Abstract
The availability of genome sequences from 16 anopheline species provides unprecedented opportunities to study the evolution of reproductive traits relevant for malaria transmission. In Anopheles gambiae, a likely candidate for sexual selection is male 20-hydroxyecdysone (20E). Sexual transfer of this steroid hormone as part of a mating plug dramatically changes female physiological processes intimately tied to vectorial capacity. By combining phenotypic studies with ancestral state reconstructions and phylogenetic analyses, we show that mating plug transfer and male 20E synthesis are both derived characters that have coevolved in anophelines, driving the adaptation of a female 20E-interacting protein that promotes oogenesis via mechanisms also favoring Plasmodium survival. Our data reveal coevolutionary dynamics of reproductive traits between the sexes likely to have shaped the ability of anophelines to transmit malaria.
Anophelines are the only mosquitoes capable of transmitting human malaria, a disease that affects hundreds of millions of people and causes approximately 600,000 fatalities annually (1). The vectorial capacity of different anopheline species, a metric of their ability to transmit the Plasmodium parasites that cause malaria, is governed by multiple elements of mosquito biology. These include longevity, blood-feeding preferences, immune responses to the parasites, and mosquito population densities, the latter largely determined by the mosquito's reproductive success (2). Across most anopheline species, females are monandrous, with reproduction relying on a single mating event that generally occurs in swarms (3). Our knowledge on the reproductive biology of this mosquito genus derives principally from studies in Anopheles gambiae, the primary malaria vector. In this species, males coagulate seminal secretions produced by the male accessory glands (MAGs) into a mating plug that is transferred to females during mating, delivering also large titers of the steroid hormone 20-hydroxyecdysone (20E) (4, 5). Sexual transfer of 20E has profound effects on female physiology as it induces a cascade of events that render A. gambiae females refractory to further copulation while increasing egg production and triggering egg laying (5, 6). The mating-induced increase in oogenesis is mediated by vitellogenic lipid transporters that are also regulated by lower levels of 20E produced by the female after a blood meal (7). Interestingly, these lipid transporters facilitate Plasmodium development by reducing the parasite-killing ability of the mosquito immune system, providing a link between reproductive and immune processes (8). Additionally, the 20E-regulated monandrous mating system positively affects female longevity—a key feature of malaria transmission given the lengthy Plasmodium cycle within the mosquito—by decreasing predation risks associated with swarming behavior (3) and avoiding the fitness costs associated with multiple matings in other insects (9–11). Therefore, 20E in A. gambiae modulates multiple physiological processes that are highly relevant to the mosquito's competence to transmit malaria: reproductive success, parasite development, and longevity.
Mating plug formation and 20E synthesis in the MAGs are not observed in other mosquito genera or in the fruit fly Drosophila melanogaster (3,5,12), suggesting that these traits are potential targets of sexual selection that have either been lost by other insects or gained in anophelines. Given their fundamental relation to fitness, ejaculate characters are often subject to rapid change driven by coevolutionary interactions between the sexes (13,14). Importantly, the evolution of novel reproductive traits in males that induce adaptive changes in female physiology may also affect pathogen transmission in insect vectors of disease (15). Phylogenetic analyses of relevant sexual traits can therefore offer insights into how male-female co-evolutionary dynamics influence the vectorial capacity of different species.
To determine how selection has shaped the macroevolution of reproductive traits that also impact malaria transmission, we set out to establish the evolutionary trajectories of the mating plug and male 20E in anophelines, taking advantage of the recent completion of genome assemblies for 16 Anopheles species (16). We first characterized the occurrence of mating plug formation among nine geographically dispersed species, which span the three subgenera (Cellia, Anopheles, and Nyssorhynchus) that include the most relevant malaria vectors and comprise a range of evolutionary distances from A. gambiae (16). We performed mating experiments on laboratory-reared colonies, dissected female reproductive tracts from each of the nine selected species, and assessed whether females had received a mating plug. We observed considerable divergence in plug phenotypes (Fig. 1). Three African species (the previously characterized A. gambiae, plus A. arabiensis and A. funestus) and one Indian species (A. stephensi) had a solid, highly coagulated plug; three east and southeast Asian species (A. farauti, A. dirus, and A. sinensis) and one European species (A. atroparvus) exhibited a less compact, more amorphous structure—likely reflecting reduced levels of coagulation—whereas no plug was detected in the New World species A. albimanus.
Fig. 1. Mating plug phenotypes across anophelines.
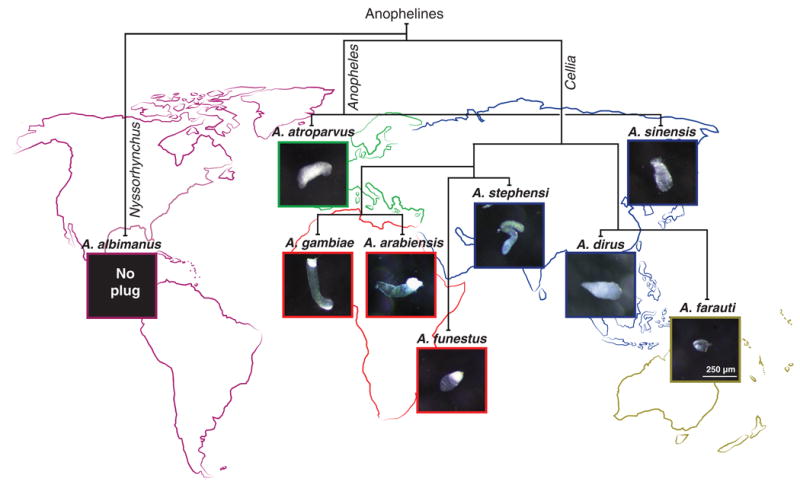
Considerable divergence in mating plug phenotypes is observed across nine geographically dispersed anopheline species that span three major subgenera (vertical branch labels). The African A. gambiae, A. arabiensis, and A. funestus and the Indian A. stephensi species transfer a structured, fully coagulated plug, whereas the east and southeast Asian species A. farauti, A. dirus, and A. sinensis, along with the European species A. atroparvus, present a less coagulated, amorphous plug phenotype. No mating plug transfer was recorded in the New World species A. albimanus. Species are placed according to their approximate geographical distribution, and the scale bar present in A. farauti applies to all. For accurate branch lengths, see fig. S2.
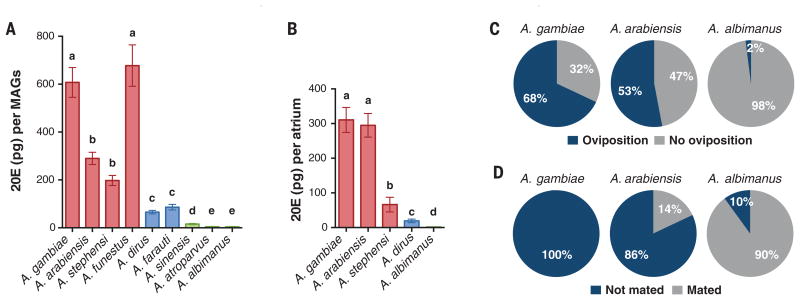
We next assessed whether 20E is produced in the MAGs of the same species and identified significantly different hormone levels through an enzyme immunoassay on MAGs from virgin males (P < 0.0001) (Fig. 2A). Although all species with a fully coagulated plug produced high titers of 20E (above 200 pg/MAGs), two species with partial plug coagulation, A. dirus and A. farauti, had intermediate hormone levels (<100 pg/MAGs), and negligible amounts (<20 pg/MAGs) were found in A. sinensis and A. atroparvus (intermediate plugs) and in the plugless A. albimanus. Sexual transfer of 20E was determined in five species for which we could reliably isolate mating events and dissect females shortly after copulation (Fig. 2B). All species transferred approximately half of the 20E content from their MAGs, with the exception of A. arabiensis, which invested the full 20E complement, suggesting a possible divergence of mating strategy in this important vector. This observed variation in hormone sexual transfer led us to assess the role of 20Ein inducing postmating responses in other anophelines. Similar to results previously obtained in A. gambiae (6), 20E injections in virgin A. arabiensis females, a species with high levels of male-transferred 20E, triggered both oviposition and refractoriness to mating (Fig. 2, C and D). Conversely, these postmating responses were not induced in A. albimanus, as expected given the lack of 20E transfer by males (Fig. 2, C and D). Consistent with these findings, a mating-induced increase in egg development was observed in A. arabiensis and A. stephensi but not in A. albimanus (fig. S1).
Fig. 2. Synthesis and transfer of 20-hydroxyecdysone (20E) and its effect on oviposition and refractoriness to mating.
(A) 20E levels in male accessory glands (MAGs) differ significantly across the nine anophelines [analysis of variance (ANOVA) F8,73 = 227.8, P < 0.0001]. Species are color-coded according to 20E levels (high, red; intermediate, blue; negligible, green). (B) 20E levels in the female atrium from five selected species dissected 1 hour postmating. A. gambiae and A. arabiensis receive significantly higher levels of 20E from males compared with the other species analyzed (ANOVA F4,32 = 80.52, P < 0.0001). In both (A) and (B), results are presented as mean values ± SEM, and letters indicate post hoc significance (Tukey's post hoc test). (C and D) 20E injections in virgin females induce oviposition after blood feeding (C) and refractoriness to mating (D) in a species that sexually transfers 20E (A. arabiensis, P < 0.0001), but not in 20E-less A. albimanus (P = ns) when compared with ethanol-injected controls (Fisher's exact test). A. gambiae results are taken from previously published data (6). Both oviposition and refractoriness data are presented as percentage relative to control females.
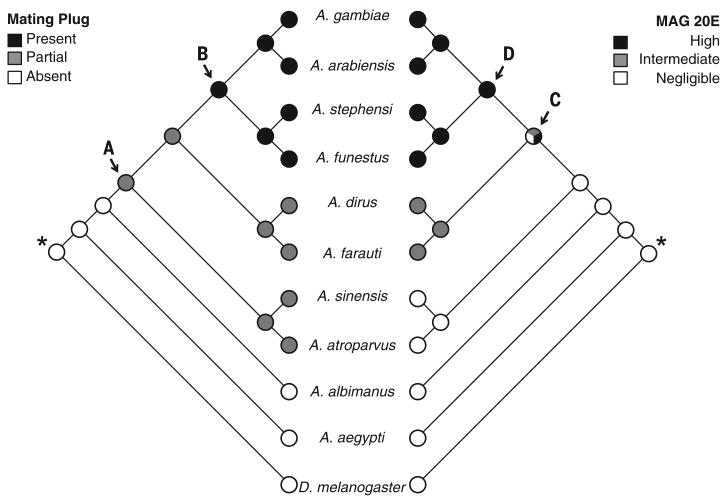
To determine whether mating plug transfer and 20E synthesis in the MAGs are ancestral or derived traits within the anophelines, we next performed ancestral state reconstructions using maximum parsimony. As outgroups, we selected two related dipterans, D. melanogaster and Aedes aegypti, for which sufficient genomic information is available to build our phylogeny (fig. S2). Phylogenetic hypotheses about species relatedness were based on maximum likelihood analysis of 4829 single-copy orthologs. Parsimony reconstructions indicated that the most recent common ancestor of the species included in our phylogeny was likely one in which males were incapable of coagulating seminal secretions and did not synthesize the steroid hormone 20E in their MAGs (Fig. 3). From this ancestor, the ability to form a plug did not evolve until after the A. albimanus lineage split from the remainder of the Anopheles (node A). Our analysis indicates that plug formation began as a partially coagulated structure, with subsequent elaboration into a fully coagulated plug occurring at node B, the common ancestor of A. gambiae, A. arabiensis, A. stephensi, and A. funestus. The production of 20E was not likely to start until node C (where all states are equally parsimonious), suggesting that 20E synthesis in the MAGs evolved shortly after plug coagulation. High titers of 20E occurred at node D, representing the same common ancestor in which full plug coagulation arose. No reversions to the plugless and 20E-less states have occurred in these extant species. The remarkable congruence in ancestral state reconstructions of plug coagulation and 20E levels was confirmed with character correlation and independent contrast analysis, which indicates strong support for a model of correlated evolution between these two characters (independent contrasts, r = 0.86, F1,10 = 26.65, P = 0.0006).
Fig. 3. Maximum parsimony multistate ancestral state reconstructions of mating plug and 20-hydroxyecdysone (20E) characters.
Mating plug phenotypes (left) and 20E titers (right) from nine anopheline species and two related dipteran species (A. aegypti and D. melanogaster) were each coded as multistate categorical characters. Reconstructions reveal that the most common ancestor to all species (indicated by *) was one that lacked a mating plug and did not produce 20E in their male accessory glands (MAGs). Labeled nodes (A to D) are discussed in the text.
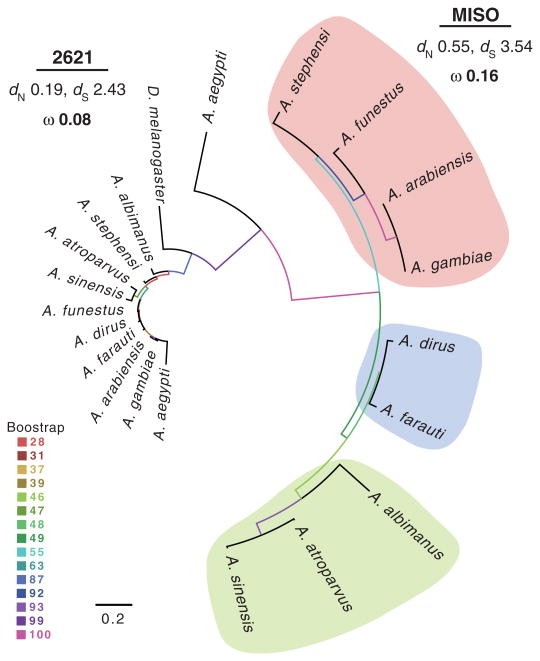
Given our finding of correlated evolution between male ejaculate characters and the observed cross-species increase in egg development as a consequence of 20E transfer, females are predicted to exhibit a pattern of parallel (or reciprocal) evolution of the reproductive traits that translate male 20E into such a fitness benefit (17–19). To explore this prediction, we performed an evolutionary analysis of the female reproductive protein Mating-Induced Stimulator of Oogenesis (MISO), which to date is the only fitness-increasing female factor known to interact with sexually transferred 20E (5). MISO in A. gambiae is strongly induced by 20E specifically in the atrium (uterus) (5). Upon induction, the interaction of MISO with 20E leads to an increase in the expression of vitellogenic lipid transporters after blood feeding, causing a boost in oogenesis by a mechanism also known to promote Plasmodium development (5, 8). We created a maximum likelihood protein phylogeny using the orthologs of MISO (AGAP002620) and its adjacent paralog (AGAP002621) in the anopheline species and the Aedes and Drosophila outgroups studied above. Although the paralog AGAP002621 was considerably conserved, MISO was highly divergent and evolved more rapidly than AGAP002621 [phylogenetic analysis by maximum likelihood (PAML) analysis: ratio of the number of nonsynonymous to synonymous substitutions (dN/dS)MISO, 0.16; dN/dSAGAP002621, 0.08] (Fig. 4). Interestingly, among the species producing high titers of 20E, A. funestus showed a greater degree of sequence similarity to A. gambiae than to A. stephensi (Fig. 4), more consistent with 20E levels (Fig. 2A) than with phylogenetic distance across these species (fig. S2). Moreover, A. albimanus, which diverged some 100 million years ago from the rest of the anophelines (16) (fig. S2), surprisingly grouped with A. sinensis and A. atroparvus (Fig. 4), the other two species with negligible 20E levels (Fig. 2A). Taken together, these data indicate that MISO may be the product of gene duplication from its ancestral paralog and thus subject to relaxed evolutionary constraints promoting acquisition of novel functions (20), potentially driven by divergence in male 20E levels. Additional elements of female physiology are likely to have evolved in response to these substantial changes in ejaculate characteristics. As an example, A. gambiae females synthesize significantly lower levels of 20E after blood feeding compared with A. albimanus (7,21), indicating a possible adaptation of female 20E to levels transferred by the male. The observed effects of the male 20E-MISO interaction in regulating egg development suggest that the evolution of sexually transferred 20E will have influenced other blood-feeding-induced processes, with possible consequences for parasite transmission. Notably, a role for ecdysone in mediating protozoan parasite development has been reported in a number of insect species [reviewed in (22)], including other vectors of human disease (23).
Fig. 4. Phylogenetic analysis of MISO and AGAP002621.
A maximum likelihood phylogeny of protein sequences from nine anophelines and two outgroup dipteran species (D. melanogaster and A. aegypti) using randomized axelerated maximum likelihood (RaxML). Across the anophelines, high sequence conservation (shorter branch lengths) is seen in the MISO paralog AGAP002621 (2621), whereas greater divergence, reflected in a higher dN and ω ratio, is exhibited by MISO. Colors are coded according to 20E levels in Fig. 2A.
Our phylogenetic approaches combined with phenotypic analyses of multiple reproductive traits provide considerable insight into a group of important disease vectors. Multiple key entomological parameters that directly affect malaria transmission are influenced by the diverse functions of sexually transferred 20E: mosquito densities via MISO-mediated increased oogenesis (5); parasite development through the expression of lipid transporters that protect Plasmodium from the mosquito immune system (8); and longevity due to reduced mating-associated fitness costs (9–11). Consequently, divergent sexual transfer of 20E across anophelines may have shaped their ability to transmit this deadly disease, and, intriguingly, all four species that transfer large levels of 20E are major malaria vectors originating from Africa and India, the regions of highest malaria burden (1). By demonstrating correlated evolution in male ejaculate characters and parallel changes in female physiology implicated in vectorial capacity, we reveal coevolutionary dynamics likely to have fundamentally influenced disease transmission to humans.
Supplementary Material
Acknowledgments
We thank E. Lund and D. Clarke for help with mosquito rearing and insectary procedures and M. Bernardi for assistance with artwork. We are grateful to D. Neafsey and N. Besansky for numerous helpful discussions and to S. Lewis, D. Neafsey, M. Mota, and members of the Catteruccia laboratory for careful reading of the manuscript. This work was sponsored in part by the following grants awarded to F.C.: a European Research Council FP7 ERC Starting Grant (grant Anorep, ID: 260897), a William F. Milton Fund grant (Harvard Medical School 2013), and an NIH grant (grant ID: NIH 1R01AI104956-01A1). S.N.M., E.G.K., A.S., and F.C. designed the experiments. P.I.H. provided experimental material, and S.N.M., E.G.K, and P.I.H. performed the experiments. S.N.M., E.G.K, A.S., and R.M.W. analyzed the data. S.N.M., E.G.K., A.S., and F.C. wrote the manuscript. S.N.M., E.G.K., and A.S. contributed equally to this study. All gene sequences are freely available via given gene identifiers from VectorBase (www.vectorbase.org). The single-copy ortholog sequences used to produce the species phylogeny are available via OrthoDB (http://cegg.unige.ch/orthodbmoz2). Protein sequence alignments employed for the species and MISO-AGAP002621 phylogenies are available via DRYAD: doi:10.5061/dryad.6f576.
Footnotes
References and Notes
- 1.World Health Organization. World Malaria Report 2014. WHO; Geneva: 2014. [Google Scholar]
- 2.MacDonald G. Bull World Health Organ. 1956;15:613–626. [PMC free article] [PubMed] [Google Scholar]
- 3.Yuval B. Annu Rev Entomol. 2006;51:413–440. doi: 10.1146/annurev.ento.51.110104.151058. [DOI] [PubMed] [Google Scholar]
- 4.Pondeville E, Maria A, Jacques JC, Bourgouin C, Dauphin-Villemant C. Proc Natl Acad Sci U S A. 2008;105:19631–19636. doi: 10.1073/pnas.0809264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldini F, et al. PLOS Biol. 2013;11:e1001695. doi: 10.1371/journal.pbio.1001695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabrieli P, et al. Proc Natl Acad Sci U S A. 2014;111:16353–16358. doi: 10.1073/pnas.1410488111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai H, Gelman DB, Palli SR. Pest Manag Sci. 2010;66:936–943. doi: 10.1002/ps.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rono MK, Whitten MM, Oulad-Abdelghani M, Levashina EA, Marois E. PLOS Biol. 2010;8:e1000434. doi: 10.1371/journal.pbio.1000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman T, Liddle LF, Kalb JM, Wolfner MF, Partridge L. Nature. 1995;373:241–244. doi: 10.1038/373241a0. [DOI] [PubMed] [Google Scholar]
- 10.Wigby S, Chapman T. Curr Biol. 2005;15:316–321. doi: 10.1016/j.cub.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 11.Dao A, et al. J Med Entomol. 2010;47:769–777. doi: 10.1603/me10052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bownes M, Dubendorfer A, Smith T. J Insect Physiol. 1984;30:823–830. [Google Scholar]
- 13.Perry JC, Sirot L, Wigby S. Trends Ecol Evol. 2013;28:414–422. doi: 10.1016/j.tree.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis SM, South A. Adv Stud Behav. 2012;44:53–97. [Google Scholar]
- 15.Lawniczak MK, et al. Trends Ecol Evol. 2007;22:48–55. doi: 10.1016/j.tree.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Neafsey DE, et al. Science. 2015;347:1258522. doi: 10.1126/science.1258522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alonzo SH, Pizzari T. Am Nat. 2010;175:174–185. doi: 10.1086/649596. [DOI] [PubMed] [Google Scholar]
- 18.Alonzo SH, Pizzari T. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120044. doi: 10.1098/rstb.2012.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.West SA, Griffin AS, Gardner A. Curr Biol. 2007;17:R661–R672. doi: 10.1016/j.cub.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Walsh B. Genetica. 2003;118:279–294. [PubMed] [Google Scholar]
- 21.Lu YH, Hagedorn HH. Int J Inver Reprod Develop. 1986;9:79–94. [Google Scholar]
- 22.Lawrence PO. In Vitro Cell Dev Biol. 1991;27:487–496. doi: 10.1007/BF02631150. [DOI] [PubMed] [Google Scholar]
- 23.Cortez MR, et al. Exp Parasitol. 2012;131:363–371. doi: 10.1016/j.exppara.2012.05.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.