Abstract
Background:
Real-life studies are needed to determine the cost-effectiveness of asthma therapies in clinical practice.
Aim:
To compare the cost-effectiveness of extrafine-particle inhaled corticosteroid (ICS) with standard size-particle ICS in the United Kingdom (UK) and United States (US).
Methods:
These retrospective matched cohort analyses used large electronic databases to study asthma-related outcomes for patients in the UK (12–60 years old; n=1730) and US (12–80 years; n=10,312) prescribed extrafine beclomethasone or fluticasone as their first ICS therapy for asthma. Patients were matched on demographic characteristics and asthma severity during 1 baseline year, and asthma control and asthma-related costs were compared during 1 outcome year.
Results:
In both the UK and US, adjusted odds of risk-domain asthma control were similar, whereas the odds of overall control (no hospitalisation or oral steroids for asthma, no antibiotics for lower respiratory infection, limited reliever use) were greater for extrafine ICS in both countries (UK odds ratio, 1.23; 95% confidence interval (CI), 1.01–1.50). Asthma-related annual costs, adjusted for baseline, were significantly lower for extrafine-particle ICS cohorts in both countries (UK difference, −£66 (95% CI,−93 to −37)). Cost-effectiveness analyses using the two measures of asthma control found 92 and 98% probabilities of extrafine-particle ICS being the preferred treatment strategy (less costly and more effective than standard size-particle ICS) in the UK, and 84 and 100% probabilities in the US.
Conclusions:
Initiating ICS therapy for asthma as extrafine-particle ICS seems the dominant treatment option (less costly and more effective) compared with standard size-particle ICS in both the UK and the US.
Introduction
Asthma is common and expensive, affecting ~1 in 12 adults in the United Kingdom (UK) and 1 in 13 adults in the United States (US).1–4 The annual economic burden of asthma was estimated recently at 1 billion pounds (£) in costs for the UK National Health Service (NHS) and 56 billion dollars ($) in direct costs and productivity losses in the US.4,5 The costs of asthma are disproportionately attributable to patients with poorly controlled disease,6,7 highlighting the importance of maintaining asthma control and minimising exacerbations, unscheduled consultations and asthma-related hospitalisations.
Inhaled corticosteroids (ICSs) are first-line therapy for the airway inflammation present in persistent asthma.8,9 Although ICSs vary in potency and formulation, little work has been done to compare the cost-effectiveness of different ICSs used in real-life among the varied patient populations seen in clinical practice. Real-life studies are particularly pertinent for economic evaluations to determine true comparative cost-effectiveness.10
In previous work,11,12 we analysed the real-life effectiveness of a small-particle ICS, extrafine beclomethasone dipropionate, with median mass aerodynamic diameter particle size of 1.1 μm,13,14 and a standard size-particle ICS, fluticasone propionate (median mass aerodynamic diameter, 2.4–3.2 μm, depending on formulation15), for patients with asthma prescribed their first ICS or stepping up their ICS therapy in the UK and US. Patients prescribed extrafine beclomethasone as their first ICS experienced similar or better asthma-related outcomes at lower doses compared with patients prescribed fluticasone. We hypothesised that these effects could result from characteristics of the extrafine-particle beclomethasone formulation and inhaler that enable improved total and peripheral lung distribution as compared with a larger-sized particle ICS.13,14
Our objective in this retrospective matched cohort study was to compare the cost-effectiveness of initiating an extrafine-particle ICS as compared with a representative standard size-particle ICS for patients ⩾12 years old with asthma in the UK and US, two countries with very different health-care systems.
Materials and Methods
Effectiveness results and costs for the US cohorts, reported previously,12 are included here in brief to support the cost-effectiveness analyses.
Study design and patients
We used de-identified patient data contained in two large, well-established databases for these matched cohort analyses. The UK study drew on data (January 1997 to June 2007) from primary care practices subscribing to the General Practice Research Database, now the Clinical Practice Research Datalink.16 The US study drew on data (2003–2010) contained in the Ingenix Normative Health Information Database, now the OptumInsight Research Database, an administrative claims database for primary and secondary (specialist and emergency) medical care.12,17
We included data for patients prescribed their first ICS for asthma by means of a pressurised metered-dose inhaler as extrafine beclomethasone (Qvar, Teva Pharmaceuticals, Petach Tikva, Israel) or fluticasone (hydrofluoroalkane or chlorofluorocarbon formulation; Flixotide, GlaxoSmithKline, Brentford, UK). Patients in the UK prescribed extrafine beclomethasone by breath-actuated inhaler were excluded from the analyses; extrafine beclomethasone breath-actuated inhalers were not available in the US.
Patients aged 12–60 years in the UK and 12–80 years in the US were eligible for the analyses.12 Each patient had complete up-to-standard data for 1 year before (baseline year) and 1 year after (outcome year) the index ICS prescription (Figure 1). We excluded patients with a diagnosis of any chronic respiratory disease other than asthma and, in the US study, patients who had received maintenance oral corticosteroid therapy during baseline and smokers ⩾60 years to minimise inclusion of patients with chronic obstructive pulmonary disease misdiagnosed as asthma.
Figure 1.
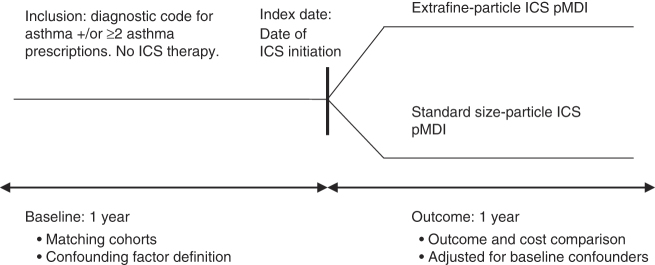
Study design. ICS, inhaled corticosteroid; pMDI, pressurised metered-dose inhaler.
Effectiveness measures
The two composite measures used to evaluate treatment effectiveness were designed to capture both risk and current control aspects of asthma control from the data sets (details in Supplementary Appendix), as per consensus recommendations.18–20 We defined risk-domain asthma control as the absence of all of the following during the outcome year: (1) hospital attendance for asthma, including inpatient, unscheduled outpatient, or emergency department; (2) general practice consultation for lower respiratory tract infection requiring antibiotics; and (3) an acute course of oral corticosteroids. (Hospital attendance for asthma included all events with a lower respiratory code—i.e., including all asthma codes and lower respiratory tract infection codes.) Overall control (impairment and risk domains of asthma control) was defined as risk-domain asthma control plus the fourth criterion of average daily use of ⩽2 puffs of salbutamol (⩽200 μg in the UK and ⩽180 μg in the US study; Supplementary Table S1).
Resource use and costs
Information on asthma-related health-care resource use during baseline and outcome years was extracted from the data sets and used to calculate annual direct costs, including costs for all asthma- and lower respiratory-related drugs, lower respiratory primary care consultations and respiratory-related hospital attendance. Drug costs included those for ICSs, short-acting β2-agonists (SABAs), long-acting β2-agonists (LABAs), combination ICS-LABA inhalers, leukotriene receptor antagonists, theophylline, oral corticosteroids and antibiotics.
The designated price year for the UK was 2007. Prices were derived from national sources of cost data: namely, unit costs for general practice resource use published by the Personal Social Services Research Unit; British National Formulary for prescription use; and NHS Reference Costs for hospital attendance.21–23
The designated price year for the US was 2010. Actual claimed costs from the database, including both health plan and patient costs, were adjusted to 2010 US$ using the Consumer Price Index for medical care.24 To account for the switch to more expensive hydrofluoroalkane inhalers in 2008, we used a standard 2010 price for SABA inhalers.
Cost-effectiveness analysis
The composite outcome measures and cost-effectiveness analyses were prespecified according to the standard operating procedures of the research group.25 The analyses were carried out using IBM SPSS Statistics versions 19 and 20 (SPSS Statistics, IBM, Somers, NY, USA), SAS versions 9.2 and 9.3 (SAS Institute, Marlow, UK) and Microsoft Excel 2007 (Microsoft, Bellevue, WA, USA). Statistically significant results were defined as P⩽0.05.
We matched patients sequentially on the following criteria: sex, age and criteria indicative of asthma severity at baseline, including number of acute oral corticosteroid prescriptions during the year; mean daily SABA dose; and (for the US only) number of asthma consultations with no acute oral corticosteroid prescription (details in Supplementary Appendix). Matching ratios, chosen to maximise patient numbers and statistical power for comparisons, were 1:1 for the UK and 1:3 for the US for extrafine-particle versus standard size-particle ICS cohorts.12
Summary statistics for baseline and unadjusted effectiveness variables were tabulated by matched treatment cohort and compared using conditional logistic regression. Total asthma-related and lower respiratory-related health-care costs (hereafter called asthma-related costs) were adjusted to 2007 (UK) or 2010 (US) prices, summed for baseline and outcome years and compared between cohorts using conditional logistic regression. Although the distributions were skewed, we report the arithmetic mean costs, as recommended by Thompson et al.,26 because means can be multiplied by a target population to estimate total costs, the main outcome of interest for policy-makers and providers. The adjusted odds of achieving risk-domain asthma control and overall control were compared between matched cohorts using conditional binary logistic regression models, with standard size-particle ICS as the reference treatment. Asthma control status was used as the dependent variable, with treatment and potential confounding factors (Supplementary Table S2) as explanatory variables.
Expected differences in adjusted mean costs were modelled using generalised linear models with gamma distributions to best fit the data; expected differences in adjusted proportions of patients achieving asthma control were modelled using binomial distributions.26 The 95% confidence intervals (CIs) were determined by bootstrapping methods, using 1,000 random samples taken, with replacement, from the data set27; differences (relative to standard size-particle ICS) were displayed graphically on a cost-effectiveness plane. Further information on the methods of analysis is detailed in the Supplementary Appendix.
Results
Patients
After matching, there were 865 patients in each UK treatment cohort (ages 12–60 years). The two cohorts were well matched for baseline demographic and asthma-related characteristics, with no clinically important differences (Table 1, Supplementary Table S3).
Table 1. Baseline characteristics of patients in the extrafine-particle ICS and standard size-particle ICS cohorts in the UK.
| Characteristic |
UK study population
|
|
|---|---|---|
| Extrafine ICS (n=865) | SSP ICS (n=865) | |
|
Matching criteria
| ||
| Female sex | 516 (60) | 516 (60) |
| Age at index date, years, mean (s.d.) | 38 (13) | 38 (14) |
| Age 12–60 years | 865 (100) | 865 (100) |
| Nonsmokers aged ⩾60–80 years | n/a | n/a |
|
Oral corticosteroid courses
| ||
| 0 | 721 (83) | 721 (83) |
| 1 | 118 (14) | 118 (14) |
| 2 | 22 (3) | 22 (3) |
| 3 | 3 (0) | 3 (0) |
| ⩾4 | 1 (0) | 1 (0) |
|
Mean daily SABA dose (μg/day) | ||
| 0 | 410 (47) | 410 (47) |
| 1–100 | 266 (31) | 266 (31) |
| 101–200 | 125 (15) | 125 (15) |
| 201–300 | 32 (4) | 32 (4) |
| 301–400 | 10 (1) | 10 (1) |
| >400 | 22 (3) | 22 (3) |
|
Asthma consultation/no oral corticosteroids
a
| ||
| 0 | 468 (54) | 467 (54) |
| 1 | 326 (38) | 298 (35) |
| ⩾2 | 71 (8) | 100 (12) |
|
Effectiveness measures
| ||
| Risk-domain asthma control | 607 (70) | 610 (71) |
| 1 severe exacerbationb | 108 (13) | 114 (13) |
| ⩾2 severe exacerbations | 20 (2) | 16 (2) |
| ⩾1 course of antibiotics for LRTI | 169 (20) | 166 (19) |
| ⩾1 hospital attendance for asthma | 7 (1) | 9 (1) |
| Overall control (risk and impairment) | 560 (65) | 560 (65) |
Data are n (%) unless otherwise stated. Percentages may not add up to 100% because of rounding.
Abbreviations: LRTI, lower respiratory tract infection; n/a, not applicable; OCS, oral corticosteroid course; SABA, short-acting β-agonist; s.d., standard deviation; SSP ICS, standard size-particle inhaled corticosteroid.
Matching criterion for US study only.
A severe exacerbation was defined as an acute course of oral corticosteroids or unscheduled hospital admission or emergency department attendance for asthma.
Baseline characteristics of the 2,578 and 7,734 patients in the US extrafine- and standard size-particle ICS cohorts were also well matched, as previously described (see Supplementary Table S4).12 The UK and US cohorts had similar sex and age distributions, with an additional 7% of US patients who were non-smokers ⩾60–80 years old. During the baseline period, the US patients were more likely to have received oral corticosteroids and were less likely to have achieved either risk-domain or overall control than the UK patients. In the UK similar proportions of patients in the two cohorts were prescribed a spacer; in the US a larger proportion of patients prescribed standard size-particle ICS, compared with those prescribed extrafine beclomethasone, were prescribed spacers during baseline and/or outcome year (7% vs. 9%; P=0.006; Supplementary Table S5); however, the difference between cohorts was small and likely not clinically significant.
Effectiveness
The percentage of patients achieving risk-domain asthma control in the UK study increased from 70% at baseline in both cohorts to 84% and 82% in extrafine-particle ICS and standard size-particle ICS cohorts, respectively, during the outcome year. There was no significant difference between cohorts in the odds of achieving risk-domain asthma control, with an adjusted odds ratio (OR) of 1.20 (95% CI, 0.93–1.57) for extrafine-particle ICS relative to standard size-particle ICS. The percentage of patients achieving overall control decreased from 65% at baseline in both cohorts to 63% and 58% during the outcome year in extrafine- and standard size-particle ICS cohorts, respectively. Patients in the extrafine-particle ICS cohort had significantly higher odds of achieving overall control (adjusted OR, 1.23; 95% CI, 1.01–1.50).
Effectiveness results for the US cohorts have been described in detail elsewhere.12 In brief, the adjusted odds of achieving risk-domain asthma control were similar for the two cohorts (adjusted OR, 1.05; 95% CI, 0.96–1.15), whereas the odds of overall control during the outcome year significantly favoured extrafine-particle ICS (adjusted OR, 1.19; 95% CI, 1.08–1.31).12
Resource use and costs
In the UK as in the US,12 extrafine beclomethasone was prescribed at significantly lower doses than fluticasone on the index date (median (interquartile range), 200 (100–200) vs. 500 (200–500) μg/day in the UK, P<0.001). During the outcome year, the median (interquartile range) daily ICS dose exposure was also lower in extrafine beclomethasone compared with fluticasone cohorts at 110 (55–181) vs. 115 (41–247) μg/day in the UK and 44 (22–98) vs. 72 (36–195) μg/day in the US, respectively (P<0.001 for both), although the difference was likely not clinically significant in the UK.
In the UK, mean asthma-related costs during the baseline year were comparable between cohorts (Supplementary Table S6). Over the outcome year, the mean total adjusted asthma-related costs were significantly lower in the extrafine-particle ICS cohort both with and without the inclusion of ICS costs (Table 2); the unadjusted costs are depicted in Supplementary Table S7.
Table 2. Outcome year asthma-related health-care costs after patients were initiated on ICS treatment (cost/patient per year) in the UK.
| Asthma-related resource |
UK study population (costs in 2007 UK£)
|
||
|---|---|---|---|
| Extrafine ICS (n=865) | SSP ICS (n=865) | P value a | |
| Asthma-related medication | 121 (311) | 174 (421) | 0.004 |
| Asthma-related medication, excluding ICS | 71 (301) | 89 (384) | 0.29 |
| Asthma-related primary care consultation | 25 (38) | 30 (48) | 0.008 |
| Total asthma-related hospitalisations | 9 (68) | 14 (71) | 0.17 |
| Asthma-related inpatient | 5 (63) | 4 (58) | 0.76 |
| Asthma-related outpatient | 4 (26) | 9 (39) | 0.001 |
| Asthma-related emergency department | 0 | 0 | n/a |
| Asthma-related—other medical | 0 | 0 | n/a |
| Total unadjusted asthma-related costs, including ICS | 155 (329) | 218 (440) | 0.001 |
| Total unadjusted asthma-related costs, excluding ICS | 104 (315) | 132 (398) | 0.12 |
| Total adjusted costs per patientb (95% CI) | 145 (131–160) | 211 (190–232) | <0.001 |
| Total adjusted costs per patient, excluding ICS costsb (95% CI) | 100 (89–112) | 134 (116–154) | 0.006 |
Asthma-related costs included all costs for lower respiratory-related health-care resource use. Values are mean (s.d.) unless otherwise noted. Mean values are reported, despite substantially skewed distributions, because mean values can be multiplied by a target population to estimate total costs and thus are of most interest for policy makers and providers. US costs are in Supplementary Table S8.
Abbreviations: CI, confidence interval; n/a, not applicable; s.d., standard deviation; SSP ICS, standard size-particle inhaled corticosteroid.
Conditional logistic regression.
Adjusted for baseline asthma-related health-care costs.
In the US, drug and consultation costs during the baseline year were significantly higher for patients in the extrafine-particle ICS cohort, whereas total annual asthma-related costs were comparable because of lower hospitalisation costs (Supplementary Table S6). During the outcome year, the unadjusted asthma-related costs were similar for both cohorts, as previously described (see Supplementary Table S8).12
Cost-effectiveness
In the UK as in the US,12 total asthma-related costs during the outcome year, when adjusted for baseline confounders, were significantly lower for patients prescribed extrafine-particle ICS compared with standard size-particle ICS (Table 2). In the UK, the adjusted mean difference (95% CI) of £66 (£37–£93) was driven mainly by significantly lower ICS costs in the extrafine-particle ICS cohort (Tables 2 and 3).
Table 3. Incremental cost-effectiveness analysis: effectiveness determined by risk-domain asthma control and overall control (risk and impairment).
|
UK study population
|
US study population
|
|||
|---|---|---|---|---|
| Extrafine ICS (n=865) | SSP ICS (n=865) | Extrafine ICS (n=2578) | SSP ICS (n=7734) | |
| Adjusted OR for risk-domain asthma control | 1.20 (0.93–1.57)a | 1.00 | 1.05 (0.96–1.15)b | 1.00 |
| Adjusted proportion controlled | 0.68 (0.60–0.76)a | 0.64 (0.56–0.72) | 0.52 (0.50–0.55)b | 0.51 (0.49–0.53) |
| Difference relative to SSP ICS | 0.04 (−0.02–0.10) | — | 0.01 (−0.01–0.04) | — |
| Adjusted OR for overall control | 1.23 (1.01–1.50)c | 1.00 | 1.19 (1.08–1.31)d | 1.00 |
| Adjusted proportion controlled | 0.50 (0.49–0.56)c | 0.45 (0.40–0.50) | 0.32 (0.31–0.34)d | 0.29 (0.27–0.30) |
| Difference relative to SSP ICS | 0.05 (0.001–0.10) | — | 0.04 (0.02–0.06) | — |
| Adjusted mean asthma-related costs per patient per yeare | £145 (131–160) | £211 (190–232) | $1869 ($1727–2032) | $2259 ($2111–2404) |
| Difference relative to SSP ICS | −£66 (−93–−37) | — | −$390 (−$620–−$165) | |
95% confidence intervals (ranges in parentheses), other than those for ORs, were found using bootstrapping methods with 1000 random samples.
Costs are in 2007 UK£ and 2010 US$.
Abbreviations: OR, odds ratio; SSP ICS, standard size-particle inhaled corticosteroid.
Adjusted for gastro-oesophageal reflux (GERD) diagnosis, number of consultations for lower respiratory tract infection (LRTI) resulting in a prescription for antibiotics and number of non-asthma-related consultations.
Adjusted for GERD diagnosis and/or therapy and numbers of paracetamol prescriptions, non-asthma-related consultations in primary care and lower respiratory-related hospitalisations and referrals.
Adjusted for GERD diagnosis, socioeconomic status and number of prescriptions for short-acting β-agonist.
Adjusted for GERD diagnosis and/or therapy, year of index date, and numbers of paracetamol prescriptions, prescriptions for asthma/allergies, and lower respiratory-related hospitalisations and referrals.
Adjusted for baseline asthma-related health-care costs (logged).
The cost-effectiveness planes show the spread of the estimated differences in cost and effectiveness based on 1000 replicated samples (Figures 2 and 3). With regard to risk-domain asthma control, the primary effectiveness measure, there was a 92% (UK) or 84% (US) probability that extrafine-particle ICS was the preferred treatment strategy (less costly and more effective) and an 8% (UK) or 16% (US) probability that extrafine-particle ICS was less costly but less effective (a trade-off; Figures 2a and 3a). With regard to the overall control measure, after adjusting for potential confounders, in the UK there was a 98% probability that extrafine-particle ICS was the preferred treatment strategy, and a 2% probability that extrafine-particle ICS was less costly but less effective (Figure 2b). In the US, extrafine-particle ICS was less costly (100%) and more effective (100%) and thus the dominant treatment (Figure 3b).
Figure 2.
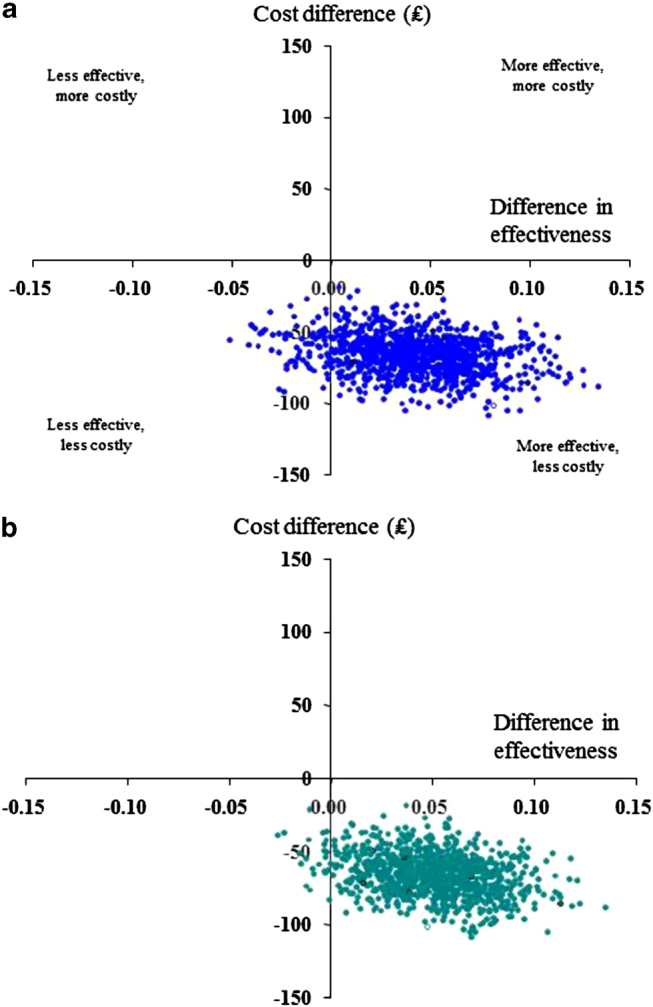
UK Study: Cost-effectiveness planes for extrafine-particle ICS relative to standard size-particle ICS matched cohort (adjusted results): effectiveness based on (a) risk-domain asthma control and (b) overall control (risk and impairment). The horizontal axis divides probable costs (more expensive above, less expensive below) and the vertical axis divides the probable effectiveness (less on the left, more on the right).
Figure 3.
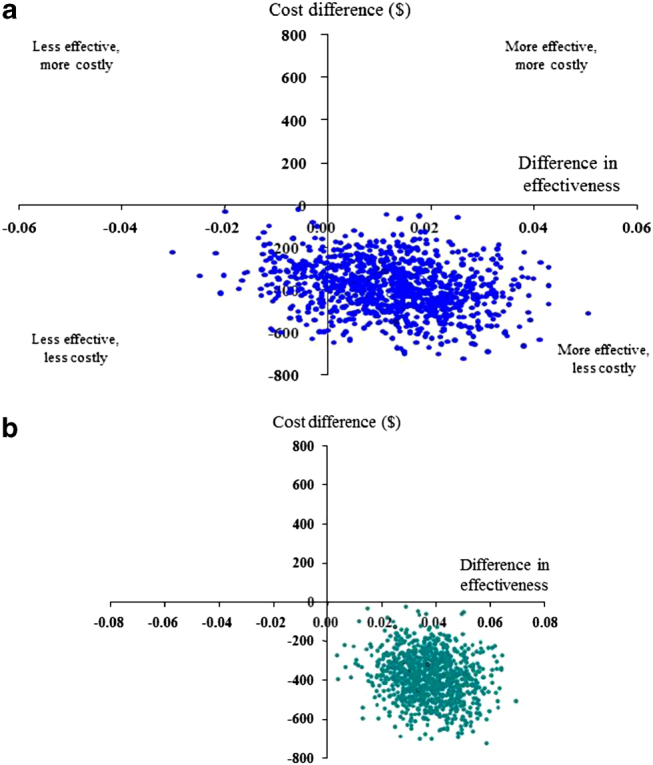
US Study: Cost-effectiveness planes for extrafine-particle ICS relative to standard size-particle ICS matched cohort (adjusted results): effectiveness based on (a) risk-domain asthma control and (b) overall control (risk and impairment).
Discussion
Main findings
The results of these cost-effectiveness analyses indicate that extrafine-particle ICS is likely to be the dominant treatment strategy (less costly and more effective), as compared with standard size-particle ICS, in both the UK and the US for patients prescribed ICS for the first time for asthma. In the UK, the probabilities of extrafine-particle ICS being the preferred strategy with regard to cost-effectiveness for risk-domain asthma control and overall control were 92% and 98%, respectively; in the US, the probabilities were 84% and 100%.
To our knowledge, this is the first comparative cost-effectiveness assessment of two ICSs in asthma performed using the same metrics in two countries with very different health-care systems. The direct asthma- and lower respiratory-related health-care costs, adjusted for baseline costs, were significantly lower for UK and US patients12 treated with extrafine-particle ICS with or without the inclusion of ICS costs. The health-care systems in the UK and US, however, are structured differently and, indeed, the absolute costs were very different between the two countries, notably lower for the UK primary care population than for US patients.
Interpretation of findings in relation to previously published work
For the UK, the mean unadjusted total asthma-related cost of £155–218/year (depending on treatment cohort) was similar to the annual cost to the NHS of £166/year (or £332 over 2 years) reported for patients prescribed ICS in a recent UK pragmatic trial.28 With regard to effectiveness findings, as compared with our prior study that included children as young as 5 years,11 the proportions of patients achieving asthma control were roughly similar, whereas the adjusted odds of risk-domain asthma control were not significantly different between treatment cohorts in the present study (as we had found in the prior study11).
For the US, this study’s categorised costs were generally in line with those reported recently by Barnett and Nurmagambetov,5 with the exception of respiratory-related medication costs, which were lower by about $850/year in each cohort. Possible explanations for the lower medication costs in our analyses are (1) the exclusion of in-hospital medications from the study database and (2) the inclusion of patients ⩾65 years of age (3% of all patients) who used health-care resources covered by Medicare that were not captured by the database. Our study’s total costs ($1952–2369, depending on cohort) are thus lower than the previously reported total per-patient annual incremental cost of $3259 for asthma in 2009 US$.5,12
There are few economic evaluations comparing ICSs of different particle sizes as monotherapy for asthma.29–31 In a 1-year pragmatic, open-label study of adults with asthma, extrafine beclomethasone was a cost-effective alternative when administered at half the dose of the larger-particle chlorofluorocarbon beclomethasone in the US, UK, Belgium and the Netherlands.32 More recently, in a 1-year US database study, significantly lower drug and total medical costs and fewer emergency department visits were reported with extrafine beclomethasone compared with fluticasone for adults and children (5–64 years old) with persistent asthma.33 Prior studies comparing extrafine beclomethasone and fluticasone were short-term (3–12 weeks) randomised trials that did not evaluate cost-effectiveness.34
Strengths and limitations of this study
Our study has several potential limitations. As for any observational study using a large database, the analyses relied on the accuracy and completeness of data recording. Some data were not available for all patients, including socioeconomic status and body mass index, both factors linked to asthma control; thus, we cannot rule out the possibility of unrecognised confounding factors. However, our matching process paired patients in each cohort using several criteria reflective of asthma severity and control at baseline, with subsequent adjustments for minor residual confounding.
We used composite measures of asthma control to capture all key elements of control (impairment and risk) that were available from the database. The risk-domain asthma control measure included the absence of criteria defining severe exacerbations,18 which are important drivers of asthma-related costs. However, a study limitation is that the databases, and thus our outcome measures, do not capture symptoms or lung function measurements. We included SABA prescriptions as a proxy for symptoms in the ‘overall control’ measure, because SABA use reflects symptom control. The control cutoff point of a mean SABA use of ⩽2 puffs/day corresponds to the level 2 category (2 of 4, with 1 being the best controlled) of the validated approach of Schatz et al. 20 for SABA canister dispensing to assess asthma symptom control. The definitions of the outcome measures are inherently related to the use of health-care resources; therefore, it is unsurprising that costs and outcome in these analyses work in the same direction. For the US analyses, the later index dates of patients in the extrafine-particle ICS cohort12 might have biased comparative costs, increasing the cost of extrafine-particle ICS and decreasing the cost–benefit relative to standard size-particle ICS, despite the fact that costs were adjusted to 2010 dollars, because of the general increases in costs over time.
A strength of these analyses is the consistency of results across effectiveness measures and between the two countries. Moreover, the large sample sizes reassure us of the robustness of the findings. We evaluated outcomes over 12 months to eliminate seasonal variations and evaluate long-term effects, as recommended by expert group consensus.18,19 An important strength is the fact that cost and effectiveness data were drawn from large numbers of patients in real-life clinical practice, as opposed to economic modelling based on non-representative clinical trial data, which is the most common approach. Studied patients thus included smokers, patients with comorbidities and potentially those with poor adherence or poor inhaler technique, enhancing the applicability of the findings to real-life practice.
Implications for future research, policy and practice
Calculations of indirect costs would have been of interest, as patients with asthma have significantly higher work loss than those without,35 and recent estimates put lost productivity because of asthma at 8–12% of the total societal burden of asthma in the US.5 Moreover, continuing research is needed to better characterise the role of small airway disease in asthma and the benefits of extrafine-particle ICS for treating both large and small airway inflammation.36 Comparisons of other extrafine- and larger-particle ICS are needed, and our findings should not be extended beyond this comparison of extrafine beclomethasone and fluticasone.
A direct comparison between clinical outcomes in the US and UK would be inappropriate because of differences between the databases and health-care systems. However, several observations are intriguing. The percentage of patients meeting the asthma control measures was much lower, for both control measures, in the US12 than in the UK during baseline as well as outcome. We do not have an obvious explanation for this observation but have marked it as a topic for further study. In the US there was a greater disparity than in the UK between dose prescribed at the index date and dose exposure during the outcome year, possibly indicating poor adherence to therapy. A change in therapy in the form of an increase in ICS dose or additional therapy was more frequent in the US than in the UK (see Supplementary Appendix). The percentages of patients achieving risk-domain asthma control increased from baseline during the outcome year in both treatment cohorts in the UK and US, although the increase was small (1%) in the US.12 However, the percentages achieving overall control decreased, likely driven by increased SABA prescriptions during the outcome year, as noted in our prior studies,11,12 for patients newly diagnosed with persistent asthma who were prescribed additional SABA together with ICS.
Conclusions
Real-life comparative effectiveness and cost-effectiveness analyses are needed to guide practical decision making with regard to asthma therapies. These analyses serve as preliminary evidence of the relative cost-effectiveness of extrafine-particle ICS and suggest that initiating ICS therapy for asthma as extrafine-particle ICS is the dominant treatment option (less costly and more effective) as compared with standard size-particle ICS for adults and children ⩾12 years old in both the UK and US. Further studies, including prospective pragmatic trials, are warranted to investigate the comparative effectiveness and cost-effectiveness of other examples of extrafine- and larger-particle ICS.
Acknowledgments
We thank Professor Neil Barnes for his contributions to the early discussions when planning the analyses.
RJM has done consultancy work and received travel support for attendance at advisory boards for Teva, and has received research grants from MedImmune and the NHLBI and royalties from UpToDate.
DP has Board Membership with Aerocrine, Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis and Teva; he is a consultant at Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Meda, Mundipharma, Napp, Novartis, Pfizer and Teva; he has received grants from or has grants pending with UK National Health Service, British Lung Foundation, Aerocrine, AstraZeneca, Boehringer Ingelheim, Chiesi, Eli Lilly, GlaxoSmithKline, Meda, Merck, Mundipharma, Novartis, Orion, Pfizer, Respiratory Effectiveness Group, Takeda, Teva and Zentiva; he has received payments for lectures or speaker fees from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Meda, Merck, Mundipharma, Novartis, Pfizer, SkyePharma, Takeda and Teva; he has received payment for manuscript preparation from Mundipharma and Teva; he has patents (planned, pending or issued) from AKL Ltd.; he has received payment for the development of educational materials from GlaxoSmithKline and Novartis; he has stock or stock options in the form of shares in AKL Ltd, which produces phytopharmaceuticals; he owns 80% of Research in Real Life Ltd and its subsidiary social enterprise Optimum Patient Care; he has received payment for travel/accommodations/meeting expenses from Aerocrine, Boehringer Ingelheim, Mundipharma, Napp, Novartis and Teva; he has received funding for patient enrolment or completion of research from Almirall, Chiesi, Teva and Zentiva; he is a peer reviewer for grant committees such as Medical Research Council (2014), Efficacy and Mechanism Evaluation programme (2012) and HTA (2014); he has also received unrestricted funding for investigator-initiated studies from Aerocrine, AKL Ltd, Almirall, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis, Orion, Takeda, Teva and Zentiva.
NR received fees for speaking, organising education and research or consulting from Aerocrine, Nycomed, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, MEDA, MSD, Mundipharma, Novartis, Pfizer and Teva, and research funds from Boehringer Ingelheim, Pfizer, Novartis, Nycomed.
EI reports receiving consulting fees from Cowen & Co, Infinity Pharmaceuticals, Merck, Regeneron Pharmaceuticals and TEVA Specialty Pharmaceutical; fees for Expert Testimony from Campbell, Campbell, Edwards & Conroy, Ficksman & Conley and Ryan Ryan Deluca LLP; lecturing fees from Merck; travel grant support from TEVA Specialty Pharmaceuticals; and grant support to his institution from Amgen, i3 Research (Biota).
WMCvA is member of the Medical Advisory Boards of Mundipharma BV, Astra-Zeneca and Teva and received a travelling grant from Teva and a speaker’s fee from Forest.
JG received honoraria from Novartis as a member of an advisory board for an asthma medication and received honoraria from GlaxoSmithKline for advice on an asthma medication study design.
In the past 3 years, The University of Groningen has received money for DSP regarding an unrestricted educational grant for research from AstraZeneca, Chiesi; travel to ERS and/or ATS has been partially funded by AstraZeneca, Chiesi, GSK, Nycomed; fees for consultancies were given to the University of Groningen by AstraZeneca, Boeringer Ingelheim, Chiesi, GSK, Nycomed and TEVA; travel and lectures in China were paid by Chiesi.
TWG reports the following: board membership on the American Board of Pediatrics Pediatric Pulmonary Subboard; being a consultant for Teva, MAP Pharmaceuticals, GSK and Merck; receiving grant funding through CDC, NIH and DHHS; being a subinvestigator for Altus Pharmaceuticals, Inspire Pharmaceuticals, Abbott Laboratories, Array Biopharma, Teva, Mylan, Forest Research Institute, F. Hoffman-LaRoche, GSK, Medimmune, KaloBios Pharmaceuticals, Vertex Pharmaceuticals, Roxane Laboratories and CompleWare Corporation, CF Foundation Therapeutics and Roche/Genetech; receiving travel support to research meetings from Merck-Schering Plough; and royalities from UptoDate.
EVH is a consultant for RiRL and has received payment for manuscript preparation from Merck and TevaFrance.
AB and JvZ are employees of RiRL, which has conducted paid research in respiratory disease on behalf of the following organisations over the past 5 years: Aerocrine, AKL Ltd, Almirall, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Meda, Mundipharma, Napp, Novartis, Orion, Takeda, Teva and Zentiva.
GC is speaker/advisor/consultant for Teva, MedImmune, Alitair, Dey, Mylan and Novartis.
References
- Simpson CR, Sheikh A. Trends in the epidemiology of asthma in England: a national study of 333,294 patients. J R Soc Med. 2010;103:98–106. doi: 10.1258/jrsm.2009.090348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahran HS, Bailey C, Garbe P. Vital signs: asthma prevalence, disease characteristics, and self-management education --- United States, 2001--2009. MMWR. 2011;60:547–552. [PubMed] [Google Scholar]
- Meltzer EO, Blaiss MS, Nathan RA, Doherty DE, Murphy KR, Stoloff SW. Asthma burden in the United States: results of the 2009 Asthma Insight and Management survey. Allergy Asthma Proc. 2012;33:36–46. doi: 10.2500/aap.2011.32.3519. [DOI] [PubMed] [Google Scholar]
- Asthma UK. Asthma facts and FAQs , updated 2013. Available from http://www.asthma.org.uk/asthma-facts-and-statistics (accessed 14 January 2014).
- Barnett SB, Nurmagambetov TA. Costs of asthma in the United States: 2002–2007. J Allergy Clin Immunol. 2011;127:145–152. doi: 10.1016/j.jaci.2010.10.020. [DOI] [PubMed] [Google Scholar]
- Sadatsafavi M, Lynd L, Marra C, Carleton B, Tan WC, Sullivan S. Direct health care costs associated with asthma in British Columbia. Can Respir J. 2010;17:74–80. doi: 10.1155/2010/361071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accordini S, Corsico AG, Braggion M, Gerbase MW, Gislason D, Gulsvik A. The cost of persistent asthma in Europe: an international population-based study in adults. Int Arch Allergy Immunol. 2013;160:93–101. doi: 10.1159/000338998. [DOI] [PubMed] [Google Scholar]
- National Asthma Education and Prevention Program . Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma, 2007. Available from http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.pdf (accessed 14 January 2014).
- British Thoracic Society (BTS), Scottish Intercollegiate Guidelines Network (SIGN) . British Guideline on the Management of Asthma, May 2008, revised Jan 2012. Available from http://www.sign.ac.uk/guidelines/fulltext/101/index.html (accessed 14 January 2014).
- Jarvinen TL, Sievanen H, Kannus P, Jokihaara J, Khan KM. The true cost of pharmacological disease prevention. BMJ. 2011;342:d2175. doi: 10.1136/bmj.d2175. [DOI] [PubMed] [Google Scholar]
- Price D, Martin RJ, Barnes N, Dorinsky P, Israel E, Roche N. Prescribing practices and asthma control with hydrofluoroalkane-beclomethasone and fluticasone: a real-world observational study. J Allergy Clin Immunol. 2010;126:511–518. doi: 10.1016/j.jaci.2010.06.040. [DOI] [PubMed] [Google Scholar]
- Colice G, Martin RJ, Israel E, Roche N, Barnes N, Burden A. Asthma outcomes and costs of therapy with extrafine beclomethasone and fluticasone. J Allergy Clin Immunol. 2013;132:45–54. doi: 10.1016/j.jaci.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Leach C, Colice GL, Luskin A. Particle size of inhaled corticosteroids: does it matter? J Allergy Clin Immunol. 2009;124:S88–S93. doi: 10.1016/j.jaci.2009.09.050. [DOI] [PubMed] [Google Scholar]
- Leach CL, Davidson PJ, Hasselquist BE, Boudreau RJ. Lung deposition of hydrofluoroalkane-134a beclomethasone is greater than that of chlorofluorocarbon fluticasone and chlorofluorocarbon beclomethasone : a cross-over study in healthy volunteers. Chest. 2002;122:510–516. doi: 10.1378/chest.122.2.510. [DOI] [PubMed] [Google Scholar]
- Cripps A, Riebe M, Schulze M, Woodhouse R. Pharmaceutical transition to non-CFC pressurized metered dose inhalers. Respir Med. 2000;94 (Suppl B):S3–S9. [PubMed] [Google Scholar]
- Clinical Practice Research Datalink . Available from http://www.cprd.com/home/ (accessed 14 January 2014.
- OptumInsight Research Database . Available from http://www.optuminsight.com/life-sciences/solutions/real-world-evidence/data-assets/retrospective-database/overview/ (accessed 14 January 2014).
- Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- Akinbami LJ, Sullivan SD, Campbell JD, Grundmeier RW, Hartert TV, Lee TA. Asthma outcomes: healthcare utilization and costs. J Allergy Clin Immunol. 2012;129:S49–S64. doi: 10.1016/j.jaci.2011.12.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz M, Zeiger RS, Vollmer WM, Mosen D, Apter AJ, Stibolt TB. Validation of a beta-agonist long-term asthma control scale derived from computerized pharmacy data. J Allergy Clin Immunol. 2006;117:995–1000. doi: 10.1016/j.jaci.2006.01.053. [DOI] [PubMed] [Google Scholar]
- Personal Social Services Research Unit . Unit costs of health and social care 2007. Available from http://www.pssru.ac.uk/project-pages/unit-costs/2007/index.php (accessed 14 January 2014).
- British National Formulary. v . British Medical Association and the Royal Pharmaceutical Society of Great Britain. London, UK; 2007. [Google Scholar]
- Department of Health . NHS reference costs 2007–2008. Available from http://webarchive.nationalarchives.gov.uk/+/www.dh.gov.uk/en/Managingyourorganisation/Financeandplanning/NHScostingmanual/index.htm (accessed 14 January 2014).
- Bureau of Labor Statistics 2010 , Consumer Price Index. Available from http://www.bls.gov/schedule/archives/cpi_nr.htm#2010 (accessed 14 January 2014).
- Research in Real Life: Standard Operating Procedures . Available from http://www.optimumpatientcare.org/Docs/SOP%20Observational%20Database%20Studies.pdf (accessed 14 January 2014).
- Thompson SG, Nixon RM, Grieve R. Addressing the issues that arise in analysing multicentre cost data, with application to a multinational study. J Health Econ. 2006;25:1015–1028. doi: 10.1016/j.jhealeco.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Briggs AH, Wonderling DE, Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Econ. 1997;6:327–340. doi: 10.1002/(sici)1099-1050(199707)6:4<327::aid-hec282>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Wilson EC, Sims EJ, Musgrave SD, Shepstone L, Blyth A, Murdoch J. Cost effectiveness of leukotriene receptor antagonists versus inhaled corticosteroids for initial asthma controller therapy: a pragmatic trial. Pharmacoeconomics. 2010;28:585–595. doi: 10.2165/11537550-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Shepherd J, Rogers G, Anderson R, Main C, Thompson-Coon J, Hartwell D. Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in adults and children aged 12 years and over. Health Technol Assess. 2008;12:1–360. doi: 10.3310/hta12190. [DOI] [PubMed] [Google Scholar]
- Bahadori K, Quon BS, Doyle-Waters MM, Marra C, Fitzgerald JM. A systematic review of economic evaluations of therapy in asthma. J Asthma Allergy. 2010;3:33–42. doi: 10.2147/jaa.s11038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih YC, Mauskopf J, Borker R. A cost-effectiveness analysis of first-line controller therapies for persistent asthma. Pharmacoeconomics. 2007;25:577–590. doi: 10.2165/00019053-200725070-00004. [DOI] [PubMed] [Google Scholar]
- Price D, Haughney J, Duerden M, Nicholls C, Moseley C. The cost effectiveness of chlorofluorocarbon-free beclomethasone dipropionate in the treatment of chronic asthma: a cost model based on a 1-year pragmatic, randomised clinical study. Pharmacoeconomics. 2002;20:653–664. doi: 10.2165/00019053-200220100-00002. [DOI] [PubMed] [Google Scholar]
- Lage MJ, Gross GN, Brewster C, Spalitto A. Outcomes and costs of patients with persistent asthma treated with beclomethasone dipropionate hydrofluoroalkane or fluticasone propionate. Adv Ther. 2009;26:762–775. doi: 10.1007/s12325-009-0056-z. [DOI] [PubMed] [Google Scholar]
- Lasserson TJ, Cates CK, Jones AB, Steele EH, White J. Fluticasone versus HFA-beclomethasone dipropionate for chronic asthma in adults and children. Cochrane Database Syst Rev. 2006. [DOI] [PMC free article] [PubMed]
- Shenolikar R, Song X, Anderson JA, Chu BC, Cantrell CR. Costs of asthma among US working adults. Am J Manag Care. 2011;17:409–416. [PubMed] [Google Scholar]
- Lipworth B. Targeting the small airways asthma phenotype: if we can reach it, should we treat it? Ann Allergy Asthma Immunol. 2013;110:233–239. doi: 10.1016/j.anai.2013.02.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


