Abstract
Mutations in COCH cause autosomal dominant non-syndromic hearing loss with variable degrees of clinical onset and vestibular malfunction. We selected eight uncharacterized mutations and performed immunocytochemical and Western blot analyses to track cochlin through the secretory pathway. We then performed a comprehensive analysis of clinical information from DFNA9 patients with all 21 known COCH mutations in conjunction with cellular and molecular findings to identify genotype-phenotype correlations. Our studies revealed that five mutants were not secreted into the media: two vWFA domain mutants, which were not transported from the ER to Golgi complex and formed high-molecular-weight aggregates in cell lysates; and three LCCL domain mutants, which were detected as intracellular dimeric cochlins. Mutant cochlins that were not secreted and accumulated in cells result in earlier age of onset of hearing defects. In addition, individuals with LCCL domain mutations show accompanying vestibular dysfunction, whereas those with vWFA domain mutations exhibit predominantly hearing loss. This is the first report showing failure of mutant cochlin transport through the secretory pathway, abolishment of cochlin secretion, and formation and retention of dimers and large multimeric intracellular aggregates, and high correlation with earlier onset and progression of hearing loss in individuals with these DFNA9-causing mutations.
Keywords: DFNA9, COCH, cochlin, secretion, aggregation, misfolding protein, genotype-phenotype correlation
Introduction
COCH (coagulation factor C homology; OMIM 603196), encoding the secreted protein cochlin, contains an N-terminal signal peptide (SP), an LCCL (Limulus factor C, cochlin, and late gestation lung protein Lgl1) domain, two von Willebrand factor A-like (vWFA) domains, and two short intervening domains (ivd) (Fig 1). The LCCL module is an autonomously folding domain, with a central α-helix wrapped by two β-sheets, and thought to serve host defense functions (Liepinsh, et al., 2001; Trexler, et al., 2000). vWFA domains are found in a number of secreted and extracellular matrix proteins, and are all known to bind other proteins such as fibrillar collagens, glycoproteins, and proteoglycans (Kommareddi, et al., 2007; Nagy, et al., 2008; Sadler, 1998).
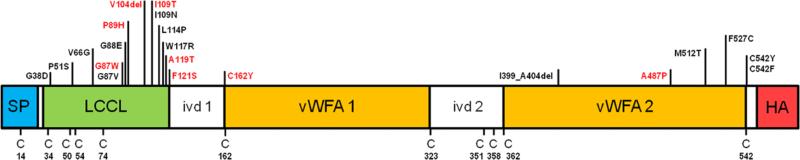
Figure 1.
Schematic representation of cochlin domain structure with an N-terminal signal peptide (SP), followed by a Limulus factor C, cochlin, and late gestation lung protein, Lgl1 (LCCL) domain, two von Willebrand factor A-like (vWFA) domains, and two short lengths of intervening domains (ivd1 and ivd2). A total of 21 mutations have been reported, eight of which (marked red) were investigated in this study. Three HA epitope tags were added at the C-terminal end of the COCH sequences (C=cysteine residue).
Mutations in COCH are causative of autosomal dominant non-syndromic hearing loss, DFNA9, which has a late onset (ranging from 2nd to 7th decade of life) and progressive presentation, with variable degrees of vestibular malfunction such as dizziness, vertigo, and instability in the dark. To date, 21 COCH mutations (19 missense and two in-frame deletions) have been reported throughout the world (Chen, et al., 2013; Cho, et al., 2012; Choi, et al., 2013; Collin, et al., 2006; de Kok, et al., 1999; Dodson, et al., 2012; Faletra, et al., 2011; Gallant, et al., 2013; Gao, et al., 2013; Hildebrand, et al., 2010; Kamarinos, et al., 2001; Nagy, et al., 2004; Pauw, et al., 2007a; Pauw, et al., 2007b; Robertson, et al., 1998; Street, et al., 2005; Usami, et al., 2003; Yuan, et al., 2008). The true world-wide incidence of COCH mutations is not known, as systematic genetic screening for this and other genes resulting in late-onset disorders is typically not performed. Rather, mutations have been discovered and reported largely in research studies based on large pedigrees. However, we believe that the prevalence of COCH mutations may be quite high, given their presence in individuals throughout four continents (with widely different ethnicities), in addition to the finding of several distinct mutations in the Netherlands alone. Although these mutations are thought to act in a dominant negative fashion, with a gain of deleterious function of the mutant cochlin, they may exert pathological effects through various different molecular mechanisms, which may account for differences in clinical features and presentation among individuals with DFNA9 mutations.
A unique and characteristic histopathological DFNA9 finding is presence of abundant cochlin-staining eosinophilic deposits in the spiral ligament and spiral limbus in the cochlea, and stroma underlying vestibular sensory epithelia, with substantial loss of cellularity in these compartments (Robertson, et al., 2006; Robertson, et al., 1998). Accumulation of misfolded mutant cochlins has been implicated in aggregate formation. Several studies have shown misfolding of the LCCL domain as a result of missenses and deletion mutations in this domain (Liepinsh, et al., 2001; Nagy, et al., 2004; Trexler, et al., 2000). Furthermore, some LCCL domain mutations can induce dimerization of mutant cochlins and heterodimer formation of mutant and wild-type cochlins (Yao, et al., 2010). Previously, we also demonstrated that two vWFA domain mutations (p.F527C and p.C542Y) result in high-molecular-weight cochlin aggregate formation in cells, but with secretion of monomeric mutant cochlin similar to that in wild-type (Cho, et al., 2012).
While studies using Coch−/− and CochG88E/G88E mouse models have been performed, exact pathological mechanisms of different COCH mutations and differences among DFNA9 patients in clinical manifestations of hearing loss and vestibular dysfunction remain unclear (Jones, et al., 2011; Makishima, et al., 2005; Robertson, et al., 2008).
In this study, we selected eight mutations whose functions have not been previously characterized: c.259G>T (p.G87W), rs149903169:C>A (p.P89H), c.311_313del (p.V104del), c.326T>C (p.I109T), c.355G>A (p.A119T), c.362T>C (p.F121S), c.485G>A (p.C162Y), and c.1459G>C (p.A487P) (six in the LCCL and two in vWFA domains; NM_001135058.1; http://www.ncbi.nlm.nih.gov/) (Collin, et al., 2006; Dodson, et al., 2012; Faletra, et al., 2011; Gao, et al., 2013; Hildebrand, et al., 2010; Nagy, et al., 2004; Pauw, et al., 2007b; Usami, et al., 2003) (Fig 1). We investigated subcellular localization within the secretory pathway, post-translational processing, secretion, dimerization, and aggregate formation of these mutant cochlins, in comparison to wild-type, to elucidate pathogenic mechanisms of the different COCH mutations. In addition, we performed a comprehensive review of clinical information available for individuals with DFNA9 in relation to molecular and functional data on all 21 different COCH mutations known to date, to assess genotype-phenotype correlations.
Materials and methods
Expression of wild-type and mutant cochlins
To express wild-type cochlin, a pcDNA3 vector containing human wild-type COCH cDNA with three C-terminal hemagglutinin (HA) epitope tags was used (Robertson, et al., 2003). Eight mutations, p.G87W, p.P89H, p.V104del, p.I109T, p.A119T, p.F121S, p.C162Y, and p.A487P (Collin, et al., 2006; Dodson, et al., 2012; Faletra, et al., 2011; Gao, et al., 2013; Hildebrand, et al., 2010; Nagy, et al., 2004; Pauw, et al., 2007b; Usami, et al., 2003) were generated using the QuikChange site-directed mutagenesis kit (Stratagene Inc., La Jolla, CA, USA) according to the manufacturer's protocol. HeLa cells were transiently transfected with the COCH constructs using the Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol.
Immunocytochemistry
Between 24 hours and 48 hours after transfection, cells were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 15 minutes at room temperature (RT), washed three times in PBS, permeabilized in 0.1% Triton X-100 in PBS for 30 minutes at RT and blocked in 5% normal goat serum (NGS) and 0.1% Triton X-100 in PBS for one hour. Subsequently, they were incubated with primary antibodies diluted in 5% NGS blocking solution for 12 hours at 4°C, washed three times in PBS, and incubated with secondary antibodies diluted in blocking solution for one hour at RT. After washing in PBS, cells were counterstained with 4,6-diamidine-2-phenylindole dihydrochloride (DAPI) for one minute to stain nuclei and mounted on slides and visualized using a Zeiss DE/AX10 Imager A1 fluorescence microscope system (Carl Zeiss, Oberkochen, Germany). A rabbit anti-HA monoclonal antibody (Cell Signaling Technology, Danvers, MA, USA) was used to identify cochlin expressed by transfected cells. Mouse anti-KDEL monoclonal (Enzo Life Sciences, Farmingdale, NY, USA) and mouse anti-human Golgin-97 antibodies (Invitrogen, Carlsbad, CA, USA) were used as primary antibodies to detect the endoplasmic reticulum (ER) and Golgi apparatus, respectively. Goat anti-mouse Alexa 488 (Zymed, San Francisco, CA, USA) and goat anti-rabbit Alexa 555 (Zymed, San Francisco, CA, USA) were used as secondary antibodies.
Immunoprecipitation and Western blot analysis
At 48 hours after transfection, culture media from transfected cells were collected for immunoprecipitation, and the transfected HeLa cells were washed twice with cold PBS and solubilized in lysis buffer containing 1X protease inhibitor cocktail (Calbiochem, La Jolla, CA, USA) for 30 minutes at 4°C. Subsequently, samples were centrifuged at 13000 × g for 30 minutes, and supernatants were incubated with rabbit anti-HA monoclonal antibody (Cell Signaling Technology, Danvers, MA, USA) bound to Protein G Dynabeads (Invitrogen, Carlsbad, CA, USA) for one hour at RT. Bound cochlin complexes were eluted using 20 μl of 50 mM glycine (pH 2.8) and neutralized with 20 μl of 1 M Tris-HCl (pH 7.5). Cell lysates and immunoprecipitation products were separated by non-reducing and reducing sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE; 8% gels) followed by immunoblotting, as previously described (Street, et al., 2005). Mouse anti-HA monoclonal antibody (Cell Signaling Technology, Danvers, MA, USA) and goat anti-mouse IgG-HRP (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used for Western blot analysis. An anti-β-actin antibody (Cell Signaling Technology, Danvers, MA, USA) and goat anti-rabbit IgG-HRP (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used to identify β-actin, as a quantitative control.
N-linked glycosylation analysis
The NetNGlyc 1.0 server (http://www.cbs.dtu.dk/services/NetNGlyc/) was used for in silico prediction of N-linked glycosylation sites in the wild-type and eight mutant cochlins. At 48 hours after transfection, cell lysates were denatured in glycoprotein denaturing buffer at 98°C for 10 minutes, and digested with N-glycosidase F (PNGase F) at 37°C for one hour according to the manufacturer's protocol (New England Biolabs, Beverley, MA, USA). Proteins were separated by SDS-PAGE (8% gels) followed by immunoblotting as previously described.
Statistics
Statistical analyses were performed with a commercial software package (Statistical Package for the Social Sciences 13.0 for Windows; SPSS, Chicago, IL, USA). The correlation of the amounts of accumulated mutant cochlins and the age at onset of hearing loss was analyzed by Spearman correlation analysis.
Results
Intracellular localization of wild-type and mutant cochlins
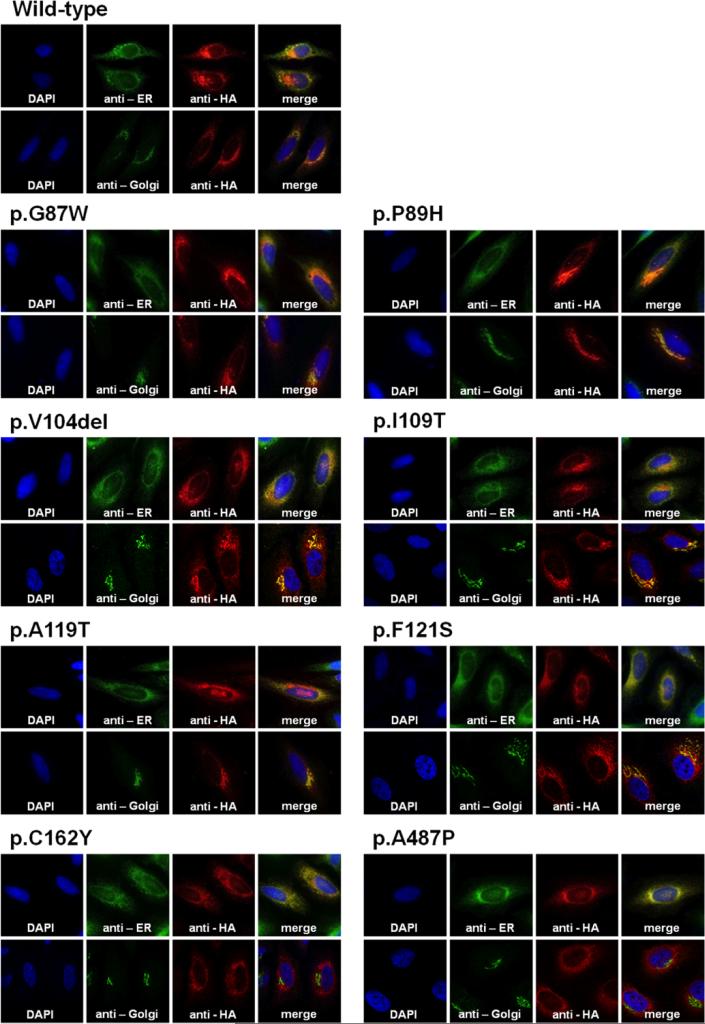
To determine if mutant cochlins are secreted normally from cells, we tracked subcellular localization of HA epitope-tagged wild-type and mutant cochlins by immunostaining transfected HeLa cells (Fig 2). Wild-type cochlin was detected in the perinuclear region, as a reticular formation in the ER, and particularly compacted in the Golgi complex, in agreement with previous reports (Cho, et al., 2012; Grabski, et al., 2003; Robertson, et al., 2003). Likewise, cochlins containing the p.G87W, p.P89H, p.V104del, p.I109T, p.A119T, or p.F121S mutations, all located in the LCCL domain, showed localization in the ER and Golgi complex. However, cochlins containing the p.C162Y or p.A487P mutation, located in the vWFA domains, also localized in the ER but did not colocalize with the Golgin-97 antibody signal in the Golgi complex. These results indicate a disruption in normal cellular trafficking, by failure of transport of p.C162Y and p.A487P mutant cochlins from the ER to the Golgi complex.
Figure 2.
Immunocytochemical analysis of HeLa cells transiently transfected with wild-type or mutant cochlins. Cochlin localization was detected by immunostaining with an Alexa 555-conjugated anti-HA antibody (red). The ER and Golgi complex were immunostained with Alexa 488 (green) using anti-KDEL and anti-Golgin-97 antibodies, respectively. Nuclei were counterstained with DAPI (cyan). Wild-type, p.G87W, p.P89H, p.V104del, p.I109T, p.A119T, and p.F121S each colocalized with the ER and Golgi complex markers, but both p.C162Y and p.A487P did not colocalize with the Golgi complex markers, indicating lack of transport of these mutant cochlins from ER to the Golgi apparatus.
Secretion and accumulation of wild-type and mutant cochlins
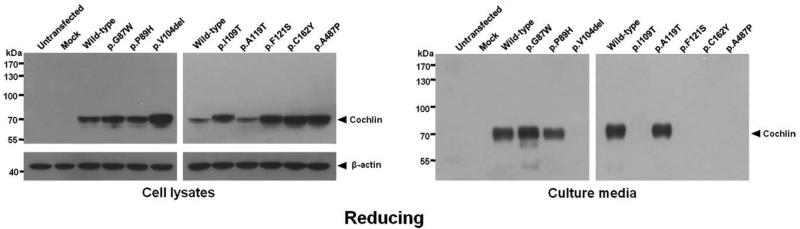
To investigate intracellular and secreted levels of wild-type and mutant cochlins, we performed Western blot analysis of transfected cell lysates and of culture media from transfected cells. In the reduced state (Fig 3), full-length, wild-type cochlin was observed (~68 kDa) in both cell lysates and media. Mutant cochlins were also detected in reduced cell lysates, at ~68 kDa, except for a slightly larger p.I109T cochlin, likely a result of presence of additional protein modifications (see N-linked glycosylation experiment results). Higher levels of p.V104del, p.I109T, p.F121S, p.C162Y, and p.A487P mutant cochlins as compared to wild-type cochlin, were detected as stronger band intensities in these mutants. Interestingly, these same mutant cochlins were completely absent in the culture media, indicating that they were not secreted, whereas p.G87W, p.P89H, and p.A119T mutants were detected in the media similarly to wild-type cochlin. Therefore, increased amounts of intracellular mutant cochlins were closely associated with impaired secretion of these proteins from the cell.
Figure 3.
Western blot analysis of cell lysates and culture media of HeLa cells transiently transfected with HA-tagged COCH under reducing gel electrophoresis conditions. Intracellularly, all cochlins (both wild-type and all mutants) were produced, but different band intensities of cochlins with various mutations were detected, in relation to β-actin internal control. This indicates successful transfection and expression of all cochlins, but different levels of intracellular cochlins. However, in culture media of transfected cells, cochlin bands were detected only for wild-type and three mutants, but not the other five mutants, indicating lack of secretion of these cochlins. Untransfected HeLa cells and HeLa cells transfected with the pcDNA3 empty vector (mock) were used as negative controls.
Protein misfolding and aggregation
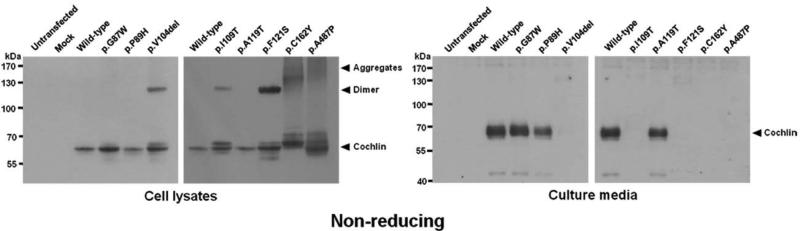
To investigate dimer and aggregate formation and other possible protein folding problems, cell lysates and media from transfected cells were analyzed under non-reducing conditions (Fig 4). In the non-reduced state, in which structure is maintained, for example by disulfide bonds, wild-type cochlin migrated faster, appearing as a smaller size (~60 kDa) than in the reduced state (~68 kDa). The p.C162Y cochlin exhibited a band shift (slower migration) similar to that of p.C542F and p.C542Y (Cho, et al., 2012; Street, et al., 2005), likely as a result of loss of the cysteine residue and disruption of an intramolecular disulfide bond present in the wild-type.
Figure 4.
Western blot analysis of cell lysates and culture media from HeLa cells transiently transfected with HA-tagged COCH under non-reducing gel electrophoresis conditions. Additional dimeric cochlin bands were observed for p.V104del, p.I109T, and p.F121S. High-molecular-weight aggregates were detected for p.C162Y and p.A487P mutant cochlins. No aggregates were detected in culture media, and five out of eight mutant cochlins tested were not secreted. The five mutants that failed to be secreted were the same ones that showed dimer and aggregate formation in cell lysates.
The p.V104del, p.I109T, and p.F121S cochlins, which contain mutations in the LCCL domain, were not detected in the media, and formed additional, larger-sized cochlins (~130 kDa, consistent with dimeric cochlins) in cell lysates. The p.C162Y and p.A487P cochlins, which contain mutations in the vWFA 1 and vWFA 2 domains, respectively, also failed to be secreted into the media and were detected as larger-sized multimeric (>130 kDa) smeared bands in the cell lysates. These high-molecular-weight protein aggregates are predicted to result from the presence of unpaired cysteines, which promote aggregation through aberrant covalent and disulfide bonds in the p.C162Y mutation, and the p.A487P mutation also likely affects normal folding and structure of cochlin, resulting in aggregation. Therefore, our results indicate that concomitant with intracellular aggregation (both dimeric and multimeric), there was complete lack of secretion of these mutant cochlins.
N-linked glycosylation
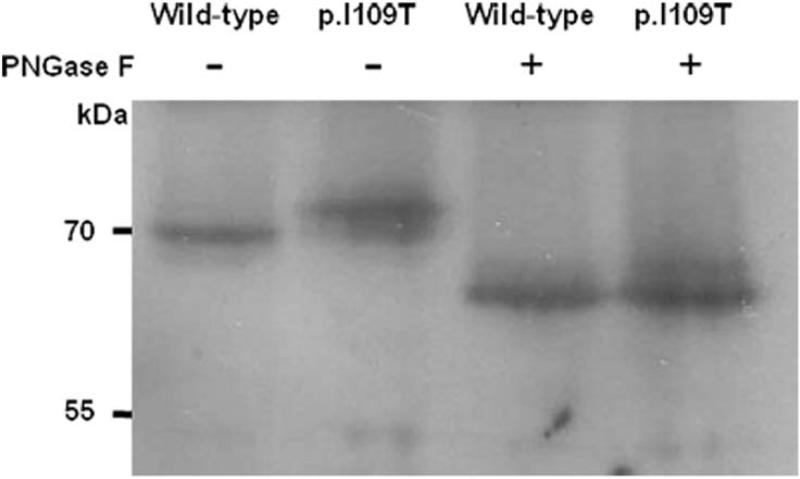
In reducing conditions, the apparent size of the p.I109T cochlin was slightly larger than that of the other cochlins (Fig 3 and 5). In the p.I109T mutant, the amino acid residues N-G-I are substituted by N-G-T, making it consistent with the consensus sequence for N-linked glycosylation (NXS/T), in which X can be any amino acid except proline (Bause and Legler, 1981; Gavel and von Heijne, 1990). The NetNGlyc 1.0 server was used to predict an additional N-linked glycosylation site at position asparagine 107 (N-G-T) in the p.I109T cochlin (data not shown). Wild-type cochlin was previously characterized as having two N-linked glycosylation consensus sites, showing reduction in size after PNGase F digestion (Robertson, et al., 2003). To compare N-linked glycosylation in wild-type and p.I109T cochlins, we performed PNGase F digestion, followed by Western blot analysis (Fig 5). Wild-type and p.I109T cochlins were detected as the same size after PNGase F digestion, representing a larger band shift for the p.I109T mutant cochlin, indicating that it has acquired an additional N-linked glycosylation site.
Figure 5.
Western blot analysis of wild-type and p.I109T cell lysates under reducing gel electrophoresis conditions, with and without PNGase F digestion (–, PNGase F untreated; +, PNGase F treated). The predicted additional N-linked glycosylation site in the p.109T mutant cochlin was confirmed with the observation of a larger band shift of the mutant vs. wild-type cochlin after digestion.
Genotype-phenotype correlation in DFNA9 hearing loss and vestibular disorder
We performed a comprehensive review and analysis of previously collected and reported clinical information on individuals and families with all 21 currently known COCH mutations (14 in the LCCL domain and seven in the vWFA domains), in addition to molecular characteristics of mutant cochlins from our new findings in this study and from past reports (Baek, et al., 2010; Chen, et al., 2013; Cho, et al., 2012; Choi, et al., 2013; Collin, et al., 2006; de Kok, et al., 1999; Dodson, et al., 2012; Faletra, et al., 2011; Fransen and Van Camp, 1999; Fransen, et al., 2001; Gallant, et al., 2013; Gao, et al., 2013; Grabski, et al., 2003; Hildebrand, et al., 2010; Hildebrand, et al., 2009; Kamarinos, et al., 2001; Kemperman, et al., 2005; Nagy, et al., 2004; Pauw, et al., 2007a; Pauw, et al., 2007b; Robertson, et al., 2003; Robertson, et al., 1998; Street, et al., 2005; Usami, et al., 2003; Yao, et al., 2010; Yuan, et al., 2008). These results are summarized in Table 1. We then performed the following correlational analyses, identifying four interesting trends:
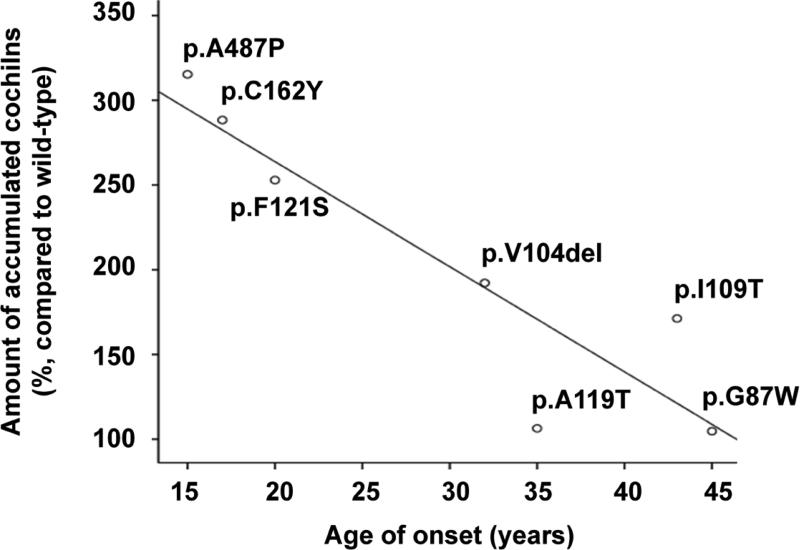
In reduced form, intensities of wild-type and mutant cochlin bands, as detected by Western blot analysis (Fig 3), were measured and relatively quantitated using Image J (http://rsb.info.nih.gov/ij/), and subsequently correlated with age of onset in DFNA9 patients with COCH mutations (Table S1, Fig 6). Statistical analysis revealed that the amount of accumulated mutant cochlins was negatively correlated with the age at onset of hearing loss. We confirmed statistical significance for the different mutations tested (r = −0.964, p<0.001). The one outlier represents an individual with a p.P89H mutation (in the LCCL domain) with an atypical presentation of unilateral (left-sided) congenital hearing loss and an ipsilateral enlarged vestibular aqueduct, described in a previous report (Dodson, et al., 2012), and could therefore indicate presence of other causative genetic or environmental factors.
Some LCCL domain mutations resulted in formation of dimeric cochlins, whereas both vWFA domain mutants (p.C162Y and p.A487P) analyzed in this report and two vWFA mutants (p.F527C, p.C542Y) analyzed in our previous report (Cho et al., 2012) formed higher-molecular-weight aggregates in cells.
LCCL domain mutations cause hearing defects that are accompanied by vestibular dysfunction, while vWFA domain mutations cause predominantly hearing loss.
The age of onset of hearing loss among individuals with vWFA domain mutations is earlier than that of those with LCCL domain mutations.
Table 1.
Clinical information and characteristics of individuals with reported COCH mutations
| Clinical information | Molecular analysis of mutant cochlin | |||||||
|---|---|---|---|---|---|---|---|---|
| Domain | Mutation | Ethnicity | Age of onset | Vestibular disordera | ER to Golgi transport | Secretion in media | Dimer or aggregates in cell lysates | Referencesa |
| LCCL | p.G38D | Korean | N/A | N/A | N/A | N/A | N/A | [Choi et al., 2013] |
| p.P51S | Dutch, Belgian, USA | 4th - 6th decade | +++ | Yes | Yes | dimer | [de Kok et al., 1999] | |
| p.V66G | USA | 2nd - 3rd decade | Yes | Yes | dimer | [Robertson et al., 1998] | ||
| p.G87V | Chinese | 5th decade | +++ | N/A | N/A | N/A | [Chen et al., 2013] | |
| p.G87W | Dutch | 5th decade | +++ | Yesb | Yes | - | [Collin et al., 2006] | |
| p.G88E | USA, Dutch | 5th - 6th decade | +- | Yes | Yes | dimer | [Kemperman et al., 2005] | |
| p.P89H | USA | congenital | N/A | Yes | Yes | - | [Dodson et al., 2012] | |
| p.V104del | Hungarian | 4th decade | +++ | Yes | No | dimer | [Nagy et al., 2004] | |
| p.I109N | Australian | 4th - 5th decade | +++ | Yes | Yes | N/A | [Kamarinos et al., 2001] | |
| p.I109T | Dutch | 5th decadeb | ++ | Yes | No | dimer | [Pauw et al., 2007b] | |
| p.L114P | Korean | N/A | N/A | N/A | N/A | N/A | [Choi et al., 2013] | |
| p.W117R | USA, Korean | 4th - 5th decade | +- | Yes | Yes | - | [Robertson et al., 1998] | |
| p.A119T | Japanese | 4th decade | +++ | Yes | Yes | - | [Usami et al., 2003] | |
| p.F121S | USA | 2nd - 3rd decade | +++ | Yes | No | dimer | [Hildebrand et al., 2010] | |
| vWFA 1 | p.C162Y | Chinese | 2nd decade | -- | No | No | aggregates | [Gao et al., 2013] |
| vWFA 2 | p.I399_A404del | USA | 2nd decade | -- | N/A | N/A | N/A | [Gallant et al., 2013] |
| p.A487P | Italian | 2nd decade | +- | No | No | aggregates | [Faletra et al., 2011] | |
| p.M512T | Chinese | 5th decade | -- | Yes | Yes | - | [Yuan et al., 2008] | |
| p.F527C | Korean | 3rd decadeb | -- | Yes | Yes | aggregates | [Cho et al., 2012] | |
| p.C542F | USA | 2nd decadeb | +- | Yes | Yes | N/A | [Street et al., 2005] | |
| p.C542Y | Chinese | 2nd - 5th decade | -- | Yes | Yes | aggregates | [Yuan et al., 2008] | |
The primary reference for each mutation is listed.
As the age of onset of auditory symptom in the pedigree is not specified on the previous reports, we represented the age of the youngest family member affected with hearing loss and/or vestibular disorder.
The results obtained in this study are presented in bold.
+++, present in all; ++, present in most; +-, present in some; --, none; N/A, not available.
Figure 6.
The correlation between relative amounts of accumulated mutant cochlins in cells and the age of onset in the mutations of COCH. The only outlier (p.P89H) represented one individual with an atypical presentation of unilateral hearing loss.
Interestingly, cochlins with vWFA domain mutations had the two highest band intensities in reduced form, indicative of increased intracellular cochlin retention as compared to those with LCCL domain mutations. These vWFA domain mutants were the same ones exhibiting high-molecular-weight intracellular aggregates (prominent smears above 130 kDa, up to greater than 170 kDa) in non-reduced form, and individuals with these vWFA domain COCH mutations had the earliest ages of onset of hearing loss among individuals with other DFNA9 mutations.
Discussion
Since the identification of COCH as the causative gene for DFNA9 in 1998, 21 COCH mutations have been reported, and several mechanisms by which COCH mutations cause hearing defects and vestibular disorder have been proposed (Chen, et al., 2013; Cho, et al., 2012; Choi, et al., 2013; Collin, et al., 2006; de Kok, et al., 1999; Dodson, et al., 2012; Faletra, et al., 2011; Gallant, et al., 2013; Gao, et al., 2013; Hildebrand, et al., 2010; Kamarinos, et al., 2001; Nagy, et al., 2004; Pauw, et al., 2007b; Robertson, et al., 1998; Street, et al., 2005; Usami, et al., 2003; Yao, et al., 2010; Yuan, et al., 2008). However, the exact function of cochlin and the role of cochlin mutations in hearing loss and vestibular dysfunction remain to be elucidated fully.
In this study, we first assessed various properties of eight previously uncharacterized mutant cochlins as compared to wild-type, through mammalian cell transfection experiments. We have demonstrated that LCCL domain mutant cochlins (p.G87W, p.P89H, p.V104del, p.I109T, p.A119T, and p.F121S) appear to be localized normally in the ER and Golgi complex. Of these, p.G87W, p.P89H, and p.A119T cochlin mutants were secreted into the media, whereas p.V104del, p.I109T, and p.F121S failed to be secreted and showed accumulation in cells in dimeric form. We also showed that p.C162Y and p.A487P mutants (in vWFA1 and vWFA2 domains, respectively) were not transported from the ER to the Golgi complex, and consequently failed to be secreted, but rather accumulated within cells. In contrast to LCCL domain mutants, showing dimerization, vWFA domain mutants were detected as additional smeared bands of larger, multimeric sizes (>130 kDa).
Although previous reports have shown formation of dimers and multimers of cochlins containing other LCCL mutations (p.P51S, p.V66G, p.G88E, p.W117R), these mutant cochlins did not show impaired secretion (Grabski, et al., 2003; Robertson, et al., 2003; Street, et al., 2005; Yao, et al., 2010). Also, while we previously demonstrated formation of high-molecular-weight aggregates for other vWFA domain mutants (p.F527C and p.C542Y), these mutant cochlins were secreted normally from the cell in monomeric form (Cho, et al., 2012). In our current study, analysis of previously uncharacterized LCCL domain and vWFA domain mutants revealed not only increased intracellular levels of mutant cochlins, with further formation and intracellular retention of higher-molecular-weight multimers, but also failure to progress through the normal secretory pathway, leading to absence of secreted mutant cochlins.
We evaluated the correlation between clinical history from DFNA9 patients and the molecular and biochemical data for cochlins with the corresponding COCH mutations (Table 1). Interestingly, individuals with the p.C162Y, p.I399_A404del, p.A487P, p.F527C, p.C542F, and p.C542Y mutations exhibited an early age of onset (average 2nd – 3rd decade), and all of these mutant cochlins, with the exception of p.I399_A404del and p.C542F, formed high-molecular-weight aggregates in cells. However, individuals with the p.M512T mutation, which did not accumulate in cells, showed a later age of onset (5th decade). Although multimerization of the p.C542F cochlin has not been evaluated, this mutant may also lead to aggregate formation, given that substitution of the cysteine residue in p.C542F will likely yield an abnormal cochlin structure, as has been observed for p.C542Y. These results indicate that accumulation of high-molecular-weight aggregates in cells and failure of cochlin secretion result in early onset of hearing loss in DFNA9 patients with vWFA domain mutations.
Another interesting correlation which has previously been noted (Street, et al., 2005), and further confirmed in our study, is presence of vestibular symptoms, (in particular those which are self-reported), in addition to hearing loss in cases with COCH mutations in the LCCL domain, as opposed to predominantly hearing deficits in cases with vWFA domain mutations. Cochlin is known to undergo post-translational cleavage between the LCCL and vWFA1 domains, resulting in release of the p16 kDa peptide consisting of the N-terminal LCCL module (Ikezono, et al., 2004; Robertson, et al., 2003). Further studies are warranted to elucidate the mechanism by which mutants in the LCCL domain result in vestibular malfunction in addition to hearing loss.
We demonstrated that the p.I109T mutant contains an additional N-linked glycosylation site at asparagine 107 and accumulated intracellularly as dimeric cochlin and was not secreted. N-linked glycosylation is known to be an essential factor for some secretory proteins and plays a role in promoting protein folding, sorting, transport, and quality control through mechanisms such as endoplasmic reticulum-associated protein degradation (ERAD) and lysosomal targeting (Helenius and Aebi, 2001). It is possible that this additional N-linked glycosylation leads to alterations in the normal secretory pathway. The other COCH mutation at the same I109 residue, namely I109N, which does not result in additional glycosylation, was secreted normally from cells (Grabski, et al., 2003; Kamarinos, et al., 2001). However, of the five mutant cochlins that accumulated in cell lysates, the p.I109T mutation appeared to result in the mildest form of accumulation and latest age of onset in DFNA9-affected individuals. These results suggest that the p.I109T mutant is more susceptible to N-linked glycosylation-related protein degradation than the p.V104del and p.F121S mutant proteins.
In contrast, our molecular and functional analyses of COCH mutations, p.G87W, p.P89H, and p.A119T, revealed no differences between mutant and wild-type proteins, indicating disruption of other processes involving cochlin function. One possibility is altered cochlin interactions with other secreted proteins. Cochlin domains likely bind to, or associate with, other components of the extracellular matrix, as shown by its high affinity for types I, II, and IV collagens (Nagy, et al., 2008). Cochlin has also been shown to have a role in innate immunity in follicular dendritic cells in the spleen and lymph nodes by post-translational cleavage and secretion of the LCCL domain p16kDa peptide, a phenomenon initially observed in the cochlea (Ikezono, et al., 2004; Robertson, et al., 2003), resulting in amplification of cytokines and recruitment of immune effector cells mediated by a rise in tumor necrosis factor-alpha (Py, et al., 2013). In addition, cochlin itself is a target of autoantibodies and of cochlin-specific T cell mediated immunity in some cases of autoimmune sensorineural hearing loss (Baek, et al., 2006; Boulassel, et al., 2001). It remains to be determined as to how these various properties of cochlin may be involved in pathology in the presence of COCH mutations.
In summary, we investigated various cellular and molecular properties of eight previously uncharacterized mutant cochlins, and identified a novel pathogenic mechanism of COCH mutations as failure of protein secretion concomitant with intracellular cochlin accumulation and aggregate formation. Furthermore, we performed an analysis of clinical presentation and symptoms of DFNA9 patients carrying 21 different COCH mutations, in correlation with specific molecular characteristics of these various mutant cochlins. These results illustrate that although all known COCH mutations are missense or in-frame-deletions, all with likely gain-of-function of the mutant protein, they exhibit differences in their characteristics and outcomes. We believe these findings provide not only valuable genotype-phenotype information, but also interesting pathogenic mechanisms in cellular transport, misfolding, aggregation, secretion, and post-translational processing as a result of the different COCH mutations. In addition, these studies can provide further insight into other disorders where protein aggregation is a major feature of the pathology.
Supplementary Material
Acknowledgments
This work was supported by the Korea Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI12C1004 to U.K.Kim and A111345 to K.Y.Lee) and National Institutes of Health grants (R01 DC003402 to C.C.M.)
REFERENCES
- Baek JI, Cho HJ, Choi SJ, Kim LS, Zhao C, Sagong BR, Kim UK, Jeong SW. The Trp117Arg mutation of the COCH gene causes deafness in Koreans. Clin Genet. 2010;77(4):399–403. doi: 10.1111/j.1399-0004.2009.01362.x. [DOI] [PubMed] [Google Scholar]
- Baek MJ, Park HM, Johnson JM, Altuntas CZ, Jane-Wit D, Jaini R, Solares CA, Thomas DM, Ball EJ, Robertson NG. Increased frequencies of cochlin-specific T cells in patients with autoimmune sensorineural hearing loss. J Immunol. 2006;177(6):4203–10. doi: 10.4049/jimmunol.177.6.4203. others. [DOI] [PubMed] [Google Scholar]
- Bause E, Legler G. The role of the hydroxy amino acid in the triplet sequence Asn-Xaa-Thr(Ser) for the N-glycosylation step during glycoprotein biosynthesis. Biochem J. 1981;195(3):639–44. doi: 10.1042/bj1950639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulassel MR, Tomasi JP, Deggouj N, Gersdorff M. COCH5B2 is a target antigen of anti-inner ear antibodies in autoimmune inner ear diseases. Otology & Neurotology. 2001;22(5):614–618. doi: 10.1097/00129492-200109000-00009. [DOI] [PubMed] [Google Scholar]
- Chen DY, Chai YC, Yang T, Wu H. Clinical characterization of a novel COCH mutation G87V in a Chinese DFNA9 family. Int J Pediatr Otorhinolaryngol. 2013;77(10):1711–5. doi: 10.1016/j.ijporl.2013.07.031. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Park HJ, Trexler M, Venselaar H, Lee KY, Robertson NG, Baek JI, Kang BS, Morton CC, Vriend G. A novel COCH mutation associated with autosomal dominant nonsyndromic hearing loss disrupts the structural stability of the vWFA2 domain. J Mol Med (Berl) 2012;90(11):1321–31. doi: 10.1007/s00109-012-0911-2. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BY, Park G, Gim J, Kim AR, Kim BJ, Kim HS, Park JH, Park T, Oh SH, Han KH. Diagnostic application of targeted resequencing for familial nonsyndromic hearing loss. PLoS One. 2013;8(8):e68692. doi: 10.1371/journal.pone.0068692. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin RW, Pauw RJ, Schoots J, Huygen PL, Hoefsloot LH, Cremers CW, Kremer H. Identification of a novel COCH mutation, G87W, causing autosomal dominant hearing impairment (DFNA9). Am J Med Genet A. 2006;140(16):1791–4. doi: 10.1002/ajmg.a.31354. [DOI] [PubMed] [Google Scholar]
- de Kok YJ, Bom SJ, Brunt TM, Kemperman MH, van Beusekom E, van der Velde-Visser SD, Robertson NG, Morton CC, Huygen PL, Verhagen WI. A Pro51Ser mutation in the COCH gene is associated with late onset autosomal dominant progressive sensorineural hearing loss with vestibular defects. Hum Mol Genet. 1999;8(2):361–6. doi: 10.1093/hmg/8.2.361. others. [DOI] [PubMed] [Google Scholar]
- Dodson KM, Georgolios A, Barr N, Nguyen B, Sismanis A, Arnos KS, Norris VW, Chapman D, Nance WE, Pandya A. Etiology of unilateral hearing loss in a national hereditary deafness repository. Am J Otolaryngol. 2012;33(5):590–4. doi: 10.1016/j.amjoto.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Faletra F, Pirastu N, Athanasakis E, Somaschini A, Pianigiani G, Gasparini P. A novel mutation in the vWFA2 domain of the COCH gene in an Italian DFNA9 family. Audiological Medicine. 2011;9(1):4–7. [Google Scholar]
- Fransen E, Van Camp G. The COCH gene: a frequent cause of hearing impairment and vestibular dysfunction? Br J Audiol. 1999;33(5):297–302. doi: 10.3109/03005369909090113. [DOI] [PubMed] [Google Scholar]
- Fransen E, Verstreken M, Bom SJ, Lemaire F, Kemperman MH, De Kok YJ, Wuyts FL, Verhagen WI, Huygen PL, McGuirt WT. A common ancestor for COCH related cochleovestibular (DFNA9) patients in Belgium and The Netherlands bearing the P51S mutation. J Med Genet. 2001;38(1):61–5. doi: 10.1136/jmg.38.1.61. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant E, Francey L, Fetting H, Kaur M, Hakonarson H, Clark D, Devoto M, Krantz ID. Novel COCH mutation in a family with autosomal dominant late onset sensorineural hearing impairment and tinnitus. Am J Otolaryngol. 2013;34(3):230–5. doi: 10.1016/j.amjoto.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Gao J, Xue J, Chen L, Ke X, Qi Y, Liu Y. Whole exome sequencing identifies a novel DFNA9 mutation, C162Y. Clin Genet. 2013;83(5):477–81. doi: 10.1111/cge.12006. [DOI] [PubMed] [Google Scholar]
- Gavel Y, von Heijne G. Sequence differences between glycosylated and non glycosylated Asn-X-Thr/Ser acceptor sites: implications for protein engineering. Protein Eng. 1990;3(5):433–42. doi: 10.1093/protein/3.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabski R, Szul T, Sasaki T, Timpl R, Mayne R, Hicks B, Sztul E. Mutations in COCH that result in non-syndromic autosomal dominant deafness (DFNA9) affect matrix deposition of cochlin. Hum Genet. 2003;113(5):406–16. doi: 10.1007/s00439-003-0992-7. [DOI] [PubMed] [Google Scholar]
- Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science. 2001;291(5512):2364–9. doi: 10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]
- Hildebrand MS, Gandolfo L, Shearer AE, Webster JA, Jensen M, Kimberling WJ, Stephan D, Huygen PL, Smith RJ, Bahlo M. A novel mutation in COCH-implications for genotype-phenotype correlations in DFNA9 hearing loss. Laryngoscope. 2010;120(12):2489–93. doi: 10.1002/lary.21159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand MS, Tack D, Deluca A, Hur IA, Van Rybroek JM, McMordie SJ, Muilenburg A, Hoskinson DP, Van Camp G, Pensak ML. Mutation in the COCH gene is associated with superior semicircular canal dehiscence. Am J Med Genet A. 2009;149A(2):280–5. doi: 10.1002/ajmg.a.32618. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikezono T, Shindo S, Li L, Omori A, Ichinose S, Watanabe A, Kobayashi T, Pawankar R, Yagi T. Identification of a novel Cochlin isoform in the perilymph: insights to Cochlin function and the pathogenesis of DFNA9. Biochem Biophys Res Commun. 2004;314(2):440–6. doi: 10.1016/j.bbrc.2003.12.106. [DOI] [PubMed] [Google Scholar]
- Jones SM, Robertson NG, Given S, Giersch AB, Liberman MC, Morton CC. Hearing and vestibular deficits in the Coch(−/−) null mouse model: comparison to the Coch(G88E/G88E) mouse and to DFNA9 hearing and balance disorder. Hear Res. 2011;272(1-2):42–8. doi: 10.1016/j.heares.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarinos M, McGill J, Lynch M, Dahl H. Identification of a novel COCH mutation, I109N, highlights the similar clinical features observed in DFNA9 families. Hum Mutat. 2001;17(4):351. doi: 10.1002/humu.37. [DOI] [PubMed] [Google Scholar]
- Kemperman MH, De Leenheer EM, Huygen PL, van Duijnhoven G, Morton CC, Robertson NG, Cremers FP, Kremer H, Cremers CW. Audiometric, vestibular, and genetic aspects of a DFNA9 family with a G88E COCH mutation. Otol Neurotol. 2005;26(5):926–33. doi: 10.1097/01.mao.0000185062.12458.87. [DOI] [PubMed] [Google Scholar]
- Kommareddi PK, Nair TS, Raphael Y, Telian SA, Kim AH, Arts HA, El-Kashlan HK, Carey TE. Cochlin isoforms and their interaction with CTL2 (SLC44A2) in the inner ear. J Assoc Res Otolaryngol. 2007;8(4):435–46. doi: 10.1007/s10162-007-0099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepinsh E, Trexler M, Kaikkonen A, Weigelt J, Banyai L, Patthy L, Otting G. NMR structure of the LCCL domain and implications for DFNA9 deafness disorder. EMBO J. 2001;20(19):5347–53. doi: 10.1093/emboj/20.19.5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makishima T, Rodriguez CI, Robertson NG, Morton CC, Stewart CL, Griffith AJ. Targeted disruption of mouse Coch provides functional evidence that DFNA9 hearing loss is not a COCH haploinsufficiency disorder. Hum Genet. 2005;118(1):29–34. doi: 10.1007/s00439-005-0001-4. [DOI] [PubMed] [Google Scholar]
- Nagy I, Horvath M, Trexler M, Repassy G, Patthy L. A novel COCH mutation, V104del, impairs folding of the LCCL domain of cochlin and causes progressive hearing loss. J Med Genet. 2004;41(1):e9. doi: 10.1136/jmg.2003.012286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy I, Trexler M, Patthy L. The second von Willebrand type A domain of cochlin has high affinity for type I, type II and type IV collagens. FEBS Lett. 2008;582(29):4003–7. doi: 10.1016/j.febslet.2008.10.050. [DOI] [PubMed] [Google Scholar]
- Pauw RJ, Collin RW, Huygen PL, Hoefsloot LH, Kremer H, Cremers CW. Clinical characteristics of a Dutch DFNA9 family with a novel COCH mutation, G87W. Audiol Neurootol. 2007a;12(2):77–84. doi: 10.1159/000097794. [DOI] [PubMed] [Google Scholar]
- Pauw RJ, Huygen PL, Collin RW, Cruysberg JR, Hoefsloot LH, Kremer H, Cremers CW. Phenotype description of a novel DFNA9/COCH mutation, I109T. Ann Otol Rhinol Laryngol. 2007b;116(5):349–57. doi: 10.1177/000348940711600506. [DOI] [PubMed] [Google Scholar]
- Py BF, Gonzalez SF, Long K, Kim MS, Kim YA, Zhu H, Yao J, Degauque N, Villet R, Ymele-Leki P. Cochlin produced by follicular dendritic cells promotes antibacterial innate immunity. Immunity. 2013;38(5):1063–72. doi: 10.1016/j.immuni.2013.01.015. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson NG, Cremers CW, Huygen PL, Ikezono T, Krastins B, Kremer H, Kuo SF, Liberman MC, Merchant SN, Miller CE. Cochlin immunostaining of inner ear pathologic deposits and proteomic analysis in DFNA9 deafness and vestibular dysfunction. Hum Mol Genet. 2006;15(7):1071–85. doi: 10.1093/hmg/ddl022. others. [DOI] [PubMed] [Google Scholar]
- Robertson NG, Hamaker SA, Patriub V, Aster JC, Morton CC. Subcellular localisation, secretion, and post-translational processing of normal cochlin, and of mutants causing the sensorineural deafness and vestibular disorder, DFNA9. J Med Genet. 2003;40(7):479–86. doi: 10.1136/jmg.40.7.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson NG, Jones SM, Sivakumaran TA, Giersch AB, Jurado SA, Call LM, Miller CE, Maison SF, Liberman MC, Morton CC. A targeted Coch missense mutation: a knock-in mouse model for DFNA9 late-onset hearing loss and vestibular dysfunction. Hum Mol Genet. 2008;17(21):3426–34. doi: 10.1093/hmg/ddn236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson NG, Lu L, Heller S, Merchant SN, Eavey RD, McKenna M, Nadol JB, Jr., Miyamoto RT, Linthicum FH, Jr., Lubianca Neto JF. Mutations in a novel cochlear gene cause DFNA9, a human nonsyndromic deafness with vestibular dysfunction. Nat Genet. 1998;20(3):299–303. doi: 10.1038/3118. others. [DOI] [PubMed] [Google Scholar]
- Sadler JE. Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem. 1998;67:395–424. doi: 10.1146/annurev.biochem.67.1.395. [DOI] [PubMed] [Google Scholar]
- Street VA, Kallman JC, Robertson NG, Kuo SF, Morton CC, Phillips JO. A novel DFNA9 mutation in the vWFA2 domain of COCH alters a conserved cysteine residue and intrachain disulfide bond formation resulting in progressive hearing loss and site-specific vestibular and central oculomotor dysfunction. Am J Med Genet A. 2005;139A(2):86–95. doi: 10.1002/ajmg.a.30980. [DOI] [PubMed] [Google Scholar]
- Trexler M, Banyai L, Patthy L. The LCCL module. Eur J Biochem. 2000;267(18):5751–7. doi: 10.1046/j.1432-1327.2000.01641.x. [DOI] [PubMed] [Google Scholar]
- Usami S, Takahashi K, Yuge I, Ohtsuka A, Namba A, Abe S, Fransen E, Patthy L, Otting G, Van Camp G. Mutations in the COCH gene are a frequent cause of autosomal dominant progressive cochleo-vestibular dysfunction, but not of Meniere's disease. Eur J Hum Genet. 2003;11(10):744–8. doi: 10.1038/sj.ejhg.5201043. [DOI] [PubMed] [Google Scholar]
- Yao J, Py BF, Zhu H, Bao J, Yuan J. Role of protein misfolding in DFNA9 hearing loss. J Biol Chem. 2010;285(20):14909–19. doi: 10.1074/jbc.M110.106724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan HJ, Han DY, Sun Q, Yan D, Sun HJ, Tao R, Cheng J, Qin W, Angeli S, Ouyang XM. Novel mutations in the vWFA2 domain of COCH in two Chinese DFNA9 families. Clin Genet. 2008;73(4):391–4. doi: 10.1111/j.1399-0004.2008.00972.x. others. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.