Abstract
The study has analysed the action of histamine in the rabbit venous system and evaluated its potential role in contraction during increased venous pressure. We have found that a great variety exists in histamine sensitivity and H1-histamine receptor expression in various types of rabbit veins. Veins of the extremities (saphenous vein, femoral vein, axillary vein) and abdomen (common iliac vein, inferior vena cava) responded to histamine by a prominent, concentration-dependent force generation, whereas great thoracic veins (subclavian vein, superior vena cavas, intrathoracic part of inferior vena cava) and a pelvic vein (external iliac vein) exhibited slight sensitivity to exogenous histamine. The lack of reactivity to histamine was not due to increased activity of nitric oxide synthase (NOS) or heme oxygenase-1. H1-histamine receptor expression of veins correlated well with the histamine-induced contractions. Voltage-dependent calcium channels mediated mainly the histamine-induced force generation of saphenous vein, whereas it did not act in the inferior vena cava. In contrast, the receptor-operated channels were not involved in this response in either vein. Tyrosine phosphorylation occurred markedly in response to histamine in the saphenous vein, but not in the inferior vena cava. Histamine induced a prominent ρ kinase activation in both vessels. Protein kinase C and mitogen-activated protein kinase (MAPK) were not implicated in the histamine-induced intracellular calcium sensitization. Importantly, transient clamping of the femoral vein in animals caused a short-term constriction, which was inhibited by H1-histamine receptor antagonist in vivo. Furthermore, a significantly greater histamine immunopositivity was detected in veins after stretching compared to the resting state. We conclude that histamine receptor density adapts to the actual requirements of the circulation, and histamine liberated by the venous wall during increased venous pressure contributes to the contraction of vessels, providing a force for the venous return.
Keywords: venous regulation, histamine, regional differences, H1-histamine receptors, ρ kinase, tyrosine phosphorylation
Introduction
Research of cardiovascular regulation is a well-established area of physiological science. However, the role of veins in this process has never received much emphasis, although about 70% of the circulating blood volume is distributed in the venous system [1–2]. The main driving force of venous return to the heart was based on the venous pump mechanism. This comprises muscle pumps in extremities, thoraco-abdominal pump vis a fronte and vis a tergo mechanisms of the heart and myogenic mechanism of veins [3–7].
It is known that veins are sensitive to a variety of neurotransmitters and autacoids and these can be produced in the wall of the veins [2, 7]. Veins have been described as eliciting contractile responses to norepinephrine [2, 8–12], epinephrine [13–18], angiotensin II [17–24] and endothelin [25–28]. In addition, purinergic mediators such as ATP are also capable of inducing significant venoconstrictions [29, 30]. However, to the best of our knowledge, there are no convincing experimental data for the role of these substances – with the exception of adrenergic neurotransmitters – in the involvement of physiology and pathophysiology of venous return [2, 31]. Vasoactivity of histamine is also well known, and it is described that this autacoid has a prominent action not only in arteries, but in veins as well [32–40].
The aim of our study was to analyse the overall mechanical activity of histamine in the rabbit venous system and to evaluate whether histamine has a potential role in venous contraction during increased venous pressure. We have found that a great variety exists in the histamine sensitivity and H1-histamine receptor expression in various types of rabbit veins, and that histamine receptor density adapts to the actual and local requirements of the circulation. On the basis of our results, it can be supposed that histamine liberated by the venous wall during increased venous pressure can play a significant role in the mechanism of venous return.
Materials and methods
Animals
Male New Zeeland (Lab-Nyul Kft, Godollo, Hungary) white rabbits were used in the study (weight: 3.2–3.8 kg). The experimental protocol has been approved by the Animal Care and Use Committee of University of Debrecen. Animals were kept in metal cages at room temperature and 12 hr light cycle. Animals had access to standard granulated food with the addition of fresh vegetables.
Contractility measurements
Animals were killed by a single bonus injection of sodium pentobarbital (60 mg/kg) into a marginal ear vein. Various veins were dissected by microscopic control and excess connective tissue excised. Vascular specimens were cut into rings approximately 4 mm long. Special care was taken to avoid contact with the luminal surface of the rings in order to preserve the endothelium. The endothelium was removed in some rings, where the luminal surface of vessels was rubbed for 10–20 sec. with a wooden stick to damage the endothelium. Rings were vertically mounted on two stainless steel triangular clips, the lower clip being attached to a moveable support, and the upper clip to a force displacement transducer in a 10-ml organ bath containing Krebs solution (37°C), the composition of which was (mmol/l): NaCl, 118; KCl, 4.7; CaCl2, 2.5; NaH2PO4, 1.0; MgCl2, 1.2; NaHCO3, 24.9 and glucose 11.5 with pH 7.4 when gassed with 95% O2 and 5% CO2 (pH 7.4). Tension of vascular strips was measured isometrically by transducer (SG-01D, Experimetria, Budapest, Hungary), and the output fed to a potentiometric recorder (SP-K2V, Riken Denshi, Tokyo, Japan). Specimens were given an initial tension of 10 mN and allowed to stabilize this tension for at least 2 hrs. Following the equilibration period, preparations were exposed to 10 μM histamine (Sigma, St. Louis, MO, USA) to reach a steady level of tension. Integrity or removal of endothelium was monitored functionally by the quality of responses to acetylcholine (0.1–10 μM, Sigma). Following a 30-min. wash-out period, a cumulative concentration-response (E/[A]) curve was constructed to histamine (1 nM to 100 μM). At the completion of the experiments, tissues were lightly blotted, measured and weighed. The cross-sectional area of each preparation was calculated using the following formula: cross-sectional area (mm2) = weight (mg)/(length (mm) × density (mg/mm3)). The density of the vessels was assumed to be 1.05 mg/mm3. Responses to histamine were then calculated as the increase in tension (mN) in response to each concentration of agonist/cross-sectional area of tissue (mm2).
Western blotting
Tissue samples were collected in lysis buffer [20 mM Tris-HCl, pH 7.4, 5 mM ethylene glycol tetraacetic acid (EGTA), 1 mM 4-(2-aminoethyl) benzensulfonyl fluoride, protease inhibitor cocktail diluted 1:100, all from Sigma] and the protein content of samples was measured by a BCA protein assay (Pierce, Rockford, IL, USA) [41–43]. The samples were subjected to SDS-PAGE (7.5% gels were loaded with 30 μg protein per lane), transferred to BioBond nitrocellulose membranes (Whatman, Maidstone, England). After the membranes were blocked with 5% non-fat milk in phosphate-buffered saline for 1 hr, the blots were probed with anti-Histamine or anti-H1-histamine receptor rabbit primary antibodies (1:200, Santa Cruz). Horseradish peroxidase-polymer-conjugated, goat anti-rabbit antibodies (1:1000, BioRad) were used as the secondary antibody and the immunoreactive bands were visualized by SuperSignal® West Pico Chemiluminescent Substrate-enhanced chemiluminescence (Pierce). Immunoblots were then subjected to densitometric analysis using an Intelligent Dark Box (Fuji, Tokyo, Japan) and the Image Pro Plus 4.5.0 software (Media Cybernetics, Silver Spring, MD, USA). To assess equal loading, membranes were stripped in 200 ml of 50 mM TRIS-HCl buffer (pH 7.5) containing 2% SDS and 0.1%-mercaptoethanol (all from Sigma) at 65°C for 1 hr and were re-probed with a rabbit cytochrome-C (CytC) antibody (Santa Cruz) followed by a similar visualization procedure as described above.
Immunohistochemistry
According to the slight modification of the original method described in Anichtchik et al. [44] and Rinne et al. [45] after excision, the samples of veins were immediately immersed in the fixative, 4% l-ethyl-3.3 (dimethylaminopropyl) carbodiimide (EDAC, Sigma) in 0.1 M phosphate buffer, pH 7.0 for 2 days followed by fixation in 4% paraformaldehyde (Sigma), embedded in paraffin and processed for immunohistochemistry. The expression of histamine was determined by horseradish-peroxidase (HRP) based method using diaminobenzidine (DAB) as a chromogene. In brief, paraffin-embedded sections (5 μm), after antigen retrieval [with 1 mg/ml protease (Sigma Aldrich, St. Louis, MO, USA) diluted in TRIS-HCl], endogenous peroxidase activity was blocked with peroxidase blocking solution (DAKO, Glostrup, Denmark) (10 min., RT). Non-specific binding was prevented by incubating the sections with Protein Block Serum Free Reagent (DAKO, 5 min., RT). The tissue sections were then incubated overnight at 4°C with the primary anti-Histamine H1-receptor antibody (1:50, Santa Cruz, Biotechnology, Santa Cruz, CA, USA). Sections were then incubated with a rabbit anti-goat HRP-polymer-conjugated secondary antibody (1:1000, BioRad). Immunoreactions were finally visualized using DAB-substrate Histamine H1-receptor antibody (1:50, Santa Cruz, Biotechnology, Santa Cruz, CA, USA). Sections were then incubated with a rabbit anti-goat HRP-polymer-conjugated secondary antibody (1:1000, BioRad). Immunoreactions were finally visualized using DAB-substrate μ (EnVision kit, DAKO) and the sections were counterstained by haematoxylin (Sigma-Aldrich) and mounted in aqueous mounting medium (DAKO).
In addition, for negative controls of the labelling procedure, primary antibodies were omitted from the procedure. Immunohistochemical images were captured and digitalized using an RT Spot Colour CCD camera (Diagnostic Instruments, Inc., Sterling Heights, MI, USA) integrated on a Nikon Eclipse 600 fluorescence and light microscope (Nikon, Tokyo, Japan). Analysis of images was carried out using Image Pro Plus 4.5 software. Identification of immunpositive pixels was based on specific colour of immunostaining. A mask was applied for immunpositive pixels and the number of positive pixels was counted in the region of the given endothelium section. Data are presented as percentage of immunpositive pixels in the selected region.
Ultrasonic echo-tracking
The experiments were performed in 12 New Zeeland rabbits weighing 3.2–3.8 kg, anesthetized with sodium pentobarbital 30 mg/kg i.v. into the marginal ear vein. The animals were placed on a heated table to maintain constant body temperature (39.0 ± 0.4° C). A 1 cm segment of right femoral vein was prepared just below the Poupart’s ligament and a tourniquet was placed gently around the vessel. After preparation of the vessel, a non-invasive ultrasound probe was positioned in the distal portion of femoral vein for measuring its internal diameter by an ultrasonic echo-tracking device (ATL HDI 5000) C8-5 transducer with 5–8 MHz probe scan frequency. Using a Doppler mode, the probe was positioned by the characteristic sound of the femoral vein and its focal zone was close to the centre of the vein. Signals of the venous walls were tagged by an electronic tracker, allowing the continuous measurement of the venous diameter. After measuring the resting internal diameter of femoral vein, a 15-sec. occlusion was applied, followed by release of the vessel clamp. One group of animals (n = 6) was treated by i.v. administration of 4 mg/kg pyrilamine (a H1-histamine receptor blocker; Sigma) before clamping. In the other group of animals (controls, n = 5), phosphate-buffered saline (PBS) was administered as a solvent.
Data analysis
Agonist-induced changes in mechanical activity were expressed as the percentage reduction of pre-drug contractile force. Individual E/[A] curve data were fitted by means of a least-square iterative computer program to a logistic function of the following form: E = Emax[A]nH/[A]nH+[EC50]nH, where E denotes the effect, Emax is the asymptote, [A] is the concentration of the agonist, EC50 is the concentration producing a half-maximal response and nH is the midpoint slope parameter.
The data are expressed as means ± S.E.M. The EC50 values were expressed as their negative base 10 logarithms (pD2 values) throughout the text. Multiple comparisons between the experimental groups were performed by one-way ANOVA with a Newman–Keuls post hoc test. Statistical significance was evaluated by Student’s t-test, where appropriate; P < 0.05 was taken as the level of significance.
Results
Regional variability of histamine-induced contractions
Histamine (10 pM to 100 μM) evoked a concentration-dependent increase of contractile force, but Emax of E/[A] was significantly different in various types of veins. Veins have substantial histamine sensitivity in the lower (saphenous vein, femoral vein) and upper extremities (axillary vein), as well as in the abdomen (common iliac vein, inferior vena cava). In contrast, the thoracic veins (thoracic part of inferior vena cava, right and left superior vena cavas, subclavian vein) and one pelvic vein, external iliac vein were virtually insensitive to histamine (Figs 1–3 and Table 1).
Fig 1.

Histamine-induced contraction in isolated rabbit vessels. Saphenous vein (open circle) (n = 12), femoral vein (full circle) (n = 7), external iliac vein (open square) (n = 9). The histamine-induced increases in tension are normalized for vessel cross-sectional area, and absolute values of the increase in tension are shown. Data represent mean ± S.E.M.
Fig 3.
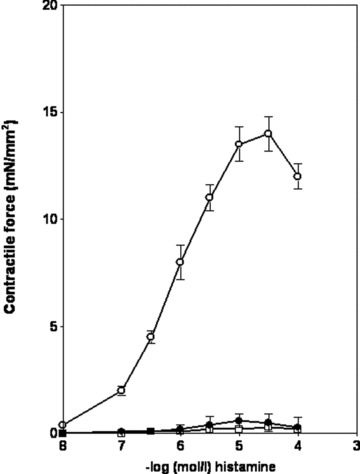
Histamine-induced contraction in isolated rabbit vessels. Axillary vein (open circle) (n = 5), right superior vena cava (full circle) (n = 7), subclavian vein (open square) (n = 5). The histamine-induced increases in tension are normalized for vessel cross-sectional area, and absolute values of the increase in tension are shown. Data represent mean ± S.E.M.
Table 1.
Effect of histamine on contractility parameters in isolated ring preparations of various rabbit veins
| Vein | n | pD2 | Emax | P (Emax) | |
|---|---|---|---|---|---|
| 1 | Inferior vena cava (abdominal part) | 10 | 6.11 ± 0.10 | 17.8 ± 1.1 | |
| 2 | Saphenous vein | 12 | 5.73 ± 0.04 | 16.2 ± 0.4 | >0.05 1 versus 2 |
| 3 | Axillary vein | 5 | 6.07 ± 0.12 | 14.7 ± 0.3 | >0.052 versus 3 |
| 4 | Femoral vein | 7 | 6.03 ± 0.08 | 10.7 ± 0.8 | <0.053 versus 4 |
| 5 | Common iliac vein | 5 | 6.29 ± 0.11 | 9.5 ± 0.6 | >0.054 versus 5 |
| 6 | Inferior vena cava (thoracic part) | 7 | 5.57 ± 0.06 | 1.5 ± 0.1 | <0.015 versus 6 |
| 7 | Right superior vena cava | 7 | 5.77 ± 0.16 | 0.6 ± 0.1 | <0.056 versus 7 |
| 8 | Subclavian vein | 5 | 5.10 ± 2.60 | 0.5 ± 0.7 | >0.057 versus 8 |
| 9 | External iliac vein | 9 | 5.74 ± 0.07 | 0.3 ± 0.01 | >0.058 versus 9 |
Data are mean values ± S.E.M.
Analysis of statistical significance was performed with variance analysis (ANOVA) with the Newman–Keuls post-hoc test.
n: number of experiments; pD2: negative base 10 logarithms of EC50 values; Emax: maximum effect (mN/mm2); P: statistical significance.
Fig 2.
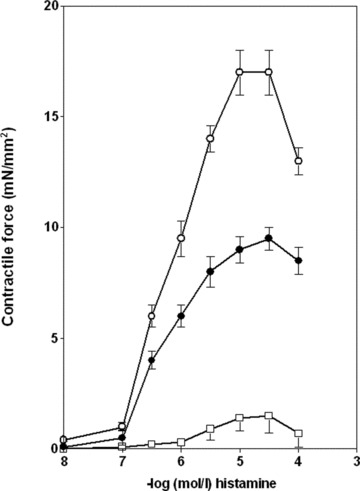
Histamine-induced contraction in isolated rabbit vessels. Abdominal part (open circle) of inferior vena cava (n = 10), common iliac vein (full circle) (n = 5), intrathoracic part (open square) of inferior vena cava (n = 7). The histamine-induced increases in tension are normalized for vessel cross-sectional area, and absolute values of the increase in tension are shown. Data represent mean ± S.E.M.
Role of nitric oxide or CO liberation in the histamine resistance
To analyse the action of a possibly increased nitric oxide or carbon monoxide release in the reduced histamine-induced contraction of special veins, after constructing the first concentration-response curve and a washout period, external iliac veins (n = 5) and right superior vena cavas (n = 6) were exposed to 100 μM NG-nitro-L-arginine (L-NOARG) to block NOS activity. After the blocking of NOS enzymes, no significant differences were observed in histamine-induced responses of both veins. Similar results were detected if the above specimens were incubated by the inhibitor of heme oxygenase-1 Zn-protoporphyrine IX (ZnPP) of 20 μM (data not shown).
Immunoblotting of H1-histamine receptor proteins
H1-histamine receptors were expressed in high density in saphenous vein and inferior vena cava, whereas expression level of these receptors was low in internal iliac vein and right and left superior vena cavas (Fig. 4).
Fig 4.
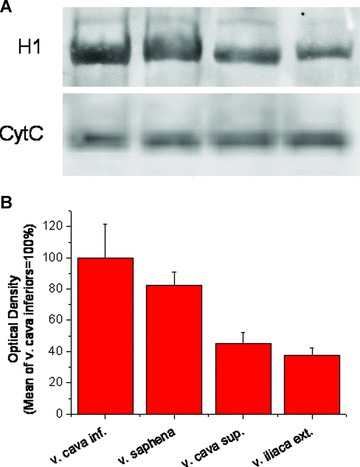
H1 histamine receptor expression in various rabbit veins. (A) Specific H1 immunopositive bands at ∼55 kD of a representative immunoblot and corresponding CytC staining. (B) Optical density of H1 immunopositive bands, normalized to CytC. n ≥ 3 in each group.
Differences in signalling mechanism of histamine-sensitive veins
In these experiments, the signalling of two high histamine-sensitive veins (saphenous vein, inferior vena cava) was compared. The possible role of membrane calcium channels (voltage-dependent calcium channels [VOC] and receptor-operated channels [ROC]), intracellular calcium stores and enzymes involved in calcium sensitization (ρ-kinase, tyrosine kinase, MAPK, protein kinase C) were studied. In saphenous vein, experiments with nifedipine and SKF-96365 demonstrated that histamine activates mainly VOC, whereas the role of ROC is negligible. In the inferior vena cava, the roles of both types of calcium channels are minor in mechanical activity induced by histamine (Fig. 5).
Fig 5.
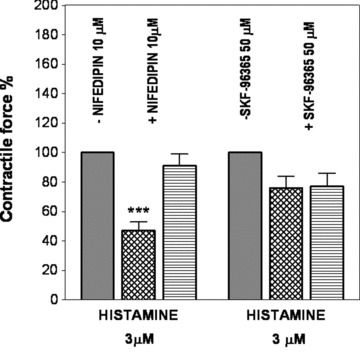
Role of voltage-operated (nifedipine treatment) and receptor operated (SKF-96365 treatment) channels in the histamine-induced contraction of rabbit saphenous vein (grid columns; n = both 6) and abdominal part of inferior vena cava (hatched columns; n = both 5). Contractile force is expressed in % of control. ***P < 0.001.
For investigating the role of the possible implication of Ca2+ sensitization pathways, we studied the action of various protein kinases previously known to be involved in Ca2+ sensitization process. Thus the effects of inhibitors of ρ kinase, protein kinase C, MAPK and tyrosine kinase were investigated. ρ kinase inhibitors (50 μM HA-1077 or Y-27632 10 μM) all virtually completely inhibited the histamine-induced force generation in both saphenous vein and inferior vena cava ring preparations (Figs 6–7). Tyrosine phosphorylation was studied by application of genistein 50 μM. Strong difference was observed between the two types of vessels in the genistein-induced inhibition of histamine contraction. In saphenous veins, genistein induced a 43% inhibition, whereas it was practically ineffective in inferior vena cava indicating the diverse implication of tyrosine phosphorylation in histamine’s actions in the above vessels. The inhibition of protein kinase C by calphostin 0.2 μM or inhibition of MAPK by PD-098059 10 μM did not influence significantly the development of force in both saphenous veins and the inferior vena cava (data not shown).
Fig 6.
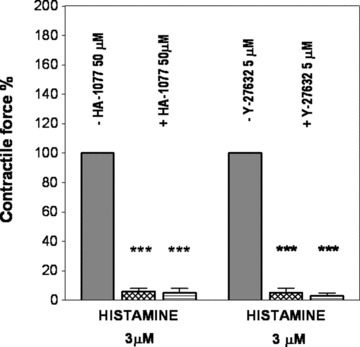
Effects of inhibition of ρ kinase by 50 μM HA-1077 (n = both 5) or 5 μM Y-27632 (n = both 4) on the histamine-induced contraction in rabbit saphenous vein (grid columns) and abdominal part of inferior vena cava (hatched columns). Contractile force is expressed in % of control. ***P < 0.001.
Fig 7.
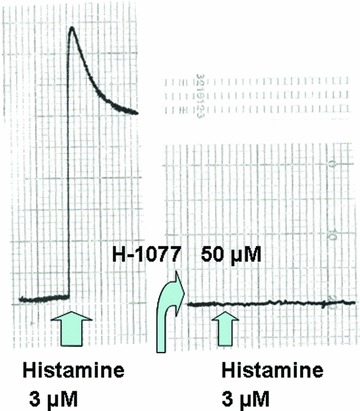
Original record of the action of ρ kinase inhibition (50 μM H-1077) on the histamine-induced force generation in rabbit saphenous vein.
Effects of transient clamping/release on the femoral vein in vivo
In anesthetized rabbits, the femoral vein just below the Poupart’s ligand was clamped for 20 sec., followed by a release. In controls, after clamping, an immediate slight constriction was monitored by an echo-tracking device. This constriction became more prominent after releasing the clamping, then returned to the pre-clamping value. In another group of animals, 4 mg/kg pyrilamine, a specific H1-histamine receptor blocker was injected into the marginal ear vein. This induced an initial dilation of the femoral vein, which then returned to the control value. When clamping, the femoral vein was not constricted, but dilated and this venodilation became pronounced after the release of the clamp (Fig. 8).
Fig 8.
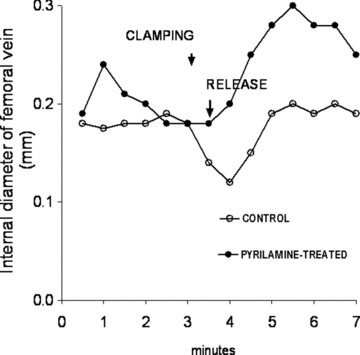
Typical clamping/release studies in anesthetized rabbit under control condition and after administration of 4 mg/kg i.v. pyrilamine.
Effect of stretch of femoral vein on the histamine distribution and content
Rabbits were anesthetized and saphenous vein and inferior vena cava were prepared. One part of the proximal region of these veins was cut and immersed in 4% EDAC, embedded in paraffin and processed for immunohistochemistry (non-stretched veins). A silicon catheter was inserted into the proximal end of the veins and PBS was injected into the vein by a 80–100 mmHg controlled pressure for 20 sec. After this procedure, the intact part of vein was excised and used for immunohistochemistry following the above protocol (stretched veins). Histamine staining was more intensive on stretched saphenous vein and inferior vena cava than on corresponding controls suggesting a causative relationship between stretching and histamine release (Fig. 9).
Fig 9.
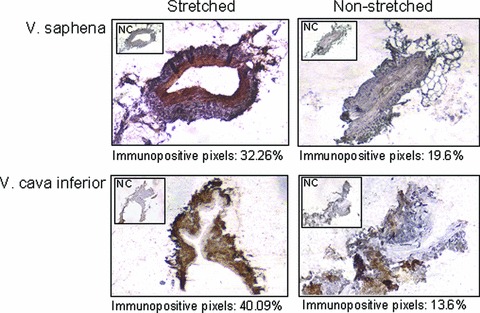
Histamine immunostaining in stretched and non-stretched veins of rabbit. Histamine was visualized using DAB chromogen (brown). Immunopositive pixels were selected and counted on images using Image Pro Plus 4.5 software. NC: negative control.
Study of histamine liberation from mast cells
In isolated saphenous veins, experiments were carried out to study the possible cellular basis of histamine release. The mast cell activator compound, 48/80, at a concentration of 300 μM, was not able to induce contraction both in endothelium intact and denuded saphenous vein rings (data not shown).
Discussion
The major findings of this study are the followings: (i) Histamine sensitivity of rabbit veins shows an extreme variability. Veins of the extremities (saphenous vein, femoral vein, axillary vein) and abdominal veins (common iliac vein, inferior vena cava) respond to histamine by a prominent, concentration-dependent force generation, whereas great thoracic veins (subclavian vein, superior vena cavas, intrathoracic parts of the inferior vena cava) and a pelvic vein (external iliac vein) displayed minimum sensitivity to exogenous histamine. The concentration-response curve for histamine (ii) was not influenced by mechanical deprivation of endothelial layer. The low histamine sensitivity (iii) of selected veins (external iliac vein, superior vena cava) was not changed by the inhibition of NOS or heme oxigenase-1 enzyme by L-NOARG or Zn protoporphyrine-IX, respectively. H1-histamine receptor expression (iv) of the selected high and low histamine-sensitivity veins correlated well with the histamine-induced force generation and (v) there are some differences in excitation-contraction coupling between the histamine-reactive vessels saphenous vein and inferior vena cava. Although VOC play a significant role in the histamine-induced force generation of saphenous vein, they do not in the inferior vena cava. The role of ROC is very slight in both types of veins. Tyrosine phosphorylation is profoundly involved in the saphenous vein, but not in the inferior vena cava. Histamine induces a prominent ρ kinase activation in both vessels. Protein kinase C and MAPK are not implicated in the histamine-induced intracellular calcium sensitization in any of the vessels studied. In vivo experiments (vi) of transient clamping of the femoral vein caused a short-term constriction followed by a moderate dilation. Following the blockade of H1-histamine receptors, no constriction was demonstrated and (vii) in veins with great sensitivity to histamine (saphenous vein, inferior vena cava) a significantly greater histamine immunopositivity was detected after stretching than in resting state. As a pharmacological approach, it seems (viii) that after stretched conditions, histamine release probably is not originated from mast cells, but the origin of its release from endothelial or non-mast cells cannot be excluded.
It is an important question in the physiology and pathophysiology whether active vasoconstriction plays an important role in venous return and raising of end-diastolic volume. It is generally accepted that in orthostasis, the increase of myogenic tone and an increment of sympathetic tone have a great significance in the regulation of venous blood flow [2, 6, 7, 46]. To the best of our knowledge, our present paper is the first to describe the potential role of histamine as an autacoid in the active vasoconstriction of veins, emphasizing the involvement of histamine in the venous return. We have shown that by increasing the venous pressure by clamping of the proximal part of the saphenous vein, a possible histamine-induced transient vasoconstriction develops. Histamine liberation from the vessel wall was convincingly documented by immunohistology, where after stretching of saphenous vein and inferior vena cava a strong increase in histamine immunoreactivity can be detected. The overall results of the present study emphasize that the venous network is extremely plastic at the point of view of histamine-induced regulation. In the proximal parts of the body, in extremities, but also in the abdomen, the histamine receptor expression and the maximum contractile response to histamine are very high, whereas in the thorax (because of the changes of intrathoracic pressure) the veins are virtually insensitive to histamine. Regional variabilities for the sensitivity of rabbit veins were described earlier by Tsuru et al. [39], but these authors did not detect such practically inactive veins, as our present study did. This variability is physiologically quite logical, because the thoraco-abdominal venous pump is effectively operating and no additional venoconstriction is needed for venous return in this region. It is not completely understood that the insensitivity of the external iliac vein operates in the pelvic part. It might ensure a reservoir or buffer function preventing the simultaneous constriction of all veins in the lower extremities and the abdomen, providing as a ‘fountain pump’ and making the venous return continuous. It is mentioned that in this region of the body, the plasticity of the histamine sensitivity is noteworthy. In some experiments, we observed arteriolization of the external iliac vein when forming a side-to-side anastomosis between the femoral vein and artery, the external iliac vein became highly sensitive to histamine. In addition, we have observed that under certain physiological and pathological circumstances (post-partum period, intrapelvic tumours), a clear-cut shift can be observed from the external iliac vein toward common iliac vein, i.e. the external iliac vein responds well to histamine, but the common iliac artery lost its histamine sensitivity (Galajda and Szentmiklósi: unpublished data). This phenomenon can be high significant, mainly in women during pregnancy.
Histamine is an endogenous substance that is widely distributed in various tissues and has been involved in a variety of physiological and pathophysiological processes. This autacoid is implicated in the initial phase of an anaphylactic reaction and acts on various smooth muscles including vascular. The biological action of histamine operates by three different surface receptors, i.e. H1-, H2- and H3-receptors. We have analysed only the role of H1-histamine receptors, as H2 and H3 antagonists have very slight or negligible action on the H1-histamine receptor-induced contractions ([39, 47], Galajda and Szentmiklosi: unpublished data). In our experiments, regional differences in H1-histamine receptor expression and signalling mechanism were also shown. Histamine-induced contractions and H1-histamine receptor expressions are well correlated in sensitive and non-sensitive veins. As far as the regional variability of signalling mechanism is concerned, the role of the activation of voltage-operated calcium channels is significant in histamine-induced saphenous vein contraction, but not in inferior vena cava. A special emphasis was placed on the analysis of histamine-related intracellular calcium sensitizing mechanisms. In vessels, a variety of protein kinases are implicated in Ca2+ sensitization mechanisms: ρ kinase, protein kinase C, mitogen-activated protein kinase and tyrosine phosphorylation [48–51]. In our study, tyrosine phosphorylation had a significant role in the Ca2+ sensitization mechanism underlying the histamine-induced contractions of saphenous vein, but no involvement was detected in inferior vena cava. Activation of ρ kinase was strongly implicated in the histamine-induced Ca2+ sensitization in both veins. In our experiments, endothelium did not play any role in the histamine-induced contraction. In histamine-insensitive veins, the slight reactivity of the specimens can be due mainly to low H1-histamine receptor density, and no role of a possible increased liberation of nitric oxide or CO was supposed. However, we did not investigate the potential role of peroxynitrate as an oxidative and nitrosative stressor, but we cannot rule out its possible contribution to histamine-induced venorelaxation [52].
In histochemical studies, we convincingly demonstrated that stretching of the veins induces a remarkable histamine release in the vascular tissue. The source of histamine is possibly not the vascular mast cells, but in this case liberation of non-mast cell histamine [53] could be suggested. In vivo experiments by inducing venoconstriction during clamping of the femoral vein, and the fact that this process could be reversed by pyrilamine, a selective H1-histamine receptor blocker, confirmed our theory that depending on where the vein wall is stressed, this induces histamine liberation followed by a venoconstriction. This process might be a ‘helper’ in addition to the musculovenous pump and the myogenic tone.
It should be emphasized that these results could also be observed in human veins obtained from multidonors and the variability of the histamine sensitivity, histamine receptor expression and excitation-contraction coupling of various veins are also operating (Galajda and Szentmiklósi: unpublished results). Furthermore, detailed studies are needed to clarify the physiological and pathophysiological significance of these results, but on the basis of our study it can be suggested that histamine release after stretching the vein wall may play a significant role in the regulation of venous circulation and widen the implication of this autacoid in a variety of physiological processes.
Acknowledgments
We thank Ildikó Juhász, Krisztina Dobák and Katalin Nagy for their excellent technical assistance. This work was supported by grants from the Medical Research Council, Hungary (ETT 586/2006), from OTKA (K-72315), Hungary, and TAMOP 4.2.2-08/1-2008-0007, TAMOP 4.2.1/B-09/1/KONV-2010-0007, ETT-147/2009, OTKA-K75883, OTKA-K83478 and MTA-DE-11003 projects. The project is implemented through the New Hungary Development Plan, co-financed by the European Social Fund.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Rothe CF. In: Venous system: physiology of the capacitance vessels. Shepherd JT, Abboud FM, editors. Washington, DC: Am. Physiol. Soc; 1983. Sect. 2, Vol. III, Chap. 13, p. 397, 452. [Google Scholar]
- 2.Pang CCY. Autonomic control of the venous system in health and disease. Effects of drugs. Pharmacol Ther. 2001;90:179–230. doi: 10.1016/s0163-7258(01)00138-3. [DOI] [PubMed] [Google Scholar]
- 3.Donegan JF. The physiology of veins. J Physiol. 1921;55:226–45. doi: 10.1113/jphysiol.1921.sp001964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ludbrook J. The musculovenous pumps of the human lower limb. Am Heart J. 1966;71:635–41. doi: 10.1016/0002-8703(66)90313-9. [DOI] [PubMed] [Google Scholar]
- 5.Noordergraaf A. Circulatory system dynamics. New York: Academic Press; 1978. pp. 157–81. [Google Scholar]
- 6.Rothe CF. Physiology of venous return. An unappreciated boost to the heart. Arch Intern Med. 1986;146:977–82. [PubMed] [Google Scholar]
- 7.Monos E, Berczi V, Nadasy G. Local control of veins: biomechanical, metabolic, and humoral aspects. Physiol Rev. 1995;75:611–66. doi: 10.1152/physrev.1995.75.3.611. [DOI] [PubMed] [Google Scholar]
- 8.Burch GE, DePasquale NP. The effect of norepinephrine on the digital veins. Am Heart J. 1960;60:915–23. doi: 10.1016/0002-8703(60)90123-x. [DOI] [PubMed] [Google Scholar]
- 9.Cohen ML, Wiley KS. Comparison of arteries with longitudinal and circular venous muscle from the rat. Am J Physiol. 1977;232:H131–9. doi: 10.1152/ajpheart.1977.232.2.H131. [DOI] [PubMed] [Google Scholar]
- 10.Sticht FD, Wood WB, Cardoso SS, et al. Effect of prazosin on veins: inhibition of norepinephrine venoconstriction in dog forelimb. Drug Dev Res. 1983;3:393–488. [Google Scholar]
- 11.Hohmann M, Keve TM, Osol G, et al. Norepinephrine sensitivity of mesenteric veins in pregnant rats. Am J Physiol. 1990;259:R753–9. doi: 10.1152/ajpregu.1990.259.4.R753. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Dyer DC. Characterization of alpha-adrenoceptors mediating contraction in isolated ovine umbilical vein. Eur J Pharmacol. 1991;197:63–7. doi: 10.1016/0014-2999(91)90365-w. [DOI] [PubMed] [Google Scholar]
- 13.Errasti AE, Werneck de Avellar MC, Daray FM, et al. Human umbilical vein vasoconstriction induced by epinephrine acting on alpha1B-adrenoceptor subtype. Am J Obstet Gynecol. 2003;189:1472–80. doi: 10.1067/s0002-9378(03)00646-x. [DOI] [PubMed] [Google Scholar]
- 14.Miller JW, Streeten DH. Vascular responsiveness to norepinephrine in sympathicotonic orthostatic intolerance. J Lab Clin Med. 1990;115:549–58. [PubMed] [Google Scholar]
- 15.Nilsson H. Adrenergic nervous control of resistance and capacitance vessels. Studies on isolated blood vessels from the rat. Acta Physiol Scand. Suppl. 1985;541:1–34. [PubMed] [Google Scholar]
- 16.Arneklo-Nobin B, Owman C. Adrenergic and serotoninergic mechanisms in human hand arteries and veins studied by fluorescence histochemistry and in vitro pharmacology. Blood Vessels. 1985;22:1–12. [PubMed] [Google Scholar]
- 17.Savino EA, Taquini AC., Jr Effects of adrenaline, angiotensin, potassium and calcium on lanthanum treated portal veins. Arch Int Pharmacodyn Ther. 1977;226:100–8. [PubMed] [Google Scholar]
- 18.Mantel R, Taquini AC, Jr, Savino EA. Effects of adrenaline, angiotensin and calcium on spontaneously active and potassium-depolarized rat portal veins. Arch Int Physiol Biochim. 1975;83:211–9. doi: 10.3109/13813457509081865. [DOI] [PubMed] [Google Scholar]
- 19.Gurzu B, Costuleanu M, Slatineanu SM, et al. Are multiple angiotensin receptor types involved in angiotensin (1–7) actions on isolated rat portal vein. J Renin Angiotensin Aldosterone Syst. 2005;6:90–5. doi: 10.3317/jraas.2005.015. [DOI] [PubMed] [Google Scholar]
- 20.Fernandes L, Loiola RA, Tostes RC, et al. Angiotensin II-induced venoconstriction involves both AT1 and AT2 receptors and is counterbalanced by nitric oxide. Peptides. 2005;26:2458–63. doi: 10.1016/j.peptides.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 21.Li Q, Feenstra M, Pfaffendorf M, et al. Comparative vasoconstrictor effects of angiotensin II, III, and IV in human isolated saphenous vein. J Cardiovasc Pharmacol. 1997;29:451–6. doi: 10.1097/00005344-199704000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Pelet C, Mironneau C, Rakotoarisoa L, et al. Angiotensin II receptor subtypes and contractile responses in portal vein smooth muscle. Eur J Pharmacol. 1995;279:15–24. doi: 10.1016/0014-2999(95)00125-5. [DOI] [PubMed] [Google Scholar]
- 23.Zhang JS, Van Meel JC, Pfaffendorf M, et al. Characterization of the angiotensin II-receptor subtype in the longitudinal smooth muscle of the rat portal vein. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:220–4. doi: 10.1007/BF00169271. [DOI] [PubMed] [Google Scholar]
- 24.Blair-West JR, McKenzie JS, McKinley MJ. The actions of angiotensin II on the isolated portal vein of the rat. Eur J Pharmacol. 1971;15:221–30. doi: 10.1016/0014-2999(71)90177-4. [DOI] [PubMed] [Google Scholar]
- 25.Bogoni G, Rizzi A, Calo G, et al. Characterization of endothelin receptors in the human umbilical artery and vein. Br J Pharmacol. 1996;119:1600–4. doi: 10.1111/j.1476-5381.1996.tb16078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mawatari K, Komori K, Kuma S, et al. Effects of serotonin and endothelin on the smooth muscle cells of autogenous vein grafts. Br J Surg. 1997;84:1419–24. [PubMed] [Google Scholar]
- 27.Wang HG, Shibamoto T, Miyahara T. Endothelin-1 selectively contracts portal vein through both ETA and ETB receptors in isolated rabbit liver. Am J Physiol. 1997;273:G1036–43. doi: 10.1152/ajpgi.1997.273.5.G1036. [DOI] [PubMed] [Google Scholar]
- 28.Rizzi A, Calo G, Battistini B, et al. Contractile activity of endothelins and their precursors in human umbilical artery and vein: identification of distinct endothelin-converting enzyme activities. J Cardiovasc Pharmacol. 1998;31:S58–61. doi: 10.1097/00005344-199800001-00019. [DOI] [PubMed] [Google Scholar]
- 29.Ziganshin AU, Khaziakhmetov DF, Ziganshina LE, et al. Contractile activity of human greater saphenous vein mediated by P2-receptors. Bull Exp Biol Med. 2003;135:23–5. doi: 10.1023/a:1023477324977. [DOI] [PubMed] [Google Scholar]
- 30.Ralevic V, Burnstock G. Roles of P2-purinoceptors in the cardiovascular system. Circulation. 1991;84:1–14. doi: 10.1161/01.cir.84.1.1. [DOI] [PubMed] [Google Scholar]
- 31.Monos E, Lorant M, Dornyei G, et al. Long-term adaptation mechanisms in extremity veins supporting orthostatic tolerance. News Physiol Sci. 2003;18:210–4. doi: 10.1152/nips.01447.2003. [DOI] [PubMed] [Google Scholar]
- 32.Okamura T, Yamazaki M, Toda N. Responses to histamine and acetylcholine in isolated monkey mesenteric veins versus arteries. Cardiovasc Res. 1994;28:667–72. doi: 10.1093/cvr/28.5.667. [DOI] [PubMed] [Google Scholar]
- 33.Mikkelsen E, Sakr AM, Jespersen LT. Studies on the effect of histamine in isolated human pulmonary arteries and veins. Acta Pharmacol Toxicol. 1984;54:86–93. doi: 10.1111/j.1600-0773.1984.tb01900.x. [DOI] [PubMed] [Google Scholar]
- 34.Schoeffter P, Godfraind T. Histamine receptors in the smooth muscle of human internal mammary artery and saphenous vein. Pharmacol Toxicol. 1989;64:64–71. doi: 10.1111/j.1600-0773.1989.tb00603.x. [DOI] [PubMed] [Google Scholar]
- 35.Diana JN, Schwinghamer J, Young S. Direct effect of histamine on arterial and venous resistance in isolated dog hindlimb. Am J Physiol. 1968;214:494–505. doi: 10.1152/ajplegacy.1968.214.3.494. [DOI] [PubMed] [Google Scholar]
- 36.Cook DA, Macleod KM. Responses of rabbit portal vein to histamine. Br J Pharmacol. 1978;62:165–70. doi: 10.1111/j.1476-5381.1978.tb08441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toshimitsu Y, Uchida K, Kojima S, et al. Histamine responses mediated via H1- and H2-receptors in the isolated portal vein of the dog. J Pharm Pharmacol. 1984;36:404–5. doi: 10.1111/j.2042-7158.1984.tb04409.x. [DOI] [PubMed] [Google Scholar]
- 38.Oriowo MA, Bevan JA. Characterization of histamine H1 and H2 receptors in the rabbit isolated ovarian artery and vein. J Cardiovasc Pharmacol. 1987;10:76–81. doi: 10.1097/00005344-198707000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Tsuru H, Kohno S, Iwata M, et al. Characterization of histamine receptors in isolated rabbit veins. J Pharmacol Exp Ther. 1987;243:696–702. [PubMed] [Google Scholar]
- 40.Bergner M, Graser T, Handschuk L, et al. Vasomotor tone of isolated porcine coronary veins in response to acetylcholine, noradrenaline, and histamine. Biomed Biochim Acta. 1988;47:775–9. [PubMed] [Google Scholar]
- 41.Bodo E, Kovacs I, Telek A, et al. Vanilloid receptor-1 (VR1) is widely expressed on various epithelial and mesenchymal cell types of human skin. J Invest Dermatol. 2004;123:410–3. doi: 10.1111/j.0022-202X.2004.23209.x. [DOI] [PubMed] [Google Scholar]
- 42.Bodo E, Biro T, Telek A, et al. A hot new twist to hair biology: involvement of vanilloid receptor-1 (VR1/TRPV1) signaling in human hair growth control. Am J Pathol. 2005;166:985–98. doi: 10.1016/S0002-9440(10)62320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Biro T, Maurer M, Modarres S, et al. Characterization of functional vanilloid receptors expressed by mast cells. Blood. 1998;91:1332–40. [PubMed] [Google Scholar]
- 44.Anichtchik OV, Rinne JO, Kalimo H, et al. An altered histaminergic innervation of the substantia nigra in Parkinson’s disease. Exp Neurol. 2000;163:20–30. doi: 10.1006/exnr.2000.7362. [DOI] [PubMed] [Google Scholar]
- 45.Rinne JO, Anichtchik OV, Eriksson KS, et al. Increased brain histamine levels in Parkinson’s disease but not in multiple system atrophy. J Neurochem. 2002;81:954–60. doi: 10.1046/j.1471-4159.2002.00871.x. [DOI] [PubMed] [Google Scholar]
- 46.Berczi V, Greene AS, Dornyei G, et al. Venous myogenic tone: studies in human and canine vessels. Am J Physiol Heart Circ Physiol. 1992;263:H315–20. doi: 10.1152/ajpheart.1992.263.2.H315. [DOI] [PubMed] [Google Scholar]
- 47.Champion HC, Bivalacqua TJ, Lambert DG, et al. Analysis of vasoconstrictor responses to histamine in the hindlimb vascular bed of the rabbit. Am J Physiol. 1999;277:R1179–87. doi: 10.1152/ajpregu.1999.277.4.R1179. [DOI] [PubMed] [Google Scholar]
- 48.Fukata Y, Amano M, Kaibuchi K. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol Sci. 2001;22:32–9. doi: 10.1016/s0165-6147(00)01596-0. [DOI] [PubMed] [Google Scholar]
- 49.Walsh MP. Calmodulin and the regulation of smooth muscle contraction. Mol Cell Biochem. 1994;135:21–41. doi: 10.1007/BF00925958. [DOI] [PubMed] [Google Scholar]
- 50.Somlyo AP, Wu X, Walker LA, et al. Pharmacomechanical coupling: the role of calcium, G-proteins, kinases and phosphatases. Rev Physiol Biochem Pharmacol. 1999;134:201–34. doi: 10.1007/3-540-64753-8_5. [DOI] [PubMed] [Google Scholar]
- 51.Hughes AD, Wijetunge S. Role of tyrosine phosphorylation in excitation-contraction coupling in vascular smooth muscle. Acta Physiol Scand. 1998;164:457–69. doi: 10.1046/j.1365-201X.1998.00446.x. [DOI] [PubMed] [Google Scholar]
- 52.Ferdinandy P. Peroxynitrite: just an oxidative/nitrosative stressor or a physiological regulator as well. Br J Pharmacol. 2006;148:1–3. doi: 10.1038/sj.bjp.0706693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gruetter CA, Bailey B, Easterling L, et al. Release of non-mast cell histamine from rat aorta. Life Sci. 2002;70:1709–17. doi: 10.1016/s0024-3205(02)01488-1. [DOI] [PubMed] [Google Scholar]


