Abstract
Background
An Ebola Virus Disease (EVD) epidemic of unprecedented magnitude is ongoing in West Africa, affecting for the first time large urban centers like Conakry, the capital of Guinea.
Methods
Interviews of EVD patients, relatives and neighbors and laboratory databases were used to reconstruct EVD chains of transmission in Conakry, from March to August 2014.
Findings
Out of 193 confirmed and probable EVD cases reported in Conakry, Boffa and Télimélé, 152 (79%) were positioned in the chains of transmission. In March, non-Health Care Workers cases infected on average 2.3 (95% CI: 1.6, 3.2) persons, breaking down into 1.4 (95% CI: 0.9, 2.2) persons in the community, 0.4 (95% CI: 0.1, 0.9) in the hospital and 0.5 (95% CI: 0.2, 1.0) at funerals. Following implementation of infection control in April, the reproduction number in the hospital and at funerals reduced below 0.1. In the community, the reproduction number, which was positively correlated with patients viremia, dropped by 50% for hospitalized cases but remained unchanged for those not hospitalized. Hospital and funeral transmission represented 35% (7/20) and 15% (3/20) of all transmissions in March; but only 9% (11/128) and 4% (5/128) from April onward. Overall, 82% (119/145) of transmission occurred in the community and 72% (105/145) between family members. Simulations showed that a 10% increase in hospitalizations could have reduced the length of chains by 26% (95% CI: 4%, 45%).
Interpretation
Monitoring chains of transmission is critical to evaluate and optimize local control strategies for EVD. In Conakry, interventions had the potential to stop the epidemic but reintroductions of the disease and lack of cooperation of a small number of families led to prolonged low-level spread, highlighting challenges of EVD control in large urban centers.
Funding
Labex IBEID, Reacting, PREDEMICS, NIGMS MIDAS initiative, Institut Pasteur de Dakar.
Introduction
An epidemic of Ebola Virus Disease (EVD) of unprecedented magnitude has been ongoing in West Africa for about a year. As of November 26th 2014, 15935 probable, confirmed and suspected cases and 5689 deaths were reported1 with a case fatality ratio estimated to 70% 2. Guinea, Liberia and Sierra Leone are the most affected countries although Nigeria and Senegal also reported cases. This epidemic was declared a public health emergency of international concern3 in August 2014. Outside Africa, the USA and Spain have reported nosocomial transmission2.
Transmission of EVD occurs by direct contact with body fluids of symptomatic cases. Caring of patients at the hospital, family or community levels or touching bodies at funerals are important routes of infection. Since patients become infectious after 11 days (range: 2–21 days)2 of incubation on average, contacts that have been exposed to Ebola virus can be identified, monitored and when symptomatic be isolated to limit spread. Therefore, multi-faceted control strategies against EVD involving tight infection control in the hospitals and at funerals, active case finding and isolation, identification and follow up of their contacts are believed to be sufficient to stop EVD epidemics4. However, there is currently general agreement that drastic improvement in control measures will be required to put the current EVD epidemic to an end2. As more resources become available, it is essential that strategic decisions to control the epidemic are informed by experience gained in the field.
Clinical manifestations, case fatality rates and key time periods have already been described2, but a detailed quantification of the routes of transmission and the impact of specific interventions is still lacking. Because of the absence of adequate data, it has indeed only been possible to characterize overall growth in case numbers (e.g. doubling times, overall reproduction numbers)2, 5 and many questions therefore remain unanswered. What are the relative contributions of hospitals and funerals to spread? What has been the impact of infection control in these settings? What is the effect of hospitalization on transmission in the community? Do high population densities in urban centers increase opportunities for transmission? How does mobility in these areas affect spread and control of EVD?
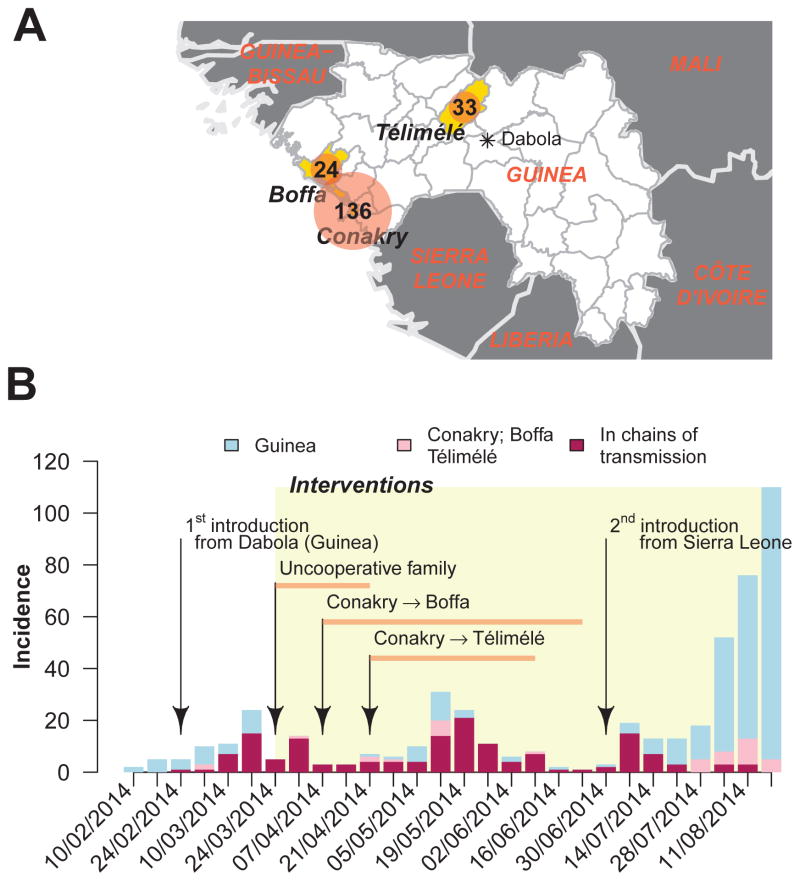
Here, we make a detailed description of EVD chains of transmission to investigate some of these questions using Conakry, the capital city of Guinea (Figure 1A) and the first urban center ever affected by EVD, as case study. From March to August 2014, Conakry was affected by three consecutive EVD epidemic waves (Figure 1B), which led to two new foci in Boffa and Telimélé (Figure 1A). We investigated the role of the different modes of transmission and the impact of control measures in these three prefectures during this time period.
Figure 1. Epidemiological context.
A. Map of Guinea. Conakry, the capital city of Guinea is highlighted along with two prefectures, Telimélé and Boffa, the chains of transmission of Conakry expanded to. The total number of probable and confirmed cases reported from March to August 2014 is also provided. B. Epidemic curve of probable and confirmed EVD cases for Guinea (grey) and for the prefectures of Conakry/Telimélé/Boffa (pink). The number of cases appearing in the chains of transmission of Conakry/Telimélé/Boffa is indicated in red. From the end of March, control measures including the opening of a treatment center, social mobilization among HCW, secured burial by professional staff were implemented.
Material and Methods
Case definitions
WHO case definitions1 were used for suspected, probable and confirmed EVD cases (see Supplementary Material). Diagnostics was performed using either real time RT-PCR or serology methods for patients that were identified more than 10 days after the date of onset and for whom RT-PCR were negative 6, 7. We restricted our analysis to probable and confirmed cases. In our analysis, probable cases corresponded to patients that meet the clinical definition, had an epidemiological link with a confirmed patient but died and were buried before a sample could be collected.
Laboratory work and epidemiological investigations
Three complementary datasets were used: i) the linelist of all confirmed and probable cases in Guinea (as of September 16th 2014); ii) the laboratory database compiling socio-economic, outcome of infection, symptoms, course of infection and viral load information; and iii) the results of additional epidemiological investigations of confirmed and probable cases that provided further insights on chains of transmission.
The linelist has already been described in detail elsewhere2. In short, a standard case investigation form was used to collect clinical and demographic data for all confirmed, probable and suspected EVD cases identified through clinical care and contact tracing in Guinea. When possible, cases were also interviewed to document contacts with other EVD cases as well as exposures at funerals.
In addition, relatives and neighbors of identified cases were interviewed to identify additional cases, collect additional contextual information, validate and complement information provided by cases in particular about possible contacts and sources of infection of cases. On the basis of the data gathered during these investigations, chains of transmission were established.
For the subset of cases appearing in these chains, information from the three datasets was merged in a single dataset, with the following variables: patient ID, status (confirmed, probable), date of symptom onset, start/end of hospitalization, date of death, occupation (HCW or not), district, age, sex, patient ID(s) of possible infector(s), start/end dates of possible contacts with infector(s), indicator for family relationship with possible infector(s) (=1 if they had the same family name or if a family relationship was documented), indicator for nosocomial transmission, attending the funeral of an EVD case (ID of possible infector(s), date(s) of the funerals), viremia.
Reconstructing the transmission tree
Most cases had a single known source of exposure. For the few cases with multiple possible infectors/contexts of infection, statistical techniques were developed to handle uncertainty about the source.
For each EVD case in the tree, we applied a simple algorithm to determine the exhaustive list of exposure occurrences where the ID of the possible infector, the date of exposure and the context (community, hospital or funeral) defined an exposure occurrence. In short, for each pair of case and possible infector, we proceeded as follows. If the start/end dates of contact were available, we used them to define the period of exposure. Otherwise, for community cases, this period started with symptom onset in the infector and ended when the infector was hospitalized. HCWs and nosocomial cases were exposed to non-HCW cases only during the hospital stay of their infector and to HCW cases from symptom onset in the HCW. Funeral attendances were added to the list of exposure occurrences.
The incubation period was estimated at maximum likelihood by fitting a Gamma distribution to the interval-censored dates of infection, conditional on infection on one of the exposure occurrences and assuming each exposure occurrence contributed equally to the likelihood (see Supplementary Material). The serial interval distribution was fitted in the same way.
Based on the exposure occurrences and the incubation period, it was possible to draw fully resolved transmission trees. We did this by sampling for each case one of the exposure occurrences, proportionally to the probability of the associated incubation period (see Supplementary Material). From the analysis of these resolved trees, we computed for each case the context-specific reproduction number, i.e. the number of persons they infected in the community, in the hospital and at funerals, respectively. We also computed the overall reproduction number, i.e. the total number of persons they infected, which is the sum of the context-specific reproduction numbers (see Supplementary Material). We analyzed by Poisson regression the community reproduction number as a function of viremia measured in the week following symptom onset, adjusting for the time period (March vs after March) and delay from symptom onset to sample collection.
Finally, we evaluated how the transmission tree would have been modified had more cases been hospitalized. For each resolved tree, we randomly drew a number of cases that had not been hospitalized and assumed they had been. We then assumed that persons infected by these cases in the community avoided infection if their date of infection occurred after the new date of hospitalization of the infector, and that all subsequent transmissions were avoided as well.
Technical details are provided in the Supplementary Material.
Ethical Considerations
This study is based on data collected during surveillance and response activities for EVD in Guinea. All information on individual patients has been anonymized for presentation.
Role of funders
Funders had no role in data collection, interpretation, writing of the manuscript or the decision to submit. OF, PYB, AAS and SC had access to all the data and had final responsibility for the decision to submit for publication.
Results
Case patients
The chains of transmission in Conakry expanded to Boffa and Telimélé prefectures (Figure 1A). 147 confirmed and 46 probable cases were identified in these three prefectures (Table 1). Of these, 152 (79%) were positioned in the transmission tree. Cases in the tree were 34.4 (standard deviation SD=17.2) years old on average, the proportion of women was 45% (N= 68) and 14% (N=21) were HCWs. Most cases were hospitalized during the course of the disease (N=123, 81%). Cases not placed in the transmission tree had similar characteristics. The case fatality ratio was 54% (95% CI: 49%, 63%).
Table 1.
Characteristics of probable and confirmed cases in the three prefectures of Conakry, Boffa and Telimélé, Guinea, March-August 2014. Information is provided for cases that appear in the transmission tree and for those that do not.
| In the transmission tree (N=152) | Not in the transmission tree (N=41) | |
|---|---|---|
| Age (yrs, m (SD)) | 34.4 (17.2) | 32.2 (13.0) |
| Sex (F) | 45% (68) | 50% (20) |
| HCW | 14% (21) | 15% (6) |
| Confirmed cases | 79% (120) | 85% (35) |
| Exposure | ||
| Possible infector | ||
| unknown+ | 5% (7) | - |
| single | 84% (127) | - |
| multiple | 12% (18) | - |
| Context of exposure* | ||
| Nb of contexts where exposed | ||
| 1 context | 90% (131) | - |
| 2 contexts | 10% (14) | - |
| Exposed at least once in | ||
| Community | 84% (122) | - |
| Hospital | 13% (19) | - |
| Funeral | 13% (19) | - |
| Familial exposure* | 72% (105) | - |
| Hospitalized | 81% (123) | 70% (20) |
| Delay onset to hospitalization (days, m (SD)) for those hospitalized | 5.0 (3.9) | 3.9 (3.1) |
| Dead | 54% (82) | 45% (18) |
| Delay onset to death (days, m (SD)) for those who died | 8.9 (4.0) | 8.8 (2.7) |
Cases with an unknown source of infection or with a source of infection outside the study area initiated chains of transmission (yellow dots in Figure 2D).
Computed for cases with at least one known possible infector.
The transmission tree was well resolved with 127 (84%) cases having a single possible infector and 131 (90%) being exposed in a single context (Table 1). Seven cases with no known infector or with an infector outside the study area initiated the chains of transmission.
Context of transmission
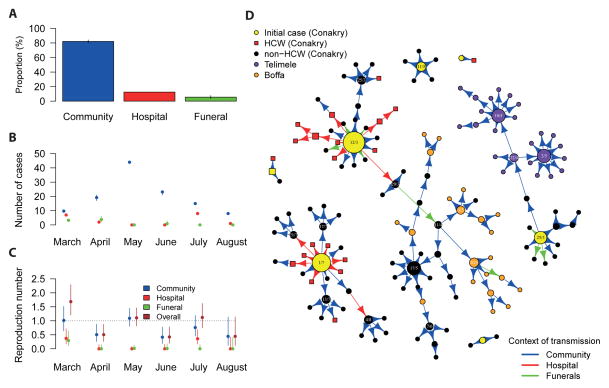
Figures 2A–C characterize EVD transmission in the areas under study, with an example of resolved tree being presented in Figure 2D.
Figure 2. Transmission tree.
A. Proportion of cases infected in the community, at hospital and at funerals. B. Number of cases infected in each context, by month of symptom onset. C. Context-specific and overall reproduction numbers by month of symptom onset. The reproduction number is the mean number of persons infected by a case. D. A fully resolved transmission tree. Each point represents an EVD case. The size of the point is proportional to overall reproduction number R. Dates of symptom onset are indicated in the figure for cases with R≥3. HCWs are represented by squares. The color of the arrow indicates the context of transmission. Cases were stratified by their date of symptom onset.
Eighty four percent (122/145), 13% (19/145) and 13% (19/145) of cases were exposed at least once in the community, in hospital and at funerals, respectively (Table 1). After adjusting for multiple sources of infection, we estimate that 82% (119/145), 12% (18/145) and 6% (8/145) of transmissions occurred in the community, in the hospital and at funerals, respectively (Figure 2A).
Transmission between family members represented 81% (95% CI: 80%, 82%) of transmissions in the community and 86% (95% CI: 75%, 90%) of those at funerals. Overall, 72% (105/145) of transmissions occurred between family members.
The number of infections in the hospital varied substantially over time (Figure 2B). There were two hospital super-spreading events: one in March, after which numbers dropped to low levels, and a second one in July (Figures 2B, 2D).
Reproduction numbers
Figure 2C shows context-specific and overall reproduction numbers from March to August. In March, a case generated on average 1.0 (95% CI: 0.6, 1.5) secondary cases in the community, 0.4 (95% CI: 0.2, 0.7) in the hospital and 0.3 (95% CI: 0.1, 0.6) at funerals, leading to an overall reproduction number R of 1.7 (95% CI: 1.2, 2.3). However, in the following months, the overall reproduction number fluctuated between 0.4 and 1.1.
Averaging across the whole study period, the overall reproduction number of cases aged ≥20 years was twice larger than that of those aged <20 years although the difference was not significant (1.0, 95% CI 0.8, 1.2 vs 0.5, 95% CI 0.2, 1.0). No significant difference was observed between male (0.8, 95% CI 0.6, 1.1) and female (1.1, 95% CI 0.8, 1.2) cases.
The longest chain in Conakry had 10 generations as opposed to 3 and 4 in Boffa and Telimélé, respectively (Figure 2D).
Impact of hospitalization and of infection control on transmission
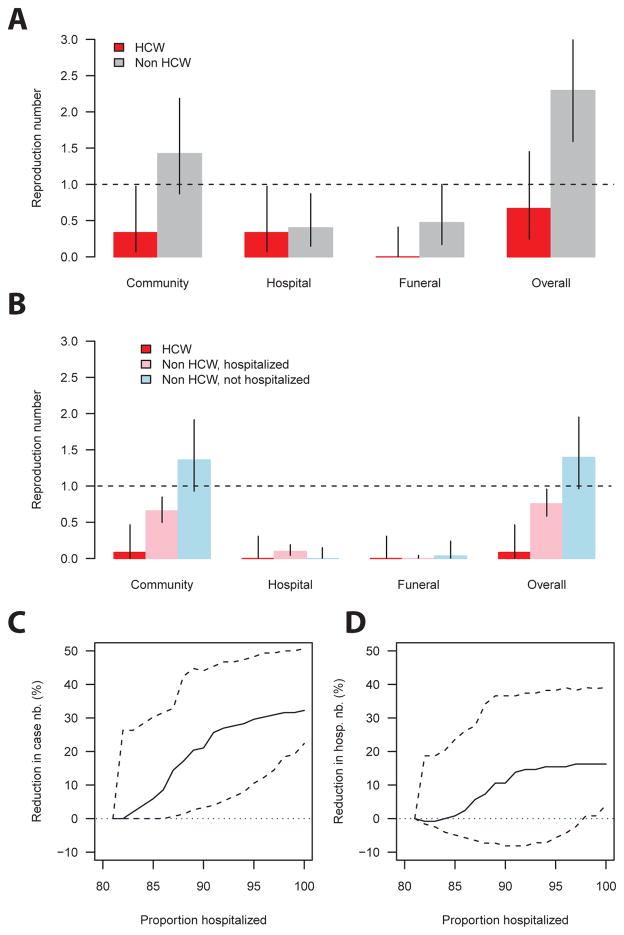
Figure 3 presents context-specific and overall reproduction numbers for non-HCW cases in March (i.e. before infection control was in place; Figure 3A) and afterwards (Figure 3B). In March, their reproduction number was 1.4 (95% CI: 0.9, 2.2) in the community, 0.4 (95% CI: 0.1, 0.9) in the hospital, and 0.5 (95% CI: 0.2, 1.0) at funeral, yielding an overall reproduction number of 2.3 (95% CI: 1.6, 3.2) (Figure 3A). Following the implementation of infection control (Figure 1B), the reproduction number in the hospital and at funerals became close to null (≤0.1) (Figure 3B). As a consequence, although hospital and funeral transmission represented 35% (7/20) and 15% (3/20) of all transmissions in March, their contributions dropped to 9% (11/128) and 4% (5/128), respectively, from April onward. The reproduction number in the community dropped by 50% to 0.7 (95% CI: 0.5, 0.8) for hospitalized cases but remained unchanged for those not hospitalized (1.4, 95% CI: 0.9, 1.9). The contribution of HCWs to EVD spread was limited throughout the epidemic, dropping almost to zero from April onwards (Figure 3A–B). The community reproduction number doubled (1.8; 95% CI: 1.1, 3.0) with an increase of 1 log10 unit in viremia (see Supplementary Material).
Figure 3. Impact of interventions.
A. Context-specific and overall reproduction numbers for HCW and non-HCW case patients in March, before infection control was implemented. B. Context-specific and overall reproduction numbers for HCW and for non-HCW case patients who were/were not hospitalized from April to August, i.e. after infection control was implemented. C. Estimated reduction in the size of the chain as a function of the proportion of hospitalized cases. D. Estimated reduction in the number of hospitalized cases as a function of the proportion of hospitalized cases. Cases were stratified by their date of symptom onset.
Figures 3C–D show how chains of transmission would have been affected if more cases had been hospitalized. Increasing the hospitalization rate from 81% to 91% would have reduced the size of the chains and the number of hospitalized cases by 26% (95% CI: 4%, 45%) (Figure 3C) and 14% (95% CI: −8%, 37%), respectively (Figure 3D).
Time intervals
In agreement with other studies2, the incubation period was estimated to 9.9 (95% CI, 9.0, 11.0) days on average (SD: 5.5 days, 95% CI 4.7, 6.5) and the serial interval to 14.2 days (95% CI: 13.1, 15.5) (SD: 7.1 days, 95% CI 6.2, 8.2). The delays from onset to hospitalization and from onset to death were 5.0 (SD=3.9) and 8.9 (SD=4.0) days, respectively (Table 1).
Discussion
The detailed investigation of EVD chains of transmission is crucial to understand where control measures are effective and where they need to be reinforced, as well as to inform on the best use of additional resources. From the analysis of such data, we found that in Conakry, the first large urban center ever affected by EVD: (1) hospital transmission was elevated in March 2014 but quickly declined to relatively low levels; (2) the contribution of HCWs to spread was limited; (3) transmission at funerals played a minor role once controls were in place; (4) a majority of transmissions took place in the community and between family members; (5) Hospitalizing cases substantially reduced the number of persons they infected in the community; (6) Cases with elevated viremia infected on average more people in the community.
Two sources of concern were raised when the first cases of EVD were reported in Conakry: (1) Would high population densities increase opportunities for transmission? (2) Would the large number of persons travelling to/from Conakry generate frequent re-introductions of EVD to the city and facilitate seeding of cases to other regions? The risk of an increased potential for local transmission did not materialize in Conakry, March to August 2014. Although we estimated the reproduction number was large early in the epidemic, as in other places (R in the range 1.4–2.3)2, interventions managed to quickly reduce it to relatively low levels (R=0.4–1.1) (Figure 2). There was therefore no exponential growth in incidence in Conakry (Figure 1B) and it is expected the epidemic would have stopped in the absence of re-introductions or resistance to interventions. However, infections in Conakry have been maintained for nine months now. Following an initial introduction from Dabola (Figure 1), the first epidemic wave was relatively well contained but transmission remained latent in an uncooperative family. This led to the second epidemic wave. The third epidemic wave was caused by a re-introduction of EVD from Sierra Leone. This sequence of events highlights the difficulties of controlling EVD epidemics, particularly in large urban centers that are more likely to see reintroductions of EVD due to population mobility, making it imperative to uphold precautionary measures for long time periods. Our study also documented how chains of transmission starting in Conakry expanded to two other areas of Guinea. More detailed analyses of spatial spread relying on mobility and demographic data8 will be required to make a more definitive assessment of the contribution of urban centers to EVD spread.
Infection controls in hospital settings and at funerals appear to have been relatively effective. The second hospital super-spreading event that occurred in July was due to a loosening of hospital control at a time when the local epidemic was believed to be largely over and emphasizes again the need for controls to be maintained for prolonged periods. Hospitalizing cases effectively reduced the number of persons they infected in the community and we found that chains of transmission could have been reduced by a quarter (although 95% CIs are large) had hospitalizations been 10% higher. These results support the objective to increase the capacity of EVD treatment centers both to care for EVD patients and to stop transmission or strategies designed to isolate cases in their community9. In parallel, it is essential that communication campaigns reinforce social mobilization and adherence to control measures. We found a positive correlation between the viremia of an EVD case patient and the number of persons he/she infected in the community. Delay from symptom onset to sample collection did not appear to be a confounding factor here (see Supplementary Material) but other confounding factors cannot be excluded. Further research is needed to assess the policy implications of this positive correlation, for example in terms of optimization of contact tracing strategies.
It is important to put our results into the wider context of the EVD epidemic in West Africa. From April to August, infection control in Conakry was sufficient to avoid sustained exponential growth in case numbers. In areas that failed to achieve such objective, transmission will likely present different characteristics. For example, funerals will likely have a larger contribution to overall spread, closer to what we found early on for Conakry (15% of all transmissions; 21% of transmissions due to non-HCW cases). Interestingly, our description of the epidemic in Conakry may provide realistic targets for control programs in other areas. Another specificity of the epidemic in Conakry is that the reported proportion of hospitalized cases was high (80% of cases positioned in the transmission tree; 70% of all cases reported in Conakry). These elevated rates may be indicative of the relative good compliance of populations in Conakry; but are likely to somewhat overestimate true hospitalization rates in the area as cases that are not hospitalized may have a higher probability of being underreported. Unfortunately, the strength of the bias is hard to quantify. If controls during burials are more commonly implemented for hospitalized cases, the contribution of funerals to overall spread might be more important in populations with lower hospitalization rates.
As for most other epidemiological investigations of EVD in West Africa, a proportion of EVD cases in Conakry are likely to be unreported but it is hard to estimate this proportion in the absence of independent data. It is critical studies are designed to assess reporting rates, for example based on capture-recapture methods. The proportion of cases with a known source in Conakry appears to have been relatively high. This might be explained by the fact the epidemic remained of a manageable size with less than 10 cases per week for most of the epidemic (Figure 1). Similarly, the transmission tree in Nigeria which was made of 20 cases in July-August was also well resolved10. A large proportion of cases positioned in the transmission tree (83%) had only one known source of infection. This again might be explained by the manageable size of the epidemic and the fact that the reproduction number remained below/around 1 from April to August (Figure 2). We did not have access to data documenting the number of contacts followed up per EVD case, which is a limitation of the study.
Reconstructing chains of transmission is always a challenge and prone to mistakes. Here we used two layers of analyses to resolve EVD chains of transmission in Conakry. Field epidemiologists made a first synthesis of all the epidemiological data they had collected and listed for each case the most probable sources of infection and contexts of infection. Second, for the relatively small proportion of cases exposed to multiple sources (17% exposed to multiple infectors, 10% exposed in multiple contexts), simple statistical and mathematical models integrated this information along with data about the timing of events, the incubation period and further contextual information to probabilistically allocate them to one of the sources. The algorithm remained deliberately simple, using Uniform priors for possible exposure occurrences (i.e. we did not use information about the context of infection or family-membership to modify the allocation probability a priori). Since this step only concerned a small proportion of cases, our estimates of the contribution of hospitals and funerals were relatively robust to these modelling assumptions (see Supplementary Materials). In the future, we aim to use both viral sequences collected in case patients and the epidemiological data presented here to validate the reconstruction of the tree and determine what can be gained from adding sequence data to the analysis11.
A detailed description of chains of transmission has made it possible to evaluate the impact of controls measures implemented in Conakry. Because situations in the field are very diverse, this effort should be reproduced in other areas affected by EVD so as to best inform local decision making.
Supplementary Material
Box research in context.
We searched Medline, journal sites and Plos current outbreaks for publications in English regarding transmission in the current Ebola epidemic in West Africa (Keywords : Ebola; transmission; 2014; West Africa). Estimation of the reproduction number of the epidemic in different locations has been the most studied, yielding values in the range 1.4–3.2, 12–14 Early estimates were obtained using incidence or death data reported to WHO by the affected countries, but detailed data were lacking to apportion transmission to different settings known to be of interest (community, hospital, funeral). Analysis of small chain of transmission (20 cases) in Nigeria showed that transmission was heterogeneous and involved a large number of HCWs.10
Interpretation.
This study presents the most detailed description to date of a large chain of transmission of EVD (n=152), in Conakry, the capital city of Guinea, in 2014. The study makes it possible to characterize transmission in the different contexts of interest and to make a detailed assessment of the impact of control measures on transmission in this epidemic. It shows how transmission changed with time, with a reduced role for funerals after implementation of prevention measures. Healthcare workers were at increased risk of infection, but contributed little to transmission. Early hospitalisation led to less transmission in the community. Altogether, it shows that interventions in Conakry had the potential to stop the epidemic but reintroductions of the disease and lack of cooperation of a small number of families led to prolonged low-level spread, highlighting challenges of EVD control in large urban centers.
Acknowledgments
The research leading to these results received funding from Labex IBEID, Reacting (INSERM), the European Union Seventh Framework Programme (FP7/2007-2013) under Grant Agreement nu278433-PREDEMICS, the NIGMS MIDAS initiative, Institut Pasteur de Dakar.
Footnotes
Author contributions
OF, EH, OF, NM, BS, SK, TG, EHIB, LK, AAS collected the data; OF, PYB, CL, AAS, SC analyzed the data; OF, PYB, AAS, SC wrote the first draft; all authors reviewed and edited the manuscript.
References
- 1.WHO. [Last accessed on 2 Dec 2014. 2014];Ebola response roadmap situation report. 2014 Nov 26; Available at http://apps.who.int/iris/bitstream/10665/144498/1/roadmapsitrep_26Nov2014_eng.pdf?ua=1.
- 2.W. H. O. Ebola Response Team. Ebola Virus Disease in West Africa - The First 9 Months of the Epidemic and Forward Projections. The New England journal of medicine. 2014 doi: 10.1056/NEJMoa1411100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. WHO Statement on the Meeting of the International Health Regulations Emergency Committee Regarding the 2014 Ebola Outbreak in West Africa. [Last accessed on 22 Oct 2014];WHO statement. 2014 Aug 8; Available at http://www.who.int/mediacentre/news/statements/2014/ebola-20140808/en.
- 4.Fraser C, Riley S, Anderson RM, Ferguson NM. Factors that make an infectious disease outbreak controllable. Proc Natl Acad Sci U S A. 2004;101(16):6146–51. doi: 10.1073/pnas.0307506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisman D, Khoo E, Tuite A. Early epidemic dynamics of the West African 2014 Ebola outbreak: Estimates derived with a simple two-parameter model. PLoS Currents Outbreaks. 2014 doi: 10.1371/currents.outbreaks.89c0d3783f36958d96ebbae97348d571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weidmann M, Muhlberger E, Hufert FT. Rapid detection protocol for filoviruses. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2004;30(1):94–9. doi: 10.1016/j.jcv.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Ksiazek TG, Rollin PE, Williams AJ, et al. Clinical virology of Ebola hemorrhagic fever (EHF): virus, virus antigen, and IgG and IgM antibody findings among EHF patients in Kikwit, Democratic Republic of the Congo, 1995. The Journal of infectious diseases. 1999;179 (Suppl 1):S177–87. doi: 10.1086/514321. [DOI] [PubMed] [Google Scholar]
- 8.Wesolowski A, Buckee C, Bengtsson L, Wetter E, Lu X, AJT Commentary: Containing the Ebola Outbreak – the Potential and Challenge of Mobile Network Data. PLOS Currents Outbreaks. 2014 doi: 10.1371/currents.outbreaks.0177e7fcf52217b8b634376e2f3efc5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitty CJ, Farrar J, Ferguson N, et al. Infectious disease: tough choices to reduce Ebola transmission. Nature. 2014;515(7526):192–4. doi: 10.1038/515192a. [DOI] [PubMed] [Google Scholar]
- 10.Fasina F, Shittu A, Lazarus D, et al. Transmission dynamics and control of Ebola virus disease outbreak in Nigeria, July to September 2014. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2014;19(40) doi: 10.2807/1560-7917.es2014.19.40.20920. [DOI] [PubMed] [Google Scholar]
- 11.Jombart T, Cori A, Didelot X, Cauchemez S, Fraser C, Ferguson N. Bayesian reconstruction of disease outbreaks by combining epidemiologic and genomic data. PLoS computational biology. 2014;10(1):e1003457. doi: 10.1371/journal.pcbi.1003457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Althaus C. Estimating the reproduction number of Ebola Virus (EBOV) during the 2014 outbreak in West Africa. PLoS Currents Outbreaks. 2014:1. doi: 10.1371/currents.outbreaks.91afb5e0f279e7f29e7056095255b288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomes M, Pastore Y, Piontti A, Rossi L, et al. Assessing the International Spreading Risk Associated with the 2014 West African Ebola Outbreak. PLoS Currents Outbreaks. 2014 doi: 10.1371/currents.outbreaks.cd818f63d40e24aef769dda7df9e0da5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandey A, Atkins KE, Medlock J, et al. Strategies for containing Ebola in West Africa. Science. 2014;346(6212):991–5. doi: 10.1126/science.1260612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.