Abstract
Objective
We propose that metastatic epithelial ovarian cancer (EOC) is a potential therapeutic target for the oncolytic agent, Myxoma virus (MYXV).
Methods
Primary EOC cells were isolated from patient ascites and cultured as adherent cells or in suspension using Ultra Low-Attachment dishes. MYXV expressing green fluorescent protein was used to infect cells and spheroids. Infection was monitored by fluorescence microscopy, viral titering and immunoblotting for M-T7 and M130 virus protein expression, and cell viability by alamarBlue assay. Akti-1/2 (5 μM) and rapamycin (20 nM) were used to assay the role of PI3K–AKT signaling in mediating MYXV infection.
Results
Ascites-derived EOC cells grown in adherent culture are effectively killed by MYXV infection. EOC cells grown in suspension to form three-dimensional EOC spheroids readily permit MYXV entry into cells, yet are protected from the cytopathic effects of late MYXV infection. Upon reattachment (to model secondary metastasis), EOC spheroids are re sensitized to MYXV-mediated oncolysis. The critical determinant that facilitates efficient MYXV infection is the presence of an activated PI3K-AKT signaling pathway. Treatment with the specific AKT inhibitor Akti-1/2 reduces infection of monolayer EOC cells and spheroids. Direct infection of freshly collected ascites demonstrated that 54.5% of patient samples were sensitive to MYXV-mediated oncolytic cell killing. We also demonstrate that factor(s) present in ascites may negatively impact MYXV infection and oncolysis of EOC cells, which may be due to a down-regulation in endogenous AKT activity.
Conclusions
Differential activity of AKT serves as the mechanistic basis for regulating MYXV-mediated oncolysis of EOC spheroids during key steps of the metastatic program. In addition, we provide the first evidence that MYXV oncolytic therapy may be efficacious for a significant proportion of ovarian cancer patients with metastatic disease.
Keywords: Ovarian cancer, Ascites, Spheroid, Oncolytic virus, Myxoma virus, AKT kinase
Introduction
Ovarian cancer is the sixth most prevalent cancer in women and the most lethal of the gynecologic malignancies [1]. Epithelial ovarian cancer (EOC) constitutes approximately 90% of ovarian tumors, and is believed to originate from the ovarian surface epithelium (OSE) [2] although evidence for alternative origins is emerging [3]. Metastasis of EOC is unique in that it typically spreads by direct dissemination or shedding of cancer cells from the primary tumor site into the peritoneal space and secondary tumor formation on serosal surfaces of the peritoneal cavity [4]. Although about three-quarters of EOC patients treated with chemotherapy are initially responsive, most of these women will ultimately relapse with chemo-resistant disease. Thus, it is critical that we develop new therapeutics to better eradicate meta-static EOC cells.
Clinical application of viruses for cancer treatment has been attempted for nearly a century with sporadic success, but there has been a resurgence of virotherapy for cancer over the last two decades [5]. The premise of viral oncolysis is that naturally-occurring or genetically engineered viruses will undergo their replicative lytic cycle preferentially within cancer cells. This selective tropism of oncolytic viruses for cancer cells is in large part due to deficient anti viral responses, genetic mutations, and aberrant signaling pathways which are inherent to cancer cells, yet function properly in normal cells [6]. Thus, cancer cells, including those from EOC, have the potential to be targeted and eliminated by oncolytic viruses while leaving normal cells unscathed. For example, conditionally replicating recombinant human adenovirus strategies were the most extensively studied in EOC [7]. Other viruses have been applied to EOC including reovirus [8], mea sles and mumps virus [9] and vesicular stomatitis virus [10,11]; and some have progressed to clinical trials for EOC [12 15]. Thus, there remains continued interest in identifying and developing potent oncolytic viral therapies for EOC [16].
A recent player in the viral therapy arena is the rabbit specific poxvirus, Myxoma virus (MYXV) [17]. Like all poxviruses, MYXV is able to bind and enter a wide variety of mammalian cells [18]. Current data indicates that productive MXV infection is highly dependent upon the ability of the virus to take control of specific signaling molecules in the host cell [19]. Specifically, MYXV encodes multiple host range fac tors, including M T5, which regulates tropism to cancer cells based on the presence of activated PI3K AKT signaling, a pathway commonly mutated or up regulated in human cancers [19,20]. MYXV infectivity profiles in human cancer cells can be divided into three distinct catego ries, designated as Types I (permissive), II (semi permissive, i.e. requires M T5 host range factor) or III (restrictive) [19,21]. Targeted inhibition of certain phosphatidylinositol 3 kinase (PI3K) AKT pathway components can boost AKT activity through feedback mechanisms, thus enhancing MYXV infectivity and improving oncolytic efficacy in both in vitro and in vivo cancer models [22 25].
To date only two reports indicate that MYXV can infect immortal ized human EOC cell lines [21,26], but there is no data indicating that this virus can infect primary human EOC cells isolated directly from pa tients. Thus, we sought to test the efficacy of MYXV mediated cell killing of ascites derived EOC cells using suspension culture and three dimensional multicellular spheroids as a model for metastatic disease. Herein, we describe novel data regarding differential MYXV oncolysis in adherent EOC cells compared with spheroids, which impinges on the underlying level of PI3K AKT pathway activity. In addition, we pro vide the first evidence of the therapeutic potential for MYXV oncolytic action on malignant ascites directly isolated from EOC patients.
Materials and methods
Cell culture
The human ovarian cancer cell lines SKOV3 and OVCAR3 were purchased from the American Type Culture Collection (ATCC; Manassas, VA) and cultured in Dulbecco's modified Eagle's medium (DMEM) (Wi sent, St. Bruno, Canada) supplemented with 5% fetal bovine serum (FBS; Wisent). OVCA429 cells (gift of Dr. Barbara Vanderhyden, OHRI) were maintained in alpha MEM with 5% FBS and 1 × non essential amino acids. OVCAR3 cells were purchased from ATCC and cultured in RPMI medium containing 5% FBS. The 4306 mouse ovarian cancer cell line (gift of Dr. Daniela Dinulescu, Brigham & Women's Hospital) and baby green monkey kidney (BGMK) cells were cultured in DMEM supple mented with 5% FBS. Cells were maintained in a 37 °C humidified atmo sphere of 95% air and 5% CO2.
Ascitic fluid collected from chemotherapy naïve patients at time of paracentesis or debulking surgery was used to generate primary human EOC cell cultures as described previously [27]. Primary cultures of ascites cells or spheroids isolated from ascites were main tained in a 1:1 mixture of MCDB 105 medium: medium 199 medium (Sigma, St. Lewis, MO) supplemented with 10% FBS (Wisent) and 50 μg/mL penicillin streptomycin (Wisent). Clarified ascites fluid was prepared by centrifugation at 1500 rpm for 5 min to remove cells and used immediately, or stored at − 80 °C for subsequent use. All work with patient materials has been reviewed and approved by The University of Western Ontario Human Subjects Research Ethics Board. Adherent cells were maintained on tissue culture treated polystyrene (Sarstedt, Newton, NC). Non adherent cells and spheroids were maintained on Ultra Low Attachment (ULA®) cultureware (Corning, Corning, NY), which is coated with a hydrophilic, neutrally charged hydrogel to prevent cell attachment. Single cell suspensions of 1 × 105 cells/mL were seeded to ULA® plates to establish suspension cultures and to form spheroids. Spheroids were reattached to standard tissue culture plastic by transferring spheroids in a volume of b 200 μL from individual wells of 24 well ULA® cluster dishes to individual wells of 6 well tissue culture treated plates containing 2 mL of com plete growth medium.
Myxoma virus infection
Preparation and titering of MYXV (i.e. vMyxGFP [28]) using BGMK cells were performed as described previously [29]. For preparation of UV inactivated MYXV, virus stock was UV irradiated for 5 min using a Stratalinker UV crosslinker (Stratagene); this incubation time was deter mined empirically to inactivate vMyxGFP to undetectable fluorescence in BGMK cells at MOI of 10.
Primary EOC cells and cell lines were infected with MYXV by add ing serially diluted virus (diluted in growth media) to cells to achieve an MOI of 0.1, 1, or 10, as indicated in each experiment. For infection of adherent monolayer EOC cells, cells were seeded 24 h prior to MYXV infection. For infection of EOC cells in suspension culture, MYXV was added immediately upon seeding cells to wells of ULA® plates. For infection of EOC spheroids, cells were seeded to wells of ULA® plates, allowed to form spheroids for 3 days, and then treated with MYXV. Virus infection was monitored over 24 96 h by phase contrast and fluorescence microscopy using an Olympus IX70 inverted microscope and photos were acquired using ImagePro image capture software.
Direct infection of patient derived ascites with MYXV was per formed by transferring 2 mL of fluid into each well of a 6 well tissue culture treated dish, followed by addition of 105, 106 or 107 ffu, as de termined by titering in BGMK cells, or the equivalent of 107 ffu of UV irradiated control virus. Two wells remained as negative controls without any virus treatment. After 3 4 days, ascites fluid was replaced with MCDB 105:M199 medium containing 10% FBS and 50 μg/mL penicillin/streptomycin, as per standard primary culture of EOC cells, and replaced every 3 4 days as needed. The experiment ter minated when cells in the negative control wells reached 100% confluence, at which point cells were stained using the Hema 3 Stain kit.
Viability assays
Cell viability of MYXV infected cells and spheroids was assessed using alamarBlue® (Invitrogen, Burlington, ON) as per manufacturer's instructions. For adherent monolayer cells, medium was replaced with alamarBlue® reagent diluted 1:20 in complete medium, incubated for 4 h at 37 °C in a humidified atmosphere of 95% air and 5% CO2, and then fluorescence was quantified with a Wallac plate reader using 560/590 nm excitation/emission filter settings. For suspension and spheroid cultures, 50% of the culture medium (500 μL per well of 24 well plate) was removed carefully and an equal volume of alamar Blue® reagent diluted 1:10 in complete medium was added; analysis was performed as described above.
Immunoblotting
Cell lysates were obtained using a modified radioimmunoprecipi tation assay (RIPA) buffer [50 nM HEPES pH7.4, 150 nM NaCl, 10% glycerol, 1.5 mM MgCl2, 1 nM EGTA, 1 nM sodium orthovanadate, 10 mM sodium pyrophosphate, 10 mM NaF, 1% Triton X 100, 1% sodium deoxycholate, 0.1% SDS, 1 mM PMSF, 1× protease inhibitor cocktail (Roche, Laval, QC)] as described previously[30]. To ensure complete dis sociation of spheroids, lysates were vortexed several times during lysis on ice prior to high speed centrifugation. Protein concentration of each lysate was determined by Bradford assay using Protein Assay Dye Reagent (BioRad, Mississauga, ON). Total protein (10 30 μg) was resolved by 8% or 14% SDS PAGE, transferred to a polyvinylidene difluoride mem brane (PVDF; Roche), blocked with 5% skim milk in Tris buffered saline with Tween 20 [TBST; 10 mM Tris HCl, pH 8.0, 150 mM NaCl, 0.1% Tween 20]. Membranes were washed in TBST and incubated overnight at 4 °C with specific antibodies (1:1000 in 5% skim milk/TBST or 5% BSA/TBST as per manufacturer's recommendations). Immunoreactive bands were visualized by incubating for 1 h at room temperature with a peroxidase conjugated anti rabbit or anti mouse IgG (1:10 000 in 1% skim milk/TBST; GE Healthcare) followed by enhanced chemilumines cence reagent (ECL Plus; GE Healthcare) and exposure to Kodak Biomax X ray film (GE Healthcare).
Antibodies and other reagents
Antibodies against phospho AKT Ser473 (#9271) and phospho p70S6K (#9234) were obtained from Cell Signaling Technology (Danvers, MA). Antibodies against total Akt1/2/3 (H 136; sc 8312) and p70S6K (S 04; sc100423) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against M T7 and M130 have been described previously [20,31]. Anti actin antibody (A2066; Sigma) served as a pro tein loading control. AKT inhibitor VIII (Akti 1/2; EMD/Calbiochem) and rapamycin (R0395; Sigma) were prepared according to manufacturer's instructions.
Spheroid dispersion assay
Spheroids were transferred in a volume of b 200 μL from individual wells of 24 well ULA® cluster dishes to individual wells of 6 well tissue culture treated plates containing 2 mL of complete growth medium. Reattachment was allowed to proceed for up to three days with images cap tured during this time by phase contrast and fluorescence microscopy using an Olympus inverted microscope and ImagePro image capture soft ware. After 3 days, dispersing spheroids were fixed and stained using Hema 3 Stain kit (Fisher Scientific) and images were captured of inde pendent reattached spheroids using an Olympus stereomicroscope and ImagePro image capture software. The dispersion area was quantified for each image using NIH ImageJ (NIH, Bethesda, MD) area measurement tool.
Results
MYXV infection of EOC cells and spheroids
There is limited evidence in the literature that MYXV is capable of infecting established human ovarian cancer cell lines indicating that they are either permissive or semi permissive to complete oncolysis [21,26]. To significantly add to these data, we performed direct analysis of primary EOC cells derived from patient ascites and have also broadened this approach by applying MYXV infection studies to both adherent as well as suspension and spheroid culture systems to mimic EOC dissemi nation in ascites fluid in vitro. We have determined empirically that seed ing 50,000 cells per milliliter using Ultra Low Attachment (ULA®) tissue culture plastic results in multi cellular spheroids that closely resemble those observed in patient derived ascites. Ovarian cancer cell lines, such as SkOV3, OVCA429, OVCAR3 and 4306 cells used in this study, can also be used to generate spheroids using the same approach.
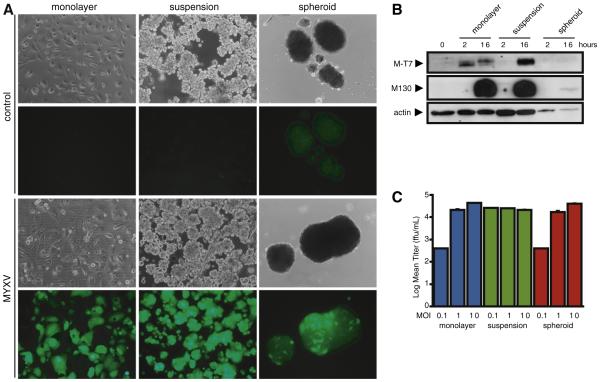
Adherent primary EOC cells are readily infected by MYXV within 24 h at an MOI of 1, as evidenced by visualization of green fluorescent protein (GFP) expression from the vMyxGFP recombinant virus, and eventually undergo oncolysis as demonstrated by cytopathic effect by 72 h post infection (Fig. 1A). Entry of MYXV into suspension cells is also efficient and comparable to adherent cells, with all visible cells and small aggregates exhibiting GFP positive fluorescence (MOI of 1 at 24 h post infection). Interestingly, under these same infection con ditions, established EOC spheroids have a markedly reduced ability to be infected by MYXV. This phenomenon is diminished over time with EOC cells comprising large spheroids efficiently infected by MYXV (91.8% ± 8.4%) as quantified by disaggregating multicellular spheroids using trypsin digestion 72 h post infection and visualizing fluorescent single cells that had formerly comprised the interior of spheroids (Supplementary Fig. S1).
Fig. 1.
EOC spheroids have reduced MYXV infection kinetics compared to monolayer and suspension cultures. (A) Adherent monolayer, suspension cells, and 3-day EOC spheroids were infected with MYXV (MOI of 1), or UV-inactivated MYXV as a control. Phase contrast and fluorescence images were captured 72 h post-infection to assess MYXV infection. Cytopathic effect was noted in MYXV-infected adherent monolayer cultures as characterized by rounding up of cells. (B) Western blotting was performed to determine expression of MYXV-specific M-T7 (early) and M130 (late) proteins. Reduced expression of both M-T7 and M130 was observed in MYXV-infected EOC spheroids compared to monolayer and suspension cultures. Actin served as a loading control. (C) MYXV was collected at 96 h post-infection of monolayer, suspension and spheroid cultures and titered using BGMK cells. Comparable levels of infectious MYXV were collected in each culture condition.
We used Western blot detection of two virus specific proteins, M T7, an early protein[20] and M130, a late protein [31] to further confirm that MYXV was infecting primary EOC cells in monolayer, suspension and spheroids and to examine the apparent retarded ki netics of infection in established spheroids. Both adherent and sus pension EOC cells expressed M T7 and M130 proteins within hours after infection, with a slightly faster rate of early protein synthesis after infection in adherent cells as evidenced by detectable M T7 ex pression (Fig. 1B). By 16 h, however, both adherent and suspension cells exhibit detectable M T7 expression and robust M130 protein levels. In contrast, expression of both M T7 and M130 proteins is largely undetectable 16 h after infection of established EOC spheroids. This reduced expression was observed in primary EOC cells (n = 2), the SkOV3 cell line, and in the 4306 mouse ovarian tumor cell line (data not shown).
These results indicate that, compared to adherent and suspension cells, EOC spheroids exhibit early resistance to MYXV infection. After prolonged infection, however, the entire EOC spheroid became GFP positive. In order to determine whether there was virus replication and progeny virus production, infectious particles were quantified after complete infection. Lysates and culture supernatant were collected from adherent, suspension and spheroid cultures 96 h after MYXV in fection and titered using permissive baby green monkey kidney (BGMK) cells. Although the kinetics of MYXV infection progression appeared delayed in EOC spheroids, there was no difference in final MYXV production among the three culture conditions (Fig. 1C).
Given that single EOC cells were readily infected in adherent and suspension culture, yet large spheroids showed delayed progression of infection, we postulated that MYXV penetration into spheroids was largely determined by the overall size of the multicellular aggre gates. By using three different amounts of MYXV (MOI of 0.1, 1 and 10), we infected established EOC spheroids of various sizes ranging from 10 to 100 μm in diameter, and observed an inverse correlation between the rate of MYXV infection and increasing spheroid size (Supplementary Fig. S2).
EOC spheroids are resistant to MYXV mediated cell killing
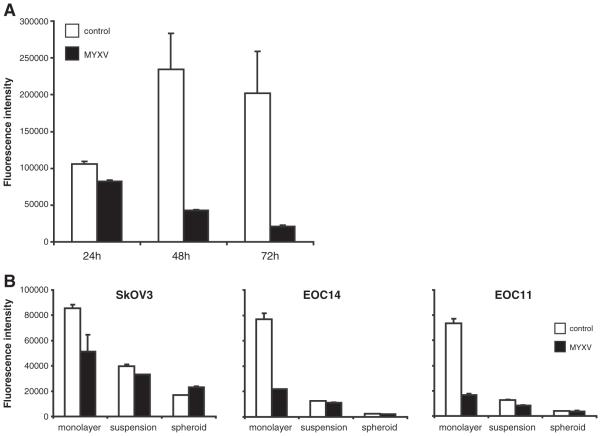
Primary human EOC cells and ovarian cancer cell lines are readily infected and exhibit cytopathic effects when grown as adherent cul tures. To determine that this results in reduced viability and oncolytic cell death, we performed alamarBlue® cell viability assays over 3 days post infection. Adherent primary EOC cells displayed a marked reduction in cell viability over the 3 days post infection, compared to the continued growth and viability of UV irradiated MYXV treated cells (Fig. 2A). This reduced viability was observed in MYXV infected SkOV3 cell line and the 4306 mouse ovarian tumor cell line, as well (Fig. 2B, and data not shown). To determine if EOC cells in suspension and as spheroids are also sensitive to MYXV mediated cell killing, we performed alamarBlue® assays on cells seeded to ULA® plates after 3 days of infection. In fact, we did not observe any further reduction in cell viability due to MYXV infection (Fig. 2B). This indicates that, although MYXV can enter and produce virus in EOC suspension cells and spheroids, it is unable to reduce the overall bulk viability (i.e. induce oncolysis) of cells under these conditions.
Fig. 2.
MYXV infection does not affect viability of EOC cells in suspension or as spheroids. (A) Cell viability was assessed using alamarBlue® reagent after 24, 48 and 72 h of MYXV infection (MOI of 1) in primary EOC cells grown in monolayer culture. MYXV infection decreased cell viability as compared to cells treated with UV-irradiated MYXV control. (B) Cell viability was assessed after 72 h of MYXV infection (MOI of 1) in SkOV3 cells and two ascites-derived primary EOC samples (EOC14 and EOC11) grown in monolayer, suspension and spheroid cultures. MYXV infection decreased cell viability in all monolayer EOC cell samples, yet no effect was observed in suspension and spheroid cultures.
Reattached EOC spheroids regain sensitivity to MYXV oncolysis
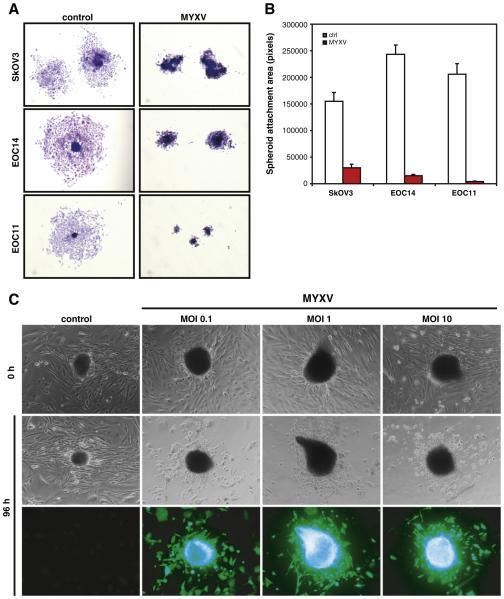
The ability of EOC cells and spheroids to establish and grow as sec ondary metastases in the peritoneal cavity requires successful attach ment to the surface of abdominal organs. To determine whether MYXV infected EOC spheroids are capable of performing this step of the metastatic process, we re seeded infected EOC spheroids to standard tissue culture plastic. EOC spheroids derived from the SkOV3 cell line and primary EOC cells when treated with UV irradiated MYXV readily attached and cells efficiently dispersed from the central spheroid after 3 days (Fig. 3A). In contrast, EOC spheroids that were infected with live MYXV while in suspension had a marked reduction in their ability to both adhere and disperse upon reattachment (Fig. 3A,B). SkOV3 spheroid cell dispersion was reduced by 80.7% by MYXV infection, and this was even more pronounced in primary EOC cells with a 93.9 98.0% reduction compared to mock infected controls (Fig. 3B).
Fig. 3.
MYXV readily infects and kills attached EOC spheroids. (A) SkOV3, EOC14 and EOC11 spheroids were infected with MYXV (MOI of 1) or UV-irradiated control (ctrl) and 72 h post-infection were transferred to standard tissue culture plastic to facilitate reattachment. Three days post-reattachment attached spheroids were fixed and stained to visualize EOC cell dispersion. MYXV infection substantially reduced the number of cells migrating away from the attached spheroids compared to control infected spheroids. (B) Dispersion area was quantified from images of 12–20 attached spheroids per sample and treatment using ImageJ software. (C) Primary EOC spheroids were plated to standard tissue culture plastic to facilitate reattachment, and subsequently infected with MYXV (MOI of 0.1, 1 and 10) or UV-irradiated control (ctrl). Phase contrast images were captured prior to infection and at 96 h post-infection, along with fluorescence microscopy to visualize infected cells. Cytopathic effect was evident in the dispersing cells; the attached, intact spheroids permitted MYXV entry, as well.
We then sought to determine whether EOC spheroids that have al ready attached and are actively growing and dispersing are now capable of being infected by MYXV, as well. Established EOC spheroids were seeded to standard tissue culture plastic and allowed to reattach and disperse for 72 h before initiation of infection with MYXV or control virus. Both the spheroid (still present in most cases) as well as dispersing EOC cells were readily infected by MYXV even at a low MOI of infection. However, only the adherent cells emanating from the spheroid were rendered susceptible to MYXV mediated cell killing as evidenced by cytopathic effect (Fig. 3C).
MYXV mediated oncolysis requires active AKT signaling
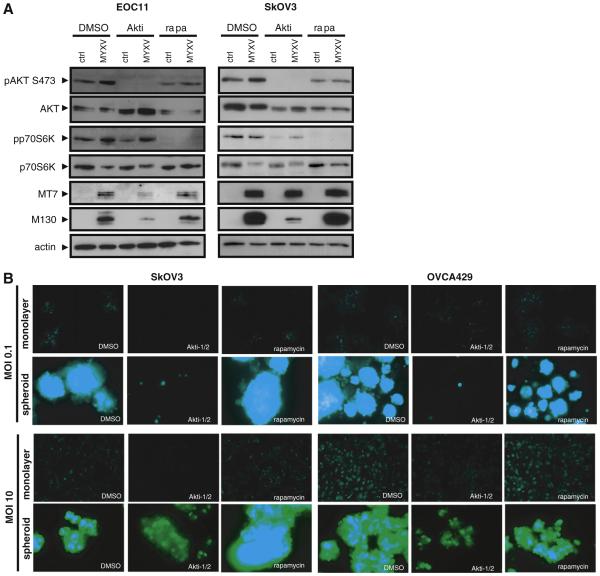
Wang and colleagues [21] originally showed that activated AKT kinase activity is required for efficient MYXV mediated oncolysis of human cancer cells. Interestingly, our laboratory has demonstrated recently that endogenous AKT activity is reduced in EOC cells upon spheroid formation [32], thus providing a potential explanation for the reduced kinetics of MYXV infection in spheroids and the attenuated oncolytic response. To directly determine whether MYXV infection influences AKT activity in EOC cells, we prepared total protein lysates from control and MYXV infected cells for immunoblot analysis. AKT phosphorylation at serine 473, a modification indicative of activation of this pathway, is induced within 2 h post infection (Supplementary Fig. S3a); this is observed in both adherent and suspension EOC cells, but delayed in EOC spheroids (Supplementary Fig. S3b) MYXV infection was confirmed by detection of early M T7 and late M130 viral protein expression. The increased level of AKT phosphorylation in adherent primary EOC cells in response to MYXV infection was blocked by treatment with the specific AKT kinase inhibitor Akti 1/2 (5 μM; Fig. 4A). This was further reflected by reduced M T7 and M130 protein expression levels. However, we observed that even in the presence of the AKT inhibitor, MYXV infection was still capable of in creasing p70 S6 kinase phosphorylation. This suggests that MYXV can utilize an alternative, AKT independent mechanism for activation of p70 S6 kinase during infection.
Fig. 4.
AKT activity is required for efficient MYXV infection of EOC cells and spheroids. (A) Western blotting was performed to determine expression of phosphorylated AKT kinase and phosphorylated p70 S6 kinase at 24 h post-MYXV infection of primary EOC11 cells and SkOV3 cells grown in monolayer culture and treated with the AKT inhibitor Akti-1/2 (5 μM), the mTOR inhibitor rapamycin (20 nM), or DMSO vehicle control. Phosphorylated AKT was completely absent in Akti-1/2 treated cells and this markedly decreased MYXV infection as determined by M-T7 and M130 expression. MYXV infection increased phospho-p70S6 kinase, which is unaltered by Akti-1/2 treatment. Rapamycin treatment did not affect MYXV infection, but was effective at reducing phospho-p70 S6 kinase levels to below detection. Actin served as a loading control. (B) SkOV3 and OVCA429 monolayer cells and spheroids were infected with MYXV (MOI of 0.1 and 10) treated with the AKT inhibitor Akti-1/2 (5 μM), the mTOR inhibitor rapamycin (20 nM), or DMSO vehicle control. Images were captured by fluorescence microscopy at 96 h post-infection for MOI of 0.1 and 24 h post-infection at an MOI of 10. Treatment with Akti-1/2 decreased the efficiency of MYXV infection in both monolayer and spheroids when compared to rapamycin treatment and DMSO controls as determined by intensity and degree of fluorescence.
Rapamycin has been shown to increase MYXV infection of human cancer cells, particularly those cells that are restrictive to MYXV oncoly sis, due to compensatory feedback activation of AKT [22,33]. To deter mine whether rapamycin may enhance MYXV oncolysis, we treated primary EOC cells and cell lines with rapamycin (20 nM) and performed immunoblot assays to assess AKT activity. As expected, rapamycin treat ment was effective at reducing phosphorylated p70 S6 kinase levels (both basal and MYXV induced), indicating that the drug was functional under our culture conditions (Fig. 4A). However, for the most part, rapa mycin had little effect on AKT phosphorylation after MYXV infection as well as on the expression of MYXV proteins M T7 and M130.
To determine the requirement of functional AKT activity in EOC cells and spheroids during MYXV infection, adherent EOC cells or established spheroids were infected with MYXV in the presence of 5 μM Akti 1/2 or DMSO vehicle control. We consistently observed that Akti 1/2 treatment reduced MYXV infection (as observed by GFP expression using fluorescence microscopy) of both adherent pri mary EOC cells and spheroids. This was tested in three different cell lines (SkOV3, OVCA429, OVCAR3) and with two different doses of MYXV (MOI of 0.1 and 10) (Fig. 4B, and data not shown). Contrary to the results of blocking AKT activity, inhibition of mTOR activity using rapamycin did not have a detectable effect on MYXV infection in terms of promoting or inhibiting infection (Fig. 4B). This correlates well with the immunoblotting results for M T7 and M130 expression, where rapamycin did not exhibit any effect (Fig. 4A). Taken together these data imply that MYXV infection of primary EOC cells and spheroids requires AKT activation; however, the positive effect of rapamycin on this process observed in other classes of cancer cells does not appear opera tive in human EOC cells or spheroids.
Efficacy of MYXV oncolysis on direct EOC ascites samples
To function as a therapeutic MYXV must infect EOC tumor cells within the peritoneal cavity and likely within the environment of ma lignant ascites fluid. We assessed the effect of MYXV treatment on 22 independent patient ascites samples immediately upon collection. Within this cohort of patient samples, 16 represent women diagnosed and clinically confirmed to have EOC (Table 1). As expected, the major ity of samples represent EOC of the most common histotype of serous adenocarcinoma. However, we received and tested ascites from other gynecologic malignancies (n = 5) within this sample set. Ascites fluid containing tumor cells was seeded to tissue culture dishes and immedi ately treated with three increasing doses of MYXV [105, 106 and 107 foci forming units (ffu) per 35 mm well] or with the equivalent of a 107 dose of UV irradiated control virus. Note that the concentration and viability of EOC cells are unknown since we seeded ascites directly; therefore, we do not refer to MOI, but rather number of viral infectious units applied per well of ascites fluid (2 mL volume per well). From this experiment, we observed that 12 out of 22 samples demonstrated a qualitative and quantitative level of response to MYXV infection, as compared with controls (Table 1 and Supplementary Fig. S4). From this sample set, 3 were considered highly sensitive as illustrated by efficacy at all three MYXV doses, 3 were moderately sensitive, and 6 were weakly sensitive (Table 1). Among the 22 samples tested in this exper iment, 10 appeared resistant to MYXV infection, at least to the doses that were used. With respect to clinical data, however, there does not appear to be a correlation between MYXV oncolytic sensitivity and tumor histotype, stage or grade.
Table 1.
Summary of clinical data for ascites samples used in direct MYXV infection experiments.
| Sample | Age | Site | Stage | Histotype and grade | Infectivity scorea |
|---|---|---|---|---|---|
| EOC59 | 47 | Ovarian | IIIc | Serous adenoca, grade 3 | + |
| EOC61 | 78 | Ovarian | IIIc | Serous adenoca, grade 3 | − |
| EOC65 | 67 | Ovarian | IV | Serous adenoca, grade 3 | − |
| EOC67 | 51 | Ovarian | IIIc | Serous adenoca, grade 2 | +++ |
| EOC72 | 77 | Ovarian | IIIc | Serous adenoca, grade 3 | + |
| EOC74 | 69 | CMML, ITPb | n.a. | n.a. | ++ |
| EOC75 | 77 | Ovarian | IIIc | High grade pap serous | + |
| EOC81 | 69 | Ovarian | n.a. | Mucinous borderline | − |
| EOC83 | 69 | Fallopian tube | IV | Poorly differentiated pap adenoca |
++ |
| EOC84 | 68 | Endometrium | IV | Serous adenoca | − |
| EOC85 | 49 | Ovarian | IIIc | Pap serous, grade 3 | − |
| EOC95 | 79 | Peritoneal carcinomatosisc |
n.d. | n.d. | + |
| EOC96 | 52 | Ovarian | IIIc | Serous adenoca, grade 3 | − |
| EOC98 | 51 | Ovarian | IIIc | Serous adenoca, grade 3 | ++ |
| EOC100 | 65 | Endometrium | IV | High-grade carcinoma | − |
| EOC101 | 43 | Ovarian | IIIc | Serous adenoca, grade 3 | +++ |
| EOC103 | 64 | Ovarian | IIIc | Carcinosarcoma | + |
| EOC106 | 61 | Ovarian | IIIc | Serous adenoca, grade 1 | − |
| EOC107 | 46 | Cervix | II | Mucinous adenoca, intestinal type |
+ |
| EOC108 | 70 | Ovarian | IIIc | Serous adenoca, grade 3 | − |
| EOC109 | 56 | Ovarian | IIIc | Endometrioid adenoca, grade 2 | − |
| EOC110 | 45 | Endometrium and ovariand |
IIb | Endometrioid adenocarcinoma, grade 1 ovarian adenoca, grade 1 |
+++ |
n.a.: not applicable
n.d.: not determined
Infectivity Score: −, no infection at all three doses; +, infection at one (highest) dose; ++, infection at two doses; +++, infection at all three doses; see Supplementary Fig. S3.
CMML, ITP: chronic myelomonocytic leukemia with idiopathic thrombocytopenia purpura.
Patient EOC95 diagnosed with peritoneal carcinomatosis, likely ovarian; chemotherapy only, therefore was not staged.
Patient EOC110 had synchronous primaries of the endometrium and right ovary; staging is based on endometrium.
To further investigate the mechanisms underlying differential sen sitivity of primary EOC samples to MYXV infection, we performed western blotting against phosphorylated AKT on protein isolated from a subset of the clinical specimens. We selected four representing MYXV resistant samples (EOC61, EOC65, EOC81 and EOC100) and six that were sensitive to MYXV oncolysis to different degrees (low: EOC75 and EOC103; intermediate: EOC74 and EOC98; high: EOC67 and EOC101). From this selective analysis, however, we did not ob serve any correlation in endogenous expression levels of either phos phorylated AKT or total AKT protein and the inherent sensitivity to MYXV mediated oncolysis by direct infection of patient ascites (Supplementary Fig. S5).
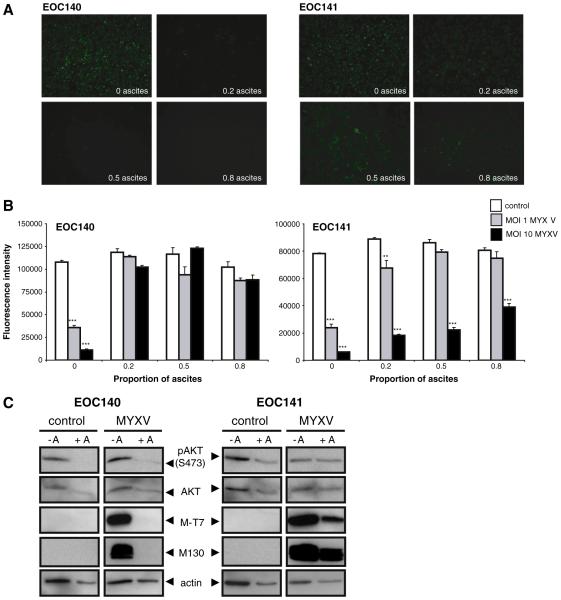
We then postulated that acellular factors in the ascites fluid may impact the ability of MYXV to infect EOC cells. To test this experimen tally, we selected two independent patient samples and their matched clarified ascites fluid (i.e. cellular constituents were removed by centri fugation) and performed a MYXV infection with increasing concentra tion of ascites fluid. Indeed, MYXV infection (MOI of 1 and 10) was dramatically reduced in EOC140 in the presence of clarified ascites at all concentrations tested (Fig. 5A). In EOC141, infection and MYXV mediated oncolysis were largely unaffected by the presence of clarified ascites, although a dose dependent increase in cell viability was ob served at an MOI of 1 with increasing ascites concentration (Fig. 5A, B). Analysis of phosphorylated AKT levels in response to ascites treat ment indicated that down regulation of AKT activity may contribute, at least in part, to the decreased MYXV oncolysis observed in EOC140 as compared to EOC141 (Fig. 5C). MYXV infection was confirmed by detection of M T7 and M130 proteins in EOC141 lysates in both the presence and absence of ascites, whereas these viral proteins were only detected in EOC140 lysates in the absence of ascites treatment.
Fig. 5.
Ascites may reduce the efficiency of MYXV infection and oncolysis of primary EOC cells. Two independent primary EOC samples (EOC140 and EOC141) were infected with MYXV at an MOI of 1 and 10, or UV-inactivated control virus, while in the presence of clarified ascites (0, 20, 50 or 80% diluted in complete media) which was matched to the same patient. (A) Marked reduction of MYXV infection (MOI 10) was observed in EOC140 at all ascites concentrations tested when compared with complete media alone control (0 as- cites); ascites had little to no observable effect on infection in EOC141. (B) MYXV-mediated oncolysis of EOC140 cells was inhibited by the presence of ascites at all concentrations tested for both MYXV doses when compared with complete media alone control, as determined by alamarBlue® assay after 3 days of treatment. Reduction in EOC141 cell viability by MYXV oncolysis was largely unaffected at an MOI of 10, whereas oncolysis at the lower dose (MOI 1) was inhibited by increasing concentration of ascites. Note that ascites fluid alone (control, UV-inactivated virus) did not affect the overall viability of EOC cells over the 3-day treatment period at all concentrations tested. (C) Western blot analysis was per- formed using lysates from EOC140 and EOC141 cells to determine phospho-AKT (Ser473) and total AKT protein expression levels in the presence (+ A) or absence ( A) of ascites (20% in complete media) after 24-hour treatment and concomitant infection with MYXV at an MOI of 1. Detection of M-T7 and M130 proteins confirmed MYXV infection of EOC cells in the absence of ascites, whereas M-T7 and M130 were only present in EOC141 cells in the presence of ascites, which correlates with cell viability data.
Taken together, these data imply that a subset of EOC, and perhaps other gynecologic malignancies, are sensitive to MYXV oncolysis, with the important implication that intracellular AKT activity levels in con cert with putative acellular mediators present in malignant ascites can impact its potential as an anticancer therapeutic.
Discussion
Our results provide the first evidence of MYXV mediated oncolytic effects using primary human EOC cells. Previously, a few reports have demonstrated that select established human ovarian cancer cell lines are infected by MYXV [21,26], however direct testing of MYXV using primary tumor material had been lacking. Access and application of viral oncolytics using patient derived tumor cells are a common hurdle of most studies both in fundamental and preclinical research. Even more importantly, we have tested MYXV efficacy using a relevant cell culture model system that mimics the mechanisms of metastasis, as well as tumor pathobiology in a three dimensional context. By using this novel system to perform MYXV oncolytic testing in cell culture, we have discovered unique characteristics that directly impact the life cycle of MYXV infection and its potential efficacy as an anti cancer therapeutic.
Metastasis of EOC by direct dissemination of tumor cells into the peritoneal cavity and subsequent formation of multicellular spheroids is thought to be a critical step in the spread of malignant cells [34]. We have recently observed that EOC cells present in multicellular spheroids undergo a state of dormancy where cell proliferation is markedly reduced [32], a phenotype which would render EOC spher oids relatively resistant to standard cytotoxic chemotherapeutics. Our results herein indicate that dormant EOC spheroids are effectively tar geted by MYXV; however, this occurs with slower kinetics and does not result in efficient cell death until reattachment occurs. Interest ingly, our data also points to the notion that AKT activity may be in part responsible for efficient establishment of infection or spread of MYXV, since EOC spheroids, which possess decreased endogenous levels of phospho AKT, experience reduced kinetics of MYXV infec tion. Furthermore, we have data clearly demonstrating that AKT ac tivity is required during EOC spheroid reattachment particularly for re initiation of cell proliferation [32]. This result supports the notion that reactivation of AKT activity is likely the essential requirement for oncolytic efficacy of MYXV in EOC spheroids. Thus, it is not sur prising that MYXV, already present in the infected yet dormant EOC cells, is able to efficiently complete the oncolytic program once AKT activity is re established upon spheroid reattachment. This phenom enon is also relevant to EOC pathogenesis, since reattachment of spheroids is a likely mechanism of EOC secondary metastasis [34]. Thus, these data clearly demonstrate the potential therapeutic efficacy of MYXV infection in targeting and killing secondary EOC metastases.
There is a well established significant role for the PI3K AKT mTOR pathway in human EOC [35 38]. Genes encoding several components of this pathway are commonly mutated in EOC tumors. For example, amplification of the genes PIK3CA, encoding the catalytic subunit of PI3K, and AKT2, its downstream kinase, are frequently observed [29 31]. Inactivating mutations in the PTEN tumor suppressor gene has also been documented, particularly in endometrioid ovarian can cers [39]. We postulate that the differential sensitivity of some ovarian tumors to MYXV infection is related to the underlying status of this pathway. Perhaps genetic analysis of PI3K AKT pathway genes within EOC samples, or determination of endogenous AKT activity levels, would predict whether malignant EOC cells are sensitive or restrictive to MYXV infection. Indeed, the classification of sensitive (Type I) and re strictive (Type III) cells in response to MYXV oncolytic infection has its mechanistic basis upon the activity level of AKT kinase [19,21]. Others have demonstrated that treatment with rapamycin can render Type III restrictive cancer cells sensitive to MYXV oncolysis due to upregulation of AKT kinase activity by feedback signaling [22,33]. Although we did not observe significant effects of rapamycin treatment on MYXV infec tion of EOC cells and spheroids, there is the potential to use mTOR inhib itors or similar drugs to modulate AKT activity and increase sensitivity of primary EOC cells and spheroids to MYXV infection.
Our results support the established connection between AKT ac tivity and MYXV mediated oncolysis, however we have additional data from direct infection of patient ascites cells that there is a subset of EOC samples, which are apparently insensitive to MYXV. It is pos sible that some ascites samples harbor additional factors or character istics that inhibit or reduce the ability of MYXV to infect the EOC cells and spheroids present in the ascites [16]. For example, a number of complement protein precursors have been identified in the ascites that may contribute to an innate antiviral immune response against MYXV [40]; this potential alternative complement pathway has been observed to affect adenovirus infection in other systems [41]. Al ternatively, it has been observed that ascites treatment of EOC cells and ovarian cancer cell lines can regulate AKT activity to promote cell survival in the presence of pro apoptotic stimuli [42]. Our results suggest that some ascites may have the opposite effect of down regulating AKT activity in EOC cells, thereby rendering cells less sen sitive to MYXV infection and oncolysis. An alternative explanation, however, is that underlying mutations in other dominant oncogenic pathways may be present in the unresponsive EOC samples. For ex ample, recent data indicates that all high grade EOC of the serous his totype carry mutations in TP53 [43,44]. Alternatively, low grade EOC tumors often possess oncogenic mutations in KRAS and BRAF [45]. Therefore, it may be more effective to utilize other oncolytic viruses that target these types of mutations (e.g. reovirus for KRAS muta tions). It is of interest to test a panel of different oncolytic vectors against primary human EOC samples derived from patient ascites, and correlate therapeutic efficacy with mutational analysis of putative underlying disease causing genes such as AKT2, PTEN, PIK3CA, TP53, BRAF and KRAS. This would provide a personalized cancer therapy ap proach to the use of oncolytic viruses in EOC, which could be amplified with combination therapy using multiple oncolytic viruses and chemo therapeutics in sequence [46,47].
Another intriguing and emerging concept is that metastatic EOC cells and spheroids may represent a reservoir of ovarian cancer initi ating cells [48 52]. Collection and isolation of ovarian cancer initiat ing cells from patient ascites have been successful by a number of groups using cell surface markers, such as CD133+ [52], CD44+/ CD24+/EpCAM+ [49], CD44+/CD117+ [48], and CD44+/MyD88+ [50], or by verapamil sensitive side population cells [51]. This has in creased relevance with regard to targeted killing by MYXV, since the idea of oncolytic viral purging of pluripotent cancer stem cells using MYXV has been recently applied in the context of human myeloid leukemia [53]. We propose that MYXV application directly to malig nant ascites may function in the same way, and provide an effective way to target ovarian cancer stem cells, perhaps those residing within dormant EOC spheroids and secondary metastases. Future studies would involve selection and culture of ovarian cancer initiating cells from EOC patient ascites and directly assaying their sensitivity to MYXV oncolysis.
Supplementary materials related to this article can be found on line at doi:10.1016/j.ygyno.2012.01.048.
Supplementary Material
Acknowledgments
We acknowledge the essential contribution of our colleagues the gynecologic oncology surgeons Monique Bertrand, Akira Sugimoto, and Michel Préfontaine at the London Regional Cancer Program in providing all clinical specimens used in this study, and Christine Gawlik for assistance with retrieval of clinical data. We thank Joe Mymryk for critical appraisal of this manuscript. We are also extremely grateful to the women with ovarian cancer who generously donated their ascites samples to support our research. This research was supported by funding from the Small Grants Program at the London Regional Cancer Program. R. Correa is supported by a graduate scholarship from the Strategic Training Program in Cancer Research and Technology Transfer Pro gram with funds from Canadian Institutes for Health Research. Laboratory of G. McFadden is supported by an NCI operating grant R01 CA138541 01.
Footnotes
Conflict of interest statement
G. McFadden holds inventor's intellectual property for Myxoma virus as an oncolytic virotherapeutic. The other authors declare they have no conflicts of interest.
References
- [1].Society CC, Canada S, Canada PHAo. Canadian Cancer Statistics. 2011 2011. [Google Scholar]
- [2].Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev. 2001;22(2):255–88. doi: 10.1210/edrv.22.2.0422. [DOI] [PubMed] [Google Scholar]
- [3].Levanon K, Crum C, Drapkin R. New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J Clin Oncol. 2008;26(32):5284–93. doi: 10.1200/JCO.2008.18.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ahmed N, Thompson EW, Quinn MA. Epithelial-mesenchymal interconversions in normal ovarian surface epithelium and ovarian carcinomas: an exception to the norm. J Cell Physiol. 2007;213(3):581–8. doi: 10.1002/jcp.21240. [DOI] [PubMed] [Google Scholar]
- [5].Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract. 2007;4(2):101–17. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- [6].Kirn D, Martuza RL, Zwiebel J. Replication-selective virotherapy for cancer: biological principles, risk management and future directions. Nat Med. 2001;7(7):781–7. doi: 10.1038/89901. [DOI] [PubMed] [Google Scholar]
- [7].Matthews KS, Alvarez RD, Curiel DT. Advancements in adenoviral based virotherapy for ovarian cancer. Adv Drug Deliv Rev. 2009;61(10):836–41. doi: 10.1016/j.addr.2009.04.012. [DOI] [PubMed] [Google Scholar]
- [8].Hirasawa K, Nishikawa SG, Norman KL, Alain T, Kossakowska A, Lee PW. Oncolytic reovirus against ovarian and colon cancer. Cancer Res. 2002;62(6):1696–701. [PubMed] [Google Scholar]
- [9].Myers R, Greiner S, Harvey M, Soeffker D, Frenzke M, Abraham K, et al. Oncolytic activities of approved mumps and measles vaccines for therapy of ovarian cancer. Cancer Gene Ther. 2005;12(7):593–9. doi: 10.1038/sj.cgt.7700823. [DOI] [PubMed] [Google Scholar]
- [10].Lin X, Chen X, Wei Y, Zhao J, Fan L, Wen Y, et al. Efficient inhibition of intraperitoneal human ovarian cancer growth and prolonged survival by gene transfer of vesicular stomatitis virus matrix protein in nude mice. Gynecol Oncol. 2007;104(3):540–6. doi: 10.1016/j.ygyno.2006.09.022. [DOI] [PubMed] [Google Scholar]
- [11].Nguyen TL, Abdelbary H, Arguello M, Breitbach C, Leveille S, Diallo JS, et al. Chemical targeting of the innate antiviral response by histone deacetylase inhibitors renders refractory cancers sensitive to viral oncolysis. Proc Natl Acad Sci U S A. 2008;105(39):14981–6. doi: 10.1073/pnas.0803988105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Oncolytic virus therapy in treating patientswith progressive, recurrent, or refractory ovarian epithelial cancer or primary peritoneal cancer. ClinicalTrialsgov identifier: NCT00408590; 2008 http://clinicaltrials.gov/ct2/show/related/NCT00408590. [Google Scholar]
- [13].Vasey PA, Shulman LN, Campos S, Davis J, Gore M, Johnston S, et al. Phase I trial of intraperitoneal injection of the E1B-55-kd-gene-deleted adenovirus ONYX-015 (dl1520) given on days 1 through 5 every 3 weeks in patients with recurrent/refractory epithelial ovarian cancer. J Clin Oncol. 2002;20(6):1562–9. doi: 10.1200/JCO.2002.20.6.1562. [DOI] [PubMed] [Google Scholar]
- [14].Galanis E, Hartmann LC, Cliby WA, Long HJ, Peethambaram PP, Barrette BA, et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010;70(3):875–82. doi: 10.1158/0008-5472.CAN-09-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Breitbach CJ, Burke J, Jonker D, Stephenson J, Haas AR, Chow LQ, et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011;477(7362):99–102. doi: 10.1038/nature10358. [DOI] [PubMed] [Google Scholar]
- [16].Hartkopf AD, Fehm T, Wallwiener D, Lauer U. Oncolytic virotherapy of gynecologic malignancies. Gynecol Oncol. 2011;120(2):302–10. doi: 10.1016/j.ygyno.2010.10.031. [DOI] [PubMed] [Google Scholar]
- [17].Stanford MM, McFadden G. Myxoma virus and oncolytic virotherapy: a new biologic weapon in the war against cancer. Expert Opin Biol Ther. 2007;7(9):1415–25. doi: 10.1517/14712598.7.9.1415. [DOI] [PubMed] [Google Scholar]
- [18].McFadden G. Poxvirus tropism. Nat Rev Microbiol. 2005;3(3):201–13. doi: 10.1038/nrmicro1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Werden SJ, McFadden G. The role of cell signaling in poxvirus tropism: the case of the M-T5 host range protein of myxoma virus. Biochim Biophys Acta. 2008;1784(1):228–37. doi: 10.1016/j.bbapap.2007.08.001. [DOI] [PubMed] [Google Scholar]
- [20].Barrett JW, Alston LR, Wang F, Stanford MM, Gilbert PA, Gao X, et al. Identification of host range mutants of myxoma virus with altered oncolytic potential in human glioma cells. J Neurovirol. 2007;13(6):549–60. doi: 10.1080/13550280701591526. [DOI] [PubMed] [Google Scholar]
- [21].Wang G, Barrett JW, Stanford M, Werden SJ, Johnston JB, Gao X, et al. Infection of human cancer cells with myxoma virus requires Akt activation via interaction with a viral ankyrin-repeat host range factor. Proc Natl Acad Sci U S A. 2006;103(12):4640–5. doi: 10.1073/pnas.0509341103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lun XQ, Zhou H, Alain T, Sun B, Wang L, Barrett JW, et al. Targeting human medulloblastoma: oncolytic virotherapy with myxoma virus is enhanced by rapamycin. Cancer Res. 2007;67(18):8818–27. doi: 10.1158/0008-5472.CAN-07-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Stanford MM, Shaban M, Barrett JW, Werden SJ, Gilbert PA, Bondy-Denomy J, et al. Myxoma virus oncolysis of primary and metastatic B16F10 mouse tumors in vivo. Mol Ther. 2008;16(1):52–9. doi: 10.1038/sj.mt.6300348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Werden SJ, Lanchbury J, Shattuck D, Neff C, Dufford M, McFadden G. The myxoma virus m-t5 ankyrin repeat host range protein is a novel adaptor that coordinately links the cellular signaling pathways mediated by Akt and Skp1 in virus-infected cells. J Virol. 2009;83(23):12068–83. doi: 10.1128/JVI.00963-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Werden SJ, McFadden G. Pharmacological manipulation of the akt signaling pathway regulates myxoma virus replication and tropism in human cancer cells. J Virol. 2010;84(7):3287–302. doi: 10.1128/JVI.02020-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sypula J, Wang F, Ma Y, Bell JC, McFadden G. Myxoma virus tropism in human tumor cells. Gene Ther Mol Biol. 2004;8:103–14. [Google Scholar]
- [27].Shepherd TG, Theriault BL, Campbell EJ, Nachtigal MW. Primary culture of ovarian surface epithelial cells and ascites-derived ovarian cancer cells from patients. Nat Protoc. 2006;1(6):2643–9. doi: 10.1038/nprot.2006.328. [DOI] [PubMed] [Google Scholar]
- [28].Barrett JW, Sypula J, Wang F, Alston LR, Shao Z, Gao X, et al. M135R is a novel cell surface virulence factor of myxoma virus. J Virol. 2007;81(1):106–14. doi: 10.1128/JVI.01633-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Smallwood SE, Rahman MM, Smith DW, McFadden G. Myxoma virus: propagation, puri?cation, quantification, and storage. Current protocols in microbiology. 2010 doi: 10.1002/9780471729259.mc14a01s17. Chapter 14:Unit 14A 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shepherd TG, Mujoomdar ML, Nachtigal MW. Constitutive activation of BMP signalling abrogates experimentalmetastasis of OVCA429 cells via reduced cell adhesion. J Ovarian Res. 2010;3:5. doi: 10.1186/1757-2215-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Barrett JW, Werden SJ, Wang F, McKillop WM, Jimenez J, Villeneuve D, et al. Myxoma virus M130R is a novel virulence factor required for lethal myxomatosis in rabbits. Virus Res. 2009;144(1-2):258–65. doi: 10.1016/j.virusres.2009.05.009. [DOI] [PubMed] [Google Scholar]
- [32].Correa RJ, Peart T, Valdes YR, DiMattia GE, Shepherd TG. Modulation of AKT activity is associated with reversible dormancy in ascites-derived epithelial ovarian cancer spheroids. Carcinogenesis. 2012;33:49–58. doi: 10.1093/carcin/bgr241. [DOI] [PubMed] [Google Scholar]
- [33].Stanford MM, Barrett JW, Nazarian SH, Werden S, McFadden G. Oncolytic virotherapy synergism with signaling inhibitors: rapamycin increases myxoma virus tropism for human tumor cells. J Virol. 2007;81(3):1251–60. doi: 10.1128/JVI.01408-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Shield K, Ackland ML, Ahmed N, Rice GE. Multicellular spheroids in ovarian cancer metastases: biology and pathology. Gynecol Oncol. 2009;113:143–8. doi: 10.1016/j.ygyno.2008.11.032. [DOI] [PubMed] [Google Scholar]
- [35].Levine DA, Bogomolniy F, Yee CJ, Lash A, Barakat RR, Borgen PI, et al. Frequent mutation of the PIK3CA gene in ovarian and breast cancers. Clin Cancer Res. 2005;11(8):2875–8. doi: 10.1158/1078-0432.CCR-04-2142. [DOI] [PubMed] [Google Scholar]
- [36].Shayesteh L, Lu Y, Kuo WL, Baldocchi R, Godfrey T, Collins C, et al. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 1999;21(1):99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- [37].Nakayama K, Nakayama N, Kurman RJ, Cope L, Pohl G, Samuels Y, et al. Sequence mutations and amplification of PIK3CA and AKT2 genes in purified ovarian serous neoplasms. Cancer Biol Ther. 2006;5(7):779–85. doi: 10.4161/cbt.5.7.2751. [DOI] [PubMed] [Google Scholar]
- [38].Carpten JD, Faber AL, Horn C, Donoho GP, Briggs SL, Robbins CM, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448(7152):439–44. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- [39].Obata K, Morland SJ, Watson RH, Hitchcock A, Chenevix-Trench G, Thomas EJ, et al. Frequent PTEN/MMAC mutations in endometrioid but not serous or mucinous epithelial ovarian tumors. Cancer Res. 1998;58(10):2095–7. [PubMed] [Google Scholar]
- [40].Kuk C, Kulasingam V, Gunawardana CG, Smith CR, Batruch I, Diamandis EP. Mining the ovarian cancer ascites proteome for potential ovarian cancer biomarkers. Mol Cell Proteomics. 2009;8(4):661–9. doi: 10.1074/mcp.M800313-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jiang H, Wang Z, Serra D, Frank MM, Amalfitano A. Recombinant adenovirus vectors activate the alternative complement pathway, leading to the binding of human complement protein C3 independent of anti-ad antibodies. Mol Ther. 2004;10(6):1140–2. doi: 10.1016/j.ymthe.2004.08.015. [DOI] [PubMed] [Google Scholar]
- [42].Lane D, Goncharenko-Khaider N, Rancourt C, Piche A. Ovarian cancer ascites protects from TRAIL-induced cell death through alphavbeta5 integrin-mediated focal adhesion kinase and Akt activation. Oncogene. 2010;29(24):3519–31. doi: 10.1038/onc.2010.107. [DOI] [PubMed] [Google Scholar]
- [43].Bowtell DD. The genesis and evolution of high-grade serous ovarian cancer. Nat Rev Cancer. 2010;10:803–8. doi: 10.1038/nrc2946. [DOI] [PubMed] [Google Scholar]
- [44].Network TCGAR Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Singer G, Oldt R, III, Cohen Y, Wang BG, Sidransky D, Kurman RJ, et al. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst. 2003;95(6):484–6. doi: 10.1093/jnci/95.6.484. [DOI] [PubMed] [Google Scholar]
- [46].Ottolino-Perry K, Diallo JS, Lichty BD, Bell JC, McCart JA. Intelligent design: combination therapy with oncolytic viruses. Mol Ther. 2010;18(2):251–63. doi: 10.1038/mt.2009.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Le Boeuf F, Bell JC. United virus: the oncolytic tag-team against cancer! Cytokine Growth Factor Rev. 2010;21(2-3):205–11. doi: 10.1016/j.cytogfr.2010.02.008. [DOI] [PubMed] [Google Scholar]
- [48].Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68(11):4311–20. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wei X, Dombkowski D, Meirelles K, Pieretti-Vanmarcke R, Szotek PP, Chang HL, et al. Mullerian inhibiting substance preferentially inhibits stem/progenitors in human ovarian cancer cell lines compared with chemotherapeutics. Proc Natl Acad Sci U S A. 2010;107(44):18874–9. doi: 10.1073/pnas.1012667107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Alvero AB, Chen R, Fu HH, Montagna M, Schwartz PE, Rutherford T, et al. Molecular phenotyping of human ovarian cancer stem cells unravels the mechanisms for repair and chemoresistance. Cell Cycle. 2009;8(1):158–66. doi: 10.4161/cc.8.1.7533. Georgetown, Tex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, et al. Ovarian cancer side population de?nes cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci U S A. 2006;103(30):11154–9. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Stewart JM, Shaw PA, Gedye C, Bernardini MQ, Neel BG, Ailles LE. Phenotypic heterogeneity and instability of human ovarian tumor-initiating cells. Proc Natl Acad Sci U S A. 2011;108(16):6468–73. doi: 10.1073/pnas.1005529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Rahman MM, Madlambayan GJ, Cogle CR, McFadden G. Oncolytic viral purging of leukemic hematopoietic stem and progenitor cells with Myxoma virus. Cytokine Growth Factor Rev. 2010;21(2-3):169–75. doi: 10.1016/j.cytogfr.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.