Abstract
The use of stem cells has opened new prospects for the treatment of orthopaedic conditions characterized by large bone defects. However, many issues still exist to which answers are needed before routine, large-scale application becomes possible. Bone marrow stromal cells (MSC), which are clonogenic, multipotential precursors present in the bone marrow stroma, are generally employed for bone regeneration. Stem cells with multilineage differentiation similar to MSC have also been demonstrated in adipose tissue, peripheral blood, umbilical cord and amniotic fluid. Each source presents its own advantages and drawbacks. Unfortunately, no unique surface antigen is expressed by MSC, and this hampers simple MSC enrichment from heterogeneous populations. MSC are identified through a combination of physical, morphological and functional assays. Different in vitro and in vivo models have been described for the research on bone stem cells. These models should predict the in vivo bone healing capacity of MSC and if the induced osteogenesis is similar to the physiological one. Although stem cells offer an exciting possibility of a renewable source of cells and tissues for replacement, orthopaedic applications often represent case reports whereas controlled randomized trials are still lacking. Further biological aspects of bone stem cells should be elucidated and a general consensus on the best models, protocols and proper use of scaffolds and growth factors should be achieved.
Keywords: stem cell, bone, mesenchymal stromal cell, adipose stem cell, embryonic stem cell, tissue engineering, osteoblast, osteogenic differentiation
Introduction
The use of bone stem cells has opened new prospects for the treatment of orthopaedic conditions characterized by large bone defects. An increasing number of in vitro and in vivo studies, and a few clinical investigations, have reported on the use of stem cells for bone regeneration so far. However, many questions of concern regarding bone stem cell usage exist to which answers are needed and before translating the basic biological knowledge and applying it into clinical practice, it is imperative that these issues are addressed. The Workshop ‘Bone Stem Cells’, organized in Bertinoro (Italy) on 7–10 October 2009, aimed to answer some of these questions. Particular issues addressed were the most suitable source of stem cell for bone regeneration, specific markers for the identification of adult bone stem cells, as well as the most suitable in vitro and in vivo models in order to predict clinical behaviour.
Sources of stem cells for bone regeneration
A stem cell is a cell from the embryo, foetus or adult that is capable of asymmetric cellular divisions to parent and daughter cells and thus has the capacity of self-renewal and multipotent differentiation into specialized cells of the body. The most widely used sources of bone stem cells along with their advantages and drawbacks can be found in Table 1.
Table 1.
Advantages and disadvantages of the different sources of mesenchymal stem cells (MSC)
| Source | Advantages | Disadvantages |
|---|---|---|
| Embryonic stem cell | Infinite lifespan, totipotency unlimited supply | Ethical concern, teratomas, requirement of animal-derived components during culture |
| Induced pluripotent stem cells | Infinite lifespan, totipotency, unlimited supply, absence of ethical concern | Extremely specialized technique |
| Bone marrow mesenchymal stromal cell | Absence of ethical concern | Slow proliferation rate, limited life span, high variability in osteogenic potential, progressive loss of differentiative ability, immunogenicity |
| Adipose tissue-derived stem cells | Absence of ethical concern, availability through procedure acceptable by patients | Immunogenicity, not yet fully understood differences with MSC |
| Bone stem cells from peripheral blood | Absence of ethical concern, availability through procedure acceptable by patients | Low number, immunogenicity, not yet fully understood differences with MSC |
| Stem cells from the umbilical cord | Absence of ethical concern | Immunogenicity? Not yet fully understood differences with MSC |
| Stem cells from amniotic fluid | Absence of ethical concern, high proliferative capacity, high differentiation potential | Immunogenicity? Not yet fully understood differences with MSC |
| Foetal osteoblasts | Easy availability, fast growth rate, differentiation into mature osteoblasts, low immunogenicity, banking facility |
Immunogenicity refers to homologous use.
Embryonic stem cells (ESC) derive from the inner cells of the blastocyst and are characterized by high telomerase expression, normal and stable karyotype and the ability to form cells belonging to any of the three germ layers [1–3]. The ethical issue that impedes the application of hESC could be resolved through pluripotent (hESC-like) cells that do not require fertilization for their generation, but can be isolated from embryos developed directly from oocytes via parthenogenesis [4] or perhaps via somatic cell nuclear transfer. Recently, human-induced pluripotent stem cells were generated by the ectopic expression of ESC-specific transcription factors in somatic cells [5, 6].
Mesenchymal stem cells (MSC) derived from bone marrow are responsible for the maintenance of osteoblasts, osteocytes and bone lining cells throughout life and can be considered characteristic adult stem cells, and indeed cells with similar properties reside in virtually all postnatal organs and tissues. MSC have been isolated from bone marrow of iliac crest, femur [7], periosteum [8] and synovium [9]. Late plastic adherent MSC have recently shown to differentiate into osteoblasts [10].
Stem cells capable of multilineage differentiation similar to MSC were demonstrated in adipose tissue (ADSC) [11]. ADSC differentiated into functional osteoblasts when cultured on titanium [12], hydroxyapatite, cancellous human bone fragments or deproteinized bovine bone granules [13, 14].
In addition to the endothelial progenitor cells, circulating osteoblast-lineage cells were also isolated from human peripheral blood [15] and these cells formed ectopic bone when implanted in tissues in vivo[16].
MSC with osteogenic potential are present in the umbilical blood [17], umbilical cord perivascular cells [18] and Wharton’s jelly [19].
A variety of different stem cell populations were described in human amniotic fluid (AFSC) [20], which, in turn, formed embryoid bodies [21], showed high proliferative capacity and differentiate along the adipogenic, osteogenic, myogenic, endothelial, neurogenic and hepatic pathways [22]. Human AFSC seeded onto scaffolds and cultured in osteogenic medium supported the formation of highly mineralized tissue upon implantation in immunodeficient mice, without teratoma formation [23].
Human foetal bone cells may be an alternative to MSC. Foetal osteoblasts have a doubling time comparable to MSC but start calcium deposition earlier [24, 25]. When seeded on porous scaffolds, they promoted bone repair in rats with critical size defects [26].
Stem cells, similar to bone marrow-derived MSC, were demonstrated in dental pulp [27]. Five different types of human dental stem/progenitor cells were isolated and characterized: dental pulp stem cells (DPSCs) (Fig. 1), stem cells from exfoliated deciduous teeth (SHED), periodontal ligament stem cells (PDLSCs), stem cells from apical papilla (SCAP) and dental follicle progenitor cells (DFPCs) [28]. Adult dental pulp contains precursors capable of forming odontoblasts [29]. Dental pulp progenitors have not been clearly identified but some data suggest that pericytes, which are able to differentiate into osteoblasts, could also differentiate into odontoblasts [30–32]. Recent findings demonstrated the isolation of mesenchymal progenitors from the pulp of human deciduous incisor. These cells were named SHED and exhibited a high plasticity because they could differentiate into neurons, adipocytes, osteoblasts and odontoblasts [33]. The periodontal ligament is a specialized tissue that contains STRO-1 positive cells and progenitors which can be activated to self-renew and regenerate other tissues such as cementum and alveolar bone [34]. Stem cells from the apical part of the human dental papilla exhibit a higher rate of proliferation and appear more effective than PDLSC for tooth formation [35]. Stem cells from the dental follicle express the stem cells markers Notch1, STRO-1 and Nestin [36]. Dental stem cells can differentiate into odontoblasts, adipocytes, neuronal-like cells, glial cells, osteoblasts, chondrocytes, melanocytes, myotubes and endothelial cells [37].
Fig 1.
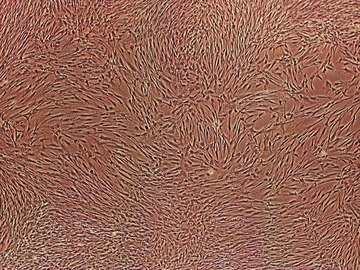
Cultured dental pulp stem cells (DPSCs) show a mesenchymal stromal cell (MSC)-like spindle shape morphology.
Markers for the identification of adult bone stem cells
Effective methods of cell sorting and cell enrichment are useful for isolating homogeneous cell preparations [38, 39]. However, the enrichment of MSC from a heterogeneous population is hampered by the lack of specific and unique markers. There is a general consensus that human MSC do not express the hematopoietic markers CD45, CD34, CD14 or CD11, the adhesion molecules CD31, CD18 (leucocyte function-associated antigen-1) or CD56 (neuronal cell adhesion molecule-1), rather, they express a set of non-specific markers: CD105 (SH2), CD73 (SH3/4), CD44, CD90 (Thy-1), CD71, STRO-1, CD106, CD166, ICAM-1, SB-10, CD29 [40] and CD146 [41]. None of these markers, either alone or in combination, are specific enough to achieve a high enrichment level from a heterogeneous population. STRO-1, which was extensively evaluated for the identification of bone stem cells, is not strictly specific because it is expressed in erythroid precursors [42].
MSC were found to express ESC transcription factors, such as OCT4, NANOG and SOX2 [43], which are associated with maintenance of multipotency. The stage-specific embryonic antigen-4 (SSEA-4), previously thought to be exclusively present on ESC, erythrocytes and some neural cells, were found in adult mesenchymal stem cell and can also be used to prospectively isolate MSC [44].
MSC have unique characteristics that prevent them from participation in prominent alloreactivity induction. MSC lack major histocompatibility complex MHC-II, show only minimal MHC-I expression and do not express the costimulatory molecules CD40, CD80 or CD86 [45].
Surface marker expressions may be influenced by species differences [46], tissue source [47, 48], isolation and culture methods [49]. In vitro expression of some markers by MSC did not always correlate with their expression patterns in vivo[50] and was influenced by the microenvironment [51].
Molecular aspects of bone stem cells
The specific differentiation pathways of MSC are guided in a complex multigenic process [52] under the control of key regulatory factors and transcriptional factors, such as runt-related transcription factor 2 (Runx2) and osterix [53].
Through microarray analysis of MSC differentiation into osteogenic lineage different gene clusters were associated with distinct stages, i.e. proliferation, matrix maturation and mineralization. The zinc finger and BTB domain containing 16 protein (ZNF145) has been identified as an upstream regulator of Runx2, with a crucial role in the initiation of osteoblastic differentiation [54]. The homeobox gene MSX2, which is implicated in osteoprogenitor cell function, was up-regulated during the whole period of differentiation. Alkaline phosphatase (ALP), collagen Type X alpha 1, bone morphogenetic protein 1, insulin-like growth factor 2, bone sialoprotein, periostin, C-type lectin domain family 3, osteoprotegerin and osteonectin were up-regulated during the intermediate and late phases of the osteogenic differentiation. Osteocalcin, which appeared only during matrix formation and mineralization phase, was a late marker [55], similarly to ID4, a member of the ID transcription factor family involved in cell cycle control [56], CRYAB (crystallin-αB), a small heat shock protein, [57] and SORT1 (sortillin 1), a multiligand type I receptor, that has been shown to promote extracellular matrix maturation [58].
Other factors implicated in the regulation of osteogenic differentiation of MSC include ATF4, SATB2, transforming growth factor-β (TGF-β), Hedgehog, fibroblast growth factor (FGF), ephrin and mitogen-activated protein kinases [59]. Osteocalcin, dermatopontin (DPT) and histamine receptor (HR1) are known downstream target for the vitamin D receptor (VDR) transcription complex, one of the drivers of osteoblast differentiation process [60]. Genes related to angiogenesis, through an angiopoietin and a vascular endothelial growth factor (VEGF)-dependent pathway, and to nervous system development, such as brain-derived neurotrophic factor, are up-regulated in MSC. A role of MSC in supporting vascularization and development of neuronal-like structures has been hypothesized [55, 57].
In parallel to gene expression analysis as a tool for understanding the molecular mechanisms underlying cell commitment, the study of post-transcriptional regulation has recently intensified. microRNAs (miRNAs) are small non-coding RNAs which bind the 3’UTR of mRNAs bearing fully or partially complementary sequences, therefore leading to either transcript degradation or translation inhibition [61]. Several authors have identified critical roles for miRNAs in bone cell differentiation and activity. Li et al. showed that BMP2-induced osteogenic differentiation of C2C12 cells is mediated both by the expression of miRNAs which inhibit muscle genes and by the down-regulation of 22 miRNAs which target osteoblast-specific genes constituting program of osteogenesis inhibition. In particular, miR-133 directly interacts with the master transcription factor Runx2 whereas miR-135 targets Smad5, a key mediator in osteogenic signal transduction [62]. A recent study revealed that bone marrow-derived MSC express miR-204/211 and that the level of these small non-coding RNAs increased when adipogenesis was induced; miR-204 is able to attenuate Runx2, thereby preventing osteogenesis, whereas the use of anti-miR-204 oligo increases ALP activity [63]. Micro-R-29b has been found to be up-regulated during the differentiation of MC3T3 mouse pre-osteoblastic cells; its expression inhibits a number of negative regulators of osteoblast differentiation which affect Smad, ERK, p38 MAPK and Wnt signalling pathways. Furthermore, miR-29b directly targets several collagen genes (COL1A1, COL5A3 and COL4A2), preventing fibrosis and facilitating extracellular matrix mineralization at late stages of differentiation [64]. Micro-R-29a and -29c interact with osteonectin mRNA and their increased expression during osteoblast differentiation in vitro correlates with the reduction of osteonectin protein during the mineralization process [65]. By employing a library of miRNA inhibitors, Schoolmeesters et al. identified miR-148b, miR-26a and miR-489 as key regulators of MSC osteogenic differentiation [66]. In a recent study, in vitro and in vivo evidence ascribed a critical role to miR-2861 in osteoblast differentiation. miR-2861 is able to induce osteoblast differentiation by targeting histone deacetylase 5 (HDAC5), which deacetylates Runx2, therefore enhancing its degradation. Moreover, the authors found that a homozygous mutation in the miR-2861 precursor which blocks the expression of the mature microRNA, contributed to the development of primary osteoporosis in two related adolescents [67].
Signaling pathways of osteoblast differentiation
β-Catenin, a central component of the cadherin cell adhesion complex, has an essential role in the Wingless/Wnt signaling pathway for osteoblast differentiation [68]. Wnt pathway is initiated through the binding of a Wnt ligand to the seven-transmembrane domain-spanning Frizzled receptor and the low-density lipoprotein receptor-related protein 5 and 6 (LRP5/6) co-receptors. As a consequence, cytosolic b-catenin escapes phosphorylation by glycogen synthase kinase (GSK)-3 β, which phosphorylation would be followed by degradation in the ubiquitin/proteasome pathway. As a result of Wnt pathway activation the now stabilized b-catenin translocates to the nucleus, binds to T cell factor/lymphoid enhancer binding factor (TCF/LEF) transcription factors, and regulates downstream gene expression. In human beings, the mutations in the Wnt co-receptor LRP5 lead to decreased or increased canonical Wnt signaling, which result in osteoporosis or a high bone mass phenotype, respectively. LRP5 mutations and the level of Wnt signalling were shown to determine the differentiation of hMSC into osteoblasts or adipocytes. Particularly, activating mutation T253I of LRP5 enhanced osteogenesis and inhibited adipogenesis, whereas inactivating mutation T244M of LRP5 exerted opposite effects [69].
Control of bone remodelling by the nervous system
Bone remodelling is also under the influence of central and peripheral neural control [70]. Neuropeptide Y (NPY) and its receptors Y1 and Y2 were demonstrated in bone tissue. Osteoblasts, osteocytes and chondrocytes do not only synthesize NPY, but also peptidylglycine α-amidating monooxygenase (PAM), the enzyme responsible for NPY activation. NPY increased Y2 receptor mRNA in the mouse pre-osteoblast cell line MC3T3-E1, and decreased Y1 receptor mRNA of MSC. NPY significantly enhanced ALP activity and osteocalcin, but decreased calcium deposition [71]. In transthyretin (TTR) knockout mouse which overexpresses NPY as a result of PAM overexpression, increased bone mineral density and trabecular volume were observed. Simultaneously, significant increase of PAM expression in MSC occurred during osteogenic differentiation. Osteoblasts from TTR knockout mice showed higher ALP activity and higher osteopontin and osteocalcin mRNA expression, suggesting that increased neuropeptide Y content was related to increased bone mass [72].
In vivo models
One of the crucial aspects of bone engineering is the prediction of the bone healing capacity of the produced constructs. Although in vitro formation of mineral-like foci is generally considered indicative of an osteoprogenitor phenotype, a strict correlation between in vitro calcium deposition and in vivo mineralization was not consistently demonstrated [73]. To assess this correlation, ESC and ADSC were seeded on CultiSpher S microcarriers and then implanted into ectopic sites. Only heterogeneous bone formation, limited to specific areas, was obtained [74].
Moreover, in vivo models should evaluate if the osteogenesis induced by tissue engineering is similar to the physiological bone formation. A bone engineering approach should probably mimic the endochondral ossification, which is the physiological process for the repair of trabecular bone defects below the articular cartilage layer in long bones. An experimental model of this approach involved in vitro chondrocyte differentiation of mouse ESC seeded on a ceramic scaffold. After differentiation, the samples were implanted subcutaneously into immunodeficient mice. The implanted cartilage tissue matured, became hypertrophic and was ultimately replaced by bone tissue. This result demonstrated that a cartilage matrix was required for bone formation by ESC [75].
A similar approach taking use of chondrogenic pre-induction before implantation was also successful for MSC. A 6-week chondrogenic pre-induction of MSC seeded on β-TCP granules, before subcutaneous implantation into immunocompromised mice, resulted in endochondral ossification yielding ectopic bone. On the contrary, unprimed MSC underwent intramembranous bone formation. The origin of new bone from chondrogenic primed MSC most likely results of transdifferentiation of chondrocytes to osteoblasts, or of direct osteogenesis of cartilage-resident MSC [76].
Orthopaedic applications of bone stem cell technology
The orthopaedic application of bone stem cells may be of use when bone repair is impeded because of acquired or congenital [77] pseudoarthrosis, non-unions, large bone resections, significant traumas, local infection, previous irradiation or compromised vascularity.
In orthopaedics bone stem cells could be used in bone tissue engineering. There are five major types of bone engineering: (1) local stimulation of osteoprogenitor cells, (2) homing of osteoprogenitor cells, (3) transplantation of autologous osteoprogenitor cells to augment the local population, (4) transplantation of osteoprogenitor cells after culture expansion and (5) transplantation of fully formed tissue [78].
Local stimulation of proliferation and differentiation of resident osteoprogenitors requires a sufficient number of functionally active cells. Examples of local stimulation include the delivery of autologous or recombinant growth factors, biophysical stimulation, systemic pharmacological treatments and implantation of acellular scaffolds, where osteoprogenitor cells can adhere, proliferate and differentiate. The stimulation of local osteoprogenitors has the advantage that the regenerated tissue will have the same biological properties as the lost tissue [79]. However, local stimulation will most likely fail if subjected to debilitating healing conditions, such as infections, diabetes or necrosis which perhaps caused the bone defect in the first place.
Homing of osteoprogenitors refers to the recruitment of cells from the systemic circulation. In fact, it was recently demonstrated that osteogenic progenitor cells may travel through the systemic circulation [80]. According to this hypothesis, stem cell homing is a physiological process that may become the focus for new therapies to enhance the recruitment of osteo progenitors at bone repair sites.
Transplantation of autologous osteoprogenitors to augment the local population would be useful in clinical situations when complete healing is impeded by a local deficiency of stem cells. Transplantation of autologous bone marrow cells, after concentration by centrifugation, is an example of cell transplantation.
Transplantation of osteoprogenitor cells after culture expansion is similar to the technique already applied clinically for cartilage repair [81]. In vitro expansion generates a large number of osteoprogenitors, however, some variables can affect the clinical outcome, such as cell source, culture conditions and implantation procedure [82].
From the first report in 2001 concerning the implantation of MSC seeded on bioceramic scaffold in three patients with large bone defects [83], a number of clinical studies addressing engineered grafts in bone repair have been described, however, only few were prospective, randomized and controlled. A prospective, randomized, controlled study on the healing of high tibial osteotomy showed that MSC mixed with platelet-rich plasma and lyophilized bone chips had a higher osteogenic potential than lyophilized bone chips alone or in combination with platelet-rich plasma [84].
The use of MSC has also been proposed in bone marrow transplantation for the treatment of skeletal genetic diseases, such as osteogenesis imperfecta (OI) whereby transplanted donor cells migrate to the bone and form osteoblasts. OI patients with bone marrow transplantation showed new dense bone formation, an increase in total body bone mineral content, increases in growth velocity and reduced frequency of bone fracture [85]. In utero transplantation of adult MSC is a promising approach, as demonstrated in heterozygous BrtlIV knockin mice with a classical glycine substitution in type I collagen [alpha1(I)-Gly349Cys], dominant trait transmission and characterized by a phenotype similar to moderately severe and lethal OI [86].
Conclusions
Without question, critical aspects of bone engineering, which may hamper a wider application, undoubtedly are the most suitable source of stem cell, specific markers for their identification and clear orthopaedic indications. Although adult bone marrow derived cells have been characterized and are extensively used, they have a limited developmental potential and loose their ability to proliferate and differentiate over time, thus presenting considerable drawbacks. On the other hand, embryonic stem cells have infinite lifespan and are unlimited in supply; however their use is not presently allowed in many countries because of ethical concerns. Stem cells from cord blood or from amniotic fluid are promising alternatives but more comprehensive knowledge of the differences with bone marrow cells needs to be achieved. Adipose tissue derived cells have been better investigated and could represent a useful cell population for bone engineering, even though some differences with bone marrow derived stromal cells should be taken into account. Foetal osteoblasts could have both the advantages of being more easily available than adult bone marrow cells and raising less ethical concerns than embryonic cells, but this approach requires further characterization.
Efforts should be focused on how to guide a stem cell from an uncommitted state to a more differentiated phenotype. No specific marker has been identified for osteogenic differentiation of adult stem cells. Moreover, it is necessary to compare in vitro and in vivo evidence.
Thus far, orthopaedic applications have been reported as case reports and controlled randomised trials are still lacking. Nevertheless, before drawing conclusive results from this clinical research, further biological aspects of bone stem cells should be elucidated and a general consensus on the best models, protocols and proper use of scaffolds and growth factors should be achieved.
Acknowledgments
This review is an initiative of REMEDIC, a Network on Regenerative Medicine of the European Science Foundation. The Workshop ‘Bone Stem Cells’, organized in Bertinoro (Italy) on 7–10 October 2009, was carried out jointly by REMEDIC and by the European Orthopaedic Research Society (EORS). The authors were supported by the Istituto Ortopedico Rizzoli, ‘Ricerca Corrente’ and by the Regione Emilia-Romagna ‘Progetto di Ricerca Regione-Université 2007–2009: Regenerative Medicine in Osteo-articular Diseases’. The authors thank Ms. Lucy Scioscia for her help in editing English.
Conflict of interest
The authors confirm that there are no conflicts of interest.
References
- 1.Hyslop LA, Armstrong L, Stojkovic M, et al. Human embryonic stem cells: biology and clinical implications. Expert Rev Mol Med. 2005;7:1–21. doi: 10.1017/S1462399405009804. [DOI] [PubMed] [Google Scholar]
- 2.Gertow K, Wolbank S, Rozell B, et al. Organized development from human embryonic stem cells after injection into immunodeficient mice. Stem Cells Dev. 2004;13:421–35. doi: 10.1089/scd.2004.13.421. [DOI] [PubMed] [Google Scholar]
- 3.Martin MJ, Muotri A, Gage F, et al. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11:228–32. doi: 10.1038/nm1181. [DOI] [PubMed] [Google Scholar]
- 4.Kim K, Lerou P, Yabuuchi A, et al. Histocompatible embryonic stem cells by parthenogenesis. Science. 2007;315:482–6. doi: 10.1126/science.1133542. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 7.Ciapetti G, Ambrosio L, Marletta G, et al. Human bone marrow stromal cells: in vitro expansion and differentiation for bone engineering. Biomaterials. 2006;27:6150–60. doi: 10.1016/j.biomaterials.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 8.Kawase T, Okuda K, Kogami H, et al. Characterization of human cultured periosteal sheets expressing bone-forming potential: in vitro and in vivo animal studies. J Tissue Eng Regen Med. 2009;3:218–29. doi: 10.1002/term.156. [DOI] [PubMed] [Google Scholar]
- 9.De Bari C, Dell’Accio F, Tylzanowski P, et al. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–42. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 10.Leonardi E, Ciapetti G, Baglío SR, et al. Osteogenic properties of late adherent subpopulations of human bone marrow stromal cells. Histochem Cell Biol. 2009;132:547–57. doi: 10.1007/s00418-009-0633-x. [DOI] [PubMed] [Google Scholar]
- 11.Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 12.Tognarini I, Sorace S, Zonefrati R, et al. In vitro differentiation of human mesenchymal stem cells on Ti6Al4V surfaces. Biomaterials. 2008;29:809–24. doi: 10.1016/j.biomaterials.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 13.Rimondini L, Mele S. Stem cell technologies for tissue regeneration in dentistry. Minerva Stomatol. 2009;58:483–500. [PubMed] [Google Scholar]
- 14.De Girolamo L, Sartori MF, Arrigoni E, et al. Human adipose-derived stem cells as future tools in tissue regeneration: osteogenic differentiation and cell-scaffold interaction. Int J Artif Organs. 2008;31:467–79. doi: 10.1177/039139880803100602. [DOI] [PubMed] [Google Scholar]
- 15.Otsuru S, Tamai K, Yamazaki T, et al. Circulating bone marrow derived osteoblast progenitor cells are recruited to the bone-forming site by the CXCR4/stromal cell-derived factor-1 pathway. Stem Cells. 2008;26:223–34. doi: 10.1634/stemcells.2007-0515. [DOI] [PubMed] [Google Scholar]
- 16.Otsuru S, Tamai K, Yamazaki T, et al. Bone marrow-derived osteoblast progenitor cells in circulating blood contribute to ectopic bone formation in mice. Biochem Biophys Res Commun. 2007;354:453–8. doi: 10.1016/j.bbrc.2006.12.226. [DOI] [PubMed] [Google Scholar]
- 17.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–42. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 18.Sarugaser R, Lickorish D, Baksh D, et al. Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells. 2005;23:220–9. doi: 10.1634/stemcells.2004-0166. [DOI] [PubMed] [Google Scholar]
- 19.Penolazzi L, Vecchiatini R, Bignardi S, et al. Influence of obstetric factors on osteogenic potential of umbilical cord-derived mesenchymal stem cells. Reprod Biol Endocrinol. 2009;7:106. doi: 10.1186/1477-7827-7-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prusa AR, Marton E, Rosner M, et al. Oct4 expressing cells in human amniotic fluid: a new source for stem cell research. Hum Reprod. 2003;18:1489–93. doi: 10.1093/humrep/deg279. [DOI] [PubMed] [Google Scholar]
- 21.Valli A, Rosner M, Fuchs C, et al. Embryoid body formation of human amniotic fluid stem cells depends on mTOR. Oncogene. 2010;29:966–77. doi: 10.1038/onc.2009.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bossolasco P, Montemurro T, Cova L, et al. Molecular and phenotypic characterization of human amniotic fluid cells and their differentiation potential. Cell Res. 2006;16:329–36. doi: 10.1038/sj.cr.7310043. [DOI] [PubMed] [Google Scholar]
- 23.De Coppi P, Bartsch G, Jr, Siddiqui MM, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100–6. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 24.Montjovent MO, Burri N, Mark S, et al. Fetal bone cells for tissue engineering. Bone. 2004;35:1323–33. doi: 10.1016/j.bone.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Montjovent MO, Bocelli-Tyndall C, Scaletta C, et al. In vitro characterization of immune-related properties of human fetal bone cells for potential tissue engineering applications. Tissue Eng Part A. 2009;15:1523–32. doi: 10.1089/ten.tea.2008.0222. [DOI] [PubMed] [Google Scholar]
- 26.Montjovent MO, Mark S, Mathieu L, et al. Human fetal bone cells associated with ceramic reinforced PLA scaffolds for tissue engineering. Bone. 2008;42:554–64. doi: 10.1016/j.bone.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 27.D’Aquino R, Graziano A, Sampaolesi M, et al. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007;14:1162–71. doi: 10.1038/sj.cdd.4402121. [DOI] [PubMed] [Google Scholar]
- 28.Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.About I, Mitsiadis TA. Molecular aspects of tooth pathogenesis and repair: in vivo and in vitro models. Adv Dent Res. 2001;15:59–62. doi: 10.1177/08959374010150011501. [DOI] [PubMed] [Google Scholar]
- 30.Alliot-Licht B, Bluteau G, Magne D, et al. Dexamethasone stimulates differentiation of odontoblast-like cells in human dental pulp cultures. Cell Tissue Res. 2005;321:391–400. doi: 10.1007/s00441-005-1115-7. [DOI] [PubMed] [Google Scholar]
- 31.Lovschall H, Mitsiadis TA, Poulsen K, et al. Coexpression of Notch3 and Rgs5 in the pericyte-vascular smooth muscle cell axis in response to pulp injury. Int J Dev Biol. 2007;51:715–21. doi: 10.1387/ijdb.072393hl. [DOI] [PubMed] [Google Scholar]
- 32.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 33.Miura M, Gronthos S, Zhao M, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807–12. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seo BM, Miura M, Gronthos S, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–55. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 35.Bluteau G, Luder HU, De Bari C, et al. Stem cells for tooth engineering. Eur Cell Mater. 2008;16:1–9. doi: 10.22203/ecm.v016a01. [DOI] [PubMed] [Google Scholar]
- 36.Morsczeck C, Moehl C, Götz W, et al. In vitro differentiation of human dental follicle cells with dexamethasone and insulin. Cell Biol Int. 2005;29:567–75. doi: 10.1016/j.cellbi.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 37.Petrovic V, Stefanovic V. Dental tissue–new source for stem cells. Scientific World J. 2009;9:1167–77. doi: 10.1100/tsw.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu H, Toh WS, Lu K, et al. A subpopulation of mesenchymal stromal cells with high osteogenic potential. J Cell Mol Med. 2009;13:2436–47. doi: 10.1111/j.1582-4934.2009.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roda B, Lanzoni G, Alviano F, et al. A novel stem cell tag-less sorting method. Stem Cell Rev. 2009;5:420–7. doi: 10.1007/s12015-009-9088-7. [DOI] [PubMed] [Google Scholar]
- 40.Chamberlain G, Fox J, Ashton B, et al. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–49. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 41.Sorrentino A, Ferracin M, Castelli G, et al. Isolation and characterization of CD1461 multipotent mesenchymal stromal cells. Exp Hematol. 2008;36:1035–46. doi: 10.1016/j.exphem.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- 43.Greco SJ, Liu K, Rameshwar P. Functional similarities among genes regulated by OCT4 in human mesenchymal and embryonic stem cells. Stem Cells. 2007;25:3143–54. doi: 10.1634/stemcells.2007-0351. [DOI] [PubMed] [Google Scholar]
- 44.Gang EJ, Bosnakovski D, Figueiredo CA, et al. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109:1743–5. doi: 10.1182/blood-2005-11-010504. [DOI] [PubMed] [Google Scholar]
- 45.Tse WT, Pendleton JD, Beyer WM, et al. Suppression of allogeneic T-cell proliferation by human marrow stromal cells:implications in transplantation. Transplantation. 2003;75:389–97. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 46.Javazon EH, Colter DC, Schwarz EJ, et al. Rat marrow stromal cells are more sensitive to plating density and expand more rapidly from single-cell-derived colonies than human marrow stromal cells. Stem Cells. 2001;19:219–25. doi: 10.1634/stemcells.19-3-219. [DOI] [PubMed] [Google Scholar]
- 47.Martins AA, Paiva A, Morgado JM, et al. Quantification and immunophenotypic characterization of bone marrow and umbilical cord blood mesenchymal stem cells by multicolor flow cytometry. Transplant Proc. 2009;41:943–6. doi: 10.1016/j.transproceed.2009.01.059. [DOI] [PubMed] [Google Scholar]
- 48.De Ugarte DA, Morizono K, Elbarbary A, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–9. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 49.Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: paradoxes of passaging. Exp Hematol. 2004;32:414–25. doi: 10.1016/j.exphem.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 50.Kaiser S, Hackanson B, Follo M, et al. BM cells giving rise to MSC in culture have a heterogeneous CD34 and CD45phenotype. Cytotherapy. 2007;9:439–50. doi: 10.1080/14653240701358445. [DOI] [PubMed] [Google Scholar]
- 51.Fromigué O, Hamidouche Z, Chateauvieux S, et al. Distinct osteoblastic differentiation potential of murine fetal liver and bone marrow stroma-derived mesenchymal stem cells. J Cell Biochem. 2008;104:620–8. doi: 10.1002/jcb.21648. [DOI] [PubMed] [Google Scholar]
- 52.Menicanin D, Bartold PM, Zannettino AC, et al. Genomic profiling of mesenchymal stem cells. Stem Cell Rev. 2009;5:36–50. doi: 10.1007/s12015-009-9056-2. [DOI] [PubMed] [Google Scholar]
- 53.Lian JB, Stein GS, Javed A, et al. Networks and hubs for the transcriptional control of osteoblastogenesis. Rev Endocr Metab Disord. 2006;7:1–16. doi: 10.1007/s11154-006-9001-5. [DOI] [PubMed] [Google Scholar]
- 54.Ikeda R, Yoshida K, Tsukahara S, et al. The promyelotic leukemia zinc finger promotes osteoblastic differentiation of human mesenchymal stem cells as an upstream regulator of CBFA1. J Biol Chem. 2005;280:8523–30. doi: 10.1074/jbc.M409442200. [DOI] [PubMed] [Google Scholar]
- 55.Granchi D, Ochoa G, Leonardi E, et al. Gene expression patterns related to osteogenic differentiation of bone marrow-derived mesenchymal stem cells during ex vivo expansion. Tissue Eng Part C Methods. 2010;16:511–24. doi: 10.1089/ten.TEC.2009.0405. [DOI] [PubMed] [Google Scholar]
- 56.Ruzinova MB, Benezra R. Id proteins in development, cell cycle and cancer. Trends Cell Biol. 2003;13:410–8. doi: 10.1016/s0962-8924(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 57.Kulterer B, Friedl G, Jandrositz A, et al. Gene expression profiling of human mesenchymal stem cells derived from bone marrow during expansion and osteoblast differentiation. BMC Genomics. 2007;8:70. doi: 10.1186/1471-2164-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maeda S, Nobukuni T, Shimo-Onoda K, et al. Sortilin is upregulated during osteoblastic differentiation of mesenchymal stem cells and promotes extracellular matrix mineralization. J Cell Physiol. 2002;193:73–9. doi: 10.1002/jcp.10151. [DOI] [PubMed] [Google Scholar]
- 59.Huang W, Yang S, Shao J, et al. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front Biosci. 2007;12:3068–92. doi: 10.2741/2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Christakos S, Dhawan P, Liu Y, et al. New insights into the mechanisms of vitamin D action. J Cell Biochem. 2003;88:695–705. doi: 10.1002/jcb.10423. [DOI] [PubMed] [Google Scholar]
- 61.Le Sage C, Agami R. Immense promises for tiny molecules: uncovering miRNA functions. Cell Cycle. 2006;5:1415–21. doi: 10.4161/cc.5.13.2890. [DOI] [PubMed] [Google Scholar]
- 62.Li Z, Hassan MQ, Volinia S, et al. A microRNA signature for a BMP2-induced osteoblast lineage commitment program. Proc Natl Acad Sci U S A. 2008;105:13906–11. doi: 10.1073/pnas.0804438105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang J, Zhao L, Xing L, et al. MicroRNA-204 regulates Runx2 protein expression and mesenchymal progenitor cell differentiation. Stem Cells. 2010;28:357–64. doi: 10.1002/stem.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Z, Hassan MQ, Jafferji M, et al. Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. J Biol Chem. 2009;284:15676–84. doi: 10.1074/jbc.M809787200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kapinas K, Kessler CB, Delany AM. miR-29 suppression of osteonectin in osteoblasts: regulation during differentiation and by canonical Wnt signalling. J Cell Biochem. 2009;108:216–24. doi: 10.1002/jcb.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schoolmeesters A, Eklund T, Leake D, et al. Functional profiling reveals critical role for miRNA in differentiation of human mesenchymal stem cells. PLoS One. 2009;4:e5605. doi: 10.1371/journal.pone.0005605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li H, Xie H, Liu W, et al. A novel microRNA targeting HDAC5 regulates osteoblast differentiation in mice and contributes to primary osteoporosis in humans. J Clin Invest. 2009;119:3666–77. doi: 10.1172/JCI39832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006;116:1202–9. doi: 10.1172/JCI28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qiu W, Andersen TE, Bollerslev J, et al. Patients with high bone mass phenotype exhibit enhanced osteoblast differentiation and inhibition of adipogenesis of human mesenchymal stem cells. J Bone Miner Res. 2007;22:1720–31. doi: 10.1359/jbmr.070721. [DOI] [PubMed] [Google Scholar]
- 70.Chenu C, Marenzana M. Sympathetic nervous system and bone remodeling. Joint Bone Spine. 2005;72:481–3. doi: 10.1016/j.jbspin.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 71.Teixeira L, Sousa DM, Nunes AF, et al. NPY revealed as a critical modulator of osteoblast function in vitro: new insights into the role of Y1 and Y2 receptors. J Cell Biochem. 2009;107:908–16. doi: 10.1002/jcb.22194. [DOI] [PubMed] [Google Scholar]
- 72.Nunes AF, Liz MA, Franquinho F, et al. Neuropeptide Y expression and function during osteoblast differentiation—insights from transthyretin knockout mice. FEBS J. 2010;277:263–75. doi: 10.1111/j.1742-4658.2009.07482.x. [DOI] [PubMed] [Google Scholar]
- 73.Dickhut A, Pelttari K, Janicki P, et al. Calcification or dedifferentiation: requirement to lock mesenchymal stem cells in a desired differentiation stage. J Cell Physiol. 2009;219:219–26. doi: 10.1002/jcp.21673. [DOI] [PubMed] [Google Scholar]
- 74.Lippens E, Vertenten G, Gironés J, et al. Evaluation of bone regeneration with an injectable, in situ polymerizable Pluronic(R)F127 hydrogel derivative combined with autologous mesenchymal stem cells in a goat tibia defect model. Tissue Eng Part A. 2010;16:617–27. doi: 10.1089/ten.TEA.2009.0418. [DOI] [PubMed] [Google Scholar]
- 75.Jukes JM, Both SK, Leusink A, et al. Endochondral bone tissue engineering using embryonic stem cells. Proc Natl Acad Sci U S A. 2008;105:6840–5. doi: 10.1073/pnas.0711662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Janicki P, Kasten P, Kleinschmidt K, et al. Chondrogenic pre-induction of human mesenchymal stem cells on beta-TCP: enhanced bone quality by endochondral heterotopic bone formation. Acta Biomater. 2010;6:3292–301. doi: 10.1016/j.actbio.2010.01.037. [DOI] [PubMed] [Google Scholar]
- 77.Granchi D, Devescovi V, Baglío SR, et al. Biological basis for the use of autologous bone marrow stromal cells in the treatment of congenital pseudarthrosis of the tibia. Bone. 2010;46:780–8. doi: 10.1016/j.bone.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 78.Patterson TE, Kumagai K, Griffith L, et al. Cellular strategies for enhancement of fracture repair. J Bone Joint Surg Am. 2008;90(Suppl 1):111–9. doi: 10.2106/JBJS.G.01572. [DOI] [PubMed] [Google Scholar]
- 79.Meyer U, Wiesmann HP, Berr K, et al. Cell-based bone reconstruction therapies-principles of clinical approaches. Int J Oral Maxillofac Implants. 2006;21:899–906. [PubMed] [Google Scholar]
- 80.Khosla S, Eghbali-Fatourechi GZ. Circulating cells with osteogenic potential. Ann N Y Acad Sci. 2006;1068:489–97. doi: 10.1196/annals.1346.022. [DOI] [PubMed] [Google Scholar]
- 81.Van Osch GJ, Brittberg M, Dennis JE, et al. Cartilage repair: past and future-lessons for regenerative medicine. J Cell Mol Med. 2009;13:792–810. doi: 10.1111/j.1582-4934.2009.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tare RS, Babister JC, Kanczler J, et al. Skeletal stem cells: phenotype, biology and environmental niches informing tissue regeneration. Mol Cell Endocrinol. 2008;288:11–21. doi: 10.1016/j.mce.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 83.Quarto R, Mastrogiacomo M, Cancedda R, et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344:385–6. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 84.Dallari D, Savarino L, Stagni C, et al. Enhanced tibial osteotomy healing with use of bone grafts supplemented with platelet gel or platelet gel and bone marrow stromal cells. J Bone Joint Surg Am. 2007;89:2413–20. doi: 10.2106/JBJS.F.01026. [DOI] [PubMed] [Google Scholar]
- 85.Horwitz EM, Prockop DJ, Fitzpatrick LA, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–13. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 86.Panaroni C, Gioia R, Lupi A, et al. In utero transplantation of adult bone marrow decreases perinatal lethality and rescues the bone phenotype in the knockin murine model for classical, dominant osteogenesis imperfecta. Blood. 2009;114:459–68. doi: 10.1182/blood-2008-12-195859. [DOI] [PMC free article] [PubMed] [Google Scholar]


