Abstract
Previously we observed that cardiomyocyte progenitor cells (hCMPCs) isolated from the human heart differentiate spontaneously into cardiomyocytes and vascular cells when transplanted after myocardial infarction (MI) in the ischemic heart. After MI, deprivation of oxygen is the first major change in the cardiac environment. How cells handle hypoxia is highly cell type dependent. The effect of hypoxia on cardiac stem or progenitor cells remains to be elucidated. Here, we show for the first time that short- and long-term hypoxia have different effects on hCMPCs. Short-term hypoxia increased the migratory and invasive capacities of hCMPCs likely via mesenchymal transformation. Although long-term exposure to low oxygen levels did not induce differentiation of hCMPCs into mature cardiomyocytes or endothelial cells, it did increase their proliferation, stimulated the secretome of the cells which was shifted to a more anti-inflammatory profile and dampened the migration by altering matrix metalloproteinase (MMP) modulators. Interestingly, hypoxia greatly induced the expression of the extracellular matrix modulator thrombospondin-2 (TSP-2). Knockdown of TSP-2 resulted in increased proliferation, migration and MMP activity. In conclusion, short exposure to hypoxia increases migratory and invasive capacities of hCMPCs and prolonged exposure induces proliferation, an angiogenic secretion profile and dampens migration, likely controlled by TSP-2.
Keywords: cardiomyocyte progenitor cells; hypoxia, proliferation; thrombospondin-2; migration
Introduction
Over the last years, it has been established that the human heart harbours populations of cardiac progenitor cells that reside in the interstitial space in the myocardium [1–4]. These progenitor cells are thought to participate in maintenance and repair of the heart muscle [5, 6]. Therefore, progenitor cells need to (i) proliferate, (ii) migrate out of their niche and (iii) locally form new cardiac tissue by differentiating into cardiomyocytes, endothelial cells and vascular smooth muscle cells. The cues for these three processes are provided by the immediate environment of the progenitor cells. After myocardial infarction (MI) extensive regeneration of the ischemic area is required. Progenitor cells in the heart itself, or introduced via cell transplantation, will immediately be exposed to hypoxia. Oxygen tension is an important stimulus that has received increasing recognition for its ability to influence gene expression and to affect the characteristics of various types of progenitor cells [7–9]. Although a few studies describe the use of oxygen as a signalling molecule that can influence the survival, proliferation and differentiation of cells, the effect of oxygen on cardiac progenitor cell behaviour is not yet studied.
Under hypoxic conditions enhanced proliferation has been reported for bone marrow-derived mesenchymal stem cells [10, 11], skeletal muscle satellite cells [7, 12, 13] and central nervous system derived stem cells [12, 14, 15]. In the same stem cell populations reduced apoptosis was observed under low oxygen conditions [12, 14, 16]. Interestingly, the effects of hypoxia on differentiated cell types are more diverse; for example, cardiomyocytes show increased apoptosis under hypoxia [17], whereas endothelial cells are able to survive a hypoxic environment [18]. However, analysing ischemia/reperfusion induced cell death in rat hearts showed that hypoxia activated programmed cell death occurs in cardiomyocytes and endothelial cells in vitro and in the injured myocardium [19].
Besides survival and proliferation also migration of cells can be influenced by the oxygen tension as shown for bone marrow cells [16] and endothelial cells [20]. Additionally, in some situations hypoxia is reported to affect cell differentiation: skeletal muscle differentiation was enhanced in low oxygen conditions [13], whereas e.g. adipocyte differentiation was reduced [13, 21]. In this respect, oxygen tension can play an important role in the processes underlying cardiac repair; proliferation, migration and differentiation of cardiac progenitor cells. Alternatively, it may influence the therapeutic potential of cardiac progenitor cells as a source for cell-based therapy. Therefore it is important to understand how these progenitor cells respond to a hypoxic environment.
Previously, we have reported that a population of human cardiomyocyte progenitor cells (hCMPCs) can be isolated from the adult and foetal heart [3, 22]. In vitro these hCMPCs maintain their progenitor cell characteristics under normal culture conditions. However, when the cells are stimulated in vitro with 5-azacytidine and transforming growth factor (TGF)-β, hCMPCs change into beating cardiomyocytes with organized sarcomeric structures, a ventricular like action potential and capable of gap junctional communication [3, 23]. Culturing of CMPCs on matrigel with the addition of vascular endothelial growth factor resulted in the formation of vessel-like structures and the differentiation into endothelial cells and smooth muscle cells [3, 23]. Interestingly, when undifferentiated hCMPCs were injected into the infarcted mouse heart, we observed that these cells proliferate, migrate through the tissue and locally differentiate into cardiomyocytes and vessel-like structures [24]. This observation is particularly important because it shows that the ischemic heart provides the correct environmental cues for regeneration. Additionally, injection of hCMPCs stimulated the formation of mouse blood vessels in the infarcted area and infarct border zone, showing a paracrine effect of progenitor cells on their surroundings [24]. This makes hCMPCs an excellent in vitro model to study the effects of environmental factors on cardiac stem cell populations.
Hypoxia is one of the most prominent factors after a MI. We therefore suggest that hypoxia will (i) stimulate the proliferation and migration of hCMPCs, (ii) affect the ability of hCMPCs to modify their environment and (iii) stimulate the differentiation of hCMPCs into cardiac cell types.
Materials and methods
Cell culture
hCMPC
Human foetal hearts were collected after elective abortion based on individual informed consent and after approval by the Medical Ethics committee of the Leiden University Medical Center. The investigation conforms to the principles outlined in the Declaration of Helsinki. hCMPCs were isolated using magnetic cell sorting and cultured as described previously [3, 22]. For all experiments hCMPCs (passages 10–15) were grown in 0.5% foetal bovine serum (FBS, Gibco, Paisley, UK) and penicillin–streptomycin (pen–strep, 100 U/ml each, Gibco) in DMEM/M199, except for the viability and proliferation assays in which hCMPCs were cultured in DMEM/M199 containing 1% FBS and pen–strep. A total of 10,000 hCMPCs/cm2 were plated for mRNA expression, protein secretion or for production of conditioned medium (CM). To induce a low oxygen environment, cells were cultured in a hypoxic incubator (1% O2). CM was prepared by culturing hCMPCs for the indicated days under normoxic (20% O2-CM) or hypoxic conditions (1% O2-CM) and filter sterilized by passing it through a 0.2 μm filter.
HUVEC
Human umbilical vein endothelial cells (HUVECs) were isolated from umbilical cords and cultured on 1% gelatine-coated plates in EGM-2 medium (Lonza, Walkersville, MD, USA), supplemented with 10% FBS and pen–strep.
Proliferation and cell numbers
To determine the number of cells, 750 CMPCs/well were seeded on day 0 and grown for 9 days under normoxia or hypoxia and were stained with 1 μg/ml fluorescein di-acetate (Sigma, St. Louis, MO, USA). After which pictures were taken for evaluation of cell density.
Furthermore, 750 hCMPCs/well were seeded in 96-well plates and cultured up to 9 days after which proliferation was measured by BrdU incorporation ELISA according to the manufacturer’s protocol (Calbiochem, San Diego, MD, USA).
Cell viability under hypoxia and normoxia was evaluated using the thiazolyl blue tetrazolium bromide assay (MTT, Sigma). hCMPCs were seeded in 96-well tissue culture plates and after 6 and 9 days cell viability was assessed by measuring the amount of solubilized formazan using an ELISA reader at 570 nm.
Scratch assay
To determine the migration capacity of hCMPCs, we performed a scratch assay. hCMPCs were seeded in 12-well plates, and incubated under normoxic or hypoxic conditions. After 24 hrs or 9 days, scratches were made in the confluent monolayer and medium was refreshed or replaced by CM to remove dead cells due to scratching the monolayer. To analyse the migratory response, pictures were taken after 0 and 4 hrs. Quantification was performed with the ImageJ software. Analysis of the scratch at 0 hr showed that mean scratch width was comparable between all groups.
Boyden chamber assay
The chemotactic properties of hCMPCs were determined using a Boyden chamber assay. Fifty thousand hCMPCs were seeded in the upper chamber of a transwell (pore size 8.0 μM; Corning, Amsterdam, The Netherlands). Prior to migration the polycarbonate filters were coated with gelatine. In the lower chamber, unconditioned medium, 1% O2-CM or 20% O2-CM was added, and cells were allowed to migrate at 37°C. After 4 hrs, the chambers were paraformaldehyde and methanol fixed, and cells were stained with 0.4% crystal violet. Pictures were taken and migrated hCMPCs were counted using Cell^B software (Olympus, Hamburg, Germany).
Spheroid sprouting assays
Spheroids were formed as described previously [25]. A total of 500 cells/well were plated and incubated overnight in non-adherent round-bottom plates (Greiner bio one GmbH, Frickenhausen, Germany) to form spheroids. The day after the spheroids were embedded in collagen/methylcellulose gels. Following polymerization of the gel (approximately 30 min.), unconditioned or conditioned medium was added and incubated overnight. hCMPCs spheroids were used to determine the effect of hypoxia the invasive capacity of hCMPCs sprouts assay was performed in 1% and 20% O2. To analyse the effect of the hypoxia-induced secreted proteins by hCMPCs on endothelial cells, HUVEC spheroids were allowed to sprout in the presence of hCMPC CM. Pictures were taken after 24 hrs and the length and number of sprouts were quantified using Cell^B software.
Gene expression
RNA isolation and RT-qPCR
RNA was isolated using TriPure (Roche, Almere, The Netherlands) according to manufacturer’s protocol and purified using the Nucleospin RNA isolation kit (Macherey-Nagel, Düren, Germany). cDNA was synthesized from 500 ng RNA according to the protocol of the Revert™ Aid H minus first strand synthesis kit (Fermentas GmbH, St. Leon-Rot, Germany). Beacon Designer 4.0 (Premier Biosoft International, Palo Alta, CA, USA) was used to design quantitative polymerase chain reaction (qPCR) primers for β-actin, Nkx2.5, myocardin, ventricular myosin regulatory light chain (MLC-2V), α-cardiac actin, Von Willebrand factor, vascular endothelial (VE)-cadherin, platelet endothelial cell adhesion molecule, vascular endothelial growth factor receptor (VEGFR)2, endoglin, Tie-2, VEGF-A, human platelet derived growth factor BB (PDGF-BB), placental growth factor (PlGF), Snail, Slug, TWIST, matrix metalloproteinase (MMP)-2, MMP-9, tissue inhibitor of metalloproteinase (TIMP)-1, TIMP-2, thrombospondin-1 (TSP-1) and TSP-2. β-actin was measured as a housekeeping gene. Primer sequences and annealing temperatures are available on request.
Paracrine factors
Zymography
Zymography was performed as described previously [26]. In brief, CM samples were run on a 10% SDS-PAGE gel containing 0.8 mg/ml gelatine. After overnight incubation with freshly prepared Brij-35 solution, gels were stained with Coomassie, MMP-2 and MMP-9 activity was quantified using a densitometric method on a Geldoc 1000 system (Bio-Rad, Hercules, CA, USA) and Quantity One software (Bio-Rad). (Active) MMP-2 and (active) MMP-9 were identified based on their size on the zymography gel.
Angiogenesis antibody array
After 9 days of culture, CM was collected and incubated with the RayBio human angiogenesis antibody arrays 1 and 2 (Ray Biotech, Norcross, GA, USA) to determine the secretion levels of angiogenic growth factors and cytokines. Protein-antibody complexes were visualized by chemiluminescence according to the manufacturer’s protocol. The results were photographed using Geldoc 1000 system (Bio-Rad) and quantified with Quantity One software.
ELISA
We performed an ELISA for VEGF-A (Human VEGF-A ELISA, Bender MedSystems GmbH, Vienna, Austria), PlGF (PeProTech, Rocky, Hill, NJ, USA), PDGF-BB (PeProTech), monocyte chemotactic protein-1 (MCP-1; PeProTech), interleukin (IL)-8 (PeliKine Compact human IL-8 ELISA kit, Sanquin, Amsterdam, The Netherlands) and TGF-β1 (human TGF-β1, DY240, R&D Systems, Abingdon, UK) according to the manufacturer’s protocols.
Western blotting
To validate protein levels, Western blotting was performed for TSP-1 and TSP-2 on 9 days CM. CM was loaded and separated by 4–10% gradient SDS-PAGE and transferred to Hybond PVDF membranes (Amersham, Uppsala, Sweden). After blocking with 5% milk in Tris-buffered saline tween-20 (TBST), membranes were incubate overnight with TSP-2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or TSP-1 antibody (A6.1; Thermo Scientific, Fremont, CA, USA) followed by incubation with a horseradish peroxidase labelled secondary donkey anti-goat or goat antimouse antibody, respectively, for 1 hr. Chemiluminescence was induced by ECL Advanced Detection reagent (Pierce, Rockford, IL, USA) and detected by exposure to Hyperfilm ECL (Amersham).
RNA interference
hCMPCs were transduced with lentiviruses, expressing shRNAs that specifically target human TSP-2, from the MISSION RNAi library (Sigma) in the presence of 8 μg/ml polybrene. After 6 hrs the medium was refreshed, and the next day 1 μg/ml puromycin was added for selection of transduced cells. Three days after selection, an MTT and spheroid assay was performed and expression of TSP-2 was analysed by RT-PCR.
Statistical analysis
All values are represented as mean ± S.E.M. P-values <0.05 were considered significant. Student’s t-test was used to compare hypoxia and normoxia. For multiple group comparison anova with Fisher’s least significant difference (LSD) post hoc test was used.
Results
Hypoxia induces hCMPC proliferation
Because hypoxia has been shown to induce the proliferation of several progenitor cell types [7], we analysed the growth rate of hCMPCs. During the first days of culture there were no differences in the proliferation of hCMPCs cultured under normoxic or hypoxic conditions (data not shown). However from day 6 onwards, hypoxic hCMPCs exhibited a higher cell density as was shown by labelling the cell cultures with fluorescein di-acetate (Fig. 1A). This increase in cell number was further consolidated by measuring the overall cellular mitochondrial respiratory chain activity using an MTT assay. Moreover, cellular DNA synthesis, a measure of cell division, was investigated by BrdU incorporation. Both assays revealed a significantly increased proliferation under low oxygen culture conditions (Fig. 1B and C).
Fig 1.
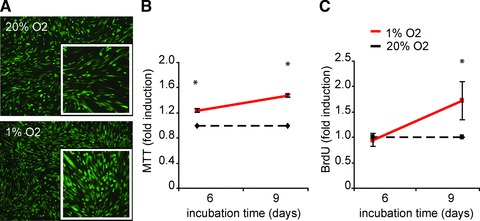
Hypoxia increases the proliferation of hCMPCs. hCMPCs were cultured under normoxic and hypoxic conditions for the indicated time intervals. An increase in the number of cells under hypoxia was visualized by staining with fluorescein di-acetate (A). Viability and cell cycle progression was quantified by MTT (B) and BrdU (C), respectively. Both assays show an increased proliferation under hypoxia. Values were normalized to normoxia of the same time-point and one representative experiment of seven is shown. *Significant difference between normoxia and hypoxia at the same time-point.
Effect of hypoxia on hCMPC differentiation and pro-angiogenic capacity
hCMPCs are able to differentiate into both cardiac and vascular cells in vitro and in vivo [3, 22, 23] and provide a unique biological system to explore the effect of hypoxia on cardiac progenitor cell differentiation. After 9 days of culture in a hypoxic environment, hCMPCs expressed the early cardiac transcription factors Nkx2.5 and myocardin indicating a commitment to the cardiac lineage. However, the sarcomeric genes MLC-2V and α-cardiac actin, which are specific for striated cardiomyocytes, were not observed. This shows that 9 days of only hypoxia is not sufficient to differentiate hCMPCs into cardiomyocytes (Fig. 2A). Low oxygen was also not sufficient to steer their differentiation into endothelial cells; only increased Tie-2 mRNA expression levels were observed (Fig. 2B).
Fig 2.
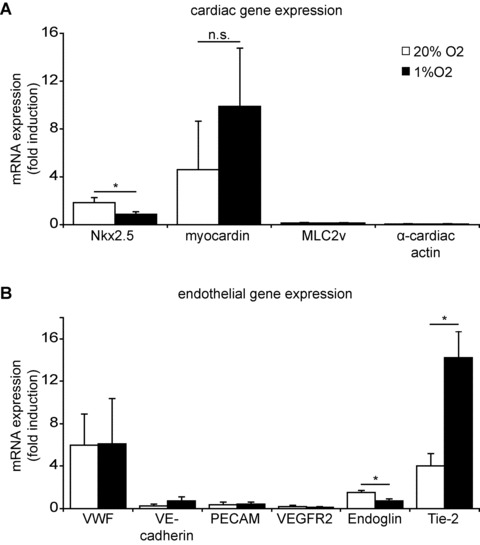
Hypoxia does not induce differentiation of hCMPCs. Nine days of hypoxic culture did not induce expression of cardiomyocyte specific genes, as shown by RT-qPCR (A). Analysis of endothelial specific genes showed that there is no differentiation of hCMPCs into vascular cells during this incubation period (B). β-actin was measured as a housekeeping gene, and data were normalized to day 0 expression. *Significant difference between normoxia and hypoxia.
Although hypoxia did not induce vascular differentiation of hCMPCs themselves, it may affect paracrine factors released by hCMPCs that can stimulate neo-angiogenesis in the injured myocardium [24]. To gain more insight into the pro-angiogenic activity of hypoxic hCMPCs, we analysed the secretome of hCMPCs cultured under hypoxic or normoxic conditions using an angiogenic antibody array. Several proteins were found to be differentially expressed, which we confirmed using ELISA and RT-qPCR. Although the release of large amounts of MCP-1 (Fig. 3A), active TGF-β (Fig. 3B) and IL-8 (Fig. 3C) was decreased when hCMPCs were cultured under hypoxic conditions, low oxygen stimulated the secretion of VEGF-A (Fig. 3D) and increased VEGF-A mRNA expression (Fig. S1A). Although the expression of PDGF-BB mRNA increased under hypoxia (Fig. S1B), there was no difference in its secretion (Fig. 3E). hCMPCs secrete more PlGF under normoxic conditions than under hypoxic conditions. (Fig. 3F), as was observed for PlGF mRNA expression (Fig. S1C). Thus although hypoxia did not induce differentiation of hCMPCs into cardiovascular cells, it did enhance the secretion of pro-angiogenic growth factors and cytokines by hCMPCs.
Fig 3.
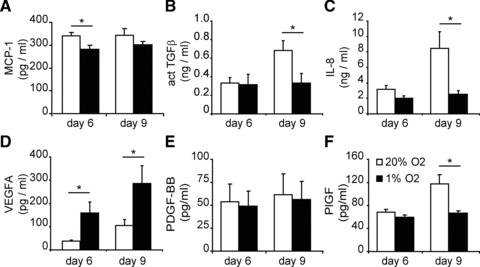
Hypoxia influences secretion of factors by hCMPCs. To determine the secretion of proteins, medium was conditioned by culturing cells for 6 or 9 days under hypoxia or normoxia. To measure protein levels, ELISA was performed. Hypoxia decreased the secretion of MCP-1 (A), TGF-β (B) and IL-8 (C). The secretion of VEGF-A (D) was induced by hypoxia, whereas PDGF-BB secretion remained unaffected (E). PlGF secretion was increased during prolonged normoxic culture and remained unaffected by hypoxia (F). Average of five experiments is shown. *Significant difference between normoxia and hypoxia at the same time-point.
Hypoxia stimulated the migration and invasion of hCMPCs
The ability of progenitor cells to move through (scar) tissue is important for cells to reach the area that requires repair. Therefore we analysed the migration and invasion capacity of hCMPCs under hypoxia. Using a 2D scratch assay, we observed increased motility of hypoxic hCMPCs, resulting in a closure of 86%, whereas under normoxic conditions 41% was closed (Fig. 4A and B).
Fig 4.
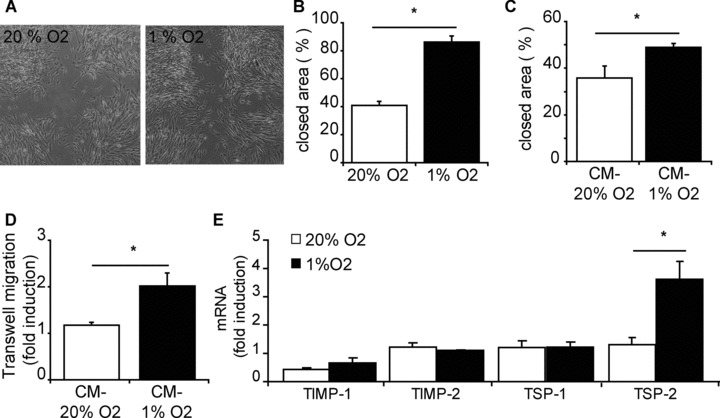
Migration capacity of hCMPCs is enhanced by hypoxia. hCMPCs secrete factors under hypoxia that improve the migration. (A) Representative pictures of cell migration after 4 hrs in scratch assay; hCMPCs migrate faster under hypoxia than under normoxia. (B) Quantification of migration in (A). (C) 1% O2-CM induced more migration than 20% O2-CM when the scratch assay was performed in 20% O2. (D) A Boyden chamber assay shows increased migration of hCMPCs towards 1% O2-CM. The number of migrated cells was normalized to unconditioned medium. (E) Gene expression of remodelers of ECM after 1 day of culturing was not significant influenced by hypoxia, except for TSP-2 mRNA expression which was increased under hypoxia. β-actin was measured as a housekeeping gene, and data were normalized to day 0 expression. *Significant difference between normoxia and hypoxia.
To determine whether hCMPCs secrete factors that would influence their migration, we performed a scratch assay in the presence of CM of normoxically or hypoxically grown hCMPCs. In the presence of 1% O2-CM, hCMPCs were able to close 49% of the scratch whereas in the presence of 20% O2-CM only 36% was closed (Fig. 4C). Additionally, using a Boyden chamber assay, we observed that significantly more hCMPCs migrated towards 1% O2-CM when compared to 20% O2-CM (Fig. 4D). These data suggest that hypoxic CM increased the mobility of hCMPCs and that under hypoxia secreted factors have chemotactic properties.
Because migration is facilitated by the breakdown of extracellular matrix (ECM), we investigated whether hCMPC migration was affected by changes in ECM remodellers. After a short, 24 hr exposure, to hypoxic conditions, there was no change in the expressions of TIMP-1, TIMP-2 and TSP-1 (Fig. 4E) or the secretion of MMP-2 (data not shown). However, there was a significant induction of TSP-2 by hypoxia. Another contribution to enhanced migration can be the mesenchymal transformation of cells to a more migratory phenotype. We observed that after 24 hrs of exposure to hypoxia, hCMPCs have increased expression of Snail, Slug and TWIST (Fig. S2), genes known to be induced during mesenchymal transformation.
To determine the effect of hypoxia on the invasiveness of hCMPCs, we embedded these cells as spheroids in a 3D collagen gel. hCMPCs successfully formed spheroids that were able to form sprouts (Fig. 5A). When performing this assay under hypoxic conditions, we observed a significant increase in the number of sprouts invading the collagen layer and a decrease in the average length of the hypoxic sprouts (Fig. 5A–C).
Fig 5.
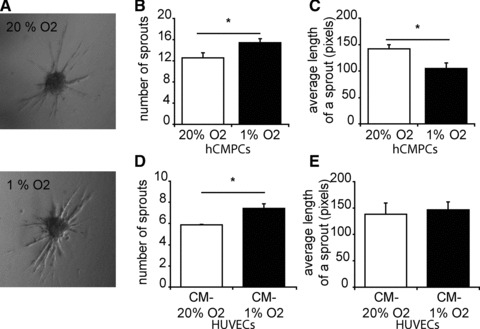
Hypoxia increases the invasion capacity of hCMPCs, and stimulates the production of pro-angiogenic factors. (A) hCMPC spheroids, embedded in collagen, invade collagen to form sprouts. Representative pictures of hCMPC spheroid sprouting under normoxia and hypoxia are shown. (B) The number of sprouts per spheroid increased under hypoxic conditions, whereas the average length of a sprout decreased (C). (D) CM of hCMPCs positively influences the sprouting capacity of endothelial cells. The number of sprouts per spheroid increased when HUVEC spheroids are cultured in the presence of 1% O2-CM, the length of a sprout was not affected (E).
Because hCMPC are shown to have a paracrine effect on their surroundings, we examined the effect of hCMPCs secreted proteins on the sprouting capacity of endothelial cells. HUVEC spheroids were formed, and subsequently grown in the presence of hypoxic or normoxic CM of hCMPCs. 1% O2-CM induced significantly more sprouts than 20% O2-CM (Fig. 5D). However, there was no difference in the mean length of a HUVEC sprout between the conditions (Fig. 5E). These data suggest that under hypoxia, hCMPCs release paracrine factors that induce migration and invasion of both cardiac progenitor cells and endothelial cells.
Prolonged exposure to low oxygen tension inhibits hCMPC growth and migration
Because repair of ischemic damage of cardiac tissue takes longer than 1 day we also explored the effects of prolonged (6 and 9 days) exposure to hypoxia on the different aspects of migration. Because ECM modulators are important for the migration of (progenitor) cells through cardiac tissue, we determined the effect hypoxia had of MMP-2 and -9, and the MMP inhibitors TIMP and TSP [27, 28]. Using zymography and RT-qPCR we observed that hCMPCs express high levels MMP-2 mRNA and secreted large amounts of MMP-2 protein (Fig. 6A and B). Culturing hCMPCs under hypoxic conditions did not affect the MMP-2 gene expression nor the MMP-2 protein secretion (Fig. 6A and B). MMP-9 protein or mRNA levels were below the detection level in all conditions analysed (data not shown).
Fig 6.
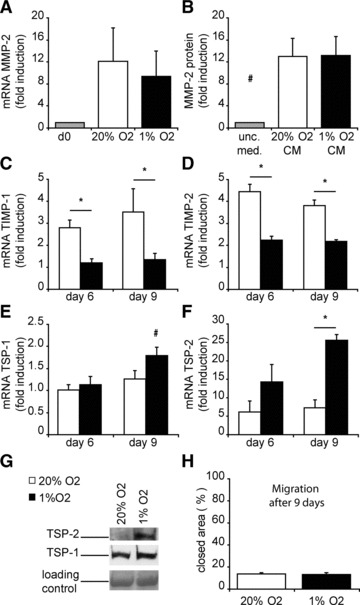
Long-term exposure to hypoxia inhibits CMPC migration via increased expression of the ECM modulator TSP-2. (A) The expression of MMP-2 in cells was determined via RT-qPCR and was not influenced by hypoxic conditions. (B) Secretion of MMP-2 in CM was demonstrated by zymography. The expression TIMP-1 (C), TIMP-2 (D), TSP-1 (E) and TSP-2 (F) was measured by RT-qPCR. (G) Secretion of TSP-1 and TSP-2 is increased when hCMPCs are exposed to hypoxia for 9 days. (H) After 9 days of culture, migration in a scratch assay was determined, and showed no difference between hypoxia and normoxia. For all genes, expression was normalized to day 0 and β-actin was measured as a housekeeping gene. #Significant difference compared to all groups; *significant difference between two groups.
The expression of TIMP-1 and TIMP-2 increased in time in both hCMPCs cultures compared to day 0 (Fig. 6C and D), but the increase in expression was less pronounced when hCMPCs were grown under hypoxic conditions. Although TSP-1 significantly increased under hypoxia (Fig. 6E and G), the most striking difference in expression level of the matrix modulators was observed for TSP-2 mRNA, increasing 26-fold after 9 days of culture under hypoxic conditions (Fig. 6F), which was translated in an increased secretion of TSP-2 under hypoxia (Fig. 6G).
To investigate whether these observed changes in ECM remodelers affect cellular behaviour, we performed a scratch assay after prolonged culture. After 9 days of normoxic culture, hCMPCs migrate slower (Fig. 6H) compared to 1 day (Fig. 4B), and only half of the area is closed 4 hrs after scratching of the monolayer. The difference under hypoxic conditions is even more striking. After short-term exposure to hypoxia, hCMPCs were able to close 86% of the scratched area. In contrast, long-term culture under low oxygen led to a 15% closure of the area, in an equal time-span (Fig. 6H).
Thus, prolonged low oxygen levels inhibit TIMPs whereas the expression especially of TSP-2 is increased and hCMPC migration is significantly reduced.
TSP-2 controls the proliferation and migration of hCMPCs
Because hypoxia strongly induced TSP-2 expression, we explored the function of TSP-2 by lentiviral knockdown of TSP-2 levels in hCMPCs (Fig. 7A). As previously observed, hypoxia induced the proliferation of hCMPCs transduced with an empty vector control. Interestingly, TSP-2 knockdown cells showed enhanced proliferation under normoxia and now had the same growth rate as TSP-2 cells grown under hypoxia (Fig. 7B). Migration of shTSP-2-hCMPCs was significantly enhanced under both normoxic and hypoxic conditions and as observed for cell proliferation, hypoxia no longer increased the migration of the shTSP-2-hCMPCs (Fig. 7C). Finally, we analysed the role of TSP-2 knockdown on migration and invasion in a 3D spheroid assay. Interestingly, knockdown of TSP-2 altered the capacity of hCMPCs to form spheroids, leading to very loose irregular aggregates (Fig. 7D) that easily dissociated into loose cells when seeded into the collagen matrix. From those that were correctly embedded into the 3D collagen gel, we found 21% of the TSP-2 knockdown hCMPC spheroids on the bottom of the well-compared to 10% of the control spheroids (Fig. 7E). Normally, spheroids remain embedded within the collagen matrix and only the invading cells locally modulate the collagen. TSP-2 knockdown spheroids degraded the collagen extensively, resulting in a loose more liquid matrix causing them to drop to the bottom, attached and spread out. MMP-2 and MMP-9 are important proteases for collagen break down. Therefore, we analysed the secretion of these MMPs using zymography. Although knockdown of TSP-2 resulted in a slight increase in MMP-2 secretion (Fig. 7F and G), a more striking effect was seen for MMP-9. Although MMP-9 was hardly detectable in empty vector transduced hCMPCs, TSP-2 knockdown resulted in enhanced secretion of MMP-9 (Fig. 7F and H).
Fig 7.
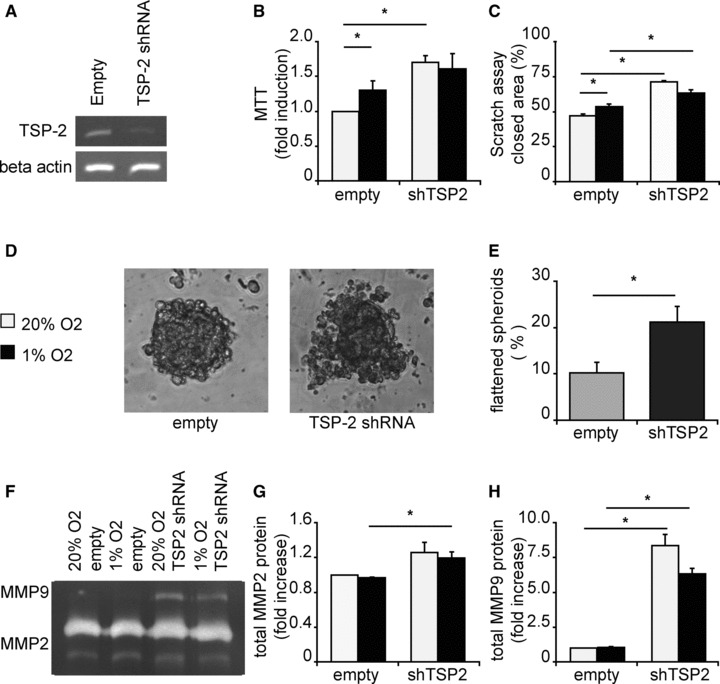
Knockdown of thrombospondin-2 increased proliferation and migration of hCMPCs. (A) TSP-2 was knocked down using RNAi lentiviruses and decreased TSP-2 expression in hCMPCs with approximately 70%. (B) Knockdown of TSP-2 resulted in an increased cell number measured with MTT. One representative experiment of four is shown. (C) Knockdown of TSP-2 induced the migration of hCMPCs in a scratch assay, and hypoxia did not increase the migration in TSP-2 knockdown cells. (D) Representative pictures of spheroids from hCMPCs transduced with empty vector or shTSP-2. (E) Knockdown of TSP-2 resulted in more spheroids that broke down the matrix and spread out on the bottom of the well. (F) Representative picture of a zymogram loaded with 9 days CM of TSP-2 knockdown or empty vector hCMPCs. (G) Quantification of MMP-2 in (F), normalized to unconditioned medium. (H) Quantification of MMP-9 in (F); knockdown of TSP-2 greatly induced the secretion of MMP-9. *Significant difference between two groups.
Discussion
The aim of this study was to investigate the effect of low oxygen tension on the cellular behaviour of hCMPCs. We found that short exposure to hypoxia enhanced the migration and invasive capacity of the cells. In contrast, prolonged hypoxia inhibited cell migration, but induced the proliferation of hCMPCs. Importantly low oxygen tension increased the production of pro-angiogenic and anti-inflammatory factors by hCMPCs, and affected matrix degradation.
The primary response of human cardiac progenitor cells exposed to an ischemic environment is an enhanced migration. This improved motility may be partially explained by an increase in growth factor expression like VEGF, which has been reported to influence the migration of endothelial cells [29] and is regulated by hypoxia [30, 31]. Induced migration was also described for hypoxic CM of MSCs and adipo-derived stem cells, which was at least partly explained by the up-regulation of VEGF [8, 32]. In our experiments, medium conditioned by hypoxic cultured hCMPCs stimulated the migration of hCMPCs more than normoxic CM. This corresponded with the presence of higher levels of VEGF in the medium. Intriguingly, the overall migration level using hypoxic CM was lower compared to the migration performed under complete hypoxia. This means that the effect can only partially be explained by hypoxia induced secretion of growth factors, and a portion of the effect has to be attributed to a change in cellular characteristics.
Short-term hypoxia indeed induced the expression of Slug and Snail, genes known to be involved in mesenchymal transition. This phenomenon is well described for epithelial cells to become more migratory [33]. A recent study shows that Snail expression increases in low oxygen culture and enhances the motility of cancer cells [34].
Prolonged exposure of hCMPCs to a hypoxic environment inhibited cell migration. This could be explained by the increased production of inhibitors of matrix degradation we observed. Cell migration requires controlled degradation of the ECM by MMPs and their modulators [35] and the expression of these MMPs and their modulators are cell type and tissue specific [36]. After MI mainly the roles of MMP-2, MMP-9, TIMP-1, TIMP-2, TSP-1 and TSP-2 are explored [36–38]. The expression of ECM modulators in progenitor cells is not fully elucidated, but there are several reports that show an increased expression of mainly MMP-2 and TIMP-1 [39–43]. Disturbing the balance between ECM production, degradation, and remodelling can result in an impaired migration of progenitor cells. We observed that hypoxia affect the expression of TIMP-1 and TIMP-2 and increased the expression of TSP-1 and TSP-2, factors known to decrease MMP activity [38]. Especially the levels of TSP-2 were tremendously increased in long-term hypoxic hCMPCs. The role of TSP-2 in migration has been established previously by analysing fibroblast from TSP-2 deficient mice, MacLauchlan and coworkers described enhanced migration of these cells. Furthermore, increased wound healing was observed in TSP-2-null mice, which was mediated by increased activity of MMP-2 and MMP-9 [44]. Interestingly, in our experiments knockdown of TSP-2 resulted in increased proliferation and enhanced the migration of hCMPCs grown in 20% O2 to similar levels as hypoxic cultures. This could suggest that after prolonged hypoxia TSP-2 levels serve as a negative feedback loop to dampen the migration of cardiac progenitor cells.
Although it is necessary for cardiac progenitor cells to migrate, the balance in matrix modulation needs to be tightly controlled to preserve tissue integrity and cohesiveness. Too much degradation might cause tissue rupture whereas too little will result in fibrosis [28, 45, 46]. Although hardly any MMP-9 was present in control hCMPCs, knockdown of TSP-2 increased the activity of MMP-9, most likely explaining the increased migratory capacity of the TSP-2 knockdown hCMPCs. This increased MMP-9 activity could also justify why spheroids made of TSP-2 knockdown cells efficiently degrade the collagen matrix and cannot form sprouts.
Another feature that became evident after prolonged exposure to hypoxia is a positive effect on proliferation of hCMPCs. Previous in vitro studies have shown that exposure to low oxygen can both inhibit or induce proliferation of cells, depending on the experimental set-up, maturity of the cells and studied cell type [7, 10, 12, 47–49]. However, there is increasing evidence that hypoxia promotes the proliferation of progenitor cells such as adult skeletal muscle satellite cells [7, 12, 13], bone marrow derived mesenchymal stem cells [10, 11, 50] and the CD34+ bone marrow progenitor population [12, 49].
Besides cellular behaviour, hypoxia can also modulate gene transcription. Hypoxic hCMPCs showed increased expression and secretion of VEGF-A, and lowered PlGF levels. How PlGF influences angiogenesis is not clear and depending on the cell type studied [51], absence of PlGF was shown to reduce VEGF-induced vascular leakage in microvessels [52, 53], and important for the formation of new stable vessels [51], which is in agreement with our data. Interestingly, we observed that the hypoxia-stimulated secretion of angiogenic factors was able to influence not only the migratory response of hCMPCs both in a scratch, as well as a transwell assay, it could also affect the capacity of endothelial cells to form new vascular sprouts. When HUVECs were embedded as spheroids in a 3D collagen gel, adding CM of hCMPCs grown in 1% oxygen resulted in more sprouts.
The critical importance of VEGF signalling for repair of the injured myocardium was elegantly shown by Thirunavukkarasu and coworkers [54]. They demonstrated that in vivo precondition reduced infarct size and cellular apoptosis only in wild-type mice, but not in VEGFR1+/− animals. This significant decrease of the post-ischemic myocardial function in the heterozygous animals demonstrated the cardioprotective function through the VEGF signalling pathway. This would imply that hCMPCs could positively influence their surroundings by stimulating neo-angiogenesis, and it could explain why we observed an increased number of mouse vessels and improved heart function when hCMPCs were injected into the infarcted mouse heart [24, 55].
After 9 days of hypoxia we also observed a decreased production of IL-8, MCP-1 and TGF-β. Reduction of these pro-inflammatory cytokines may dampen an inflammatory response e.g. after MI [56]. IL-8 is has been implicated in a number of inflammatory diseases involving neutrophil activation [56]. MCP-1 is involved in the pro-inflammatory response after injury, and is an important factor in the pathogenesis of myocardial ischemia reperfusion injury, atherosclerosis and a variety of inflammatory diseases, whose pathogenesis is known to involve infiltration and activation of monocytes and lymphocytes [57, 58]. Also TGF-β is pro-inflammatory [56]. TGF-β1 elicits a direct chemotactic response from neutrophils and monocytes, which may be important for the movement of infiltrating leucocytes into injured tissue [59, 60]. Therefore the down-regulation of IL-8, MCP-1 and TGF-β production under hypoxia suggests that hCMPCs change their secretion profile from pro-inflammatory under normoxia to an anti-inflammatory profile under hypoxia. This was further supported by the up-regulation of TSP-1 under hypoxia. In vivo after MI, TSP-1 is suggested to serve as a ‘barrier’ to limit the extension of the inflammatory response and granulation tissue into the non-infarcted areas [61].
Transplantation of hCMPCs into the ischemic mouse myocardium induced their spontaneous in vivo differentiation into cardiomyocytes and vascular cells, as observed 3 months after transplantation [24]. Here, we investigated if hypoxia alone is sufficient to induce this differentiation of hCMPCs in vitro and found that 9 days of hypoxia did not induce vascular differentiation. We have previously reported that differentiation of hCMPCs into the cardiomyogenic lineage starts with an increase in Nkx2.5 expression and as differentiation proceeds the expression of Nkx2.5 decreases again. The presence of Nkx2.5 and increase in myocardin expression we observed after 9 days of culture in low oxygen indicated that there is a commitment to differentiate into the myogenic lineage, but the absence of e.g. cardiac actin shows that only hypoxia is not sufficient for full differentiation. Most likely other growth factors and cytokines are needed to achieve this. Because TGF-β is able to shift the phenotype of fibroblasts to myofibroblasts, the active collagen-secreting cells involved in scarring [62], the hypoxia-induced TGF-β1 down-regulation by the hCMPCs may suggest that these cells positively affect post-MI adverse remodelling.
Although this study is performed in vitro, it may have some implication for future in vivo applicability. hCMPCs are a unique population that exhibit in vitro and in vivo differentiation into cardiac cell types [3, 24]. This makes them an interesting population for future use in cardiovascular regenerative medicine. However, more information on how these cells behave under stressful conditions may provide new approaches to stimulate the endogenous cardiac cell population. In this regard the findings of this study are of interest, because they suggest that the primary response of these progenitor cells is to migrate and proliferate. Furthermore, the presence of these cells in ischemic conditions influences the secretion of paracrine factors that can positively affect the neighbouring cells, and surrounding tissue and matrix [8, 24, 55, 63].
In conclusion, we show that hCMPCs is a progenitor cell population able to withstand low oxygen tension. A low oxygen environment stimulated hCMPCs proliferation, migration and angiogenic secretion profile. Hypoxia induced TSP-2 expression in cardiac progenitor cells, affecting MMP-2 and MMP-9 and thereby the degradation of the ECM.
Acknowledgments
We thank Zhen Liu, Corina Metz, Joost Sluijter, Maarten van Dinther and Erik Meulmeester for their technical assistance and Peter ten Dijke and Noortje Bax for careful reading of the manuscript. This work was supported by a VIDI grant (016.056.319) from the Netherlands Organization for Scientific Research (NWO), SmartCare project of Biomedical Materials Program (BMM) and Center for Biomedical Genetics.
Conflict of interest
The authors confirm that there are no conflicts of interest.
Supporting Information
Hypoxia influences expression of paracrinefactors by hCMPCs. mRNA expression of VEGF-A (A) and PDGF-BB(B) and PlGF (C) was measured by RT-qPCR. Geneexpression patterns of five experiments using different primaryisolations are shown. Gene expression was normalized to day 0 andβ-actin was measured as a housekeeping gene.
Hypoxia induces expression of EMT markers byhCMPCs. EMT markers expressed by hCMPCs after 1 day culture undernormoxia and hypoxia were measured by RT-qPCR. Average of fourexperiments was represented in (A) for Snail, Slug andTWIST. Hypoxia resulted in a positive trend of Snail (B),Slug (C) and TWIST (D) mRNA expression. Gene expressionpatterns of four experiments using different primary isolations areshown. Gene expression was normalized to day 0 and β-actin wasmeasured as a housekeeping gene.
References
- 1.Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. Proc Natl Acad Sci USA. 2007;104:14068–73. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Messina E, De Angelis L, Frati G, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–21. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 3.Goumans MJ, de Boer TP, Smits AM, et al. TGF-beta1 induces efficient differentiation of human cardiomyocyte progenitor cells into functional cardiomyocytes in vitro. Stem Cell Res. 2007;1:138–49. doi: 10.1016/j.scr.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 4.van Oorschot AA, Smits AM, Goumans MJ. Stem cells: the building blocks to repair the injured heart. Panminerva Med. 2010;52:97–110. [PubMed] [Google Scholar]
- 5.Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kajstura J, Urbanek K, Perl S, et al. Cardiomyogenesis in the adult human heart. Circ Res. 2010;107:305–15. doi: 10.1161/CIRCRESAHA.110.223024. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Kook SH, Son YO, Lee KY, et al. Hypoxia affects positively the proliferation of bovine satellite cells and their myogenic differentiation through up-regulation of MyoD. Cell Biol Int. 2008;32:871–8. doi: 10.1016/j.cellbi.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 8.Nakanishi C, Yamagishi M, Yamahara K, et al. Activation of cardiac progenitor cells through paracrine effects of mesenchymal stem cells. Biochem Biophys Res Commun. 2008;374:11–6. doi: 10.1016/j.bbrc.2008.06.074. [DOI] [PubMed] [Google Scholar]
- 9.Oberringer M, Meins C, Bubel M, et al. In vitro wounding: effects of hypoxia and transforming growth factor beta1 on proliferation, migration and myofibroblastic differentiation in an endothelial cell-fibroblast co-culture model. J Mol Histol. 2008;39:37–47. doi: 10.1007/s10735-007-9124-3. [DOI] [PubMed] [Google Scholar]
- 10.Grayson WL, Zhao F, Bunnell B, et al. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358:948–53. doi: 10.1016/j.bbrc.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 11.Lennon DP, Edmison JM, Caplan AI. Cultivation of rat marrow-derived mesenchymal stem cells in reduced oxygen tension: effects on in vitro and in vivo osteochondrogenesis. J Cell Physiol. 2001;187:345–55. doi: 10.1002/jcp.1081. [DOI] [PubMed] [Google Scholar]
- 12.Csete M. Oxygen in the cultivation of stem cells. Ann NY Acad Sci. 2005;1049:1–8. doi: 10.1196/annals.1334.001. [DOI] [PubMed] [Google Scholar]
- 13.Csete M, Walikonis J, Slawny N, et al. Oxygen-mediated regulation of skeletal muscle satellite cell proliferation and adipogenesis in culture. J Cell Physiol. 2001;189:189–96. doi: 10.1002/jcp.10016. [DOI] [PubMed] [Google Scholar]
- 14.Studer L, Csete M, Lee SH, et al. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci. 2000;20:7377–83. doi: 10.1523/JNEUROSCI.20-19-07377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horie N, So K, Moriya T, et al. Effects of oxygen concentration on the proliferation and differentiation of mouse neural stem cells in vitro. Cell Mol Neurobiol. 2008;28:833–45. doi: 10.1007/s10571-007-9237-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ju SY, Cho KA, Cho SJ, et al. Effect of hypoxic treatment on bone marrow cells that are able to migrate to the injured liver. Cell Biol Int. 2009;33:31–5. doi: 10.1016/j.cellbi.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L. Prenatal hypoxia and cardiac programming. J Soc Gynecol Investig. 2005;12:2–13. doi: 10.1016/j.jsgi.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Rabelink TJ, de Boer HC, de Koning EJ, et al. Endothelial progenitor cells: more than an inflammatory response. Arterioscler Thromb Vasc Biol. 2004;24:834–8. doi: 10.1161/01.ATV.0000124891.57581.9f. [DOI] [PubMed] [Google Scholar]
- 19.Juhasz B, Der P, Szodoray P, et al. Adrenocorticotrope hormone fragment (4–10) attenuates the ischemia/reperfusion-induced cardiac injury in isolated rat hearts. Antioxid Redox Signal. 2007;9:1851–61. doi: 10.1089/ars.2006.1535. [DOI] [PubMed] [Google Scholar]
- 20.Liesmaa I, Leskinen HK, Kokkonen JO, et al. Hypoxia-induced expression of bradykinin type-2 receptors in endothelial cells triggers NO production, cell migration, and angiogenesis. J Cell Physiol. 2009;221:359–66. doi: 10.1002/jcp.21861. [DOI] [PubMed] [Google Scholar]
- 21.Lin Q, Lee YJ, Yun Z. Differentiation arrest by hypoxia. J Biol Chem. 2006;281:30678–83. doi: 10.1074/jbc.C600120200. [DOI] [PubMed] [Google Scholar]
- 22.Smits AM, van Vliet P, Metz CH, et al. Human cardiomyocyte progenitor cells differentiate into functional mature cardiomyocytes: an in vitro model for studying human cardiac physiology and pathophysiology. Nat Protoc. 2009;4:232–43. doi: 10.1038/nprot.2008.229. [DOI] [PubMed] [Google Scholar]
- 23.van Vliet P, Roccio M, Smits AM, et al. Progenitor cells isolated from the human heart: a potential cell source for regenerative therapy. Neth Heart J. 2008;16:163–9. doi: 10.1007/BF03086138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smits AM, van Laake LW, den Ouden K, et al. Human cardiomyocyte progenitor cell transplantation preserves long-term function of the infarcted mouse myocardium. Cardiovasc Res. 2009;83:527–35. doi: 10.1093/cvr/cvp146. [DOI] [PubMed] [Google Scholar]
- 25.Liu Z, Kobayashi K, van Dinther M, et al. VEGF and inhibitors of TGF{beta} type-I receptor kinase synergistically promote blood-vessel formation by inducing {alpha}5-integrin expression. J Cell Sci. 2009;122:3294–302. doi: 10.1242/jcs.048942. [DOI] [PubMed] [Google Scholar]
- 26.Pasterkamp G, Schoneveld AH, Hijnen DJ, et al. Atherosclerotic arterial remodeling and the localization of macrophages and matrix metalloproteases 1, 2 and 9 in the human coronary artery. Atherosclerosis. 2000;150:245–53. doi: 10.1016/s0021-9150(99)00371-8. [DOI] [PubMed] [Google Scholar]
- 27.Blankesteijn WM, Creemers E, Lutgens E, et al. Dynamics of cardiac wound healing following myocardial infarction: observations in genetically altered mice. Acta Physiol Scand. 2001;173:75–82. doi: 10.1046/j.1365-201X.2001.00887.x. [DOI] [PubMed] [Google Scholar]
- 28.Schroen B, Heymans S, Sharma U, et al. Thrombospondin-2 is essential for myocardial matrix integrity: increased expression identifies failure-prone cardiac hypertrophy. Circ Res. 2004;95:515–22. doi: 10.1161/01.RES.0000141019.20332.3e. [DOI] [PubMed] [Google Scholar]
- 29.Rousseau S, Houle F, Kotanides H, et al. Vascular endothelial growth factor (VEGF)-driven actin-based motility is mediated by VEGFR2 and requires concerted activation of stress-activated protein kinase 2 (SAPK2/p38) and geldanamycin-sensitive phosphorylation of focal adhesion kinase. J Biol Chem. 2000;275:10661–72. doi: 10.1074/jbc.275.14.10661. [DOI] [PubMed] [Google Scholar]
- 30.Fischer S, Clauss M, Wiesnet M, et al. Hypoxia induces permeability in brain microvessel endothelial cells via VEGF and NO. Am J Physiol. 1999;276:C812–20. doi: 10.1152/ajpcell.1999.276.4.C812. [DOI] [PubMed] [Google Scholar]
- 31.Namiki A, Brogi E, Kearney M, et al. Hypoxia induces vascular endothelial growth factor in cultured human endothelial cells. J Biol Chem. 1995;270:31189–95. doi: 10.1074/jbc.270.52.31189. [DOI] [PubMed] [Google Scholar]
- 32.Lee EY, Xia Y, Kim WS, et al. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen. 2009;17:540–7. doi: 10.1111/j.1524-475X.2009.00499.x. [DOI] [PubMed] [Google Scholar]
- 33.Jayachandran A, Konigshoff M, Yu H, et al. SNAI transcription factors mediate epithelial-mesenchymal transition in lung fibrosis. Thorax. 2009;64:1053–61. doi: 10.1136/thx.2009.121798. [DOI] [PubMed] [Google Scholar]
- 34.Lundgren K, Nordenskjold B, Landberg G. Hypoxia, Snail and incomplete epithelial-mesenchymal transition in breast cancer. Br J Cancer. 2009;101:1769–81. doi: 10.1038/sj.bjc.6605369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.VanSaun MN, Matrisian LM. Matrix metalloproteinases and cellular motility in development and disease. Birth Defects Res C Embryo Today. 2006;78:69–79. doi: 10.1002/bdrc.20061. [DOI] [PubMed] [Google Scholar]
- 36.Bellayr I, Mu X, Li Y. Biochemical insights into the role of matrix metalloproteinases in regeneration: challenges and recent developments. Future Med Chem. 2009;1:1095–111. doi: 10.4155/fmc.09.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Creemers EE, Cleutjens JP, Smits JF, et al. Matrix metalloproteinase inhibition after myocardial infarction: a new approach to prevent heart failure. Circ Res. 2001;89:201–10. doi: 10.1161/hh1501.094396. [DOI] [PubMed] [Google Scholar]
- 38.Vanhoutte D, Schellings M, Pinto Y, et al. Relevance of matrix metalloproteinases and their inhibitors after myocardial infarction: a temporal and spatial window. Cardiovasc Res. 2006;69:604–13. doi: 10.1016/j.cardiores.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Mannello F, Tonti GA, Bagnara GP, et al. Role and function of matrix metalloproteinases in the differentiation and biological characterization of mesenchymal stem cells. Stem Cells. 2006;24:475–81. doi: 10.1634/stemcells.2005-0333. [DOI] [PubMed] [Google Scholar]
- 40.Steingen C, Brenig F, Baumgartner L, et al. Characterization of key mechanisms in transmigration and invasion of mesenchymal stem cells. J Mol Cell Cardiol. 2008;44:1072–84. doi: 10.1016/j.yjmcc.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 41.Xu X, Xu Z, Xu Y, et al. Effects of mesenchymal stem cell transplantation on extracellular matrix after myocardial infarction in rats. Coron Artery Dis. 2005;16:245–55. doi: 10.1097/00019501-200506000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Mias C, Lairez O, Trouche E, et al. Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem Cells. 2009;27:2734–43. doi: 10.1002/stem.169. [DOI] [PubMed] [Google Scholar]
- 43.Perez-Ilzarbe M, Agbulut O, Pelacho B, et al. Characterization of the paracrine effects of human skeletal myoblasts transplanted in infarcted myocardium. Eur J Heart Fail. 2008;10:1065–72. doi: 10.1016/j.ejheart.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Maclauchlan S, Skokos EA, Agah A, et al. Enhanced angiogenesis and reduced contraction in thrombospondin-2-null wounds is associated with increased levels of matrix metalloproteinases-2 and -9, and soluble VEGF. J Histochem Cytochem. 2009;57:301–13. doi: 10.1369/jhc.2008.952689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Creemers EE, Pinto YM. Molecular mechanisms that control interstitial fibrosis in the pressure overloaded heart. Cardiovasc Res. 2011;89:265–72. doi: 10.1093/cvr/cvq308. [DOI] [PubMed] [Google Scholar]
- 46.Kyriakides TR, Wulsin D, Skokos EA, et al. Mice that lack matrix metalloproteinase-9 display delayed wound healing associated with delayed reepithelization and disordered collagen fibrillogenesis. Matrix Biol. 2009;28:65–73. doi: 10.1016/j.matbio.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goda N, Dozier SJ, Johnson RS. HIF-1 in cell cycle regulation, apoptosis, and tumor progression. Antioxid Redox Signal. 2003;5:467–73. doi: 10.1089/152308603768295212. [DOI] [PubMed] [Google Scholar]
- 48.Marx J. Cell biology. How cells endure low oxygen. Science. 2004;303:1454–6. doi: 10.1126/science.303.5663.1454. [DOI] [PubMed] [Google Scholar]
- 49.Reykdal S, Abboud C, Liesveld J. Effect of nitric oxide production and oxygen tension on progenitor preservation in ex vivo culture. Exp Hematol. 1999;27:441–50. doi: 10.1016/s0301-472x(98)00030-7. [DOI] [PubMed] [Google Scholar]
- 50.Potier E, Ferreira E, Andriamanalijaona R, et al. Hypoxia affects mesenchymal stromal cell osteogenic differentiation and angiogenic factor expression. Bone. 2007;40:1078–87. doi: 10.1016/j.bone.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 51.Gobble RM, Groesch KA, Chang M, et al. Differential regulation of human PlGF gene expression in trophoblast and nontrophoblast cells by oxygen tension. Placenta. 2009;30:869–75. doi: 10.1016/j.placenta.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carmeliet P, Moons L, Luttun A, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–83. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 53.Luttun A, Brusselmans K, Fukao H, et al. Loss of placental growth factor protects mice against vascular permeability in pathological conditions. Biochem Biophys Res Commun. 2002;295:428–34. doi: 10.1016/s0006-291x(02)00677-0. [DOI] [PubMed] [Google Scholar]
- 54.Thirunavukkarasu M, Penumathsa SV, Koneru S, et al. Resveratrol alleviates cardiac dysfunction in streptozotocin-induced diabetes: role of nitric oxide, thioredoxin, and heme oxygenase. Free Radic Biol Med. 2007;43:720–9. doi: 10.1016/j.freeradbiomed.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Winter EM, van Oorschot AA, Hogers B, et al. A new direction for cardiac regeneration therapy: application of synergistically acting epicardium-derived cells and cardiomyocyte progenitor cells. Circ Heart Fail. 2009;2:643–53. doi: 10.1161/CIRCHEARTFAILURE.108.843722. [DOI] [PubMed] [Google Scholar]
- 56.Frangogiannis NG. Chemokines in the ischemic myocardium: from inflammation to fibrosis. Inflamm Res. 2004;53:585–95. doi: 10.1007/s00011-004-1298-5. [DOI] [PubMed] [Google Scholar]
- 57.Galindo M, Santiago B, Alcami J, et al. Hypoxia induces expression of the chemokines monocyte chemoattractant protein-1 (MCP-1) and IL-8 in human dermal fibroblasts. Clin Exp Immunol. 2001;123:36–41. doi: 10.1046/j.1365-2249.2001.01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boyle JJ. Macrophage activation in atherosclerosis: pathogenesis and pharmacology of plaque rupture. Curr Vasc Pharmacol. 2005;3:63–8. doi: 10.2174/1570161052773861. [DOI] [PubMed] [Google Scholar]
- 59.Wahl SM, Hunt DA, Wakefield LM, et al. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci USA. 1987;84:5788–92. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fava RA, Olsen NJ, Postlethwaite AE, et al. Transforming growth factor beta 1 (TGF-beta 1) induced neutrophil recruitment to synovial tissues: implications for TGF-beta-driven synovial inflammation and hyperplasia. J Exp Med. 1991;173:1121–32. doi: 10.1084/jem.173.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frangogiannis NG, Ren G, Dewald O, et al. Critical role of endogenous thrombospondin-1 in preventing expansion of healing myocardial infarcts. Circulation. 2005;111:2935–42. doi: 10.1161/CIRCULATIONAHA.104.510354. [DOI] [PubMed] [Google Scholar]
- 62.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gnecchi M, He H, Noiseux N, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–9. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hypoxia influences expression of paracrinefactors by hCMPCs. mRNA expression of VEGF-A (A) and PDGF-BB(B) and PlGF (C) was measured by RT-qPCR. Geneexpression patterns of five experiments using different primaryisolations are shown. Gene expression was normalized to day 0 andβ-actin was measured as a housekeeping gene.
Hypoxia induces expression of EMT markers byhCMPCs. EMT markers expressed by hCMPCs after 1 day culture undernormoxia and hypoxia were measured by RT-qPCR. Average of fourexperiments was represented in (A) for Snail, Slug andTWIST. Hypoxia resulted in a positive trend of Snail (B),Slug (C) and TWIST (D) mRNA expression. Gene expressionpatterns of four experiments using different primary isolations areshown. Gene expression was normalized to day 0 and β-actin wasmeasured as a housekeeping gene.


