Abstract
Neuroendocrine tumours (NETs) may occur at many sites in the body although the majority occur within the gastroenteropancreatic axis. Non-gastroenteropancreatic NETs encompass phaeochromocytomas and paragangliomas, medullary thyroid carcinoma, anterior pituitary tumour, broncho-pulmonary NETs and parathyroid tumours. Like most endocrine tumours, NETs also express somatostatin (SST) receptors (subtypes 1–5) whose ligand SST is known to inhibit endocrine and exocrine secretions and have anti-tumour effects. In the light of this knowledge, the idea of using SST analogues in the treatment of NETs has become increasingly popular and new studies have centred upon the development of new SST analogues. We attempt to review SST receptor (SSTR) biology primarily in neuroendocrine tissues, focusing on pituitary tumours. A full data search was performed through PubMed over the years 2000–2009 with keywords ‘somatostatin, molecular biology, somatostatin receptors, somatostatin signalling, NET, pituitary’ and all relevant publications have been included, together with selected publications prior to that date. SSTR signalling in non-neuroendocrine solid tumours is beyond the scope of this review. SST is a potent anti-proliferative and anti-secretory agent for some NETs. The successful therapeutic use of SST analogues in the treatment of these tumours depends on a thorough understanding of the diverse effects of SSTR subtypes in different tissues and cell types. Further studies will focus on critical points of SSTR biology such as homo- and heterodimerization of SSTRs and the differences between post-receptor signalling pathways of SSTR subtypes.
Keywords: somatostatin, somatostatin receptor, molecular biology, receptor signalling
Introduction
Somatostatin (SST) is a cyclic peptide and a notable physiological regulator of neuroendocrine function across multiple organ systems [1]. It is produced by the hypothalamus, throughout the central nervous system (CNS), and in different peripheral organs including gastrointestinal tract (GIT) and pancreas[1–3]. The SST gene is located in chromosome 3q28 and encodes a 116 amino acid preprohormone (preprosomatostatin) which contains the 92 amino acid SST prohormone (prosomatostatin) [4]. Prosomatostatin is the precursor peptide of the two biologically active SST forms: 14 amino acid long SST-14 and amino-terminus extended SST-28 [5]. The biological roles of the two SST isoforms strongly overlap and the relative proportions of SST-14 to SST-28 change between different tissues [6, 7]. SST-14 is the predominant form in the brain (including the hypothalamus), whereas SST-28 is the major form in the GIT, especially the duodenum and jejunum [8].
SST has a broad range of biological actions including inhibition of exocrine secretions (gastric acid production, pancreatic enzyme, bile and colonic fluid secretion) and endocrine secretions in the pituitary (GH, TSH, prolactin), pancreas (insulin, gastrin, glucagon) and GIT (cholecystokinin, vasoactive intestinal peptide and secretin) [3, 9–12]. In the CNS, SST is highly expressed in the cortex, lateral septum, extended amygdala, reticular nucleus of the thalamus, hippocampus and many brain stem nuclei where it acts as a neurotransmitter and neuromodulator [8, 13]. The hippocampal SST receptor (SSTR)4 modulates memory formation by diminishing hippocampus-based spatial function while enhancing striatum-dependent behaviour. SSTR4-mediated regulation of neuronal activity in the hippocampus appears to depend on both competitive and cooperative interactions with SSTR2 [14]. In a study by Craft and colleagues [15], SST analogue infusion was shown to improve memory in patients with Alzheimer disease. Moreover, SST is inhibitory on cell survival and angiogenesis and has anti-proliferative activity on normal and neoplastic pituitary cells [16, 17], cancer cell lines [18, 19] and tumours in experimental animals [20]. There are five SSTR subtypes, each encoded by five separate genes on five separate chromosomes: 14, 17, 22, 20 and 16, respectively [4, 10, 12]. Sequence homology is 39–57% among the five subtypes [12]. SSTRs belong to the G-protein coupled receptor (GPCR) superfamily and are extensively distributed throughout many tissues ranging from the CNS to the pancreas and gut, and also in pituitary, kidney, thyroid, lung and immune cells [19], besides their presence in various cancer cells. The majority of tumours (and also normal tissues) express SSTR2, followed by SSTR1, SSTR5 and SSTR3, whereas SSTR4 is the least expressed subtype [19, 21, 22]. There are two different isoforms of SSTR2 (SSTR2A and SSTR2B) produced via alternative splicing [23, 24] and SSTR2B is almost unexpressed in human beings [9]. SSTRs bind natural peptides, SST-14 and SST-28, with similar high affinity (nM range) [10]. However, SSTR5 has a 10-fold higher affinity for SST-28 compared to SSTR2A or SSTR3, which have a higher affinity to SST-14 [25].
Investigations on SSTR signalling and determining the specific function of each SSTR pose difficulty due to various factors in experimental settings. First, the different SSTR subtypes can coexist in the same tissue and even on the same cell at different densities, and which type of signalling becomes predominant in the given cell depends on the cell-specific distribution of SSTR subtypes and signalling elements [4]. Second, experimental studies on SSTRs are generally performed in cell lines from different species and the significance of the findings of these studies for normal and malignant human tissues remains to be determined. Fortunately, a remarkable degree of structural conservation across species has been reported for SSTRs [13]. SSTR1 is the most highly conserved, with 97% identity between human beings and rat, whereas the sequence of SSTR5 is the most divergent, with 81% identity between human beings and rat [13]. There is 92%, 86% and 89% identity between human and rat SSTR2, SSTR3 and SSTR4, respectively [6, 26]. The main structural and functional features of SSTRs, including their signalling, endocytosis and recycling, have been extensively characterized using both primary cell cultures and, especially, cell lines stably transfected with different receptors from diverse species, and hence these data need careful interpretation [12, 25]. Third, it is now evident that GPCR function is highly dependent upon the cellular environment in which GPCRs are expressed, resulting in tissue-specific responses, even those originating from the same GPCR subtype [27]. In recent years, researchers have been obliged to find correlations between recombinant and native receptors and to ascribe specific functions to individual receptor subtypes in their own environment [28]. Fourth, although receptor-specific SST analogues have been generated, the discovery of receptor homo- and heterodimerization has added another level of complexity to the understanding of post-receptor events. SSTRs can form heterodimers with dopamine receptors [29], other SSTR subtypes [30], opioid receptors [31] or epidermal growth factor (EGF) receptors [32], which generate receptor oligomers with unique pharmacological profiles. However, it still has not been elucidated whether all these potential interactions do actually occur in the native cell, and, if they occur, what their precise functional relevance and importance are [33]. Fifth, it is known that, SSTR coupling to a given pathway can be strongly influenced by the ligand used [34–36], which is due to distinct conformations of the receptor/ligand complexes [37] induced by different SST analogues. Sixth and lastly, internalization, desensitization and/or receptor crosstalk [38, 39] are all known to occur in SSTRs, which may well have an impact on post-receptor signalling events. In addition, at the cellular level, crosstalk between signalling pathways may occur. All these factors might also explain the different downstream effects observed in SSTR signalling in different experimental settings.
Neuroendocrine tumours (NETs) are derived from a wide spectrum of different cell population and include, e.g. carcinoids, pituitary tumours, phaeochromocytomas, paragangliomas, medullary thyroid carcinomas and gastroenteropancreatic tumours such as insulinomas, gastrinomas and VIPomas [40]. This review will focus on the anti-tumour actions of SST, SSTR signalling pathways and the significance of our increasing knowledge on SST tissue receptor biology in terms of imaging and treatment of NETs. Cortistatin, a natural SSTR ligand which has both similar and different actions compared to SST, will not be included in this review (for detailed information regarding roles of cortistatin and SST, the review by Gahete et al. is recommended).
The somatostatin receptor as a GPCR
GPCRs are characterized by a core of seven transmembrane a-helices connected by three intra- and three extracellular loops [4]. The effector proteins of GPCR are heterotrimeric G proteins, which are composed of α, β and δ subunits. SSTRs belong to the GPCR superfamily and rhodopsin-like GPCR subclass [41]. SSTRs first activate a G protein which then modulates several downstream second messenger systems after binding [42]. Activated G protein depends on the SSTR subtype, and three isoforms of the inhibitory G proteins (Gαi1–3) are all coupled to the different SSTRs [43]. However, using an mRNA anti-sense strategy SSTR2 was reported to couple to the Gαo2/β2/γ3 complex to control Ca++ channel activity in pituitary cells [44, 45], and SSTR3 was also identified to couple to Gαo, Gα14 and α16[46].
Recent evidence indicates that, within the GPCR superfamily, there are mechanisms to increase receptor variability involving generation of splicing variants with less than seven transmembrane domains (TMDs) [47, 48]. Such truncated receptors, which may possess their own function or regulate the function of their respective long, canonical receptor isoforms, are frequently associated with tumour pathology [47, 48]. The family of SSTRs is known to uniquely comprise intronless genes except SSTR2. The only SST subtype variants known so far are the long (SSTR2A) and short (SSTR2B) isoforms of the SSTR2 gene, generated due to the presence of a cryptic splice site [49, 50]. Recently, Durán-Prado and colleagues have reported the first evidence for the existence of two functional human SSTR5 truncated isoforms of five and four TMDs, termed sst5TMD5 and sst5TMD4. These isoforms showed a unique expression pattern in normal tissues as well as in different pituitary tumour types, and display distinct functional responses to SST [51].
Binding of SST to its corresponding GPCR (SSTRs) triggers a cyclical activation and inactivation process in the G protein whereas the signal is transduced intracellularly. GPCRs are components of multiprotein networks, called ‘receptosomes’, which are organized around scaffolding proteins [28]. In this regard, GPCR-interacting proteins (GIPs), regulators of G-protein signalling (RGS) and GPCR kinases (GRKs) are the main proteins that are known to effect GPCR signalling after ligand binding. GIPs are transmembrane or cytosolic proteins which may alter either binding or functional responses of GPCR resulting in an abundance of potential receptor–protein connections [52]. RGS proteins have been found to regulate GPCR responses by binding to and stimulating the GTPase activity of the receptor-activated GTP-bound Gα[53]. Although GIPs and RGS have been found to interact with distinct recombinant SSTRs [54, 55], the real functional role of these complexes in native systems with respect to signal transduction is still not very well documented. SST binding is known to be rapidly followed by phosphorylation of SSTR1, SSTR2A and SSTR3 by GRKs [56–58]. The GRK family consists of six serine–threonine kinases that specifically bind to and phosphorylate agonist-activated GPCRs [59, 60]. GRKs play key roles in the fundamental pathways leading to phosphorylation-dependent GPCR desensitization, endocytosis, intracellular trafficking and resensitization, as well as in the modulation of important intracellular signalling cascades by GPCR [61]. Receptor phosphorylation by GRKs results in the recruitment of cytoplasmic proteins called arrestins in a receptor subtype-specific manner [62–64]. Arrestins are proteins involved in intracellular vesicle trafficking. In general, binding of arrestins to an activated, phosphorylated GPCR blocks further interaction between the receptors and G proteins, and thus results in the desensitization of G-protein mediated signalling. In addition, arrestins play a key role in directing GPCRs to clathrin-coated vesicles and thus preparing them for endocytosis [65]. The consequent receptor internalization removes GPCRs from the cell surface so that they are no longer available for agonist stimulation. This is a second mechanism for GPCR desensitization, and the concentration of different arrestins as well as GRK subtypes influences the extent of receptor internalization [66]. However, not all SSTRs internalize equally after agonist binding [67, 68]. SSTR2, SSTR3 and SSTR5 are internalized to a much higher extent than SSTR1 or SSTR4 after stimulation [25]. However, SSTR2 is rapidly recycled to the plasma membrane and does not enter any degradative pathway [25]. Desensitization and internalization are not induced by SSTR2 antagonists [69], but there is a growing body of evidence on agonist-induced desensitization and/or internalization of the SSTR2 subtypes, which have been assessed in several cell lines and tissues [21, 67, 70]. After activation and internalization through a clathrin-dependent pathway, SSTR3 and SSTR5 rapidly dissociate from arrestin and undergo ubiquitin-dependent lysosomal degradation, preventing plasma membrane recycling [64]. Arrestins have also been shown to play an important role in G-protein independent GPCR signalling by recruiting cytosolic molecules to the receptor–arrestin complex [71]. The precise role of receptor phosphorylation and arrestin binding in the desensitization, trafficking and signalling of different SSTR subtypes has not been fully elucidated as yet, but they probably play a critical role in SSTR function [65]. As desensitization is known to occur a few minutes after ligand binding, it is expected to have a strong impact in experimental settings in which second messenger systems are being evaluated [56, 72, 73].
Not only the nature of the SSTR subtypes present in a particular cellular environment but also the nature of the agonist is a critical determinant of the tissue response to SST [65]. In GPCRs, including SSTR signalling, a classical two-state model was accepted for a long time. This model proposed that the binding of an agonist shifted an equilibrium from ‘the inactive’, basal conformation of a receptor to ‘the active’ conformation by stabilizing the latter [74, 75]. According to this model the relative potencies of agonists for inducing any two biological effects from a single receptor would be the same [74, 75]. In studies evaluating receptor dynamics, analogue activities were generally retrieved from only one or two measurements; such as inhibition of hormone secretion or modulation of second messenger production [65]. Then these measurements were used to indicate the overall potency and efficacy of the analogues under investigation [65]. However, with this model it was not possible to explain the effect of many agonists which might induce only some of the possible responses following receptor activation, or to activate two effectors with different relative potencies [65]. New terms including ‘functionally selective agonism’ have been used to describe the idea that agonists can selectively activate different signalling pathways and responses via a single GPCR [65]. Described in detail in an elegant review by Schonbrunn, such selective signalling is explained by a model in which agonists not only exhibit different affinities for a receptor, but they also stabilize different active receptor conformations [65]. These differentially activated receptor structures determine the interaction of the receptor with the cytoplasmic proteins, including G proteins, kinases and phosphatases, and additionally its regulation by GRKs or arrestins [65]. Thus, this model predicts that in any given cell type, the relative potencies or efficacies of agonists for various biological effects may differ even though their actions are triggered by a single receptor subtype [65]. Meanwhile, it is clear that signalling by, and regulation of, SSTR2, SSTR4 and SSTR5, are sensitive to the nature of the specific activating agonist [65]. Thus, a basic and simple assumption that all agonists are functionally equivalent and produce the same spectrum of effects at individual SSTRs can not be accepted any longer [65].
Receptor homodimerization and oligomerization of SSTRs are other critical issues, as these processes have been shown to modify some properties of the receptors, such as ligand binding affinity, signal transduction, desensitization and up-regulation [30, 76–78]. It has been demonstrated that (excepting SSTR1) SSTRs transfected in different cell lines form homodimers and some form heterodimers with other SSTR subtypes and with different families of GPCR [29, 30, 79] (Table 1). Furthermore, GPCR dimerization is also regulated by ligand binding, yet not all receptors function similarly [80, 81]. Even in the same family, the behaviour of receptors can be opposite, as reported for SSTR2 and SSTR5, whose dimerization decreases or increases, respectively, after ligand binding [82, 83]. Interestingly, interactions among SSTRs, in other words heterodimerization between each other, seem to be highly selective, as not all the potential combinations occur. Nevertheless, not all the possibilities of SSTR heterodimers have been explored, because the interactions characterized so far are between murine SSTR2 and SSTR3 [76], human SSTR1 and SSTR5 [30], human SSTR4 and SSTR5 [84] and human SSTR2 and SSTR5, some being demonstrated under certain conditions [85]. Heterodimerization among these SSTR subtypes causes both distinct effects on SSTR functioning and modifies the endocytic process of these receptors [33]. An example for this may be the interaction between SSTR2 and SSTR3, which results in both inactivation and impaired internalization of SSTR3 [76]. Cotransfection of human SSTR1 and SSTR5 in Chinese hamster ovary (CHO)-K1 cells results in an increased ligand affinity for SST [30]. On the other hand, internalization of this heterodimer is better in response to a specific SSTR1 ligand and smaller in response to a specific SSTR5 ligand compared to monomer forms of these receptors [30]. Stabilization of human SSTR2 and SSTR5 heterodimers was shown to occur following selective activation of SSTR2 but not human SSTR5 or their concurrent stimulation [85]. Moreover, an increased recycling rate and a greater propensity of SSTR2 to signal and induce growth inhibition following its heterodimerization with SSTR5 were observed [86]. Heterodimerization of SSTRs is not limited to occur between SSTR subtypes. Murine SSTR2 has been shown to heterodimerize with the μ-opioid receptor, MOR1, in cotransfected human embryonic kidney (HEK)-293 cells. Although this interaction does not alter their signalling properties it induces a cross-modulation of the desensitization and internalization of both receptors [31]. Another example is heterodimerization of the dopamine receptor 2 (D2R) and SSTR5. In the basal state, in the absence of ligands, these receptors do not interact: D2R forms dimers and SSTR5 remains monomeric [29]. However, treatment with ligands for any of the receptors dramatically induces heterodimerization and enhanced inhibition of cAMP formation occurs [29]. Binding of quinpirole, a D2R agonist, increases binding affinity of SST by 3000%, whereas a D2R antagonist, sulpiride, decreases binding affinity for the peptide by 80%[29]. An interaction between the D2R and the human SSTR2 has been also reported, which does not seem to occur under basal conditions, but is induced by ligand binding [87]. Their interaction results in a heterodimer with an increased affinity for dopamine and increased signalling via the D2R receptor [87]. However, SSTR2 pharmacology and signalling are not altered by heterodimerization with the D2R, but its endocytic rate is increased as a consequence of this interaction [87]. Additionally, heterodimerization of SSTRs appears to confer the properties of one receptor subtype onto the dimer; SSTR1 is internalized and up-regulated by octreotide when heterodimerized with SSTR5 [30], and the SSTR3/SSTR2A heterodimer has the pharmacological effects of the SSTR2A receptor [76]. However, because of the fact that the dimerization of SSTRs has been observed in recombinant systems and experimental settings, it is not clear as to what degree it can be extended to native systems [33].
Table 1.
Summary of the studies on receptor oligomerization
| Study | Findings |
|---|---|
| Rocheville et al.[29] | In the absence of ligands, D2R forms dimers and SSTR5 remains monomeric. Treatment with ligands for any of the receptors induces heterodimerization of D2R and SSTR5. |
| Rocheville et al.[30] | Cotransfection of CHO-K1 cells results in an increased ligand affinity for SST and heterodimerization between human SSTR1 and SSTR5. |
| Pfeiffer et al.[31] | Direct evidence for heterodimerization of SSTR2A and MOR1 in HEK-293 cells, interaction does not alter signalling properties, induces a cross-modulation of the desensitization and internalization of both receptors. |
| Pfeiffer et al.[76] | SSTR2A and SSTR3 exist as homodimers at the plasma membrane in HEK-293 cells. Heterodimerization of SSTR2A and SSTR3 results in a new receptor with a pharmacological and functional profile resembling that of the SSTR2A. |
| Duran-Prado et al.[78] | Porcine SSTR2 is a potent inhibitory receptor displaying unique features of agonist-dependent dimerization, dissociation, internalization and re-association. |
| Grant et al.[82] | Agonist-dependent dissociation of self-associated human SSTR2 stably expressed in CHO-K1 and HEK-293 cells occurring in a concentration-dependent manner. |
| Grant et al.[83] | Human SSTR5 could both homodimerize and heterodimerize with human SSTR1 in the presence of SST, activation of human SSTR5 but not human SSTR1 is necessary for heterodimeric assembly in live cells. Human SSTR1 remained monomeric when expressed alone regardless of agonist exposure in live cells. |
| Somvanshi et al.[84] | Heterodimerization between human SSTR4/human SSTR5, but not between human SSTR4/human SSTR1. |
| Grant et al.[85] | SSTR2 and SSTR5 heterodimerize. Stabilization of human SSTR2 and SSTR5 heterodimers was shown to occur following selective activation of SSTR2 but not human SSTR5 or their concurrent stimulation. Heterodimerization increases the recycling rate of internalized SSTR2 by destabilizing its interaction with -arrestin. |
| Sharif et al.[86] | Increased recycling rate and a greater propensity of SSTR2 to signal and induce growth inhibition following its heterodimerization with SSTR5. |
| Baragli et al.[87] | Interaction between the D2R and the human SSTR2 does not seem to occur under basal conditions, but is induced by ligand binding. SSTR2 pharmacology and signalling are not altered by heterodimerization with the D2R, but its endocytic rate is increased as a consequence of this interaction. Ligand interaction results in a heterodimer with an increased affinity for dopamine and increased signalling via the D2R. |
Abbreviations: CHO-K1, Chinese hamster ovary cells; D2R, dopamin receptor 2; (HEK)-293, human embryonic kidney cells; MOR1, m-opioid receptor.
Anti-tumour effects of somatostatin
There are a number of mechanisms responsible for the anti-tumour actions of SST [88]. These are the direct blockade of cell cycle progression through the activation of phosphotyrosine phosphatases (PTPs), the indirect influence on tumour growth mediated by the inhibition of the production of growth factors that sustain tumour development, and an anti-angiogenic effect that involves the regulation of the activity of both endothelial cells and monocytes. Some of the key points in direct and indirect anti-tumour actions of SST will be summarized in this section.
In general, SSTR1, SSTR2, SSTR4 and SSTR5 produce cytostatic effects through similar downstream effector pathways, whereas SSTR2 and SSTR3 induces pro-apoptotic (cytotoxic) signals when activated [79, 89, 90] (Table 2) (for detailed information see next section). SST suppresses insulin-like growth factor (IGF)-I serum levels through a direct inhibition of its gene expression or through the inhibition of GH secretion from pituitary and the consequent reduction of the GH-stimulated IGF-1 production in liver. Moreover, SST analogues inhibit the secretion of autocrine/paracrine effectors of tumour cell survival such as the IGF-1 and -2, EGF, interleukin-6 and the transforming growth factor family. The attenuation of secretion of such survival factors in the tumour microenvironment accordingly establishes an autocrine/paracrine anti-proliferative effect [4].
Table 2.
Summary of SST action [4]
| Anti-mitotic (cytostatic) effects by SSTR1, SSTR2, SSTR4 and SSTR5 signalling |
| Apoptotic (cytotoxic) effects by SSTR2 and SSTR3 signalling |
| Direct blocking of autocrine/paracrine ‘survival factor’ secretion by cancer cells |
| Restoration of contact inhibition |
| Inhibition of blood vessel adhesion |
| Suppression of the GH/IGF-I axis: inhibition of GH secretion; negative regulation of IGF-I production; increased release of IGFBPs |
| Blockade of neovessel formation by vascular endothelial cells |
| Reduction of pro-angiogenic factors |
| Attenuation of monocyte activity in neoangiogenesis |
Stable transfection of pancreatic cancer cell lines with human SSTR2A was reported to induce an overexpression of the connexins 26 and 43, which resulted in formation of functional intercellular gap junctions and hence restored contact inhibition of cell proliferation [91].
During the initial stages of metastatic development, malignant cells must enter the lymphatic and systemic circulation by detaching from adjacent cells and then attaching to and disrupting the endothelial basement membrane [92, 93]. SST has been shown to reduce the adhesion of carcino-sarcoma cells to blood vessels and thus attenuate the metastatic potential of these tumours [94]. For the anti-angiogenic activity of SSTRs, three signal pathways were identified: inhibition of endothelial cell activity (proliferation, migration and invasion), inhibition of the synthesis and secretion of pro-angiogenic factors such as vascular endothelial growth factor and basic fibroblast growth factor, and inhibition of monocyte activation. Very recently, thrombospondin-1 was defined as a critical effector of the inhibitory role of SSTR2 on the neoangiogenesis and oncogenesis induced by pancreatic cancer cells [95].
Post-receptor signalling pathways
Studies in the signal transduction field have demonstrated that native SST inhibits the secretion and proliferation of both normal and neoplastic pituitary cells by inducing several intracellular pathways, depending on receptor subtype and target tissue [96]. The mechanisms whereby SSTRs transduce agonist induced messages into intracellular responses under different conditions and in different cells are complex [10]. The signalling pathways used by each receptor have not been fully elucidated yet, as different tissues and cells express different subtypes of receptors and most cells have more than one subtype of receptor. Besides, the modulated intracellular cascades may vary depending on the SST analogue, the SSTR subtype and – most importantly – according to the cell type used in that experiment [6, 68, 79, 97]. Although there are a number of specific receptor agonists, some agonists used in experimental settings can activate two receptors at the same time with different levels of affinities, which makes it difficult to clarify receptor-specific effects.
When SSTRs are activated by SST, the receptor interacts with heterotrimeric G protein which consists of α, β and γ subunits [41]. When an agonist activates GPCR, the GDP-bound Gαbβ heterotrimer interacts with the receptor and the α subunit decreases the affinity for GDP [41]. As the GTP concentration is higher in the cytoplasm, this results in substitution of GDP with GTP. Thereafter, Gα protein dissociates both from the receptor and βδ subunits and both modulate the activity of several intracellular pathways [41]. Among these there are several key enzymes, including PTPs, adenylyl cyclase (AC) and pathways including mitogen-activated protein kinase (MAPK) and phosphoinositol-3-kinase (PI3K)/Akt, which are modulated along with reduction of the Ca++ influx through voltage sensitive channels and the activation of K+ channels [98].
Phosphotyrosine phosphatases and their action on MAPK and PI3K/Akt pathways
In the human genome around 107 PTPs have been identified, which includes 38 so-called ‘classical PTPs’ with an elevated specificity for phosphotyrosines [99]. In studies using cells transfected with individual SSTR subtypes, all the five members of this receptor subfamily have been shown to couple to a number of PTPs, including the SH2 domain-containing cytosolic tyrosine phosphatases (SHP-1 and SHP-2), and the density-enhanced protein-tyrosine phosphatase-1 (DEP-1/PTPη) [38, 100]. SST also couples to protein-serine/threonine phosphatases (PP), including PP2A and PP2B (calcineurin).
The anti-proliferative action of SST-activated PTPs depends on altered growth factor signalling through the selective dephosphorylation and inactivation of their receptors, plus inhibition of one of the most important MAPK pathways for cell proliferation, namely the extracellular signal-regulated protein kinase 1/2 (ERK 1/2) pathway (Fig. 1), and control of the activity of another important downstream signalling pathway, PI3K/Akt (Fig. 2) [101]. The inhibition of the ERK 1/2 pathway can occur either indirectly via the inhibition of the growth factor tyrosine kinase receptors or directly by dephosphorylating ERK 1/2 or by hyperactivation of ERK 1/2 (see below) [102, 103]. Such altered growth factor signalling through the selective dephosphorylation and inactivation of their receptors have been reported for SHP-1 with the insulin receptor, for SHP-2 with the insulin, EGF and platelet-derived growth factor (PDGF) receptor [104–107], for DEP-1 with PDGF and the vascular endothelial growth factor receptor [108, 109] and for PTP1B with the EGF receptor [110].
Fig 1.
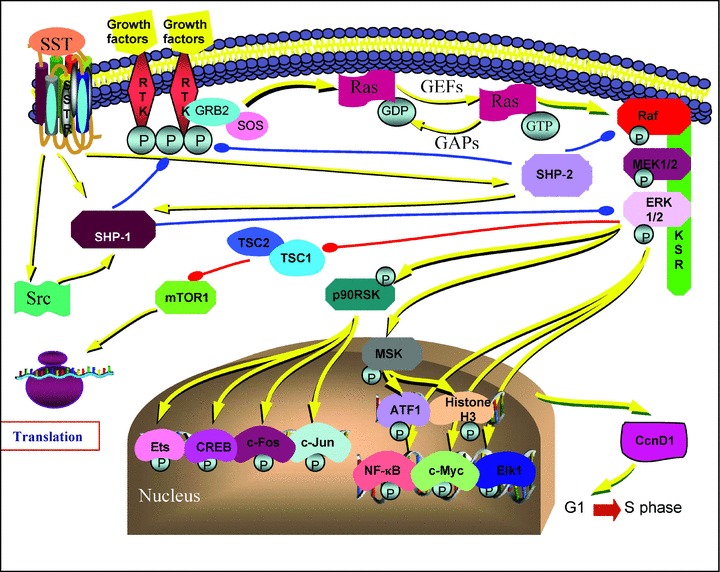
A simplified diagram of the Ras/ERK pathway and SST effects through PTPs, SHP-1 and SHP-2. Yellow lines show activation, red lines show inhibition of the corresponding protein. Note SHP-1 and SHP-2 inhibition of growth factor receptors, SHP-1 inhibition of ERK 1/2, SHP-2 inhibition of Raf kinase shown in blue lines. Src and SHP-2 activates SHP-1. As a result, SHP-1 and SHP-2 lead the cells to accumulate in G1 phase and inhibit entry in the S phase of the cell cycle. For simplicity the SSTR subtype-specific effects has not been shown separately. Abbreviations: ATF1, activating transcription factor; CcnD1, cyclin D1; CREB, cAMP responsive element binding protein; ERK, extracellular signal-regulated protein kinase; GAP, GTPase-activating protein; GEF, guanine nucleotide exchange factor; GRB2, growth factor receptor binding protein 2; KSR, kinase suppressor of Ras; mTOR, mammalian Target of Rapamycin; MEK, mitogen-activated protein/extracellular signal-regulated kinase (ERK) kinase; MSK, mitogen and stress activated kinase; NF-κB, nuclear factor-κB; p90RSK, p90 ribosomal S6 kinase; RTK, receptor tyrosine kinase; Src, cytosolic tyrosine kinase; SHP-1, SH-2 domain containing cytosolic tyrosine phosphatase 1; SHP-2, SH-2 domain containing cytosolic tyrosine phosphatase 2; SOS, mammalian son-of-sevenless; SST, somatostatin; SSTR, somatostatin receptor.
Fig 2.
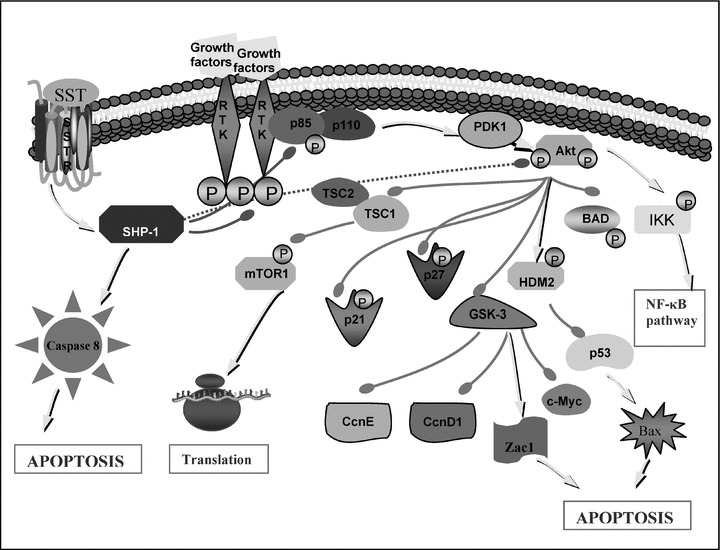
A simplified diagram of PI3K/Akt pathway and SST effects through PTP SHP-1. Arrowheads show activation, bold lines with rounded heads show inhibition of the corresponding protein. Note direct inhibition of growth factor receptor and p85 regulatory subunit of PI3K, and indirect inhibition of Akt pathway by SHP-1 shown in dotted lines. As a result, SHP-1 cause up-regulation of p21cip1/waf1 and p27kip1 and the tumour suppressor gene Zac1 and activation of caspase 8 and pro-apoptotic protein Bax. For simplicity, the SSTR subtype-specific effects has not been shown separately. Abbreviations: BAD, BCL2-antagonist of death; CcnD1, cyclin D1; CcnE, cyclin E; ERK, extracellular signal-regulated protein kinases; HDM2, human homolog of murine double minute ubiquitin ligase; GSK-3, glycogen synthase kinase-3; IKK, IκB kinase; mTOR, mammalian target of Rapamycin; NF-κB, nuclear factor-κB; p21, cyclin dependent kinase inhibitor p21Cip1/WAF1; p27, cyclin dependent kinase inhibitor p27Kip1; PDK1, phosphoinositide-dependent kinase 1; RTK, receptor tyrosine kinase; SHP-1, SH-2 domain containing cytosolic tyrosine phosphatase 1; SST, somatostatin; SSTR, somatostatin receptor.
SSTR activation of SHP-1 was reported to induce arrest of cell proliferation in human pituitary adenomas [111], GH3 rat pituitary tumour cells [112], in different tumour cell lines derived from pancreatic cancers (MIA-PaCa-2, PANC-1, PC-1) and from thyroid medullary carcinoma, among others [79, 101, 113–115]. SHP-1 activation is the critical step for SSTR2-mediated anti-proliferative signalling [101, 116]. Tyrosine phosphorylated SSTR2 interacts with and activates SHP-2 and Src, a cytosolic tyrosine kinase, inducing consequent SHP-1 recruitment and activation [117] (Fig. 1). In CHO cells, SST-activated SHP-1 rapidly associates to the insulin receptor causing a tyrosine dephosphorylation of both the receptor itself and its substrates (i.e. IRS-1, Shc) leading to a negative modulation of insulin mitogenic signalling [106]. Additionally, SHP-1 inhibits the ERK 1/2 pathway by directly or indirectly dephosphorylating ERK 1/2 [4], causes overexpression of the cyclin-dependent kinase inhibitor p27kip1 and an increase in hypophosphorylated retinoblastoma gene product (Rb) which, taken together, leads to inhibition of the entry in the S phase of the cell cycle and accumulation of the cells in G1 [118]. In pancreatic and pituitary tumour cells SSTR2 activation of SHP-1 was found to be involved in the p53-independent induction of apoptosis [119–121]. Additionally, a completely novel mechanism was identified in NIH3T3 (NIH Swiss mouse embryonic fibroblast cell line) cells in which the activation of SHP-1 by SSTR2 activates the transcription factor nuclear factor-κB causing an inhibition of the anti-apoptotic effects of the MAP kinase, Jun N-terminal kinase and, in turn, hyperactivation of caspase 8 and apoptosis [122]. Of interest in this regard is the finding that SSTR2 also affects the apoptosis induced by death receptors, sensitizing the cells to tumour necrosis factor (TNF)-α- and TRAIL-mediated responses through the up-regulation of their receptors: death receptor 4 and TNFR1 [119]. In essence, SHP-1 activity was also involved in SSTR3-dependent apoptosis in transfected CHO cells, but this time by involving the induction of p53 and the subsequent activation of the pro-apoptotic protein Bax [123]. In GH3 cells, SSTR2-induced activation of SHP-1 causes the dephosphorylation of p85 and hence the inhibition of PI3K activity [101]. As would be expected, inhibition of PI3K activity causes inhibition of PDK1 and Akt activities, which in turn results in the activation of the glycogen synthase kinase-3β (GSK-3β) (Fig. 2). Theodoropoulou and colleagues have reported that the enhanced GSK-3β activity up-regulated the expression of Zac1 gene, which ultimately induced growth arrest [101]. Zac1 is highly expressed in normal pituitary, mammary and ovarian glands but is down-regulated in pituitary, breast and ovarian tumours, suggesting that it might act as a tumour suppressor gene [101]. Their further study investigating the immunoreactivity of Zac1 protein in tumour tissues of patients treated with SST analogues prior to surgery has also revealed a significantly positive correlation between strong Zac1 immunoreactivity and IGF-I normalization and the presence of tumour shrinkage after SST analogue treatment [124].
In left, besides SHP-1 and SHP- 2 activity, another delayed and long-lasting PTP activity was also induced following SST treatment [125]. As opposed to SHP-1 and SHP-2 which are cytosolic, non-membrane PTPs, one of these PTPs was identified in a receptor like PTP named DEP-1 in human beings (PTPη in rats), whose tumour suppressor role had already been established [130]. It was suggested that, in endothelial cells, DEP-1/PTPη represents a possible effector of SSTR to inhibit tumoral angiogenesis and the activity of DEP-1 seems fundamental in blocking endothelial cell migration and proliferation [109, 131, 132]; the in vivo and in vitro anti-angiogenic activity of SST was dependent on the activation of PTPs [133, 134]. SSTR1-activated DEP-1 was shown to inhibit ERK 1/2 in PC C13 thyroid cells and SSTR1-, SSTR2- and SSTR5-activated DEP-1 was shown to inhibit ERK 1/2 in glioma cells [135]. In CHO-K1 cells expressing SSTR1, a large multimeric protein aggregation composed of the G protein, Jak2, SHP-2, Src and DEP-1 was observed in resting conditions in proximity to SSTR1 [136]. DEP-1 activation required the sequential activation of Jak2 (G-protein mediated), which phosphoryated SHP-2. When phosphorylated, SHP-2 shows increased activity, dissociates from the receptor and dephosphorylates the inhibitory tyrosine on Src which, activated this way, in turn, phosphorylate DEP-1 causing the sustained activity of this PTP to inactivate ERK 1/2 [136]. As activation of similar multieffector cascades by SSTR1 and SSTR2 involving a similar interaction of kinases and PTPs (Jak2, SHP-2, Src) leads to final PTP activation (SHP-1 or DEP-1), it has been suggested that this multieffector pathway could represent a common modular mechanism by which cytostatic mechanisms are induced by SST [117, 136].
The right PTP, SHP-2, was involved in the anti-proliferative activity of SST following SSTR1 [102, 125], SSTR2, SSTR3 and SSTR4 activation [126]. Besides dephosphorylating and inactivating the tyrosine kinase receptors for insulin and EGF [104, 107], SHP-2 is involved in cell growth arrest [125] (Fig. 1). It is known that the effects of ERK 1/2 on cell proliferation are also related to the duration and intensity of ERK 1/2 activation, and hyperactivation of the ERK 1/2 pathway can also cause cell cycle arrest [127]. In light of this knowledge, it is now known that the activation of both SSTR1 and SSTR2 induces cell cycle arrest via the hyperactivation of ERK 1/2 and the up-regulation of p21cip1/waf1 and p27kip1, respectively [102, 103]. In particular, the activation of SSTR1-induced ERK 1/2 pathway involved Src/SHP-2/PI3K/ras/Raf-1/MEK (mitogen-activated protein) [102], the SSTR2-regulated pathway involved SHP-1/SHP-2/PI3K/rap1 and ras/B-Raf/MEK [103] while, on the contrary, SSTR3 activation caused Raf-1 inactivation and blockade of the ERK 1/2 cascade [128, 129].
Anti-secretory action of SST through cAMP, K+ and Ca++ channels
The inhibitory effects of SST on cAMP production, and its effects on K+ and Ca++ currents, are mainly involved in the regulation of hormone secretion in different endocrine cells [19]. Decreased intracellular cAMP and Ca++ levels and increased outward K+ currents all lead to decreased hormone secretion [79].
Starting with cAMP, the negative modulation of AC activity leads to the regulation of cAMP production. This also has a negative impact on various downstream elements, in particular, protein kinase A, which is the major target for cAMP in the cell. The inhibition of AC by SST has been demonstrated in many different systems, including human pituitary adenomas [137]. All SSTR subtypes inhibit AC via the pertussis toxin-sensitive G-protein family, Gi/Go [65]. Specifically, SSTR2 decreased AC activity/cAMP accumulation in BON-1 (pancreatic NET cell line) and SH-SY5Y (neuroblastoma cell line) cells [138, 139] and in pituitary cells [140]. The stimulation of SSTR3 in CHO and HEK-293 cells decreases AC activity [141, 142]. SSTR5 decreased cAMP levels in human SH-SY5Y and BON-1 cells [138, 139] and in AtT-20 mouse pituitary corticotroph cells [34, 143–145].
SSTRs have been shown to directly (via G-protein activation of the K+ channel) or indirectly (via G-protein activation of second messenger pathways, which ultimately lead to activation of the K+ channel) activate a number of different K+ channels and increase K+ currents, leading to cell membrane hyperpolarization and a reduction in action potential frequency and duration [13, 68, 146, 147]. Specifically, SSTR2 (more efficiently than the other subtypes), SSTR3, SSTR4 and SSTR5 were shown to activate the G-protein gated inward-rectifier K+ channels [148]. However, in rat GH3 cells, SSTR1, SSTR2, SSTR4 and SSTR5 are all involved in the activation of delayed rectifying and transient outward K+ channels, although SSTR2 and SSTR4 were suggested to mediate the full activation of K+ current caused by SST [149].
There is a general agreement that SSTRs are linked to the direct inhibition of voltage-dependent Ca++ channels [28]. The SSTR1 inhibits Ca++ current in GH12C1 (rat pituitary tumour cell line) and rat insulinoma 1046–38 cells, [150] but not in GH3 cells [149]. The SSTR2 inhibits voltage-dependent Ca++ channels in certain cells, like GH12C1, RINm5F (rat insulinoma cell line), AtT-20 (a pituitary cell line) and GH3 cells [149–152] and again SSTR5 was shown to be coupled negatively to a Ca++ current in AtT-20 [152].
Other post-receptor pathways
Following the evidence showing SST-induced increase of inositol 1,4,5-trisphosphate (IP3) in bovine adrenal medullary cells [153], it is now widely accepted that SSTRs may affect the activity of phospholipase C (PLC) in native systems and, hence, modulate intracellular levels of IP3 and/or the activity of PLC-dependent protein kinase (PKC). SSTR1 was shown to mediate phospholipase PLC activation and IP3 production in CHO cells [154, 155]. SSTR2 mediates the activation of PLC in GH4C1 (rat pituitary tumour cell line) and F4C1 (rat pituitary cell line) cells [150, 154, 156]. Activation of PLC and IP3 production by SSTR4 was also observed in transfected COS (monkey kidney cell line) cells [157].
Phospholipase A2 (PLA2) is a cytoplasmic enzyme that hydrolyses triglycerol to form arachidonic acid, the metabolites of which have been shown to regulate different physiological and pathological functions [158, 159]. SST coupling negatively to arachidonate release has been shown in the rat anterior pituitary gland [160]. In contrast, SST has been suggested to generate arachidonic acid metabolites in rat GH4C1 cells, through pertussis toxin-sensitive G proteins [161]. Nevertheless, in a few cases SST was reported to induce cell proliferation through SSTR4 activation, which was observed to increase ERK 1/2 activity and, in turn, regulate PLA2 activation [162].
Moreover, also other MAPKs, more frequently associated to the induction of cell growth arrest, are regulated by SST: p38 is activated by SSTR2 and SSTR4 in CHO-K1 cells [98] and Jun N-terminal kinase is activated by SSTR5 [163].
SST was reported to regulate nitric oxide generation through the activation of both the endothelial and neuronal nitric oxide synthases (eNOS and nNOS, respectively). However, SST has been shown to have a dual effect on the release of nitric oxide. In different paradigms it has been shown to increase or inhibit nitric oxide levels via the involvement of different receptor subtypes and intracellular pathways [164]. SSTR2 caused a SHP-1-dependent dephosphorylation and activation of nNOS in mouse pancreas acini cells, which leads to increased nitric oxide production [165]. Nitric oxide is a major regulator of soluble guanylyl cyclase [28]. Guanylyl cyclase converts GTP to cyclic nucleotide guanosine monophosphate (cGMP), which is a regulatory mediator of cell proliferation [166]. The increased nitric oxide production in these cells results in activation of cGMP levels which is believed to be instrumental in growth arrest [164, 165]. cGMP exerts its effects via modulation of cGMP-dependent protein kinase (PKG), cGMP-gated ionic channels and cyclic nucleotide phosphodiesterases [167, 168]. Conversely, in a study by Cordelier and colleagues, inhibition of nNOS activity and a reduction in intracellular cGMP formation which resulted in cell growth arrest was observed in CHO cells expressing SSTR5 [169]. It was noted in this study that SSTR5 inhibits cell proliferation through SSTR5-mediated Src activation and subsequent nNOS tyrosine phosphorylation and inactivation, leading to a decrease of cGMP production and subsequent MAPK inhibition [166, 169]. SST-induced negative regulation of nitric oxide was also shown to be implicated in the inhibition of tumour angiogenesis and growth via the SSTR3-mediated negative regulation of eNOS [170].
The inhibition of Na+-H+ (NHE1) exchanger activity is responsible for anti-proliferative effects of SST in enteric endocrine cells and hepatic cells [79]. These effects are mediated by SSTR1, SSTR3 and SSTR4. Interestingly, the inhibition of NHE1 is the only intracellular signalling regulated by SSTRs that was reported to be pertussis toxin insensitive [171].
Involvement of serine–threonine phophatase in SST action was also implied in a study by Grozinsky-Glasberg and colleagues. In this study in INS-1 rat insulinoma cell line, both octreotide and the mTOR inhibitor everolimus (RAD001, Novartis, Basel, Switzerland) were shown inhibit cell proliferation and dephosphorylate tuberous sclerosis 2 (TSC2), which could imply activation of a serine–threonine phosphatase by these agents [172]. We have also shown that in the INS-1 cell line, the presence of a serine- threonine phosphatase inhibitor (okadaic acid) significantly reversed the anti-proliferative effect of octreotide (M. Cakir and A. Grossman, unpublished observations). This implies that blockade of activation of a serine–threonine phosphatase is a necessary component to the anti-proliferative activity of octreotide in this system.
Acknowledgments
A.G. has received Advisory Board and lecture fees from Novartis and Ipsen.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Bronstein MD. Acromegaly: molecular expression of somatostatin receptor subtypes and treatment outcome. In: Arzt E, Bronstein M, Guitelman M, editors. Front horm res, pituitary today: molecular, physiological and clinical aspects. Basel: Karger; 2006. pp. 129–34. [DOI] [PubMed] [Google Scholar]
- 2.Sam S, Frohman LA. Normal physiology of hypothalamic pituitary regulation. Endocrinol Metab Clin N Am. 2008;37:1–22. doi: 10.1016/j.ecl.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Reichlin S. Somatostatin. N Engl J Med. 1983;309:1495–501. doi: 10.1056/NEJM198312153092406. [DOI] [PubMed] [Google Scholar]
- 4.Msaouel P, Galanis E, Koutsilieris M. Somatostatin and somatostatin receptors: implications for neoplastic growth and cancer biology. Expert Opin Investig Drugs. 2009;18:1297–316. doi: 10.1517/13543780903176399. [DOI] [PubMed] [Google Scholar]
- 5.Shen LP, Pictet RL, Rutter WJ. Human somatostatin I: sequence of the cDNA. Proc Natl Acad Sci USA. 1982;79:4575–79. doi: 10.1073/pnas.79.15.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol. 1999;20:157–98. doi: 10.1006/frne.1999.0183. [DOI] [PubMed] [Google Scholar]
- 7.Moller LN, Stidsen CE, Hartmann B, et al. Somatostatin receptors. Biochim Biophys Acta. 2003;1616:1–84. doi: 10.1016/s0005-2736(03)00235-9. [DOI] [PubMed] [Google Scholar]
- 8.Low MJ. In: Williams textbook of endocrinology. Melmed S, editor. Philadelphia: Saunders Elsevier; 2008. pp. 85–155. [Google Scholar]
- 9.Ferone D, Gatto F, Arvigo M, et al. The clinical-molecular interface of somatostatin, dopamine and their receptors in pituitary pathophysiology. J Mol Endocrinol. 2009;42:361–70. doi: 10.1677/JME-08-0162. [DOI] [PubMed] [Google Scholar]
- 10.Pyronnet S, Bousquet C, Najib S, et al. Antitumor effects of somatostatin. Mol Cell Endocrinol. 2008;286:230–7. doi: 10.1016/j.mce.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Gahete MD, Durán-Prado M, Luque RM, et al. Are somatostatin and cortistatin two siblings in regulating endocrine secretions?In vitro work ahead. Mol Cell Endocrinol. 2008;286:128–34. doi: 10.1016/j.mce.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Siehler S, Nunn C, Hannon J, et al. Pharmacological profile of somatostatin and cortistatin receptors. Mol Cell Endocrinol. 2008;286:26–34. doi: 10.1016/j.mce.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Yang SK, Chen C. Involvement of somatostatin receptor subtypes in membrane ion channel modification by somatostatin in pituitary somatotropes. Clin Exp Pharmacol Physiol. 2007;34:1221–7. doi: 10.1111/j.1440-1681.2007.04806.x. [DOI] [PubMed] [Google Scholar]
- 15.Craft S, Asthana S, Newcomer JW, et al. Enhancement of memory in Alzheimer disease with insulin and somatostatin, but not glucose. Arch Gen Psychiatry. 1999;56:1135–40. doi: 10.1001/archpsyc.56.12.1135. [DOI] [PubMed] [Google Scholar]
- 14.Gastambide F, Lepousez G, Viollet C, et al. Cooperation between hippocampal somatostatin receptor subtypes 4 and 2: functional relevance in interactive memory systems. Hippocampus. 2009 doi: 10.1002/hipo.20680. Doi: 10.1002/hipo.20680. [DOI] [PubMed] [Google Scholar]
- 16.Cheung NW, Boyages SC. Somatostatin-14 and its analog octreotide exert a cytostatic effect on GH3 rat pituitary tumor cell proliferation via a transient G0/G1 cell cycle block. Endocrinology. 1995;136:4174–81. doi: 10.1210/endo.136.10.7664634. [DOI] [PubMed] [Google Scholar]
- 17.Lopez F, Estève JP, Buscail L, et al. Molecular mechanisms of antiproliferative effect of somatostatin: involvement of a tyrosine phosphatase. Metabolism. 1996;45:14–16. doi: 10.1016/s0026-0495(96)90071-2. [DOI] [PubMed] [Google Scholar]
- 18.Hoyer D, Bell GI, Berelowitz M, et al. Classification and nomenclature of somatostatin receptors. Trends Pharmacol Sci. 1995;16:86–88. doi: 10.1016/s0165-6147(00)88988-9. [DOI] [PubMed] [Google Scholar]
- 19.Weckbecker G, Lewis I, Albert R, et al. Opportunities in somatostatin research: biological, chemical and therapeutic aspects. Nat Rev Drug Discov. 2003;2:999–1017. doi: 10.1038/nrd1255. [DOI] [PubMed] [Google Scholar]
- 20.Bousquet C, Guillermet J, Vernejoul F, et al. Somatostatin receptors and regulation of cell proliferation. Dig Liver Dis. 2004;36:S2–7. doi: 10.1016/j.dld.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Liu Q, Cescato R, Dewi DA, et al. Receptor signaling and endocytosis are differentially regulated by somatostatin analogs. Mol Pharmacol. 2005;68:90–101. doi: 10.1124/mol.105.011767. [DOI] [PubMed] [Google Scholar]
- 22.Reubi JC, Waser B. Concomitant expression of several peptide receptors in neuroendocrine tumours: molecular basis for in vivo multireceptor tumour targeting. Eur J Nucl Med Mol Imaging. 2003;30:781–793. doi: 10.1007/s00259-003-1184-3. [DOI] [PubMed] [Google Scholar]
- 23.Missale C, Nash SR, Robinson SW, et al. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 24.Hofland LJ, Lamberts SWJ. The pathophysiological consequences of somatostatin receptor internalization and resistance. Endocr Rev. 2003;24:28–47. doi: 10.1210/er.2000-0001. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs S, Schulz S. Intracellular trafficking of somatostatin receptors. Mol Cell Endocrinol. 2008;286:58–62. doi: 10.1016/j.mce.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Reisine T, Bell GI. Molecular biology of somatostatin receptors. Endocr Rev. 1995;16:427–42. doi: 10.1210/edrv-16-4-427. [DOI] [PubMed] [Google Scholar]
- 27.Eglen RM. Emerging concepts in GPCR function-the influence of cell phenotype on GPCR pharmacology. Proc West Pharmacol Soc. 2005;48:31–4. [PubMed] [Google Scholar]
- 28.Cervia D, Bagnoli P. An update on somatostatin receptor signaling in native systems and new insights on their pathophysiology. Pharmacol Ther. 2007;116:322–41. doi: 10.1016/j.pharmthera.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 29.Rocheville M, Lange DC, Kumar U, et al. Receptors for dopamine and somatostatin: formation of hetero-oligomers with enhanced functional activity. Science. 2000;288:154–7. doi: 10.1126/science.288.5463.154. [DOI] [PubMed] [Google Scholar]
- 30.Rocheville M, Lange DC, Kumar U, et al. Subtypes of the somatostatin receptor assemble as functional homo- and heterodimers. J Biol Chem. 2000;275:7862–9. doi: 10.1074/jbc.275.11.7862. [DOI] [PubMed] [Google Scholar]
- 31.Pfeiffer M, Koch T, Schröder H, et al. Heterodimerization of somatostatin and opioid receptors cross-modulates phosphorylation, internalization, and desensitization. J Biol Chem. 2002;277:19762–72. doi: 10.1074/jbc.M110373200. [DOI] [PubMed] [Google Scholar]
- 32.Watt HL, Kharmate GD, Kumar U. Somatostatin receptors 1 and 5 heterodimerize with epidermal growth factor receptor: agonist-dependent modulation of the downstream MAPK signalling pathway in breast cancer cells. Cell Signal. 2009;21:428–39. doi: 10.1016/j.cellsig.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Durán-Prado M, Malagón MM, Gracia-Navarro F, et al. Dimerization of G protein-coupled receptors: new avenues for somatostatin receptor signalling, control and functioning. Mol Cell Endocrinol. 2008;286:63–8. doi: 10.1016/j.mce.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Cervia D, Nunn C, Fehlmann D, et al. Pharmacological characterisation of native somatostatin receptors in AtT-20 mouse tumour corticotrophs. Br J Pharmacol. 2003;139:109–21. doi: 10.1038/sj.bjp.0705235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nunn C, Cervia D, Langenegger D, et al. Comparison of functional profiles at human recombinant somatostatin sst2 receptor: simultaneous determination of intracellular Ca2+ and luciferase expression in CHO-K1 cells. Br J Pharmacol. 2004;142:150–60. doi: 10.1038/sj.bjp.0705735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cescato R, Loesch KA, Waser B, et al. Agonist-biased signaling at the sst2A receptor: the multi-somatostatin analogs KE108 and SOM230 activate and antagonize distinct signaling pathways. Mol Endocrinol. 2010;24:240–9. doi: 10.1210/me.2009-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tulipano G, Schulz S. Novel insights in somatostatin receptor physiology. Eur J Endocrinol. 2007;156:S3–11. doi: 10.1530/eje.1.02354. [DOI] [PubMed] [Google Scholar]
- 38.Lahlou H, Guillermet J, Hortala M, et al. Molecular signaling of somatostatin receptors. Ann NY Acad Sci. 2004;1014:121–31. doi: 10.1196/annals.1294.012. [DOI] [PubMed] [Google Scholar]
- 39.Waser B, Tamma ML, Cescato R, et al. Highly efficient in vivo agonist-induced internalization of sst2 receptors in somatostatin target tissues. J Nucl Med. 2009;50:936–41. doi: 10.2967/jnumed.108.061457. [DOI] [PubMed] [Google Scholar]
- 40.Koch CA, Pacak K, Chrousos GP. Endocrine tumors. In: Pizzo PA, Poplack DG, editors. Principles and practice of pediatric oncology. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 1139–72. [Google Scholar]
- 41.Gonzalez-Maeso J, Sealfon SC. Hormone signalling via G protein-coupled receptors. In: DeGroot LJ, Jameson JL, editors. Endocrinology. Philadelphia: Elsevier Saunders; 2006. pp. 177–203. [Google Scholar]
- 42.Schonbrunn A. Somatostatin action in pituitary cells involves two independent transduction mechanism. Metabolism. 1990;39:96–100. doi: 10.1016/0026-0495(90)90221-w. [DOI] [PubMed] [Google Scholar]
- 43.Meyerhof W. The elucidation of somatostatin receptor functions: a current view. Rev Physiol Biochem Pharmacol. 1998;133:55–108. doi: 10.1007/BFb0000613. [DOI] [PubMed] [Google Scholar]
- 44.Kleuss C, Scherubl H, Hescheler J, et al. Selectivity in signal transduction determined by gamma subunits of heterotrimeric G proteins. Science. 1993;259:832–34. doi: 10.1126/science.8094261. [DOI] [PubMed] [Google Scholar]
- 45.Kleuss C, Hescheler J, Ewel C, et al. Assignment of G-protein subtypes to specific receptors inducing inhibition of calcium currents. Nature. 1991;353:43–8. doi: 10.1038/353043a0. [DOI] [PubMed] [Google Scholar]
- 46.Komatsuzaki K, Murayama Y, Giambarella U, et al. A novel system that reports the G-proteins linked to a given receptor: a study of type 3 somatostatin receptor. FEBS Lett. 1997;406:165–70. doi: 10.1016/s0014-5793(97)00257-3. [DOI] [PubMed] [Google Scholar]
- 47.Havt A, Schally AV, Halmos G, et al. The expression of the pituitary growth hormone-releasing hormone receptor and its splice variants in normal and neoplastic human tissues. Proc Natl Acad Sci USA. 2005;102:17424–9. doi: 10.1073/pnas.0506844102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rekasi Z, Czompoly T, Schally AV, et al. Isolation and sequencing of cDNAs for splice variants of growth hormone-releasing hormone receptors from human cancers. Proc Natl Acad Sci USA. 2000;97:10561–6. doi: 10.1073/pnas.180313297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reisine T, Kong H, Raynor K, et al. Splice variant of the somatostatin receptor 2 subtype, somatostatin receptor 2B, couples to adenylyl cyclase. Mol Pharmacol. 1993;44:1016–20. [PubMed] [Google Scholar]
- 50.Vanetti M, Kouba M, Wang X, et al. Cloning and expression of a novel mouse somatostatin receptor (SSTR2B) FEBS Lett. 1992;311:290–4. doi: 10.1016/0014-5793(92)81122-3. [DOI] [PubMed] [Google Scholar]
- 51.Durán-Prado M, Gahete MD, Martìnez-Fuentes AJ, et al. Identification and characterization of two novel truncated but functional isoforms of the somatostatin receptor subtype 5 differentially present in pituitary tumors. J Clin Endocrinol Metab. 2009;94:2634–43. doi: 10.1210/jc.2008-2564. [DOI] [PubMed] [Google Scholar]
- 52.Bockaert J, Fagni L, Dumuis A, et al. GPCR interacting proteins (GIP) Pharmacol Ther. 2004;103:203–21. doi: 10.1016/j.pharmthera.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 53.Jean-Baptiste G, Yang Z, Greenwood MT. Regulatory mechanisms involved in modulating RGS function. Cell Mol Life Sci. 2006;63:1969–85. doi: 10.1007/s00018-006-6066-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zitzer H, Honck HH, Bachner D, et al. Somatostatin receptor interacting protein defines a novel family of multidomain proteins present in human and rodent brain. J Biol Chem. 1999;274:32997–3001. doi: 10.1074/jbc.274.46.32997. [DOI] [PubMed] [Google Scholar]
- 55.Kong JL, Panetta R, Song W, et al. Inhibition of somatostatin receptor 5-signaling by mammalian regulators of G-protein signaling (RGS) in yeast. Biochim Biophys Acta. 2002;1542:95–105. doi: 10.1016/s0167-4889(01)00170-7. [DOI] [PubMed] [Google Scholar]
- 56.Elberg G, Hipkin RW, Schonbrunn A. Homologous and heterologous regulation of somatostatin receptor 2. Mol Endocrinol. 2002;16:2502–14. doi: 10.1210/me.2002-0207. [DOI] [PubMed] [Google Scholar]
- 57.Liu Q, Schonbrunn A. Agonist-induced phosphorylation of somatostatin receptor subtype 1 (sst1). Relationship to desensitization and internalization. J Biol Chem. 2001;276:3709–17. doi: 10.1074/jbc.M008873200. [DOI] [PubMed] [Google Scholar]
- 58.Roth A, Kreienkamp HJ, Meyerhof W, et al. Phosphorylation of four amino acid residues in the carboxyl terminus of the rat somatostatin receptor subtype 3 is crucial for its desensitization and internalization. J Biol Chem. 1997;272:23769–74. doi: 10.1074/jbc.272.38.23769. [DOI] [PubMed] [Google Scholar]
- 59.Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Ann Rev Physiol. 2007;69:451–82. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 60.Reiter E, Lefkowitz RJ. GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab. 2006;17:159–65. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 61.Ribas C, Penela P, Murga C, et al. The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. Biochim Biophys Acta. 2006;1768:913–22. doi: 10.1016/j.bbamem.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 62.Brasselet S, Guillen S, Vincent JP, et al. Beta-Arrestin is involved in the desensitization but not in the internalization of the somatostatin receptor 2A expressed in CHO cells. FEBS Lett. 2002;516:124–8. doi: 10.1016/s0014-5793(02)02517-6. [DOI] [PubMed] [Google Scholar]
- 63.Kreuzer OJ, Krisch B, Dery O, et al. Agonist mediated endocytosis of rat somatostatin receptor subtype 3 involves beta-arrestin and clathrin coated vesicles. J Neuroendocrinol. 2001;13:279–87. doi: 10.1046/j.1365-2826.2001.00630.x. [DOI] [PubMed] [Google Scholar]
- 64.Tulipano G, Stumm R, Pfeiffer M, et al. Differential beta-arrestin trafficking and endosomal sorting of somatostatin receptor subtypes. J Biol Chem. 2004;279:21374–82. doi: 10.1074/jbc.M313522200. [DOI] [PubMed] [Google Scholar]
- 65.Schonbrunn A. Selective agonism in somatostatin receptor signaling and regulation. Mol Cell Endocrinol. 2008;286:35–9. doi: 10.1016/j.mce.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Menard L, Ferguson SS, Zhang J, et al. Synergistic regulation of beta2-adrenergic receptor sequestration: intracellular complement of beta-adrenergic receptor kinase and beta-arrestin determine kinetics of internalization. Mol Pharmacol. 1997;51:800–8. [PubMed] [Google Scholar]
- 67.Csaba Z, Dournaud P. Cellular biology of somatostatin receptors. Neuropeptides. 2001;35:1–23. doi: 10.1054/npep.2001.0848. [DOI] [PubMed] [Google Scholar]
- 68.Olias G, Viollet C, Kusserow H, et al. Regulation and function of somatostatin receptors. J Neurochem. 2004;89:1057–91. doi: 10.1111/j.1471-4159.2004.02402.x. [DOI] [PubMed] [Google Scholar]
- 69.Cescato R, Schulz S, Waser B, et al. Internalization of sst2, sst3, and sst5 receptors: effects of somatostatin agonists and antagonists. J Nucl Med. 2006;47:502–11. [PubMed] [Google Scholar]
- 70.Csaba Z, Simon A, Helboe L, et al. Neurochemical characterization of receptor-expressing cell populations by in vivo agonist-induced internalization: insights from the somatostatin sst2A receptor. J Comp Neurol. 2002;454:192–9. doi: 10.1002/cne.10430. [DOI] [PubMed] [Google Scholar]
- 71.Dewire SM, Ahn S, Lefkowitz RJ, et al. Beta-Arrestins and cell signaling. Ann Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 72.Engstrom M, Savola JM, Wurster S. Differential efficacies of somatostatin receptor agonists for G-protein activation and desensitization of somatostatin receptor subtype 4-mediated responses. J Pharmacol Exp Ther. 2006;316:1262–68. doi: 10.1124/jpet.105.094128. [DOI] [PubMed] [Google Scholar]
- 73.Hipkin RW, Friedman J, Clark RB, et al. Agonist-induced desensitization, internalization, and phosphorylation of the sst2a somatostatin receptor. J Biolog Chem. 1997;272:13869–76. doi: 10.1074/jbc.272.21.13869. [DOI] [PubMed] [Google Scholar]
- 74.Kenakin T. Principles: receptor theory in pharmacology. Trends Pharmacol Sci. 2004;25:186–92. doi: 10.1016/j.tips.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 75.Perez DM, Karnik SS. Multiple signaling states of G-protein-coupled receptors. Pharmacol Rev. 2005;57:147–61. doi: 10.1124/pr.57.2.2. [DOI] [PubMed] [Google Scholar]
- 76.Pfeiffer M, Koch T, Schroder H, et al. Homo- and heterodimerization of somatostatin receptor subtypes. Inactivation of sst(3) receptor function by heterodimerization with sst(2A) J Biol Chem. 2001;276:14027–36. doi: 10.1074/jbc.M006084200. [DOI] [PubMed] [Google Scholar]
- 77.Patel RC, Kumar U, Lamb DC, et al. Ligand binding to somatostatin receptors induces receptor-specific oligomer formation in live cells. Proc Natl Acad Sci USA. 2002;99:3294–9. doi: 10.1073/pnas.042705099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duran-Prado M, Bucharles C, Gonzalez BJ, et al. Porcine somatostatin receptor 2 displays typical pharmacological sst2 features but unique dynamics of homodimerization and internalization. Endocrinology. 2007;148:411–21. doi: 10.1210/en.2006-0920. [DOI] [PubMed] [Google Scholar]
- 79.Florio T. Molecular mechanisms of the antiproliferative activity of somatostatin receptors (SSTRs) in neuroendocrine tumors. Front Biosci. 2008;13:822–40. doi: 10.2741/2722. [DOI] [PubMed] [Google Scholar]
- 80.Milligan G. G protein-coupled receptor dimerization: function and ligand pharmacology. Mol Pharmacol. 2004;66:1–7. doi: 10.1124/mol.104.000497.. [DOI] [PubMed] [Google Scholar]
- 81.Pfleger KD, Eidne KA. Monitoring the formation of dynamic G protein- coupled receptor-protein complexes in living cells. Biochem J. 2005;385:625–37. doi: 10.1042/BJ20041361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grant M, Collier B, Kumar U. Agonist-dependent dissociation of human somatostatin receptor 2 dimers: a role in receptor trafficking. J Biol Chem. 2004;279:36179–83. doi: 10.1074/jbc.M407310200. [DOI] [PubMed] [Google Scholar]
- 83.Grant M, Patel RC, Kumar U. The role of subtype-specific ligand binding and the C-tail domain in dimer formation of human somatostatin receptors. J Biol Chem. 2004;279:38636–43. doi: 10.1074/jbc.M406276200. [DOI] [PubMed] [Google Scholar]
- 84.Somvanshi RK, Billova S, Kharmate G, et al. C-tail mediated modulation of somatostatin receptor type-4 homo- and heterodimerizations and signaling. Cell Signal. 2009;21:1396–414. doi: 10.1016/j.cellsig.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 85.Grant M, Alturaihi H, Jaquet P, et al. Cell growth inhibition and functioning of human somatostatin receptor type 2 are modulated by receptor heterodimerization. Mol Endocrinol. 2008;22:2278–92. doi: 10.1210/me.2007-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sharif N, Gendron L, Wowchuk J, et al. Coexpression of somatostatin receptor subtype 5 affects internalization and trafficking of somatostatin receptor subtype 2. Endocrinology. 2007;148:2095–105. doi: 10.1210/en.2006-1266. [DOI] [PubMed] [Google Scholar]
- 87.Baragli A, Alturaihi H, Watt HL, et al. Heterooligomerization of human dopamine receptor 2 and somatostatin receptor 2 Co-immunoprecipitation and fluorescence resonance energy transfer analysis. Cell Signal. 2007;19:2304–16. doi: 10.1016/j.cellsig.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 88.Reubi JC, Laissue JA. Multiple actions of somatostatin in neoplastic disease. Trends Pharmacol Sci. 1995;16:110–5. doi: 10.1016/s0165-6147(00)88992-0. [DOI] [PubMed] [Google Scholar]
- 89.Sharma K, Srikant CB. Induction of wild-type p53, Bax, and acidic endonuclease during somatostatin-signaled apoptosis in MCF-7 human breast cancer cells. Int J Cancer. 1998;76:259–66. doi: 10.1002/(sici)1097-0215(19980413)76:2<259::aid-ijc14>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 90.Srikant CB. Cell cycle dependent induction of apoptosis by somatostatin analog SMS 201–995 in AtT-20 mouse pituitary cells. Biochem Biophys Res Commun. 1995;209:400–6. doi: 10.1006/bbrc.1995.1517. [DOI] [PubMed] [Google Scholar]
- 91.Lahlou H, Fanjul M, Pradayrol L, et al. Restoration of functional gap junctions through internal ribosome entry site-dependent synthesis of endogenous connexins in density-inhibited cancer cells. Mol Cell Biol. 2005;25:4034–45. doi: 10.1128/MCB.25.10.4034-4045.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Msaouel P, Pissimissis N, Halapas A, et al. Mechanisms of bone metastasis in prostate cancer: clinical implications. Best Pract Res Clin Endocrinol Metab. 2008;22:341–55. doi: 10.1016/j.beem.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 93.Koutsilieris M. Skeletal metastases in advanced prostate cancer: cell biology and therapy. Crit Rev Oncol Hematol. 1995;18:51–64. doi: 10.1016/1040-8428(94)00122-a. [DOI] [PubMed] [Google Scholar]
- 94.Gastpar H, Zoltobrocki M, Weissgerber PW. Res Exp Med. Vol. 182. (Berl): 1983. The inhibition of cancer cell stickiness by somatostatin; pp. 1–6. [DOI] [PubMed] [Google Scholar]
- 95.Laklai H, Laval S, Dumartin L, et al. Thrombospondin-1 is a critical effector of oncosuppressive activity of sst2 somatostatin receptor on pancreatic cancer. Proc Natl Acad Sci USA. 2009;106:17769–74. doi: 10.1073/pnas.0908674106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ferjoux G, Bousquet C, Cordelier P, et al. Signal transduction of somatostatin receptors negatively controlling cell proliferation. J Physiol. 2000;94:205–10. doi: 10.1016/s0928-4257(00)00206-0. [DOI] [PubMed] [Google Scholar]
- 97.Alderton F, Humphrey PP, Sellers LA. High-intensity p38 kinase activity is critical for p21(cip1) induction and the antiproliferative function of G(i) protein-coupled receptors. Mol Pharmacol. 2001;59:1119–28. doi: 10.1124/mol.59.5.1119. [DOI] [PubMed] [Google Scholar]
- 98.Florio T. Somatostatin/somatostatin receptor signalling: phosphotyrosine phosphatases. Mol Cell Endocrinol. 2008;286:40–8. doi: 10.1016/j.mce.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 99.Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol. 2006;7:833–46. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- 100.Pan MG, Florio T, Stork PJ. G protein activation of a hormone-stimulated phosphatase in human tumor cells. Science. 1992;256:1215–7. doi: 10.1126/science.256.5060.1215. [DOI] [PubMed] [Google Scholar]
- 101.Theodoropoulou M, Zhang J, Laupheimer S, et al. Octreotide, a somatostatin analogue, mediates its antiproliferative action in pituitary tumor cells by altering phosphatidylinositol 3-kinase signaling and inducing Zac1 expression. Cancer Res. 2006;66:1576–82. doi: 10.1158/0008-5472.CAN-05-1189. [DOI] [PubMed] [Google Scholar]
- 102.Florio T, Yao H, Carey KD, et al. Somatostatin activation of mitogen-activated protein kinase via somatostatin receptor 1 (SSTR1) Mol Endocrinol. 1999;13:24–37. doi: 10.1210/mend.13.1.0224. [DOI] [PubMed] [Google Scholar]
- 103.Lahlou H, Saint-Laurent N, Esteve JP, et al. sst2 Somatostatin receptor inhibits cell proliferation through Ras-, Rap1-, and B-Raf-dependent ERK2 activation. J Biol Chem. 2003;278:39356–71. doi: 10.1074/jbc.M304524200. [DOI] [PubMed] [Google Scholar]
- 104.Held-Feindt J, Forstreuter F, Pufe T, et al. Influence of the somatostatin receptor sst2 on growth factor signal cascades in human glioma cells. Brain Res Mol Brain Res. 2001;87:12–21. doi: 10.1016/s0169-328x(00)00225-4. [DOI] [PubMed] [Google Scholar]
- 105.Cattaneo MG, Scita G, Vicentini LM. Somatostatin inhibits PDGF-stimulated Ras activation in human neuroblastoma cells. FEBS Lett. 1999;459:64–8. doi: 10.1016/s0014-5793(99)01218-1. [DOI] [PubMed] [Google Scholar]
- 106.Bousquet C, Delesque N, Lopez F, et al. sst2 somatostatin receptor mediates negative regulation of insulin receptor signaling through the tyrosine phosphatase SHP-1. J Biol Chem. 1998;273:7099–106. doi: 10.1074/jbc.273.12.7099. [DOI] [PubMed] [Google Scholar]
- 107.Florio T, Arena S, Thellung S, et al. The activation of the phosphotyrosine phosphatase eta (r-PTP eta) is responsible for the somatostatin inhibition of PCCl3 thyroid cell proliferation. Mol Endocrinol. 2001;15:1838–52. doi: 10.1210/mend.15.10.0713. [DOI] [PubMed] [Google Scholar]
- 108.Kovalenko M, Denner K, Sandström J, et al. Site-selective dephosphorylation of the platelet-derived growth factor beta-receptor by the receptor-like protein-tyrosine phosphatase DEP-1. J Biol Chem. 2000;275:16219–26. doi: 10.1074/jbc.275.21.16219. [DOI] [PubMed] [Google Scholar]
- 109.Grazia Lampugnani M, Zanetti A, Corada M, et al. Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. J Cell Biol. 2003;161:793–804. doi: 10.1083/jcb.200209019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Milarski KL, Zhu G, Pearl CG, et al. Sequence specificity in recognition of the epidermal growth factor receptor by protein tyrosine phosphatase 1B. J Biol Chem. 1993;268:23634–9. [PubMed] [Google Scholar]
- 111.Florio T, Thellung S, Corsaro A, et al. Characterization of the intracellular mechanisms mediating somatostatin and lanreotide inhibition of DNA synthesis and growth hormone release from dispersed human GH-secreting pituitary adenoma cells in vitro. Clin Endocrinol. 2003;59:115–28. doi: 10.1046/j.1365-2265.2003.01811.x. [DOI] [PubMed] [Google Scholar]
- 112.Dasgupta P, Singh AT, Mukherjee R. Antiproliferative and GH inhibitory activity of chimeric peptides consisting of GHRP-6 and somatostatin. Biochem Biophys Res Commun. 1999;259:379–84. doi: 10.1006/bbrc.1999.0773. [DOI] [PubMed] [Google Scholar]
- 113.Thangaraju M, Sharma K, Liu D, et al. Interdependent regulation of intracellular acidification and SHP-1 in apoptosis. Cancer Res. 1999;59:1649–54. [PubMed] [Google Scholar]
- 114.Zapata PD, Ropero RM, Valencia AM, et al. Autocrine regulation of human prostate carcinoma cell proliferation by somatostatin through the modulation of the SH2 domain containing protein tyrosine phosphatase (SHP)-1. J Clin Endocrinol Metab. 2002;87:915–26. doi: 10.1210/jcem.87.2.8194. [DOI] [PubMed] [Google Scholar]
- 115.Zatelli MC, Piccin D, Tagliati F, et al. SRC homology-2-containing protein tyrosine phosphatase-1 restrains cell proliferation in human medullary thyroid carcinoma. Endocrinology. 2005;146:2692–8. doi: 10.1210/en.2005-0001. [DOI] [PubMed] [Google Scholar]
- 116.Lopez F, Estève JP, Buscail L, et al. The tyrosine phosphatase SHP-1 associates with the sst2 somatostatin receptor and is an essential component of sst2-mediated inhibitory growth signaling. J Biol Chem. 1997;272:24448–54. doi: 10.1074/jbc.272.39.24448. [DOI] [PubMed] [Google Scholar]
- 117.Ferjoux G, Lopez F, Esteve JP, et al. Critical role of Src and SHP-2 in sst2 somatostatin receptor-mediated activation of SHP-1 and inhibition of cell proliferation. Mol Biol Cell. 2003;14:3911–28. doi: 10.1091/mbc.E03-02-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pagès P, Benali N, Saint-Laurent N, et al. sst2 somatostatin receptor mediates cell cycle arrest and induction of p27(Kip1). Evidence for the role of SHP-1. J Biol Chem. 1999;274:15186–93. doi: 10.1074/jbc.274.21.15186. [DOI] [PubMed] [Google Scholar]
- 119.Guillermet J, Saint-Laurent N, Rochaix P, et al. Somatostatin receptor subtype 2 sensitizes human pancreatic cancer cells to death ligand-induced apoptosis. Proc Natl Acad Sci USA. 2003;100:155–60. doi: 10.1073/pnas.0136771100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Teijeiro R, Rios R, Costoya JA, et al. Activation of human somatostatin receptor 2 promotes apoptosis through a mechanism that is independent from induction of p53. Cell Physiol Biochem. 2002;12:31–8. doi: 10.1159/000047824. [DOI] [PubMed] [Google Scholar]
- 121.Ferrante E, Pellegrini C, Bondioni S, et al. Octreotide promotes apoptosis in human somatotroph tumor cells by activating somatostatin receptor type 2. Endocr Relat Cancer. 2006;13:955–62. doi: 10.1677/erc.1.01191. [DOI] [PubMed] [Google Scholar]
- 122.Guillermet-Guibert J, Saint-Laurent N, Davenne L, et al. Novel synergistic mechanism for sst2 somatostatin and TNFalpha receptors to induce apoptosis: crosstalk between NF-kappaB and JNK pathways. Cell Death Differ. 2007;14:197–208. doi: 10.1038/sj.cdd.4401939. [DOI] [PubMed] [Google Scholar]
- 123.Sharma K, Patel YC, Srikant CB. Subtype-selective induction of wild-type p53 and apoptosis, but not cell cycle arrest, by human somatostatin receptor 3. Mol Endocrinol. 1996;10:1688–96. doi: 10.1210/mend.10.12.8961277. [DOI] [PubMed] [Google Scholar]
- 124.Theodoropoulou M, Tichomirowa MA, Sievers C, et al. Tumor ZAC1 expression is associated with the response to somatostatin analog therapy in patients with acromegaly. Int J Cancer. 2009;125:2122–6. doi: 10.1002/ijc.24602. [DOI] [PubMed] [Google Scholar]
- 125.Florio T, Thellung S, Arena S, et al. Somatostatin receptor 1 (SSTR1)-mediated inhibition of cell proliferation correlates with the activation of the MAP kinase cascade: role of the phosphotyrosine phosphatase SHP-2. J Physiol. 2000;94:239–50. doi: 10.1016/s0928-4257(00)00214-x. [DOI] [PubMed] [Google Scholar]
- 126.Reardon DB, Dent P, Wood SL, et al. Activation in vitro of somatostatin receptor subtypes 2, 3, or 4 stimulates protein tyrosine phosphatase activity in membranes from transfected Ras-transformed NIH 3T3 cells: coexpression with catalytically inactive SHP-2 blocks responsiveness. Mol Endocrinol. 1997;11:1062–9. doi: 10.1210/mend.11.8.9960. [DOI] [PubMed] [Google Scholar]
- 127.Murphy LO, Smith S, Chen RH, et al. Molecular interpretation of ERK signal duration by immediate early gene products. Nat Cell Biol. 2002;4:556–64. doi: 10.1038/ncb822. [DOI] [PubMed] [Google Scholar]
- 128.Dent P, Wang Y, Gu YZ, et al. S49 cells endogenously Express subtype 2 somatostatin receptors which couple to increase protein tyrosine phosphatase activity in membranes and down-regulate Raf-1 activity in situ. Cell Signal. 1997;9:539–49. doi: 10.1016/s0898-6568(97)00048-x. [DOI] [PubMed] [Google Scholar]
- 129.Reardon DB, Wood SL, Brautigan DL, et al. Activation of a protein tyrosine phosphatase and inactivation of Raf-1 by somatostatin. Biochem J. 1999;314:401–4. doi: 10.1042/bj3140401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ostman A, Hellberg C, Bohmer FD. Protein-tyrosine phosphatases and cancer. Nat Rev Cancer. 2006;6:307–20. doi: 10.1038/nrc1837. [DOI] [PubMed] [Google Scholar]
- 131.Micke P, Hackbusch D, Mercan S, et al. Regulation of tyrosine phosphatases in the adventitia during vascular remodelling. Biochem Biophys Res Commun. 2009;382:678–84. doi: 10.1016/j.bbrc.2009.03.078. [DOI] [PubMed] [Google Scholar]
- 132.Kappert K, Paulsson J, Sparwel J, et al. Dynamic changes in the expression of DEP-1 and other PDGF receptor-antagonizing PTPs during onset and termination of neointima formation. FASEB J. 2007;21:523–34. doi: 10.1096/fj.06-6219com. [DOI] [PubMed] [Google Scholar]
- 133.Albini A, Florio T, Giunciuglio D, et al. Somatostatin controls Kaposi’s sarcoma tumor growth through inhibition of angiogenesis. FASEB J. 1999;13:647–55. doi: 10.1096/fasebj.13.6.647. [DOI] [PubMed] [Google Scholar]
- 134.Badway AC, West FM, Tente SM, et al. Somatostatin regulates intracellular signaling in human carotid endothelial cells. Biochem Biophys Res Commun. 2004;319:1222–7. doi: 10.1016/j.bbrc.2004.05.110. [DOI] [PubMed] [Google Scholar]
- 135.Barbieri F, Pattarozzi A, Gatti M, et al. Somatostatin receptors 1, 2, and 5 cooperate in the somatostatin inhibition of C6 glioma cell proliferation in vitro via a phosphotyrosine phosphatase-eta-dependent inhibition of extracellularly regulated kinase-1/2. Endocrinology. 2008;149:4736–46. doi: 10.1210/en.2007-1762. [DOI] [PubMed] [Google Scholar]
- 136.Arena S, Pattarozzi A, Massa A, et al. An intracellular multi-effector complex mediates somatostatin receptor 1 activation of phospho-tyrosine phosphatase eta. Mol Endocrinol. 2007;21:229–46. doi: 10.1210/me.2006-0081. [DOI] [PubMed] [Google Scholar]
- 137.Bertherat J, Chanson P, Dewailly D, et al. Somatostatin receptors, adenylate cyclase activity, and growth hormone (GH) response to octreotide in GH-secreting adenomas. J Clin Endocrinol Metab. 1993;77:1577–83. doi: 10.1210/jcem.77.6.7903312. [DOI] [PubMed] [Google Scholar]
- 138.Cattaneo MG, Taylor JE, Culler MD, et al. Selective stimulation of somatostatin receptor subtypes: differential effects on Ras/MAP kinase pathway and cell proliferation in human neuroblastoma cells. FEBS Lett. 2000;481:271–6. doi: 10.1016/s0014-5793(00)02012-3. [DOI] [PubMed] [Google Scholar]
- 139.Ludvigse E, Stridsberg M, Taylor JE, et al. Subtype selective interactions of somatostatin and somatostatin analogs with sst1, sst2, and sst5 in BON-1 cells. Med Oncol. 2004;21:285–96. doi: 10.1385/MO:21:3:285. [DOI] [PubMed] [Google Scholar]
- 140.Djordjijevic D, Zhang J, Priam M, et al. Effect of 17beta-estradiol on somatostatin receptor expression and inhibitory effects on growth hormone and prolactin release in rat pituitary cell cultures. Endocrinology. 1998;139:2272–7. doi: 10.1210/endo.139.5.5990. [DOI] [PubMed] [Google Scholar]
- 141.Law SF, Yasuda K, Bell GI, et al. Gi alpha 3 and G(o) alpha selectively associate with the cloned somatostatin receptor subtype SSTR2. J Biol Chem. 1993;268:10721–7. [PubMed] [Google Scholar]
- 142.Law SF, Zaina S, Sweet R, et al. Gi alpha 1 selectively couples somatostatin receptor subtype 3 to adenylyl cyclase: identification of the functional domains of this alpha subunit necessary for mediating the inhibition by somatostatin of cAMP formation. Mol Pharmacol. 1994;45:587–90. [PubMed] [Google Scholar]
- 143.Strowski MZ, Dashkevicz MP, Parmar RM, et al. Somatostatin receptor subtypes 2 and 5 inhibit corticotropin-releasing hormone-stimulated adrenocorticotropin secretion from AtT-20 cells. Neuroendocrinology. 2002;75:339–46. doi: 10.1159/000059430. [DOI] [PubMed] [Google Scholar]
- 144.Cervia D, Fehlmann D, Hoyer D. Native somatostatin sst2 and sst5 receptors functionally coupled to G(i/o)-protein, but not to the serum response element in AtT-20 mouse tumour corticotrophs. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:578–87. doi: 10.1007/s00210-003-0752-1. [DOI] [PubMed] [Google Scholar]
- 145.Cervia D, Langenegger D, Schuepbach E, et al. Binding and functional properties of the novel somatostatin analogue KE 108 at native mouse somatostatin receptors. Neuropharmacology. 2005;48:881–93. doi: 10.1016/j.neuropharm.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 146.Birnbaumer L, Abramowitz J, Yatani A, et al. Roles of G proteins in coupling of receptors to ionic channels and other effector systems. Crit Rev Biochem Mol Biol. 1990;25:225–44. doi: 10.3109/10409239009090610. [DOI] [PubMed] [Google Scholar]
- 147.Yatani A, Birnbaumer L, Brown AM. Direct coupling of the somatostatin receptor to potassium channels by a G protein. Metabolism. 1990;39:91–5. doi: 10.1016/0026-0495(90)90220-7. [DOI] [PubMed] [Google Scholar]
- 148.Kreienkamp HJ, Honck HH, Richter D. Coupling of rat somatostatin receptor subtypes to a Gprotein gated inwardly rectifying potassium channel (GIRK1) FEBS Lett. 1997;419:92–4. doi: 10.1016/s0014-5793(97)01437-3. [DOI] [PubMed] [Google Scholar]
- 149.Yang SK, Parkington HC, Blake AD, et al. Somatostatin increases voltage-gated K+ currents in GH3 cells through activation of multiple somatostatin receptors. Endocrinology. 2005;146:4975–84. doi: 10.1210/en.2005-0696. [DOI] [PubMed] [Google Scholar]
- 150.Chen L, Fitzpatrick VD, Vandlen RL, et al. Both overlapping and distinct signaling pathways for somatostatin receptor subtypes SSTR1 and SSTR2 in pituitary cells. J Biol Chem. 1997;272:18666–72. doi: 10.1074/jbc.272.30.18666. [DOI] [PubMed] [Google Scholar]
- 151.Fujii Y, Gonoi T, Yamada Y, et al. Somatostatin receptor subtype SSTR2 mediates the inhibition of highvoltage-activated calcium channels by somatostatin and its analogue SMS 201–995. FEBS Lett. 1994;355:117–20. doi: 10.1016/0014-5793(94)01159-1. [DOI] [PubMed] [Google Scholar]
- 152.Tallent M, Liapakis G, O’Carroll AM, et al. Somatostatin receptor subtypes SSTR2 and SSTR5 couple negatively to an L-type Ca2+ current in the pituitary cell line AtT-20. Neuroscience. 1996;71:1073–81. doi: 10.1016/0306-4522(95)00510-2. [DOI] [PubMed] [Google Scholar]
- 153.Moeller I, Bunn SJ, Marley PD. Actions of somatostatin on perfused bovine adrenal glands and cultured bovine adrenal medullary cells. Brain Res. 1989;484:192–202. doi: 10.1016/0006-8993(89)90362-4. [DOI] [PubMed] [Google Scholar]
- 154.Tomura H, Okajima F, Akbar M, et al. Transfected human somatostatin receptor type 2, SSTR2, not only inhibits adenylate cyclase but also stimulates phospholipase C and Ca2+ mobilization. Biochem Biophys Res Commun. 1994;200:986–92. doi: 10.1006/bbrc.1994.1547. [DOI] [PubMed] [Google Scholar]
- 155.Kubota A, Yamada Y, Kagimoto S, et al. Multiple effector coupling of somatostatin receptor subtype SSTR1. Biochem Biophys Res Commun. 1994;204:176–86. doi: 10.1006/bbrc.1994.2442. [DOI] [PubMed] [Google Scholar]
- 156.Hipkin RW, Wang Y, Schonbrunn A. Protein kinase C activation stimulates the phosphorylation and internalization of the sst2A somatostatin receptor. J Biol Chem. 2000;275:5591–9. doi: 10.1074/jbc.275.8.5591. [DOI] [PubMed] [Google Scholar]
- 157.Akbar M, Okajima F, Tomura H, et al. Phospholipase C activation and Ca2+ mobilization by cloned human somatostatin receptor subtypes 1–5, in transfected COS-7 cells. FEBS Lett. 1994;348:192–6. doi: 10.1016/0014-5793(94)00603-2. [DOI] [PubMed] [Google Scholar]
- 158.Hirabayashi T, Murayama T, Shimizu T. Regulatory mechanism and physiological role of cytosolic phospholipase A2. Biol Pharm Bull. 2004;27:1168–73. doi: 10.1248/bpb.27.1168. [DOI] [PubMed] [Google Scholar]
- 159.Sun GY, Xu J, Jensen MD, et al. Phospholipase A2 in the central nervous system: implications for neurodegenerative diseases. J Lipid Res. 2004;45:205–13. doi: 10.1194/jlr.R300016-JLR200. [DOI] [PubMed] [Google Scholar]
- 160.Judd AM, Koike K, MacLeod RM. GRF increases release of growth hormone and arachidonate from anterior pituitary cells. Am J Physiol. 1985;248:E438–42. doi: 10.1152/ajpendo.1985.248.4.E438. [DOI] [PubMed] [Google Scholar]
- 161.Duerson K, White RE, Jiang F, et al. Somatostatin stimulates BKCa channels in rat pituitary tumor cells through lipoxygenase metabolites of arachidonic acid. Neuropharmacology. 1996;35:949–61. doi: 10.1016/0028-3908(96)00131-1. [DOI] [PubMed] [Google Scholar]
- 162.Sellers LA, Feniuk W, Humphrey PP, et al. Activated G protein-coupled receptor induces tyrosine phosphorylation of STAT3 and agonist-selective serine phosphorylation via sustained stimulation of mitogen-activated protein kinase. Resultant effects on cell proliferation. J Biol Chem. 1999;274:16423–30. doi: 10.1074/jbc.274.23.16423. [DOI] [PubMed] [Google Scholar]
- 163.Komatsuzaki K, Terashita K, Kinane TB, et al. Somatostatin type V receptor activates c-Jun N terminal kinases via G alpha(12) family G proteins. Biochem Biophys Res Commun. 2001;289:1211–7. doi: 10.1006/bbrc.2001.6085. [DOI] [PubMed] [Google Scholar]
- 164.Thermos K. Novel signals mediating the functions of somatostatin: the emerging role of NO/cGMP. Mol Cell Endocrinol. 2008;286:49–57. doi: 10.1016/j.mce.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 165.Lopez F, Ferjoux G, Cordelier P, et al. Neuronal nitric oxide synthase: a substrate for SHP-1 involved in sst2 somatostatin receptor growth inhibitory signaling. FASEB J. 2001;15:2300–2. doi: 10.1096/fj.00-0867fje. [DOI] [PubMed] [Google Scholar]
- 166.Cordelier P, Estève JP, Bousquet C, et al. Characterization of the antiproliferative signal mediated by the somatostatin receptor subtype sst5. Proc Natl Acad Sci USA. 1997;94:9343–8. doi: 10.1073/pnas.94.17.9343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.McDonald LJ, Murad F. Nitric oxide and cyclic GMP signaling. Proc Soc Exp Biol Med. 1996;211:1–6. doi: 10.3181/00379727-211-43950a. [DOI] [PubMed] [Google Scholar]
- 168.Corbin JD, Francis SH. Cyclic GMP phosphodiesterase-5: target of sildenafil. J Biol Chem. 1999;274:13729–32. doi: 10.1074/jbc.274.20.13729. [DOI] [PubMed] [Google Scholar]
- 169.Cordelier P, Estève JP, Najib S, et al. Regulation of neuronal nitric-oxide synthase activity by somatostatin analogs following SST5 somatostatin receptor activation. J Biol Chem. 2006;281:19156–71. doi: 10.1074/jbc.M602024200. [DOI] [PubMed] [Google Scholar]
- 170.Florio T, Morini M, Villa V, et al. Somatostatin inhibits tumor angiogenesis and growth via somatostatin receptor-3-mediated regulation of endothelial nitric oxide synthase and mitogen-activated protein kinase activities. Endocrinology. 2003;144:1574–84. doi: 10.1210/en.2002-220949. [DOI] [PubMed] [Google Scholar]
- 171.Hou C, Gilbert RL, Barber DL. Subtype specific signaling mechanisms of somatostatin receptors SSTR1 and SSTR2. J Biol Chem. 1994;269:10357–62. [PubMed] [Google Scholar]
- 172.Grozinsky-Glasberg S, Franchi G, Teng M, et al. Octreotide and the mTOR inhibitor RAD001 (everolimus) block proliferation and interact with the Akt-mTOR-p70S6K pathway in a neuro-endocrine tumour cell Line. Neuroendocrinology. 2008;87:168–81. doi: 10.1159/000111501. [DOI] [PubMed] [Google Scholar]


