Abstract
Blood vessels are highly organized and complex structure, which are far more than simple tubes conducting the blood to almost any tissue of the body. The fine structure of the wall of blood vessels has been studied previously using the electron microscope, but the presence the telocytes associated with vasculature, a specific new cellular entity, has not been studied in depth. Interestingly, telocytes have been recently found in the epicardium, myocardium, endocardium, human term placenta, duodenal lamina propria and pleura. We show the presence of telocytes located on the extracellular matrix of blood vessels (arterioles, venules and capillaries) by immunohistochemistry and transmission electron microscopy. Also, we demonstrated the first evidence of a primary cilium in telocytes. Several functions have been proposed for these cells. Here, the telocyte-blood vessels cell proximity, the relationship between telocytes, exosomes and nervous trunks may have a special significance.
Keywords: telocytes, primary cilium, vasculature, transmission electron microscopy
Introduction
Blood vessels are highly organized and complex structure, which are far more than simple tubes conducting the blood to almost any tissue of the body. They are able to autonomously regulate the blood flow, thus providing the tissues an optimal support of oxygen and nutrients and an efficient removal of waste products [1]. The blood supply of the duodenum is confusing because of the diverse possibilities of origin, distribution and individual variations [2].
The wall of the large vessels usually contains endothelial cells, inner circular and outer longitudinal smooth muscle layers, nerve cells, connective tissue and collagen and elastic fibres. The fine structure of the wall of blood vessels has been studied previously using the electron microscope [3], but the presence the telocytes associated with vasculature, a specific new cellular entity, has not been studied in depth. Interestingly, telocytes have been recently found in the epicardium [4], myocardium [5–7], endocardium [8], human term placenta [9], duodenal lamina propria [10] and pleura [11].
Because of electron microscopy and histochemical techniques as well as the possibilities of investigation at the single cell level, the interest in the investigation of the features, function and localization of telocytes have increased. These cells have a small oval body, mainly occupied by the nucleus, surrounded by a small amount of cytoplasm. The perinuclear cytoplasm is rich in mitochondria, contains a small Golgi apparatus, as well as elements of rough and smooth endoplasmic reticulum and cytoskeletal elements (thin and intermediate filaments). As main characteristic, telocytes have two or three thin and long processes, telopodes, with dichotomic branching pattern [12].
In this paper, we show the presence of telocytes located on the extracellular matrix of blood vessels (arterioles, venules and capillaries) by immunohistochemistry and transmission electron microscopy (TEM). Also, we demonstrated the first evidence of a primary cilium in telocytes. Several functions have been proposed for these cells. Here, the telocyte-blood vessels cell proximity, the relationship between telocyte and nervous trunks and the presence of primary cilium may have a special significance.
Materials and methods
Animal use
Six adult Wistar rats, 3 months old, weighing between 180 and 210 g (The Jackson Laboratory, Bar Harbor, ME, USA), were used in accordance with institutional guidelines (Ethics Advisory Commission for animal Experimentation, PI 36/10). Each animal had ad libitum access to food and water and was fed on a complete and balanced standard laboratory diet (Teklad 4% rat diet 7001; Harlan Teklad, Madison, WI, USA). They were housed in temperature controlled rooms (20 ± 1°C) and natural light.
All rats were anaesthetized, killed and perfused intracardially with the specific fixative of the technique described earlier.
Immunohistochemistry
The duodenum samples (n = 6) were fixed for 6 hrs with formol saline solution at 10% pH 7. Immunohistochemical staining was performed on paraffin sections 5 μm thick using the immunohistochemistry EnVision® (Dako, Carpinteria, CA, USA) method. The primary antibody used in this study was: polyclonal rabbit anti–CD117/c-Kit (1:50, ABIN188397; Antibodies-Online, Aachen, Germany). Antibody was diluted with Dako diluent (S2022; Dako). The tissue sections were deparaffined in xylene (10 min. twice) and rehydrated in a graded ethanol series up to distilled water. Before all assays, for heat-induced antigen retrieval, the samples were treated during 6 min. in an 800 W microwave oven with 10% citrate buffer (S2031; Dako) in distilled water and at 360 W for five additional minutes. After washing twice with PBS, 3 min., the sections were treated with endogenous peroxidase blocking (S2001; Dako) for 20 min., washed in distilled water and blocking buffer [100 ml PBS, 2 ml triton X100, 0.25 ml BSA (A4503; Sigma-Aldrich, St. Louis, MO, USA)], for 3 min., twice. The blocking was repeated for a second time. The sections were incubated with the primary antibody solution for 30 min. followed by a rinse in PBS, for 3 min., twice. Sections not incubated with the primary antibody were used as negative control. The visualization was made by incubating with Envision® peroxidase-based visualization kit (K5007; Dako) during 30 min., washed twice in PBS, for 3 min. according to manufacturer’s directions. To confirm the presence of immunocomplexes, 3,3′-diaminobenzidine was used as chromogene and hydrogen peroxide as substrate. The samples were washed twice in distilled water, contrasted with Mayer’s haematoxylin for 7 min., washed in tap water for 15 min., dehydrated in a graded series of ethanol, cleared in xylene and cover slipped with DPX. Digital microscope images were captured by means of an Olympus BX 51 microscope.
Transmission electron microscopy (TEM)
Duodenum samples (about 1–1.5 mm3) were washed in phosphate buffer and fixed with 2.5% glutaraldehyde and 2% paraformaldehyde. The pieces were fixed overnight at room temperature in the same fixative, washed in 0.1 M phosphate buffer for 5 min., post-fixed with 2% osmium, rinsed, dehydrated in graded acetones (30%, 50%, 70% with 2% uranyl-acetate, 90%, 100%), cleared in propylene oxide and embedded in araldite (Durcupan, Fluka AG, Buchs SG, Switzerland). Semi-thin sections (1.5 μm) were cut with a diamond knife and stained lightly with 1% toluidine blue. Later, ultrathin (0.08 μm) sections were cut with a diamond knife, collected on Formvar coated single-slot grids, counterstained with 1% uranyl acetate and with Reynold’s lead citrate for 10 min. and examined under a FEI Tecnai G2 Spirit TEM. The images were achieved with Advanced Microscopy Techniques, Corp.’s charge-coupled device (CCD) (Danvers, MA, USA) imaging system. Semi-thin sections (≤1 μm thick) were stained with toluidine blue and examined by light microscopy (Olympus BX51 microscope; Olympus Imaging Corporation, Tokyo, Japan).
Results
Light microscopy
By immunohistochemistry, cell expressing the stemness marker c-kit could be found in all the types of blood vessels studied: arterioles, venules and capillaries. c-kit was expressed in cells located just behind the muscle layer surrounding blood vessels (Fig. 1A). Sections containing archetypal myenteric plexus interstitial cell of Cajal (ICC-MP) from the digestive tract were used as positive controls (Fig. 1B).
Fig 1.
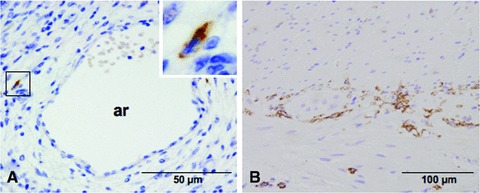
(A) Duodenal arteriole from Wistar rat. Positive immunostaining to CD117/c-kit in perivascular localization. Inset: Cell with fusiform body is intensely c-kit positive. (B) Positive control in rat jejunum. Nuclei were counterstained with Mayer haematoxylin. ar: arteriole.
Light microscopy on semi-thin sections
Toluidine blue staining revealed the general morphology of telocyte: the fusiform or triangular cells bodies and the emerging cytoplasmic processes, telopodes, that surround capillary (Fig. 2A) and arteriole (Fig. 2B). The moniliform aspect and sinuous trajectory of telopodes is evident. However, with the exception of nucleus, no other subcellular component (organelle) can be really recognized inside the telocytes with this tissue processing and the given resolving power.
Fig 2.
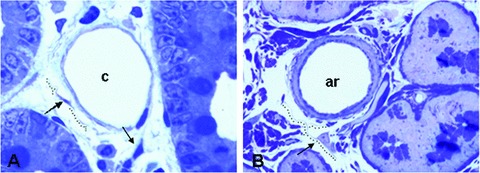
Light microscopy of toluidine-blue stained semithin sections of the duodenum Wistar rats. A typical capillary (A) and arteriole (B) circumscribed by telocytes (arrows). Objective 40 × .
Transmission electron microscopy (TEM)
Using electron microscopy, we observed that telocytes have been clearly found in the loose connective tissue surrounding the arterioles, venules and capillaries. Telocytes associated with the vasculature were morphologically similar to those previously found in other locations. In arterioles, the telocytes often send telopodes bordering the tunica adventitia (Fig. 3A). The adjacent telopodes may establish junctional complexes (Fig. 3B) and show small dilatations (podoms) that accommodate rough endoplasmic reticulum (Fig. 3C). In relation to venules and capillaries, the telocytes were located parallel with the longitudinal axis of the smooth muscle cells of the vessel wall (Fig. 4A and B, respectively). Also, the telopodes can form a wide network around the blood vessels (Fig. 4C).
Fig 3.

Electron micrograph showing the close relationship between arteriole and telocytes. (A) Telocyte covering collagen and elastic fibres present in the tunica adventitia. (B and C) Higher magnification of boxed areas in A. (B) Gap junction connecting the processes of two telopodes. (C) Two dilatations, podoms (pd1 and pd2) which accommodate rough endoplasmic reticulum. ar: arteriole; smcs: smooth muscle cells; tp: telopodes; nt: nervous trunk; TC: telocyte; pd: podom.
Fig 4.
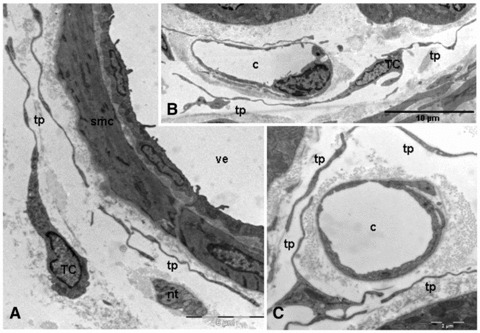
TEM images of telopodes next to a venule and capillary. (A) Several telopodes bordering the smooth muscle cells of a venule. (B) The capillary is partially surrounded by long telopodes. (C) Numerous telopodes encloses a capillary. smc: smooth muscle cell; tp: telopodes; nt: nervous trunk; TC: telocyte; c: capillary; ve: venule.
Vascular telocytes showed typical features: small cellular body; long, thin and moniliform prolongations with dichotomic branching pattern; mitochondria, well-developed Golgi apparatus and polyribosomes. In addition, we have identified a novel cytoplasmic component; a deep invagination that is continuous with the plasma membrane, the cilium-pit, which accommodate the primary cilium (Fig. 5A and B). The cilium that extends into the extracellular matrix showed a structure 9 + 0 (absence of the central filaments) and a length of about 2–3 μm (Fig. 6A and B).
Fig 5.
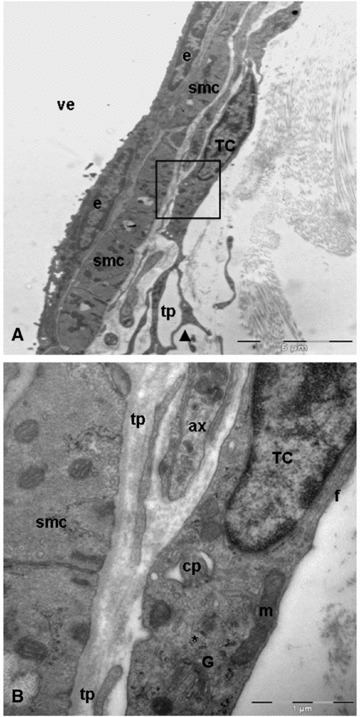
Transmission electron microscopy images of telocyte in close apposition to wall of venule. (A) A typical example of telocyte: long, thin and moniliform processes with dichotomous pattern (arrowhead) of branching. Black square illustrating a higher magnification of telocyte from A. (B) The perinuclear cytoplasm contains well-developed Golgi apparatus (G), mitochondria (m), numerous polyribosomes (asterisk) and intermediate filaments (f). Note the deep invagination (cilium-pit) corresponding to primary cilium. smc: smooth muscle cells; tp: telopodes; ax: axon; TC: telocyte; e: endothelial cell; cp: cilium-pit; pd: podom; ve: venule.
Fig 6.
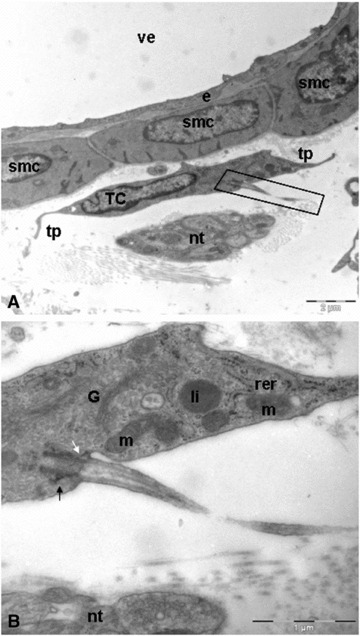
Duodenal venule. (A) Telocyte located in parallel between the cells of the vessel wall and a nerve trunk. Note as the cytoplasm of telocyte establishes interactions with vascular smooth muscle. Black rectangular illustrating the primary cilium. (B) Longitudinally section. Some telocytes exhibit a primary cilium. The basal body resides at the base of the cilium-pit and is associated with its membrane via the distal appendages (white arrow). Below the distal appendages are the subdistal appendages (black arrow). smc: smooth muscle cells; tp: telopodes; nt: nervous trunk; TC: telocyte; e: endothelial cell; ve: venule; G: golgi apparatus; m: mitochondria; rer: rough endoplasmic reticulum; li: lipid drop.
Sometimes, we observed small membrane vesicles secreted by telocytes into the extracellular compartment called exosomes (Fig. 7).
Fig 7.

TEM. Isolated secretory vesicle with exosome morphology (arrow) from telocyte was located close to endothelial cell. nt: nervous trunk; TC: telocyte; c: capillary.
Discussion
In the vascular system, we characterize a cell population does not correspond to any of the other cell types present in the vessels wall: endothelial cells, smooth muscle cells (SMCs), fibroblasts, inflammatory cells, and pericytes. Cells with irregular bodies and numerous thin long processes, interstitial cells of Cajal (ICCs) or interstitial Cajal-like cells (ICLCs), have been described in the vasculature [13–14]: rat mesenteric artery [15], human pulmonary veins [16] and rabbit portal vein [17]. Not all the above cases, the ICCs were positive for c-kit. Using electron microscopy and exclusion criteria defined by Popescu [12], the results we obtained so far prove that it has many phenotypic and morphologic features related to the archetypal telocytes.
Although in the last papers we have witnessed an increased interest and knowledge about telocytes and their relation to blood vessels, several unsolved issues regarding these cells should be emphasized. Apart from several studies reporting the presence of telopodes in close proximity to blood vessel, there are virtually no studies to address the properties of telocytes in the vasculature.
Telopodes and interstitial network
Popescu suggested that telocytes seem to form an interstitial network in endocardium [8], human term placenta [9] and in pleura [18]. In the first case, the telocytes were connected by gap junctions in a network extending into the placental stroma between concentric layers formed by SMC and myofibroblasts around large blood vessels. However, their telopodes were often connected through a ‘plug and socket’ complex, as revealed by electron tomography, in case of the pleura. We also observed this interstitial network surrounding the blood vessels where the telopodes were connected by gap junctions.
Primary cilium of telocytes
As mentioned in this paper, the ultrastructural identity of telocytes is well established and unequivocal. However, we have identified a relevant feature in these cells, the presence of a single non-motile cilium called primary cilium. Primary cilia contain a 9+0 axoneme, consisting of nine outer doublet microtubules but lacking the central pair of microtubules that is found in the 9+2 axonemes of most motile cilia. Except for nodal cilia, primary cilia are thought to lack axonemal dyneins and be immotile [19].
A recent study has suggested that in cells displaying an internal primary cilium, the cilium extend towards the cell surface though a channel lined with a membrane that in its mature form is continuous with the plasma membrane and only a portion of the cilium extends free of the cell margin and into the cellular environment. They have designated this cilium filled channel the cilium-pit and the space between the cilium and the walls of the pit the cilium reservoir. The cilium reservoir is continuous with the extracellular environment [20].
This structure has also been reported in interstitial cells of Cajal by our research group [21–22]. Since the discovery of this organelle in 1898 [23], three major hypotheses for their function have been put forth. The first is that they are vestigial organelles inherited from an ancestor whose cells had motile cilia, and that they now have no purpose [24]. A second hypothesis is that primary cilia are closely linked with the cell cycle. In post-mitotic cells, the centrosome, which is composed of two centrioles surrounded by pericentriolar material, migrates to the cell surface, and one of the centrioles differentiates into a basal body that nucleates microtubules to give rise to the primary cilium. During mitosis, the primary cilium is disassembled and the centrosome organizes the bipolar spindle [25].
Such a role would not be incompatible with the third hypothesis, which is that primary cilia are sensory organelles [26]. This hypothesis is suggested by the fact that, first, cilia are clearly used as sensory organelles in lower eukaryotes; second, the sensory structures of the vertebrate visual and olfactory systems are modified cilia and third, the primary cilium has emerged as key organelle, for example, in hedgehog signal transduction in mammalian systems [19, 27]. The primary cilium of telocytes associated with the vasculature may be particularly important in signalling processes within the vascular niche.
Telocytes as progenitor cells
Recently it has been suggested that telocytes could be considered an early step in the differentiation process of the mesenchymal cells, a subpopulation of progenitor cells among the stromal cells of human placenta [9]. In fact, besides being c-kit-positive, the telocytes coexpressed other stem-cell markers including CD34, CD44 [28] and nestin [29]. On the other hand, the primary cilium is necessary for chemically induced differentiation of human mesenchymal stem cells [30]. Then, based on the previous considerations, we can add a new phenotypic characteristic, the primary cilium, and support the previous hypothesis given by Suciu [9].
Vascular niche
Some previous studies demonstrate that, for example, ICC processes might act as ‘cellular’ guides for immune cells that arrive via blood stream [31] or telocytes have ‘strategic’ positioning in a tissue, in between blood capillaries and their specific target cells (e.g. smooth muscle cells, cardiomyocytes) and in close contact with nerve ending [12]. Vascular cells are key elements of different cellular niches, for example in the adult hippocampus [32], the bone marrow [33], the intestine and skin [34]. Based on our observations, we think that ‘mesenchymal cell niche’ is composed of telocytes, nerve fibres and blood vessels. Telocytes whose somas did not contact blood vessels frequently extended their telopodes towards the vasculature and nervous trunks.
The presence of exosomes seems to be a conserved feature in the telocytes, regardless of their location. An increasing body of evidence indicates that they play a pivotal role in cell-to-cell communication. Indeed, they may directly stimulate target cells by receptor-mediated interactions or may transfer from the cell of origin various bioactive molecules including membrane receptors, proteins, mRNAs, microRNAs and organelles [35]. Although other roles have been suggested, such as, immune system modulation [36].
We suggest that blood vessels and nerve fibres can create a favourable microenvironment (‘niche’) in the differentiation process of mesenchymal precursor cells so, the telocytes could secrete exosomes with the aim to modulate their microenvironment.
In conclusion, this study has revealed the characteristics of telocytes in duodenal vasculature. We considered that the telocytes might contribute to the regulation of microvascular niche and to participate in heterocellular communication mediated by gap junctions and exosomes. Of particular interest is the presence of primary cilium. At least for the moment, we strongly believe in previous hypothesis that telocytes may be a subpopulation of mesenchymal progenitor cells.
Acknowledgments
This research has received financial support from Aragon Institute of Health Sciences (I+CS) (PIPAMER 10/001) and the European Social Fund (ESF), DGA (B83).
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Eble JA, Niland S. The extracellular matrix of blood vessels. Curr Pharm Des. 2009;15:1385–400. doi: 10.2174/138161209787846757. [DOI] [PubMed] [Google Scholar]
- 2.Skandalakis LJ, Skandalakis JE, Skandalakis PN. Surgical anatomy and technique: a pocket manual. 3. New York: Springer Science+Business Media LLC; 2009. Duodenum; pp. 333–46. [Google Scholar]
- 3.Gabella G. Asymmetric distribution of dense bands in muscle cells of mammalian arterioles. J Ultrastruct Res. 1983;84:24–33. doi: 10.1016/s0022-5320(83)90083-7. [DOI] [PubMed] [Google Scholar]
- 4.Popescu LM, Manole CG, Gherghiceanu M, et al. Telocytes in human epicardium. J Cell Mol Med. 2010;14:2085–93. doi: 10.1111/j.1582-4934.2010.01129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kostin S. Myocardial telocytes: a specific new cellular entity. J Cell Mol Med. 2010;14:1917–21. doi: 10.1111/j.1582-4934.2010.01111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suciu L, Nicolescu MI, Popescu LM. Cardiac telocytes: serial dynamic images in cell culture. J Cell Mol Med. 2010;14:2687–92. doi: 10.1111/j.1582-4934.2010.01185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou J, Zhang Y, Wen X, et al. Telocytes accompanying cardiomyocyte in primary culture: two- and three-dimensional culture environment. J Cell Mol Med. 2010;14:2641–5. doi: 10.1111/j.1582-4934.2010.01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gherghiceanu M, Manole CG, Popescu LM. Telocytes in endocardium: electron microscope evidence. J Cell Mol Med. 2010;14:2330–4. doi: 10.1111/j.1582-4934.2010.01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suciu L, Popescu LM, Gherghiceanu M, et al. Telocytes in human term placenta: morphology and phenotype. Cells Tissues Organs. 2010;192:325–39. doi: 10.1159/000319467. [DOI] [PubMed] [Google Scholar]
- 10.Carmona IC, Bartolomé MJ, Escribano CJ. Identification of telocytes in the lamina propria of rat duodenum: transmission electron microscopy. J Cell Mol Med. 2011;15:26–30. doi: 10.1111/j.1582-4934.2010.01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hinescu ME, Gherghiceanu M, Suciu L, et al. Telocytes in pleura: two- and three-dimensional imaging by transmission electron microscopy. Cell Tissue Res. 2011;343:389–97. doi: 10.1007/s00441-010-1095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Popescu LM, Faussone-Pellegrini MS. TELOCYTES – a case of serendipity: the winding way from interstitial cells of Cajal (ICC), via interstitial Cajal-like cells (ICLC) to TELOCYTES. J Cell Mol Med. 2010;14:729–40. doi: 10.1111/j.1582-4934.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harhun MI, Pucovský V, Povstyan OV, et al. Interstitial cells in the vasculature. Cell Mol Med. 2005;9:232–43. doi: 10.1111/j.1582-4934.2005.tb00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolton TB, Gordienko DV, Povstyan OV, et al. Smooth muscle cells and interstitial cells of blood vessels. Cell Calcium. 2004;35:643–57. doi: 10.1016/j.ceca.2004.01.018. Review. [DOI] [PubMed] [Google Scholar]
- 15.Formey A, Buscemi L, Boittin FX, et al. Identification and functional response of interstitial Cajal-like cells from rat mesenteric artery. Cell Tissue Res. 2011;343:509–19. doi: 10.1007/s00441-010-1114-1. [DOI] [PubMed] [Google Scholar]
- 16.Gherghiceanu M, Hinescu ME, Andrei F, et al. Interstitial Cajal-like cells (ICLC) in myocardial sleeves of human pulmonary veins. J Cell Mol Med. 2008;12:1777–81. doi: 10.1111/j.1582-4934.2008.00444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Povstyan OV, Gordienko DV, Harhun MI, et al. Identification of interstitial cells of Cajal in the rabbit portal vein. Cell Calcium. 2003;33:223–39. doi: 10.1016/s0143-4160(02)00197-5. [DOI] [PubMed] [Google Scholar]
- 18.Hinescu ME, Gherghiceanu M, Suciu L, et al. Telocytes in pleura: two- and three-dimensional imaging by transmission electron microscopy. Cell Tissue Res. 2011;343:389–97. doi: 10.1007/s00441-010-1095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pazour GJ, Witman GB. The vertebrate primary cilium is a sensory organelle. Curr Opin Cell Biol. 2003;15:105–10. doi: 10.1016/s0955-0674(02)00012-1. [DOI] [PubMed] [Google Scholar]
- 20.Moser JJ, Fritzler MJ, Ou Y, et al. The PCM-basal body/primary cilium coalition. Semin Cell Dev Biol. 2010;21:148–55. doi: 10.1016/j.semcdb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Junquera C, Martìnez-Ciriano C, Castiella T, et al. Immunohistochemical and ultrastructural characteristics of interstitial cells of Cajal in the rabbit duodenum. Presence of a single cilium. J Cell Mol Med. 2007;11:776–87. doi: 10.1111/j.1582-4934.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Junquera Escribano C, Cantarero Carmona I, Luesma Bartolomé MJ, et al. The primary cilium: a relevant characteristic in interstitial cells of rat duodenum enteric plexus. Histol Histopathol. 2011;26:461–70. doi: 10.14670/HH-26.461. [DOI] [PubMed] [Google Scholar]
- 23.Zimermman KW. Beiträge zur Kenntniss einiger drüsen und epithelien [English translation: Contributions to knowledge of some glands and epithelium] Arch Mikr Anat. 1898;52:552–706. [Google Scholar]
- 24.Sorokin S. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J Cell Biol. 1962;15:363–77. doi: 10.1083/jcb.15.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plotnikova OV, Golemis EA, Pugacheva EN. Cell cycle-dependent ciliogenesis and cancer. Cancer Res. 2008;68:2058–61. doi: 10.1158/0008-5472.CAN-07-5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singla V, Reiter JF. The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science. 2006;313:629–33. doi: 10.1126/science.1124534. Review. [DOI] [PubMed] [Google Scholar]
- 27.Eggenschwiler JT, Anderson KV. Cilia and developmental signaling. Annu. Rev. Cell Dev. Biol. 2007;23:345–73. doi: 10.1146/annurev.cellbio.23.090506.123249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blondheim NR, Levy YS, Ben-Zur T, et al. Human mesenchymal stem cells express neural genes, suggesting a neural predisposition. Stem Cells Dev. 2006;15:141–64. doi: 10.1089/scd.2006.15.141. [DOI] [PubMed] [Google Scholar]
- 29.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–95. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 30.Tummala P, Arnsdorf EJ, Jacobs CR. The role of primary cilia in mesenchymal stem cell differentiation: a pivotal switch in guiding lineage commitment. Cell Mol Bioeng. 2010;3:207–12. doi: 10.1007/s12195-010-0127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Popescu LM, Gherghiceanu M, Cretoiu D, et al. The connective connection: interstitial cells of Cajal (ICC) and ICC-like cells establish synapses with immunoreactive cells. Electron microscope study in situ. J Cell Mol Med. 2005;9:714–30. doi: 10.1111/j.1582-4934.2005.tb00502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–94. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 33.Kiel MJ, Yilmaz OH, Iwashita T, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–21. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 34.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–78. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 35.Camussi G, Deregibus MC, Bruno S, et al. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78:838–48. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 36.Stoorvogel W, Kleijmeer MJ, Geuze HJ, et al. The biogenesis and functions of exosomes. Traffic. 2002;3:321–30. doi: 10.1034/j.1600-0854.2002.30502.x. Review. [DOI] [PubMed] [Google Scholar]


