Abstract
Purpose.
We previously reported that extracellular matrix composition (fibrin versus collagen) modulates the pattern of corneal fibroblast spreading and migration in 3-D culture. In this study, we investigate the role of thrombin and cell contractility in mediating these differences in cell behavior.
Methods.
To assess cell spreading, corneal fibroblasts were plated on top of fibrillar collagen and fibrin matrices. To assess 3-dimensional cell migration, compacted collagen matrices seeded with corneal fibroblasts were embedded inside acellular collagen or fibrin matrices. Constructs were cultured in serum-free media containing platelet-derived growth factor (PDGF), with or without thrombin, the Rho kinase inhibitor Y-27632, and/or the myosin II inhibitor blebbistatin. We used 3-dimensional and 4-dimensional imaging to assess cell mechanical behavior, connectivity and cytoskeletal organization.
Results.
Thrombin stimulated increased contractility of corneal fibroblasts. Thrombin also induced Rho kinase–dependent clustering of cells plated on top of compliant collagen matrices, but not on rigid substrates. In contrast, cells on fibrin matrices coalesced into clusters even when Rho kinase was inhibited. In nested matrices, cells always migrated independently through collagen, even in the presence of thrombin. In contrast, cells migrating into fibrin formed an interconnected network. Both Y-27632 and blebbistatin reduced the migration rate in fibrin, but cells continued to migrate collectively.
Conclusions.
The results suggest that while thrombin-induced actomyosin contraction can induce clustering of fibroblasts plated on top of compliant collagen matrices, it does not induce collective cell migration inside 3-D collagen constructs. Furthermore, increased contractility is not required for clustering or collective migration of corneal fibroblasts interacting with fibin.
Keywords: extracellular matrix, corneal keratocytes, cell mechanics, thrombin, 3-D culture
Thrombin induces Rho kinase–dependent clustering of corneal fibroblasts on top of compliant collagen matrices, but not collective migration within 3-D collagen constructs. In contrast, fibrin induces clustering and collective migration of corneal fibroblasts that is Rho kinase–independent.
Introduction
The cornea is an optically clear tissue that forms the front surface of the eye, and accounts for nearly two-thirds of its refractive power. The corneal stroma, which makes up 90% of corneal thickness, is a highly ordered structure with lamellae consisting of uniformly thin collagen fibrils organized into a pseudo-hexagonal lattice that is critical to maintenance of corneal transparency. Corneal stromal cells (keratocytes) reside between the collagen lamellae, and are responsible for secreting ECM components required to maintain normal corneal structure and function (i.e., transparency and biomechanical stability).1–3 From a mechanical standpoint, resting keratocytes are considered quiescent; they do not express stress fibers or generate substantial contractile forces.4,5 Since it is directly exposed to environmental conditions, the cornea is susceptible to physical and chemical injuries. Because of its accessibility and optical power, it also is the target for numerous refractive surgical procedures, such as photorefractive keratectomy (PRK) and LASIK.
During wound healing following injury or surgery, quiescent corneal keratocytes generally become activated, and transform into fibroblast and myofibroblast repair phenotypes.6–9 Corneal fibroblasts proliferate, develop intracellular stress fibers, migrate into the wound, and reorganize the extracellular matrix (ECM) through the application of mechanical forces. Corneal myofibroblasts express α-smooth muscle actin, generate even stronger forces on the matrix, and synthesize a disorganized fibrotic ECM.8,9 Together, these processes can alter corneal shape and transparency.10–12 Thus, a better understanding of the underlying cellular and molecular mechanisms that regulate the transformation and biomechanical activities of corneal keratocytes could lead to more effective approaches to modulating the wound healing response in vivo.
Most studies investigating corneal keratocyte differentiation have been performed using rigid, 2-dimensional (2-D) substrates. However, keratocytes reside within a complex 3-dimensional (3-D) extracellular matrix in vivo, and significant differences in cell morphology, adhesion organization, and mechanical behavior have been identified between 2-D and 3-D culture models.13–15 Furthermore, unlike rigid 2-D substrates, 3-D models also allow assessment of cellular force generation and cell-induced matrix reorganization; biomechanical activities that are critically involved in the migratory, contractile, and remodeling phases of wound healing. In a recent study, we demonstrated for the first time to our knowledge that ECM composition can modulate the mechanism of corneal fibroblast spreading and migration in 3-D culture. Specifically, whereas corneal fibroblasts generally move independently within 3-D collagen matrices, fibrin induces a switch to an interconnected, collective mode of cell spreading and migration, which is independent of differences in ECM stiffness.16 The mode of cell migration (individual versus collective) has been shown to have a dramatic influence on the pattern of polarization, force generation, and tissue organization in other systems, and also has a pivotal role in cancer invasion.17–19 Thus, a better understanding of the factors that mediate the switch between individual and collective cell migration in 3-D culture could have broad significance.
Previous studies by others have shown that stimulating cell contractility using serum or the phospholipid lysophosphatidic acid (LPA) can induce clustering of dermal fibroblasts plated on top of compliant collagen matrices.20,21 However, the role of cell contractility in fibrin-induced clustering and collective cell migration has not been investigated to our knowledge. In addition to cleaving fibrinogen to form fibrin fibers, thrombin has been shown to stimulate contractility in a variety of cell types.22–26 Previous studies have confirmed that the cornea contains and synthesizes prothrombin, as well as factors required for conversion of prothrombin to form thrombin.27 However, the impact of thrombin on corneal keratocyte mechanical behavior has not been assessed previously to our knowledge.
The goal of this study was to assess the roles of thrombin and cell contractility in mediating collective spreading and migration of fibroblasts interacting with fibrin and collagen matrices. The results suggested that, while thrombin-induced actomyosin contraction can induce clustering of fibroblasts plated on top of compliant collagen matrices, it does not induce collective cell migration inside a 3-D collagen construct. Furthermore, increased contractility is not required for clustering or collective migration of corneal keratocytes or fibroblasts interacting with fibrin. This interplay between cell contractility, and matrix composition, stiffness, and dimensionality may influence cell behavior during wound healing, development, tumor invasion, and repopulation of engineered tissues.
Methods
Materials
Dulbecco's modified Eagle's medium (DMEM) and 0.25% trypsin/EDTA solution were purchased from Invitrogen (Gaithersburg, MD, USA). Platelet-derived growth factor BB isotype (PDGF) was obtained from Upstate Biotechnology, Inc. (Lake Placid, NY, USA). Fetal bovine serum (FBS), fatty acid-free and fraction V BSA, RPMI 1640 vitamin solution, HEPES, sodium bicarbonate, and thrombin from human plasma were obtained from Sigma-Aldrich Corp. (St. Louis, MO, USA). Penicillin, streptomycin, and amphotericin B were obtained from Lonza, Inc. (Walkersville, MD, USA). Type I rat tail collagen was purchased from BD Biosciences (Bedford, MA, USA). Alexa Fluor Phalloidin 488 and propidium iodide (PI) were obtained from Molecular Probes, Inc. (Eugene, OR, USA). The RNase (DNase free) was purchased from Roche (Indianapolis, IN, USA). The Y-27632 and blebbistatin were purchased from Calbiochem (San Diego, CA, USA).
Cell Culture
A previously published human corneal fibroblast cell line (HTK cells) was used.28,29 Fibroblasts were maintained in tissue culture flasks with DMEM containing 10% FBS and supplemented with 1% penicillin/streptomycin/amphotericin B. In some experiments, primary cultures of corneal keratocytes (NRK cells) also were used. Corneal keratocytes were isolated from rabbit eyes obtained from a slaughterhouse (Pel Freez, Rogers, AR, USA) as described previously.4,30 The NRK cells were maintained in serum-free media composed of DMEM containing pyruvate, HEPES, 1% RPMI vitamin mix, 1% ×100 MEM nonessential amino acids, 100 μg/mL ascorbic acid, and 1% penicillin/streptomycin/amphotericin B to maintain the keratocyte phenotype.4,31
Global Matrix Contraction Assay
To assess the effects of thrombin on cell contractility, contraction of restrained collagen matrices was measured.32 A neutralized collagen solution was prepared by mixing high concentration rat tail type I collagen with 0.1 N NaOH, ×10 DMEM, and H2O to achieve a final concentration of 2 mg/ml. A 14-mm diameter circular score was made within each well of 12-well culture plates, and 200 μL of the collagen solution containing 5 × 104 cells was poured into each circle. Samples then were allowed to polymerize for 30 minutes in a humidified incubator (37°C, 5% CO2), and basal media supplemented with 5 mg/mL BSA and 50 ng/mL PDGF was added to stimulate cell spreading and migration. After overnight incubation to allow cell spreading, the media was changed to basal media or basal media supplemented with 0.5 U/mL thrombin, 50 ng/mL PDGF, or thrombin and PDGF. Since the bottom of the matrix remained attached to the dish, cell-induced contraction resulted in a decrease in matrix height.33 Height was measured by focusing on the top and bottom of each matrix after 0, 1, and 24 hours of culture using phase contrast imaging. The percentage decrease in matrix height over time then was calculated. Duplicate samples were analyzed for each condition in each experiment. Final results for each condition are the mean and standard deviation of three separate experiments.
Assessment of 2-D Cell Clustering on Fibrin or Collagen ECM
To investigate the effects of thrombin-induced contractility on fibroblast clustering, cells were plated on top of compliant 3-D collagen or fibrin matrices, or rigid, collagen- or fibrin-coated glass substrates. For experiments with cells on top of 3-D matrices, 100 μL neutralized solutions of collagen (2 mg/mL) or fibrin (1 mg/mL) were poured onto glass bottom dishes (model P35GC-1.5-14-C; MatTek, Ashland, MA, USA). The collagen solution was prepared by mixing high concentration rat tail type I collagen with 0.1 N NaOH, ×10 DMEM, and H2O to achieve a final concentration of 2 mg/ml. For fibrin matrices, fibrinogen was warmed for 20 minutes and mixed with DMEM to achieve a final concentration of 1 mg/mL, and the solution was mixed with 0.5 U/mL thrombin to initiate polymerization. Samples then were placed for 30 minutes in a humidified incubator (37°C, 5% CO2) to polymerize. Matrices then were gently rinsed twice with DMEM to remove excess thrombin. For experiments with cells plated on rigid 2-D substrates, glass bottom dishes were coated by adding a 50 μg/mL neutralized solution of collagen, and incubating for 1 hour (37°C, 5% CO2). For cell seeding, 3 mL of basal media supplemented with 5 mg/mL BSA and 50 ng/mL PDGF containing 6 × 104 cells was added to each dish. After overnight culture to allow cell spreading, media was replaced with serum-free media supplemented with 5 mg/mL BSA and 50 ng/mL PDGF with or without thrombin and/or the Rho kinase inhibitor Y-27632 (10 μM).
After 24 hours of culture, f-actin and nuclei were labeled fluorescently as detailed below. Fluorescence images were collected using an inverted microscope (Leica DMI 4000B; Leica Microsystems, Wetzlar, Germany) equipped with a digital camera (Hamamatsu Flash 4.0; Hamamatsu, Hamamatsu City, Japan). Four fields were imaged at random on each dish using a 10× objective lens. To quantify cell clustering, nearest-neighbor and connected component analyses were performed using ImageJ (Supplementary Fig. S1).34 Using the “Find Maxima” tool, point selections were automatically marked at the center of each cell nucleus. Nuclei missed by the automated process then were manually marked with the “Point Tool.” The marked points subsequently were converted to a binary mask and the coordinates written to the “Results” table in ImageJ. Using the “Graph” plugin, distances between points were computed to produce a map of connected components (chains of cells within a prescribed distance of each other) and the corresponding adjacency list, and a distance matrix. In Microsoft Excel (Microsoft Corporation, Redmond, WA, USA), the distribution of different cluster sizes was extracted from the adjacency lists, while the distribution of nearest-neighbor distances was taken from the distance matrices. Duplicate samples were analyzed for each condition in each experiment. Final results for each condition are the mean and standard deviation of at least three separate experiments.
Assessment of 3-D Cell Migration
To study the pattern and amount of 3-D cell migration, cell-populated compressed ECM constructs were nested within acellular uncompressed matrices as described previously.16,32,35 In this model, 2 mL of a neutralized collagen solution (4 mg/mL) was poured into a rectangular metal mold (3 cm length, 2 cm width, 1 cm height) and placed in a humidified incubator (37°C, 5% CO2) for 30 minutes for polymerization. Cells (6 × 106) were mixed with a second 2-mL collagen solution, which was added on top of the first collagen layer. After 30 minutes to allow collagen polymerization, the sandwich construct was compressed as described previously.32,35–37 This produced an approximately 200-μm thick construct with an acellular ECM on the bottom and a cell-populated ECM on top. The first collagen layer serves as a spacer that prevents cells from contacting the glass substrate as they migrate out of the matrix, thus ensuring that they interact with the outer fibrin or collagen ECM.
To prepare the nested constructs, 6 mm diameter buttons from compressed sandwiched matrices were cut with a trephine blade and gently placed on glass bottom dishes. Buttons then were covered with a 100 μL solution of collagen (2 mg/mL) or fibrin (1 mg/mL). After polymerization, constructs were rinsed twice with DMEM to remove excess thrombin, and 2 mL of experimental media then was added to each sample. Experiments were done using serum-free media supplemented with 5 mg/mL BSA and 50 ng/mL PDGF, with or without 0.5 U/mL thrombin and/or Rho kinase inhibitor Y-27632 (10 μM) and the myosin II inhibitor blebbistatin (20 μM). Experiments were performed using duplicate matrices for each condition, and repeated three times.
Quantitative analysis of cell migration was done as described previously.16,38 After 24 hours of culture, f-actin and nuclei were fluorescently labeled as detailed below. Images then were collected with a laser confocal microscope (Leica SP2; Leica Microsystems). An HeNe laser (633 nm) was used for confocal reflection imaging of collagen and fibrin fibrils, and Argon (488 nm) and GreNe (543 nm) lasers were used for fluorescence imaging of f-actin and PI, respectively. Images were acquired sequentially to avoid cross talk between fluorescence channels. Stacks of optical sections were acquired by changing the position of the focal plane in the z-direction with a step size of 2 to 5 μm using a 20× dry objective, or a step size of 1 μm using a 63× water immersion objective (1.2 NA, 220 μm free working distance). Maximum intensity projection images were generated from PI image stacks using Metamorph, and overlaid with reflection images. An index of cell migration then was determined by counting the number of cells (based on nuclear staining) that migrated from the inner matrix into the outer matrix of the nested constructs. Counts were collected from up to four 750-μm wide strips in each construct, at approximately 90° intervals. Each strip included the border of the button and the furthest moving cell.
Time-Lapse Imaging of Cell Spreading and Migration
For dynamic assessment of cell spreading and migration mechanics, time-lapse imaging was performed on additional samples using a Nikon TE300 inverted microscope (TE300; Nikon, Tokyo, Japan) equipped with an environmental chamber (In Vivo Scientific, St. Louis, MO, USA) as described previously.39,40 Hardware was controlled using a PC running Nikon Elements software. The Z-stacks of images were taken at the border between the inner and outer matrices of nested constructs at 10-minute intervals using a 20× dry objective with Nomarski DIC, or a 10× dry phase contrast objective. Imaging was done for up to 72 hours. To create movies of cell movements, single z-plane images were selected at each time point, so that the same cells were in focus for the entire sequence. MetaMorph software version 7.7 (Molecular Devices, Inc., Sunnyvale, CA, USA) was used to generate movies of cell movements.
Fluorescent Labeling
Cell labeling was done by fixing cells with 3% paraformaldehyde in phosphate buffer, PBS, for 10 minutes, and permeabilizing with 0.5% Triton X-100 in PBS for 15 minutes. Subsequently samples were washed for 30 minutes with PBS. For f-actin labeling, cells were incubated with Alexa Fluor 488 Phalloidin (1:150 ratio) for 60 minutes and then washed for 30 minutes to remove excess label. For nuclear staining, after f-actin labeling, samples were incubated for 30 minutes with PI (1:100 ratio) in PBS containing 1:100 RNase (DNase free). All staining procedures were performed in the MatTek culture dishes to avoid cell or matrix distortion.
Statistical Analysis
Statistical analysis was performed using the analysis module within Sigmaplot (version 12.5; Systat Software, Inc., San Jose, CA, USA). An ANOVA was used to compare group means. Post hoc multiple comparisons between groups were performed using the Holm–Sidak method.
Results
Thrombin Stimulates Fibroblast Contractility and Induces Clustering on Top of Compliant Collagen Matrices
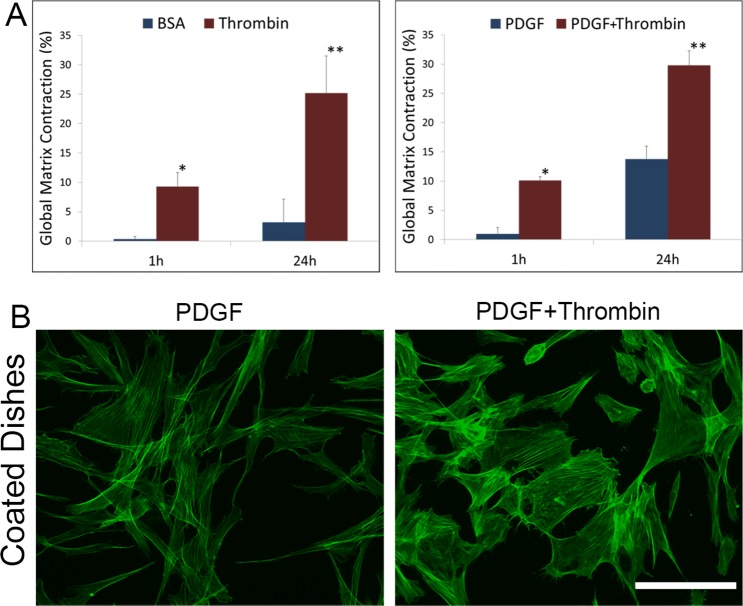
Previous studies have confirmed that the cornea contains and synthesizes prothrombin, as well as factors required for conversion of prothrombin to form thrombin.27 However, the impact of thrombin on keratocyte contractility and mechanical behavior has not been assessed previously. As shown in Figure 1A, the addition of thrombin to basal media or media containing PDGF induced a significant increase in global matrix contraction of restrained 3-D collagen matrices. To assess cell morphology and cytoskeletal organization, corneal fibroblasts also were cultured on collagen-coated dishes. As shown in Figure 1B, cells incubated with thrombin had a broader morphology and developed more stress fibers than cells cultured in PDGF alone, consistent with an increase in cell contractility.
Figure 1.
Thrombin stimulates corneal fibroblast contractility. (A) Global matrix contraction (decrease in matrix height) was significantly higher at 1 and 24 hours after adding thrombin to the media (*P < 0.05, **P < 0.01, repeated measures ANOVA). (B) When fibroblasts were plated on rigid substrates, f-actin labeling showed an increase in stress fiber formation and a decrease in the number of dendritic processes in thrombin-containing media. Scale bar: 50 μm.
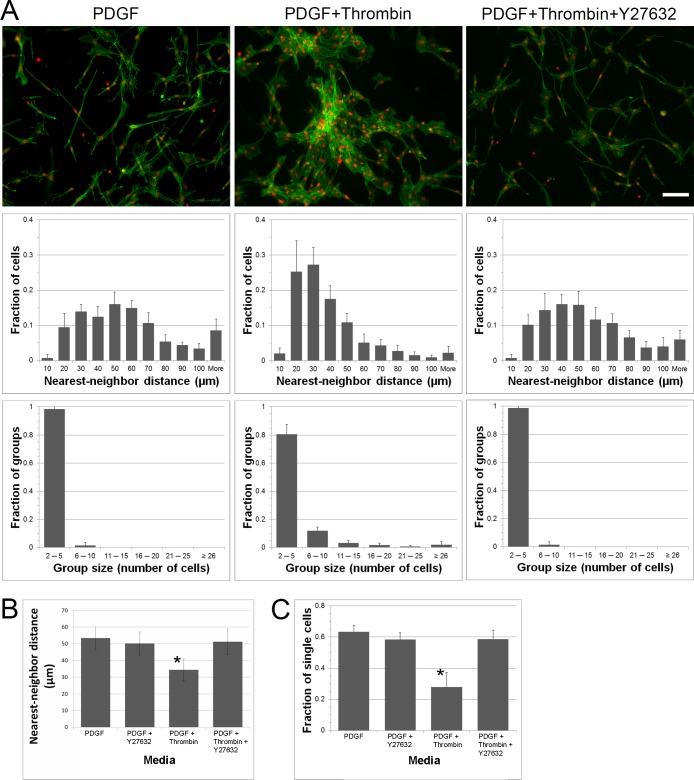
Despite stimulating fibroblast contractility, cell clusters did not form in response to thrombin on rigid 2-D substrates. In contrast, when corneal fibroblasts were cultured on top of 2-mg/mL collagen matrices, the addition of thrombin induced cell clustering (Fig. 2A, compare columns 1 and 2). For quantitative analysis, nearest neighbor distances and cluster sizes were calculated. Thrombin induced a shift in the histogram of nearest neighbor distances to smaller values (Fig. 2A, second row), and the formation of larger cell groups (Fig. 2A, third row); both of these are indicators of cell clustering.34
Figure 2.
Cell contractility induces clustering on compliant collagen matrices. (A) Representative images of cells grown on top of collagen matrices and cultured with PDGF, PDGF + thrombin, and PDGF + thrombin + Y-27632 are shown in the first row. Each image corresponds to the two graphs below it. Cells incubated with PDGF + thrombin clustered, however, when Y-27632 was present, cluster formation was blocked. The distribution of nearest-neighbor distances is displayed in the first row of graphs. A nearest-neighbor distance is the distance between the center of one cell nucleus and that of its closest neighbor. The frequency of group sizes is displayed in the second row of graphs. Chains of neighboring cells within a distance of 40 μm were grouped together. All data are means ± SD (n = 5 experiments). Scale bar: 100 μm. (B) Summary of nearest neighbor analysis for cells on collagen matrices (all 5 experiments combined). The average nearest-neighbor distances was less in PDGF + thrombin (*P < 0.05, ANOVA). (C) Summary of cluster analysis for cells on collagen matrices (all 5 experiments combined). The fraction of cells with no neighbors closer than 40 μm was less in PDGF + thrombin (*P < 0.05, ANOVA).
Thrombin-Induced Clustering Is Dependent on Rho Kinase
To evaluate if thrombin-induced clustering of corneal fibroblasts was dependent on Rho activation, we used the specific Rho kinase inhibitor Y-27632. As shown in Figure 2A, thrombin-induced cluster formation was inhibited by Y-27632 (top row, compare columns 2 and 3). The shift in the histogram of nearest neighbor distances and the formation of larger cell clusters induced by thrombin were blocked by inhibiting Rho kinase (rows 2 and 3). These quantitative results are summarized in Figures 2B and 2C, which show a statistically significant decrease in the average nearest neighbor distance and the number of isolated (nonclustered) cells in thrombin compared to all other conditions tested.
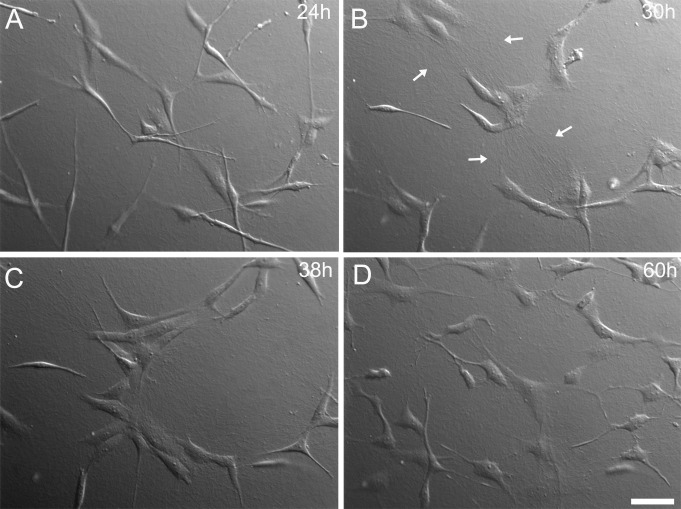
To gain further insights into the mechanism of thrombin-induced clustering, time-lapse differential interference contrast (DIC) imaging was performed. Cells on collagen matrices incubated with PDGF moved randomly and did not form stable clusters (Fig. 3A, Supplementary Movie S1). However, following addition of thrombin, cells gradually moved toward each other to form clusters (Figs. 3B and 3C; Supplementary Movie S2). During cluster formation, collagen fibers were displaced, and lines of tension between and around cells were observed, indicating an increase in cell contractile force (Fig. 3B, arrows). Following addition of Y-27632, cells that were grouped began to separate and move apart (Fig. 3D, Supplementary Movie S3). Cells also become elongated and develop dendritic process following Rho kinase inhibition. Taken together, these results demonstrated that Rho kinase–dependent contractile forces are necessary to form and maintain corneal fibroblast clusters in response to thrombin.
Figure 3.
Dynamic assessment of thrombin-induced clustering. When observed under DIC time-lapse imaging, transient collagen fibril reorganization appears to directly impact the process of fibroblast clustering on top of collagen matrices. (A) Image was taken just before the addition of thrombin after 24 hours of incubation in PDGF. (B) and (C) Images were taken at 30 and 38 hours, respectively. The thrombin-induced cellular force generation displaces the matrix substrate so as to pull cells toward each other. (B) Arrows denote regions of aligned collagen that form between cells during clustering. (D) Subsequent treatment with Y-27632 (at 48 hours) induces the breakup of clusters and development of a more dendritic morphology. Scale bar: 50 μm.
Thrombin Does Not Induce Collective Cell Migration in 3-D Collagen Matrices
Since thrombin induces cluster formation on top of collagen matrices, we decided to investigate whether thrombin could stimulate collective cell migration within nested collagen matrices. Nested collagen matrices were prepared and cultured with media containing PDGF or PDGF plus thrombin. Interestingly, cells migrated individually through the collagen ECM, even in the presence of thrombin (Fig. 4). Once cells escaped from the inner matrix, they moved in a random walk pattern and neither stable interactions nor grouping of cells was observed (Supplementary Movie S4). These data demonstrated that although thrombin stimulates Rho kinase–dependent clustering of corneal fibroblasts on top of collagen matrices, it does not induce collective cell migration through 3-D collagen matrices.
Figure 4.
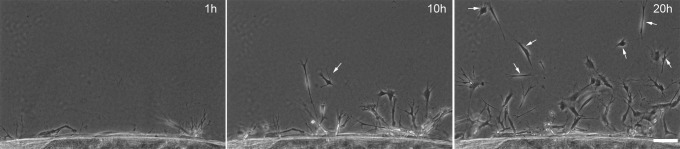
Thrombin does not induce collective cell migration in 3-D collagen matrices. Phase contrast images from time-lapse videos of fibroblasts incubated in PDGF + thrombin in nested collagen matrices. Cells migrated independently and no cell streaming or collective migration was observed. Arrows denote isolated cells within the migratory front. Scale bar: 100 μm.
Cell Clustering on Fibrin Matrices Is Not Dependent on Cell Contractility
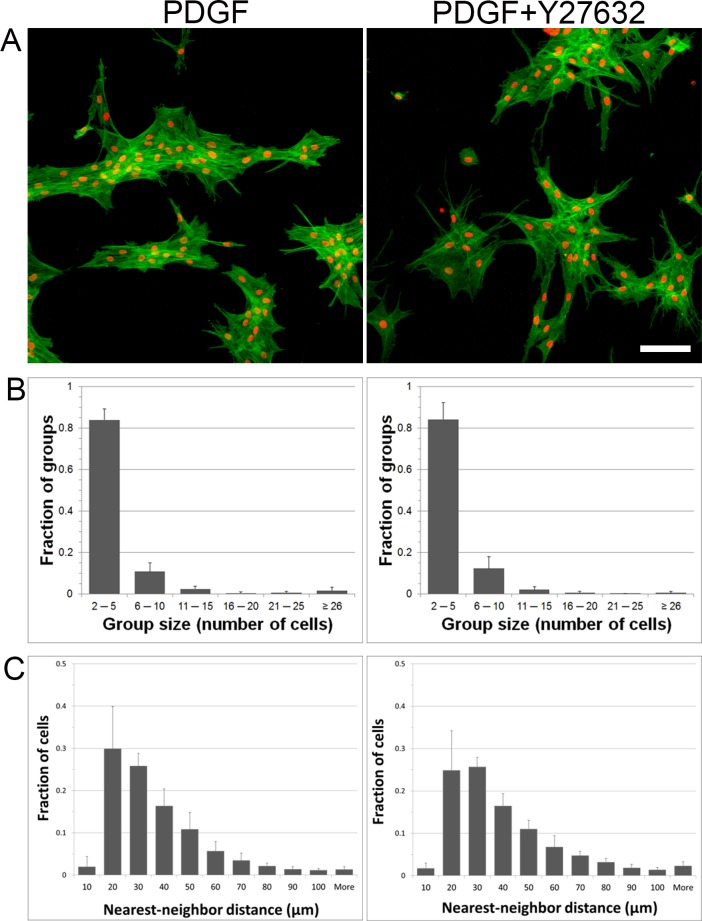
As shown above, thrombin can stimulate fibroblast clustering on top of compliant collagen matrices by increasing cell contractility. Our previously published results show that cells cultured on fibrin matrices also form clusters, and this was associated with an increase in stress fiber formation. Thus, to determine whether fibrin-induced clustering is dependent on cell contractility, cells were cultured on top of fibrin matrices and incubated with and without the addition of Y-27632. Blocking contractility of cells on fibrin matrices inhibited stress fiber formation; however, cells still formed clusters and remained interconnected (Fig. 5). Both the nearest neighbor distances and group sizes were similar for cells cultured with PDGF and those cultured with PDGF plus Y-27632. Taken together, these results suggest that cell contractility is not required for corneal fibroblast clustering on top of fibrin ECM.
Figure 5.
Blocking cell contractility does not inhibit clustering of cells on fibrin matrices. (A) F-actin and nuclear staining of cells cultured overnight on top of fibrin matrices with media containing PDGF ± Y-27632. (B) Distribution of cluster sizes under the two different culture conditions. (C) Distribution of nearest neighbor distances under the two different culture conditions. Data are the average ± SD of three separate experiments with duplicate matrices for each condition. Scale bar: 50 μm.
Fibrin-Induced Collective Cell Migration Is Not Dependent on Cell Contractility
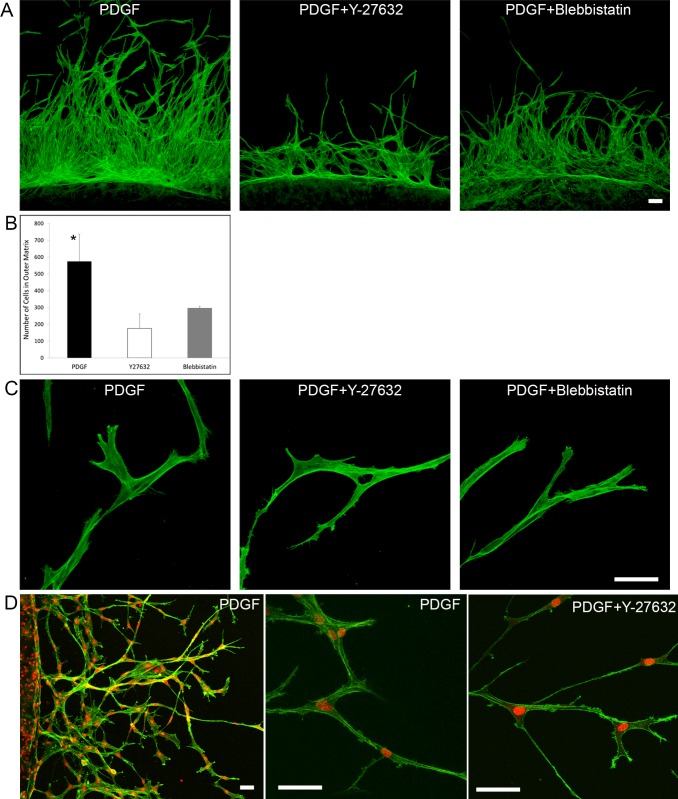
We previously demonstrated that fibrin induces an interconnected, collective mode of cell spreading and migration in 3-D culture.16 To evaluate if cell contractility could have a role inducing collective cell migration through fibrin ECM, we used Y-27632 and blebbistatin (a myosin II inhibitor) to block cellular force generation. Although the amount of cell migration decreased when matrices were cultured with Y-27632 or blebbistatin (Figs. 6A, 6B), the pattern of migration did not change; cells still remained interconnected during migration (Fig. 6C, Supplementary Movie S5). Under all conditions tested, corneal fibroblasts migrating into 3-D fibrin matrices moved into the outer fibrin ECM while maintaining connection to cells in the inner matrix at the rear. Other cells followed behind these cells along the same paths, producing lines of interconnected cells. As migration into fibrin proceeded, adjacent cells having lateral protrusions became interconnected, resulting in the formation of a mesh-like structure (Supplementary Movie S5).
Figure 6.
Blocking cell contractility does not change the pattern of cell migration through fibrin matrices. Maximum intensity projection images of migrating cells in nested fibrin matrices. (A) F-actin staining of migrating cells in fibrin nested matrices. Cells were cultured with PDGF, PDGF + Y27632, or PDGF + blebbistatin for 48 hours. Inhibitors reduced the cell migration rate, but they did not change the pattern of migration. Scale bars: 50 μm. (B) Graph from the same experiment showing cell counts in the outer matrix after 48 hours of incubation (*P < 0.05 compared to Y-27632 and blebbistatin, ANOVA). (C) Higher magnification images of migrating cells confirming that cells still were interconnected when cell contractile forces were blocked. Scale bar: 50 μm. (D) Primary rabbit corneal keratocytes showing same pattern of collective migration when cultured in fibrin nested matrices. Higher magnification shows interconnected streams of cells that form during migration (center), even in the presence of Y-27632 (right). Scale bar: 50 μm.
To further assess the role of cell contractility on fibrin-induced collective cell migration, we performed experiments using primary rabbit corneal keratocytes maintained in serum-free media, which do not generate significant forces on the ECM when cultured in PDGF.32,38 Corneal keratocytes also migrated collectively and remained interconnected within the fibrin matrix, even in the presence of Y-27632. These data further demonstrated that collective cell migration induced by fibrin matrices is not induced by increased cell contractility.
Discussion
The mechanical activity of corneal keratocytes (the generation of cellular forces and the application of these physical forces to the ECM) has a central role in mediating fundamental processes, such as developmental morphogenesis and wound healing. We previously reported that extracellular matrix composition can modulate the mechanics of corneal fibroblast spreading and migration. Specifically, cells interacting with collagen matrices develop dendritic processes and move independently, whereas cells interacting with fibrin matrices develop stress fibers and form an interconnected meshwork.16 Previous studies by others have shown that changes in contractility can modulate the mechanics of cell spreading on collagen ECM. Specifically, dermal fibroblasts plated on top of collagen matrices move independently when cultured in a promigratory growth factor environment (such as PDGF), whereas they form clusters when cultured in a procontractile growth factor environment (such as serum or LPA).20,21 Thrombin is used to cleave fibrinogen when preparing fibrin matrices, and stimulates contractility in a variety of cell types.22–26 In this study, the addition of thrombin to basal media or media containing PDGF induced a significant increase in global matrix contraction of restrained 3-D collagen matrices. When cultured on rigid substrates, cells incubated with thrombin also developed more stress fibers than cells cultured in PDGF alone. These results demonstrated for the first time to our knowledge that thrombin stimulates corneal fibroblast contractility.
When corneal fibroblasts were cultured on top of compliant collagen matrices, the addition of thrombin induced cell clustering. In contrast, cell clusters did not form in response to thrombin on rigid 2-D substrates. Similar results were obtained when dermal fibroblasts were plated on top of 3-D collagen matrices (unpublished observation), where the addition of thrombin induced clustering on compliant collagen matrices, but the amount of clustering was reduced on more rigid matrices. Overall, these results are consistent with the recent findings of Grinnell et al.,21 showing that dermal fibroblasts on collagen matrices can form aggregates when they are in a procontractile growth factor environment, but that the amount of clustering is inversely related to matrix stiffness. Vascular endothelial cells also tend to aggregate on compliant substrates, but remain separated on stiffer substrates.41 On rigid substrates, contractile forces are transmitted to the ECM, but do not produce significant displacement. In contrast, on compliant matrices, cellular forces deform the ECM, thereby pulling neighboring cells toward one another.42
In addition to cleaving fibrinogen to form fibrin fibers, thrombin can activate protease-activated receptors (PARs), a family of the G-protein coupled receptors (GPCRs) implicated in the regulation of several cellular processes, including inflammation and coagulation in wound healing.43 The protease-activated receptor-1 has been identified in all 3 layers of the cornea.27 Activation of PARs has been shown to activate the small GTPase RhoA in a variety of cell types.44–47 Activated RhoA binds to and activates Rho kinase, which inhibits myosin light chain (MLC) phosphatase, resulting in elevated MLC phosphorylation and increased cell contractility.22,48–56 To evaluate if thrombin-induced clustering of corneal fibroblasts was dependent on Rho activation, we used the specific Rho kinase inhibitor Y-27632. Quantitative analysis of static images demonstrated that thrombin-induced cluster formation was inhibited by Y-27632. Furthermore, time-lapse imaging demonstrated that following addition of Y-27632, cells that were grouped together following culture in thrombin separated and moved apart. Cells also become elongated and develop dendritic process following Rho kinase inhibition, which is characteristic of cells in a low-tension state.15,57 Taken together, these results demonstrated that Rho kinase–dependent contractile forces are necessary to form and maintain corneal fibroblast clusters in response to thrombin. Note that clustering of dermal fibroblasts on top of collagen matrices following culture in serum or LPA also is dependent on Rho kinase activation.20 In the case of thrombin, Rho kinase is most likely activated by PARs.
Migration of corneal keratocytes is involved in matrix patterning during developmental morphogenesis and also is required for repopulation of corneal tissue following wounding due to injury or surgery.13,58 Since thrombin induced cluster formation on top of collagen matrices, we decided to investigate whether thrombin could induce a switch from individual to collective cell migration within nested collagen matrices. Interestingly, cells continued to migrate individually through collagen ECM in the presence of thrombin. Thus, although thrombin stimulates Rho kinase–dependent clustering of corneal fibroblasts when plated on top of collagen matrices, it does not induce collective cell migration within 3-D collagen matrices. This is consistent with previous results demonstrating that corneal keratocytes migrate individually through collagen ECM when cultured in 10% FBS, despite increased cell contractility.38
Taken together, the data suggest that collagen matrix dimensionality may modulate the impact of cell contractility on fibroblast clustering. Cells plated on top of 3-D collagen matrices all attach to fibrils at or near the surface of the ECM. In this case, the mechanical forces generated by adjacent cells produce stresses and strains in the same 2-D plane. This results in cell–cell mechanical communication and amplification of local remodeling between cells. Cells can cluster either by pulling neighboring cells toward them via the ECM, or by migrating toward adjacent cells along the lines of increased tension between them (durotaxis). In contrast to this 2-D surface, cells migrating into the outer matrix in the 3-D nested construct are not necessarily in the same plane; thus cell-induced strain on the matrix can have less of an impact on neighboring cells. In addition, there are no cells in the outer matrix into which the fibroblast are migrating. This allows cells entering the outer matrix to move away from the cells behind them as they generate tractional forces at their leading edge. Finally, cells in 3-D matrices are surrounded by the ECM. Narrow pores in the ECM can create a physical barrier between cells, which also potentially could limit clustering.59
Previous studies have shown that following full-thickness corneal incisions in the rabbit (which produce a fibrin clot), fibroblasts form an interconnected mesh as they migrate into the wound space, and these interconnections are hypothesized to mediate force transduction during wound contraction.60,61 We previously demonstrated that fibrin also induces an interconnected, collective mode of cell spreading and migration in 3-D culture.16 Interestingly, fibrin-induced clustering is associated with the formation of stress fibers within corneal fibroblasts, suggesting increased contractility.16 Thus, to evaluate if cell contractility could have a role inducing collective cell migration through fibrin ECM, we used Y-27632 or blebbistatin (a myosin II inhibitor) to block cellular force generation.
Blocking contractility of cells on fibrin matrices inhibited stress fiber formation; however, cells still formed clusters and remained interconnected. Similarly, when using nested matrices, although the amount of cell migration decreased when force generation was inhibited, cells still remained interconnected during migration when cultured with Y-27632 or blebbistatin. During migration, interconnected chains of cells moved into the outer fibrin ECM while maintaining connection to cells in the inner matrix at the rear. This pattern of collective cell migration has been termed “multicellular streaming,”62 which is thought to have a role in directional guidance of neural crest cells during embryonic development.63–65 We also performed experiments using primary rabbit corneal keratocytes maintained in serum-free media. These cells are mechanically quiescent; that is, they do not exert significant contractile force, even when cultured in PDGF.30 The pattern of collective cell migration in fibrin was the same for corneal keratocytes as it was for fibroblasts; cells migrated collectively and remained interconnected within the fibrin matrix, even in the presence of Y-27632. Taken together, the data indicated that the collective cell spreading and migration induced by fibrin matrices is not dependent on increased cell contractility.
Overall, these data demonstrated how the interplay between cell contractility, and matrix composition, stiffness, and dimensionality may influence corneal fibroblast behavior during wound healing, development, and repopulation of engineered tissues.66 Taken together, the data suggested that, while thrombin-induced actomyosin contraction can induce clustering of fibroblasts on top of compliant collagen matrices, it does not induce collective migration of corneal fibroblasts inside 3-D collagen constructs. Furthermore, neither clustering nor collective migration of cells interacting with fibrin is dependent on cell contractility. Since data from our previous study indicates that differences in ECM stiffness are not the underlying cause for the difference in cell connectivity observed between collagen and fibrin matrices,16 it instead may be regulated by the interplay between cell–cell and cell–substrate adhesion, integrin expression and/or protease activity.
One candidate for mediating these changes is fibronectin. Fibronectin is an adhesive protein secreted by cells that facilitates binding to other ECM proteins (including fibrin),67–69 and it is expressed by corneal fibroblasts during clustering on top of fibrin ECM.16 Fibronectin synthesis has been shown to contribute to endothelial cell network formation and tubulogenesis in vitro.41,70 Furthermore, blocking fibronectin secretion or interfering with the integrin protein α5β1 prevents contraction-induced cluster formation on compliant collagen matrices.20 Fibronectin can also have a role in mediating cell–cell adhesion, by serving as a bridge for integrin–integrin interactions between cells.18 For example, α5β1 integrin binds to fibronectin along interfaces between ovarian carcinoma cells71 or fibroblasts,72 and blocking of β1-integrin function in migrating multicellular melanoma clusters leads to loss of cell-cell cohesion, cell detachment and transition to single-cell migration.17 Future studies are needed to investigate the possible roles of fibronectin and α5β1 integrin in mediating collective cell migration through fibrin ECM.
Acknowledgments
Supported in part by National Institutes of Health (NIH; Bethesda, MD, USA) Grants R01 EY 013322, NIH P30 EY020799, and an unrestricted grant from Research to Prevent Blindness, Inc., New York, New York, United States.
Disclosure: M. Miron-Mendoza, None; E. Graham, None; P. Kivanany, None; J. Quiring, None; W.M. Petroll, None
References
- 1. Hassell JR, Birk DE. The molecular basis of corneal transparency. Exp Eye Res. 2010; 91: 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chakravarti S, Petroll WM, Hassell J, et al. Corneal opacity in lumican-null mice: defects in collagen fibril structure and packing in the posterior stroma. Invest Ophthalmol Vis Sci. 2000; 41: 3365–3373. [PMC free article] [PubMed] [Google Scholar]
- 3. Funderburgh JL, Mann MM, Funderburgh ML. Keratocyte phenotype mediates proteoglycan structure: a role for fibroblasts in corneal fibrosis. J Biol Chem. 2003; 278: 45629–45637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jester JV, Barry PA, Lind GJ, Petroll WM, Garana R, Cavanagh HD. Corneal keratocytes: in situ and in vitro organization of cytoskeletal contractile proteins. Invest Ophthalmol Vis Sci. 1994; 35: 730–743. [PubMed] [Google Scholar]
- 5. Lakshman N, Kim A, Petroll WM. Characterization of corneal keratocyte morphology and mechanical activity within 3-D collagen matrices. Exp Eye Res. 2010; 90: 350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: the role of the myofibroblast. Prog Retinal Eye Res. 1999; 18: 311–356. [DOI] [PubMed] [Google Scholar]
- 7. Stramer BM, Zieske JD, Jung J-C, Austin JS, Fini ME. Molecular mechanisms controlling the fibrotic repair phenotype in cornea: implications for surgical outcomes. Invest Ophthalmol Vis Sci. 2003; 44: 4237–4246. [DOI] [PubMed] [Google Scholar]
- 8. Jester JV, Huang J, Barry-Lane PA, Kao WW, Petroll WM, Cavanagh HD. Transforming growth factor(beta)-mediated corneal myofibroblast differentiation requires actin and fibronectin assembly. Invest Ophthalmol Vis Sci. 1999; 40: 1959–1967. [PubMed] [Google Scholar]
- 9. Blalock TD, Duncan MR, Varela JC, et al. Connective tissue growth factor expression and action in human corneal fibroblast cultures and rat corneas after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2003; 44: 1879–1887. [DOI] [PubMed] [Google Scholar]
- 10. Moller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Stromal wound healing explains refractive instability and haze development after photorefractive keratectomy: a 1-year confocal microscopic study. Ophthalmology. 2000; 107: 1235–1245. [DOI] [PubMed] [Google Scholar]
- 11. Dupps WJ, Wilson SE. Biomechanics and wound healing in the cornea. Exp Eye Res. 2006; 83: 709–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruberti JW, Roy AS, Roberts CJ. Corneal biomechanics and biomaterials. Annu Rev Biomed Eng. 2011; 13: 269–295. [DOI] [PubMed] [Google Scholar]
- 13. Tomasek JJ, Hay ED, Fujiwara K. Collagen modulates cell shape and cytoskeleton of embryonic corneal and fibroma fibroblasts: distribution of actin, a-actinin and myosin. Dev Biol. 1982; 92: 107–122. [DOI] [PubMed] [Google Scholar]
- 14. Cukierman E, Pankov R, Yamada KM. Cell interactions with three-dimensional matrices. Curr Opin Cell Biol. 2002; 14: 633–639. [DOI] [PubMed] [Google Scholar]
- 15. Grinnell F, Petroll WM. Cell motility and mechanics in three-dimensional collagen matrices. Annu Rev Cell Dev Biol. 2010; 26: 335–361. [DOI] [PubMed] [Google Scholar]
- 16. Miron-Mendoza M, Lin X, Ma L, Ririe P, Petroll WM. Individual versus collective fibroblast spreading and migration: regulation by matrix composition in 3D culture. Exp Eye Res. 2012; 99: 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hegerfeldt Y, Tusch M, Brocker E-B, Friedl P. Collective cell movement in primary melanoma explants: plasticity of cell-cell interaction, b1-integrin function, and migration strategies. Cancer Res. 2002; 62: 2125–2130. [PubMed] [Google Scholar]
- 18. Ilina O, Friedl P. Mechanisms of collective cell migration at a glance. J Cell Sci. 2009; 122: 3203–3208. [DOI] [PubMed] [Google Scholar]
- 19. Friedl P, Zallen JA. Dynamics of cell-cell and cell-matrix interactions in morphogenesis, regeneration and cancer. Curr Opin Cell Biol. 2010; 22: 557–559. [DOI] [PubMed] [Google Scholar]
- 20. da Rocha-Azevedo B, Ho C-H, Grinnell F. Fibroblast cluster formation on 3D collagen matrices requires cell contraction dependent fibronectin matrix organization. Exp Cell Res. 2013; 319: 546–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rhee S, Ho CH, Grinnell F. Promigratory and procontractile growth factor environments differentially regulate cell morphogenesis. Exp Cell Res. 2010; 316: 232–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Satpathy M, Gallagher P, Lizotte-Waniewski M, Srinivas SP. Thrombin-induced phosphorylation of the regulatory light chain of myosin II in cultured bovine corneal endothelial cells. Exp Eye Res. 2004; 79: 477–486. [DOI] [PubMed] [Google Scholar]
- 23. Kolodney MS, Wysolmerski RB. Isometric contraction by fibroblasts and endothelial cells in tissue culture. J Cell Biol. 1992; 117: 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Essler M, Amano M, Kruse HJ, Kaibuchi K, Weber PC, Aepfelbacher M. Thrombin inactivates myosin light chain phosphatase via Rho and its target Rho kinase in human endothelial cells. J Biol Chem. 1998; 273: 21867–21874. [DOI] [PubMed] [Google Scholar]
- 25. Bogatkevich GS, Tourkina E, Silver RM, Ludwicka-Bradley A. Thrombin differentiates normal lung fibroblasts to a myofibroblast phenotype via the proteolytically activated receptor-1 and a protein kinase C-dependent pathway. J Biol Chem. 2001; 276: 45184–45192. [DOI] [PubMed] [Google Scholar]
- 26. Fitzgibbon J, Morrison JJ, Smith TJ, O'Brien M. Modulation of human uterine smooth muscle cell collagen contractility by thrombin, Y-27632, TNF alpha and indomethacin. Reprod Biol Endocrinol. 2009; 7: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ayala A, Warejcka DJ, Olague-Marchan M, Twining SS. Corneal activation of prothrombin to form thrombin, independent of vascular injury. Invest Ophthalmol Vis Sci. 2007; 48: 134–143. [DOI] [PubMed] [Google Scholar]
- 28. Jester JV, Huang J, Fisher S, et al. Myofibroblast differentiation of normal human keratocytes and hTERT, extended-life, human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2003; 44: 1850–1858. [DOI] [PubMed] [Google Scholar]
- 29. Petroll WM, Ma L, Kim A, Ly L, Vishwanath M. Dynamic assessment of fibroblast mechanical activity during Rac-induced cell spreading in 3-D culture. J Cell Physiol. 2008; 217: 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lakshman N, Petroll WM. Growth factor regulation of corneal keratocyte mechanical phenotypes in 3-D collagen matrices. Invest Ophthalmol Vis Sci. 2012; 53: 1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jester JV, Chang J-H. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp Eye Res. 2003; 77: 581–592. [DOI] [PubMed] [Google Scholar]
- 32. Kim A, Lakshman N, Karamichos D, Petroll WM. Growth factor regulation of corneal keratocyte differentiation and migration in compressed collagen matrices. Invest Ophthalmol Vis Sci. 2010; 51: 864–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grinnell F. Fibroblast-collagen matrix contraction: growth-factor signalling and mechanical loading. Trends Cell Biol. 2000; 10: 362–365. [DOI] [PubMed] [Google Scholar]
- 34. Pope MD, Graham NA, Huang BK, Asthagiri AR. Automated quantitative analysis of epithelial cell scatter. Cell Adh Migr. 2008; 2: 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Karamichos D, Lakshman N, Petroll WM. An experimental model for assessing fibroblast migration in 3-D collagen matrices. Cell Motil Cytoskel. 2009; 66: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brown RA, Wiseman M, Chuo C-B, Cheema U, Nazhat SN. Ultrarapid engineering of biomimetic materials and tissues: fabrication of nano- and microstructures by plastic compression. Adv Funct Mater. 2005; 15: 1762–1770. [Google Scholar]
- 37. Neel EAA, Cheema U, Knowles JC, Brown RA, Nazhat SN. Use of multiple unconfined compression for control of collagen gel scaffold density and mechanical properties. Soft Matter. 2006; 2: 986–992. [DOI] [PubMed] [Google Scholar]
- 38. Kim A, Zhou C, Lakshman N, Petroll WM. Corneal stromal cells use both high- and low-contractility migration mechanisms in 3-D collagen matrices. Exp Cell Res. 2012; 318: 741–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhou C, Petroll WM. Rho kinase regulation of fibroblast migratory mechanics in fibrillar collagen matrices. Cell Mol Bioeng. 2010; 3: 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhou C, Petroll WM. MMP regulation of corneal keratocyte motility and mechanics in 3-D collagen matrices. Exp Eye Res. 2014; 121: 147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Reinhart-King CA. How matrix properties control the self-assembly and maintenance of tissues. Ann Biomed Eng. 2011; 39: 1849–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Guo W-H, Frey MT, Burnham NA, Wang Y-L. Substrate rigidity regulates the formation and maintenance of tissues. Biophys J. 2006; 90: 2213–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000; 407: 258–264. [DOI] [PubMed] [Google Scholar]
- 44. Gudermann T, Steinritz D. STIMulating stress fibers in endothelial cells. Sci Signal. 2013; 6: pe8. [DOI] [PubMed] [Google Scholar]
- 45. Gadepalli R, Kotla S, Heckle MR, Verma SK, Singh NK, Rao GN. Novel role for p21-activated kinase 2 in thrombin-induced monocyte migration. J Biol Chem. 2013; 288: 30815–30831. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46. Zigler M, Kamiya T, Brantley EC, Villares GJ, Bar-Eli M. PAR-1 and thrombin: the ties that bind the microenvironment to melanoma metastasis. Cancer Res. 2011; 71: 6561–6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pilcher BK, Kim DW, Carney DH, Tomasek JJ. Thrombin stimulates fibroblast-mediated collagen lattice contraction by its proteolytically activated receptor. Exp Cell Res. 1994; 211: 368–373. [DOI] [PubMed] [Google Scholar]
- 48. Kimura K, Ito M, Amano M, et al. Regulation of mysoin phosphatase by rho and rho-associated kinase (Rho-kinase). Science. 1996; 273: 245–248. [DOI] [PubMed] [Google Scholar]
- 49. Chrzanowska-Wodnicka C, Burridge K. Tyrosine phosphorylation is involved in reorganization of the actin cytoskeleton in response to serum or LPA stimulation. J Cell Sci. 1994; 107: 3643–3564. [DOI] [PubMed] [Google Scholar]
- 50. Parizi M, Howard EW, Tomasek JJ. Regulation of LPA-promoted myofibroblast contraction: role of rho, myosin light chain kinase, and myosin light chain phosphatase. Exp Cell Res. 2000; 254: 210–220. [DOI] [PubMed] [Google Scholar]
- 51. Amano M, Ito M, Kimura K, et al. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem. 1996; 271: 20246–20249. [DOI] [PubMed] [Google Scholar]
- 52. Amano M, Chihara K, Nakamura N, et al. Myosin II activation promotes neurite retraction during the action of Rho and Rho-kinase. Genes Cells. 1998; 3: 177–188. [DOI] [PubMed] [Google Scholar]
- 53. Tomasek JJ, McConathy WJ, Checovich WJ, Mosher DF. Lipoproteins promote fibroblast-mediated collagen lattice contraction. Mol Biol Cell. 1992; 3: 234A. [Google Scholar]
- 54. Sanderson PL, Morris AM, Stanley JK, Fahmy NRM. Lipids and Dupuytren's disease. J Bone Joint Surg. 1992; 74B: 923–927. [DOI] [PubMed] [Google Scholar]
- 55. Kolodney MS, Elson EL. Correlation of myosin light chain phosphorylation with isometric contraction of fibroblasts. J Biol Chem. 1993; 268: 23850–23855. [PubMed] [Google Scholar]
- 56. Rayan GM, Parizi M, Tomasek JJ. Pharmacologic regulation of Dupuytren's fibroblast contraction in vitro. J Hand Surg. 1996; 21: 1065–1070. [DOI] [PubMed] [Google Scholar]
- 57. Grinnell F, Ho C-H, Tamariz E, Lee DJ, Skuta G. Dendritic fibroblasts in three-dimensional collagen matrices. Mol Biol Cell. 2003; 14: 384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Netto MV, Mohan RR, Ambrosio R Jr, Hutcheon AE, Zieske JD, Wilson SE. Wound healing in the cornea: a review of refractive surgery complications and new prospects for therapy. Cornea. 2005; 24: 509–522. [DOI] [PubMed] [Google Scholar]
- 59. Friedl P, Wolf K. Tube travel: the role of proteases in individual and collective cancer cell invasion. Cancer Res. 2008; 68: 7247–7249. [DOI] [PubMed] [Google Scholar]
- 60. Jester JV, Petroll WM, Barry PA, Cavanagh HD. Temporal, 3-dimensional, cellular anatomy of corneal wound tissue. J Anat. 1995; 186: 301–311. [PMC free article] [PubMed] [Google Scholar]
- 61. Petroll WM, Cavanagh HD, Barry P, Andrews P, Jester JV. Quantitative analysis of stress fiber orientation during corneal wound contraction. J Cell Sci. 1993; 104: 353–363. [DOI] [PubMed] [Google Scholar]
- 62. Friedl P, Wolf K. Plasticity of cell migration: a multiscale tuning model. J Cell Biol. 2009; 199: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Teddy JM, Kulesa PM. In vivo evidence for short-and long-range cell communication in cranial neural crest cells. Development. 2004; 131: 6141–6151. [DOI] [PubMed] [Google Scholar]
- 64. Kasemeier-Kulesa JC, Kulesa PM, Lefcort F. Imaging neural crest cell dynamics during formation of dorsal root ganglia and sympathetic ganglia. Development. 2005; 132: 235–245. [DOI] [PubMed] [Google Scholar]
- 65. Davis EM, Trinkaus JP. Significance of cell-to-cell contacts or the directional movement of neural crest cells within a hydrate collagen lattice. J Embryol Exp Morph. 1981; 63: 29–51. [PubMed] [Google Scholar]
- 66. Petroll WM, Miron-Mendoza M. Mechanical interactions and crosstalk between corneal keratocytes and the extracellular matrix. Exp Eye Res. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Singh P, Carraher C, Schwarzbauer JE. Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol. 2010; 26: 397–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Grinnell F, Feld M, Minter D. Fibroblast adhesion to fibronectin and fibrin substrata: requirement for cold-insoluble globulin (plasma fibronectin). Cell. 1980; 19: 517–525. [DOI] [PubMed] [Google Scholar]
- 69. Greiling D, Clark RA. Fibronectin provides a conduit for fibroblast transmigration from collagenous stroma into fibrin clot provisional matrix. J Cell Sci. 1997; 100: 861–870. [DOI] [PubMed] [Google Scholar]
- 70. Zhou X, Rowe RG, Hiraoke N, et al. Fibronectin fibrillogenesis regulates three-dimensional neovessel formation. Gene Dev. 2008; 22: 1231–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Casey RC, Burleson KM, Skubitz KM, et al. Beta 1-integrins regulate the formation and adhesion of ovarian carcinoma multicellular spheroids. Am J Pathol. 2001; 159: 2071–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Salmenpera P, Kankuri E, Bizik J, et al. Formation and activation of fibroblast spheroids depend on fibronectin-integrin interaction. Exp Cell Res. 2008; 314: 3444–3452. [DOI] [PubMed] [Google Scholar]