Abstract
Telocytes (TC) are interstitial cells with telopodes (Tp). These prolongations (Tp) are quite unique: very long (several tens of micrometres) and very thin (≤0.5 μm), with moniliform aspect: thin segments (podomeres) alternating with dilations (podoms). To avoid any confusion, TC were previously named interstitial Cajal-like cells (ICLC). Myocardial TC were repeatedly documented by electron microscopy, immunohistochemistry and immunofluorescence. TC form a network by their Tp, either in situ or in vitro. Cardiac TC are (completely) different of ‘classic’ fibroblasts or fibrocytes. We hereby present a synopsis of monitoring, by time-lapse videomicroscopy, of Tp network development in cell culture. We used a protocol that favoured interstitial cell selection from adult mouse myocardium. Videomicroscopy showed dynamic interactions of neighbour TC during the network formation. During their movement, TC leave behind distal segments (podomeres) of their Tp as guiding marks for the neighbouring cells to follow during network rearrangement.
Keywords: telocytes, telopodes, podomeres, podoms, interstitial cells, time-lapse videomicroscopy, ICLC
Recently, a new cell type – the telocyte (TC) – has been documented in the interstitium of several cavitary and non-cavitary organs [1, 2], including humans and other vertebrates’ myocardium [3, 4]. As a distinct feature, TC extend long, slender processes – telopodes (Tp) – to embrace the myocardial precursors and presumably form a three-dimensional network throughout the entire heart [5], as already demonstrated in atria [6–8], ventricles [9, 10], myocardial sleeves [11–13], subepicardium [14–16] and subendocardium [17]. Cardiac TC differ from archetypal fibroblasts or fibrocytes. The phenotype of TC is characterized by CD34, c-kit, vimentin and caveolin-1 positivity. The existence of similar cells (c-kit+) in the heart was reported [18–21].
TC might play a role in myocardial development by guiding cardiac muscle precursor cells to form the correct three-dimensional myocardial architecture [22]. In vitro studies of mixed cell cultures showed that TC organize into networks that provide the force to guide the assembly of cardiomyocytes into larger clusters [23, 24].
We present a series of chronological snapshots extracted from a time-lapse videomicroscopy recording of a mouse myocardium cell culture highly enriched in TC.
Adult hearts were obtained from three C57 black mice (12 months old), treated with 1000 U/kg heparin.
This study was approved by the Bioethics Committee of the ‘Victor Babes’ National Institute of Pathology, Bucharest, according to generally accepted international standards.
In order to obtain cell cultures highly enriched in interstitial cells, mice hearts were dissected under a stereomicroscope and mechanically minced into small pieces of about 1 mm3, followed by enzymatic dissociation. First, tissue fragments were incubated for 15 min. on a rocking table in 250 U/ml collagenase II (Sigma, St. Louis, MO, USA) at 37°C, and then the supernatant containing dissociated cells was collected and collagenase activity was inhibited with ice-cold HBSS. The procedure was repeated twice; in the mean time, the collected supernatant was stored in ice. The suspension containing dissociative cells was washed by centrifugation and the cells were again suspended in DMEM culture medium supplemented with 10% foetal calf serum and 100 U/ml penicillin – 100 mg/ml streptomycin (both from Sigma). The resulting single cell suspension was plated in 35 cm2 Petri dishes. The medium was changed after 24 hrs and the culture was transferred in the controlled environment of Nikon Biostation IM (Nikon Instruments Europe B.V., Amstelveen, Netherlands). TC behaviour was monitored by time-lapse videomicroscopy. Images were captured every 10 min. for 48 hrs. The video signal was digitally processed and the video was produced at 800 × 600 pixels in size. The video runs at three frames per second – 96 sec. of running video time corresponds to about 48 hrs of elapsed time. Methylene blue vital staining and immunofluorescence were used for differential diagnosis with fibroblasts/fibrocytes.
Figure 1 shows that some cells in the 48-hr-old culture, which correspond to the TC morphological profile, extend very long and thin Tp that reach out and temporarily interconnect with similar cells in a primary network-like layout. Cell culture confirms repetitive Tp structure shown in electron microscopy (EM) studies [1], of alternating thin segments (podomeres) and dilations (podoms). The mobility, as well as the plasticity of Tp, is based on this moniliform ‘design’.
Fig 1.
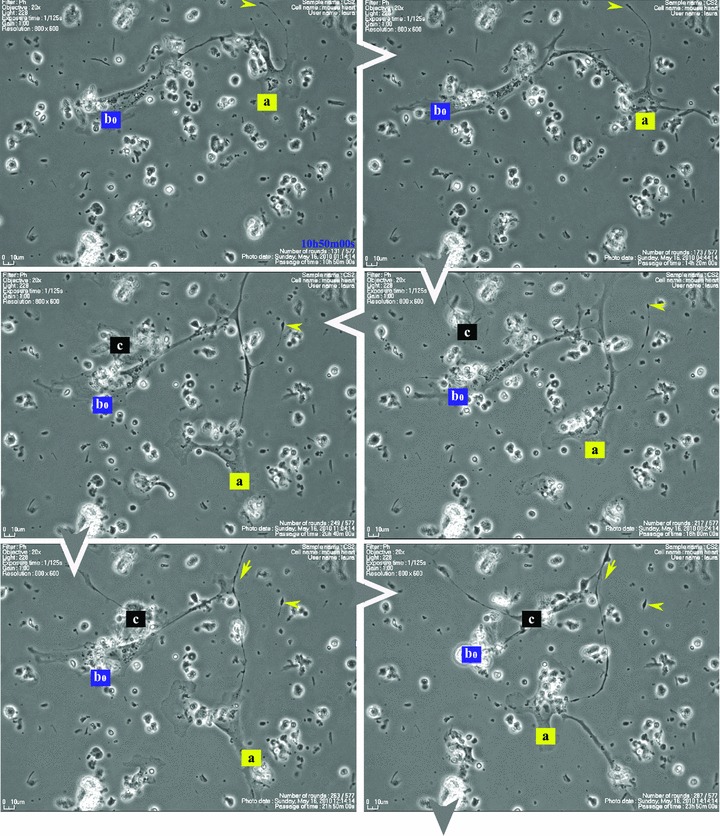
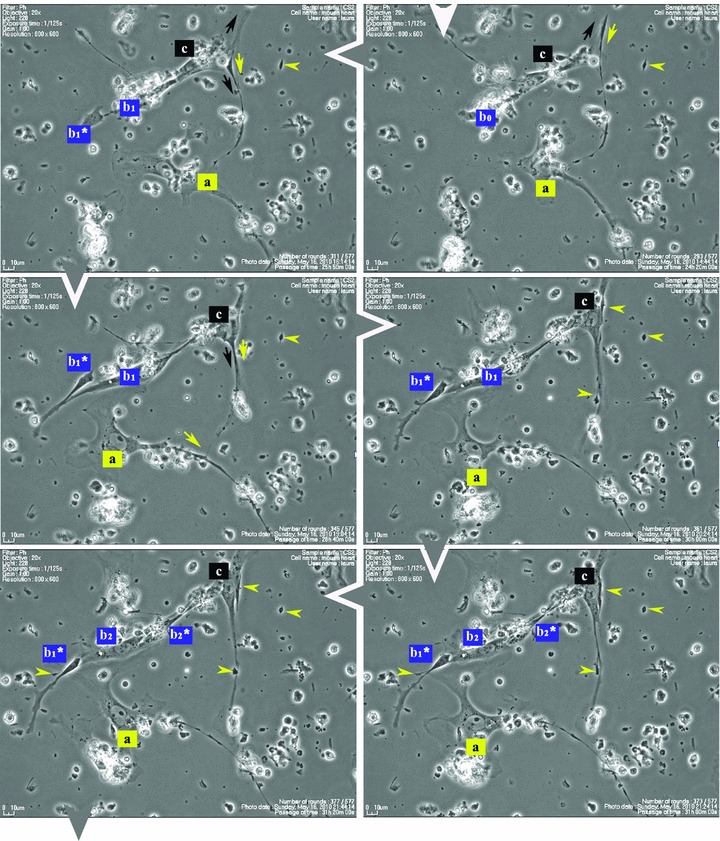
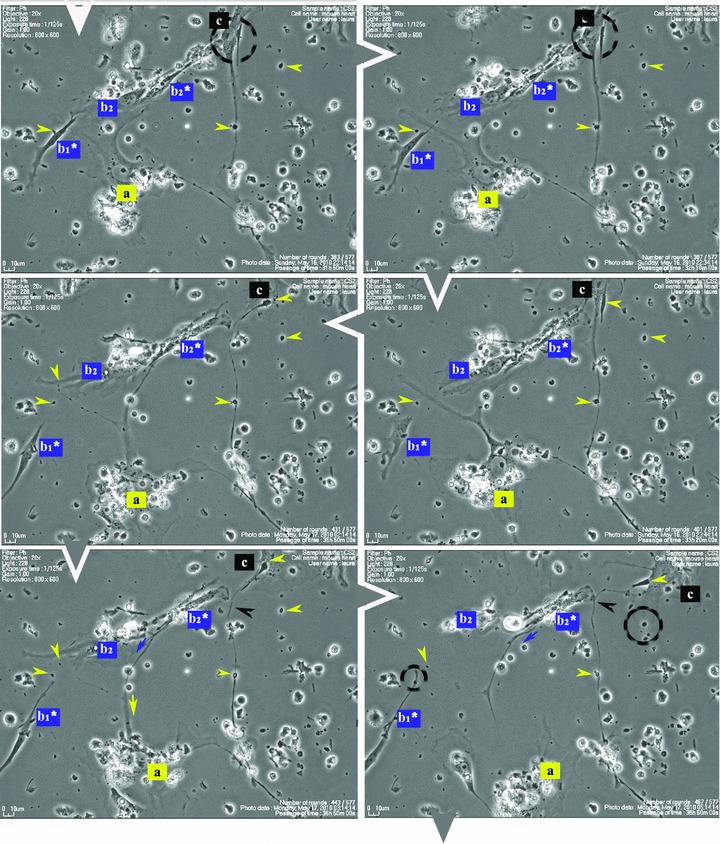
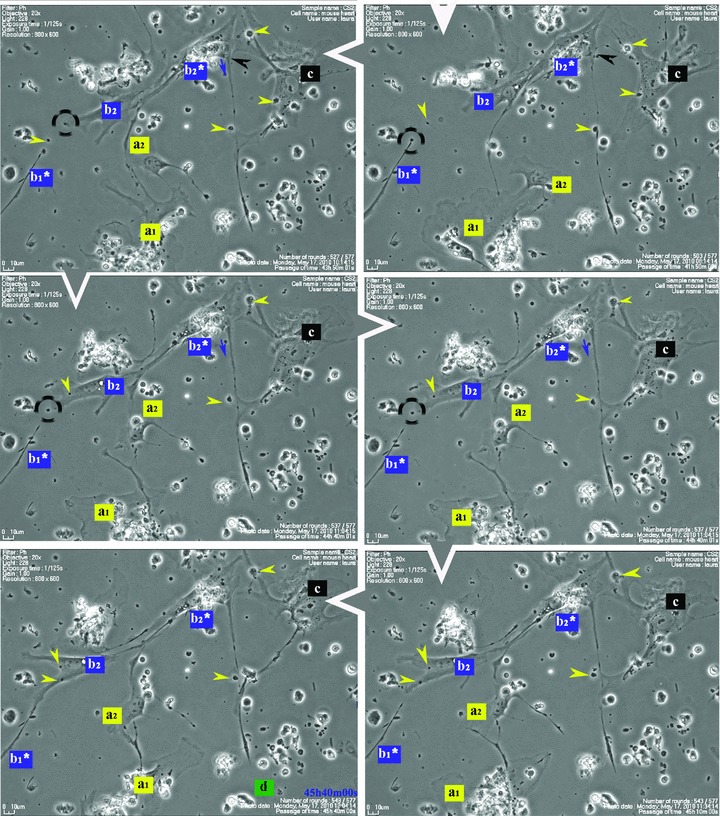
Serial snapshots selected from a time-lapse videomicroscopy recording of adult mouse myocardial interstitial cell culture. The chronological order of sequences is indicated by white arrowheads at the edge of each frame. TC were designated a, b, c and d in order of appearance in the microscopic field. They start structuring a network. The a TC (yellow) is the most active cell, scouting the area and planting marks (yellow arrowheads) out of its own regressing Tp (yellow arrows), for the other cells to follow. TC b2* and c are using those retracting Tp as guiding wires to grow their own cell projections (black and blue arrows). Following marks left by TC a (enclosed areas and yellow arrowheads), TC c leaves an entire Tp (black arrowhead) as a guiding mark for b2* and upcoming d cell. Note: a and b cells undergo cell division; daughter cells were assigned numeric indices.
Figure 1 (and Video S1) suggest that Tp are functioning as guiding wires for nascent Tp of neighbouring cells. The video also indicates that TC, as they scout the perimeter, dispose of some distal segments of their Tp (yellow arrowheads in Fig. 1), which remain stabilized in specific areas during the dynamic process of Tp growth and regression. The segments left behind, like snow traces, continue to be used (enclosed areas in Fig. 1) as guiding marks during the migration of adjoining TC.
This method of cellular-driven guidance represents a particular type of long-distance intercellular communication during the dynamic process of network rearrangement. Further studies are needed in order to accurately identify the molecular mechanisms of this podomere-dependent cell migration.
We considered [3, 15, 22] that TC assist the migration and differentiation of cardiac myocyte progenitors resulting from either resident or exogenous stem cells in cardiac stem cells niches. Taking into account that the TC in the primary culture came from adult mouse hearts, we may take their behaviour as proof that the mature heart contains a population of interstitial cells – TC – which retain the ability to form a three-dimensional scaffold for the heart. In our opinion, TC actions are essential for managing the heart’s significant ‘growth reserve’[25] and coordinating the replacement of myocyte, as well as non-myocyte compartments.
Acknowledgments
We thank Dr. M. Leabu, T. M. Regalia and C. Niculite for their constant help.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Video S1. Time-lapse videomicroscopy recording of 48 hrs of TC behaviour in cell culture. This video runs at three frames per second: 96 sec. of running video time corresponds to about 48 hrs of elapsed time. Distal podomeres detach from Tp of TC, and their presence guides the elongation and migration of Tp belonging to other TC, as preliminary stages in the formation of a complex multi-cellular network.
For supplementary materials on TC, please see http://www.telocytes.com.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Popescu LM, Faussone-Pellegrini MS. Telocytes – a case of serendipity: the winding way from interstitial cells of Cajal (ICC), via interstitial Cajal-like cells (ICLC) to Telocytes. J Cell Mol Med. 14:729–40. doi: 10.1111/j.1582-4934.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suciu L, Popescu LM, Gherghiceanu M, et al. Telocytes in human term placenta: morphology and phenotype. Cells Tissues Organs. 2010;192:325–39. doi: 10.1159/000319467. [DOI] [PubMed] [Google Scholar]
- 3.Popescu LM, Gherghiceanu M, Kostin S. Telocytes and heart renewing. In: Wang P, Kuo C-H, Takeda N, Singal PK, et al., editors. Adaptation biology and medicine. New Delhi, India: Narosa Publishing House Pvt. Ltd; 2010. pp. 17–39. [Google Scholar]
- 4.Kostin S. Myocardial telocytes: a specific new cellular entity. J Cell Mol Med. 2010;14:1917–21. doi: 10.1111/j.1582-4934.2010.01111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faussone-Pellegrini MS, Bani D. Relationships between telocytes and cardiomyocytes during pre- and post-natal life. J Cell Mol Med. 2010;14:1061–3. doi: 10.1111/j.1582-4934.2010.01074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kostin S, Popescu LM. A distinct type of cell in myocardium: interstitial Cajal-like cells (ICLCs) J Cell Mol Med. 2009;13:295–308. doi: 10.1111/j.1582-4934.2008.00668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hinescu ME, Gherghiceanu M, Mandache E, et al. Interstitial Cajal-like cells (ICLC) in atrial myocardium: ultrastructural and immunohistochemical characterization. J Cell Mol Med. 2006;10:243–57. doi: 10.1111/j.1582-4934.2006.tb00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hinescu ME, Popescu LM. Interstitial Cajal-like cells (ICLC) in human atrial myocardium. J Cell Mol Med. 2005;9:972–5. doi: 10.1111/j.1582-4934.2005.tb00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Popescu LM, Gherghiceanu M, Hinescu ME, et al. Insights into the interstitium of ventricular myocardium: interstitial Cajal-like cells (ICLC) J Cell Mol Med. 2006;10:429–58. doi: 10.1111/j.1582-4934.2006.tb00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandache E, Popescu LM, Gherghiceanu M. Myocardial interstitial Cajal-like cells (ICLC) and their nanostructural relationships with intercalated discs: shed vesicles as intermediates. J Cell Mol Med. 2007;11:1175–84. doi: 10.1111/j.1582-4934.2007.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morel E, Meyronet D, Thivolet-Bejuy F, et al. Identification and distribution of interstitial Cajal cells in human pulmonary veins. Heart Rhythm. 2008;5:1063–7. doi: 10.1016/j.hrthm.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 12.Gherghiceanu M, Hinescu ME, Andrei F, et al. Interstitial Cajal-like cells (ICLC) in myocardial sleeves of human pulmonary veins. J Cell Mol Med. 2008;12:1777–81. doi: 10.1111/j.1582-4934.2008.00444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen BL, Fishbein MC, Chen LS, et al. Histopathological substrate for chronic atrial fibrillation in humans. Heart Rhythm. 2009;6:454–60. doi: 10.1016/j.hrthm.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suciu L, Popescu LM, Regalia T, et al. Epicardium: interstitial Cajal-like cells (ICLC) highlighted by immunofluorescence. J Cell Mol Med. 2009;13:771–7. doi: 10.1111/j.1582-4934.2009.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popescu LM, Gherghiceanu M, Manole CG, et al. Cardiac renewing: interstitial Cajal-like cells nurse cardiomyocyte progenitors in epicardial stem cell niches. J Cell Mol Med. 2009;13:866–86. doi: 10.1111/j.1582-4934.2009.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popescu LM, Manole CG, Gherghiceanu M, et al. Telocytes in human epicardium. J Cell Mol Med. 2010;14:2085–93. doi: 10.1111/j.1582-4934.2010.01129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gherghiceanu M, Manole CG, Popescu LM. Telocytes in endocardium: electron microscope evidence. J Cell Mol Med. 2010;14:2330–4. doi: 10.1111/j.1582-4934.2010.01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tallini YN, Greene KS, Craven M, et al. c-kit expression identifies cardiovascular precursors in the neonatal heart. Proc Natl Acad Sci USA. 2009;106:1808–13. doi: 10.1073/pnas.0808920106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castaldo C, Di Meglio F, Nurzynska D, et al. CD117-positive cells in adult human heart are localized in the subepicardium, and their activation is associated with laminin-1 and alpha6 integrin expression. Stem Cells. 2008;26:1723–31. doi: 10.1634/stemcells.2007-0732. [DOI] [PubMed] [Google Scholar]
- 20.Limana F, Zacheo A, Mocini D, et al. Identification of myocardial and vascular precursor cells in human and mouse epicardium. Circ Res. 2007;101:1255–65. doi: 10.1161/CIRCRESAHA.107.150755. [DOI] [PubMed] [Google Scholar]
- 21.Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. Proc Natl Acad Sci USA. 2007;104:14068–73. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gherghiceanu M, Popescu LM. Cardiomyocyte precursors and telocytes in epicardial stem cell niche: electron microscope images. J Cell Mol Med. 2010;14:871–7. doi: 10.1111/j.1582-4934.2010.01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bani D, Formigli L, Gherghiceanu M, et al. Telocytes as supporting cells for myocardial tissue organization in developing and adult heart. J Cell Mol Med. 2010;14:2531–8. doi: 10.1111/j.1582-4934.2010.01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou J, Zhang Y, Wen X, et al. Telocytes accompaning cardiomyocyte in primary culture: two and three dimensional culture environment. J Cell Mol Med. 2010 doi: 10.1111/j.1582-4934.2010.01186.x. Doi: 10.1111/j.1582-4934.2010.01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kajstura J, Urbanek K, Perl S, et al. Cardiomyogenesis in the adult human heart. Circ Res. 2010;107:305–15. doi: 10.1161/CIRCRESAHA.110.223024. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


