Abstract
Glycosylation-enabling genes are thought to comprise approximately 1–2 % of the human genome, thus, it is not surprising that more than 100 genetic disorders have been identified in this complex multi-pathway cellular process. Recent advances in next generation sequencing technology (NGS) have led to the discovery of genetic causes of many new disorders and importantly highlighted the broad phenotypes that occur. Here we will focus on two glycosylation pathways that involve lipids; glycosylphosphatidylinositol (GPI) anchors and glycosphingolipids (GSL) with emphasis on the specific gene defects, their biochemical properties, and their expanding clinical spectra. These disorders involve the intersection of two pathways: lipids and carbohydrates. Studies of both pathways were founded on structural biochemistry. Those methods and their more refined and sensitive descendants can both identify the specific genes that cause the disorders and validate the importance of the specific mutations.
Overview of glycosylphosphatidylinositol (GPI) anchor related disorders
Attention for human glycosylation disorders has grown considerably in the last several years. A new glycosylation disorder was reported, on average, every 17 days in 2013, in large part due to extensive sequencing of patient exomes and genomes (Freeze et al. 2014). Most of this attention focused on defects in the N-glycosylation pathway and those that cause congenital muscular dystrophies by impairing the synthesis of O-mannose glycans found on α-dystroglycan. Disorders also occur in the synthesis of two lipid-based pathways, the glycosylphosphatidylinositol (GPI) anchors and glycosphinolipids (GSL). In this review we will focus on these groups of disorders, point out phenotypes and biochemical analysis that can help identify gene candidates and confirm the pathogenicity of mutations.
Glycosylphosphatidylinositol (GPI) anchoring of proteins is a highly conserved process that all eukaryotes require, and for this review, we will focus on the mammalian systems.
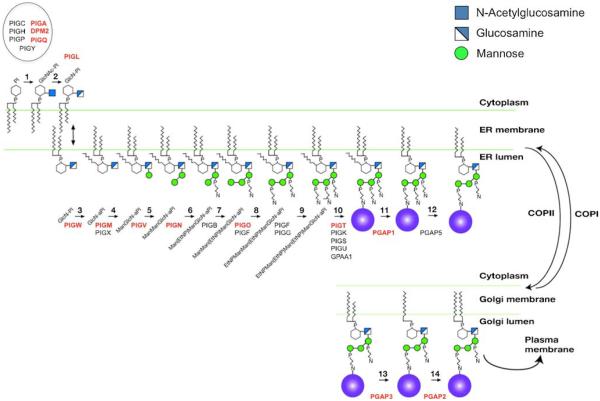
The GPI pathway can be simplified into three stages (Fig. 1). The first is synthesis of GPI precursor molecules in the ER membrane. Its synthesis begins on the cytoplasmic side of ER and requires at least seven proteins (PIGA, PIGC, PIGH, PIGP, PIGQ, PIGY, DPM2) that initiate transferofan α-N-Acetylglucosamine (GlcNAc) from UDP-GlcNAc to the phosphatidylinositol (PI) lipid (Ferguson et al. 2009). Once this GlcNAc is added, it undergoes an unusual step of de-N-acetylation by PIGL to form glucosamine-PI (GlcN-PI). This allows it to be flipped to the luminal side of the ER (Watanabe et al. 1999). Once it is correctly positioned within the ER lumen, an acyl chain is added to the inositol ring (via PIGW) to form GlcN-aPI (Murakami et al. 2003). The first of three mannose moieties is extended on to the GlcN by PIGM and PIGX (Maeda et al. 2001; Ashida et al. 2005). PIGV adds the second mannose, which is followed by the transfer of the first ethanolamine-phosphate by PIGN (Kang et al. 2005; Hong et al. 1999). The final mannose is added by PIGB, then PIGO, PIGF, and PIGG catalyze the addition of the second and third ethanolamine phosphates (Takahashi et al. 1996; Hong et al. 2000).
Fig. 1.
GPI anchor pathway in mammals. Schematic showing the mammalian GPI anchor pathway and the proteins involved for each step. Steps that involve genetic disorders are highlighted in Red. (Permission to use this figure was granted by The American Journal of Human Genetics)
The second stage involves cleavage of a carboxy—terminal GPI—signal peptide and simultaneous transfer of this entire precursor GPI to newly synthesized proteins, both occurring in the lumen of the ER (Fujita and Kinoshita 2012).
A multi-protein transamidase complex composed of PIGT, PIGK, PIGS, PIGU, and GPAA1 carries out the recognition of the appropriate peptide, transfer of the GPI anchor onto the protein, and cleavage of the C-terminal GPI-target consensus sequence (Ohishi et al. 2001).
In the third and final stage, the protein bound GPI anchor undergoes further modification. First, the acyl chain is removed by PGAP1 followed by the PGAP5-dependent removal of the ethanolamine-phosphate side chain from the second mannose (Tanaka et al. 2004; Fujita et al. 2009). These modifications allow for GPI-anchored protein sorting at ER exit sites. Within the Golgi, PGAP3 removes an unsaturated fatty acid at the sn-2 position, and subsequently PGAP2 facilitates its substitution with a saturated fatty acid (Maeda et al. 2007). This exchange is believed to be essential for incorporation of GPI-modified proteins into lipid rafts. Once a functional GPI anchor is attached to a protein, it can regulate several processes including GPI-AP sorting, trafficking, and function.
Many of the protein components required for GPI-AP synthesis and maturation were well known for many years based on work using mutant Chinese hamster ovary (CHO) cell lines (Maeda et al. 2006). However, it has only been within the last few years that the majority of human disorders have been identified thanks to advances in NGS.
Prior to this the only deficiency within GPI biosynthetic pathway was caused by somatic mutations in PIGA. This resulted in a rare hematological disorder called paroxysmal nocturnal haemoglobinuria (PNH) in which red blood cells break down earlier than normal due to defective self-protection against complement (Takeda et al. 1993).
Since then, several genetic disorders, mostly autosomal recessive, have been identified in at least 12 genes (Table 1), mostly through genetic mapping or whole exome sequencing (Freeze et al. 2014). Clinical phenotypes are often variable and in some instances multiple phenotypes can be seen within the same mutated gene (Table 1).
Table 1.
List of human genetic disorders involving the glycosylphosphatidylinositol (GPI) anchor and glycosphingolipids (GSL) pathways
| Disorder | Gene | Function | Disorder Omim |
Gene Omim |
Main clinical features |
|---|---|---|---|---|---|
| Glycosylphosphatidylinositol | disorders | ||||
| X-linked GPI-anchor deficiency |
PIGA | GlcNAc-PI synthesis protein | 300868 | 311770 | Dysmorphism, Hy, Sz, variable CNS, cardiac, urinary systems, early death |
| Paroxysmal nocturnal hemoglobinuria (Somatic mutation) |
PIGA | GlcNAc-PI synthesis protein | 300818 | 311770 | Complement-mediated hemolysis |
| CHIME syndrome | PIGL | GlcNAc-PI de-N acetylase | 280000 | 605947 | ID, colobomas, heart defect, early-onset ichthyosiform dermatosis, ear anomalies(conductive hearing loss) |
| Autosomal recessive GPI-anchor deficiency |
PIGM | First α-mannosyltransferase in GPI biosynthesis |
610293 | 610273 | Sz, portal vein thrombosis, portal hypertension |
| Autosomal recessive GPI-anchor deficiency |
PIGN | GPI ethanolamine phosphate transferase |
614080 | 606097 | Severe neurologic impairment, Sz, lack of development, multiple congenital anomalies, early death |
| Hyperphosphatasia mental retardation syndrome |
PIGO | GPI ethanolamine phosphate transferase |
614749 | 614730 | Hyperphosphatasia with mental retardation syndrome 2 (HPMRS) |
| Autosomal recessive GPI-anchor deficiency |
PIGQ | GlcNAc-PI synthesis protein | 605754 | Severe DD, SZ, early death | |
| Autosomal recessive GPI-anchor deficiency |
PIGT | GPI transamidase complex | 615398 | 615399 | ID, Hy, Sz, abnormal skelet al., endocrine, ophthalmologic abnormalities and hypophosphatasia |
| Hyperphosphatasia mental retardation syndrome |
PIGV | Second α-Mannosyltransferase in GPI biosynthesis |
239300 | 610274 | Hyperphosphatasia with mental retardation syndrome 1 (HPMRS) |
| West syndrome and hyperphosphatasia with mental retardation syndrome |
PIGW | Acylates the inositol ring of phosphatidylinositol in GPI-anchor biosynthesis |
610272 | 610275 | West syndrome, hyperphosphatasia with mental retardation syndrome |
| Autosomal recessive GPI-anchor deficiency |
PGAP1 | Lipid remodeling steps of GPI-anchor maturation |
615802 | 611655 | ID with encephalopathy |
| Hyperphosphatasia mental retardation syndrome |
PGAP2 | Lipid remodeling steps of GPI-anchor maturation |
614207 | 615187 | Hyperphosphatasia with mental retardation syndrome 3 (HPMRS) |
| Hyperphosphatasia mental retardation syndrome |
PGAP3 | Lipid remodeling steps of GPI-anchor maturation |
615716 | 611801 | Hyperphosphatasia with mental retardation syndrome 4 (HPMRS) |
| Glycosphingolipid disorders | |||||
| Amish infantile epilepsy Glc-Cer synthase (GM3) |
ST3GAL5 | Sia2,3 Galβ1,4 | 609056 | 604402 | Infantile-onset epilepsy, developmental stagnation, blindness |
| Salt and pepper syndrome Glc-Cer synthase (GM3) |
ST3GAL5 | Sia2,3 Galβ1,4 | 604402 | Severe I.D, epilepsy, scoliosis, altered dermal pigmentation, choreoathetosis, dysmorphic features |
|
| Complex hereditary spastic paraplegia |
B4GALNT1 | β-1,4 GalNAc Transferase 1 | 609195 | 601873 | Early-onset spastic paraplegia, I.D, cerebellar ataxia, and peripheral neuropathy, cortical atrophy and white matter hyperintensity |
| Non-syndromic I.D. | |||||
| West syndrome | ST3GAL3 | N-Acetyllactosaminide α-2,3 Sialyltransferase |
611090 615006 |
606494 | NSID (Non-syndromic intellectual disability), Infantile spasms, hypsarrhythmia |
ID -Intellectual disability, Sz – Seizures, Hy – Hypotonia, M – Microcephaly, DD -Developmental delay, NSID -Non-syndromic intellectual disability
For example, mutations in PIGA not only cause PNH, but can also cause at least four other phenotypically distinct disorders (1) X-linked syndrome associated with neurodegeneration, cutaneous abnormalities, and systemic iron overload, (2) multiple congenital anomalies-hypotonia-seizures syndrome 2, (3) a severe syndromic form of X-linked intellectual disability, and (4) early-onset epileptic encephalopathies (EOEEs) (Swoboda et al. 2014; Johnston et al. 2012; Belet et al. 2014; Kato et al. 2014). In addition, PIGT can also cause both a recessive intellectual disability syndrome as well as a form of spontaneous PNH (Kvarnung et al. 2013; Krawitz et al. 2013a, b). Why mutations in the same gene cause vastly different disorders is unclear, however it may be due to the severity of the mutation, and/or how that mutation affects interactions between other GPI biosynthetic proteins.
The second step of the GPI-anchor pathway involves PIGL catalyzing de-N-acetylation of GlcNAc-PI -> GlcN-PI and mutations in PIGL cause CHIME syndrome that is characterized by intellectual disability, colobomas, heart defect, early-onset ichthyosiform dermatosis, and ear anomalies (conductive hearing loss) (Ng et al. 2012). PIGN adds an ethanolamine phosphate to the first mannose on the GPI anchor. Two independent reports involving nine cases show that mutations in PIGN cause multiple congenital anomalies — hypotonia seizures syndrome in which six of the nine individuals died (Ohba et al. 2014; Maydan et al. 2011). PIGQ is involved in the early steps of synthesis and transfer of GlcNAc to phosphatidylinositol and mutations were identified in a clinical exome sequencing study of individuals with Ohtahara syndrome severe early onset epilepsy (Martin et al. 2014). More recently individuals harboring mutations in PGAP1, a deacylase that removes an acyl-chain from the inositol of GPI anchors, were identified to cause encephalopathy and non-specific autosomal recessive intellectual disability (Murakami et al. 2014). Mutations in PIGO, PIGV, PIGW, PGAP2, PGAP3 can all cause hyperphosphatasia mental retardation syndrome (HMRS) (Krawitz et al. 2012; Krawitz et al. 2010; Chiyonobu et al. 2014; Hansen et al. 2013; Krawitz et al. 2013a, 2013b; Howard et al. 2014).
It is unclear why some forms of GPI-AP deficiencies result in hyperphosphatasia while others do not. One study suggests that a functional GPI transamidase recognizes the incomplete GPI on cell surface expressed alkaline phosphatase (ALP) and cleaves the C-terminal GPI attachment signal peptide allowing for secretion of soluble ALP. This only occurs when the incomplete GPI contains mannose, other mutants that have shorter GPI lacking mannose had ALP that was efficiently degraded (Murakami et al. 2012).
One treatable form of GPI-AP deficiency involves a deficiency in PIGM, which encodes the first α -Mannosyltransferase in GPI biosynthesis. The mutation causes, portal vein thrombosis, portal hypertension with epilepsy. Only a single family has been reported with mutations in PIGM, however the mutation results in hypoacetylation of the PIGM promoter that disrupts binding of the transcription factor Sp1 to its consensus promoter motif (Almeida et al. 2006). More importantly, treatment with the histone deacetylase inhibitor butyrate caused complete remission of the intractable seizures (Almeida et al. 2007).
Two groups reported that the seizure activity seen in GPI-AP deficiencies could be effectively treated by pyridoxine (Thompson et al. 2006; Kuki et al. 2013).
One limitation in the identification of cases related to GPI deficiencies is the lack of facile and clear biomarkers. This is partly supported by the fact that nearly all the deficiencies have been identified or solved by NGS and not by screening biomarkers. However, screening can be performed using several GPI-AP such as CD59, CD24, CD16, and a non-lysing, mutated form of proaerolysin (FLAER) (Yanget et al. 2013). Yet these do not always show clear difference and cell type specificity can be an issue since not all markers are expressed across all cell types. Granulocytes appear to be the most sensitive cell type for detecting GPI related disorders (Brodsky et al. 2000). To be confident in a diagnosis of a GPI-AP deficiency, it is best to test several markers (i.e., CD16, CD24, CD55, CD59, and FLEAR) on a few different cell types (granulocytes and erythrocytes) since markers are differentially expressed on cells. However, the impact of specific mutations in the pathway can be assessed by using one of the many available step-specific mutant CHO lines (Maeda et al. 2006).
Given the variable clinical characteristics of GPI-AP deficiencies, testing every suspected individual could be difficult. However, FACS analysis is commonly used in clinics for other genetic disorders and it seems worthwhile to test for GPI-AP deficiencies. Especially in those individuals presenting with intellectual disability in combination with hyperphosphatasia or even unexplained neurological problems including epilepsy.
Overview of glycosphingolipids (GSL) related disorders
Glycosphingolipids are highly conserved types of glycolipid molecules and display a wide range of structural complexity. Here we will focus solely on the mammalian glycosphingolipid system.
Glycosphingolipids are composed of a ceramide motif together with a long chain alcohol called sphingosine in an amide linkage to a fatty acid (Schnaar et al. 2009). Variations in the ceramide molecule such as length and chemical modification can have dramatic influence on how subsequently added glycans are presented in the membrane, but it also creates great diversity and complexity across GSL species since differences occur in both the ceramide moiety and the glycan itself.
Traditionally, classification of the glycosphingolipid is based more on the glycan composition and not the particular ceramide.
For most GSL, biosynthesis begins on the cytoplasmic side of the ER where ceramide is formed, but it is quickly equilibrated and trafficked to the Golgi. In the case of glucosylceramide (GlcCer), a glucose molecule is attached to ceramide on the cytoplasmic side of the ER, and then traffics to the early Golgi where it is flipped into the lumen for further extension by various glycosyltransferases (Schnaar et al. 2009).
However, galactoceramide (GalCer), which is the most abundantly expressed GSL in the brain, takes a different path. It is synthesized in the luminal portion of the ER and is then trafficked to the Golgi where it may or may not undergo sulfation to form sulfatide (Schnaar et al. 2009).
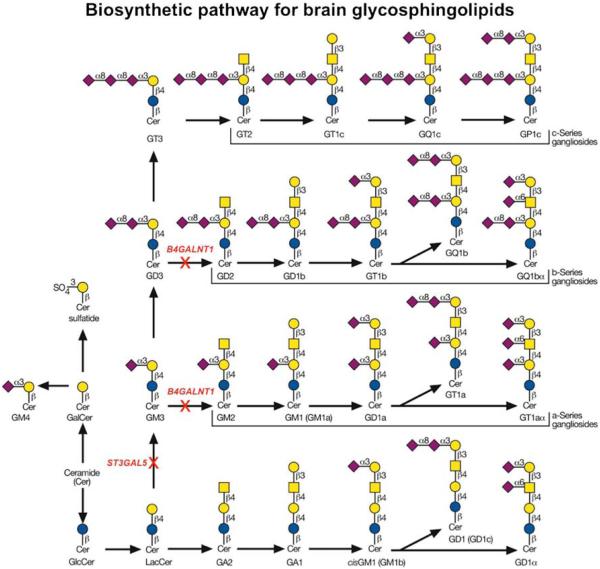
These are just two examples of simple yet abundant GSL; more complex GSL involve glycan extension and branching that requires various glycosyltransferases and sugar nucleotide donors. Those GSL that eventually undergo sialyation are called “gangliosides” (Fig. 2).
Fig. 2.
GSL biosynthetic pathway for brain glycosphingolipids Sche-in red. Permission was granted to use a modified version of figure matic showing the major pathway for biosynthesis of brain 10.2 from chapter 10 Essentials of Glycobiology, second ed., CSHLP, glycosphingolipids. Steps that involve genetic disorders are highlighted 2009
Thus far, only three human genetic disorders involving GSL have been identified, and all involve ganglioside biosynthesis (Table 1). It is very likely that more disorders will be identified via next generation sequencing, as seen with GPI-synthesis.
The first disorder was identified utilizing linkage analysis of a large old order Amish family where affected individuals presented with infantile-onset symptomatic epilepsy syndrome associated with developmental stagnation and blindness (Simpson et al. 2004). Sequencing genes within the candidate interval identified a non-sense mutation in ST3GAL5 (also known as GM3 synthase or SIAT9.) ST3GAL5 encodes a sialyltransferase which functions in the initial step in the biosynthesis of the simplest ganglioside (GM3) using the precursor intermediate, lactosylceramide. Importantly, the authors show that serum samples from affected individuals had a complete lack of GM3 ganglioside and increased lactosylceramide, and its alternate derivatives (Simpson et al. 2004) (Fig. 2).
More recently, Boccuto et al. identified another disorder involving mutations in ST3GAL5 termed “salt and pepper syndrome” that is characterized by altered dermal pigmentation with severe intellectual disability, epilepsy, scoliosis, choreoathetosis and dysmorphic facial features. As in the original report from Simpson et al., glycolipid analysis confirmed a complete lack of GM3 ganglioside in patient fibroblasts (Boccuto et al. 2014).
The second disorder involves mutations in B4GALNT1 (also known as GM2/GD2 synthase) and causes a form of hereditary spastic paraplegia, specifically HSP subtype 26 (Harlalka et al. 2013; Boukhris et al. 2013; Wakil et al. 2013). Analysis of ten families with 30 affected individuals showed mild to moderate cognitive impairment and developmental delay with an early-onset progressive spasticity due to axonal degeneration. These patients were usually ambulatory, but some were wheelchair bound in later life.
B4GALNT1 transfers a GalNAc molecule onto GM3 and GD3 precursors in the biosynthesis of GM2 and GD2 glycosphingolipids (Fig. 2). Biochemical characterization of patient samples confirmed the lack of both GM2 and GD2 products with an expected accumulation of the precursors GM3 and GD3.
ST3GAL3 is responsible for synthesis of more complex gangliosides and may be involved in higher cognitive function since four sets of consanguineous parents with 12 affected patients all showed IQ <40 that tracked to a loss of enzyme activity or mislocalization of the enzyme. In a separate study, patients with West syndrome showing infantile seizure disorder with developmental delay were also found to have mutations in ST3GAL3. The encoded enzyme can modify both gangliosides along with N-and O-glycans, so it is possible that these patients have variable phenotypes based on effects on different substrates (Schnaar et al. 2014; Edvardson et al. 2013; Hu et al. 2011).
It is not surprising that these GSL disorders present with neurologically-related phenotypes given that these gangliosides are abundantly expressed in the central nervous system. In fact 80 % of all brain glycans are found in GSL (Tettamanti et al. 1973) and many of these are complex gangliosides. Given the large number of genes required to produce these complex GSL structures, it seems a near certainty that genetic disorders in other steps of GSL biosynthesis will be identified.
There is no easy marker that has been developed for GSL deficiencies. It is possible to detect GSL in the blood, but this is not a routine procedure. If more defects are identified in this pathway, it might be a fruitful area to explore for diagnostic confirmation and as a biomarker to monitor future therapeutics.
To date more than 100 glycosylation related disorders, covering eight distinct pathways, have been identified (Freeze et al. 2014). Many solved via painstaking biochemical analysis of patient fibroblast and before the age of next generation sequencing. However, the era of high throughput NGS sequencing is here to stay as witnessed by the treasure trove of newly solved genetic disorders, but ultimately it will take a combination of NGS and detailed biochemical characterization of these genes to truly understand how mutations in those genes cause disease.
Footnotes
Presented at the workshop “Diagnostic Approach, and Classification of IEM Affecting the Synthesis and Catabolism of Complex Lipids” in Paris, France, June 14-15, 2013.
Compliance with ethics guidelines
Informed consent: This article does not contain any studies with human or animal subjects performed by the any of the authors.
Conflict of interest: None.
References
- Almeida AM, Murakami Y, Layton DM, et al. Hypomorphic promoter mutation in PIGM causes inherited glycosylphosphatidylinositol deficiency. Nat Med. 2006;12(7):846–851. doi: 10.1038/nm1410. [DOI] [PubMed] [Google Scholar]
- Almeida AM, Murakami Y, Baker A, et al. Targeted therapy for inherited GPI deficiency. N Engl J Med. 2007;356(16):1641–1647. doi: 10.1056/NEJMoa063369. [DOI] [PubMed] [Google Scholar]
- Ashida H, Hong Y, Murakami Y, et al. Mammalian PIG-X and yeast Pbn1p are the essential components of glycosylphosphatidylinositol-mannosyltransferase I. Mol Biol Cell. 2005;16(3):1439–1448. doi: 10.1091/mbc.E04-09-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belet S, Fieremans N, Yuan X, et al. Early frameshift mutation in PIGA identified in a large XLID family without neonatal lethality. Hum Mutat. 2014;35(3):350–355. doi: 10.1002/humu.22498. [DOI] [PubMed] [Google Scholar]
- Boccuto L, Aoki K, Flanagan-Steet H, et al. A mutation in a ganglioside biosynthetic enzyme, ST3GAL5, results in salt & pepper syndrome, a neurocutaneous disorder with altered glycolipid and glycoprotein glycosylation. Hum Mol Genet. 2014;23(2):418–433. doi: 10.1093/hmg/ddt434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukhris A, Schule R, Loureiro JL, et al. Alteration of ganglioside biosynthesis responsible for complex hereditary spastic paraplegia. Am J Hum Genet. 2013;93(1):118–123. doi: 10.1016/j.ajhg.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky RA, Mukhina GL, Li S, et al. Improved detection and characterization of paroxysmal nocturnal hemoglobinuria using fluorescent aerolysin. Am J Clin Pathol. 2000;114(3):459–466. doi: 10.1093/ajcp/114.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiyonobu T, Inoue N, Morimoto M, Kinoshita T, Murakami Y. Glycosylphosphatidylinositol (GPI) anchor deficiency caused by mutations in PIGW is associated with West syndrome and hyperphosphatasia with mental retardation syndrome. J Med Genet. 2014;51(3):203–207. doi: 10.1136/jmedgenet-2013-102156. [DOI] [PubMed] [Google Scholar]
- Edvardson S, Baumann AM, Muhlenhoff M, et al. West syndrome caused by ST3Gal-III deficiency. Epilepsia. 2013;54(2):e24–e27. doi: 10.1111/epi.12050. [DOI] [PubMed] [Google Scholar]
- Ferguson MAJ, Kinoshita T, Hart GW. Glycosylphosphatidylinositol Anchors. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of glycobiology. Cold Spring Harbor; New York: 2009. [PubMed] [Google Scholar]
- Freeze HH, Chong JX, Bamshad MJ, Ng BG. Solving glycosylation disorders: fundamental approaches reveal complicated pathways. Am J Hum Genet. 2014;94(2):161–175. doi: 10.1016/j.ajhg.2013.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Kinoshita T. GPI-anchor remodeling: potential functions of GPI-anchors in intracellular trafficking and membrane dynamics. Biochim Biophys Acta. 2012;1821(8):1050–1058. doi: 10.1016/j.bbalip.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Fujita M, Maeda Y, Ra M, Yamaguchi Y, Taguchi R, Kinoshita T. GPI glycan remodeling by PGAP5 regulates transport of GPI-anchored proteins from the ER to the Golgi. Cell. 2009;139(2):352–365. doi: 10.1016/j.cell.2009.08.040. [DOI] [PubMed] [Google Scholar]
- Hansen L, Tawamie H, Murakami Y, et al. Hypomorphic mutations in PGAP2, encoding a GPI-anchor-remodeling protein, cause autosomal-recessive intellectual disability. Am J Hum Genet. 2013;92(4):575–583. doi: 10.1016/j.ajhg.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, Maeda Y, Watanabe R, et al. Pig-n, a mammalian homologue of yeast Mcd4p, is involved in transferring phosphoethanolamine to the first mannose of the glycosylphosphatidylinositol. J Biol Chem. 1999;274(49):35099–35106. doi: 10.1074/jbc.274.49.35099. [DOI] [PubMed] [Google Scholar]
- Hong Y, Maeda Y, Watanabe R, Inoue N, Ohishi K, Kinoshita T. Requirement of PIG-F and PIG-O for transferring phosphoethanolamine to the third mannose in glycosylphosphatidylinositol. J Biol Chem. 2000;275(27):20911–20919. doi: 10.1074/jbc.M001913200. [DOI] [PubMed] [Google Scholar]
- Howard MF, Murakami Y, Pagnamenta AT, et al. Mutations in PGAP3 impair GPI-anchor maturation, causing a subtype of hyperphosphatasia with mental retardation. Am J Hum Genet. 2014;94(2):278–287. doi: 10.1016/j.ajhg.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Eggers K, Chen W, et al. ST3GAL3 mutations impair the development of higher cognitive functions. Am J Hum Genet. 2011;89(3):407–414. doi: 10.1016/j.ajhg.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JJ, Gropman AL, Sapp JC, et al. The phenotype of a germline mutation in PIGA: the gene somatically mutated in paroxysmal nocturnal hemoglobinuria. Am J Hum Genet. 2012;90(2):295–300. doi: 10.1016/j.ajhg.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JY, Hong Y, Ashida H, et al. PIG-V involved in transferring the second mannose in glycosylphosphatidylinositol. J Biol Chem. 2005;280(10):9489–9497. doi: 10.1074/jbc.M413867200. [DOI] [PubMed] [Google Scholar]
- Kato M, Saitsu H, Murakami Y, et al. PIGA mutations cause early-onset epileptic encephalopathies and distinctive features. Neurology. 2014;82(18):1587–1596. doi: 10.1212/WNL.0000000000000389. [DOI] [PubMed] [Google Scholar]
- Krawitz PM, Schweiger MR, Rodelsperger C, et al. Identity-by-descent filtering of exome sequence data identifies PIGV mutations in hyperphosphatasia mental retardation syndrome. Nat Genet. 2010;42(10):827–829. doi: 10.1038/ng.653. [DOI] [PubMed] [Google Scholar]
- Krawitz PM, Murakami Y, Hecht J, et al. Mutations in PIGO, a member of the GPI-anchor-synthesis pathway, cause hyperphosphatasia with mental retardation. Am J Hum Genet. 2012;91(1):146–151. doi: 10.1016/j.ajhg.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawitz PM, Hochsmann B, Murakami Y, et al. A case of paroxysmal nocturnal hemoglobinuria caused by a germline mutation and a somatic mutation in PIGT. Blood. 2013a;122(7):1312–1315. doi: 10.1182/blood-2013-01-481499. [DOI] [PubMed] [Google Scholar]
- Krawitz PM, Murakami Y, Riess A, et al. PGAP2 mutations, affecting the GPI-anchor-synthesis pathway, cause hyperphosphatasia with mental retardation syndrome. Am J Hum Genet. 2013b;92(4):584–589. doi: 10.1016/j.ajhg.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuki I, Takahashi Y, Okazaki S, et al. Vitamin B6-responsive epilepsy due to inherited GPI deficiency. Neurology. 2013;81(16):1467–1469. doi: 10.1212/WNL.0b013e3182a8411a. [DOI] [PubMed] [Google Scholar]
- Kvarnung M, Nilsson D, Lindstrand A, et al. A novel intellectual disability syndrome caused by GPI anchor deficiency due to homozygous mutations in PIGT. J Med Genet. 2013;50(8):521–528. doi: 10.1136/jmedgenet-2013-101654. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Watanabe R, Harris CL, et al. PIG-M transfers the first mannose to glycosylphosphatidylinositol on the lumenal side of the ER. EMBO J. 2001;20(1–2):250–261. doi: 10.1093/emboj/20.1.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y, Ashida H, Kinoshita T. CHO glycosylation mutants: GPI anchor. Methods Enzymol. 2006;416:182–205. doi: 10.1016/S0076-6879(06)16012-7. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Tashima Y, Houjou T, et al. Fatty acid remodeling of GPI-anchored proteins is required for their raft association. Mol Biol Cell. 2007;18(4):1497–1506. doi: 10.1091/mbc.E06-10-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin HC, Kim GE, Pagnamenta AT, et al. Clinical whole-genome sequencing in severe early-onset epilepsy reveals new genes and improves molecular diagnosis. Hum Mol Genet. 2014 doi: 10.1093/hmg/ddu030. doi:10.1093/hmg/ddu199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maydan G, Noyman I, Har-Zahav A, et al. Multiple congenital anomalies-hypotonia-seizures syndrome is caused by a mutation in PIGN. J Med Genet. 2011;48(6):383–389. doi: 10.1136/jmg.2010.087114. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Siripanyapinyo U, Hong Y, et al. PIG-W is critical for inositol acylation but not for flipping of glycosylphosphatidylinositol-anchor. Mol Biol Cell. 2003;14(10):4285–4295. doi: 10.1091/mbc.E03-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y, Kanzawa N, Saito K, et al. Mechanism for release of alkaline phosphatase caused by glycosylphosphatidylinositol deficiency in patients with hyperphosphatasia mental retardation syndrome. J Biol Chem. 2012;287(9):6318–6825. doi: 10.1074/jbc.M111.331090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y, Tawamie H, Maeda Y, et al. Null mutation in PGAP1 impairing Gpi-anchor maturation in patients with intellectual disability and encephalopathy. PLoS Genet. 2014;10(5):e1004320. doi: 10.1371/journal.pgen.1004320. doi:10.1371/journal.pgen.1004320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng BG, Hackmann K, Jones MA, et al. Mutations in the glycosylphosphatidylinositol gene PIGL cause CHIME syndrome. Am J Hum Genet. 2012;90(4):685–688. doi: 10.1016/j.ajhg.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba C, Okamoto N, Murakami Y, et al. PIGN mutations cause congenital anomalies, developmental delay, hypotonia, epilepsy, and progressive cerebellar atrophy. Neurogenetics. 2014;15(2):85–92. doi: 10.1007/s10048-013-0384-7. [DOI] [PubMed] [Google Scholar]
- Ohishi K, Inoue N, Kinoshita T. PIG-S and PIG-T, essential for GPI anchor attachment to proteins, form a complex with GAA1 and GPI8. EMBO J. 2001;20(15):4088–4098. doi: 10.1093/emboj/20.15.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaar RL, Suzuki A, Stanley P. Glycosphingolipids. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of glycobiology. Cold Spring Harbor; New York: 2009. [PubMed] [Google Scholar]
- Schnaar RL, Gerardy-Schahn R, Hildebrandt H. Sialic acids in the brain: gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol Rev. 2014;94(2):461–518. doi: 10.1152/physrev.00033.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson MA, Cross H, Proukakis C, et al. Infantile-onset symptomatic epilepsy syndrome caused by a homozygous loss-of-function mutation of GM3 synthase. Nat Genet. 2004;36(11):1225–1229. doi: 10.1038/ng1460. [DOI] [PubMed] [Google Scholar]
- Swoboda KJ, Margraf RL, Carey JC, et al. A novel germline PIGA mutation in Ferro-Cerebro-Cutaneous syndrome: a neurodegenerative X-linked epileptic encephalopathy with systemic iron-overload. Am J Med Genet A. 2014;164A(1):17–28. doi: 10.1002/ajmg.a.36189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Inoue N, Ohishi K, et al. PIG-B, a membrane protein of the endoplasmic reticulum with a large lumenal domain, is involved in transferring the third mannose of the GPI anchor. EMBO J. 1996;15(16):4254–4261. [PMC free article] [PubMed] [Google Scholar]
- Takeda J, Miyata T, Kawagoe K, et al. Deficiency of the GPI anchor caused by a somatic mutation of the PIG-A gene in paroxysmal nocturnal hemoglobinuria. Cell. 1993;73(4):703–711. doi: 10.1016/0092-8674(93)90250-t. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Maeda Y, Tashima Y, Kinoshita T. Inositol deacylation of glycosylphosphatidylinositol-anchored proteins is mediated by mammalian PGAP1 and yeast Bst1p. J Biol Chem. 2004;279(14):14256–14263. doi: 10.1074/jbc.M313755200. [DOI] [PubMed] [Google Scholar]
- Tettamanti G, Bonali F, Marchesini S, Zambotti V. A new procedure for the extraction, purification and fractionation of brain gangliosides. Biochim Biophys Acta. 1973;296(1):160–170. doi: 10.1016/0005-2760(73)90055-6. [DOI] [PubMed] [Google Scholar]
- Thompson MD, Killoran A, Percy ME, Nezarati M, Cole DE, Hwang PA. Hyperphosphatasia with neurologic deficit: a pyridoxine-responsive seizure disorder? Pediatr Neurol. 2006;34(4):303–307. doi: 10.1016/j.pediatrneurol.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Wakil SM, Monies DM, Ramzan K, et al. Novel B4GALNT1 mutations in a complicated form of hereditary spastic paraplegia. Clin Genet. 2013 doi: 10.1111/cge.12312. doi:10.1111/cge.12312. [DOI] [PubMed] [Google Scholar]
- Watanabe R, Ohishi K, Maeda Y, Nakamura N, Kinoshita T. Mammalian PIG-L and its yeast homologue Gpi12p are N-acetylglucosaminylphosphatidylinositol de-N-acetylases essential in glycosylphosphatidylinositol biosynthesis. Biochem J. 1999;339:185–192. Pt 1. [PMC free article] [PubMed] [Google Scholar]
- Yang HS, Yang M, Li X, Tugulea S, Dong H. Diagnosis of paroxysmal nocturnal hemoglobinuria in peripheral blood and bone marrow with six-color flow cytometry. Biomark Med. 2013;7(1):99–111. doi: 10.2217/bmm.12.80. [DOI] [PubMed] [Google Scholar]