Abstract
Intracerebral haemorrhage (ICH) can lead to secondary insults and severe neurological deficits. Transplantation of neural stem cells (NSCs) was suggested as an alternative to improve ICH-induced neurological dysfunction. The present study aimed at investigating the therapeutic role and long-term survival of foetal NSCs and potential role of foetal NSCs-produced factors in ICH. Our results demonstrated that foetal NSCs could differentiate into neural axons and dendrites and astrocytes in both in vitro and in vivo conditions, demonstrated by positive double or triple staining with Hoechst, neuronal specific nuclear protein, neurofilaments and glial fibrillary acidic protein. Intracerebral transplantation of foetal NSCs 3 days after ICH induction by intrastriatal administration of bacterial collagenase could improve the functional performance in the limb-placing test and shorten the duration of the recovery from ICH-induced neural disorders. The foetal NSCs may also produce neurotrophic and/or neuroprotective factors during culture, because the culture medium alone could partially improve functional performance. Thus, our data suggest that the foetal NSCs may be one of the therapeutic candidates for ICH.
Keywords: foetal neural stem cells, differentiation, transplantation, neuron, astrocytes, intracerebral haemorrhage
Introduction
The intracerebral haemorrhage (ICH) is responsible for about 15% of acute stroke in hospital [1], with 45% of mortality rate in 30 days, of which most survivors have neurological disability [2]. The ICH-induced haematoma causes a direct mechanical destruction of brain tissue, followed by inflammation. There is no efficient and specific therapy for such neurological damage. Neural stem cells (NSCs) with the ability of proliferation and differentiation have been considered as a potential therapy for neurological diseases such as stroke, Parkinson disease, Huntington disease, amyotrophic lateral sclerosis and spinal cord injury [3–5].
NSCs has been identified in the embryonic and adult human brain [6, 7] and also in neurogenic sites, e.g. the subventricular zone in the wall of the lateral ventricle and the subgranular zone of the hippocampal dendate gyrus of adult mammalian brains [4, 8, 9]. However, the central nervous system can hardly regenerate after injury, suggesting the lack of endogenous NSCs and favourable microenvironment for the survival and differentiation of NSCs. Transplantation of exogenous NSCs was suggested as an alternative to treat brain damage. Experimental evidence showed that transplantation of NSCs or neural progenitor cells could improve survival rate of animal with stroke and ameliorate neurological deficits [10–12], although the exact mechanism remains unclear. In the present study, we isolated, cultured and differentiated foetal NSCs of the mouse into neurons and glial cells to show that NSCs can act as a potential source of functional cells. Furthermore, foetal NSCs of the mouse were transplanted to animals with collagenase-induced ICH to investigate differentiation and function. We found that neurons and astrocytes appeared around the injured brain after the transplantation of foetal NSCs into the intrastriatal, accompanied with an improvement of functional deficits. The neurons derived from transplanted NSCs formed axons and dendrites, suggesting that NSCs transplantation can be used as an alternative of therapies for ICH.
Materials and methods
NSCs isolation and differentiation
The cortex of 14-day-old Sprague-Dawley foetal rat was dissected and mechanically dissociated into single cells. Isolated cells were seeded and cultured in DMEM/F12 supplemented with 20 ng/ml epidermal growth factor, 20 ng/ml basic fibroblast growth factor, 1X N2,1X L-glutamine, 1X B27, 100 U/ml penicillin and 100 U/ml streptomycin at 37°C, 5% CO2, for 6 days. After that, the cells were transferred to polylysine coated 6-well plates and seeded in DMEM/F12 supplemented with 10% foetal bovine serum for additional 7 days. The cells were then harvested and fixed with 4% paraformaldehyde for 20 min. and rinsed with phosphate-buffered saline (PBS) for morphological examination. On the other hand, cells were incubated for 30 min. in the Tris-buffered solution with 1% goat serum, 1% bovine serum albumin and 0.5% Triton X-100. After the blockage of nonspecific binding, the cells were permeabilized and followed by a 60 min. incubation with anti-microtubule-associated protein 2 (MAP-2) antibody as the neuronal marker of brain tissue (1:1000 rabbit, Chemicon, Temecula, CA, USA) and anti-glial fibrillary acidic protein (GFAP) antibody as the specific marker for astrocytes in central nervous system, (1:400 mouse, Chemicon), respectively, at room temperature. Cells were rinsed with PBS thrice and incubated with the goat anti-rabbit IgG1-Cy3 (1:200, Chemicon) and goat antimouse IgG1-FITC (1:100, Chemicon). The cells were stained with Hoechst33342 (10 μg/ml, Sigma), rinsed with PBS thrice, mounted in Vectashield and examined under a fluorescence microscope (Olympus IX51–12PH) and a laser confocal fluorescence microscope (Olympus FV1000).
Induction of ICH
Forty male Sprague Dawley rats, weighing 280 to 300 g, were used in the present study. All experimental procedures were approved by the Animal Care Committee of the University School of Medicine. ICH was induced by stereotaxic, intrastriatal administration of bacterial collagenase as described previously [13]. In brief, rats were intraperitoneally injected with 10% chloral hydrate (0.4 ml/100 g) and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA). A burr hole was made through which a 30-gauge needle was inserted into the striatum (0.1 mm posterior, 5.0 mm ventral and 2.0 mm lateral to the bregma). The solution of collagenase VII (0.075 U in 0.5 μl volume, Sigma) was injected into the striatum with a micro infusion syringe with a fixed needle (Hamilton 87942, Hamilton, Allston, MA, USA) at a rate of 0.25 μl/min. for 2 min. through an infusion pump (Bioanalytical Systems, West Lafayette, IN, USA). The needle was left in place for an additional 5 min. after injection to avoid reflux. Animals were maintained in a separate cage with free access to food and water under a 12 hr light–dark cycle. The intracerebral haematoma was examined after collagenase infusion.
NSCs labelling and transplantation
Animals were randomly divided into three groups 3 days after the induction of ICH: intracerebral transplantation of foetal NSCs labelled with Hoechst (n = 13) as therapeutic group, culture medium alone (n = 13) as control group of potential mediators and proteins or PBS (n = 13) as control group of manipulation. Foetal NSCs were cultured with Hoechst 33342 at a final concentration of 10 μg/ml for 30 min., rinsed twice with PBS and mechanically dissociated into the cell suspension. The needle was inserted into the bone hole at 5 mm below the surface of the skull and then 5 μl cell suspension was injected at the final cell concentration of 2 × 107 cells/100 μl, 3 days after introduction ICH. The needle was drawn out slowly 10 min. after cell transplantation. The control animals received the same process of injection with the same volume of culture medium or and PBS, respectively.
Behavioural testing
Behavioural testing was performed by two researchers without the knowledge of treatments 3 days after the introduction of ICH and every 7 days after cell transplantation for 31 days. The modified limb placing test consisted of two limb placing tasks to assess the sensorimotor integration of the forelimb and the hindlimb by checking responses to tactile and proprioceptive stimulation as described previously [14]. The mouse was suspended 10 cm over a table and the stretch of the forelimbs towards the table was observed and evaluated: normal stretch as 0, flexion with a delay (2 sec.) and/or incomplete as 1 score, abnormal flexion as 2 score. The mouse was positioned along the edge of the table and the forelimbs were suspended over the edge to allow free movement. Each forelimb (forelimb, second task; hindlimb, third task) was gently pulled down to check the retrieval and placement. The mouse was then placed towards the table edge to check the lateral placement of the forelimb. The score of 9 meant maximal neurological deficit, whereas 0 points normal.
NSCs differentiation in the brain
Animals were anesthetized and perfused with 100 ml cold PBS and 100 ml of 4% paraformaldehyde in 0.1 M phosphate buffer through the heart 28 days after cell transplantation. The brains were fixed for 24 hrs, cryoprotected in 30% sucrose for 24 hrs and then sectioned at 10 μm thickness using cryostat (Leica CM1900, Leica Microsystems, Nussloch, Germany) at –18°C. Five sections were selected from the needle entry site identifiable on the brain surface. On basis of pre-labelling of NSCs with Hoechst 33342, brain sections were stained with specific marker antibodies to identify transplanted NSCs and other cell types, including anti-NeuN, a neuronal specific nuclear protein, (1:200 mouse, Chemicon) and anti-neurofilament (NF), a neuron important structural protein, antibodies (1:200 mouse, Chemicon) for the neuron and anti-GFAP antibodies (1:400 rabbit, Chemicon) for astrocytes, overnight at 4°C. Sections were rinsed with PBS and then incubated with the goat anti-rabbit IgG1-Cy3 (1:200, Chemicon) and goat antimouse IgG1-FITC (1:100, Chemicon) for 60 min. at room temperature. Sections were rinsed with PBS, covered with cover slips with Vectashield for immunofluorescence, and detected under a fluorescence microscope (Olympus IX51-12PH, Center Valley, PA, USA).
Statistical analysis
Experimental design and workflow was summarized in Figure 1. Data are presented as means ± S.E.M. The statistical significance between group comparisons for behavioural data was determined by one-way ANOVA and P-values less than 0.05 were considered as significant.
Fig 1.
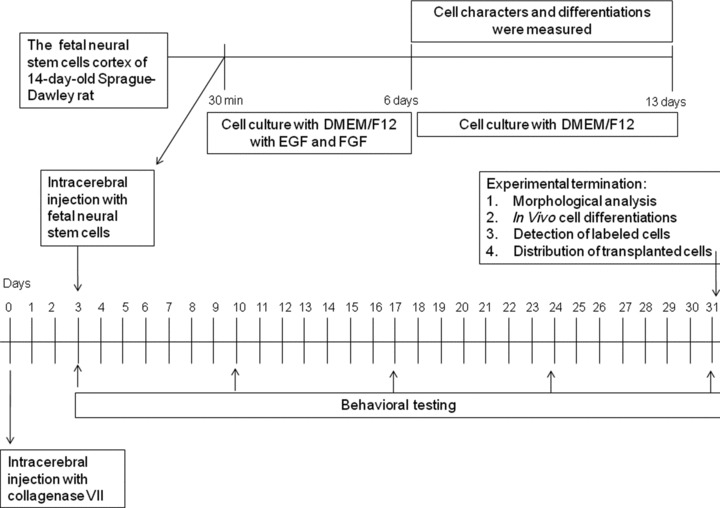
Experimental design and workflow.
Results
Mouse foetal NSCs were seeded as the separate cell on day 0 (Fig. 2A). A few cells then started to adhere on the plate and showed neurite, while some cells were still floated as single or a small mass of cells on day 3 (Fig. 2B). Most of cells grew to about 10–100 neurospheres floating as the round mass (Fig. 2C), of which the shapes were regulatory and oval with condensed cell contacts and smooth surface and became the matured neurosphere as seen at high magnification (Fig. 2D and E). The immunostaining of nestins as the marker of NSCs was performed 2 hrs after the neurosphere adhesion on the plate. Most of the neurospheres showed nestin-staining to be positive (Fig. 2F). Morphologies of NSCs changed and NSCs differentiated into neurons and astrocytes 7 days after the induction with DMEM/F12/10% foetal bovine serum.
Fig 2.
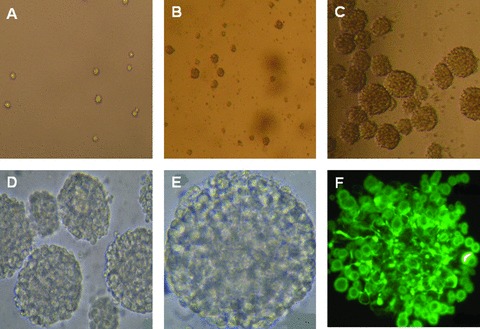
Mouse foetal NSCs were seeded and cultured on day 0 ( × 200; A), forming a few neurospheres on day 3 ( × 100; B) and a large amount of the neurospheres on days ( × 100; C). Some became matured neurosphere as seen at high magnification ( × 200 and 400; D and E, respectively). Most of the neurospheres showed nestin-staining to be positive ( × 400; F).
In order to avoid the potential influence of cellular contacts and space connections in cell differentiation, the single cell was isolated, seeded and cultured with the differentiated induction for 7 days. The cell adhered on the plate, formed neurites and differentiated into neurons and astrocytes, as shown in Figure 3. Cells with Hoechst 33342-positve nuclei showed positive to the staining of neuron-specific marker MAP-2 (Fig. 3A). The neurons formed peripheral process like extensions which became more obvious with the mono-staining of MAP-2 (Fig. 3B). The cell with clear neural axons and dendrites in the intermediate phase of differentiation from NSCs to neurons was also detected (Fig. 3C). NSCs-differentiated astrocytes were detected in microscopy with (Fig. 3D) and without GFAP staining (Fig. 3E). Some showed the matured shape of astrocytes with rich and strong GFAP staining (Fig. 3F). The cytoplasm of the astrocytes retracted towards the nucleus, forming a contracted cell body.
Fig 3.
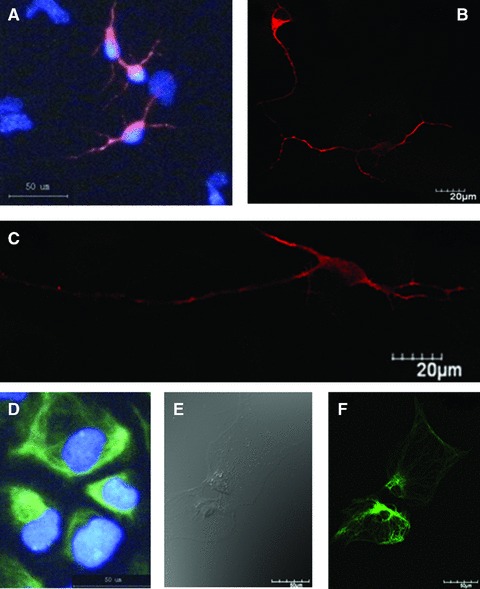
Morphologies and biomarker-specific staining of differentiated neurons and astrocytes. The cell showed positive to the staining of MAP-2 and the nuclear straining with Hoechst 33342 (A), and formed clear neurites (B) and clear neural axons and dendrites in the intermediate phase of differentiation from NSCs to neurons (C). NSCs-differentiated astrocytes were detected in the microscopy with (D) and without GFAP staining (E). Some showed the matured shape of astrocytes with rich and strong GFAP staining (F).
To evaluate and confirm the reproducibility and stability of ICH model, two animals were killed and the brains were harvested to check the formation and size of intracerebral haematoma daily for 5 days after the induction of ICH. The results showed consistent finding that the formation and size of intracerebral haematoma were clearly detectable at 6 hrs (Fig. 4A), became a clear mass on day 2 and reached the maximal 3 days after the injection of collagenase (Fig 4B). The haematoma was shaped with the clear edge, and became oval and regular with the mass effect. Behavioural test demonstrated that the dysfunction of neural system was detectable from 6 hrs and on after the introduction of ICH and became more progressive by the time. A number of limb placing tests became more obviously abnormal 2–3 days after the introduction of ICH on the right site, including the paralysis of the left (contralateral) forelimb, ipsilateral ‘circle-like’ walking, delayed or little contraction of the left hindlimb, and difficulty of passing through the narrow wooden barriers and muscular inability of the left hindlimb. Furthermore, the brain was examined using nuclear magnetic resonance imaging 3.0T (Signa HDx, GE Healthcare, Pittsburgh, PA, USA) at scanning of different sequences (AX FSE T2WI, AX FSE T1WI and AX GRE T2*WI), with the thickness layer of 1.6 mm. The normal brain showed the uniform of the density at AX FSE T2W scan (Fig. 4C), whereas ICH brain had the round lesion with low density on the right side (Fig. 4D) 3 days after the induction of ICH. At high resolution of GRE T2WI, the lesion became clearer (Fig. 4E), as compared with the FSE T1WI resolution (Fig. 4F).
Fig 4.
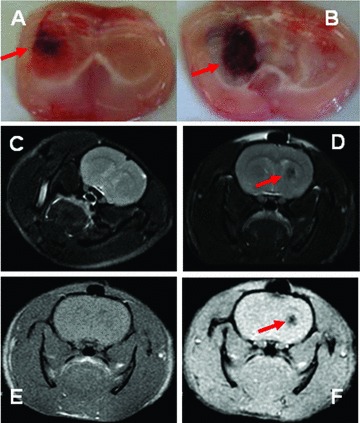
The formation of intracerebral haematoma was detectable at 6 hrs (A) and reached the maximal 3 days after the injection of collagenase (B). The examination of the nuclear magnetic resonance imaging showed the uniform of the density in normal brain at AX FSE T2W scan (C), the round lesion with low density in ICH brain (D) and the clearer lesion at high resolution of GRE T2WI (E), as compared with FSE T1WI resolution (F), 3 days after the induction of ICH. The lesions or abnormalities were pointed with red arrow.
No animals died of the operation and anaesthesia during the experiment. The limb-placing test was performed in animals receiving the intracerebral injection of foetal NSCs, culture medium alone or PBS 3, 10, 17, 24 and 31 days after the induction of ICH, as shown in Figure 5. The score of limb-placing test gradually decreased in ICH animals receiving intracerebral injection of PBS or culture medium alone by time. There was a significant difference in animals with the culture medium alone between scores on days 3 and 31 (P < 0.05). The animals transplanted with foetal NSCs showed significantly better performance on limb placement test from 3 weeks and on after the induction of ICH, as compared with those with culture medium alone or PBS (Fig. 5, P < 0.05 or 0.001, respectively). The scores of limb-placing test in animals with NSCs transplantation on both post-ICH 24 and 31 days were significantly lower than those on 3 days (P < 0.01). There was also a significance difference of scores in animals transplanted with NSCs between days 24 and 31 after ICH.
Fig 5.
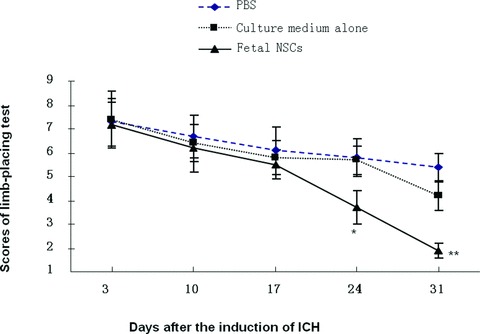
The scores of limb-placing test measured in animals with intracerebral injection of foetal NSCs, culture medium alone or PBS 3, 10, 17, 24 and 31 days after the induction of ICH. * and ** stand for the P-values less than 0.05 and 0.01, as compared with animals receiving the injection of culture medium alone or PBS at the correspondent time-points.
Hoechst-positive NSCs transplanted into ICH haemorrhage lesion were found to appear in the haemorrhage core (Fig. 6A), the border of the lesion (Fig. 6B and C) and the injection sites (Fig. 6D and E), 31 days after induction of ICH. Of those cells, transplanted foetal NSCs has positive staining with Hoechst alone (Fig. 7A and B), NeuN alone (Fig. 7C and D), GFAP alone (Fig. 7E and F) and both of Hoechst and NeuN or Hoechst and GFAP (Fig. 7G and H). The differentiated neurons had double staining of Hoechst and NeuN, whereas the astrocytes had Hoechst and GFAP. Some of differentiated neurons had neuron axon and dendrites with positive staining of Hoechst, NeurN and NF (Fig. 8). Those could be compared with eurons and nerve fibres of normal SD mouse brain (Fig. 9). The transplanted cells with NF, NeuN or both positive staining were mostly allocated in the peri-hematomal sites, where only some with GFAP positive staining appeared. Most of the Hoechst/GFAP double-positive cells were found along the border of haemorrhagic core.
Fig 6.
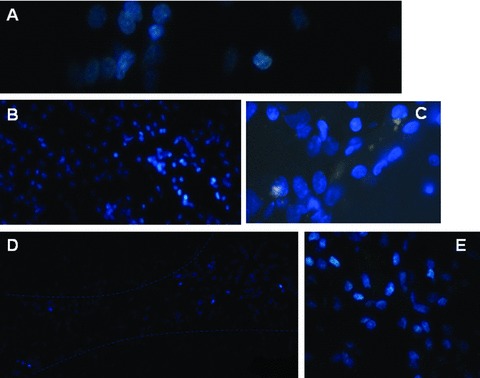
Hoechst-positive NSCs transplanted into ICH haemorrhage lesion were found to appear in the haemorrhage core ( × 400; A), the border of the lesion ( × 200 and × 400; B and C) and the injection sites ( × 100 and × 200; D and E).
Fig 7.
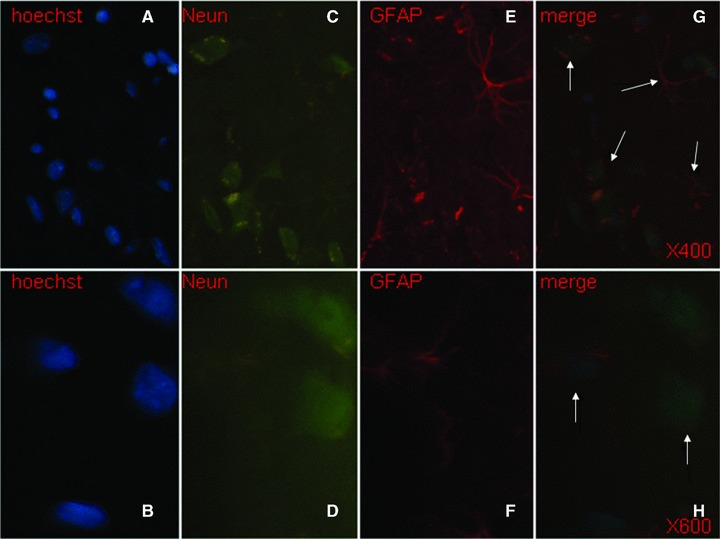
Transplanted foetal NSCs in the brain showed positive to the single staining with Hoechst (blue, × 400 and × 600, A and B), NeuN (green, × 400 and × 600, C and D) and GFAP (red, × 400 and × 600, E and F), and to triple staining of Hoechst, NeuN and GFAP ( × 400 and × 600, G and H). The differentiated neurons had double staining of Hoechst and NeuN, whereas the astrocytes had Hoechst and GFAP, as pointed with arrows.
Fig 8.
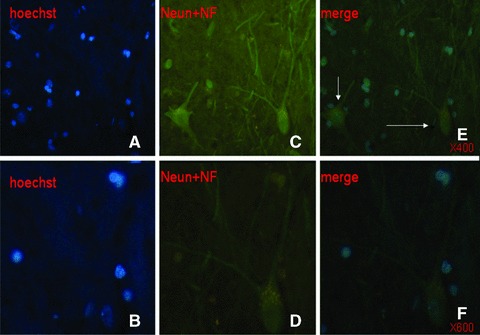
Transplanted foetal NSCs in the brain showed positive to the single staining with Hoechst (blue, × 400 and × 600, A and B), NeuN plus NF (green, × 400 and × 600, C and D) and to triple staining of Hoechst, NeuN and NF ( × 400 and × 600, E and F). The differentiated neurons with the axons and dendrites had triple positive staining of Hoechst, NeuN and NF, as pointed with arrows.
Fig 9.
Neurons and nerve fibres of normal SD mouse brain.
Discussion
The present study provided solid evidence to show that intracerebral transplantation of foetal NSCs could have therapeutic effects on the functional recovery in ICH and NSCs could differentiate into neurons and astrocytes. We propose that foetal NSCs can be one of optimal candidates for the transplantation of stem cells. Our results may contribute to the strategic consideration and plan of stem cell therapy for ICH, be helpful for clinicians to design the clinical performance and be used as reference for the practical application of foetal NSCs. Intracerebral transplantation of stem cells to treat the stroke and ICH has been explored for a decade, e.g. using bone marrow stem cells [15], NSCs [16], adipose tissue-derived stromal cells [17], umbilical cord multipotent mesenchymal stromal cells [18] and peripheral blood stem cells [19].
The efficiency of stem cell transplantation as potential therapies has been suggested to be associated with a number of influencing factors, including cell sources, ability of differentiation and proliferation, transplanting time and delivery ways, disease severity and duration, similar to other cells [17]. For example, the intravenous injection of NSCs 1 day after ICH induction could improve the functional performance for 2 weeks and those transplanted NSCs migrated selectively to the perihematomal areas and differentiated into a few neurons and most of the astrocytes [16]. It was questioned whether the effects might result from such early intervention, how many of injected NSCs were accommodated in the lesson area and how to identify the local or systemic effects. Another experiment showed that intracerebral transplantation of NSCs could be used as the late phase therapy for ICH, demonstrated by the finding that the transplantation of the HB1.F3 gene-modified human NSC line via a retroviral vector encoding v-myc 1 week after the induction of ICH could improve functional performance on the Rotarod test and limb placing after 2–8 weeks [21].
F3 per se has the ability to proliferate continuously and differentiate into cells of neuronal and glial lineage. In this particular study, transplanted human NSCs were identified in the perihematomal areas and differentiated into neurons and astrocytes, although the potential side effects and toxicity of gene modification remain unknown. In the present study, we intracerebrally transplanted the primary mouse foetal NSCs 3 days after the induction of ICH, which improved the functional performance in limb-placing test from the 2 weeks and on. Hoechst-labelled foetal NSCs transplanted into ICH haemorrhage lesion appeared in the haemorrhage core, the border of the lesion and the injecting channel, where foetal NSCs differentiated into neurons and astrocytes. It would be more helpful if the neuronal axons and dendrites after transplantation could be qualified to monitor the dynamic alternations of differentiated cells.
Our results provide the evidence that foetal NSCs without gene modification could be allocated in the brain, differentiated to neural axons and dendrites and astrocytes and survived for 31 days till the killing. Recent studies demonstrated that genetic modification with glial cell line-derived growth factor in human NSC line could increase 2- to 3-fold in cell survival of transplanted NSCs at 2 and 8 weeks after transplantation [22]. Genetic modification with Akt1 could also provide long-term improved survival (8 weeks) of hNSCs and behavioural recovery in mouse ICH model [23]. Such F3.Akt1-modified NSCs increased about 50–100% of cell survival rate at 8 weeks after transplantation, as compared to F3 NSCs. Similar findings were also observed in the intracerebral transplantation of NSC line genetically modified with the vascular epithelial growth factor [24]. Those results indicate that multiple factors are involved in the long-term survival of NSCs. However, such genetic modification of stem cells generates a number of special concerns and side effects [25] (e.g. these long-lived cells may represent a reservoir for the accumulation of proto-oncogenic lesions [26]). Our results showed that foetal NSCs were able to proliferate and differentiate into neurons and astrocytes both in the in vitro and in vivo conditions.
The mechanism by which transplanted NSCs improve the functional performance of animals with ICH remains unclear. It was suggested that such therapeutic effects of NSCs might be achieved by stimulation of endogenous progenitor cells rather than integration and differentiation of the infused NSCs [27]. The intravenously injected human processed lipoaspirate mesenchymal stem cells could improve the functional performance of animals with ICH, but could not be detected in the post mortem brain, where the number of endogenous progenitor cells increased 2-fold [27]. Intravascular injection of both mannitol and bone marrow stem cells significantly improved neurological functional outcome, and increased synaptogenesis, proliferating immature neurons and neuronal migration, as compared with either alone [28]. It implies that there are other factors involved in the effects of transplanted stem cells, demonstrated by the fact that mannitol can also open the blood–brain barrier by temporarily shrinking the tightly coupled endothelial cells and improving microcirculation [29]. It is also possible that transplanted NSCs may activate and direct endogenous repair mechanisms in the central nervous system, through exposure to specific neuronal growth factors or by inactivating inhibitory molecules [30]. Another potential is that transplanted NSCs may play an important role in ICH-induced local and systemic anti-inflammation through the immune organs, enhancing neuroprotection. It is supported by the finding that intravenous injection of NSCs could reduce ICH-induced brain oedema, leucocyte infiltrations and the activation of inflammatory mediators, which could disappear after splenectomy [31]. Although the present study hardly identifies the precise role of transplanted NSCs per se within the brain, our data indicate that the NSCs prior to transplantation may produce some neurotrophic mediators which may also play a role in the improvement of neural function like other cells [20], because the local injection of the culture medium alone could partially improve the functional performance in the limb-placing test. Previous findings demonstrated that transplanted NSCs in the damaged brain or spinal cord could replace damaged neurons and deliver therapeutic molecules to provide neuroprotection of damaged neurons [32, 33]. It was found that NSCs could release glial cell-derived neurotrophic factor, brain-derived neurotrophic factor, nerve growth factor and others [34–36].
In conclusion, the present study demonstrated that foetal NSCs could differentiate into neural axons and dendrites and astrocytes both in the in vitro and in vivo conditions. Intracerebral transplantation of foetal NSCs 3 days after the induction of ICH could improve the functional performance in the limb-placing test, have long-term survival and shorten the duration of the recovery from ICH-induced neural disorders. The foetal NSCs may also produce neurotrophic and/or neuroprotective factors during culture, because the culture medium alone improved partial functional performance. Thus, our data suggest that the foetal NSCs may be one of the optimal and therapeutic candidates for ICH.
Acknowledgments
The study was supported by the grant from the National Natural Science foundation of China (No. 30870878), the Government Agency Program of Science and Technology Department of Zhejiang Province (No: 2008C23059).
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Heiskanen O. Treatment of spontaneous intracerebral and intracerebellar hemorrhages. Stroke. 1993;24:I94–5. [PubMed] [Google Scholar]
- 2.Broderick J, Brott T, Tomsick T, et al. Management of intracerebral hemorrhage in a large metropolitan population. Neurosurgery. 1994;34:882–7. doi: 10.1227/00006123-199405000-00015. [DOI] [PubMed] [Google Scholar]
- 3.Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders-how to make it work. Nature Med. 2004;10:S42–50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- 4.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–8. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 5.McKay R. Stem cells in the central nervous system. Science. 1997;276:66–71. doi: 10.1126/science.276.5309.66. [DOI] [PubMed] [Google Scholar]
- 6.Roy NS, Nakano T, Keyoung HM, et al. Telomerase immortalization of neuronally restricted progenitor cells derived from the human fetal spinal cord. Nature Biotechnol. 2004;22:297–305. doi: 10.1038/nbt944. [DOI] [PubMed] [Google Scholar]
- 7.Sah DW, Ray J, Gage FH. Bipotent progenitor cell lines from the human CNS. Nat Biotechnol. 1997;15:574–80. doi: 10.1038/nbt0697-574. [DOI] [PubMed] [Google Scholar]
- 8.Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–89. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 9.Bjorklund A, Lindvall O. Self-repair in the brain. Nature. 2000;405:892–3. doi: 10.1038/35016175. [DOI] [PubMed] [Google Scholar]
- 10.Kelly S, Bliss TM, Shah AK, et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci USA. 2004;101:11839–44. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeong SW, Chu K, Jung KH, et al. Human neural stem cell transplantation promotes functional recovery in rats with experimental intracerebral hemorrhage. Stroke. 2003;34:2258–63. doi: 10.1161/01.STR.0000083698.20199.1F. [DOI] [PubMed] [Google Scholar]
- 12.Chu K, Kim M, Park KI, et al. Human neural stem cells improve sensorimotor deficits in the adult rat brain with experimental focal ischemia. Brain Res. 2004;1016:145–53. doi: 10.1016/j.brainres.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 13.Chesney JA, Kondoh T, Conrad JA, et al. Collagenase-induced intrastriatal hemorrhage in rats results in long-term locomotor deficits. Stroke. 1995;26:312–6. doi: 10.1161/01.str.26.2.312. [DOI] [PubMed] [Google Scholar]
- 14.Puurunen K, Jolkkonen J, Sirvio J, et al. An alpha(2)-adrenergic antagonist, atipamezole, facilitates behavioral recovery after focal cerebral ischemia in rats. Neuropharmacology. 2001;40:597–606. doi: 10.1016/s0028-3908(00)00182-9. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Li Y, Chopp M. Intracerebral transplantation of bone marrow with BDNF after MCAo in rat. Neuropharmacology. 2000;39:711–6. doi: 10.1016/s0028-3908(00)00006-x. [DOI] [PubMed] [Google Scholar]
- 16.Jeong SW, Chu K, Jung KH, et al. Human neural stem cell transplantation promotes functional recovery in rats with experimental intracerebral hemorrhage. Stroke. 2003;34:2258–63. doi: 10.1161/01.STR.0000083698.20199.1F. [DOI] [PubMed] [Google Scholar]
- 17.Kang SK, Lee DH, Bae YC, et al. Improvement of neurological deficits by intracerebral transplantation of human adipose tissue-derived stromal cells after cerebral ischemia in rats. Exp Neurol. 2003;183:355–66. doi: 10.1016/s0014-4886(03)00089-x. [DOI] [PubMed] [Google Scholar]
- 18.Liao W, Xie J, Zhong J, et al. Therapeutic effect of human umbilical cord multipotent mesenchymal stromal cells in a rat model of stroke. Transplantation. 2009;87:350–9. doi: 10.1097/TP.0b013e318195742e. [DOI] [PubMed] [Google Scholar]
- 19.Shyu WC, Lin SZ, Chiang MF, et al. Intracerebral peripheral blood stem cell (CD34+) implantation induces neuroplasticity by enhancing beta1 integrin-mediated angiogenesis in chronic stroke rats. J Neurosci. 2006;26:3444–53. doi: 10.1523/JNEUROSCI.5165-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Andersson R. Hepatocyte transplantation: a potential treatment for acute liver failure. Scand J Gastroenterol. 1995;30:193–200. doi: 10.3109/00365529509093262. [DOI] [PubMed] [Google Scholar]
- 21.Lee HJ, Kim KS, Kim EJ, et al. Brain transplantation of immortalized human neural stem cells promotes functional recovery in mouse intracerebral hemorrhage stroke model. Stem Cells. 2007;25:1204–12. doi: 10.1634/stemcells.2006-0409. [DOI] [PubMed] [Google Scholar]
- 22.Lee HJ, Park IH, Kim HJ, et al. Human neural stem cells overexpressing glial cell line-derived neurotrophic factor in experimental cerebral hemorrhage. Gene Ther. 2009;16:1066–76. doi: 10.1038/gt.2009.51. [DOI] [PubMed] [Google Scholar]
- 23.Lee HJ, Kim MK, Kim HJ, et al. Human neural stem cells genetically modified to overexpress Akt1 provide neuroprotection and functional improvement in mouse stroke model. PLoS ONE. 2009;4:e5586. doi: 10.1371/journal.pone.0005586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee HJ, Kim KS, Park IH, et al. Human neural stem cells over-expressing VEGF provide neuroprotection, angiogenesis and functional recovery in mouse stroke model. PLoS ONE. 2007;2:e156. doi: 10.1371/journal.pone.0000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baum C, Düllmann J, Li ZX, et al. Side effects of retroviral gene transfer into hematopoietic stem cells. Blood. 2003;101:2099–114. doi: 10.1182/blood-2002-07-2314. [DOI] [PubMed] [Google Scholar]
- 26.Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 27.Fatar M, Stroick M, Griebe M, et al. Lipoaspirate-derived adult mesenchymal stem cells improve functional outcome during intracerebral hemorrhage by proliferation of endogenous progenitor cell stem cells in intracerebral hemorrhages. Neurosci Lett. 2008;443:174–8. doi: 10.1016/j.neulet.2008.07.077. [DOI] [PubMed] [Google Scholar]
- 28.Seyfried DM, Han Y, Yang D, et al. Mannitol enhances delivery of marrow stromal cells to the brain after experimental intracerebral hemorrhage. Brain Res. 2008;1224:12–9. doi: 10.1016/j.brainres.2008.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mesiwala A. High-intensity focused ultrasound selectively disrupts the blood-brain barrier in vivo. Ultrasound Med Biol. 2002;28:389–400. doi: 10.1016/s0301-5629(01)00521-x. [DOI] [PubMed] [Google Scholar]
- 30.Andres RH, Guzman R, Ducray AD, et al. Cell replacement therapy for intracerebral hemorrhage. Neurosurg Focus. 2008;24:1–9. doi: 10.3171/FOC/2008/24/3-4/E15. [DOI] [PubMed] [Google Scholar]
- 31.Lee ST, Chu K, Jung KH, et al. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain. 2008;131:616–29. doi: 10.1093/brain/awm306. [DOI] [PubMed] [Google Scholar]
- 32.Lee ST, Chu K, Park JE, et al. Intravenous administration of human neural stem cells induces functional recovery in Huntington’s disease rat model. Neurosci Res. 2005;52:243–9. doi: 10.1016/j.neures.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 33.Kim SU, Park IH, Kim TH, et al. Brain transplantation of human neural stem cells transduced with tyrosine hydroxylase and GTP cyclohydrolase 1 provides functional improvement in animal models of Parkinson disease. Neuropathology. 2006;26:129–40. doi: 10.1111/j.1440-1789.2006.00688.x. [DOI] [PubMed] [Google Scholar]
- 34.Kamei N, Tanaka N, Oishi Y, et al. BDNF, NT-3, and NGF released from transplanted neural progenitor cells promote corticospinal axon growth in organotypic cocultures. Spine. 2007;32:1272–8. doi: 10.1097/BRS.0b013e318059afab. [DOI] [PubMed] [Google Scholar]
- 35.Lu P, Jones LL, Snyder EY, et al. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181:115–29. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 36.Pineda JR, Rubio N, Akerud P, et al. Neuroprotection by GDNF-secreting stem cells in a Huntington’s disease model: optical neuroimage tracking of brain-grafted cells. Gene Ther. 2007;14:118–28. doi: 10.1038/sj.gt.3302847. [DOI] [PubMed] [Google Scholar]


