Abstract
γ-Secretase is a transmembrane protease complex responsible for the processing of a multitude of type 1 transmembrane proteins, including amyloid precursor protein (APP) and Notch. A functional complex is dependent on the assembly of four proteins: presenilin (PS), nicastrin, Aph-1 and Pen-2. Little is known about how the substrates are selected by γ-secretase, but it has been suggested that γ-secretase associated proteins (GSAPs) could be of importance. For instance, it was recently reported from studies in cell lines that TMP21, a transmembrane protein involved in trafficking, binds to γ-secretase and regulates the processing of APP-derived substrates without affecting Notch cleavage. Here, we present an efficient and selective method for purification and analysis of γ-secretase and GSAPs. Microsomal membranes were prepared from rat or human brain and incubated with a γ-secretase inhibitor coupled to biotin via a long linker and a S-S bridge. After pulldown using streptavidin beads, bound proteins were eluted under reducing conditions and digested by trypsin. The tryptic peptides were subjected to LC-MS/MS analysis, and proteins were identified by sequence data from MS/MS spectra. All of the known γ-secretase components were identified. Interestingly, TMP21 and the PS associated protein syntaxin1 were associated to γ-secretase in rat brain. We suggest that the present method can be used for further studies on the composition of the γ-secretase complex.
Keywords: Alzheimer disease, γ-secretase, human and rat brain, affinity-pulldown, mass spectrometry
Introduction
γ-Secretase has due to its involvement in Alzheimer disease (AD) been extensively studied in recent years [1]. This enzyme mediates the final catalytic step that generates the amyloid β-peptide (Aβ), which assembles into neurotoxic aggregates in the human brain [2], and it has been suggested that reducing the Aβ-levels by inhibiting γ-secretase may be of therapeutic value for the treatment and prevention of AD. Four different transmembrane proteins are necessary and sufficient for the assembly of an active γ-secretase complex: presenilin (PS), nicastrin, Aph-1and Pen-2 [3]. There are two different PS genes, PSEN1 on chromosome 14 and PSEN2 on chromosome 1, and three different Aph-1 variants, suggesting that six different γ-secretase complexes could form. γ-secretase has vital functions during development, as demonstrated by PS knockout mice that are prenatally lethal. This phenotype is at least partly due to abolished signalling by Notch, which is one of many γ-secretase substrates. Besides Notch and the amyloid precursor protein (APP), γ-secretase has been shown to cleave around 50 type 1 transmembrane proteins. Thus, selective inhibition of γ-secretase processing of APP is necessary in order to avoid side effects.
γ-secretase can only cleave substrates with a relatively short ectodomain, and in the case of APP the initial cleavage is mediated by a-secretase or β-secretase [4]. α-Secretase cleaves in the middle of the Aβ-sequence, while β-secretase generates the free N-terminus of Aβ. The resulting C-terminal fragments are then processed by γ-secretase at different positions, for example between residues 49–50 liberating the APP intracellular domain (AICD), between residues 42–43 or 40–41 generating Aβ42 or Aβ40, respectively [5, 6]. Aβ42 is the most amyloidogenic and toxic variant and it is involved in AD pathogenesis [7]. There are examples of so-called γ-secretase modulators (GSMs), which decrease the Aβ42/Aβ40 ratio without any significant effects on Notch cleavage [8]. Some GSMs have been subjected to clinical trials but, although well tolerated, their efficacy is debated.
How γ-secretase processing is regulated and how the substrates are selected is a matter of intense research. The composition of the complex (e.g. whether PS1 or PS2 is present) could affect substrate selectivity and cleavage sites [9]. Moreover, there are examples of γ-secretase associated proteins (GSAPs), for instance TMP21, which regulates Ab-production without any major effects on either AICD or Notch signalling [10]. The search for GSAPs is complicated since there is a delicate balance between conditions that preserve an active γ-secretase and its interaction with GSAPs, but are harsh enough to dissolve the membranes where γ-secretase is localized.
In the present study, we designed a compound composed of a γ-secretase inhibitor coupled to biotin via a cleavable linker (GCB). We show that GCB has a high affinity for γ-secretase and can be used for efficient pulldown of γ-secretase using streptavidin (SA) beads. Importantly, γ-secretase can be eluted by cleavage of the linker by reducing agents, thereby avoiding the elution of proteins non-specifically bound to the beads. All the known γ-secretase components could be identified by liquid chromatography coupled online to tandem mass spectrometry (LC-MS/MS), as well as the previously reported GSAP TMP21, and the PS associated syntaxin1 [11] was found to be associated with γ-secretase in rat brain. Finally, we prepared and analysed membranes from human brain, and identified over 50 proteins potentially associated with γ-secretase. We conclude that the present approach is useful for further studies on γ-secretase and associated proteins.
Material and methods
Antibodies
The following antibodies were used for immunoblotting: PS1-NTF (529591; Calbiochem, Darmstadt, Germany), raised against amino acid residues 1–65 of human PS1; PS1-CTF (MAB5232; Chemicon, Billerica, MA, USA), raised against the loop (amino acid residues 263–378) of human PS1; Aph-1aL (PRB-550P; COVANCE, Berkeley, CA, USA), raised against the C-terminal region of human Aph-1aL; nicastrin (N1660, Sigma, St. Louis, MO, USA), raised against C-terminal residues 693–709 of human nicastrin; UD1 raised against the N-terminal residues ERVSNEEKLNL of Pen-2 (a gift from Dr. Jan Näslund, Karolinska Institutet); syntaxin1 (S0664, Sigma), raised against the synaptosomal plasma-membrane fraction from adult rat hippocampus; TMP21 (3999, Nordic BioSite), raised against the 18 amino acid peptide from near the centre of human TMP21.
SDS-PAGE and Western blotting
Samples were boiled in Tricine sample buffer (450 mM Tris HCl, 12% Glycerol, 4% SDS, 0.0025% Coomassie Blue G, 0.0025% Phenol Red, 50 mM DTT pH 8.45) and separated by SDS-PAGE (10–20% Tricine gels, Invitrogen, Carlsbad, CA, USA). After electrophoresis proteins were transferred to PVDF membranes (Bio-Rad, Hercules, CA, USA) and probed with specific antibodies. Immune complexes were visualized by SuperSignal West Dura enhanced chemiluminescence reagent (Pierce, Rockford, IL, USA). Hyperfilm ECL (GE Healthcare, Piscataway, NJ, USA) was used for exposure, and films were scanned using an AGFA Duoscan. For quantification, a ChemiDoc CCD camera system (Bio-Rad) was used. Bands were quantified using the Quantity One analysis software Version4.5.2 (Bio-Rad). The density of the bands was calculated as a percentage of a standard (input sample) run on each gel.
Synthesis of biotinylated inhibitor (GCB)
The synthesis of the methyl ester of the L-685,458 acid derivative was described previously [12]. The L-685,458 derivative was reacted with 10 equivalents of diamido-dPEG diamine (eChemShop, Newark, DE, USA) under EDC (N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride) and 1-hydroxybenzotriazole hydrate overnight. The resulting product was treated with 1 equivalent of EZ Link Sulfo-NHS-SS-Biotin (Pierce) overnight. The reaction mixture was purified by silica gel chromatography to give GCB.
γ-Secretase activity assay
Blastocyst-derived ES-cells deficient in PS1 and PS2, stably expressing PS1, BD8-PS1 cells, were cultured as previously described [13]. All procedures were carried out at 4°C. Cells were harvested with cell scraper, pelleted and washed once in ice cold PBS. Cells were resuspended in 9 volumes of buffer A containing 20 mM Hepes, pH 7.5, 50 mM KCL, 2 mM EGTA and Complete™ protease inhibitor cocktail (which inhibits a broad spectrum of serine, cysteine and metalloproteases, Roche Applied Science, Indianapolis, IN, USA) and sonicated on ice for 30 sec. (Sonifier 450 BRANSON, Danbury, USA). Cell debris and nuclei were removed by centrifugation at 800 g for 10 min. The resulting supernatant was pooled and centrifuged at 100,000 g for 60 min. Membrane proteins were solubilized in buffer H containing 20 mM Hepes, pH 7.4, 150 mM NaCl, 2 mM EDTA, Complete™ protease inhibitor cocktail and 1% (w/v) CHAPSO for 30 min. at 4°C. Insoluble debris was removed by centrifugation at 100,000 g for 30 min. The resulting supernatants (solubilized γ-secretase) were diluted with buffer H without CHAPSO to give a final concentration of 0.4% (w/v) CHAPSO. Protein concentration was determined by BCA protein assay (Pierce). Solubilized γ-secretase (1 mg·ml−1) was incubated in the absence or in the presence of L-685,458 or GCB for 16 hrs at 37°C. The reaction was stopped by adding RIPA (150 mM NaCl, 1.0% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris HCl, pH 8.0) and boiling for 5 min. The samples were centrifuged at 10,000 g, and Aβ40 was measured in the supernatants by ELISA (Wako Chemicals, Osaka, Japan). Background, defined as the signal in the presence of 10 μM L-685,458, was subtracted. IC50 (nM) values were calculated using the GraphPad Prism 4.02 software (GraphPad Software, La Jolla, CA, USA).
Preparation of γ-secretase enriched membranes
Sprague–Dawley rats were obtained from B&K Universal (Sollentuna, Sweden). Human brain material (40 g of frontal cortex from a non-AD case, 20 hr post-mortem time) was obtained from Huddinge Brain Bank (Huddinge, Sweden). The use of brain materials in this study was approved by the Regional Ethical Committee in Stockholm. The tissue was homogenized in buffer A (20 mM Hepes, 50 mM KCL, 2 mM EGTA, pH 7.5) containing Complete™ protease inhibitor cocktail with 25 strokes at 1500 rpm using a pestle-homogenizer. The brain homogenates were centrifuged at 1000 g to remove nuclei and cell debris. The post-nuclear supernatants were centrifuged at 10,000 g to remove mitochondria and the resulting supernatants were centrifuged at 100,000 g. The resulting microsomal pellets were resuspended in buffer A supplemented with 20% glycerol, and stored at −80°C. All centrifugation steps were carried out at 4°C.
Preparation of solubilized γ-secretase and affinity pulldown
Frozen microsomal membranes were thawed and centrifuged at 100,000 g for 30 min. Membrane proteins were solubilized in buffer A containing Complete™ protease inhibitor cocktail and 1% (w/v) CHAPSO for 30 min. at 4°C. Insoluble debris was removed by centrifugation at 100,000 g for 30 min. The resulting supernatants (solubilized γ-secretase) were diluted with buffer A without CHAPSO to give a final detergent concentration of 0.5% (w/v) CHAPSO. The samples were incubated with SA-conjugated sepharose beads (GE Healthcare) or SA-conjugated magnetic beads (Invitrogen) to remove endogenous biotinylated proteins for 16 hr at 4°C. The supernatants were recovered by centrifugation at 1000 g for 5 min. and used as starting material (input) for pulldown experiments. The samples were incubated in the presence or absence of 10 μM L-685,458 as a competing inhibitor for 10 min. at room temperature and then incubated with GCB for 30 min. at room temperature. The samples were incubated with SA-beads which were pre-equilibrated by buffer A with 0.5% CHAPSO for 2 hr at 4°C. The resin was washed 3 times with buffer A with 0.5% CHAPSO at room temperature. The captured γ-secretase complex was eluted from the resin by buffer A containing 100 mM DTT and 0.5% CHAPSO, SDS sample buffer, or 0.01% RapiGest (Waters, Milford, MA, USA) in 10 mM ammonium bicarbonate supplemented with 10 mM DTT.
Tryptic digestion and fractionation of peptides
The eluted proteins were suspended in trypsin digestion buffer (180 mM ammonium bicarbonate, 2 mM CaCl2 and 0.3% RapiGest) and digested by trypsin at 37°C overnight. The digested samples were loaded onto ZipTips C18 (Millipore, Billerica, MA, USA) after the tips had been equilibrated according to the manufacturer’s recommended procedures. The samples were washed with 0.2% formic acid (FA) in water and eluted from the ZipTips C18 with 80% acetonitrile (ACN)/0.2% FA. The eluted peptides were evaporated to dryness in a vacuum centrifuge (Maxi dry lyo, Heto-Holten AIS, Allerød, Denmark). For further fractionation, the eluted peptides were adjusted with 0.1% FA to 45% ACN and loaded onto ZipTips SCX (Millipore) according to the manufacturer’s recommendation. The samples were sequentially eluted with 40 mM ammonium formate (pH 3.0) containing 45% ACN, 80 mM ammonium formate (pH 3.0) containing 45% ACN and 5% ammonium hydroxide containing 45% ACN. The eluted peptides were evaporated to dryness in a vacuum centrifuge.
Mass spectrometry
The digested samples were dissolved in 2% ACN/0.2% FA in water and analysed on a 6330 Ion Trap LC/MS system (Agilent Technologies, Santa Clara, CA, USA). Liquid chromatography was performed on a HPLC chip-system (Agilent Technologies) using a chip with a 150 mm × 75 μm analytical column and a 160 nl enrichment column, both packed with 5 μm Zorbax 300SB-C18. Samples were loaded onto the enrichment column, using a mobile phase containing 2% ACN and 0.2% FA in water, at a flow rate of 2 μl·min−1 and washed for 3 min. Tryptic peptides were eluted into the mass spectrometer using a gradient of increasing mobile phase B at a flow rate of 200 nl·min−1. The gradient (mobile phase A: H2O/0.2% FA; mobile phase B: ACN/0.2% FA) was ramped from 3 to 26% B in 92 min., and then from 26% to 36% B in 20 min. As an alternative, the gradient was ramped from 3% to 26% B in 184 min., and then from 26% to 36% B in 40 min. The capillary voltage was set to 1950 V, the flow rate and the temperature of the dry gas were set to 4 L·min−1 and 300°C, respectively. Spectra were collected over an m/z range of 230–1 800, and the five most intense ions were subjected to MS/MS. Precursors were excluded for 30 sec. after two MS/MS spectra.
Protein identification by MASCOT Daemon software package
A list of the resulting spectra were generated using the 6300 Series Ion Trap LC/MS Software Version 6.1 (Agilent Technologies) with an intensity threshold of 1000. The compound lists were exported as Mascot generic format files (mgf). Protein identification was performed by the MASCOT Daemon software package (Matrix Science, London, UK), parameters used in the database search were: database, NCBInr; taxonomy, Homo sapiens or Mammalia; maximum missed cleavages, 1; variable modification, methionine oxidization; monoisotopic mass; unrestricted protein mass, 2.0 Da fragment ion mass tolerance; 0.3 Da MS/MS tolerances; peptide charge state, 2+,3+. Statistically significant peptide data acquired from samples incubated in the presence of competing inhibitor were subtracted from statistically significant peptides data from samples incubated in the absence of competing inhibitor. Finally, extracted ion chromatogram peptide peaks in the two samples were compared for validating the subtraction analysis.
Protein identification by SpectrumMill Proteomics Workbench
Peak lists were generated from the data obtained from each analysis using the data extractor of the SpectrumMill Proteomics Workbench version A.03.03.078 (Agilent Technologies). The MS and MS/MS data were extracted by limiting the data search to deconvoluted ions observed between 400 and 5000 Da. MS scans with similar precursor mass (±1.4 m/z) and retention time (RT) within 60 sec. were merged. Only spectra that contained sequence tag information of three or more residues were submitted for database searching. The resulting extracted data were searched against the Homo Sapiens subset of SwissProt protein database using the MS/MS search function of SpectrumMill. Search parameters included a variable modification on methionine residues (oxidized methionine), a 70% minimum matched peak intensity, ±2.5 Da tolerance on precursor ions and ±0.3 Da tolerance on product ions, one missed tryptic cleavage, and ESI-ion trap scoring parameters as defined by the search algorithm. The initial results were autovalidated using the following parameters for the ‘protein details’ mode: SPI >70% for matches with score >8 for +1, >7 for +2, and >9 for +3; SPI >90% for score >6 on +1. The autovalidation step was done in ‘peptide’ mode using criteria of a score >13 and SPI >70%. In addition, both autovalidation steps required a forward–reverse score >1 for +1 and +2, and >2 for +3 peptides. Results from select additional spectra with lower scores (>10, >70%) were accepted as valid only after manual inspection. The validated peptides were used to identify a set of proteins from which a result file was created [14]. A second round of searches with unvalidated peptide spectra was performed against the set of proteins in this result file allowing a non-specific C-terminal or a non-specific N-terminal.
Results
Characterization of the compound
The molecule was designed for efficient pulldown of γ-secretase. We coupled the commonly used inhibitor L-685,458 via a long hydrophilic linker to a cleavable biotin group (GCB) (Fig. 1A). The inhibitor and the biotin group are around 70 Å apart (as estimated by 3D ChemDraw), which is sufficient for concurrent binding of γ-secretase and SA. The inhibitory potency was tested by quantifying Aβ production from microsomal membranes prepared from BD8-PS1 cells by ELISA, and GCB showed similar IC50 as L-685,458 (Fig. 1B). Thus, we conclude that the linker-biotin part has only a minor effect on affinity, and that GCB may be suitable for pulldown of γ-secretase.
Fig 1.
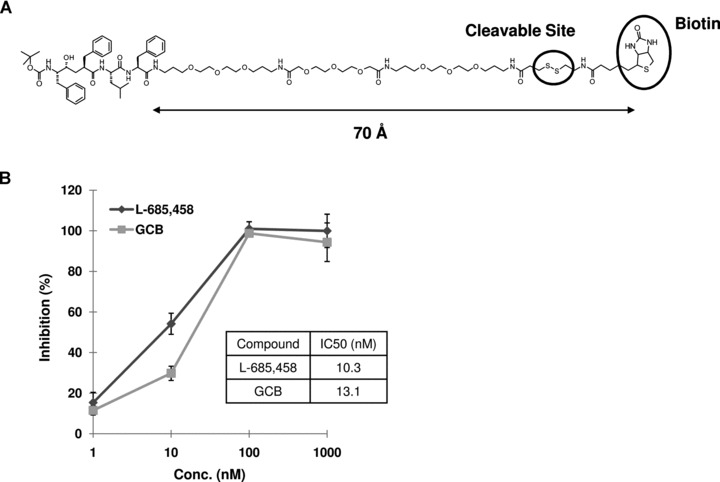
The structure of biotinylated γ-secretase inhibitor and the inhibitory potency for Aβ40 production. (A) GCB is designed with a long hydrophilic linker, a disulfide bond and a biotin group. The distance between the inhibitor part and the biotin moiety is determined by 3D ChemDraw (CambridgeSoft, Cambridge, MA, USA). (B) Dose inhibition curves of GCB and L-685,458. Inhibitory potencies were measured by the in vitro generation of Aβ40 in the endogenous substrate assay using membrane fraction from BD8-PS1cells.
Pulldown of γ-secretase from microsomal membrane preparations from rat brain
A membrane fraction enriched in γ-secretase was prepared as previously described [15]. Briefly, one rat brain was homogenized in buffer A, and subjected to centrifugation at 1000 g to remove nuclei and debris. The supernatant was centrifuged at 10,000 g in order to remove mitochondria, the resulting supernatant was centrifuged at 100,000 g for 1 hr and the pellet (P3) was collected. P3 was treated with 1% CHAPSO for 1 hr, centrifuged and the supernatant (soluble γ-secretase) was collected and used for the pulldown experiments.
In order to get a high recovery and maintain a low background, we investigated by Western blotting the recovery of the known γ-secretase components at different concentrations of GCB (Fig. 2A and B). We selected a concentration of 200 nM since higher concentrations showed only a minor increase in recovery. Two different types of SA beads were tested and were found to give similar results (data not shown). We chose to use magnetic SA-beads to ensure reproducible recovery of the beads.
Fig 2.
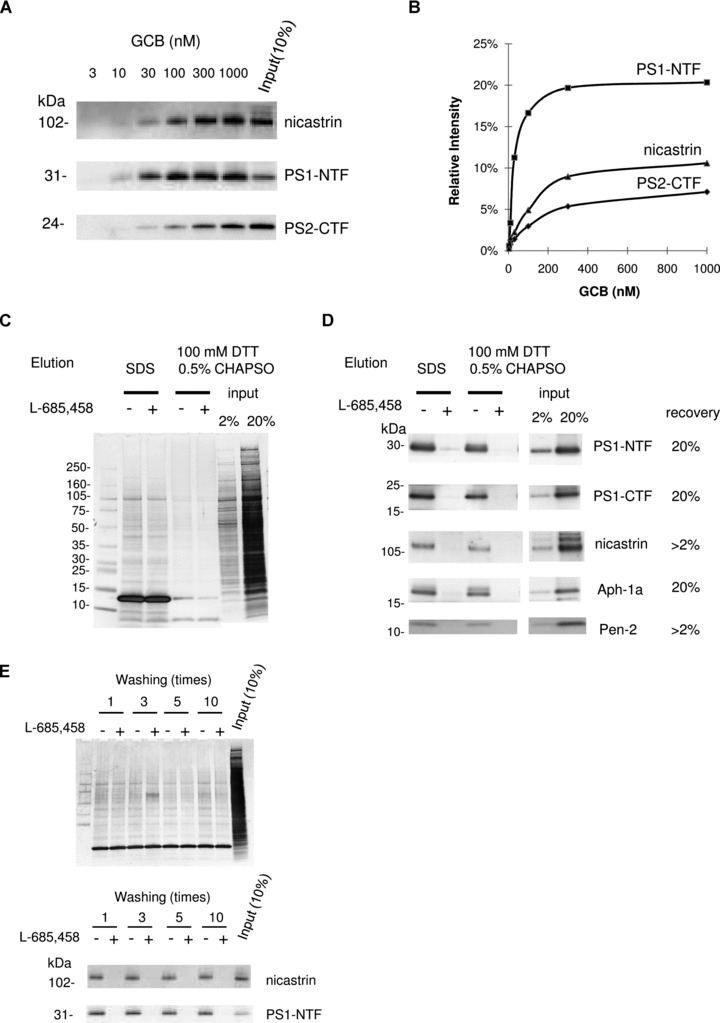
γ-secretase complex components were specifically captured by GCB pulldown from rat brain and eluted by reducing conditions. (A) Solubilized γ-secretase prepared from rat brain material was incubated with increasing concentrations of GCB and isolated with SA beads. The captured γ-secretase complex was eluted by SDS sample buffer and subject to Western blotting for the indicated γ-secretase subunit. (B) The recovery of nicastrin, PS1-NTF and PS2-CTF was estimated by quantifying the density of the respective bands and the input (10% of the total sample) by a CCD camera and appropriate software. (C) Solubilized γ-secretase prepared from rat brain material was incubated with 200 nM GCB in the presence (+) or the absence (−) of 10 μM L-685,458 and isolated with SA beads. The captured γ-secretase complex was eluted with SDS sample buffer or 100 mM DTT supplement with 0.5% CHAPSO. Eluted samples were separated by SDS-PAGE and transferred to PVDF membrane. Transferred membrane was analysed by colloidal gold staining. Elution using reducing reagent (DTT) clearly reduced non-specific binding compared to elution using SDS sample buffer. Also the elution of SA (13 kD) was reduced. (D) The same samples as in (C) were subjected to Western blotting for the indicated γ-secretase subunit. (E) Solubilized γ-secretase prepared from rat brain material was incubated with 200 nM GCB in the presence (+) or the absence (−) of 10 μM L-685,458 and isolated with SA beads. The resin was washed indicated times with buffer A with 0.5% CHAPSO at room temperature and eluted with SDS sample buffer. Eluted samples were subjected to Western blotting for the indicated γ-secretase subunit and analysed by colloidal gold staining.
Next, we investigated elution conditions and specificity. Elution with SDS sample buffer was efficient, but resulted in the elution of non-specifically bound proteins as estimated by colloidal gold staining of the membranes (Fig. 2C and D). However, the SS-linker made it possible to use mild elution conditions in the presence of a reducing agent (DTT). In this case, the background was clearly reduced (compare lanes 2 and 3 with lanes 4 and 5 in Fig. 2C) while there was only a slight reduction in recovery of the γ-secretase components (Fig. 2D). Irrespective of elution conditions, the recovery of the γ-secretase components was inhibited in presence of 10 μM L-685,458. Since we wanted to use mild and rapid washing conditions, we investigated the effect of repeated washing on the recovery of γ-secretase. The background was slightly decreased upon repeated washes, while no significant reduction in recovery was observed (Fig. 2E). We chose to wash the beads three times in the following experiments.
Liquid chromatography-mass spectrometry analysis
The samples were eluted with RapiGest (a detergent which is suitable for tryptic digestion of membrane proteins) supplemented with reducing agent, the SA beads were removed, and the samples were incubated with trypsin at 37°C overnight. RapiGest was removed according to the manufacturer’s instructions and the tryptic peptides were desalted and concentrated by using ZipTips. The peptides were extracted from the ZipTips by ACN and the samples were dried by vacuum centrifugation. The samples were diluted in water supplemented with 0.2% FA, and injected into the LC-MS/MS system. For the unbiased identification of GSAPs, we used nanoflow liquid chromatography coupled online to an electrosprayionization-ion trap mass spectrometer. The small volumes used in the LC-system and the high sensitivity of the mass spectrometer allow the identification of proteins at around one fmol. The peptides were eluted by a water/ACN gradient over 2 hrs. The masspectrometer was set to subject the five highest peaks in each MS-scan to MS/MS analysis. The cycle time was around 1 sec., and 6000 MS/MS spectra were collected in one analysis. A typical total ion chromatogram is shown in Fig. 3A.
Fig 3.
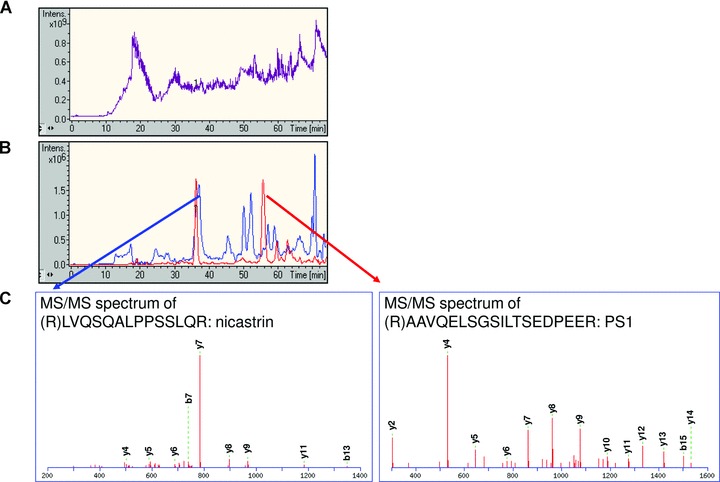
γ-secretase components were identified from LC-MS/MS analysis. Captured sample from rat brain in the absence of L-685,458 was analysed by LC-MS/MS. (A) Total ion chromatogram acquired from mass-to-charge ratio (m/z) 200 to m/z 1800. (B) Extracted ion chromatogram for m/z 1016.5 corresponding to the tryptic peptide AAVQELSGSILTSEDPEER from PS1 (red line), and for m/z 762.4 corresponding to the tryptic peptide LVQSQALPPSSLQR from nicastrin (blue line). (C) MS/MS spectrum of LVQSQALPPSSLQR and AAVQELSGSILTSEDPEER. The series of y-ions correspond to peptide fragments with an intact C-terminus (i.e. y7 = PPSSLGR), while the b-ions correspond to peptide fragments with an intact N-terminus (i.e. b7 = LVQSQAL).
Identification of γ-secretase components in rat brain
Soluble γ-secretase was prepared as described above from 20 rat brains and subjected to GCB-pulldown in the absence or in the presence of 10 μM L-685,458 (50-fold excess). The samples were eluted, digested, and injected into the LC-MS/MS system. The MS/MS spectra were subjected to protein database search using the Mascot software. In the non-competed sample, PS and nicastrin were readily identified with high scores, 315 and 131, respectively (significant score = 40). In line with Western blotting data, these proteins were not identified in the competed sample. The selected ion chromatograms for one of the identified peptides from PS (AAVQELSGSILTSEDPEER) and from nicastrin (LVQSQALPPSSLQR) are presented in Fig. 3B, and the spectra of these peptides are shown in Fig. 3C.
TMP21 and syntaxin1 are associated with γ-secretase in rat brain
In the samples analysed above, TMP21 and syntaxin1 were identified with high scores (TMP21 = 416 and syntaxin1 = 119), clearly above the significance level of 40. The selected ion chromatograms corresponding to the identified peptides showed competition by L-685,458 (Fig. 4A and B). The competition of these proteins was validated by Western blotting (Fig. 4C and D). Hence, we suggest that these proteins can interact with γ-secretase in rat brain.
Fig 4.
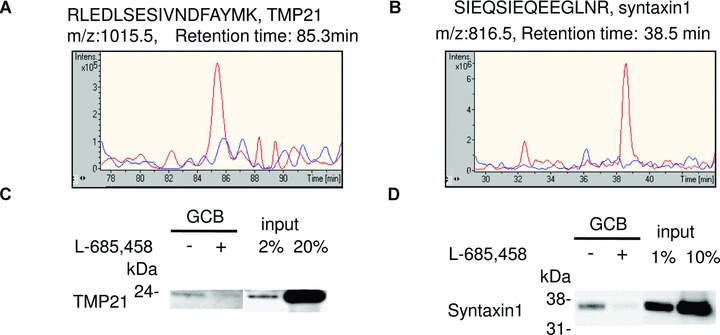
Syntaxin1 and TMP21 are GSAPs in rat brain. Solubilized γ-secretase prepared from rat brain were incubated with 200 nM GCB in the presence (+) or the absence (−) of 10 μM L-685,458 and isolated with SA beads. (A) The captured samples from rat brain in the presence (blue line) or the absence (red line) of 10 μM L-685,458 were digested with trypsin and analysed by LC-MS/MS. Extracted ion chromatogram for m/z 1015.5 corresponding to the tryptic peptide RLEDLSESIVNDFAYMK from TMP21. (B) Extracted ion chromatogram for m/z 816.5 corresponding to the tryptic peptide SIEQSIEQEEGLNR from syntaxin1. (C, D) The captured samples were eluted with SDS sample buffer and subjected to Western blotting for TMP21 (C) and syntaxin1 (D).
Several different proteins could be associated with γ-secretase in rat brain
In total, 91 proteins were uniquely identified in the sample without competing inhibitor. Most of these were integral membrane proteins (49%) or membrane associated proteins (29%) according to the annotation in UniProtKB/SwissProt database (Fig. 5). These proteins are presently evaluated with respect to association and their effect on Aβ40 and Aβ42 production.
Fig 5.
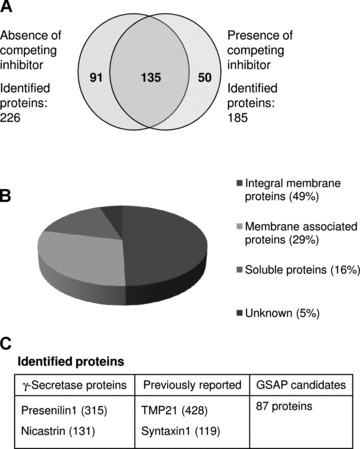
Summary of identified proteins from rat brain. (A) A total of 91 proteins were uniquely identified from samples in the absence of L-685,458 after subtraction. The subtraction analysis was performed based on statistically significant peptides data. (B) Distribution of the relative number of hydrophobic (integral membrane proteins and membrane associated proteins) and soluble proteins. (C) Identified proteins were classified in three categories. Reported proteins were identified only in the absence of competing inhibitor. The number in parenthesis represents MASCOT score.
Pulldown of γ-secretase from microsomal membrane preparations from human brain
Microsomal membranes from human frontal cortex were prepared and extracted as described above, and the optimized conditions were used for pulldown of γ-secretase. The recovery of the γ-secretase components was similar as for rat, and the competition with L-685,458 was efficient (Fig. 6). Thus, we conclude that the present approach is efficient and can be used for purification of γ-secretase also from post-mortem human brain tissue.
Fig 6.
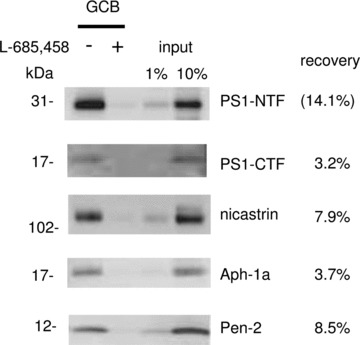
γ-Secretase complex components were specifically captured by GCB pulldown from human frozen brain material. Solubilized γ-secretase prepared from human brain (frontal cortex) was incubated with 200 nM GCB in the presence (+) or the absence (−) of 10 μM L-685,458 and isolated with SA beads. The captured γ-secretase complex was eluted by 10 mM DTT supplement with 0.01% RapiGest and subjected to Western blotting for the indicated γ-secretase subunit. The density of the bands was calculated as a percentage of a standard (input sample) run on the same gel.
Identification of γ-secretase components and associated proteins in microsomal membrane preparations from human brain
In order to increase the number of identified peptides, we fractionated the trypsinated samples by strong cation exchange (SCX) ZipTips (see materials and methods) prior to LC-MS/MS analysis. Furthermore, the LC-gradient was less steep and lasted for 4 hrs, giving a total run time of 16 hrs for each sample (4 fractions × 4 hrs LC-MS/MS analysis) (Fig. 7). In total, 174,000 MS/MS spectra were collected from four pulldown samples (two with and two without competing inhibitor). In order to process all these data and obtain a comprehensive presentation of the identified proteins, we used the SpectrumMill software, resulting in the unique identification (with a significant protein score > 12, which corresponds to a MASCOT score of around 40) of all of the known γ-secretase components in the non-competed samples. Moreover, around 50 other proteins were identified and we are currently evaluating whether these are GSAPs, and if they affect Aβ production. As an example of the GSAP candidates that we found in human brain, six proteins that were uniquely found in both experiments, or unique in one experiment and with a ratio higher than three in the other, are presented in Table 1.
Fig 7.
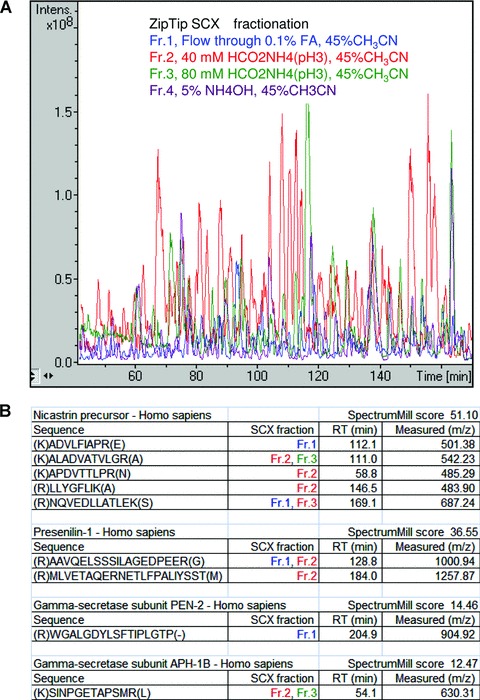
Four γ-secretase components were identified from LC-MS/MS analysis using SCX pre-fractionation. (A) Base peak chromatograms from LC-MS/MS analysis of four SCX fractions. The digested peptides from human captured sample were separated into four fractions using SCX chromatography; Fr.1, Flow through 0.1% FA, 45% CH3CN (blue line); Fr.2, 40 mM HCO2NH4(pH3), 45%CH3CN (red line); Fr.3, 80 mM HCO2NH4(pH3), 45%CH3CN (green line); and Fr.4, 5% NH4OH, 45%CH3CN (purple line). (B) List of identified peptides from the known γ-secretase components including SCX fraction number, RT, score and m/z. Protein identification was performed using the SpectrumMill software and each peptide was validated using the criteria described in material methods.
Table 1.
Membranes from human brain were prepared and subjected to affinity pulldown using GCB in the presence or absence of competing inhibitor L-685,458. The samples were eluted, digested and analysed by LC-MS/MS, and proteins were identified using the SpectrumMill software (significant hits have a protein score > 12). Six proteins uniquely found in the samples without competing inhibitor (two preparations), or uniquely found in one sample and with an intensity ratio above three in the other, are listed.
| Accession no. | Protein name | Subcellular location | TM | Ratio (Exp 1) | Ratio (Exp 2) | Protein score | No. of unique peptides |
|---|---|---|---|---|---|---|---|
| Q99805 | Transmembrane 9 superfamily member 2 precursor | Endosome membrane | 9 | Unique | 3.2 | 44 | 4 |
| P31040 | Succinate dehydrogenase [ubiquinone] flavoprotein subunit, mitochondrial precursor | Mitochondrion inner membrane | 0 | 3.4 | Unique | 41 | 4 |
| Q9HCE3 | Zinc finger protein 532 | Nucleus | 0 | Unique | 3.8 | 16 | 2 |
| Q99PL5 | Ribosome-binding protein 1 | Endoplasmic reticulum membrane | 1 | Unique | Unique | 16 | 2 |
| Q71SP7 | Fatty acid synthase | Cytoplasm, melanosome | 0 | Unique | Unique | 16 | 2 |
| Q5JRA6 | Melanoma inhibitory activity protein 3 precursor | Endoplasmic reticulum membrane | 1 | Unique | Unique | 15 | 2 |
TM = number of transmembrane regions.
PS1 is cleaved after residue 291
Previous studies have identified the N-terminus of PS1-C-terminal fragment (PS1-CTF) [16]. However, the C-terminus of PS1-N-terminal fragment (PS1-NTF) has not been reported, and therefore we subjected the MS/MS spectra to a SpectrumMill search where any residue was allowed in the C-terminus (not only lysine or arginine which terminates the peptides after tryptic cleavage). One of the identified PS1 fragments (MLVETAQERNETLFPALIYSST) ended at residue 291, which is threonine (Fig. 8). It is likely that the C-terminus of this peptide corresponds to the natural cleavage site of full length PS1. This notion is in line with previous data from N-terminal sequencing of PS-CTF from HEK293 cells [17]. Thus, we conclude that that there is no additional processing by carboxy- or amino-peptidases of the PS fragments after the initial cleavage.
Fig 8.
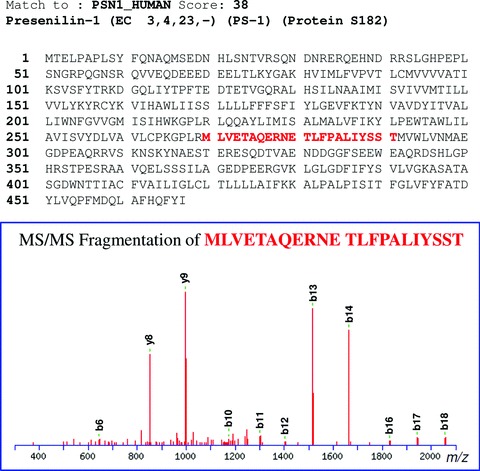
The C-terminal fragment of PS1-NTF, MLVETAQERNETLFPALIYSST, was identified in human brain. GCB was used for pulldown of γ-secretase in membranes prepared from human brain. The sample was analysed by LC-MS/MS, and MS/MS peak lists were generated using the data extractor of the SpectrumMill software. The resulting extracted data were searched against the human subset of the SwissProt protein database. Non-validated spectra were subjected to a second round of searches, allowing a non-tryptic C- or N-terminus, and the C-terminal fragment of PS1-NTF was identified. The peak list files were exported and the human SwissProt database was searched using the Mascot software. This analysis confirmed the results from SpectrumMill. The primary sequence of PS1 and the spectrum of the peptide are shown.
Discussion
γ-Secretase is implicated in AD pathogenesis since it generates Ab, which forms neurotoxic oligomers in the human brain. Inhibition of γ-secretase activity may be a treatment strategy for AD, but the situation is complicated by the fact that the enzyme is involved in the cleavage of several substrates of importance for cellular development, adhesion and signalling. Hence, a detailed knowledge of how γ-secretase activity and substrate selectivity is regulated is crucial for the development of drugs that selectively inhibits Aβ production. Most studies on γ-secretase activity and composition have been performed in cell lines, and it is likely that γ-secretase is differently regulated in brain. Here, we present a mild and efficient method for purification of γ-secretase and associated proteins from rat and human brain, and show that TMP21 and syntaxin1 are associated with γ-secretase in rat brain. Finally, we report several novel GSAPs from brain, and that PS-NTF ends at residue 291 in an active complex in human brain.
We designed a compound consisting of a γ-secretase inhibitor with a long linker connected to cleavable biotin group, GCB, with high affinity for γ-secretase. The long linker allowed concurrent binding of γ-secretase and the SA-conjugated beads that were used for pulldown. The elution of the bound complex was accomplished by using mild reducing conditions, minimizing the background from proteins non-specifically bound to the beads. For the unbiased identification of GSAPs the samples were treated with trypsin and subjected to nanoscale LC-MS/MS analysis using two different protein database search algorithms. By including ion-exchange fractionation of the tryptic peptides prior to LC-MS/MS analysis, the number of identified proteins was increased close to four times. All the known γ-secretase components were identified together with several other proteins, and the pulldown of the γ-secretase components was inhibited with competing inhibitor. Thus, we conclude that the method is suitable for purification of γ-secretase and associated proteins. However, despite our efforts to reduce the background, several proteins were uniquely identified in the competed samples. The mechanism behind this is not clear, but one possibility is that when γ-secretase is not bound to the beads, the surface of the beads is more exposed for binding to other proteins. In this study a crude membrane preparation was used as starting material, and the background may be reduced by using γ-secretase enriched fractions from for instance gradient centrifugations.
Using the present method we show that TMP21, a transmembrane protein involved in trafficking, is associated with γ-secretase in rat brain. TMP21 has previously been reported to co-purify with γ-secretase in preparations from cell lines. In that study an antibody directed to PS1 was used for immunoprecipitation (IP) followed by separation by gel electrophoresis and identification by mass spectrometry [18]. Overexpression of TMP21 had no effect on Aβ production while suppression of TMP21 expression by small interfering RNAs led to increased Aβ production. Interestingly, the silencing had no effect on the generation of AICD or Notch intracellular domain, indicating that GSAPs may be involved in the regulation of γ-secretase activity and selectivity of substrates. The direct association of TMP21 to γ-secretase was later questioned, and it was suggested that the effect of TMP21 on Aβ resulted from altered APP trafficking [19]. On the other hand, we identified TMP21 as a GSAP in rat brain using the present method. A possible explanation to this discrepancy is that only a minor amount (< 0.2%) of the total amount of TMP21 was associated with γ-secretase, and such low levels would not be detected by a less efficient assay. Another possibility is that there is a delicate balance in the TMP21/γ-secretase association that is altered by different conditions during sample preparation or varies between cell types as well as species. In our study, the lack of association in human brain may be due to the longer post-mortem time or the freeze-thaw process of the human brain material.
Another previously reported GSAP, the transmembrane glycoprotein CD147 co-purified with γ-secretase in a multi-step chromatographic procedure, and the association was verified by co-IP [20]. Similarly to TMP21, silencing of CD147 by siRNA resulted in a decreased Aβ production, while overexpression did not alter the Aβ levels. A recent study suggests that CD147 influences the Aβ levels by stimulating matrix metalloproteases that degrade Aβ[21]. In line with the latter study, we did not find an association of CD147 with γ-secretase. However, we cannot exclude the possible association of CD147 with an inactive complex since our affinity pulldown is dependent on the active site in γ-secretase.
The synaptic protein syntaxin1 was previously found to be a PS associated protein [11], but it has not been reported whether it interacts with free PS or with PS within the γ-secretase complex. Here, we show that syntaxin1 is indeed part of an active γ-secretase complex. This is of particular interest since synaptic degeneration is an early pathological hallmark in AD, and synaptic activity has been shown to increase the Aβ production [22]. Thus it is possible that synaptic proteins such as syntaxin1 regulate Aβ production by binding to γ-secretase. Our experiments showed that clearly less than 1% of the total amount of all syntaxin1 was associated with γ-secretase. However, the low degree of association is not unexpected since the main role of syntaxin1 is in the docking of synaptic vesicles to the presynaptic membrane.
The identification and characterization of GSAPs may open new possibilities for selective inhibition of Aβ-production and treatment of AD. In the preparations from human brain, we found around 50 potential GSAPs. Most of the proteins were integral membrane proteins or membrane associated proteins, but there were also some soluble proteins. Since there is a risk that some of the identified proteins are ‘false positive’ and do not affect γ-secretase, we are now evaluating their association to γ-secretase and studying their effect on Ab production. Given that γ-secretase is distributed to several subcellular compartments, can be localized to different microdomains of the membrane, and has a multitude of substrates, we suggest that there could be dozens of GSAPS.
In summary, we have shown that TMP21 and syntaxin1 are associated with γ-secretase in rat brain and suggest that the present method is suitable for the unbiased identification of GSAPs in brain.
Acknowledgments
We thank Dr. Jan Näslund (Karolinska Institutet) for the UD1 antibody. This work was supported by Dainippon Sumitomo Pharma.
References
- 1.McCarthy JV, Twomey C, Wujek P. Presenilin-dependent regulated intramembrane proteolysis and γ-secretase activity. Cell Mol Life Sci. 2009;66:1534–55. doi: 10.1007/s00018-009-8435-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardy J. Has the amyloid cascade hypothesis for Alzheimer’s disease been proved. Curr Alzheimer Res. 2006;3:71–3. doi: 10.2174/156720506775697098. [DOI] [PubMed] [Google Scholar]
- 3.Edbauer D, Winkler E, Regula JT, et al. Reconstitution of γ-secretase activity. Nat Cell Biol. 2003;5:486–8. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- 4.Cole SL, Vassar R. The role of amyloid precursor protein processing by BACE1, the beta-secretase, in Alzheimer disease pathophysiology. J Biol Chem. 2008;283:29621–5. doi: 10.1074/jbc.R800015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weidemann A, Eggert S, Reinhard FB, et al. A novel epsilon-cleavage within the transmembrane domain of the Alzheimer amyloid precursor protein demonstrates homology with Notch processing. Biochemistry. 2002;41:2825–35. doi: 10.1021/bi015794o. [DOI] [PubMed] [Google Scholar]
- 6.Qi-Takahara Y, Morishima-Kawashima M, Tanimura Y, et al. Longer forms of amyloid beta protein: implications for the mechanism of intramembrane cleavage by γ-secretase. J Neurosci. 2005;25:436–45. doi: 10.1523/JNEUROSCI.1575-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe MS. γ-secretase modulators. Curr Alzheimer Res. 2007;4:571–3. doi: 10.2174/156720507783018299. [DOI] [PubMed] [Google Scholar]
- 9.Placanica L, Tarassishin L, Yang G, et al. Pen2 and presenilin-1 modulate the dynamic equilibrium of presenilin-1 and presenilin-2 γ-secretase complexes. J Biol Chem. 2009;284:2967–77. doi: 10.1074/jbc.M807269200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou S, Zhou H, Walian PJ, Jap BK. Regulation of γ-secretase activity in Alzheimer’s disease. Biochemistry. 2007;46:2553–63. doi: 10.1021/bi602509c. [DOI] [PubMed] [Google Scholar]
- 11.Smith SK, Anderson HA, Yu G, et al. Identification of syntaxin 1A as a novel binding protein for presenilin-1. Brain Res Mol Brain Res. 2000;78:100–7. doi: 10.1016/s0169-328x(00)00079-6. [DOI] [PubMed] [Google Scholar]
- 12.Nadin A, Lopez JMS, Neduvelil JG, et al. A stereocontrolled synthesis of 2R-benzyl-5S-tert-butoxycarbonylamino-4R-(tert-butyldimethylsilanyloxy)- 6-phenyl-hexanoic acid (Phe-Phe hydroxyethylene dipeptide isostere) Tetrahedron. 2001;57:1861–4. [Google Scholar]
- 13.Farmery MR, Tjernberg LO, Pursglove SE, et al. Partial purification and characterization of γ-secretase from post-mortem human brain. J Biol Chem. 2003;278:24277–84. doi: 10.1074/jbc.M211992200. [DOI] [PubMed] [Google Scholar]
- 14.Kapp EA, Schutz F, Connolly LM, et al. An evaluation, comparison, and accurate benchmarking of several publicly available MS/MS search algorithms: sensitivity and specificity analysis. Proteomics. 2005;5:3475–90. doi: 10.1002/pmic.200500126. [DOI] [PubMed] [Google Scholar]
- 15.Frånberg J, Welander H, Aoki M, et al. Rat brain γ-secretase activity is highly influenced by detergents. Biochemistry. 2007;46:7647–54. doi: 10.1021/bi0621258. [DOI] [PubMed] [Google Scholar]
- 16.Shirotani K, Takahashi K, Ozawa K, et al. Determination of a cleavage site of presenilin 2 protein in stably transfected SH-SY5Y human neuroblastoma cell lines. Biochem Biophys Res Commun. 1997;240:728–31. doi: 10.1006/bbrc.1997.7730. [DOI] [PubMed] [Google Scholar]
- 17.Podlisny MB, Citron M, Amarante P, et al. Presenilin proteins undergo heterogeneous endoproteolysis between Thr291 and Ala299 and occur as stable N- and C-terminal fragments in normal and Alzheimer brain tissue. Neurobiol Dis. 1997;3:325–37. doi: 10.1006/nbdi.1997.0129. [DOI] [PubMed] [Google Scholar]
- 18.Chen F, Hasegawa H, Schmitt-Ulms G, et al. TMP21 is a presenilin complex component that modulates γ-secretase but not epsilon-secretase activity. Nature. 2006;440:1208–12. doi: 10.1038/nature04667. [DOI] [PubMed] [Google Scholar]
- 19.Vetrivel KS, Gong P, Bowen JW, et al. Dual roles of the transmembrane protein p23/TMP21 in the modulation of amyloid precursor protein metabolism. Mol Neurodegener. 2007;2:4. doi: 10.1186/1750-1326-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou S, Zhou H, Walian PJ, et al. CD147 is a regulatory subunit of the γ-secretase complex in Alzheimer’s disease amyloid beta-peptide production. Proc Natl Acad Sci USA. 2005;102:7499–504. doi: 10.1073/pnas.0502768102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vetrivel KS, Zhang X, Meckler X, et al. Evidence that CD147 modulation of beta-amyloid (Abeta) levels is mediated by extracellular degradation of secreted Abeta. J Biol Chem. 2008;283:19489–98. doi: 10.1074/jbc.M801037200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamenetz F, Tomita T, Hsieh H, et al. APP processing and synaptic function. Neuron. 2003;37:925–37. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]


