mol Med. 2010;14:2085–93. doi: 10.1111/j.1582-4934.2010.01129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Popescu LM, Manole E, Serboiu CS, et al. Identification of telocytes in skeletal muscle interstitium: implication for muscle regeneration. J Cell Mol Med. 2011;15:1379–92. doi: 10.1111/j.1582-4934.2011.01330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantaero I, Luesma MJ, Junquera C. The primary cilium of Telocytes in the vasculature: electron microscope imaging. J Cell Mol Med, Mar 24. 2011 doi: 10.1111/j.1582-4934.2011.01312.x. 10.1111/j.1582-4934.2011.01312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rusu MC, Pop F, Hostiuc S, et al. Extrahepatic and intrahepatic human portal interstitial Cajal cells. Anat Rec. 2011;294:1382–92. doi: 10.1002/ar.21441. [DOI] [PubMed] [Google Scholar]
- 9.Suciu L, Popescu LM, Gherghiceanu M, et al. Telocytes in human term placenta: morphology and phenotype. Cells Tissues Organs. 2010;192:325–39. doi: 10.1159/000319467. [DOI] [PubMed] [Google Scholar]
- 10.Cantarero Carmona I, Luesma Bartolom MJ, Junquera Escribano C. Identification of telocytes in the lamina propria of rat duodenum: transmission electron microscopy. J Cell Mol Med. 2011;15:26–30. doi: 10.1111/j.1582-4934.2010.01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hinescu ME, Gherghiceanu M, Suciu L, et al. Telocytes in pleura: two- and three-dimensional imaging by transmission electron microscopy. Cell Tissue Res. 2011;343:389–97. doi: 10.1007/s00441-010-1095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Popescu LM, Gherghiceanu M, Suciu LC, et al. Telocytes and putative stem cells in the lungs: electron microscopy, electron tomography and laser scanning microscopy. Cell Tissue Res. 2011 doi: 10.1007/s00441-011-1229-z. 10.1007/s00441-011-1229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai RC, Chen TS, Lim SK. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen Med. 2011;6:481–92. doi: 10.2217/rme.11.35. [DOI] [PubMed] [Google Scholar]
- 14.Bussolati G. Of GISTs and EGISTs, ICCs and ICs. Virchows Arch. 2005;447:907–8. doi: 10.1007/s00428-005-0083-3. [DOI] [PubMed] [Google Scholar]
- 15.Martignoni G, Pea M, Reghellin D, et al. PEComas: the past, the present and the future. Virchows Arch. 2008;452:119–32. doi: 10.1007/s00428-007-0509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folpe AL, Mentzel T, Lehr HA, et al. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol. 2005;29:1558–75. doi: 10.1097/01.pas.0000173232.22117.37. [DOI] [PubMed] [Google Scholar]
- 17.Perigny M, Larochelle O, Hammel P, et al. Pancreatic perivascular epithelioid cell tumour (PEComa) Ann Pathol. 2008;28:138–42. doi: 10.1016/j.annpat.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Tai Y, Wei L, Shi H. Perivascular epithelioid cell tumour of the heart in a child. Pediatr Dev Pathol. 2010;13:412–4. doi: 10.2350/09-10-0726-CR.1. [DOI] [PubMed] [Google Scholar]
- 19.Huai-yin S, Li-xin W, Lu S, et al. Perivascular epithelioid cell tumours of the laryngopharinx: three case reports and literature review. Pathol Res Pract. 2009;205:595–600. doi: 10.1016/j.prp.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Guler ML, Daniels JA, Abraham SC, et al. Expression of melanoma antigens in epithelioid gastro-intestinal stromal tumours: a potential diagnostic pitfall. Arch Pathol Lab Med. 2008;132:1302–6. doi: 10.5858/2008-132-1302-EOMAIE. [DOI] [PubMed] [Google Scholar]
- 21.Barrio MM, Bravo AI, Portela P, et al. A new epitope on human melanoma-associated antigen CD63/ME491 expressed by both primary and metastatic melanoma. Hybridoma. 1998;17:355–64. doi: 10.1089/hyb.1998.17.355. [DOI] [PubMed] [Google Scholar]
- 22.Park SH, Ro JY, Kim HS, et al. Perivascular epithelioid cell tumour of the uterus: immunohistochemical, ultrastructural and molecular study. Pathol Int. 2003;53:800–5. doi: 10.1046/j.1440-1827.2003.01557.x. [DOI] [PubMed] [Google Scholar]
- 23.Miettinen M, Sobin LH, Sarlomo-Rikala M. Immunohistochemical spectrum of GISTs at different sites and their differential diagnosis with a reference to CD117 (KIT) Mod Pathol. 2000;13:1134–42. doi: 10.1038/modpathol.3880210. [DOI] [PubMed] [Google Scholar]
- 24.Ma CK, Amin MB, Kintanar E, et al. Immunohistologic characterization of gastrointestinal stromal tumours: a study of 82 cases compared with 11 cases of leiomyomas. Mod Pathol. 1993;6:139–44. [PubMed] [Google Scholar]
- 25.Bani D, Formigli L, Gherghiceanu M, et al. Telocytes as supportino cells for myocardial tissue organization in developing and adult Hart. J Cell Mol Med. 2010;14:2531–8. doi: 10.1111/j.1582-4934.2010.01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng Y, Li H, Manole CG, et al. Telocytes in trachea and lungs. J Cell Mol Med. 2011 doi: 10.1111/j.1582-4934.2011.01404.x. 10.1111/j.1582-4934.2011.01404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Issaka RB, Oommen S, Gupta SK, et al. Vascular endothelial growth factors C and D induces proliferation of lymphangioleiomyomatosis cells through autocrine crosstalk with endothelium. Am J Pathol. 2009;175:1410–20. doi: 10.2353/ajpath.2009.080830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salto-Tellez M, Nga ME, Han HC, et al. Tissue microarrays characterise the clinical significance of a VEGF-A protein expression signature in gastrointestinal stromal tumours. Br J Cancer. 2007;96:776–82. doi: 10.1038/sj.bjc.6603551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manole CG, Cismasiu V, Gherghiceanu M, et al. Experimental acute myocardial infarction: telocytes involvement in neo-angiogenesis. J Cell Mol Med. 2011 doi: 10.1111/j.1582-4934.2011.01449.x. 10.1111/j.1582-4934.2011.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bojin FM, Gavriliuc OI, Cristea MI, et al. Telocytes within human skeletal muscle stem cell niche. J Cell Mol Med. 2011 doi: 10.1111/j.1582-4934.2011.01386.x. 10.1111/j.1582-4934.2011.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCormack FX, Inoue Y, Moss J, et al. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364:1595–606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reichardt P, Reichardt A, Pink D. Molecular targeted therapy of gastrointestinal stromal tumours. Curr Cancer Drug Targets. 2011;11:688–97. doi: 10.2174/156800911796191042. [DOI] [PubMed] [Google Scholar]
- 33.Hinescu ME, Popescu LM. Interstitial Cajal-like cells (ICLC) in human atrial myocardium. J Cell Mol Med. 2005;9:972–5. doi: 10.1111/j.1582-4934.2005.tb00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kostin S. Myocardial Telocytes: a specific new cellular entity. J Cell Mol Med. 2010;14:1917–21. doi: 10.1111/j.1582-4934.2010.01111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krikler DM, Rode J, Davies MJ, et al. Atrial myxoma: a tumour in search of its origins. Br Heart J. 1992;67:89–91. doi: 10.1136/hrt.67.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pucci A, Gagliardotto P, Zanini C, et al. Histopathologic and clinical characterization of cardiac myxoma: review of 53 cases from a single institution. Am Heart J. 2000;140:134–8. doi: 10.1067/mhj.2000.107176. [DOI] [PubMed] [Google Scholar]
- 37.Pols MS, Klumperman J. Trafficking and function of tetraspanin CD63. Exp Cell Res. 2009;315:1584–92. doi: 10.1016/j.yexcr.2008.09.020. [DOI] [PubMed] [Google Scholar]


