Abstract
Muscle tissue engineering (TE) has not yet been clinically applied because of several problems. However, the field of skeletal muscle TE has been developing tremendously and new approaches and techniques have emerged. This review will highlight recent developments in the field of nanotechnology, especially electrospun nanofibre matrices, as well as potential cell sources for muscle TE. Important developments in cardiac muscle TE and clinical studies on Duchenne muscular dystrophy (DMD) will be included to show their implications on skeletal muscle TE.
Keywords: skeletal muscle tissue engineering, satellite cells, electrospun nanofibre matrices, nanotechnology
Introduction
Tissue engineering (TE) represents a scientific approach focussing on the emulation of neo-organogenesis [1]. Research interest focuses on a variety of different tissues that might be recreated for replacement of lost tissues. After loss of skeletal muscle tissue new solutions might be brought about for the treatment of a variety of muscle diseases, including skeletal myopathies such as muscular dystrophy or spinal muscular atrophy [2, 3]. Furthermore localized loss of skeletal muscle tissue, as a result of traumatic injury, aggressive tumour ablation or prolonged denervation, e.g. are common clinical problems. In the last years, important developments have changed the field of skeletal muscle TE [4, 5].
Since Vandenburgh et al. introduced the 3D cultivation of primary myoblasts in collagen gel and generated contracting muscle tissue in vitro for the first time in 1988 [6], there have been developments in the field of skeletal muscle TE and new approaches and techniques have emerged. However, collagen as well as fibrin gels have proven unsuitable for TE due to their fast degradation in vitro[7]. With regard to material science, recent developments in the field of nanotechnology have brought up new possibilities which will be highlighted in this review. Two years after Vandenburgh’s 3D cultivation of myoblasts in collagen gels, Strohman and colleagues showed that monolayers of differentiating myoblasts detached from the membrane and formed 3D and contractible muscle tissues termed myooids [8]. Though the method of generating myooids was brought to perfection by Dennis and coworkers, the in vitro engineered muscle constructs did not exceed a diameter of 1 mm [9]. These in vitro studies led to a better understanding of myogenic differentiation and contractility. Yet, the nutrient supply for the cells in this setting depends solely on diffusion. Because diffusion capacity is limited to distances of less than 500 μm [10], nutrient supply in the central parts of engineered tissue or 3D constructs becomes deficient. Levenberg and coworkers showed that vascularized muscle tissue and recently also vascularized cardiac muscle tissue can be engineered in vitro by co-culturing myoblasts, embryonic fibroblasts and endothelial cells in a 3D polymer scaffold [11–12]. The pre-vascularized constructs showed less apoptosis of myoblasts after transplantation in vivo[12]. However, in clinical application, the transfer of larger muscle constructs to the site of the defect requires an axial vascularization of the engineered muscle tissue. Furthermore, the use of embryonic cell sources is a critical issue in medical as well as in ethical terms. Therefore, several in vivo studies [13–15] have used the microsurgical AV-loop model in the rat first described by Erol and Spira in 1980 [16] to generate an axial vascularization. In combination with secondary cell transfer to axially pre-vascularized matrices this well-established model might be the most promising step towards a future application of large tissue-engineered constructs in a clinical scenario [17]. Recently, the rat AV-loop model was translated into the sheep AV-loop model for large constructs [18–19].
Despite these encouraging developments there are further obstacles for a bench-to-bedside approach of muscle TE which have to be addressed, a suitable cell source and myogenic differentiation being the most urgent. Recent developments in the field of myogenic differentiation in vitro and in vivo will be discussed in this review, including findings from clinical Duchenne muscular dystrophy research and TE of cardiac muscle.
Matrices for skeletal muscle tissue engineering – gaining orientation
Regarding the architecture, matrices can be divided into randomly orientated scaffolds and matrices with a certain alignment (Fig. 1). Commonly used matrices with random orientation, e.g. gels and sponges, can be used for a variety of tissues. However, in case of skeletal muscle this tissue naturally consists of bundles of highly oriented muscle fibres in an extracellular 3D matrix to form an organized tissue with high cell density. The parallel orientation of muscle fibres guarantees the generation of longitudinal force after contraction that is induced by motoneuron activity [20]in vivo. Another key factor in TE is the composition of the extracellular matrix (ECM) which plays an important role in the alignment and differentiation of myoblasts [21–22]. Ideally the ECM constitutes the framework for cell adhesion and tissue growth, which includes cell proliferation and differentiation. The parallel alignment of the natural ECM in skeletal muscle tissue can be mimicked by matrix architecture. Different methods are in use to achieve parallel alignment depending on the question whether 2D or 3D matrices should be used. Regarding 2D scaffolds, alignment can be generated by electrospinning as well as microgrooving. The method of microgrooving uses either abrasives to directly grind microgrooves into the matrix as described by Shimizu et al.[23] or generating micropatterned moulds and casting the liquid matrix material onto the mould as described by Walboomers and others [24–25]. The fabrication of micropatterned matrices has been developed for in vitro analysis of cell behaviour and differentiation on aligned surfaces and showed orientated cell growth of, e.g. fibroblasts [26–27], myoblasts [28] and neural cells [29–30] along the microgrooves. This phenomenon commonly termed as ‘cell guidance theory’[24, 31] encourages and facilitates myogenic differentiation in vitro[32]. Flaibani and coworkers analysed electrical stimulation in addition to microgrooved poly-(L-lactic-acid) membranes on the differentiation of muscle precursor cells [33]. This setting increased the myogenic differentiation of myoblasts even more than cultivation on micropatterned membranes without electrical stimuli [33]. Though very valuable for in vitro studies analysing cell structure and differentiation, this 2D method is not suitable for engineering 3D muscle tissue. For engineering transplantable muscle tissue in vitro and for in vivo application of skeletal muscle TE a 3D approach is necessary.
Fig 1.
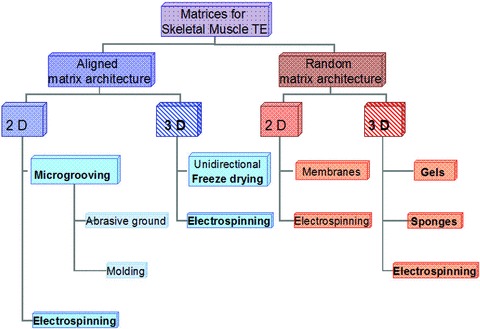
Matrices for skeletal muscle TE can be divided into aligned and random matrices. Electrospinning enables the generation of aligned and random as well as 2D and 3D matrix architecture.
Besides the electrospinning technique there are several alternative methods to generate spatially orientated structures in 3D matrices. Principally, the gradual formation of ice crystals induced by unidirectional freeze-drying of hydrogels results in highly porous scaffolds with aligned pores [34–35]. This method allows a fine adjustment of the average pore size by varying the freezing temperature [36]. A variety of different synthetic as well as biological materials can be processed as hydrogels by freeze-drying, e.g. collagen [34], fibroin [37] and composites [38]. Though these matrices have no limitations in their 3D size, the thickness and structure of matrix material between the pores is hardly controllable by the freeze-drying method. Therefore, further adjustments of the matrices including coating for controlled degradation or drug release as well as surface modification remain a challenge.
Electrospun nanofibre matrices
The electrospinning technique principally uses electrical voltage to form fibres in the range of micrometers down to a few nanometres. Though simple in its principle, electrospinning is a complex process that depends on a variety of parameters. This complexity elicits a tremendous spectrum of applications and variety of possibilities to adjust matrix properties as desired [39]. A great diversity of synthetic polymers as well as biopolymers can be used to generate electrospun nanofibres including proteins of the natural ECM such as collagen [40–41], elastin [40–41] and hyaluronic acid [42]. Matrices composed of electrospun ECM proteins uniquely mimic the structure of the natural ECM which is of utmost importance for attachment, viability and differentiation of cells seeded into nanofibrous matrices [43–45]. The aforementioned advantages of biopolymers are often combined with the higher stability of synthetic polymers such as poly-(epsilon-caprolactone). Matrices solely composed of synthetic polymers, e.g. PCL [46] or poly-(ester-urethanex) [47] show high stability but also low elasticity and sparse cell attachment. Several methods exist to enhance cell attachment to synthetic polymer nanofibres. A commonly used method is to coat the nanofibre matrix with biopolymer solution after electrospinning. In the study of Riboldi et al., microfibrous randomly spun PU matrices were coated with Matrigel™, an extract from the Engelbreth–Holm–Swarm mouse sarcoma. Due to its origin, Matrigel™ is inappropriate for a clinical setting and its use is limited to experimental models only. Alternatively, synthetic polymers can be blended with biopolymers before electrospinning (Fig. 2). PCL-collagen blend nanofibres show a significantly increased cell attachment dependant on the percentage of collagen [44]. Furthermore, co-axial electrospinning of two different polymer solutions results in fibres surrounded by the second polymer. This core-shell technique enables the processing of synthetic polymers with a shell of biopolymers. Zhang and coworkers could show that cell attachment on electrospinning core-shell nanofibres of PCL (core) and collagen (shell) is even further increased as compared to the coating procedure [48].
Fig 2.

Scanning electron microscopy: Myoblast growing on randomly electrospun 3D PCL-collagen matrix; 5000× magnification.
As mentioned before, nanofibre matrices with parallel alignment (Figs. 3 and 4) prove as ideal scaffolds for cultivating myoblasts [49]. The generation of parallel fibre alignment can be easily achieved by using a rotating mandrel to collect the nanofibres [50]. However, the pore sizes of electrospun matrices with parallel fibre alignment are hardly controllable and have ever been a great challenge. Therefore, electrospinning of aligned nanofibres into 3D scaffolds usually results in densely packed nanofibres [51] leading to poor cell infiltration of slow degradable nanofibrous scaffolds in vitro and in vivo[52]. Baker and coworkers have introduced the method of co-spinning sacrificial fibres into 3D aligned nanofibre matrices [53]. The sacrificial fibres, e.g. water-soluble poly-(ethylene-oxide), are leached out after electrospinning and leave behind interspaces where cells can easily pass through [53]. Thus the interspaces between the aligned nanofibres can be augmented without increasing the fibre diameter.
Fig 3.
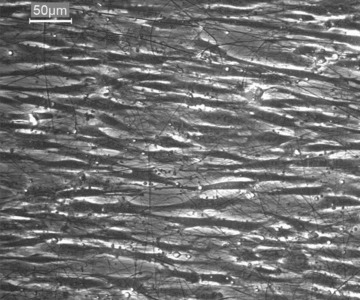
Phase contrast microscopy: Myoblasts cultured on aligned PCL-collagen nanofibres; after 2 days in culture they align themselves along the nanofibres; 320× magnification.
Fig 4.
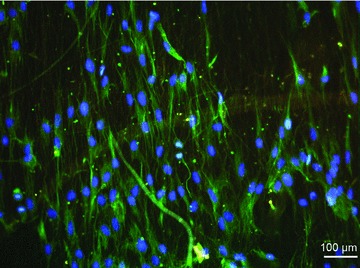
Immunofluorescence staining: Myoblasts on aligned electrospun PCL-collagen nanofibres. Green: desmin; blue: DAPI counterstaining.
Nanotechnology and smart matrices
The core-shell technique can also be used as drug delivery system (DDS), which has opened up a new opportunity to tailor ‘smart’ scaffolds with controllable drug release [54–55]. Nanoparticles and nanofibre matrices as drug delivery devices are an immensely emerging field in pharmacotherapy as well as for TE [56]. In case of Duchenne muscular dystrophy, the application of nanopolymer as DDS has already reached the stage of phase I clinical trials [57–58]. Nanofibre matrices as DDS also enable the administration of growth factors [59], angiogenic factors like VEGF [60–61], which can reduce the time of pre-vascularization of implanted matrices in vivo[62] as well as factors to enforce and promote cell differentiation. Regarding skeletal muscle TE, different factors are known to induce myoblast proliferation and myogenic differentiation, e.g. insulin-like growth factor-1 [63], nerve growth factor [64–65] or the synthetic pyrimidine MS-818 which is known as a neurotrophic factor but also accelerates muscle regeneration [66]. Smart nanofibrous matrices with controlled release of specific growth factors have already proven their potential in other fields of TE [67–68], but for skeletal muscle TE this field still has to be investigated. However, due to the risk of tumorigenesis, the administration of growth factors in clinical applications is critical. Therefore, the definite risk has to be assessed in pre-clinical in vivo studies concerning local release of growth factors through DDS over distinct time periods.
Alternatively, electrical stimulation is a well-known factor for inducing differentiation in myoblasts [69] as well as cardiomyocytes in vitro[70]. Shafy et al. proved that simultaneous implantation of a pacemaker along with myoblasts into infarcted myocard of sheep had a positive influence on differentiation of implanted myoblasts and led to better myocardial function [71]. As an alternative to the commonly used external electrical stimulation, Gaetani and coworkers analysed the influence of extremely low-frequency electromagnetic fields (ELF-EMF) on cardiac stem cells [72]. Their results show that exposure of cells to ELF-EMFs can induce differentiation of cardiac stem cells. Thus, the use of EMF could replace the implantation of pacemakers.
The electrospinning technique also provides the opportunity to generate electrically conductive matrices by spinning conductive polymers, such as poly(aniline) nanofibres (PANi). Ghasemi-Mobarakeh et al. showed that doped PANi blended with PCL and gelatine solution exhibit acceptable cell attachment [73]. Neural stem cells cultured on these PANi/PCL/gelatine-blend nanofibre scaffolds showed increased proliferation and neurite outgrowth when electrical stimulation was applied. A similar setting (PANi/gelatine blend nanofibres) was chosen by Li and coworkers to analyse the behaviour of rat cardiac myoblasts [74] on conductive nanofibrous scaffolds and by Jun and colleagues (poly(lactic-epsilon-caprolactone) [PLCL]/PANi blend nanofibres) using C2C12 murine myoblasts. Jun et al. showed that the expression of myogenin, a muscle specific protein that marks early myogenic differentiation, was significantly higher in myoblasts cultured on electrically conductive nanofibrous matrices than in myoblasts cultivated on PLCL scaffolds [75].
Cell sources for skeletal muscle TE
In opposite to the impressive developments in nanotechnology there are still great shortcomings on the cell side. Regarding the cell source, the satellite cell is the most prominent one (Fig. 5) [76–77]. The term ‘satellite cell’ is a histological description of undifferentiated cells residing between the sarcolemma and the basement membrane of muscle fibres but their exact biological characterization, their origin and differentiation potential and the question whether satellite cells are actually stem cells have been a point of discussion for a long time [78]. Today, it is common knowledge that satellite cells are characterized through the expression of the muscle-specific paired box (Pax) transcription factor Pax7 [79]. But it is also known that Pax7+ cells are a heterogeneous cell population consisting of a majority of Myf5+ cells and a minority (10%) of Myf5− cells [80]. The expression of Myf5 as an initiator of myogenic differentiation [81] marks the commitment of this cell population to the myogenic lineage [80]. Therefore, the majority of the satellite cell population actually consists of muscle precursor cells with a strong myogenic imprinting, whereas the Pax7+/Myf5− subpopulation of the satellite cells (only 10%) show stem cell properties continuously renewing the Pax7+/Myf5+ cell population. High hopes have been built on this stem cell like subpopulation regarding their proliferative capacity in vitro because these cells are capable of allowing for regeneration of large parts of the musculature in vivo[82–83]. Yet, Yaffe and coworkers have demonstrated that isolated satellite cells undergo rapid dedifferentiation in vitro after few cell cycles [84]. Boonen et al. have explained this phenomenon by the loss of the highly specific stem cell niche which preserves normal function of satellite cells in vivo[85–86]. Therefore, in vitro expansion of satellite cells does not lead to an efficient amount of cells for muscle TE. Yet, the commitment to the myogenic line of the majority of satellite cells represents an unmatched myogenic potential and implies safety concerning clinical applications without the risk of tumour genesis.
Fig 5.
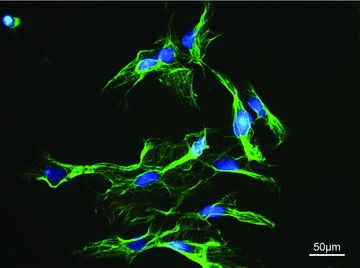
Immunofluorescence staining of myoblasts isolated from skeletal muscle of the rat in vitro. Green: desmin (muscle specific cytoskeleton), blue: nuclei, DAPI counterstain, 400× magnification.
As an alternative, mesenchymal stem cells (MSC) have been proposed for muscle TE [87]. Principally, MSCs can be derived from umbilical cord blood, bone marrow (BMSC) and adipose tissue. Though BMSCs have been commonly used, adipose-derived stem cells constitute an interesting alternative because they are easily accessible and show higher proliferation rates than BMSCs [88–89]. In addition to their high proliferation rates in vitro, MSCs have been shown to fuse with cardiomyocytes in vitro and in vivo[90]. Nevertheless, incorporation of MSC as well as transplanted satellite cells into skeletal myofibres in vivo remains a challenge. Brazelton et al. have reported an incorporation rate of 5% of transplanted MSCs into myofibres [91]. This low incorporation rate has contributed to the poor outcome of cytotherapy, i.e. the transplantation of satellite cells or MSCs with wild-type expression of dystrophin, as a treatment for DMD. Gussoni and coworkers transplanted satellite cells into defect muscle tissue of Duchenne muscular dystrophy patients in a clinical trial. Their results revealed that only 10% of the transplanted myoblasts were left after 6 months [92]. However, MSCs impose an additional paracrine effect on differentiation and thus on tissue regeneration by different cytokines [93–94]. Thereby, transplantation of MSCs and satellite cells together could possibly augment the effectiveness of muscle TE in vivo by supporting satellite cell viability and myogenic differentiation. In the field of cardiac muscle TE, Gherghiceanu and Popescu have recently shown that a kind of interstitial Cajal-like cells, which are found in different tissues in vivo[95], function as ‘nursing’ cells for differentiating cardiomyocyte progenitors (CMP) [96]. These interstitial Cajal-like cells have been renamed by Popescu and coworkers as ‘telocytes’ due to their thin prolongations [95]. Telocytes in the cardiac muscle tissue seem to encourage and guide the fusion of differentiating CMPs to adult cardiomyocytes [96]. Further investigations may show, if similar mechanisms can be found in the skeletal muscle tissue.
In 2006, Takahashi and Yamanaka [97] have shown that adult fibroblasts can be transferred into pluripotent stem cells (PSCs) which by definition show differentiation into all three germ layers in vitro and form teratoma in vivo. Thus, these induced PSCs (iPSCs) opt as an alternative source to MSCs because cell proliferation and pluripotency of iPSCs are comparable to embryonic stem cells, without including the ethical difficulties that are implied with the use of embryonic stem cells. The role iPSCs could play in the future for regenerative medicine has been reviewed recently by Lee et al.[98] as well as Amabile and Meissner [99]. In case of cardiac regenerative medicine, iPSCs have been proposed by Yuasa and Fukuda [100] for the therapy of heart failure, though studies with clinical implications are still rare. Recently, Moretti and coworkers have isolated and induced adult dermal fibroblast from patients with long-QT syndrome, a hereditary heart disease which can cause sudden cardiac death [101]. The generated iPSCs were successfully differentiated into cardiomyocytes, but also showed prolonged action potential typical for the long-QT syndrome because the cells maintained their original genotype. Hanna and his group successfully combined iPSC cell therapy with gene therapy for the treatment of sickle cell anaemia in a humanized mouse model [102]. Before transplantation the mutation causing sickle cell anaemia was corrected and the generated iPSCs were differentiated into haematopoietic progenitor cells. Thus, iPSCs have already proven their potential for regenerative medicine. However, research on iPSC is still in its early stages and many shortcomings have to be addressed before clinical applications are conceivable [103]. One of these shortcomings is the use of lenti- or retroviral vectors for transfection of the four so called ‘Yamanaka factors’– Oct4, Sox2, Klf4 and c-Myc – which are necessary for the reprogramming of adult dermal fibroblasts. To circumvent the risk of insertion mutation caused by lentiviral or retroviral transfection, Kaji et al. have used a virus-free vector for transfection [104]. Other methods for cell reprogramming like the use of microRNA are being discussed [105]. Another drawback is the risk of tumorigenicity that arises from the Yamanaka factors themselves because c-Myc and Klf4 are known oncogenes [106–107]. Nagakawa and coworkers showed that c-Myc is not mandatory for the generation of iPSCs. Though fewer iPSCs are derived through this protocol, the group could also show that the tumorigenicity of c-Myc– iPSCs is clearly lower. Whereas Okita et al. found tumours in approximately 20% of the F1 progeny mice derived from c-Myc+ iPSCs, the F1 mice derived from the Myc– iPSCs in Nagakawa’s study developed no tumours within 100 days after birth. Furthermore, Giorgetti et al. prove that iPSCs can even be generated by the use of only two factors, Oct4 and Sox2. Thus, iPSCs could play an important role in TE and regenerative medicine in the future. Apart from clinically orientated studies, further basic research is necessary to gain more security concerning tumour risk.
Conclusion
Essential problems of skeletal muscle TE such as cell expansion in vitro and myogenic differentiation in vivo are not solved to date, which hinders skeletal muscle TE from its clinical application. However, reviewing the recent developments in nanotechnology and also taking into account other fields of research, e.g. cardiac muscle TE and clinical studies on Duchenne muscular dystrophy, huge leaps have obviously taken place. These results give rise to optimism regarding the clinical application of skeletal muscle TE in future.
Acknowledgments
The authors thank the University of Erlangen for the ‘ELAN Research Fund’ and the ‘Xue Hong und Hans Georg Geis Foundation’ for supporting the research on skeletal muscle TE at the Department of Plastic and Hand Surgery, University Hospital of Erlangen, Friedrich-Alexander-University of Erlangen-Nürnberg.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Mooney DJ, Mikos AG. Growing new organs. Sci Am. 1999;280:60–5. doi: 10.1038/scientificamerican0499-60. [DOI] [PubMed] [Google Scholar]
- 2.Law PK, Goodwin TG, Fang Q, et al. Cell transplantation as an experimental treatment for Duchenne muscular dystrophy. Cell Transplant. 1993;2:485–505. doi: 10.1177/096368979300200607. [DOI] [PubMed] [Google Scholar]
- 3.Guettier-Sigrist S, Coupin G, Braun S, et al. Muscle could be the therapeutic target in SMA treatment. J Neurosci Res. 1998;53:663–9. doi: 10.1002/(SICI)1097-4547(19980915)53:6<663::AID-JNR4>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Klumpp D, Horch RE, Bitto F, et al. Skeletal muscle tissue engineering - current concepts and future perspectives. Handchir Mikrochir Plast Chir. 2010 doi: 10.1055/s-0030-1261888. DOI: 10.1055/s-0029-1234122. [DOI] [PubMed] [Google Scholar]
- 5.Bach AD, Arkudas A, Tjiawi J, et al. A new approach to tissue engineering of vascularized skeletal muscle. J Cell Mol Med. 2006;10:716–26. doi: 10.1111/j.1582-4934.2006.tb00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vandenburgh HH, Karlisch P, Farr L. Maintenance of highly contractile tissue-cultured avian skeletal myotubes in collagen gel. In Vitro Cell Dev Biol. 1988;24:166–74. doi: 10.1007/BF02623542. [DOI] [PubMed] [Google Scholar]
- 7.Beier JP, Klumpp D, Rudisile M, et al. Collagen matrices from sponge to nano: new perspectives for tissue engineering of skeletal muscle. BMC Biotechnol. 2009;9:34–47. doi: 10.1186/1472-6750-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strohman RC, Bayne E, Spector D, et al. Myogenesis and histogenesis of skeletal muscle on flexible membranes in vitro. In Vitro Cell Dev Biol. 1990;26:201–8. doi: 10.1007/BF02624113. [DOI] [PubMed] [Google Scholar]
- 9.Dennis RG, Kosnik PE, 2nd, Gilbert ME, et al. Excitability and contractility of skeletal muscle engineered from primary cultures and cell lines. Am J Physiol Cell Physiol. 2001;280:288–95. doi: 10.1152/ajpcell.2001.280.2.C288. [DOI] [PubMed] [Google Scholar]
- 10.Kannan RY, Salacinski HJ, Sales K, et al. The roles of tissue engineering and vascularisation in the development of micro-vascular networks: a review. Biomaterials. 2005;26:1857–75. doi: 10.1016/j.biomaterials.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Lesman A, Gepstein L, Levenberg S. Vascularization shaping the heart. Ann N Y Acad Sci. 2010;1188:46–51. doi: 10.1111/j.1749-6632.2009.05082.x. [DOI] [PubMed] [Google Scholar]
- 12.Levenberg S, Rouwkema J, Macdonald M, et al. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879–84. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 13.Messina A, Bortolotto SK, Cassell OC, et al. Generation of a vascularized organoid using skeletal muscle as the inductive source. FASEB J. 2005;19:1570–2. doi: 10.1096/fj.04-3241fje. [DOI] [PubMed] [Google Scholar]
- 14.Hutmacher DW, Horch RE, Loessner D, et al. Translating tissue engineering technology platforms into cancer research. J Cell Mol Med. 2009;13:1417–27. doi: 10.1111/j.1582-4934.2009.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiegel HC, Pryymachuk G, Rath S, et al. Foetal hepatocyte transplantation in a vascularized AV-Loop transplantation model in the rat. J Cell Mol Med. 2010;14:267–74. doi: 10.1111/j.1582-4934.2008.00369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erol OO, Spira M. New capillary bed formation with a surgically constructed arteriovenous fistula. Plast Reconstr Surg. 1980;66:109–15. doi: 10.1097/00006534-198007000-00021. [DOI] [PubMed] [Google Scholar]
- 17.Kneser U, Polykandriotis E, Ohnolz J, et al. Engineering of vascularized transplantable bone tissues: induction of axial vascularization in an osteoconductive matrix using an arteriovenous loop. Tissue Eng. 2006;12:1721–31. doi: 10.1089/ten.2006.12.1721. [DOI] [PubMed] [Google Scholar]
- 18.Beier JP, Horch RE, Arkudas A, et al. De novo generation of axially vascularized tissue in a large animal model. Microsurgery. 2009;29:42–51. doi: 10.1002/micr.20564. [DOI] [PubMed] [Google Scholar]
- 19.Beier JP, Horch RE, Hess A, et al. Axial vascularization of a large volume calcium phosphate ceramic bone substitute in the sheep AV loop model. J Tissue Eng Regen Med. 2010;4:216–23. doi: 10.1002/term.229. [DOI] [PubMed] [Google Scholar]
- 20.Beier JP, Horch RE, Bach AD. Tissue engineering of skeletal muscle. Minerva Biotecnologica. 2006;18:89–95. [Google Scholar]
- 21.Cassell OC, Morrison WA, Messina A, et al. The influence of extracellular matrix on the generation of vascularized, engineered, transplantable tissue. Ann N Y Acad Sci. 2001;944:429–42. doi: 10.1111/j.1749-6632.2001.tb03853.x. [DOI] [PubMed] [Google Scholar]
- 22.Boontheekul T, Hill EE, Kong HJ, et al. Regulating myoblast phenotype through controlled gel stiffness and degradation. Tissue Eng. 2007;13:1431–42. doi: 10.1089/ten.2006.0356. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu K, Fujita H, Nagamori E. Alignment of skeletal muscle myoblasts and myotubes using linear micropatterned surfaces ground with abrasives. Biotechnol Bioeng. 2009;103:631–8. doi: 10.1002/bit.22268. [DOI] [PubMed] [Google Scholar]
- 24.Walboomers XF, Jansen JA. Cell and tissue behavior on micro-grooved surfaces. Odontology. 2001;89:2–11. doi: 10.1007/s10266-001-8178-z. [DOI] [PubMed] [Google Scholar]
- 25.Kriparamanan R, Aswath P, Zhou A, et al. Nanotopography: cellular responses to nanostructured materials. J Nanosci Nanotechnol. 2006;6:1905–19. doi: 10.1166/jnn.2006.330. [DOI] [PubMed] [Google Scholar]
- 26.Meyle J, Gultig K, Wolburg H, et al. Fibroblast anchorage to microtextured surfaces. J Biomed Mater Res. 1993;27:1553–7. doi: 10.1002/jbm.820271212. [DOI] [PubMed] [Google Scholar]
- 27.Patrito N, McCague C, Norton PR, et al. Spatially controlled cell adhesion via micropatterned surface modification of poly(dimethylsiloxane) Langmuir. 2007;23:715–9. doi: 10.1021/la062007l. [DOI] [PubMed] [Google Scholar]
- 28.Huang NF, Thakar RG, Wong M, et al. Tissue engineering of muscle on micropatterned polymer films. Conf Proc IEEE Eng Med Biol Soc. 2004;7:4966–9. doi: 10.1109/IEMBS.2004.1404373. [DOI] [PubMed] [Google Scholar]
- 29.Lee W, Parpura V. Micropatterned substrates for studying astrocytes in culture. Front Neurosci. 2009;3:381–7. doi: 10.3389/neuro.01.033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin YL, Jen JC, Hsu SH, et al. Sciatic nerve repair by microgrooved nerve conduits made of chitosan-gold nanocomposites. Surg Neurol. 2008;70:9–18. doi: 10.1016/j.surneu.2008.01.057. [DOI] [PubMed] [Google Scholar]
- 31.Curtis A, Wilkinson C. Topographical control of cells. Biomaterials. 1997;18:1573–83. doi: 10.1016/s0142-9612(97)00144-0. [DOI] [PubMed] [Google Scholar]
- 32.Gingras J, Rioux RM, Cuvelier D, et al. Controlling the orientation and synaptic differentiation of myotubes with micropatterned substrates. Biophys J. 2009;97:2771–9. doi: 10.1016/j.bpj.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flaibani M, Boldrin L, Cimetta E, et al. Muscle differentiation and myotubes alignment is influenced by micropatterned surfaces and exogenous electrical stimulation. Tissue Eng A. 2009;15:2447–57. doi: 10.1089/ten.tea.2008.0301. [DOI] [PubMed] [Google Scholar]
- 34.Madaghiele M, Sannino A, Yannas IV, et al. Collagen-based matrices with axially oriented pores. J Biomed Mater Res A. 2008;85:757–67. doi: 10.1002/jbm.a.31517. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H, Hussain I, Brust M, et al. Aligned two- and three-dimensional structures by directional freezing of polymers and nanoparticles. Nat Mater. 2005;4:787–93. doi: 10.1038/nmat1487. [DOI] [PubMed] [Google Scholar]
- 36.Schoof H, Apel J, Heschel I, et al. Control of pore structure and size in freeze-dried collagen sponges. J Biomed Mater Res. 2001;58:352–7. doi: 10.1002/jbm.1028. [DOI] [PubMed] [Google Scholar]
- 37.Mandal BB, Kundu SC. Cell proliferation and migration in silk fibroin 3D scaffolds. Biomaterials. 2009;30:2956–65. doi: 10.1016/j.biomaterials.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 38.Ren L, Tsuru K, Hayakawa S, et al. Novel approach to fabricate porous gelatin-siloxane hybrids for bone tissue engineering. Biomaterials. 2002;23:4765–73. doi: 10.1016/s0142-9612(02)00226-0. [DOI] [PubMed] [Google Scholar]
- 39.Boudriot U, Dersch R, Greiner A, et al. Electrospinning approaches toward scaffold engineering–a brief overview. Artif Organs. 2006;30:785–92. doi: 10.1111/j.1525-1594.2006.00301.x. [DOI] [PubMed] [Google Scholar]
- 40.Sell SA, McClure MJ, Garg K, et al. Electrospinning of collagen/biopolymers for regenerative medicine and cardiovascular tissue engineering. Adv Drug Deliv Rev. 2009;61:1007–19. doi: 10.1016/j.addr.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 41.Buttafoco L, Kolkman NG, Engbers-Buijtenhuijs P, et al. Electrospinning of collagen and elastin for tissue engineering applications. Biomaterials. 2006;27:724–34. doi: 10.1016/j.biomaterials.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 42.Lee KY, Jeong L, Kang YO, et al. Electrospinning of polysaccharides for regenerative medicine. Adv Drug Deliv Rev. 2009;61:1020–32. doi: 10.1016/j.addr.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 43.Barnes CP, Sell SA, Boland ED, et al. Nanofiber technology: designing the next generation of tissue engineering scaffolds. Adv Drug Deliv Rev. 2007;59:1413–33. doi: 10.1016/j.addr.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 44.Schnell E, Klinkhammer K, Balzer S, et al. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-epsilon-caprolactone and a collagen/poly-epsilon-caprolactone blend. Biomaterials. 2007;28:3012–25. doi: 10.1016/j.biomaterials.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Morrison WA, Hussey AJ. Extracellular matrix as a bioactive material for soft tissue reconstruction. ANZ J Surg. 2006;76:1047. doi: 10.1111/j.1445-2197.2006.03970.x. [DOI] [PubMed] [Google Scholar]
- 46.Bolgen N, Menceloglu YZ, Acatay K, et al. In vitro and in vivo degradation of non-woven materials made of poly(epsilon-caprolactone) nanofibers prepared by electrospinning under different conditions. J Biomater Sci Polym Ed. 2005;16:1537–55. doi: 10.1163/156856205774576655. [DOI] [PubMed] [Google Scholar]
- 47.Riboldi SA, Sampaolesi M, Neuenschwander P, et al. Electrospun degradable polyesterurethane membranes: potential scaffolds for skeletal muscle tissue engineering. Biomaterials. 2005;26:4606–15. doi: 10.1016/j.biomaterials.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 48.Zhang YZ, Venugopal J, Huang ZM, et al. Characterization of the surface biocompatibility of the electrospun PCL-collagen nanofibers using fibroblasts. Biomacromolecules. 2005;6:2583–9. doi: 10.1021/bm050314k. [DOI] [PubMed] [Google Scholar]
- 49.Huang NF, Patel S, Thakar RG, et al. Myotube assembly on nanofibrous and micropatterned polymers. Nano Lett. 2006;6:537–42. doi: 10.1021/nl060060o. [DOI] [PubMed] [Google Scholar]
- 50.Ayres C, Bowlin GL, Henderson SC, et al. Modulation of anisotropy in electrospun tissue-engineering scaffolds: analysis of fiber alignment by the fast Fourier transform. Biomaterials. 2006;27:5524–34. doi: 10.1016/j.biomaterials.2006.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baker BM, Mauck RL. The effect of nanofiber alignment on the maturation of engineered meniscus constructs. Biomaterials. 2007;28:1967–77. doi: 10.1016/j.biomaterials.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Telemeco TA, Ayres C, Bowlin GL, et al. Regulation of cellular infiltration into tissue engineering scaffolds composed of submicron diameter fibrils produced by electrospinning. Acta Biomater. 2005;1:377–85. doi: 10.1016/j.actbio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 53.Baker BM, Gee AO, Metter RB, et al. The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials. 2008;29:2348–58. doi: 10.1016/j.biomaterials.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang H, Hu Y, Li Y, et al. A facile technique to prepare biodegradable coaxial electrospun nanofibers for controlled release of bioactive agents. J Control Release. 2005;108:237–43. doi: 10.1016/j.jconrel.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 55.Moroni L, De Wijn JR, Van Blitterswijk CA. Integrating novel technologies to fabricate smart scaffolds. J Biomater Sci Polym Ed. 2008;19:543–72. doi: 10.1163/156856208784089571. [DOI] [PubMed] [Google Scholar]
- 56.Sill TJ, Von Recum HA. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials. 2008;29:1989–2006. doi: 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 57.Williams JH, Schray RC, Sirsi SR, et al. Nanopolymers improve delivery of exon skipping oligonucleotides and concomitant dystrophin expression in skeletal muscle of mdx mice. BMC Biotechnol. 2008;8:35. doi: 10.1186/1472-6750-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nelson SF, Crosbie RH, Miceli MC, et al. Emerging genetic therapies to treat Duchenne muscular dystrophy. Curr Opin Neurol. 2009;22:532–8. doi: 10.1097/WCO.0b013e32832fd487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sahoo S, Ang LT, Goh JC, et al. Growth factor delivery through electrospun nanofibers in scaffolds for tissue engineering applications. J Biomed Mater Res A. 2010;93:1539–50. doi: 10.1002/jbm.a.32645. [DOI] [PubMed] [Google Scholar]
- 60.Yang F, Cho SW, Son SM, et al. Genetic engineering of human stem cells for enhanced angiogenesis using biodegradable polymeric nanoparticles. Proc Natl Acad Sci USA. 2010;107:3317–22. doi: 10.1073/pnas.0905432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yancopoulos GD, Davis S, Gale NW, et al. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–8. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 62.Arkudas A, Tjiawi J, Bleiziffer O, et al. Fibrin gel-immobilized VEGF and bFGF efficiently stimulate angiogenesis in the AV loop model. Mol Med. 2007;13:480–7. doi: 10.2119/2007-00057.Arkudas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duan C, Ren H, Gao S. Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins: roles in skeletal muscle growth and differentiation. Gen Comp Endocrinol. 2010;167:344–51. doi: 10.1016/j.ygcen.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 64.Rende M, Brizi E, Conner J, et al. Nerve growth factor (NGF) influences differentiation and proliferation of myogenic cells in vitro via TrKA. Int J Dev Neurosci. 2000;18:869–85. doi: 10.1016/s0736-5748(00)00041-1. [DOI] [PubMed] [Google Scholar]
- 65.Lavasani M, Lu A, Peng H, et al. Nerve growth factor improves the muscle regeneration capacity of muscle stem cells in dystrophic muscle. Hum Gene Ther. 2006;17:180–92. doi: 10.1089/hum.2006.17.180. [DOI] [PubMed] [Google Scholar]
- 66.Sugiyama N, Yoshimura A, Fujitsuka C, et al. Acceleration by MS-818 of early muscle regeneration and enhanced muscle recovery after surgical transection. Muscle Nerve. 2002;25:218–29. doi: 10.1002/mus.10028. [DOI] [PubMed] [Google Scholar]
- 67.Martins A, Duarte AR, Faria S, et al. Osteogenic induction of hBMSCs by electrospun scaffolds with dexamethasone release functionality. Biomaterials. 2010;31:5875–85. doi: 10.1016/j.biomaterials.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 68.Wang F, Li Z, Tamama K, et al. Fabrication and characterization of prosurvival growth factor releasing, anisotropic scaffolds for enhanced mesenchymal stem cell survival/growth and orientation. Biomacromolecules. 2009;10:2609–18. doi: 10.1021/bm900541u. [DOI] [PubMed] [Google Scholar]
- 69.Fujita H, Nedachi T, Kanzaki M. Accelerated de novo sarcomere assembly by electric pulse stimulation in C2C12 myotubes. Exp Cell Res. 2007;313:1853–65. doi: 10.1016/j.yexcr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 70.Genovese JA, Spadaccio C, Chachques E, et al. Cardiac pre-differentiation of human mesenchymal stem cells by electrostimulation. Front Biosci. 2009;14:2996–3002. doi: 10.2741/3429. [DOI] [PubMed] [Google Scholar]
- 71.Shafy A, Lavergne T, Latremouille C, et al. Association of electrostimulation with cell transplantation in ischemic heart disease. J Thorac Cardiovasc Surg. 2009;138:994–1001. doi: 10.1016/j.jtcvs.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 72.Gaetani R, Ledda M, Barile L, et al. Differentiation of human adult cardiac stem cells exposed to extremely low-frequency electromagnetic fields. Cardiovasc Res. 2009;82:411–20. doi: 10.1093/cvr/cvp067. [DOI] [PubMed] [Google Scholar]
- 73.Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, et al. Electrical stimulation of nerve cells using conductive nanofibrous scaffolds for nerve tissue engineering. Tissue Eng A. 2009;15:3605–19. doi: 10.1089/ten.TEA.2008.0689. [DOI] [PubMed] [Google Scholar]
- 74.Li M, Guo Y, Wei Y, et al. Electrospinning polyaniline-contained gelatin nanofibers for tissue engineering applications. Biomaterials. 2006;27:2705–15. doi: 10.1016/j.biomaterials.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 75.Jun I, Jeong S, Shin H. The stimulation of myoblast differentiation by electrically conductive sub-micron fibers. Biomaterials. 2009;30:2038–47. doi: 10.1016/j.biomaterials.2008.12.063. [DOI] [PubMed] [Google Scholar]
- 76.Le Grand F, Rudnicki MA. Skeletal muscle satellite cells and adult myogenesis. Curr Opin Cell Biol. 2007;19:628–33. doi: 10.1016/j.ceb.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tajbakhsh S. Stem cells to tissue: molecular, cellular and anatomical heterogeneity in skeletal muscle. Curr Opin Genet Dev. 2003;13:413–22. doi: 10.1016/s0959-437x(03)00090-x. [DOI] [PubMed] [Google Scholar]
- 78.Zammit P, Beauchamp J. The skeletal muscle satellite cell: stem cell or son of stem cell. Differentiation. 2001;68:193–204. doi: 10.1046/j.1432-0436.2001.680407.x. [DOI] [PubMed] [Google Scholar]
- 79.Seale P, Sabourin LA, Girgis-Gabardo A, et al. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–86. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 80.Kuang S, Kuroda K, Le Grand F, et al. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weintraub H, Davis R, Tapscott S, et al. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991;251:761–6. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- 82.Collins CA, Olsen I, Zammit PS, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 83.Kottlors M, Kirschner J. Elevated satellite cell number in Duchenne muscular dystrophy. Cell Tissue Res. 2010;340:541–8. doi: 10.1007/s00441-010-0976-6. [DOI] [PubMed] [Google Scholar]
- 84.Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci USA. 1968;61:477–83. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Blau HM, Brazelton TR, Weimann JM. The evolving concept of a stem cell: entity or function. Cell. 2001;105:829–41. doi: 10.1016/s0092-8674(01)00409-3. [DOI] [PubMed] [Google Scholar]
- 86.Boonen KJ, Post MJ. The muscle stem cell niche: regulation of satellite cells during regeneration. Tissue Eng B Rev. 2008;14:419–31. doi: 10.1089/ten.teb.2008.0045. [DOI] [PubMed] [Google Scholar]
- 87.Deans TL, Elisseeff JH. Stem cells in musculoskeletal engineered tissue. Curr Opin Biotechnol. 2009;20:537–44. doi: 10.1016/j.copbio.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 88.Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 89.Zhu Y, Liu T, Song K, et al. Adipose-derived stem cell: a better stem cell than BMSC. Cell Biochem Funct. 2008;26:664–75. doi: 10.1002/cbf.1488. [DOI] [PubMed] [Google Scholar]
- 90.Garbade J, Schubert A, Rastan AJ, et al. Fusion of bone marrow-derived stem cells with cardiomyocytes in a heterologous in vitro model. Eur J Cardiothorac Surg. 2005;28:685–91. doi: 10.1016/j.ejcts.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 91.Brazelton TR, Nystrom M, Blau HM. Significant differences among skeletal muscles in the incorporation of bone marrow-derived cells. Dev Biol. 2003;262:64–74. doi: 10.1016/s0012-1606(03)00357-9. [DOI] [PubMed] [Google Scholar]
- 92.Gussoni E, Blau HM, Kunkel LM. The fate of individual myoblasts after transplantation into muscles of DMD patients. Nat Med. 1997;3:970–7. doi: 10.1038/nm0997-970. [DOI] [PubMed] [Google Scholar]
- 93.Meirelles Lda S, Nardi NB. Methodology, biology and clinical applications of mesenchymal stem cells. Front Biosci. 2009;14:4281–98. doi: 10.2741/3528. [DOI] [PubMed] [Google Scholar]
- 94.Satija NK, Singh VK, Verma YK, et al. Mesenchymal stem cell-based therapy: a new paradigm in regenerative medicine. J Cell Mol Med. 2009;13:4385–402. doi: 10.1111/j.1582-4934.2009.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Popescu LM, Faussone-Pellegrini MS. Telocytes – a case of serendipity: the winding way from Interstitial cells of Cajal (ICC), via interstitial Cajal-like cells (ICLC) to Telocytes. J Cell Mol Med. 2010;14:729–40. doi: 10.1111/j.1582-4934.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gherghiceanu M, Popescu LM. Cardiomyocyte precursors and telocytes in epicardial stem cell niche: electron microscope images. J Cell Mol Med. 2010;14:871–7. doi: 10.1111/j.1582-4934.2010.01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 98.Lee H, Park J, Forget BG, et al. Induced pluripotent stem cells in regenerative medicine: an argument for continued research on human embryonic stem cells. Regen Med. 2009;4:759–69. doi: 10.2217/rme.09.46. [DOI] [PubMed] [Google Scholar]
- 99.Amabile G, Meissner A. Induced pluripotent stem cells: current progress and potential for regenerative medicine. Trends Mol Med. 2009;15:59–68. doi: 10.1016/j.molmed.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 100.Yuasa S, Fukuda K. Cardiac regenerative medicine. Circ J. 2008;72:49–55. doi: 10.1253/circj.cj-08-0378. [DOI] [PubMed] [Google Scholar]
- 101.Moretti A, Bellin M, Welling A, et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med. 2010 doi: 10.1056/NEJMoa0908679. DOI: 10.1056/NEJMoa0908679x. [DOI] [PubMed] [Google Scholar]
- 102.Hanna J, Wernig M, Markoulaki S, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–3. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 103.Chen L, Liu L. Current progress and prospects of induced pluripotent stem cells. Sci China C Life Sci. 2009;52:622–36. doi: 10.1007/s11427-009-0092-6. [DOI] [PubMed] [Google Scholar]
- 104.Kaji K, Norrby K, Paca A, et al. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–5. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sun X, Fu X, Han W, et al. Can controlled cellular reprogramming be achieved using microRNAs. Ageing Res Rev. 2010;9:475–83. doi: 10.1016/j.arr.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 106.Meyer N, Penn LZ. MYC – timeline reflecting on 25 years with MYC. Nat Rev Cancer. 2008;8:976–90. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- 107.Rowland BD, Peeper DS. KLF4, p21 and context-dependent opposing forces in cancer. Nat Rev Cancer. 2006;6:11–23. doi: 10.1038/nrc1780. [DOI] [PubMed] [Google Scholar]


