Abstract
Sleep disordered breathing (SDB) with recurrent apnea is a major health problem affecting several million adult men and women. Humans with SDB are prone to develop hypertension. Studies on rodents established that exposure to chronic intermittent hypoxia (CIH) alone is sufficient to induce hypertension similar to that seen in patients with SDB. Available evidence from studies on experimental animals suggests that catecholamines secreted from adrenal medulla (AM), an end-organ of the sympathetic nervous system is a major contributor to CIH-induced hypertension. In this article, we present an overview of our current understanding on how CIH reconfigures AM function and highlight recent findings on the underlying cellular and molecular mechanisms.
1. Introduction
Sleep disordered breathing (SDB), with recurrent apnea is a major health problem affecting several million adult men and women (Nieto et al., 2000 and Shahar et al., 2001). Recurrent apnea can be either due to obstruction of the upper airway (Obstructive Sleep Apnea, OSA) or defective generation of respiratory rhythm by the central nervous system (central apnea). People with SDB are at increased risk to develop hypertension and stroke (Nieto et al., 2000; Shahar et al., 2001 and Peppard et al., 2000). Recurrent apneas are associated with intermittent hypoxia, intermittent hypercapnia, and variation in intra-thoracic pressure. Studies on experimental animals established that exposure to chronic intermittent hypoxia (CIH) alone is sufficient to induce hypertension similar to that seen in patients with SDB (Fletcher, 2001). Considerable evidence suggests that catecholamines (CA) secreted from adrenal medulla (AM) is a major contributor to CIH-induced hypertension (Bao et al., 1997 and Peng et al., 2014). In this article, we present a brief review of how CIH affects AM function and discuss the underlying cellular and molecular mechanisms.
2. Contribution of Adrenal Medulla (AM) to CIH-induced Hypertension
Patients with SDB have a sustained elevation in blood pressure during daytime wherein apneas are absent (Carlson et al., 1993 and Somers et al., 1995) and they exhibit a more pronounced raise in blood pressure during apneic episodes than normal subjects (Stoohs & Guilleminault, 1992 and Imadojemu et al., 2002). Likewise, rats exposed to CIH, mimicking O2 saturation profiles seen in recurrent apnea patients, also developed hypertension (Fletcher et al., 1992) and displayed a marked increase in blood pressure in response to brief episodes of hypoxia as compared to normoxia exposed controls (Peng et al., 2014) (Fig. 1).
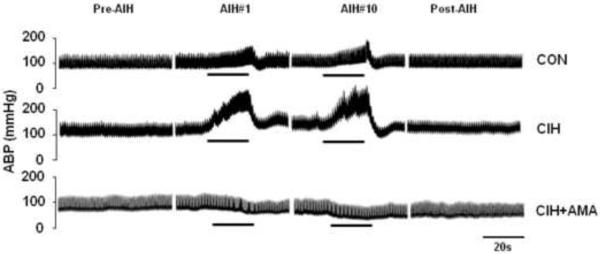
Figure 1.
Evidence for the involvement of adrenal medulla in CIH-induced increases in arterial blood pressure (ABP) in response to acute intermittent hypoxia (AIH). Three groups of rats were studied: Sham-operated and normoxia-exposed (CON); Sham-operated and exposed to CIH for 10 days (CIH) and bilateral adrenal medulla ablated (AMA) and CIH-exposed (CIH+AMA). ABP was monitored before, during and after AIH challenges. Each AIH episode consists of 12% O2 for 15 s followed by 21% O2 for 5 min. Representative changes in ABP during pre-AIH, first and 10th AIH, and post-AIH periods are shown. The black bar indicates the duration of hypoxic challenge.
Adrenal medullary chromaffin cells (AMC) synthesize and secrete catecholamines (CA) in response to a variety of stresses including hypoxia. Two studies have examined the role of CA derived from AMC in CIH-induced changes in blood pressures using adrenal medulla ablation as an experimental tool. Bilateral adrenal medullectomy prevented CIH-induced hypertension and increase in plasma catecholamines (Bao et al., 1997) as well as the abnormal elevation in blood pressure induced by acute exposure to hypoxia (Peng et al., 2014) (Fig. 1). These findings suggest that CA secreted from AM is a critical contributor to acute and chronic blood pressure elevation seen in CIH exposed rats.
3. CIH Facilitates Catecholamine Secretion from AM
The effect of CIH on CA secretion from AM was examined in age-matched adult male rats exposed to either CIH (alternating cycles of 5% O2 for 15 sec and 21% O2 for 5 min, 8 hours/day) or normoxia for 10 days (Kumar et al., 2006). Noradrenaline (NA) and adrenaline (A) effluxes were monitored from freshly prepared AM slices that are harvested from CIH and normoxia exposed rats. In CIH exposed rats, acute hypoxia evoked a robust efflux of NA and A from AM in a time-dependent manner wherein three days of CIH was ineffective whereas ten days of CIH produced a robust efflux. However, hypoxia-evoked CA efflux was absent in control rats. Unlike hypoxia, hypercapnia-evoked CA efflux was unaffected by CIH, suggesting that CIH selectively augments hypoxia-evoked CA secretion from AM. A similar CIH-induced facilitation of CA secretion by hypoxia was also observed in single AMC isolated from adult mice (Kuri et al., 2007). It is noteworthy that unlike CIH, continuous hypoxia was ineffective in facilitating CA efflux from rat AM. Collectively, these findings demonstrate that CIH exposure leads to selective facilitation of hypoxia-evoked CA release from AM of rats and mice.
3.1. Signaling mechanisms mediating the effects of CIH on AM
The magnitude of CA secretion from AMC is, in part, set by regulating the number of release-competent granules, a population that comprises the ‘readily-releasable pool' (Heinemann et al., 1993). Further, increase in intracellular Ca2+ levels is a pre-requisite for catecholamine secretion from AMC. In the following sections, the effects of CIH on readily releasable pool of secretory vesicles and calcium signaling in the AM will be presented.
3.1.1. Readily releasable pool
The effect of CIH on the readily releasable pool of secretory granules was studied in adult mice AMC using a dual-pulse protocol (Kuri et al., 2007). The results from this study showed that CIH significantly increases the number of readily releasable pool of secretory granules as compared to normoxic control. Further, CIH activated protein kinase C (PKC) in AM as evidenced by the increased phosphorylation of PKC at Thr-514 and a PKC inhibitor prevented CIH-induced increase in the number of readily releasable pool of secretory granules (Kuri et al., 2007).
3.1.2. Ca2+signaling
AMC from CIH exposed rats exhibited elevated basal [Ca2+]i and this effect was attributed to calcium mobilization via activation of ryanodine receptors (RyRs) by S-glutathionylation (Souvannakitti et al., 2010). In addition, CIH increased RyR 2 and 3 mRNA levels in rat AM. Blockade of RyRs prevented the baseline [Ca2+]i elevation suggesting that S-glutathionylation-mediated activation of RyRs contributes to augmented intracellular Ca2+ stores in CIH exposed AMC.
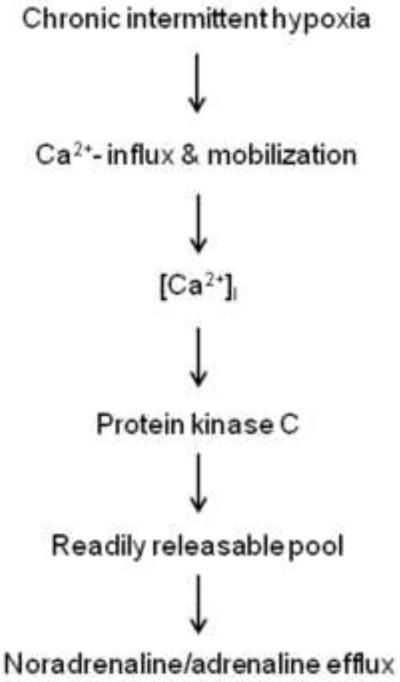
Acute hypoxia-evoked increase in [Ca2+]i was markedly enhanced in CIH treated neonatal AMC and this effect was mediated by increases in both voltage-gated Ca2+ flux and intracellular Ca2+ stores (Souvannakitti et al., 2009). CIH up regulated Cav3.1 and Cav 3.2 T-type Ca2+ channel mRNA levels and augmented T-type Ca2+ currents in neonatal rat AMC. Mibefradil, a blocker of T-type Ca2+ channels not only attenuated hypoxia-evoked [Ca2+]i elevation but also the enhanced CA secretion from AMC of CIH-treated rats (Souvannakitti et al., 2010). These findings, taken together, suggest that PKC-mediated increase in the number of readily releasable pool of secretory granules and up regulation of Ca2+ signaling pathways contributes to CIH-induced enhanced CA secretion in AM (Fig. 2).
Figure 2.
Proposed signaling mechanism(s) associated with CIH-induced facilitation of catecholamine efflux from adrenal medulla.
3.2. CIH Increases CA Levels via Activation of Tyrosine Hydroxylase
Kumar et al (2006) investigated the effect of CIH on catecholamine levels in rat AM and found that 10 days of CIH significantly increased NA and A levels as compared to normoxic controls. The mechanism(s) underlying CIH-induced increase in CA levels was investigated in Pheochromocytoma 12 (PC12) cell cultures which express dopamine (DA) as the major catecholamine. IH (consisting of 1% O2 for 15 s followed by 21% O2 for 4 min; 60 cycles) increased DA levels in PC12 cells with a concomitant increase in tyrosine hydroxylase (TH) enzyme activity, the rate-limiting enzyme in CA biosynthesis without altering TH protein levels (Kumar et al., 2003). IH-induced increase in TH activity was in part due to increased phosphorylation of serine 40, which is located at the N-terminal regulatory domain of TH. The increases in enzyme activity and serine phosphorylation of TH caused by IH were nearly absent in PC12 cells that are pre-treated with protein kinase inhibitors (Kumar et al., 2003). Taken together, these findings suggest that IH-induced raise in CA levels is in part due to increased CA synthesis via activation of TH involving serine phosphorylation.
4. Involvement of Reactive Oxygen Species (ROS)
Considerable evidence suggests that ROS signaling is an important cellular mechanism mediating the systemic responses to CIH (for references see Prabhakar et al., 2007). Consistent with this notion, CIH increased ROS levels in AM of adult (Kumar et al., 2006) and neonatal rats (Souvannakitti et al., 2009) as well as in mice (Kuri et al., 2007). The functional significance of increased ROS levels on CIH-induced changes in AM was assessed by treating rats with manganese (III) tetrakis (1-methyl-4-pyridyl) porphyrin pentachloride (MnTMPyP; 5 mg/kg, IP), a potent scavenger of ROS every day prior to CIH exposure. Treatment with MnTMPyP prevented CIH-induced changes in AM function including i) facilitation of CA efflux by hypoxia (Kumar et al., 2006; Kuri et al., 2007 and Souvannakitti et al., 2010), ii) PKC-mediated increase in the size of readily releasable pool of secretory vesicles (Kuri et al., 2007), iii) up regulation of mRNA expression of RyR 2 and 3 as well as Cav3.1 and Cav3.2 and iv) increases in S-glutathionylation of RyR2 (Souvannakitti et al., 2010). These observations provide evidence for a central role for ROS in CIH-induced functional changes in AM. The relative contribution of various forms of ROS including superoxide anion (O2.−), hydrogen peroxide (H2O2) or hydroxyl radical (OH.) to CIH-induced changes in AM, however, remains to be investigated.
4.1 CIH Tilts the Balance between Pro-oxidant and Antioxidant Enzymes
The cellular levels of ROS is governed by the balance between their generation via activation of pro-oxidant enzymes such as NADPH oxidase (Nox) family of enzymes and xanthine oxidase (XO) and their elimination by the action of antioxidant enzymes that include superoxide dismutase (SOD) 1 and 2 and catalase. Several studies have examined the effects of CIH on pro-, and anti-oxidant enzymes in AM.
4.1.1. Pro-oxidant enzymes
CIH increased Nox2 mRNA levels and Nox enzyme activity in AM (Yuan et al., 2008; Souvannakitti et al., 2010 and Peng et al., 2014). A recent study reported that XO enzyme activity is augmented in IH-treated PC12 cells and in AM of CIH exposed rats (Nanduri et al., 2013). The elevated XO activity by CIH was due to increased proteolytic conversion of xanthine dehydrogenase to XO involving a trypsin-like endoprotease and was not associated with transcriptional activation of XO (Nanduri et al., 2013). A NOX inhibitor prevented the effects of CIH on hypoxia-evoked CA secretion from AMC (Souvannakitti et al., 2010). Allopurinol, an inhibitor of XO, prevented CIH-induced ROS generation in rat AM and the elevation of plasma catecholamines (Nanduri et al., 2013). These results suggest that increased ROS derived from Nox and XO contribute to CIH-induced facilitation of CA efflux by hypoxia and elevation in plasma catecholamines.
4.1.2. Antioxidant enzymes
Unlike pro-oxidant enzymes, CIH decreased SOD2 enzyme activity in AM (Nanduri et al., 2009 and Peng et al., 2014) and in PC12 cells (Nanduri et al., 2009). CIH-induced decrease in SOD2 enzyme activity was in part due to transcriptional down regulation of SOD2 (Nanduri et al., 2009).
5. Role of HIF Transcriptional Activators in CIH-induced ROS
Hypoxia-inducible factors (HIFs) mediate transcriptional responses to low O2 (Prabhakar and Semenza 2012). HIFs consist of O2-regulated α-subunit and a constitutively expressed β-subunit. HIF-1α, the prototypical member of HIF family and HIF-2α, also known as endothelial PAS domain protein-1, are the two well-studied members of the HIF family. HIF-1 regulates transcriptional activation of pro-oxidant enzymes including Nox whereas HIF-2 governs transcriptional regulation of antioxidant enzymes that include SOD2 (Scortegagna et al., 2003; Nanduri et al., 2009 and Yuan et al., 2011).
The contribution of HIFs to CIH-induced oxidative stress and functional changes in the AM was investigated. CIH increased HIF-1α (Peng et al., 2006) and decreased HIF-2α protein levels in AM (Nanduri et al., 2009 and 2013). CIH-induced up regulation of HIF-1α mediates transcriptional activation of Nox2 in AM (Yuan et al., 2008) and the down regulation of HIF-2 by CIH leads to insufficient transcription of the gene encoding SOD2 (Nanduri et al., 2009 and 2013). These findings suggest that dysregulation of HIF-α isoform is a major molecular mechanism that governs CIH-induced imbalance in pro- and antioxidant enzymes leading to increased ROS generation which mediates the functional changes in AM.
6. Carotid Body Chemoreflex Mediates HIF-α Isoform Dysregulation by CIH
How CIH might affect HIF-α isoform expression in AM? One possibility is that the effects of CIH are due to a direct effect of alternating cycles of hypoxia and re-oxygenation on AMC. However, partial pressures of oxygen (pO2) of most tissues under normoxia lies between 60-80 mmHg (Carreau et al., 2011), which is much lower than the arterial pO2 (~100-120 mmHg). During each cycle of IH, arterial blood oxygen saturation decreases from 95% to 80% resulting in a modest drop in pO2 of about ~20-25 mmHg (Peng et al., 2014) suggesting that the effect of alternating cycles of hypoxia and re-oxygenation may not be directly sensed by most tissues including AM.
Alternatively, the effects of CIH on HIF-α isoform expression in AM could be mediated via an indirect mechanism. Considerable evidence suggests that increased neural activity is a potent regulator of transcription (Fields et al., 2005; Carulli et al., 2011 and Ganguly and Poo, 2013). Since carotid body chemoreflex is a potent regulator of AM function (Prabhakar et al., 2012) and carotid body response to hypoxia is markedly augmented by CIH (Peng et al., 2003), the role of carotid body chemoreflex in CIH-induced changes in HIF-α isoform expression and redox state in AM was investigated in carotid body ablated (CBA) rats (Peng et al., 2014). CIH-evoked HIF-α isoform dysregulation, imbalance in pro-, and antioxidant enzymes and increased ROS in AM were absent in CBA rats. In addition, these rats did not exhibit CIH-induced augmented NA and A effluxes from AM and elevation in plasma CA levels. This study further showed that the effect of the carotid body chemoreflex is mediated by increased sympathetic nerve activity to AM. These findings establish that the carotid body chemoreflex via increased sympathetic nerve activity regulates HIF-α isoform expression and the ensuing transcriptional regulation of Nox2 and SOD2 which contributes to the development of oxidative stress in AM under the setting of CIH.
7. Sympathetic Nerve Activity and HIF-α Isoform Dysregulation
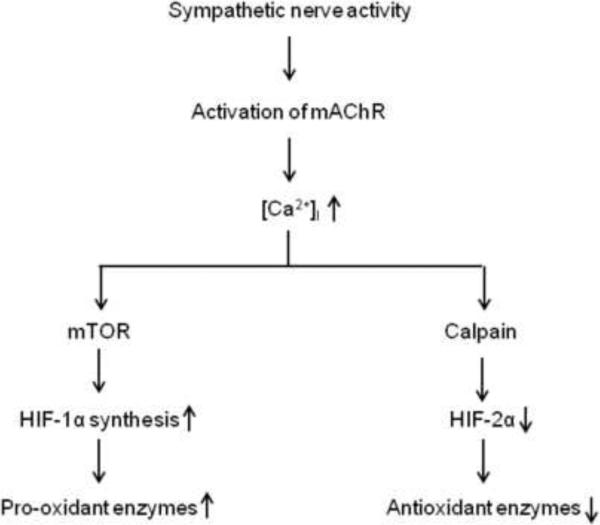
Acetylcholine is a major sympathetic neurotransmitter which activates nicotinic (nAChR) and muscarinic acetylcholine receptors (mAChR) in AMC. CIH down regulates nAChRs in AMC (Kumar et al., 2006 and Souvannakitti et al., 2010). Therefore, Peng et al (2014) examined the role of mAChRs in CIH-induced HIF-α isoform dysregulation and oxidative stress. Atropine, a broad-spectrum inhibitor of mAChRs, prevented HIF-α isoform dysregulation, imbalance in Nox and SOD2, oxidative stress, increased CA efflux by hypoxia and elevated plasma catecholamines in CIH-exposed rats. These findings suggest that increased sympathetic neural activity via mAChR activation mediates CIH-induced changes in HIF-α isoforms and redox state. Further analyses revealed that activation of mAChR, on the one hand, increases HIF-1α protein levels via increased protein synthesis by Ca2+-dependent activation of mammalian target of rapamycin (mTOR) and on the other decreases HIF-2α protein levels via Ca2+-dependent activation of calpain proteases (Peng et al., 2014) (Fig. 3). Thus far, five mAChR isoforms (M1-M5) are known and the identity of the mAChR isoform that contributes to CIH-induced HIF-α isoform dysregulation in AM remains to be established.
Figure 3.
A schematic representation of the signaling pathway linking sympathetic nerve activity and HIF-α isoform dysregulation leading to imbalance of pro- and anti-oxidant enzymes. Key: mAChR, muscarinic acetylcholine receptors; mTOR, mammalian target of rapamycin; HIF-1, hypoxia-inducible factor 1 and HIF-2, hypoxia-inducible factor 2.
Highlights.
Catecholamines secreted from adrenal medulla is a major contributor to acute and chronic blood pressure increase seen in rodents exposed to chronic intermittent hypoxia (CIH)
CIH facilitates catecholamine efflux from adrenal medulla via increasing the number of readily releasable pool of secretory granules and up regulation of Ca2+ signaling
CIH-induced altered adrenal medullary function is due to hypoxia-inducible factor (HIF)-alpha isoform dysregulation and the ensuing imbalance in prooxidant and antioxidant enzymes resulting in oxidative stress
During CIH, carotid body chemoreflex via increased sympathetic activity regulates HIF-alpha isoform expression and the ensuing transcriptional regulation of pro- and anti-oxidant enzymes which contributes to oxidative stress in adrenal medulla
Acknowledgement
This study was supported by National Institutes of Health, Heart, Lung and Blood Institute, Grant PO1-HL-90554.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bao G, Metreveli N, Li R, Taylor A, Fletcher EC. Blood pressure response to chronic episodic hypoxia: role of the sympathetic nervous system. Journal of Applied Physiology. 1997;83:95–101. doi: 10.1152/jappl.1997.83.1.95. [DOI] [PubMed] [Google Scholar]
- 2.Carulli D, Foscarin S, Rossi F. Activity-dependent plasticity and gene expression modifications in the adult CNS Frontiers in Molecular. Neuroscience. 2011;4:50. doi: 10.3389/fnmol.2011.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlson JT, Hedner J, Elam M, Ejnell H, Sellgren J, Wallin BG. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest. 1993;103:1763–1768. doi: 10.1378/chest.103.6.1763. [DOI] [PubMed] [Google Scholar]
- 4.Carreau A, El Hafny-Rahbi B, Matejuk A, Grillon C, Kieda C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. Journal of Cellular and Molecular Medicine. 2011;15:1239–1253. doi: 10.1111/j.1582-4934.2011.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fields RD, Lee PR, Cohen JE. Temporal integration of intracellular Ca2+ signaling networks in regulating gene expression by action potentials. Cell Calcium. 2005;37:433–442. doi: 10.1016/j.ceca.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Fletcher EC. Physiological consequences of intermittent hypoxia: systemic blood pressure. Journal of Applied Physiology. 2001;90:1600–1605. doi: 10.1152/jappl.2001.90.4.1600. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher EC, Lesske J, Behm R, Miller CC, 3rd, Stauss H, Unger T. Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. Journal of Applied Physiology. 1992;72:1978–1984. doi: 10.1152/jappl.1992.72.5.1978. [DOI] [PubMed] [Google Scholar]
- 8.Ganguly K, Poo MM. Activity-dependent neural plasticity from bench to bedside. Neuron. 2013;80:729–741. doi: 10.1016/j.neuron.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 9.Heinemann C, von Rüden L, Chow RH, Neher E. A two-step model of secretion control in neuroendocrine cells. Pflugers Archives. 1993;424:105–112. doi: 10.1007/BF00374600. [DOI] [PubMed] [Google Scholar]
- 10.Imadojemu VA, Gleeson K, Gray KS, Sinoway LI, Leuenberger UA. Obstructive apnea during sleep is associated with peripheral vasoconstriction American. Journal of Respiratory and Critical Care Medicine. 2002;165:61–66. doi: 10.1164/ajrccm.165.1.2009062. [DOI] [PubMed] [Google Scholar]
- 11.Kumar GK, Kim DK, Lee MS, Ramachandran R, Prabhakar NR. Activation of tyrosine hydroxylase by intermittent hypoxia: involvement of serine phosphorylation. Journal of Applied Physiology. 2003;95:536–544. doi: 10.1152/japplphysiol.00186.2003. [DOI] [PubMed] [Google Scholar]
- 12.Kumar GK, Rai V, Sharma SD, Ramakrishnan DP, Peng Y-J, Souvannakitti D, Prabhakar NR. Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. Journal of Physiology. 2006;575:229–239. doi: 10.1113/jphysiol.2006.112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuri BA, Khan SA, Chan SA, Prabhakar NR, Smith CB. Increased secretory capacity of mouse adrenal chromaffin cells by chronic intermittent hypoxia: involvement of protein kinase C. Journal of Physiology. 2007;584:313–319. doi: 10.1113/jphysiol.2007.140624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nanduri J, Vaddi DR, Khan SA, Wang N, Makerenko V, Prabhakar NR. Xanthine oxidase mediates hypoxia-inducible factor-2α degradation by intermittent hypoxia. Public Library of Science One. 2013;8:e75838. doi: 10.1371/journal.pone.0075838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nanduri J, Wang N, Yuan G, Khan SA, Souvannakitti D, Peng Y-J, Kumar GK, Garcia JA, Prabhakar NR. Intermittent hypoxia degrades HIF-2alpha via calpains resulting in oxidative stress: implications for recurrent apnea-induced morbidities. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1199–1204. doi: 10.1073/pnas.0811018106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study: Sleep Heart Health Study. Journal of American Medical Association. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 17.Peng Y-J, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10073–10078. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng Y-J, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous HIF-1 alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. Journal of Physiology. 2006;577:705–716. doi: 10.1113/jphysiol.2006.114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng Y-J, Yuan G, Khan S, Nanduri J, Makarenko VV, Reddy VD, Vasavda C, Kumar GK, Semenza GL, Prabhakar NR. Regulation of hypoxia-inducible factor-α isoforms and redox state by carotid body neural activity in rats. Journal of Physiology. 2014;592:3841–3858. doi: 10.1113/jphysiol.2014.273789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension New England. Journal of Medicine. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 21.Prabhakar NR, Semenza GL. Adaptive and maladaptive cardio respiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiological Reviews. 2012;92:967–1003. doi: 10.1152/physrev.00030.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prabhakar NR, Kumar GK, Nanduri J, Semenza GL. ROS signaling in systemic and cellular responses to chronic intermittent hypoxia. Antioxidant and Redox Signaling. 2007;9:1397–1403. doi: 10.1089/ars.2007.1732. [DOI] [PubMed] [Google Scholar]
- 23.Prabhakar NR, Kumar GK, Peng Y-J. Sympatho-adrenal activation by chronic intermittent hypoxia. Journal of Applied Physiology. 2012;113:1304–1310. doi: 10.1152/japplphysiol.00444.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, Yan LJ, Marck BT, Matsumoto AM, Shelton JM, Richardson JA, Bennett MJ, Garcia JA. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1−/− mice. Nature Genetics. 2003;35:331–340. doi: 10.1038/ng1266. [DOI] [PubMed] [Google Scholar]
- 25.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ, O'Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. American Journal of Respiratory and Critical Care Medicine. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 26.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. Journal of Clinical Investigation. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Souvannakitti D, Kumar GK, Fox A, Prabhakar NR. Neonatal intermittent hypoxia leads to long-lasting facilitation of acute hypoxia-evoked catecholamine secretion from rat chromaffin cells. Journal of Neurophysiology. 2009;101:2837–2846. doi: 10.1152/jn.00036.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Souvannakitti D, Nanduri J, Yuan G, Kumar GK, Fox AP, Prabhakar NR. NADPH oxidase-dependent regulation of T-type Ca2+ channels and ryanodine receptors mediate the augmented exocytosis of catecholamines from intermittent hypoxia treated neonatal rat chromaffin cells. Journal of Neuroscience. 2010;30:10763–10772. doi: 10.1523/JNEUROSCI.2307-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoohs R, Guilleminault C. Cardiovascular changes associated with obstructive sleep apnea syndrome. Journal of Applied Physiology. 1992;72:583–589. doi: 10.1152/jappl.1992.72.2.583. [DOI] [PubMed] [Google Scholar]
- 30.Yuan G, Khan SA, Luo W, Nanduri J, Semenza GL, Prabhakar NR. Hypoxiainducible factor 1 mediates increased expression of NADPH oxidase-2 in response to intermittent hypoxia. Journal of Cellular Physiology. 2011;226:2925–2933. doi: 10.1002/jcp.22640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1alpha expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. Journal of Cellular Physiology. 2008;217:674–685. doi: 10.1002/jcp.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]